Antioxidant and Antibacterial Potential of Passiflora edulis (Passion Fruit) at Three Ripening Stages for Waste Valorization
Abstract
1. Introduction
2. Results and Discussion
2.1. Qualitative Determination of Secondary Metabolites
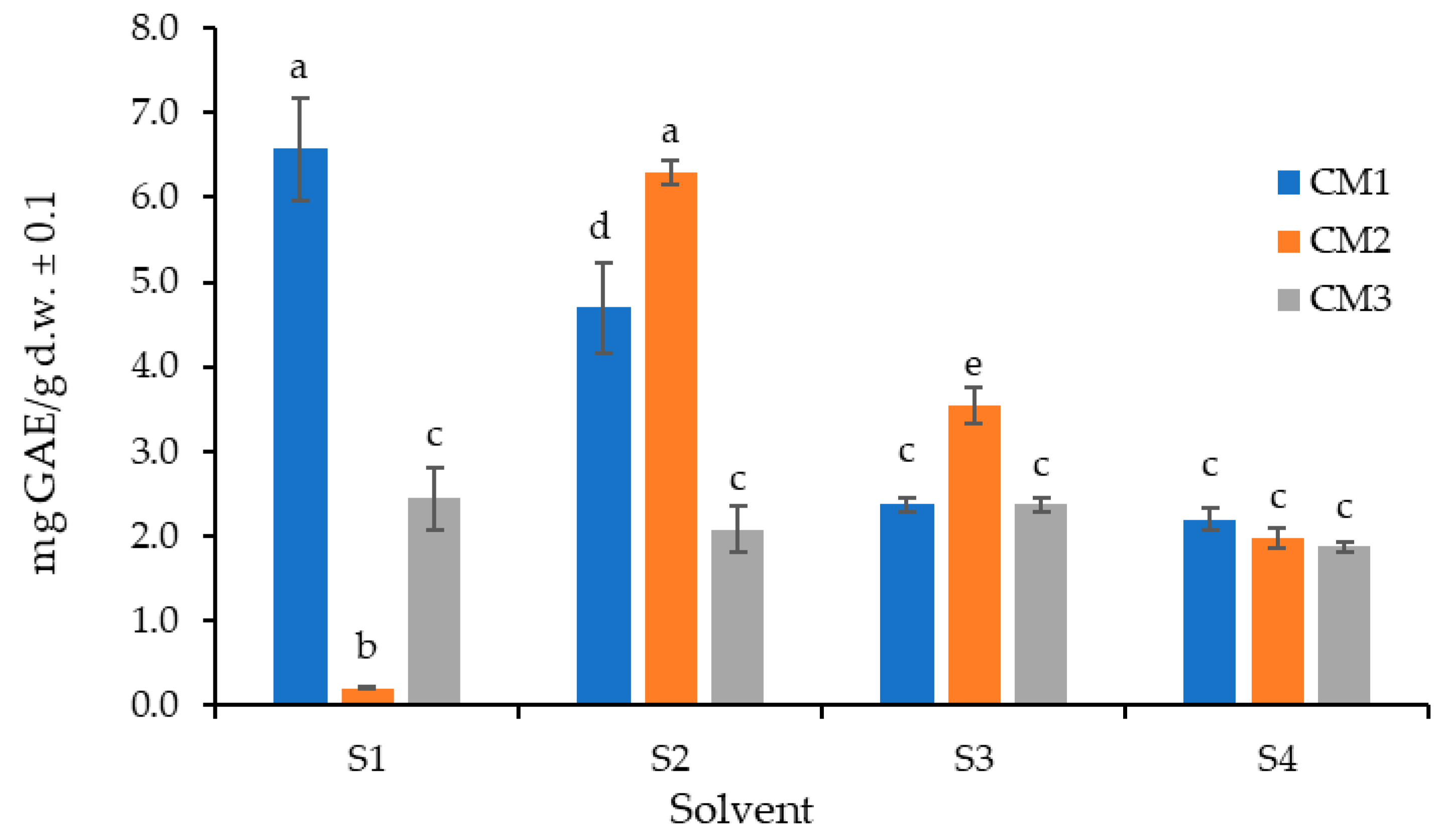
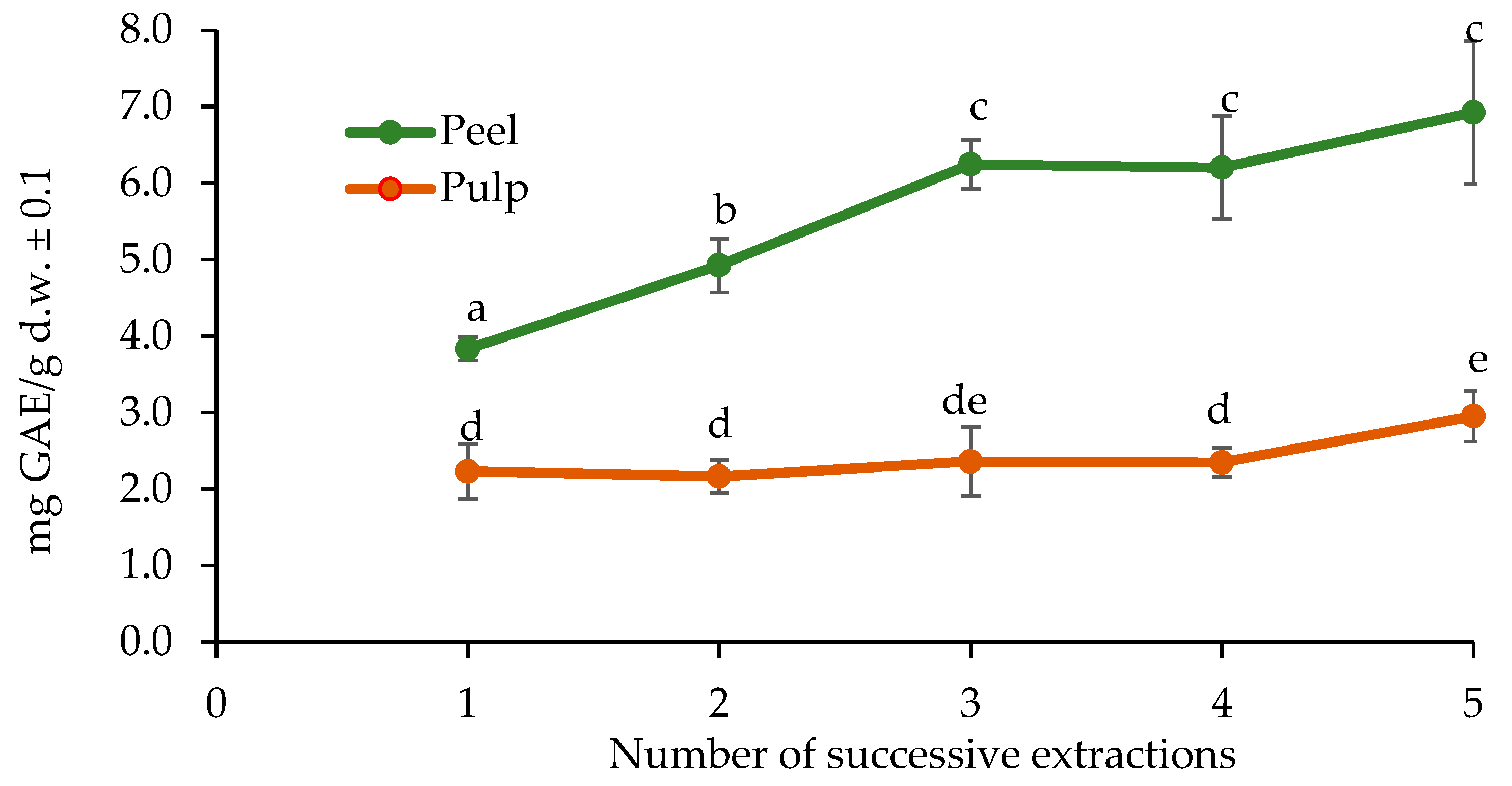
2.2. Determination of the Best Solvent and Optimal Number of Extractions
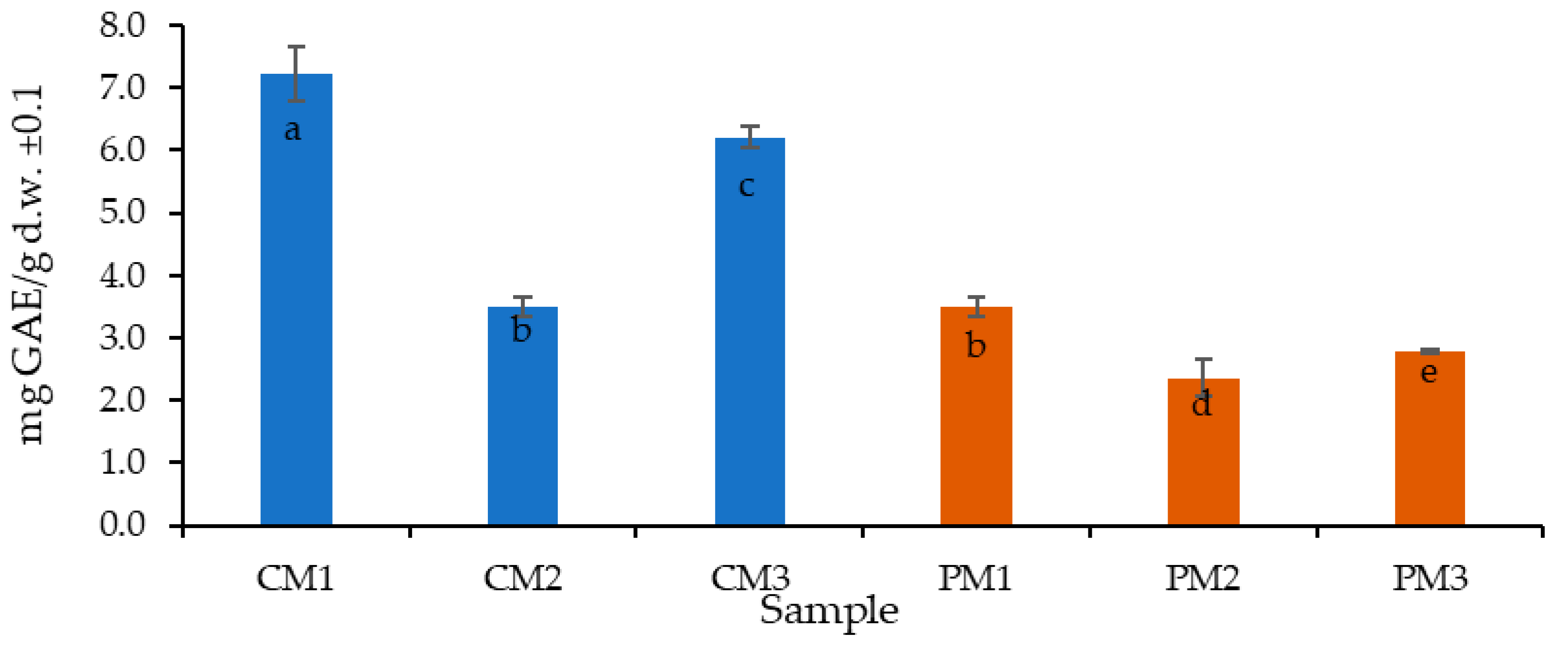
2.3. Total Polyphenol Determination by Folin–Ciocalteu
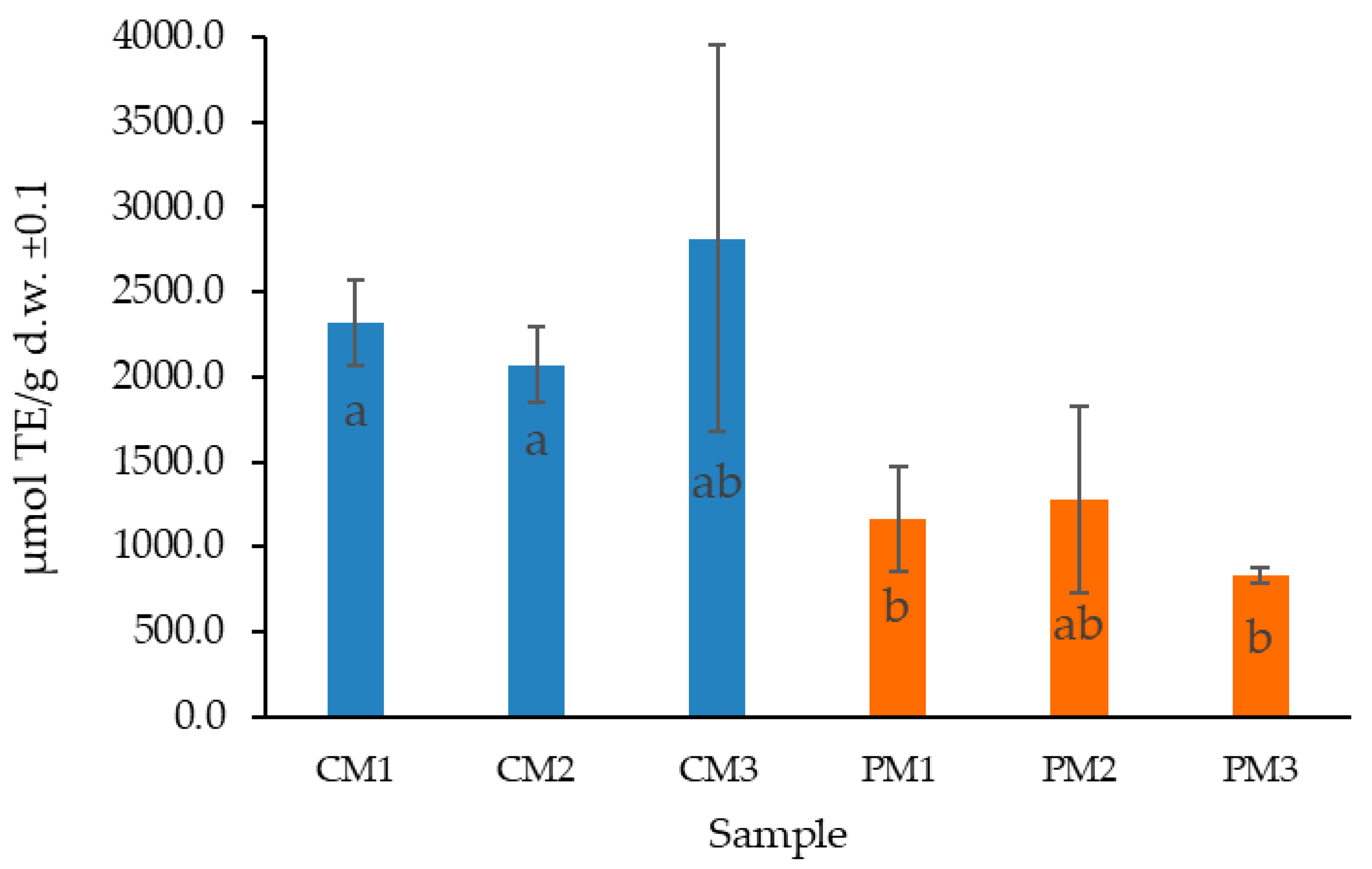
2.4. Determination of Antioxidant Activity by ORAC
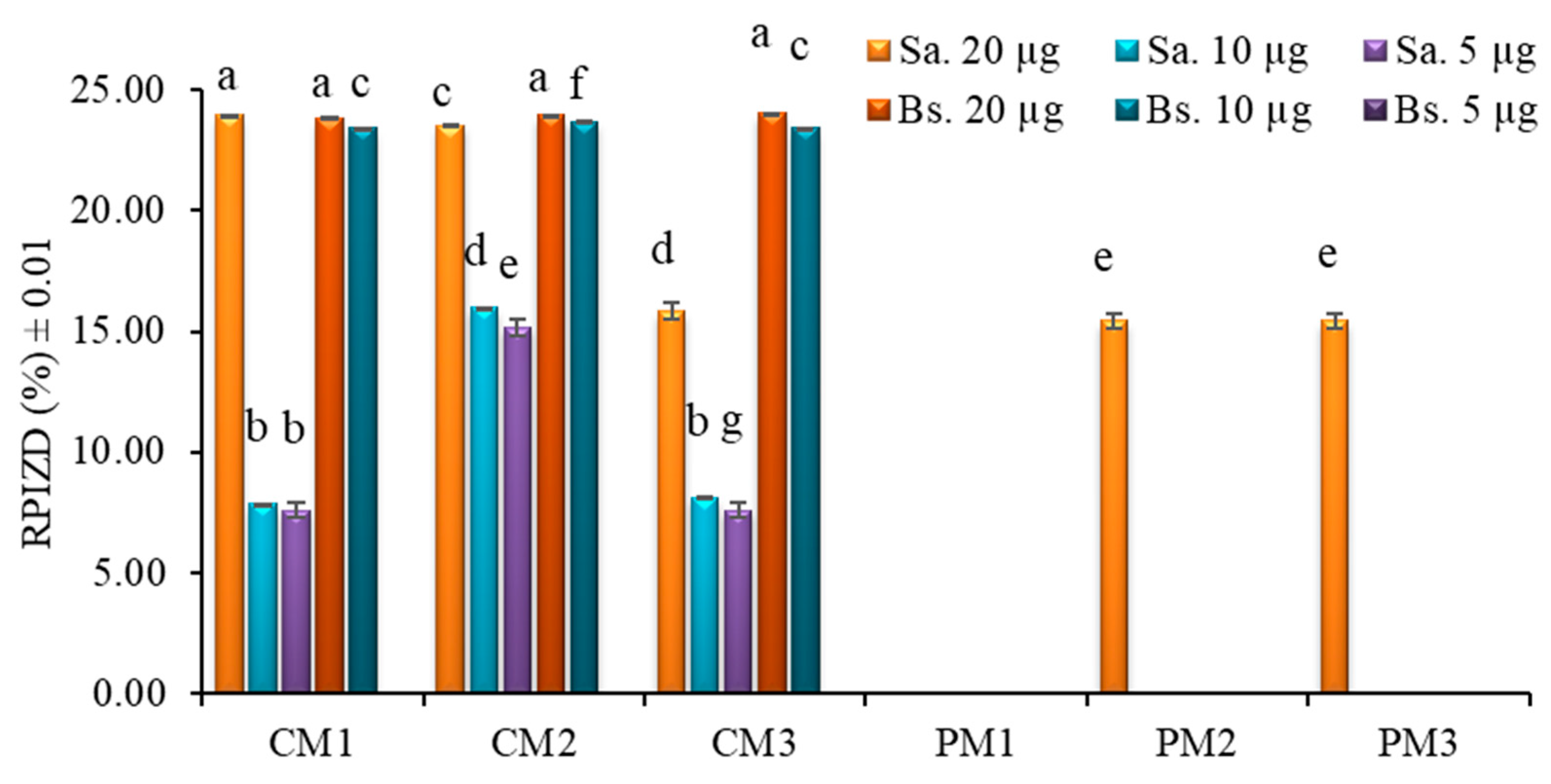
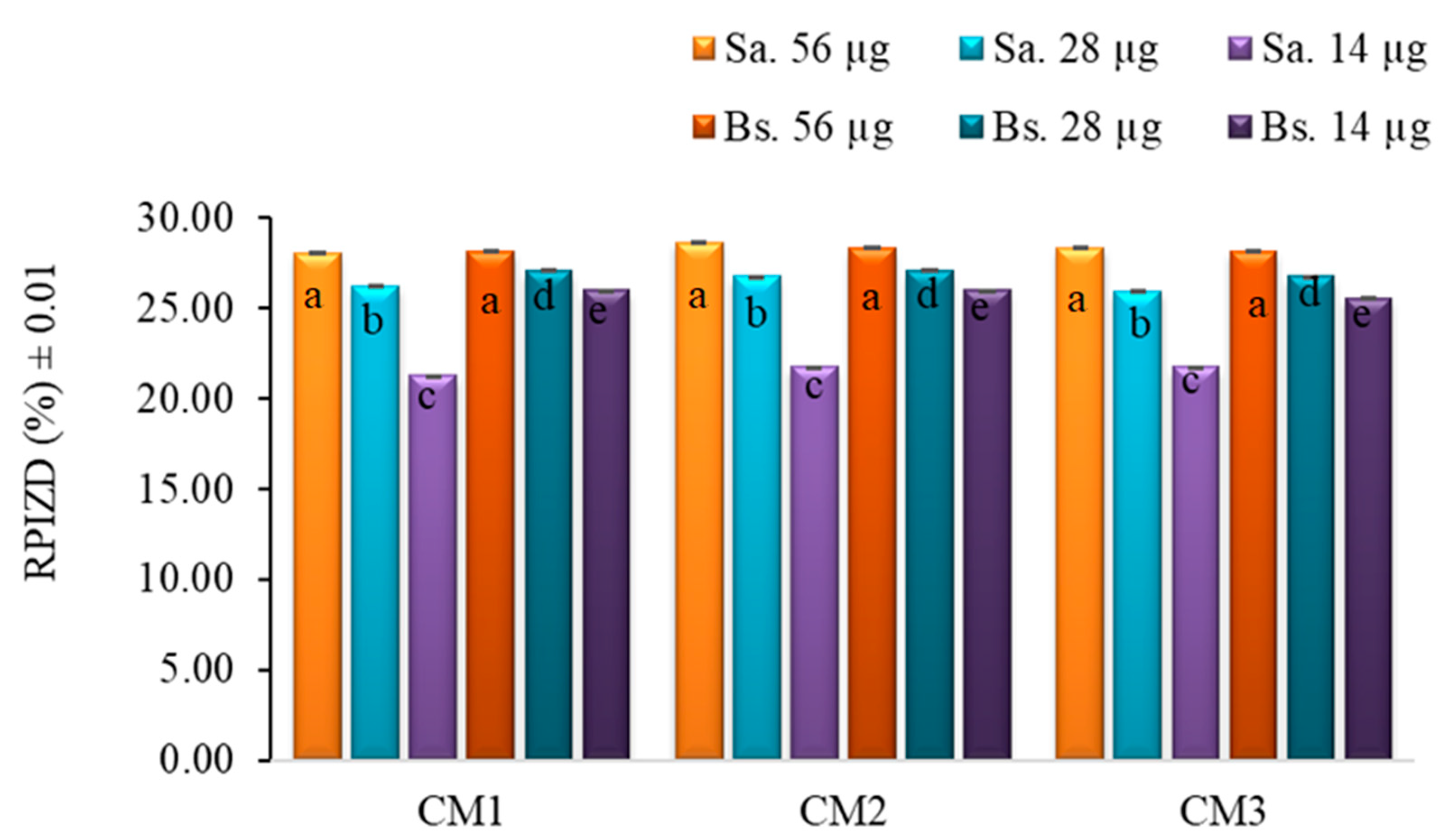
2.5. Bacterial Susceptibility Testing
3. Materials and Methods
3.1. Sample Collection and Treatment
- M1: Fruit at an early stage of ripening (some green coloration on peel; intermediate maturation);
- M2: Ripe fruit (fully colored peel; optimal for commercialization);
- M3: Overripe fruit (wrinkled or discolored peel; signs of post-climacteric stage).
3.2. Qualitative Determination of Secondary Metabolites
3.3. Determination of the Best Solvent and Optimal Number of Extractions
3.4. Total Polyphenol Determination Using Folin–Ciocalteu
3.5. Determination of Antioxidant Activity by ORAC
3.6. Bacterial Susceptibility Testing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| mg GAE/g d.w. | gallic acid equivalents per gram of dry weight |
| μmol TE/g d.w. | micromoles of Trolox equivalents per gram of dry weight |
| IZD | inhibition zone diameter |
| RPIZD | relative percentage of the inhibition zone diameter |
| TP | total polyphenol |
| MIC | minimum inhibitory concentration |
| LADOQUI | Industrial Chemistry Teaching Laboratory |
| ORAC | oxygen radical absorbance capacity |
| AA | antioxidant activity |
| TPC | total polyphenol content |
| S1 | water |
| S2 | acetone–water (7:3) |
| S3 | ethanol–water (7:3) |
| S4 | 95% ethanol |
| MS | standard deviation |
| Trolox | 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid |
| C | peel |
| P | pulp |
| M1 | pre-ripe fruit |
| M2 | ripe fruit |
| M3 | overripe fruit |
References
- Castillo, N.R.; Ambachew, D.; Melgarejo, L.M.; Blair, M.W. Morphological and Agronomic Variability among Cultivars, Landraces, and Genebank Accessions of Purple Passion Fruit, Passiflora edulis f. edulis. HortScience 2020, 55, 768–777. [Google Scholar] [CrossRef]
- Viera, W.; Shinohara, T.; Samaniego, I.; Sanada, A.; Terada, N.; Ron, L.; Suárez-Tapia, A.; Koshio, K. Phytochemical Composition and Antioxidant Activity of Passiflora spp. Germplasm Grown in Ecuador. Plants 2022, 11, 328. [Google Scholar] [CrossRef]
- da Costa, C.A.R.; Machado, G.G.L.; Rodrigues, L.J.; de Barros, H.E.A.; Natarelli, C.V.L.; Boas, E.V.d.B.V. Phenolic Compounds Profile and Antioxidant Activity of Purple Passion Fruit’s Pulp, Peel and Seed at Different Maturation Stages. Sci. Hortic. 2023, 321, 112244. [Google Scholar] [CrossRef]
- Weyya, G.; Belay, A.; Tadesse, E. Passion Fruit (Passiflora edulis Sims) by-Products as a Source of Bioactive Compounds for Non-Communicable Disease Prevention: Extraction Methods and Mechanisms of Action: A Systematic Review. Front. Nutr. 2024, 11, 1340511. [Google Scholar] [CrossRef]
- Tran, T.T.; Nguyen, A.T.; Le, T.N.H.; Dinh, T.T.N.; Ngo, T.N.M.; Nguyen, T.B.T.; Ho, Q.P.; Doan, V.H.T.; Nguyen, V.N.H. A Feasible Process for Recycling Anthocyanins and Pectin from the Waste Peels of Purple Passion Fruit (Passiflora edulis Sims) as Food Additives. CTU J. Innov. Sustain. Dev. 2025, 17, 59–68. [Google Scholar] [CrossRef]
- Franco, F.S.I. La Importancia de Los Factores Productivos y Su Impacto En Las Organizaciones Agrícolas En León Guanajuato México. EL Ágora USB 2016, 16, 393–406. [Google Scholar] [CrossRef]
- Xia, X.; Ruan, J. Analyzing Barriers for Developing a Sustainable Circular Economy in Agriculture in China Using Grey-DEMATEL Approach. Sustainability 2020, 12, 6358. [Google Scholar] [CrossRef]
- Pechlaner, G. The Sociology of Agriculture in Transition: The Political Economy of Agriculture after Biotechnology. Can. J. Sociol. 2010, 35, 243–270. [Google Scholar] [CrossRef]
- Prasad, R.; Bhattacharyya, A.; Nguyen, Q.D. Nanotechnology in Sustainable Agriculture: Recent Developments, Challenges, and Perspectives. Front. Microbiol. 2017, 8, 264976. [Google Scholar] [CrossRef]
- Jones, A.D.; Ejeta, G. A New Global Agenda for Nutrition and Health: The Importance of Agriculture and Food Systems. Bull. World Health Organ. 2016, 94, 228. [Google Scholar] [CrossRef]
- Wadhawan, N.; Kaur, J. Importance of Agriculture Intervention in Improving Nutritional Status of Women and Children: An Indian Scenario. Pharma Innov. J. 2019, 8, 387–393. [Google Scholar]
- República de Costa Rica. Sistema Costarricense de Información Jurídica; Asamblea Legislativa: San José, CA, USA, 2010. [Google Scholar]
- Alves, J.S.F.; Dos Santos Silva, A.M.; Da Silva, R.M.; Tiago, P.R.F.; De Carvalho, T.G.; De Araújo Júnior, R.F.; De Azevedo, E.P.; Lopes, N.P.; De Santis Ferreira, L.; Gavioli, E.C.; et al. In Vivo Antidepressant Effect of Passiflora edulis f. flavicarpa into Cationic Nanoparticles: Improving Bioactivity and Safety. Pharmaceutics 2020, 12, 383. [Google Scholar] [CrossRef]
- Ramaiya, S.D.; Bujang, J.B.; Zakaria, M.H.; Saupi, N. Nutritional, Mineral and Organic Acid Composition of Passion Fruit (Passiflora Species). Food Res. 2019, 3, 231–240. [Google Scholar] [CrossRef]
- Xu, M.; Li, A.; Teng, Y.; Sun, Z. Exploring the Adaptive Mechanism of Passiflora edulis in Karst Areas via an Integrative Analysis of Nutrient Elements and Transcriptional Profiles. BMC Plant Biol. 2019, 19, 185. [Google Scholar] [CrossRef]
- Escobedo Soberón, G.M. Descripción: Valorización de La Cáscara de Maracuyá (Passiflora edulis f. flavicarpa Deg.) Como Sub Producto Para Obtener Pectina Usando Como Agente Hidrolizante Ácido Cítrico; Universidad Católica Santo Toribio de Mogrovejo: Chiclayo, Peru, 2013. [Google Scholar]
- Barbieri, R.; Coppo, E.; Marchese, A.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M. Phytochemicals for Human Disease: An Update on Plant-Derived Compounds Antibacterial Activity. Microbiol. Res. 2017, 196, 44–68. [Google Scholar] [CrossRef]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.J. The Role of the Plant Antioxidant System in Drought Tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef]
- Thawabteh, A.; Juma, S.; Bader, M.; Karaman, D.; Scrano, L.; Bufo, S.A.; Karaman, R. The Biological Activity of Natural Alkaloids against Herbivores, Cancerous Cells and Pathogens. Toxins 2019, 11, 656. [Google Scholar] [CrossRef]
- Pratima, H. Antioxidant and Antibacterial Activity of Alkaloid Extract of Cucumis Trigonus ROXB. Int. J. Pharm. Pharm. Sci. 2019, 4, 44–48. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Maheshwaran, L.; Nadarajah, L.; Senadeera, S.P.N.N.; Ranaweera, C.B.; Chandana, A.K.; Pathirana, R.N. Phytochemical Testing Methodologies and Principles for Preliminary Screening/Qualitative Testing. Asian Plant Res. J. 2024, 12, 11–38. [Google Scholar] [CrossRef]
- Shanmugam, S.; Gomes, I.A.; Denadai, M.; dos Santos Lima, B.; de Souza Araújo, A.A.; Narain, N.; Neta, M.T.S.L.; Serafini, M.R.; Quintans-Júnior, L.J.; Thangaraj, P. UHPLC-QqQ-MS/MS Identification, Quantification of Polyphenols from Passiflora Subpeltata Fruit Pulp and Determination of Nutritional, Antioxidant, α-Amylase and α-Glucosidase Key Enzymes Inhibition Properties. Food Res. Int. 2018, 108, 611–620. [Google Scholar] [CrossRef]
- Shi, M.; Ali, M.M.; He, Y.; Ma, S.; Rizwan, H.M.; Yang, Q.; Li, B.; Lin, Z.; Chen, F. Flavonoids Accumulation in Fruit Peel and Expression Profiling of Related Genes in Purple (Passiflora edulis f. edulis) and Yellow (Passiflora edulis f. flavicarpa) Passion Fruits. Plants 2021, 10, 2240. [Google Scholar] [CrossRef]
- Banach, M.; Wiloch, M.; Zawada, K.; Cyplik, W.; Kujawski, W. Evaluation of Antioxidant and Anti-Inflammatory Activity of Anthocyanin-Rich Water-Soluble Aronia Dry Extracts. Molecules 2020, 25, 4055. [Google Scholar] [CrossRef]
- Rafał, I.G.; Króliczewski, B.J.; Górniak, I.; Bartoszewski, R.; Króliczewski, Á.J. Comprehensive Review of Antimicrobial Activities of Plant Flavonoids. Phytochem. Rev. 2018, 18, 241–272. [Google Scholar] [CrossRef]
- Erb, M.; Kliebenstein, D.J. Plant Secondary Metabolites as Defenses, Regulators, and Primary Metabolites: The Blurred Functional Trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef]
- Sousa, M.S.B.; Júnior, J.M.L.; Buarque, D.d.S.; Sousa, M.S.B.; Júnior, J.M.L.; Buarque, D.d.S. Plant Physiological Aspects of Phenolic Compounds; Soto-Hernández, M., García-Mateos, R., Palma-Tenango, M., Eds.; IntechOpen: Mexico City, Mexico, 2019; ISBN 978-1-78984-034-6. [Google Scholar]
- Zuorro, A.; Iannone, A.; Lavecchia, R. Water–Organic Solvent Extraction of Phenolic Antioxidants from Brewers’ Spent Grain. Processes 2019, 7, 126. [Google Scholar] [CrossRef]
- Nasr, A.; Zhou, X.; Liu, T.; Yang, J.; Zhu, G.P. Acetone-Water Mixture Is a Competent Solvent to Extract Phenolics and Antioxidants from Four Organs of Eucalyptus Camaldulensis. Turk. J. Biochem. 2019, 44, 231–239. [Google Scholar] [CrossRef]
- de Souza, C.G.; Rodrigues, T.H.S.; e Silva, L.M.A.; Ribeiro, P.R.V.; de Brito, E.S. Sequential Extraction of Flavonoids and Pectin from Yellow Passion Fruit Rind Using Pressurized Solvent or Ultrasound. J. Sci. Food Agric. 2018, 98, 1362–1368. [Google Scholar] [CrossRef]
- Gomes, S.V.F.; Portugal, L.A.; dos Anjos, J.P.; de Jesus, O.N.; de Oliveira, E.J.; David, J.P.; David, J.M. Accelerated Solvent Extraction of Phenolic Compounds Exploiting a Box-Behnken Design and Quantification of Five Flavonoids by HPLC-DAD in Passiflora Species. Microchem. J. 2017, 132, 28–35. [Google Scholar] [CrossRef]
- Obregón, P.; Obregón, F. Obtaining a Freeze-Dried Food Based on Passion Fruit (Passiflora edulis) and Camu Camu (Myrciaria Dubia). J. Agro-Ind. Sci. 2019, 1, 17–24. [Google Scholar] [CrossRef]
- Cleonice, M.; Souza, B.; Camila Queiroz, N.; Gama, S. Estudo Fitoquímico Do Maracujá-Amarelo (Passiflora edulis Sims Forma Flavicarpa O. Deg.-Passifloraceae) e Perfil Cromatográfico de Sucos de Maracujá. Rev. Eletrônica Perspect. Ciência Tecnol. 2015, 7, 16. [Google Scholar]
- Álvarez, R.; Araya, H.; Navarro-Lisboa, R.; de Dicastillo, C.L. Evaluation of Polyphenol Content and Antioxidant Capacity of Fruits and Vegetables Using a Modified Enzymatic Extraction. Food Technol. Biotechnol. 2016, 54, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Carres, L.; Mas-Capdevila, A.; Bravo, F.I.; Aragonès, G.; Muguerza, B.; Arola-Arnal, A. Optimization of a Polyphenol Extraction Method for Sweet Orange Pulp (Citrus Sinensis L.) to Identify Phenolic Compounds Consumed from Sweet Oranges. PLoS ONE 2019, 14, e0211267. [Google Scholar] [CrossRef]
- Mesén-Mora, L.D.; Carvajal-Miranda, Y.; Álvarez-Valverde, V.; Rodríguez-Rodríguez, G. Estudio Bioprospectivo, Actividad Antibiótica y Antioxidante Del Exocarpio Del Fruto de Santol (Sandoricum Koetjape). Uniciencia 2019, 33, 75–82. [Google Scholar] [CrossRef]
- Martínez-González, M.E.; Balois-Morales, R.; Alia-Tejacal, I.; Cortes-Cruz, M.A.; Palomino-Hermosillo, Y.A.; López-Gúzman, G.G.; Martínez-González, M.E.; Balois-Morales, R.; Alia-Tejacal, I.; Cortes-Cruz, M.A.; et al. Poscosecha de Frutos: Maduración y Cambios Bioquímicos. Rev. Mex. De. Cienc. Agric. 2017, 8, 4075–4087. [Google Scholar] [CrossRef]
- Martínez, R.; Torres, P.; Meneses, M.A.; Figueroa, J.G.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical, Technological and in Vitro Antioxidant Properties of Mango, Guava, Pineapple and Passion Fruit Dietary Fibre Concentrate. Food Chem. 2012, 135, 1520–1526. [Google Scholar] [CrossRef]
- Contreras-Calderón, J.; Calderón-Jaimes, L.; Guerra-Hernández, E.; García-Villanova, B. Antioxidant Capacity, Phenolic Content and Vitamin C in Pulp, Peel and Seed from 24 Exotic Fruits from Colombia. Food Res. Int. 2011, 44, 2047–2053. [Google Scholar] [CrossRef]
- Yepes, A.; Ochoa-Bautista, D.; Murillo-Arango, W.; Quintero-Saumeth, J.; Bravo, K.; Osorio, E. Purple Passion Fruit Seeds (Passiflora edulis f. Edulis Sims) as a Promising Source of Skin Anti-Aging Agents: Enzymatic, Antioxidant and Multi-Level Computational Studies. Arab. J. Chem. 2021, 14, 102905. [Google Scholar] [CrossRef]
- Oliveira, D.A.; Angonese, M.; Gomes, C.; Ferreira, S.R.S. Valorization of Passion Fruit (Passiflora edulis sp.) by-Products: Sustainable Recovery and Biological Activities. J. Supercrit. Fluids 2016, 111, 55–62. [Google Scholar] [CrossRef]
- Li, B.; Lecourt, J.; Bishop, G. Advances in Non-Destructive Early Assessment of Fruit Ripeness towards Defining Optimal Time of Harvest and Yield Prediction—A Review. Plants 2018, 7, 3. [Google Scholar] [CrossRef]
- Syedd-León, R.; Orozco, R.; Álvarez, V.; Carvajal, Y.; Rodríguez, G. Chemical and Antioxidant Charaterization of Native Corn Germplasm from Two Regions of Costa Rica: A Conservation Approach. Int. J. Food Sci. 2020, 2020, 2439541. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.W.; Ayala-Zavala, J.F.; Dhua, R.S. Genotypic Variation in Tomatoes Affecting Processing and Antioxidant Attributes. Crit. Rev. Food Sci. Nutr. 2015, 55, 1819–1835. [Google Scholar] [CrossRef] [PubMed]
- Prinsloo, G.; Nogemane, N. The Effects of Season and Water Availability on Chemical Composition, Secondary Metabolites and Biological Activity in Plants. Phytochem. Rev. 2018, 17, 889–902. [Google Scholar] [CrossRef]
- Chaparro-Roja, D.; Maldonado, E.; Franco-Londoño, C.; Urango-Marchena, A. Características Nutricionales y Antioxidantes de La Fruta Curuba Larga (Passiflora Mollissima Bailey). Perspect. En. Nutr. Humana 2015, 16, 203–212. [Google Scholar] [CrossRef]
- González, L.; Álvarez, A.; Murillo, E.; Guerra, C.; Méndez, J. Potential Uses of the Peel and Seed of Passiflora edulis Sims f. Edulis (Gulupa) from Its Chemical Characterization, Antioxidant, and Antihypertensive Functionalities. Asian J. Pharm. Clin. Res. 2019, 12, 104–112. [Google Scholar] [CrossRef]
- Cazarin, C.B.B.; Da Silva, J.K.; Colomeu, T.C.; Zollner, R.d.L.; Maróstica Junior, M.R. Capacidade Antioxidante e Composição Química Da Casca de Maracujá (Passiflora edulis). Ciência Rural. 2014, 44, 1699–1704. [Google Scholar] [CrossRef]
- Gauba, A.; Rahman, K.M. Evaluation of Antibiotic Resistance Mechanisms in Gram-Negative Bacteria. Antibiotics 2023, 12, 1590. [Google Scholar] [CrossRef]
- Varghese, M.; Balachandran, M. Antibacterial Efficiency of Carbon Dots against Gram-Positive and Gram-Negative Bacteria: A Review. J. Environ. Chem. Eng. 2021, 9, 106821. [Google Scholar] [CrossRef]
- Rizwana, H.; Al Otibi, F.; Al-Malki, N. Chemical Composition, FTIR Studies and Antibacterial Activity of Passiflora edulis f. edulis (Fruit). J. Pure Appl. Microbiol. 2019, 13, 2489–2498. [Google Scholar] [CrossRef]
- de Medeiros Gomes, J.; Terto, M.V.C.; Do Santos, S.G.; da Silva, M.S.; Tavares, J.F. Seasonal Variations of Polyphenols Content, Sun Protection Factor and Antioxidant Activity of Two Lamiaceae Species. Pharmaceutics 2021, 13, 110. [Google Scholar] [CrossRef]
- San Román Avila, D. Desarrollo de Un Método Espectrofotométrico Para La Detección de Alcaloides Pirrolizidínicos (Pas) En Mieles y Polen de Plantas Melíferas de La Península de Yucatán; CIATEJ: Guadalajara, México, 2014. [Google Scholar]
- Carvajal Rojas, L.; Uribe, Y.H.; Sierra Martínez, N.; Rueda Niño, D. Análisis Fitoquímico Preliminar de Hojas, Tallos y Semillas de Cupatá (Strychnos Schultesiana Krukoff). Colomb. For. 2008, 12, 161. [Google Scholar] [CrossRef]
- Donald, D.M.; Valencia, E.F.; Cuyos, M.; Dueñas, R. Extracción, Identificación y Evaluación de Saponinas En Agaricus Bisporus. Biotempo 2017, 5, 3–36. [Google Scholar] [CrossRef][Green Version]
- Oyola Aquino, L.D. Composición Fitoquímica y Actividad Antioxidante del Fruto de Melocactus Peruvianus; Universidad Cesar Vallejos: Trujillo, Peru, 2016. [Google Scholar]
- Carranza Vega, D.E. Determinación de Metabolitos Secundarios del Tallo de Croton Alnifolius l. (Tunga); Universidad Nacional de Trujillo: Trujillo, Peru, 2009. [Google Scholar]
- Miguel Angel, R.-G.; Antonio, A.J.; Melesio, G.-L.; Francisco, R.-F.; Juan Alfredo, M.-D.-R.; Pedro Javier, G.-M.; Jaime Alberto, M.-P.; Carmen Lizette, D.-T.-S. Identificación Cualitativa de Metabolitos Secundarios y Determinación de La Citotoxicidad de Extractos de Tempisque (Sideroxylum Capiri PITTIER). Biotecnia 2016, 18, 3–8. [Google Scholar] [CrossRef]
- Harborne, A. Phytochemical Methods a Guide to Modern Techniques of Plant Analysis; Springer Science & Business Media: London, UK, 1998; ISBN 9780412230509. [Google Scholar]
- Hudzicki, J. Kirby-Bauer Disk Diffusion Susceptibility Test Protocol|ASM.Org. Available online: https://asm.org/Protocols/Kirby-Bauer-Disk-Diffusion-Susceptibility-Test-Pro (accessed on 19 March 2025).

| Metabolite | CM1 | CM2 | CM3 | PM1 | PM2 | PM3 |
|---|---|---|---|---|---|---|
| Alkaloids | ± | ± | + | + | + | |
| Triterpenes | ++ | ++ | ++++ | + | ++ | ++++ |
| Saponins | ++++ | ++++ | ++++ | + | nd | nd |
| Tannins | ± | ± | ± | nd | nd | nd |
| Flavonoids | ++++ | ++++ | ++++ | nd | nd | nd |
| Leucoanthocyanins | nd | nd | ++++ | nd | nd | nd |
| Cardiotonic Glycosides | nd | nd | nd | nd | nd | nd |
| Quinones | nd | nd | nd | nd | nd | nd |
| Anthocyanins | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ |
| Coumarins | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quirós-Cubillo, M.; Valdés-Díaz, S.; Oviedo-Quirós, J.; Álvarez-Valverde, V.; Syedd-León, R. Antioxidant and Antibacterial Potential of Passiflora edulis (Passion Fruit) at Three Ripening Stages for Waste Valorization. Molecules 2025, 30, 3454. https://doi.org/10.3390/molecules30173454
Quirós-Cubillo M, Valdés-Díaz S, Oviedo-Quirós J, Álvarez-Valverde V, Syedd-León R. Antioxidant and Antibacterial Potential of Passiflora edulis (Passion Fruit) at Three Ripening Stages for Waste Valorization. Molecules. 2025; 30(17):3454. https://doi.org/10.3390/molecules30173454
Chicago/Turabian StyleQuirós-Cubillo, Mariela, Sandra Valdés-Díaz, Juan Oviedo-Quirós, Víctor Álvarez-Valverde, and Randall Syedd-León. 2025. "Antioxidant and Antibacterial Potential of Passiflora edulis (Passion Fruit) at Three Ripening Stages for Waste Valorization" Molecules 30, no. 17: 3454. https://doi.org/10.3390/molecules30173454
APA StyleQuirós-Cubillo, M., Valdés-Díaz, S., Oviedo-Quirós, J., Álvarez-Valverde, V., & Syedd-León, R. (2025). Antioxidant and Antibacterial Potential of Passiflora edulis (Passion Fruit) at Three Ripening Stages for Waste Valorization. Molecules, 30(17), 3454. https://doi.org/10.3390/molecules30173454







