Magnetic Nanoparticle-Based Nano-Packaging and Nano-Freezing in Food Storage Applications
Abstract
1. Introduction
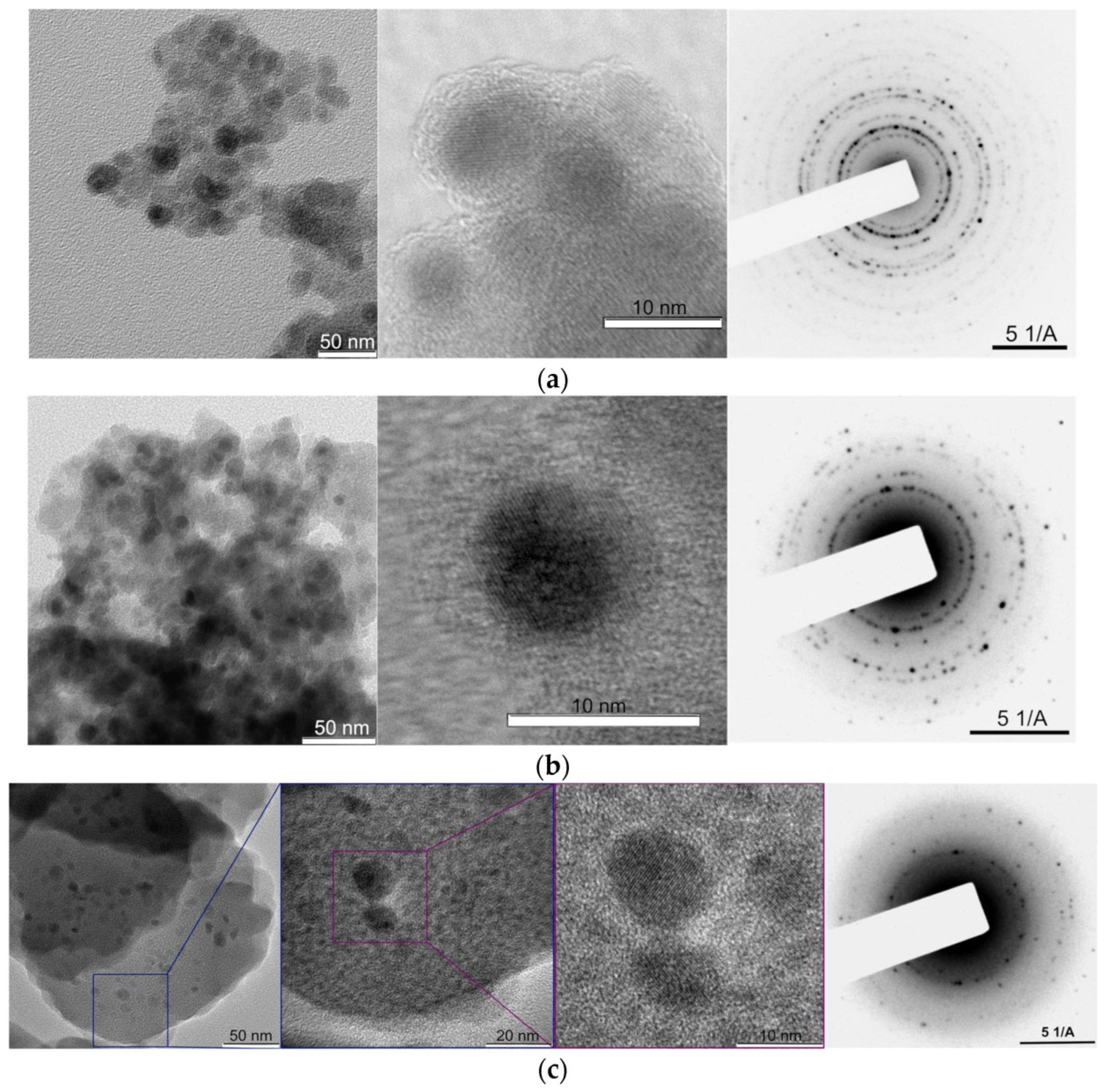
2. Fundamental Properties and Synthesis of Food-Grade MNPs
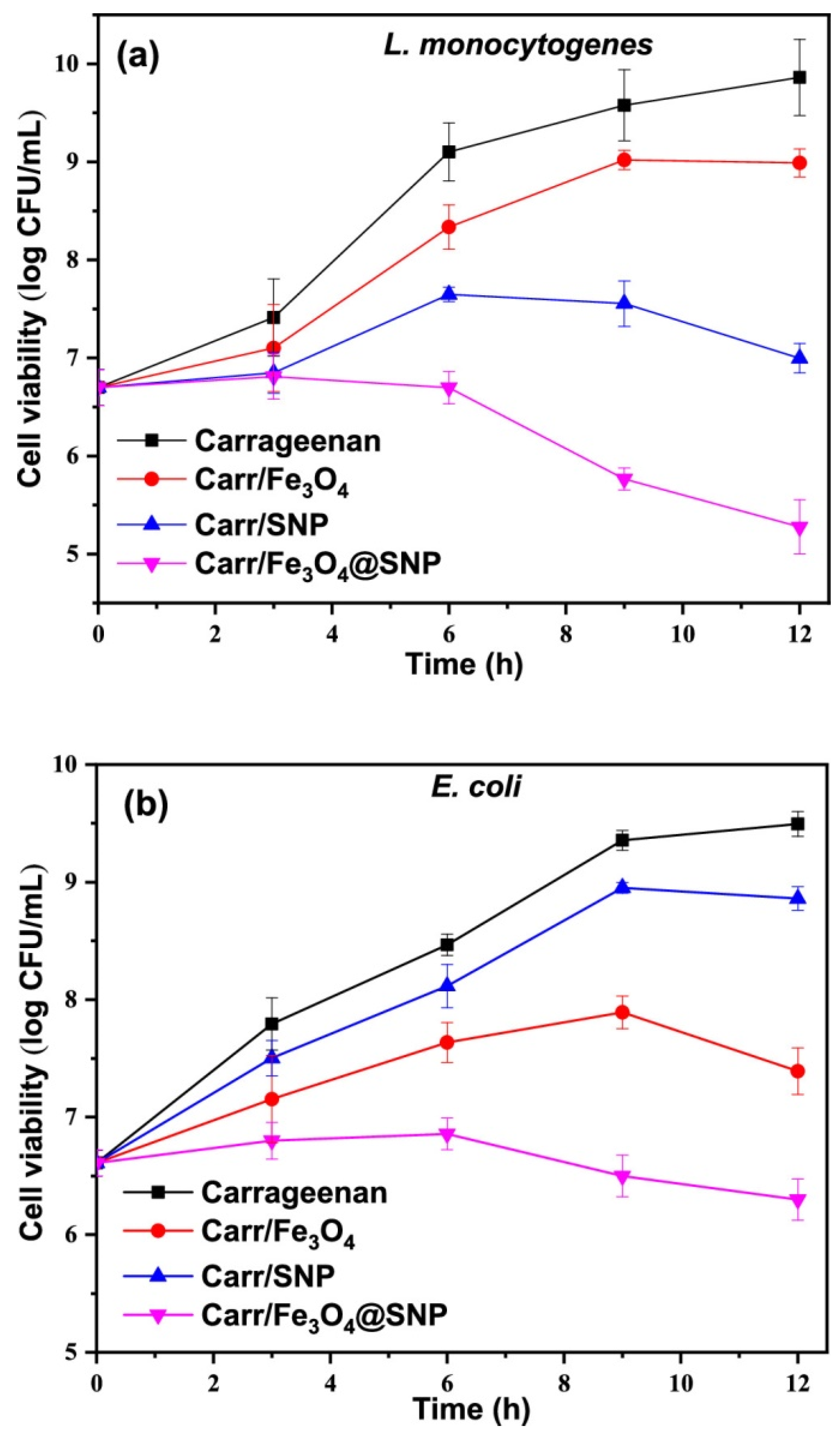
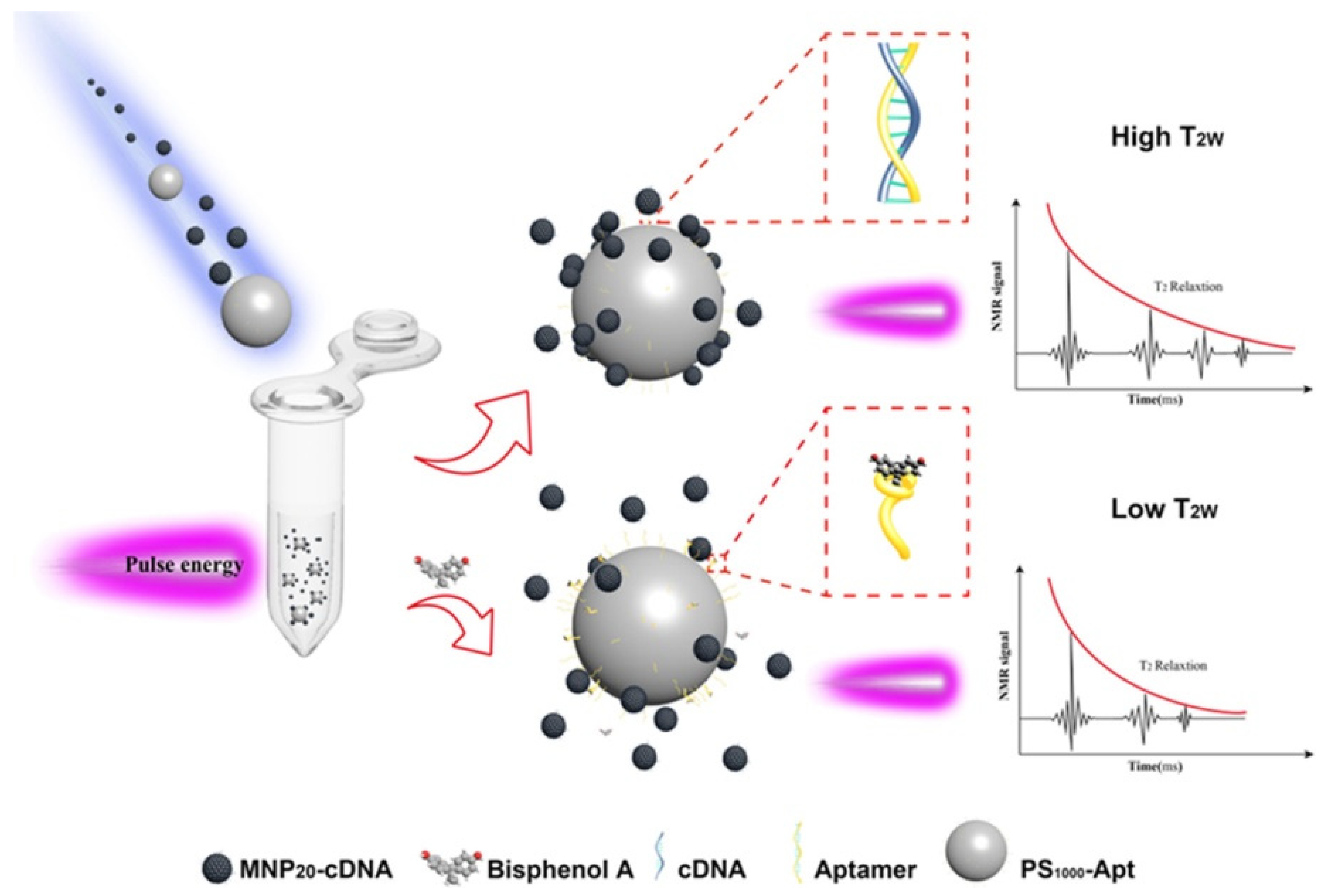
3. Nano-Packaging as Active and Intelligent Systems
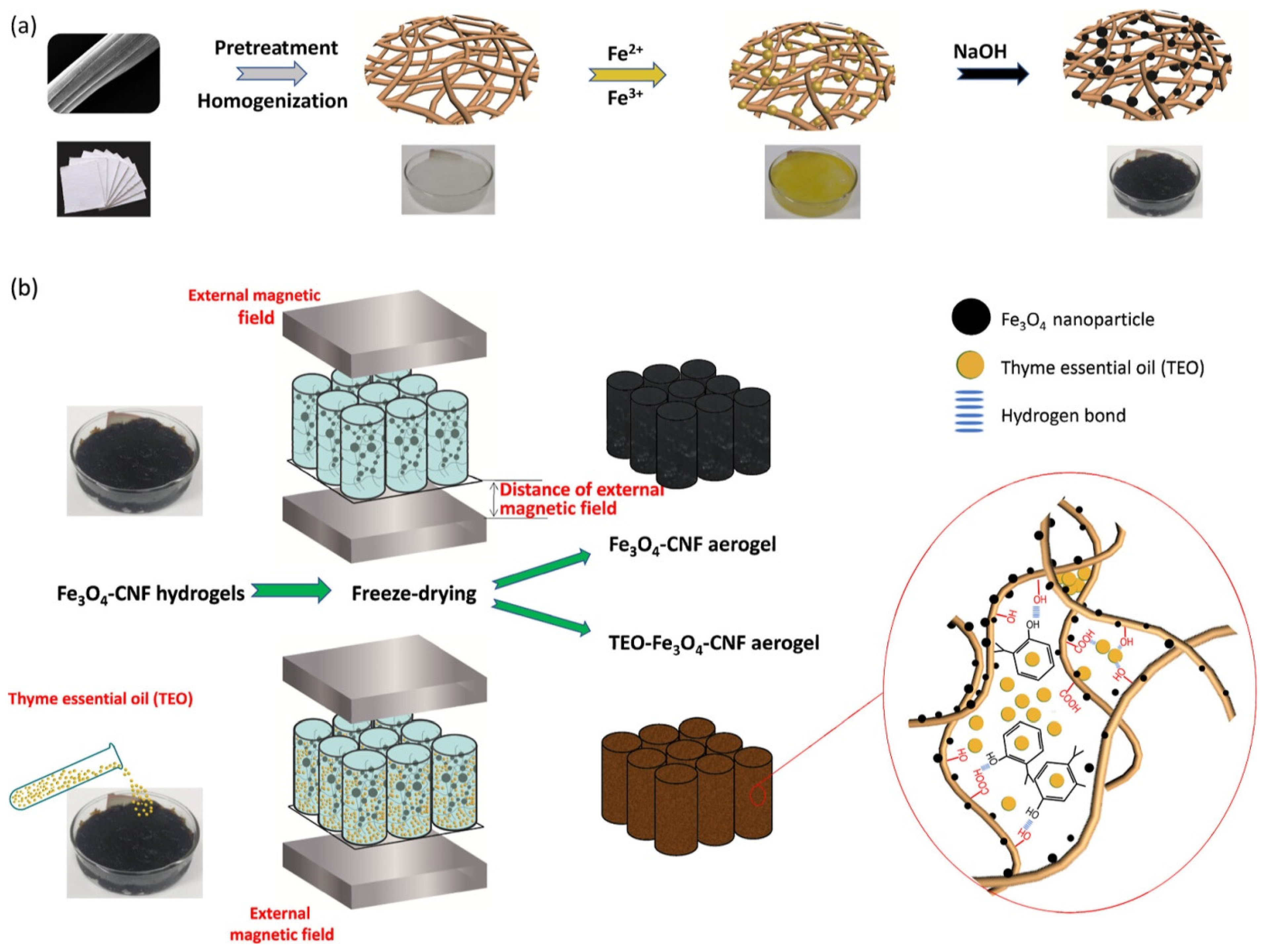
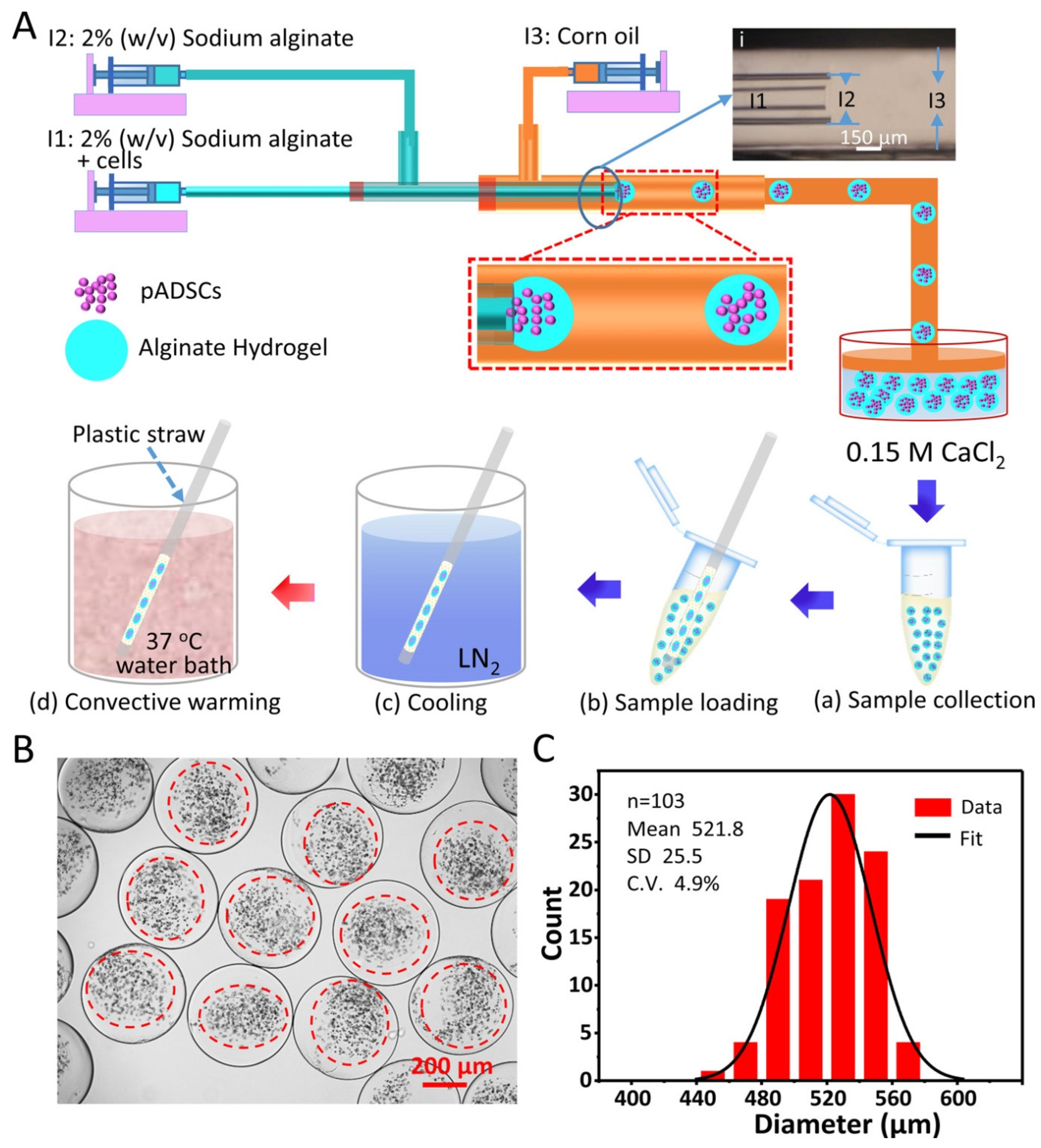
4. Nano-Freezing Technologies
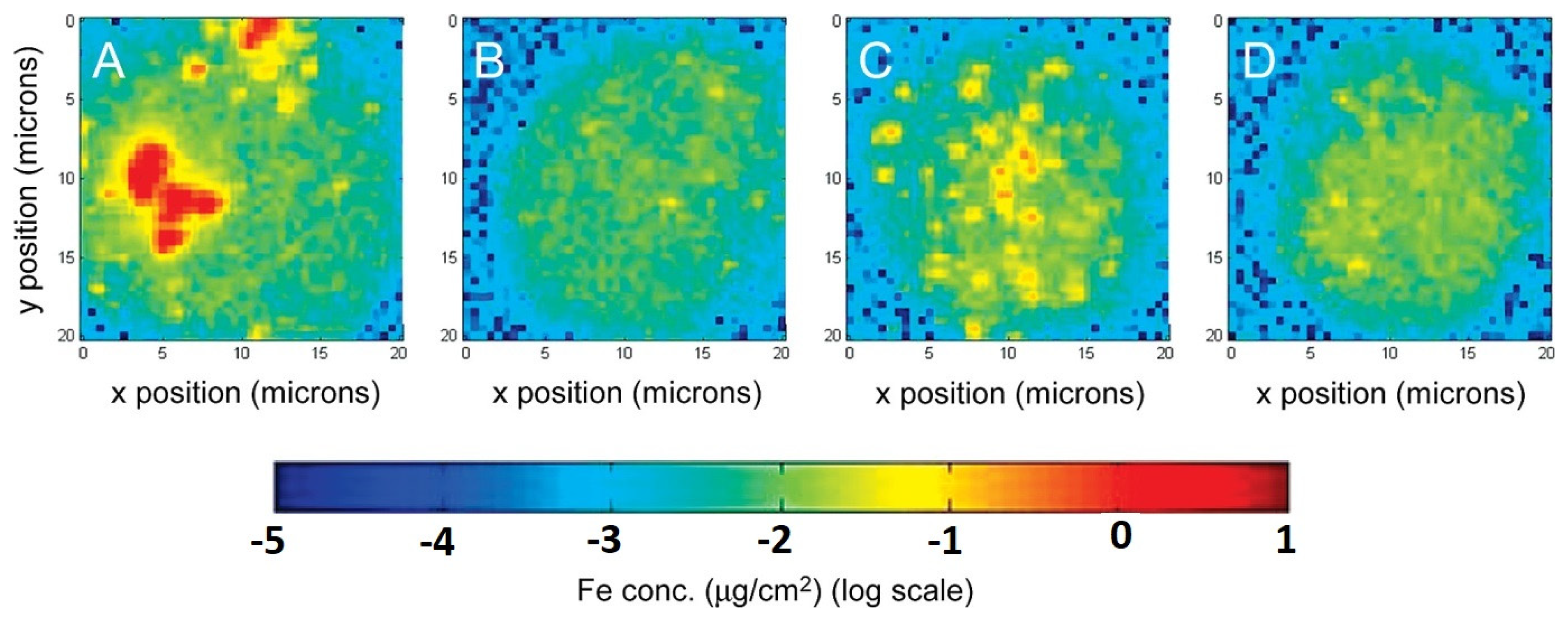
5. Toxicological Considerations of MNPs in Food Applications
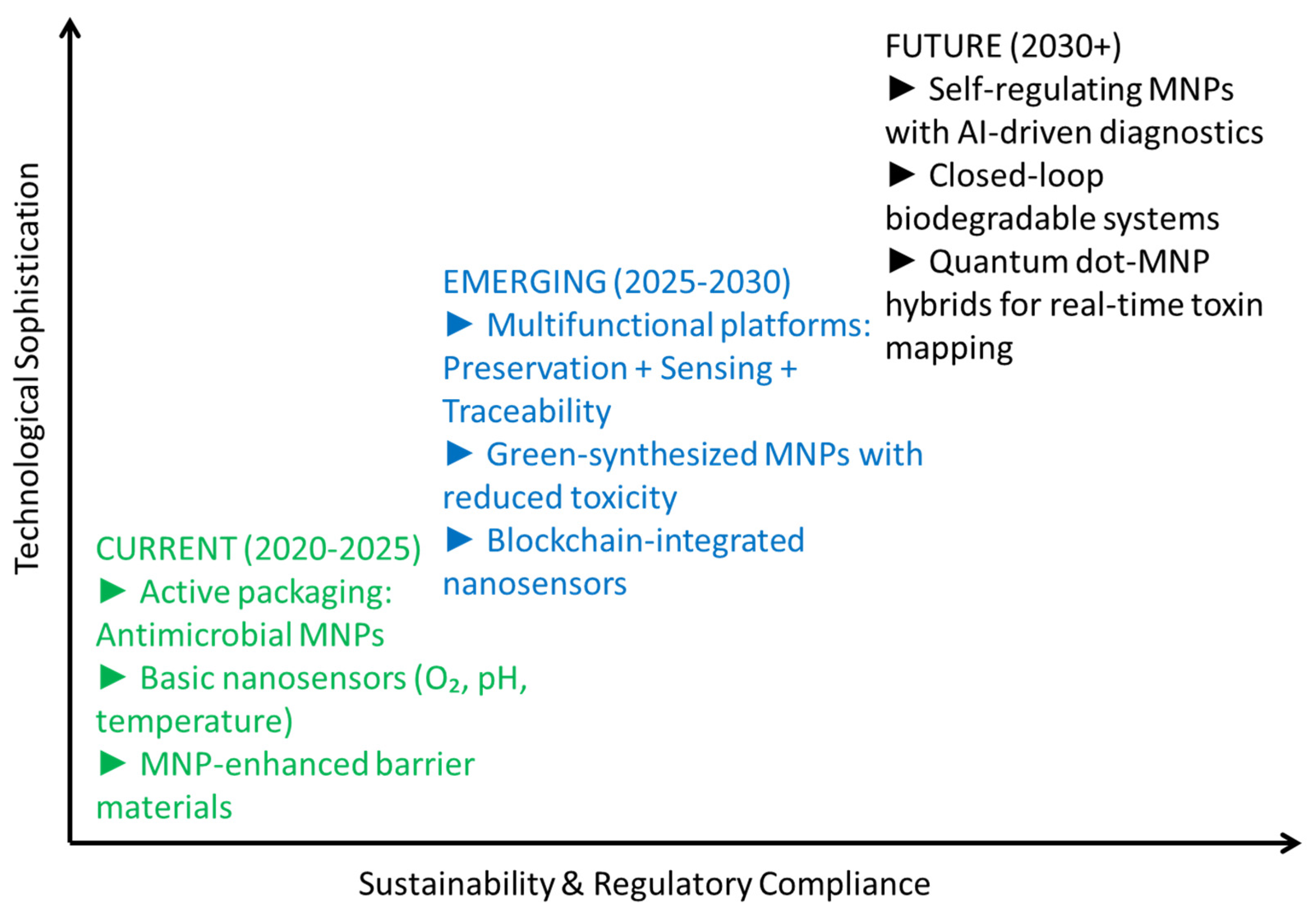
6. Summary and Future Outlook
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vesković, S. In the Global Food System: Addressing Food Losses, Waste, and Safety. In Natural Food Preservation: Controlling Loss, Advancing Safety; Springer: Berlin/Heidelberg, Germany, 2025; pp. 5–58. [Google Scholar]
- Clapp, J. The problem with growing corporate concentration and power in the global food system. Nat. Food 2021, 2, 404–408. [Google Scholar] [CrossRef]
- Myers, S.S.; Smith, M.R.; Guth, S.; Golden, C.D.; Vaitla, B.; Mueller, N.D.; Dangour, A.D.; Huybers, P. Climate change and global food systems: Potential impacts on food security and undernutrition. Annu. Rev. Public Health 2017, 38, 259–277. [Google Scholar] [CrossRef] [PubMed]
- Seekell, D.; Carr, J.; Dell’Angelo, J.; D’Odorico, P.; Fader, M.; Gephart, J.; Kummu, M.; Magliocca, N.; Porkka, M.; Puma, M. Resilience in the global food system. Environ. Res. Lett. 2017, 12, 025010. [Google Scholar] [CrossRef]
- Mehmet, O.; Yorucu, V. Human Damage to the Global Ecosystem. In From Land Disputes to Sustainable Environmental Development: A Near East Perspective; Springer: Berlin/Heidelberg, Germany, 2024; pp. 151–165. [Google Scholar]
- Qu, B.; Shao, G.; Yang, N.; Pan, K.; Xiao, Z.; Luo, Y. Revolutionizing food sustainability: Leveraging magnetic fields for food processing and preservation. Trends Food Sci. Technol. 2024, 150, 104593. [Google Scholar] [CrossRef]
- Landry, J.D.; Blanch, E.W.; Torley, P.J. Chemical indicators of atlantic salmon quality. Food Rev. Int. 2024, 40, 1426–1456. [Google Scholar] [CrossRef]
- Ganguly, S.; Margel, S. Surface-Engineered Magnetic Nanoparticles (Iron Oxides) and Their Therapeutic Applications. In Magnetic Polymer Composites and Their Emerging Applications; CRC Press: Boca Raton, FL, USA, 2024; pp. 258–288. [Google Scholar]
- Tang, Y.; Zhang, T.; Li, Y.; Wang, Q.; Zhao, W.; Nadeem, M.; Zhang, P.; Rui, Y. Magnetic Nanoparticles in Agriculture: Unraveling the Impact of Nickel Ferrite Nanoparticles on Peanut Growth and Seed Nutritional Quality. Plants 2025, 14, 1011. [Google Scholar] [CrossRef]
- Atanda, S.A.; Shaibu, R.O.; Agunbiade, F.O. Nanoparticles in agriculture: Balancing food security and environmental sustainability. Discov. Agric. 2025, 3, 26. [Google Scholar] [CrossRef]
- Althawab, S.A.; Alsulami, T.; Alzahrani, H.; Alzahrani, A. Doped carbon dots reinforced flexible nanocomposite active polymer film for antibacterial and antioxidant activities. Colloids Surf. A Physicochem. Eng. Asp. 2024, 698, 134554. [Google Scholar] [CrossRef]
- Alzahrani, A. Fluorescent carbon dots in situ polymerized biodegradable semi-interpenetrating tough hydrogel films with antioxidant and antibacterial activity for applications in food industry. Food Chem. 2024, 447, 138905. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Ahmed, S.R.; Sherazee, M.; Margel, S.; Gedanken, A.; Srinivasan, S.; Rajabzadeh, A.R. Carbon Dot Biopolymer-Based Flexible Functional Films for Antioxidant and Food Monitoring Applications. ACS Appl. Polym. Mater. 2022, 4, 9323–9340. [Google Scholar] [CrossRef]
- Das, P.; Sherazee, M.; Marvi, P.K.; Ahmed, S.R.; Gedanken, A.; Srinivasan, S.; Rajabzadeh, A.R. Waste-Derived Sustainable Fluorescent Nanocarbon-Coated Breathable Functional Fabric for Antioxidant and Antimicrobial Applications. ACS Appl. Mater. Interfaces 2023, 15, 29425–29439. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Mondal, S.; Bose, M.; Das, A.K.; Banerjee, S.; Das, N.C. Heteroatom doped photoluminescent carbon dots for sensitive detection of acetone in human fluids. Sens. Actuators B Chem. 2018, 266, 583–593. [Google Scholar] [CrossRef]
- Sakthivel, S.; Muthusamy, K.; Thangarajan, A.P.; Thiruvengadam, M.; Venkidasamy, B. Nano-based biofuel production from low-cost lignocellulose biomass: Environmental sustainability and economic approach. Bioprocess Biosyst. Eng. 2024, 47, 971–990. [Google Scholar] [CrossRef]
- Ganguly, S.; Margel, S. Bioimaging probes based on magneto-fluorescent nanoparticles. Pharmaceutics 2023, 15, 686. [Google Scholar] [CrossRef]
- Ganguly, S.; Margel, S. Magnetic polymeric conduits in biomedical applications. Micromachines 2025, 16, 174. [Google Scholar] [CrossRef]
- Escribá, P.V.; González-Ros, J.M.; Goñi, F.M.; Kinnunen, P.K.; Vigh, L.; Sánchez-Magraner, L.; Fernández, A.M.; Busquets, X.; Horváth, I.; Barceló-Coblijn, G. Membranes: A meeting point for lipids, proteins and therapies. J. Cell. Mol. Med. 2008, 12, 829–875. [Google Scholar] [CrossRef] [PubMed]
- Contreras, F.-X.; Sánchez-Magraner, L.; Alonso, A.; Goñi, F.M. Transbilayer (flip-flop) lipid motion and lipid scrambling in membranes. FEBS Lett. 2010, 584, 1779–1786. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Magraner, L.; Gumuzio, J.; Miles, J.; Quimi, N.; Martínez del Prado, P.; Abad-Villar, M.T.; Pikabea, F.; Ortega, L.; Etxezarraga, C.; Martín-Algarra, S. Functional engagement of the PD-1/PD-L1 complex but not PD-L1 expression is highly predictive of patient response to immunotherapy in non–small-cell lung cancer. J. Clin. Oncol. 2023, 41, 2561–2570. [Google Scholar] [CrossRef]
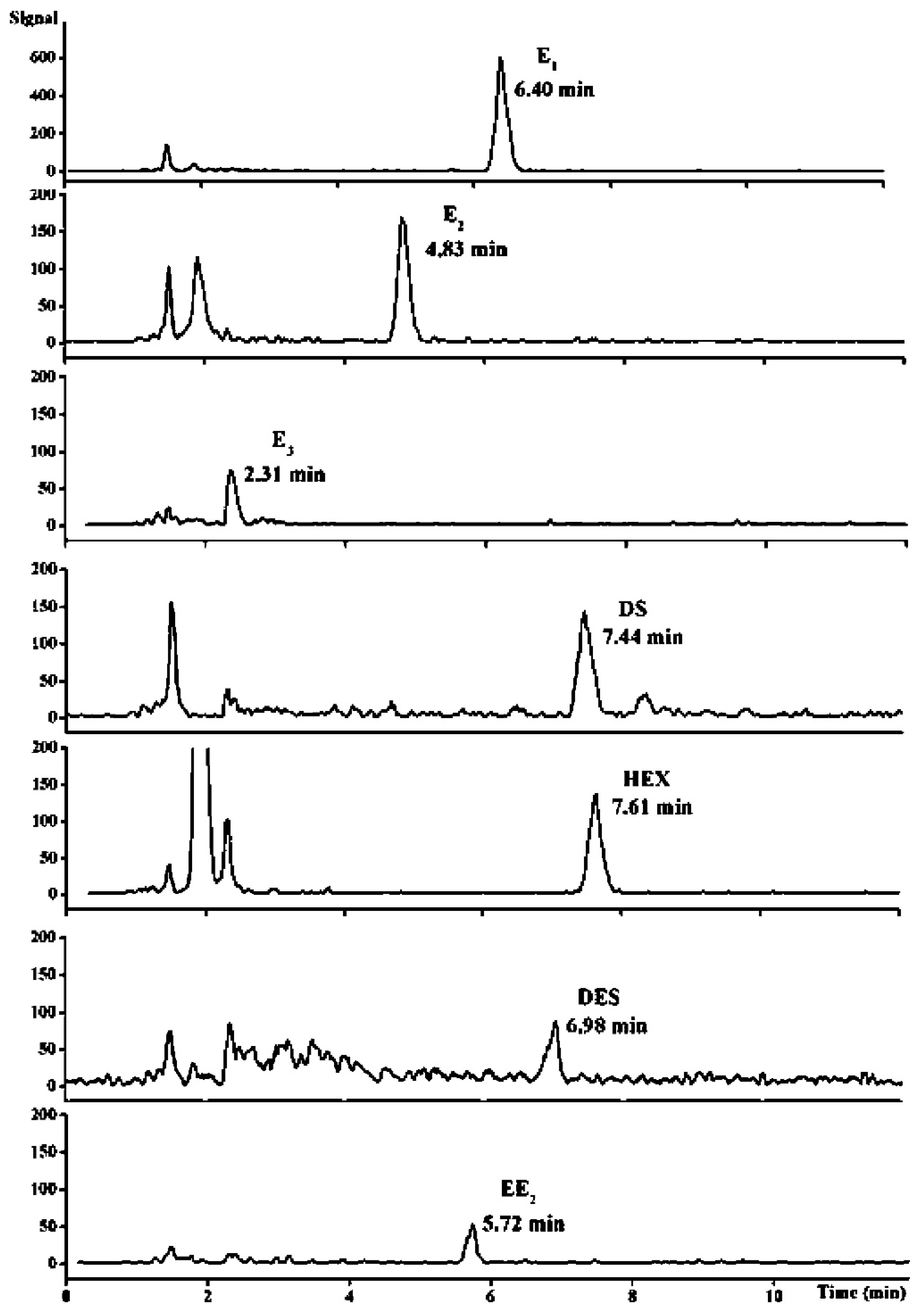
- Socas-Rodríguez, B.; Hernández-Borges, J.; Salazar, P.; Martín, M.; Rodríguez-Delgado, M.Á. Core–shell polydopamine magnetic nanoparticles as sorbent in micro-dispersive solid-phase extraction for the determination of estrogenic compounds in water samples prior to high-performance liquid chromatography–mass spectrometry analysis. J. Chromatogr. A 2015, 1397, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, V.C.; Freitas, M.; Pacheco, J.G.; Domingues, V.F.; Delerue-Matos, C. Evaluation of the QuEChERS and magnetic micro dispersive solid-phase extraction of brominated flame retardants in red fruits with determination by GC/MS. Food Chem. 2020, 309, 125572. [Google Scholar] [CrossRef]
- Velloso, C.C.V.; Torres, J.A.; Ribeiro, C. Nanotechnology for Mitigating Biological Cross-Contamination in Meat Processing. J. Food Sci. 2025, 90, e70291. [Google Scholar] [CrossRef]
- Capriotti, A.L.; Cavaliere, C.; La Barbera, G.; Montone, C.M.; Piovesana, S.; Laganà, A. Recent applications of magnetic solid-phase extraction for sample preparation. Chromatographia 2019, 82, 1251–1274. [Google Scholar] [CrossRef]
- Yang, Y.; Sheng, L.; Hang, X.; Wang, J.; Kou, G.; Ye, Y.; Ji, J.; Sun, X. Efficient Expression and Activity Optimization of Manganese Peroxidase for the Simultaneous Degradation of Aflatoxins AFB1, AFB2, AFG1, and AFG2. J. Agric. Food Chem. 2025, 73, 1608–1618. [Google Scholar] [CrossRef]
- Karimi, M.; Aboufazeli, F.; Zhad, H.R.L.Z.; Sadeghi, O.; Najafi, E. Determination of Sulfonamides in Chicken Meat by Magnetic Molecularly Imprinted Polymer Coupled to HPLC-UV. Food Anal. Methods 2014, 7, 73–80. [Google Scholar] [CrossRef]
- Vikal, S.; Gautam, Y.K.; Kumar, A.; Kumar, A.; Singh, J.; Pratap, D.; Singh, B.P.; Singh, N. Bioinspired palladium-doped manganese oxide nanocorns: A remarkable antimicrobial agent targeting phyto/animal pathogens. Sci. Rep. 2023, 13, 14039. [Google Scholar] [CrossRef]
- Liu, E.; Zhang, M.; Cui, H.; Gong, J.; Huang, Y.; Wang, J.; Cui, Y.; Dong, W.; Sun, L.; He, H. Tat-functionalized Ag-Fe3O4 nano-composites as tissue-penetrating vehicles for tumor magnetic targeting and drug delivery. Acta Pharm. Sin. B 2018, 8, 956–968. [Google Scholar] [CrossRef]
- Gloag, L.; Mehdipour, M.; Chen, D.; Tilley, R.D.; Gooding, J.J. Advances in the application of magnetic nanoparticles for sensing. Adv. Mater. 2019, 31, 1904385. [Google Scholar] [CrossRef]
- Kulkarni, M.B.; Enaganti, P.K.; Amreen, K.; Goel, S. Internet of Things enabled portable thermal management system with microfluidic platform to synthesize MnO2 nanoparticles for electrochemical sensing. Nanotechnology 2020, 31, 425504. [Google Scholar] [CrossRef] [PubMed]
- Weston, M.; Geng, S.; Chandrawati, R. Food sensors: Challenges and opportunities. Adv. Mater. Technol. 2021, 6, 2001242. [Google Scholar] [CrossRef]
- Pajic, V.; Andrejic, M.; Chatterjee, P. Enhancing cold chain logistics: A framework for advanced temperature monitoring in transportation and storage. Mechatron. Intell. Transp. Syst. 2024, 3, 16–30. [Google Scholar]
- Wang, J.; Liang, X.; Hou, H.; Deng, J.; Lin, Q.; Li, W.; Bai, J. A review on migration behavior and safety regulation of inorganic nanoparticles in food packaging: Multi-scale mechanisms, risk assessment, and innovative strategies. Food Control 2025, 178, 111490. [Google Scholar] [CrossRef]
- Bushra, R.; Ahmad, M.; Alam, K.; Seidi, F.; Qurtulen; Shakeel, S.; Song, J.; Jin, Y.; Xiao, H. Recent advances in magnetic nanoparticles: Key applications, environmental insights, and future strategies. Sustain. Mater. Technol. 2024, 40, e00985. [Google Scholar] [CrossRef]
- Li, W.; Xiao, F.; Bai, X.; Xu, H. Magnetic nanoparticles for food hazard factors sensing: Synthesis, modification and application. Chem. Eng. J. 2023, 465, 142816. [Google Scholar] [CrossRef]
- Fikiin, K. Emerging and novel freezing processes. In Frozen Food Science and Technology; Blackwell Publishing: Hoboken, NJ, USA, 2008; pp. 101–123. [Google Scholar]
- Kumari, A.; Chauhan, A.K.; Tyagi, P. Isochoric freezing: An innovative and emerging technology for retention of food quality characteristics. J. Food Process. Preserv. 2022, 46, e16704. [Google Scholar] [CrossRef]
- Isachenko, V.; Isachenko, E.; Reinsberg, J.; Montag, M.; van der Ven, K.; Dorn, C.; Roesing, B.; van der Ven, H. Cryopreservation of human ovarian tissue: Comparison of rapid and conventional freezing. Cryobiology 2007, 55, 261–268. [Google Scholar] [CrossRef]
- Hu, R.; Zhang, M.; Liu, W.; Mujumdar, A.S.; Bai, B. Novel synergistic freezing methods and technologies for enhanced food product quality: A critical review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1979–2001. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Zhang, H. Controlled freezing and freeze drying: A versatile route for porous and micro-/nano-structured materials. J. Chem. Technol. Biotechnol. 2011, 86, 172–184. [Google Scholar] [CrossRef]
- Strom, C.S.; Liu, X.Y.; Jia, Z. Antifreeze protein-induced morphological modification mechanisms linked to ice binding surface. J. Biol. Chem. 2004, 279, 32407–32417. [Google Scholar] [CrossRef]
- Lee, D.; Tang, J.; Lee, S.H.; Jun, S. Effect of oscillating magnetic fields (OMFs) and pulsed electric fields (PEFs) on supercooling preservation of atlantic salmon (Salmo salar L.) fillets. Foods 2024, 13, 2525. [Google Scholar] [CrossRef] [PubMed]
- Kalhor, A.; Ghazavi, M.; Roustaei, M.; Mirhosseini, S. Influence of nano-SiO2 on geotechnical properties of fine soils subjected to freeze-thaw cycles. Cold Reg. Sci. Technol. 2019, 161, 129–136. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, S.; Wang, Q.; Shah, S.P. Effects of nano-kaolinite clay on the freeze–thaw resistance of concrete. Cem. Concr. Compos. 2015, 62, 1–12. [Google Scholar] [CrossRef]
- Rocha-Santos, T.A. Sensors and biosensors based on magnetic nanoparticles. TrAC Trends Anal. Chem. 2014, 62, 28–36. [Google Scholar] [CrossRef]
- Darton, N.J.; Ionescu, A.; Llandro, J. Magnetic Nanoparticles in Biosensing and Medicine; Cambridge University Press: Cambridge, UK, 2019. [Google Scholar]
- Tripathy, A.; Nine, M.J.; Silva, F.S. Biosensing platform on ferrite magnetic nanoparticles: Synthesis, functionalization, mechanism and applications. Adv. Colloid Interface Sci. 2021, 290, 102380. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Kamle, M.; Shukla, S.; Mahato, D.K.; Chandra, P.; Hwang, S.K.; Kumar, P.; Huh, Y.S.; Han, Y.-K. Prospects of using nanotechnology for food preservation, safety, and security. J. Food Drug Anal. 2018, 26, 1201–1214. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.; Neelam; Grinberg, I.; Margel, S. Layer by layer controlled synthesis at room temperature of tri-modal (MRI, fluorescence and CT) core/shell superparamagnetic IO/human serum albumin nanoparticles for diagnostic applications. Polym. Adv. Technol. 2021, 32, 3909–3921. [Google Scholar] [CrossRef]
- Ganguly, S.; Das, P.; Srinivasan, S.; Rajabzadeh, A.R.; Tang, X.S.; Margel, S. Superparamagnetic amine-functionalized maghemite nanoparticles as a thixotropy promoter for hydrogels and magnetic field-driven diffusion-controlled drug release. ACS Appl. Nano Mater. 2024, 7, 5272–5286. [Google Scholar] [CrossRef]
- Ganguly, S.; Margel, S. Design of magnetic hydrogels for hyperthermia and drug delivery. Polymers 2021, 13, 4259. [Google Scholar] [CrossRef]
- Mariah, M.A.A.; Vonnie, J.M.; Erna, K.H.; Nur’Aqilah, N.M.; Huda, N.; Abdul Wahab, R.; Rovina, K. The emergence and impact of ethylene scavengers techniques in delaying the ripening of fruits and vegetables. Membranes 2022, 12, 117. [Google Scholar] [CrossRef]
- Díez, A.G.; Rincón-Iglesias, M.; Lanceros-Méndez, S.; Reguera, J.; Lizundia, E. Multicomponent magnetic nanoparticle engineering: The role of structure-property relationship in advanced applications. Mater. Today Chem. 2022, 26, 101220. [Google Scholar] [CrossRef]
- Abdulwahid, F.S.; Haider, A.J.; Al-Musawi, S. Effect of Laser Parameter on Fe3O4 NPs Formation by Pulsed Laser Ablation in Liquid. 2023. Available online: https://www.researchgate.net/publication/370200034_Effect_of_laser_parameter_on_Fe3O4_NPs_formation_by_pulsed_laser_ablation_in_liquid (accessed on 22 July 2025).
- Conesa, A.; van den Hondel Cees, A.M.J.J.; Punt Peter, J. Studies on the Production of Fungal Peroxidases in Aspergillus niger. Appl. Environ. Microbiol. 2000, 66, 3016–3023. [Google Scholar] [CrossRef]
- Xia, L.; Zhou, C.; Liu, X.; Yu, Y.; Xie, Q.; Lin, H.; Xiong, X.; Zhang, S.; Liang, W.; Shao, H. Transforming bone cancer treatment: A comprehensive review of green-synthesized metal nanoparticles. Cancer Cell Int. 2025, 25, 193. [Google Scholar] [CrossRef]
- He, J.; Gao, Y.; Li, J.; Zhang, Y.; Zhu, M.; Zhang, J.; Cao, M.; Wei, X.; Qu, M. Highly salt-resistant and all-day solar-driven interfacial evaporator based on Janus CS@ Fe3O4/SA-MF. J. Environ. Chem. Eng. 2025, 13, 117466. [Google Scholar] [CrossRef]
- Sun, M.; Sun, Q.; Li, T.; Ren, X.; Xu, Q.; Sun, Z.; Duan, J. Silica nanoparticles induce liver lipid metabolism disorder via ACSL4-mediated ferroptosis. Environ. Pollut. 2024, 359, 124590. [Google Scholar] [CrossRef] [PubMed]
- Semenov, G.; Krasnova, I.; Khvylia, S.; Balabolin, D. Freezing and freeze-drying of strawberries with an additional effect of micro-vibrations. J. Food Sci. Technol. 2021, 58, 3192–3198. [Google Scholar] [CrossRef] [PubMed]
- Bansal, D.; Bhatnagar, D.; Rana, D.; Gautam, S. Ferrite nanoparticles in food technology. In Applications of Nanostructured Ferrites; Elsevier: Amsterdam, The Netherlands, 2023; pp. 295–314. [Google Scholar]
- Wang, H.; Li, Y.; Wang, A.; Slavik, M. Rapid, sensitive, and simultaneous detection of three foodborne pathogens using magnetic nanobead-based immunoseparation and quantum dot-based multiplex immunoassay. J. Food Prot. 2011, 74, 2039–2047. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF). Scientific Opinion on the safety evaluation of the substance zinc oxide, nanoparticles, uncoated and coated with [3-(methacryloxy) propyl] trimethoxysilane, for use in food contact materials. EFSA J. 2015, 13, 4063. [Google Scholar]
- Nowak-Jary, J.; Machnicka, B. Pharmacokinetics of magnetic iron oxide nanoparticles for medical applications. J. Nanobiotechnology 2022, 20, 305. [Google Scholar] [CrossRef]
- Mahajan, R.; Suriyanarayanan, S.; Nicholls, I.A. Improved solvothermal synthesis of γ-Fe2O3 magnetic nanoparticles for SiO2 coating. Nanomaterials 2021, 11, 1889. [Google Scholar] [CrossRef]
- Di, H.-W.; Luo, Y.-L.; Xu, F.; Chen, Y.-S.; Nan, Y.-F. A Novel Route to Prepare Fe3O4/P (MAA-co-NVP) Cross-Linked Magnetic Composite Microspheres with Core–Shell Architecture by Surface-Initiated Radical Dispersion Polymerization. J. Nanosci. Nanotechnol. 2010, 10, 8210–8216. [Google Scholar] [CrossRef]
- Guasamucare, R.; Miraballes, I.; Faccio, R.; Albores, S.; Pereira, J.; Arizaga, L. Study and formulation of microemulsions for the synthesis of Fe2O3–Ag nanoparticles: Characterization and evaluation of antimicrobial activity. MRS Adv. 2024, 9, 124–129. [Google Scholar] [CrossRef]
- Joseyphus, R.J.; Shinoda, K.; Kodama, D.; Jeyadevan, B. Size controlled Fe nanoparticles through polyol process and their magnetic properties. Mater. Chem. Phys. 2010, 123, 487–493. [Google Scholar] [CrossRef]
- Naseem, K. Magnetic nanoparticles (Fe3O4 NPs) fabricated composite microgels and their applications in different fields. Rev. Chem. Eng. 2023, 39, 175–201. [Google Scholar] [CrossRef]
- Zou, L.; Huang, B.; Zheng, X.; Pan, H.; Zhang, Q.; Xie, W.; Zhao, Z.; Li, X. Microfluidic synthesis of magnetic nanoparticles in droplet-based microreactors. Mater. Chem. Phys. 2022, 276, 125384. [Google Scholar] [CrossRef]
- Khonina, T.y.G.; Demin, A.M.; Tishin, D.S.; Germov, A.Y.; Uimin, M.A.; Mekhaev, A.V.; Minin, A.S.; Karabanalov, M.S.; Mysik, A.A.; Bogdanova, E.A. Magnetic nanocomposite materials based on Fe3O4 nanoparticles with iron and silica glycerolates shell: Synthesis and characterization. Int. J. Mol. Sci. 2023, 24, 12178. [Google Scholar] [CrossRef] [PubMed]
- Sasson, E.; Malka, E.; Caspi, A.; Kanovsky, N.; Margel, S. Engineered thin coatings of cross-linked silane polymers with urea group onto polypropylene fabrics for controlled release of thymol against molds in hay. Mater. Today Chem. 2024, 37, 102009. [Google Scholar] [CrossRef]
- Malka, E.; Margel, S. Engineering of PVA/PVP hydrogels for agricultural applications. Gels 2023, 9, 895. [Google Scholar] [CrossRef]
- Sasson, E.; Agazani, O.; Malka, E.; Reches, M.; Margel, S. Engineered Cross-Linked Silane with Urea Polymer Thin Durable Coatings onto Polymeric Films for Controlled Antiviral Release of Activated Chlorine and Essential Oils. J. Funct. Biomater. 2023, 14, 270. [Google Scholar] [CrossRef]
- Ganguly, S.; Tang, X.S. 3D Printing of High Strength Thermally Stable Sustainable Lightweight Corrosion-Resistant Nanocomposite by Solvent Exchange Postprocessing. ACS Sustain. Chem. Eng. 2024, 13, 423–435. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Gao, M.; Pan, X.; Liu, G.; Chen, F.; Ma, X.; Chen, Y.; Zhang, Z. Iron Oxide Nanoparticle-Loaded Magnetic Cellulose Nanofiber Aerogel with Self-Controlled Release Property for Green Active Food Packaging. ACS Appl. Nano Mater. 2023, 6, 22373–22382. [Google Scholar] [CrossRef]
- Workineh, M.; Enyew, M. Review the extent and cause of post-harvest loss of fruits and vegetables in Ethiopia. J. Biol. Agric. Healthc. 2021, 11, 1–22. [Google Scholar]
- Bhardwaj, S.; Lata, S.; Garg, R. Application of nanotechnology for preventing postharvest losses of agriproducts. J. Hortic. Sci. Biotechnol. 2023, 98, 31–44. [Google Scholar] [CrossRef]
- Saedi, S.; Shokri, M.; Priyadarshi, R.; Rhim, J.-W. Carrageenan-Based Antimicrobial Films Integrated with Sulfur-Coated Iron Oxide Nanoparticles (Fe3O4@SNP). ACS Appl. Polym. Mater. 2021, 3, 4913–4923. [Google Scholar] [CrossRef]
- Huang, L.; Liu, S.; Ren, S.; Zhang, M.; Wang, T.; Wang, X.; Gao, Z. Magnetic Relaxation Switch Biosensors Based on Self-Assembly of Polystyrene Microspheres and Magnetic Nanoparticles for Detection of Bisphenol A. ACS Appl. Nano Mater. 2021, 4, 5963–5971. [Google Scholar] [CrossRef]
- Gao, Q.; Luo, D.; Bai, M.; Chen, Z.-W.; Feng, Y.-Q. Rapid Determination of Estrogens in Milk Samples Based on Magnetite Nanoparticles/Polypyrrole Magnetic Solid-Phase Extraction Coupled with Liquid Chromatography–Tandem Mass Spectrometry. J. Agric. Food Chem. 2011, 59, 8543–8549. [Google Scholar] [CrossRef] [PubMed]
- Otero, L.; Rodríguez, A.C.; Pérez-Mateos, M.; Sanz, P.D. Effects of magnetic fields on freezing: Application to biological products. Compr. Rev. Food Sci. Food Saf. 2016, 15, 646–667. [Google Scholar] [CrossRef] [PubMed]
- Ghoshani, M.; Sánchez, E.; Lee, S.S.; Singh, G.; Yaacoub, N.; Peddis, D.; Mozaffari, M.; Binns, C.; De Toro, J.; Normile, P. On the detection of surface spin freezing in iron oxide nanoparticles and its long-term evolution under ambient oxidation. Nanotechnology 2020, 32, 065704. [Google Scholar] [CrossRef]
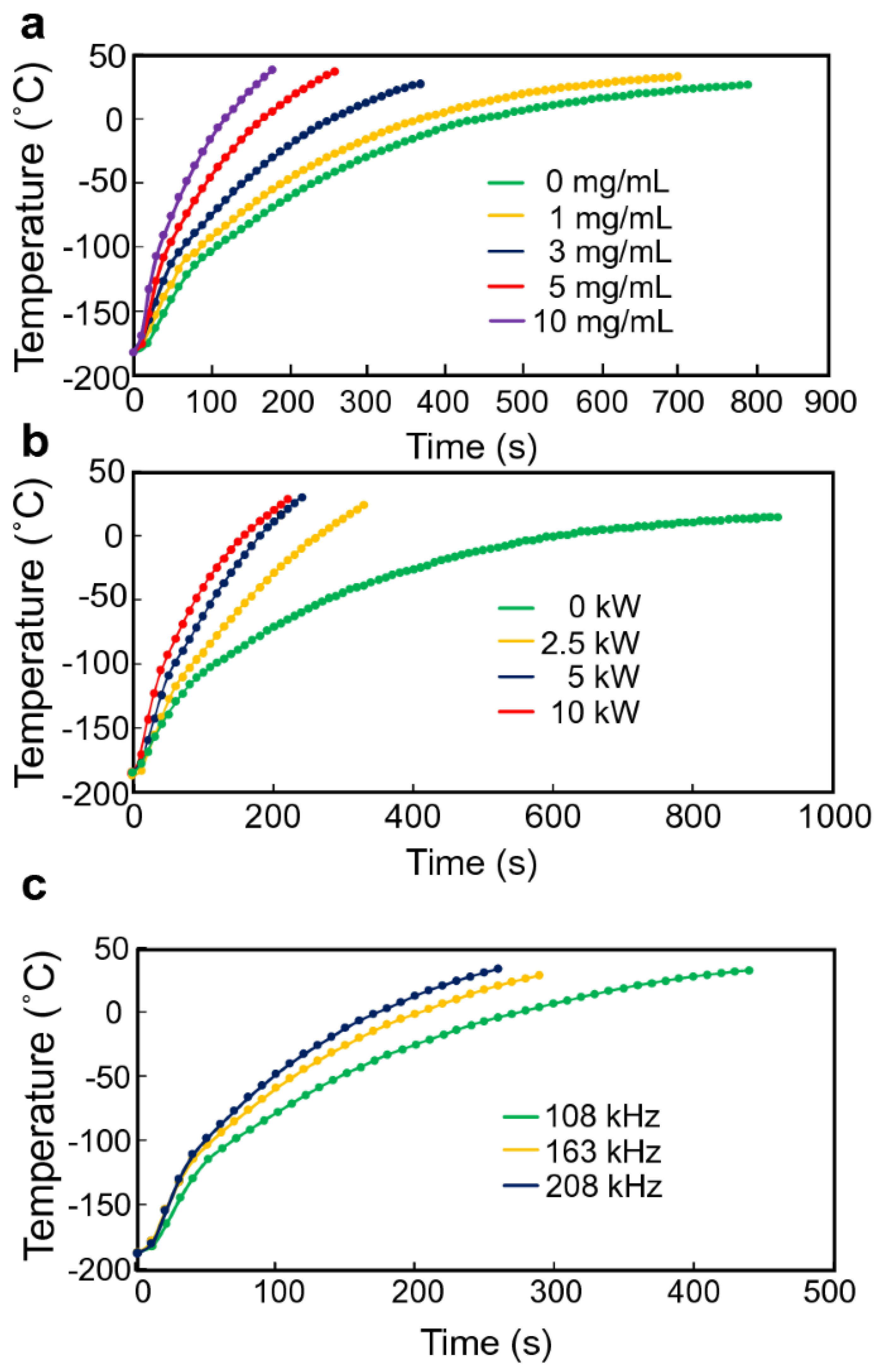
- Manuchehrabadi, N.; Gao, Z.; Zhang, J.; Ring, H.L.; Shao, Q.; Liu, F.; McDermott, M.; Fok, A.; Rabin, Y.; Brockbank, K.G. Improved tissue cryopreservation using inductive heating of magnetic nanoparticles. Sci. Transl. Med. 2017, 9, eaah4586. [Google Scholar] [CrossRef]
- Soukup, D.; Moise, S.; Céspedes, E.; Dobson, J.; Telling, N.D. In situ measurement of magnetization relaxation of internalized nanoparticles in live cells. ACS Nano 2015, 9, 231–240. [Google Scholar] [CrossRef]
- Cabrera, D.; Coene, A.; Leliaert, J.; Artés-Ibáñez, E.J.; Dupré, L.; Telling, N.D.; Teran, F.J. Dynamical magnetic response of iron oxide nanoparticles inside live cells. ACS Nano 2018, 12, 2741–2752. [Google Scholar] [CrossRef]
- Mejías, R.; Hernandez Flores, P.; Talelli, M.; Tajada-Herraiz, J.L.; Brollo, M.E.; Portilla, Y.; Morales, M.P.; Barber, D.F. Cell-promoted nanoparticle aggregation decreases nanoparticle-induced hyperthermia under an alternating magnetic field independently of nanoparticle coating, core size, and subcellular localization. ACS Appl. Mater. Interfaces 2018, 11, 340–355. [Google Scholar] [CrossRef]
- Marcus, M.; Indech, G.; Vardi, N.; Levy, I.; Smith, A.; Margel, S.; Shefi, O.; Sharoni, A. Magnetic Organization of Neural Networks via Micro-Patterned Devices. Adv. Mater. Interfaces 2020, 7, 2000055. [Google Scholar] [CrossRef]
- Corem-Salkmon, E.; Perlstein, B.; Margel, S. Design of near-infrared fluorescent bioactive conjugated functional iron oxide nanoparticles for optical detection of colon cancer. Int. J. Nanomed. 2012, 7, 5517–5527. [Google Scholar] [CrossRef]
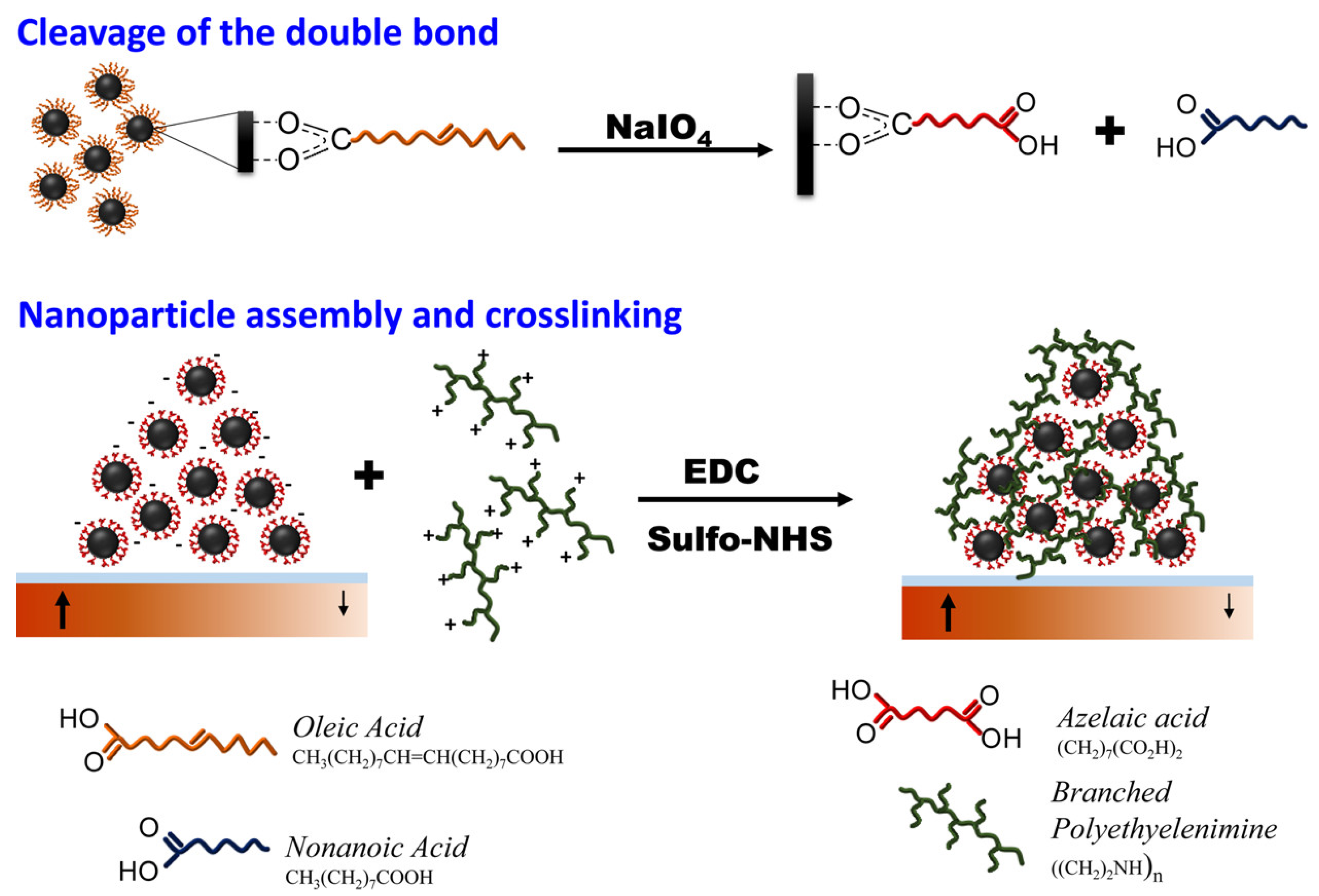
- Velez, C.; Torres-Díaz, I.; Maldonado-Camargo, L.; Rinaldi, C.; Arnold, D.P. Magnetic Assembly and Cross-Linking of Nanoparticles for Releasable Magnetic Microstructures. ACS Nano 2015, 9, 10165–10172. [Google Scholar] [CrossRef]
- Yousef, M.S.; López-Lorente, A.I.; Diaz-Jimenez, M.; Consuegra, C.; Dorado, J.; Pereira, B.; Ortiz, I.; Cárdenas, S.; Hidalgo, M. Nano-depletion of acrosome-damaged donkey sperm by using lectin peanut agglutinin (PNA)-magnetic nanoparticles. Theriogenology 2020, 151, 103–111. [Google Scholar] [CrossRef]
- Ito, A.; Yoshioka, K.; Masumoto, S.; Sato, K.; Hatae, Y.; Nakai, T.; Yamazaki, T.; Takahashi, M.; Tanoue, S.; Horie, M. Magnetic heating of nanoparticles as a scalable cryopreservation technology for human induced pluripotent stem cells. Sci. Rep. 2020, 10, 13605. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Margel, S.; Gedanken, A. Tailor made magnetic nanolights: Fabrication to cancer theranostics applications. Nanoscale Adv. 2021, 3, 6762–6796. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.; Das, P.; Saha, A.; Noked, M.; Gedanken, A.; Margel, S. Mussel-inspired polynorepinephrine/MXene-based magnetic nanohybrid for electromagnetic interference shielding in X-band and strain-sensing performance. Langmuir 2022, 38, 3936–3950. [Google Scholar] [CrossRef]
- Kesavan, M.P.; Ayyanaar, S.; Vijayakumar, V.; Dhaveethu Raja, J.; Annaraj, J.; Sakthipandi, K.; Rajesh, J. Magnetic iron oxide nanoparticles (MION s) cross-linked natural polymer-based hybrid gel beads: Controlled nano anti-TB drug delivery application. J. Biomed. Mater. Res. Part A 2018, 106, 1039–1050. [Google Scholar] [CrossRef]
- Yan, J.-F.; Liu, J. Nanocryosurgery and its mechanisms for enhancing freezing efficiency of tumor tissues. Nanomed. Nanotechnol. Biol. Med. 2008, 4, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Deng, Z.-S. Recent advances in modeling and computing the three-dimensional bioheat transfer with phase change in cryosurgical ablation of tumor tissues. Heat Transf. Res. 2013, 44, 273–302. [Google Scholar] [CrossRef]
- Ganguly, S.; Margel, S. 3D printed magnetic polymer composite hydrogels for hyperthermia and magnetic field driven structural manipulation. Prog. Polym. Sci. 2022, 131, 101574. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Q.; Liu, J.; Deng, Z.; Zhang, X.; Alifu, N.; Zhang, X.; Yu, Z.; Liu, Y.; Lan, Z. Recent advances in functionalized ferrite nanoparticles: From fundamentals to magnetic hyperthermia cancer therapy. Colloids Surf. B Biointerfaces 2024, 234, 113754. [Google Scholar] [CrossRef]
- Ganguly, S.; Kanovsky, N.; Das, P.; Gedanken, A.; Margel, S. Photopolymerized thin coating of polypyrrole/graphene nanofiber/iron oxide onto nonpolar plastic for flexible electromagnetic radiation shielding, strain sensing, and non-contact heating applications. Adv. Mater. Interfaces 2021, 8, 2101255. [Google Scholar] [CrossRef]
- Wang, W.; Li, W.; Bu, Y.; Li, X.; Zhu, W. Nano freezing–thawing of atlantic salmon fillets: Impact on thermodynamic and quality characteristics. Foods 2023, 12, 2887. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.; Das, P.; Margel, S. Containers for drug delivery. In Micro-and Nano-Containers for Smart Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 127–153. [Google Scholar]
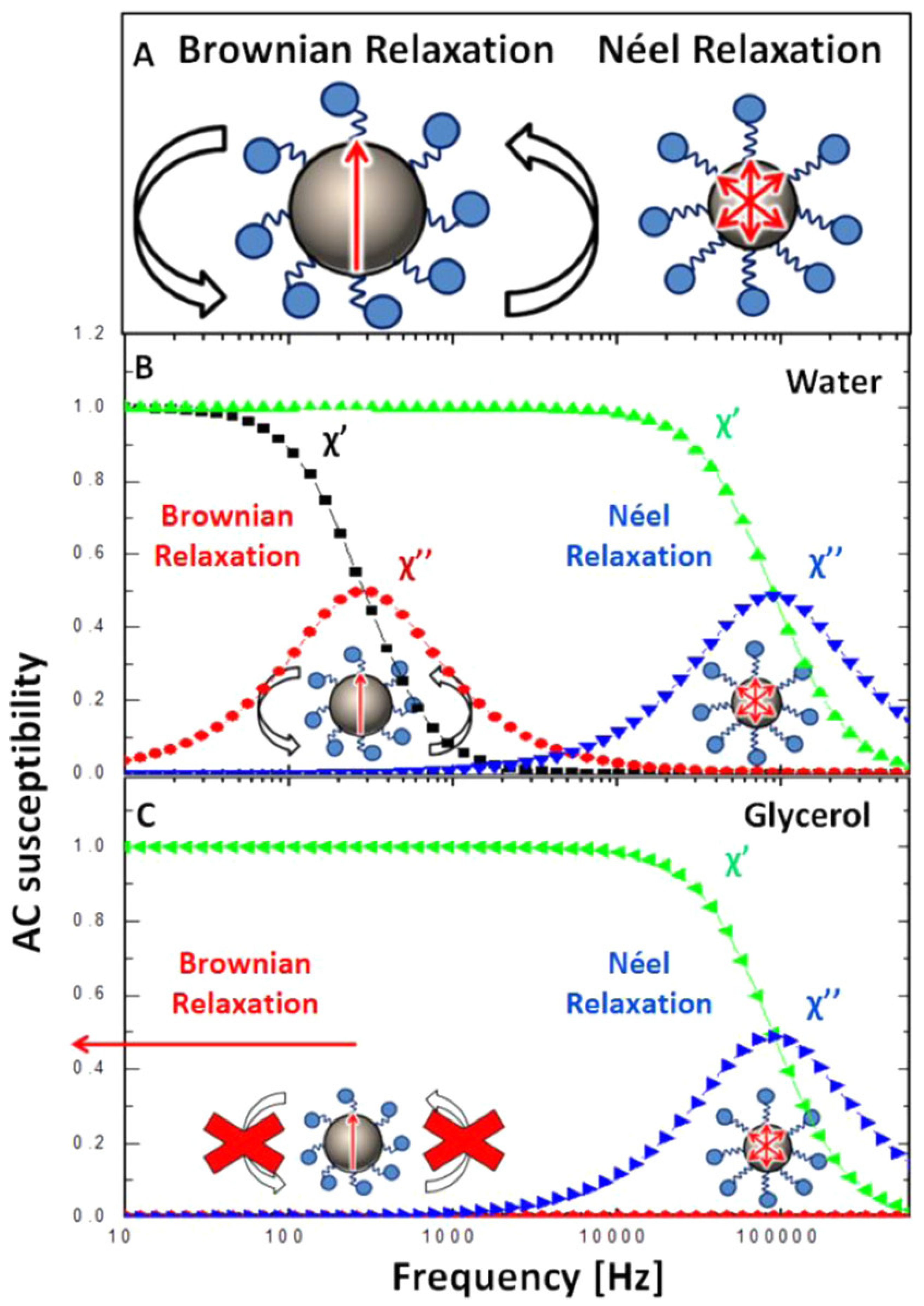
- Shah, S.A.; Reeves, D.B.; Ferguson, R.M.; Weaver, J.B.; Krishnan, K.M. Mixed Brownian alignment and Néel rotations in superparamagnetic iron oxide nanoparticle suspensions driven by an ac field. Phys. Rev. B 2015, 92, 094438. [Google Scholar] [CrossRef] [PubMed]
- Lattuada, M.; Hatton, T.A. Preparation and controlled self-assembly of Janus magnetic nanoparticles. J. Am. Chem. Soc. 2007, 129, 12878–12889. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, G.; Chen, Z.; Panhwar, F.; He, X. Dual Suppression Effect of Magnetic Induction Heating and Microencapsulation on Ice Crystallization Enables Low-Cryoprotectant Vitrification of Stem Cell–Alginate Hydrogel Constructs. ACS Appl. Mater. Interfaces 2018, 10, 16822–16835. [Google Scholar] [CrossRef]
- Cao, M.; Cao, A.; Li, Y.; Wang, W.; Wang, Y.; Cai, L. Effects of magnetic nanoparticles plus microwave on the thawing of largemouth bass (Micropterus salmoides) fillets based on iTRAQ quantitative proteomics. Food Chem. 2019, 286, 506–514. [Google Scholar] [CrossRef]
- Landmark, K.J.; DiMaggio, S.; Ward, J.; Kelly, C.; Vogt, S.; Hong, S.; Kotlyar, A.; Myc, A.; Thomas, T.P.; Penner-Hahn, J.E.; et al. Synthesis, Characterization, and In Vitro Testing of Superparamagnetic Iron Oxide Nanoparticles Targeted Using Folic Acid-Conjugated Dendrimers. ACS Nano 2008, 2, 773–783. [Google Scholar] [CrossRef]
- Tripathy, S.; Wai, N.K.; Sharma, S.; Vu, N.N.; Jaitpal, S.; Zare, F.; Singh, M.; McDaid, L.; Bhattacharyya, S.; Coté, G.L.; et al. A locked aptamer-magnetic nanoparticle assay for cardiac troponin I classification to support myocardial infarction diagnosis in resource-limited environments. Biosens. Bioelectron. 2025, 286, 117590. [Google Scholar] [CrossRef]
- Semaltianos, N.G.; Karczewski, G. Laser Synthesis of Magnetic Nanoparticles in Liquids and Application in the Fabrication of Polymer–Nanoparticle Composites. ACS Appl. Nano Mater. 2021, 4, 6407–6440. [Google Scholar] [CrossRef]
- Pavía-Sanders, A.; Zhang, S.; Flores, J.A.; Sanders, J.E.; Raymond, J.E.; Wooley, K.L. Robust Magnetic/Polymer Hybrid Nanoparticles Designed for Crude Oil Entrapment and Recovery in Aqueous Environments. ACS Nano 2013, 7, 7552–7561. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhong, T.; Zhang, Y.; Zhao, X.; Xiao, Y.; Wang, L.; Liu, X.; Zhang, X. Design, preparation, and application of magnetic nanoparticles for food safety analysis: A review of recent advances. J. Agric. Food Chem. 2021, 70, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Singh, R.; Kush, P. Biocompatibility, toxicity concerns, environmental and safety considerations, and legal aspects of functionalized magnetic nanoparticles. In Functionalized Magnetic Nanoparticles for Theranostic Applications; John Wiley & Sons: Hoboken, NJ, USA, 2024; pp. 533–558. [Google Scholar]
- Kim, J.-E.; Shin, J.-Y.; Cho, M.-H. Magnetic nanoparticles: An update of application for drug delivery and possible toxic effects. Arch. Toxicol. 2012, 86, 685–700. [Google Scholar] [CrossRef]
- Dhasmana, A.; Firdaus, S.; Singh, K.P.; Raza, S.; Jamal, Q.M.S.; Kesari, K.K.; Rahman, Q.; Lohani, M. Nanoparticles: Applications, toxicology and safety aspects. In Perspectives in Environmental Toxicology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 47–70. [Google Scholar]
- Hoseini, Z.S.; Rezaee, Z.; Derakhshani, A.; Zhang, S.Y.; SH Tehrani, S.; Taleb, M.; Ghanbari, H. Nanoparticles and Their Impact on Epigenetic Mechanisms: Insights and Implications. Adv. Ther. 2025, 8, 2500006. [Google Scholar] [CrossRef]
- Störmer, A.; Bott, J.; Kemmer, D.; Franz, R. Critical review of the migration potential of nanoparticles in food contact plastics. Trends Food Sci. Technol. 2017, 63, 39–50. [Google Scholar] [CrossRef]
- Nowak-Jary, J.; Machnicka, B. Comprehensive analysis of the potential toxicity of magnetic iron oxide nanoparticles for medical applications: Cellular mechanisms and systemic effects. Int. J. Mol. Sci. 2024, 25, 12013. [Google Scholar] [CrossRef] [PubMed]
- Reddy, L.H.; Arias, J.L.; Nicolas, J.; Couvreur, P. Magnetic nanoparticles: Design and characterization, toxicity and biocompatibility, pharmaceutical and biomedical applications. Chem. Rev. 2012, 112, 5818–5878. [Google Scholar] [CrossRef]
- Queiroz, L.G.; de Torresi, S.I.C. Metal and metal-oxide nanoparticles inducing changes in reactive oxygen species levels and enzymes of oxidative stress: A review focused on daphnids. Ecotoxicology, 2025; ahead of print. [Google Scholar]
- Genc, S.; Taghizadehghalehjoughi, A.; Yeni, Y.; Jafarizad, A.; Hacimuftuoglu, A.; Nikitovic, D.; Docea, A.O.; Mezhuev, Y.; Tsatsakis, A. Fe3O4 Nanoparticles in combination with 5-FU Exert antitumor effects superior to those of the active drug in a colon cancer cell model. Pharmaceutics 2023, 15, 245. [Google Scholar] [CrossRef]
- Mondal, M.; Chouksey, A.; Gurjar, V.; Tiwari, R.; Srivasatava, R.K.; Mishra, P.K. Micro (nano) plastics in the brain: Epigenetic perturbations in progression to neurodegenerative diseases. Neurotoxicol. Teratol. 2025, 110, 107521. [Google Scholar] [CrossRef]
- Yang, Z.; DeLoid, G.M.; Baw, J.; Zarbl, H.; Demokritou, P. Assessment of ingested micro-and nanoplastic (MNP)-mediated genotoxicity in an in vitro model of the small intestinal epithelium (SIE). Nanomaterials 2024, 14, 807. [Google Scholar] [CrossRef]
- Wang, H.-P.; Huang, X.-H.; Chen, J.-N.; Dong, M.; Nie, C.-Z.; Qin, L. Micro- and nano-plastics in food systems: Distribution, combined toxicity with environmental contaminants, and removal strategies. Chem. Eng. J. 2023, 476, 146430. [Google Scholar] [CrossRef]
- Habumugisha, T.; Zhang, Z.; Uwizewe, C.; Yan, C.; Ndayishimiye, J.C.; Rehman, A.; Zhang, X. Toxicological review of micro- and nano-plastics in aquatic environments: Risks to ecosystems, food web dynamics and human health. Ecotoxicol. Environ. Saf. 2024, 278, 116426. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Zhang, L.; Qin, Z.; Wang, T. Review of Recent Advances in Intelligent and Antibacterial Packaging for Meat Quality and Safety. Foods 2025, 14, 1157. [Google Scholar] [CrossRef] [PubMed]
- Onyeaka, H.; Passaretti, P.; Miri, T.; Al-Sharify, Z.T. The safety of nanomaterials in food production and packaging. Curr. Res. Food Sci. 2022, 5, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Seref, N.; Cufaoglu, G. Food Packaging and Chemical Migration: A Food Safety Perspective. J. Food Sci. 2025, 90, e70265. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganguly, S.; Margel, S. Magnetic Nanoparticle-Based Nano-Packaging and Nano-Freezing in Food Storage Applications. Molecules 2025, 30, 3453. https://doi.org/10.3390/molecules30173453
Ganguly S, Margel S. Magnetic Nanoparticle-Based Nano-Packaging and Nano-Freezing in Food Storage Applications. Molecules. 2025; 30(17):3453. https://doi.org/10.3390/molecules30173453
Chicago/Turabian StyleGanguly, Sayan, and Shlomo Margel. 2025. "Magnetic Nanoparticle-Based Nano-Packaging and Nano-Freezing in Food Storage Applications" Molecules 30, no. 17: 3453. https://doi.org/10.3390/molecules30173453
APA StyleGanguly, S., & Margel, S. (2025). Magnetic Nanoparticle-Based Nano-Packaging and Nano-Freezing in Food Storage Applications. Molecules, 30(17), 3453. https://doi.org/10.3390/molecules30173453





