High-Yield Vanillin Production Through RSM-Optimized Solid-State Fermentation Process from Brewer’s Spent Grains in a Single-Use Bag Bioreactor
Abstract
1. Introduction
2. Results and Discussion
2.1. Screening Scale of Solid-State Fermentation
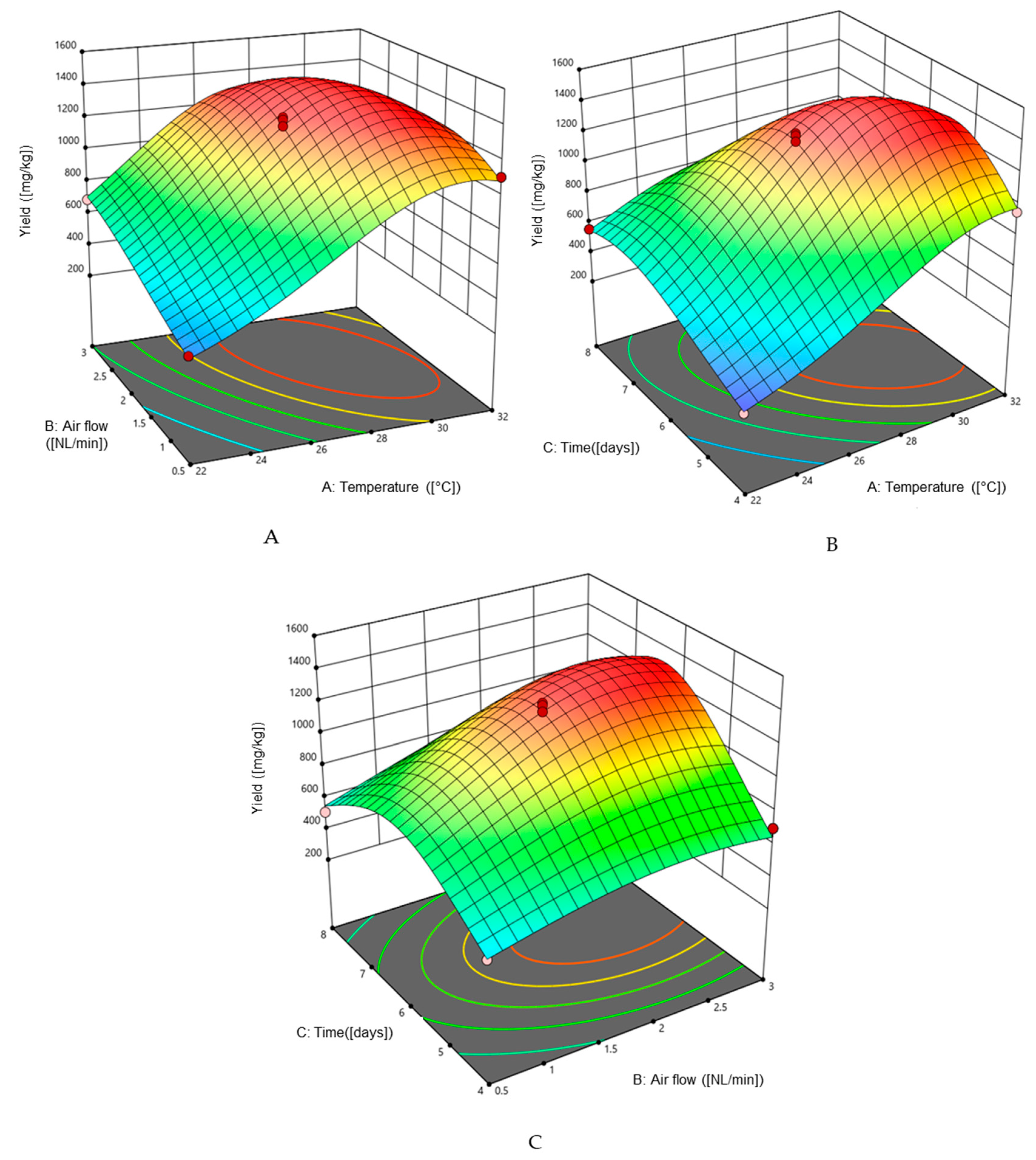
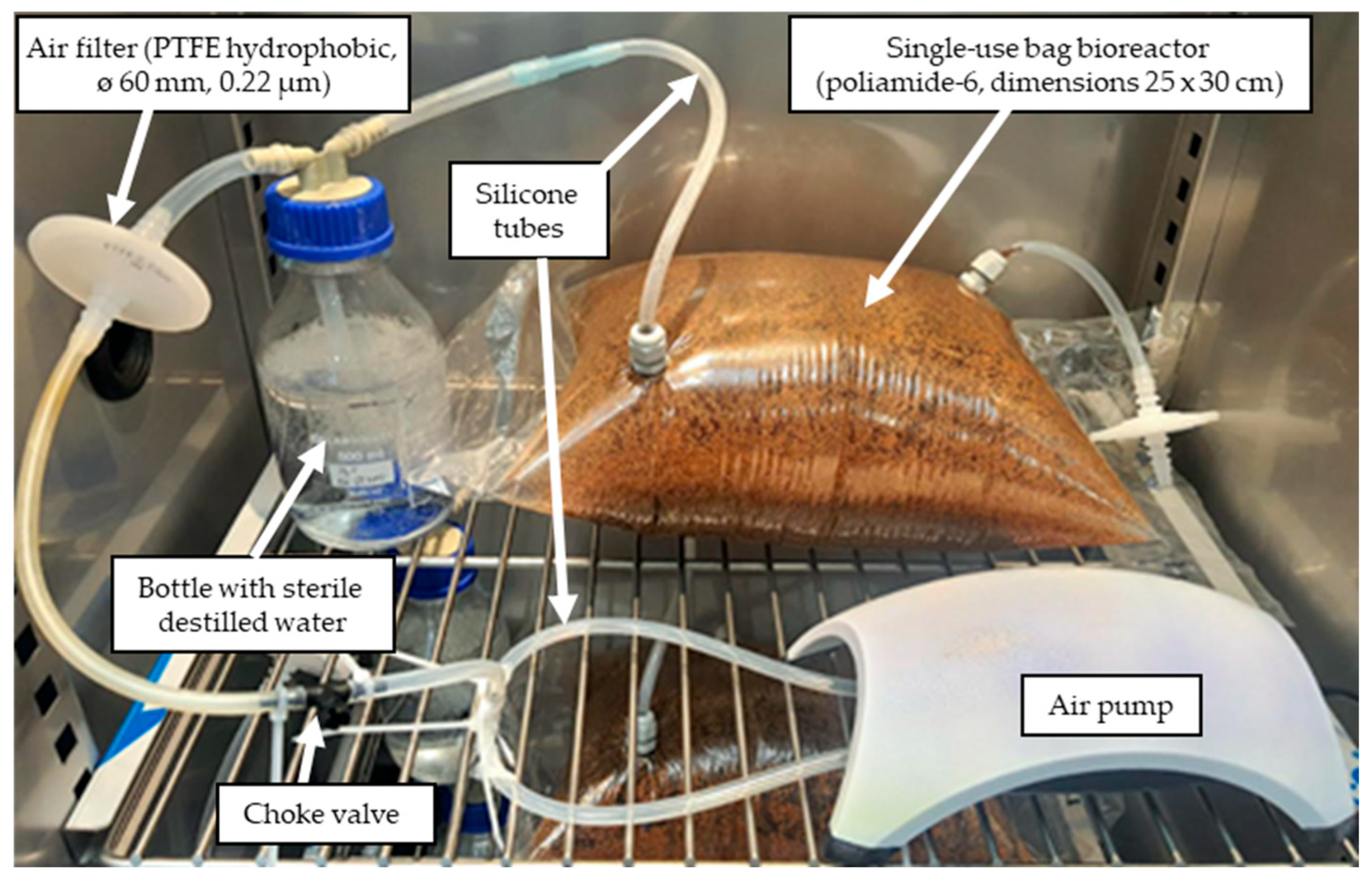
2.2. Preparative Scale of Solid-State Fermentation
+ 0.000811 × Temperature × Air flow + 0.000735 × Temperature × Time − 0.000272 × Air flow × Time +
0.000307 × Temperature2 + 0.002116 × Air flow2 + 0.002195 × Time2
3. Materials and Methods
3.1. Raw Materials and Chemicals
3.2. Microorganisms
3.3. Screening Scale of Solid-State Fermentation and Optimization of the Process Parameters by RSM
3.4. Preparative Scale of Solid-State Fermentation and Optimization of the Process Parameters by RSM
3.5. Extraction Procedure
3.6. Preparative Scale Extraction Procedure
3.7. Analysis Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olatunde, A.; Mohammed, A.; Ibrahim, M.A.; Tajuddeen, N.; Shuaibu, M.N. Vanillin: A Food Additive with Multiple Biological Activities. Eur. J. Med. Chem. Rep. 2022, 5, 100055. [Google Scholar] [CrossRef]
- Sharma, A.; Sahu, S.; Sharma, S.; Singh, G.; Arya, S.K. Valorization of Agro-Industrial Wastes into Vanillin: A Sustainable and Bio-Economical Step towards the Indigenous Production of Flavors. Biocatal. Agric. Biotechnol. 2023, 54, 102904. [Google Scholar] [CrossRef]
- Huang, W.B.; Du, C.Y.; Jiang, J.A.; Ji, Y.F. Concurrent Synthesis of Vanillin and Isovanillin. Res. Chem. Intermed. 2013, 39, 2849–2856. [Google Scholar] [CrossRef]
- D’Arrigo, P.; Rossato, L.A.M.; Strini, A.; Serra, S. From Waste to Value: Recent Insights into Producing Vanillin from Lignin. Molecules 2024, 29, 442. [Google Scholar] [CrossRef]
- Krishnan, D.; Rameshpathy, M. A Renewable Natural Resource for Ferulic Acid; An Efficient Precursor in Biotechnological Production of Vanillin and Strategies to Enhance the Yield of Bio-Vanillin from Ferulic Acid-Review. Process Biochem. 2025, 149, 181–191. [Google Scholar] [CrossRef]
- Bento-Silva, A.; Vaz Patto, M.C.; do Rosário Bronze, M. Relevance, Structure and Analysis of Ferulic Acid in Maize Cell Walls. Food Chem. 2018, 246, 360–378. [Google Scholar] [CrossRef]
- Bhanja Dey, T.; Chakraborty, S.; Jain, K.K.; Sharma, A.; Kuhad, R.C. Antioxidant Phenolics and Their Microbial Production by Submerged and Solid State Fermentation Process: A Review. Trends Food Sci. Technol. 2016, 53, 60–74. [Google Scholar] [CrossRef]
- Saeed, S.; Raza, S.Q.; Zafar, S.S.; Mujahid, H.; Irfan, M.; Mehmood, T. Microbial Conversion of Pomegranate Peels to Biovanillin Using Submerged Fermentation and Process Optimization through Statistical Design. Biomass Convers. Biorefin. 2024, 14, 679–688. [Google Scholar] [CrossRef]
- Rejani, C.T.; Radhakrishnan, S. Microbial Conversion of Vanillin from Ferulic Acid Extracted from Raw Coir Pith. Nat. Prod. Res. 2022, 36, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, P.; Banerjee, G.; Sen, S.K. Cleaner Production of Vanillin through Biotransformation of Ferulic Acid Esters from Agroresidue by Streptomyces Sannanensis. J. Clean. Prod. 2018, 182, 272–279. [Google Scholar] [CrossRef]
- Pattnaik, B.; Sarangi, P.K.; Jena, P.K.; Sahoo, H.P.; Bhatia, L. Production of Phenolic Flavoring Compounds from Sugarcane Bagasse by Lactobacillus Acidophilus MTCC 10307. Arch. Microbiol. 2022, 204, 23. [Google Scholar] [CrossRef]
- Mehmood, T.; Saeed, S.; Hussain, N.; Waseem, R. Biotransformation of Wheat Straw into Biovanillin by Solid-State Fermentation and Optimization of Conditions Parameters through Response Surface Methodology. Biomass Convers. Biorefin. 2024, 14, 7569–7578. [Google Scholar] [CrossRef]
- Bucci, P.; Martínez-Navarrete, M.; Marti-Quijal, F.J.; José Guillot, A.; Barba, F.J.; Ferrer, E.; Cantero, D.; Muñoz, R.; Melero, A. In Vivo Reduction of Skin Inflammation Using Ferulic Acid-Loaded Lipid Vesicles Derived from Brewer’s Spent Grain. Int. J. Pharm. 2024, 666, 124764. [Google Scholar] [CrossRef]
- Becker, D.; Stegmüller, S.; Richling, E. Characterization of Brewer’s Spent Grain Extracts by Tandem Mass Spectrometry and HPLC-DAD: Ferulic Acid Dehydrodimers, Phenolamides, and Oxylipins. Food Sci. Nutr. 2023, 11, 2298–2320. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, R.; Ciccoritti, R.; Ceccantoni, B.; Bellincontro, A.; Amoriello, T. Optimization of Phenolic Compound Extraction from Brewers’ Spent Grain Using Ultrasound Technologies Coupled with Response Surface Methodology. Sustainability 2022, 14, 3309. [Google Scholar] [CrossRef]
- Singh, D.; Chen, S. The White-Rot Fungus Phanerochaete Chrysosporium: Conditions for the Production of Lignin-Degrading Enzymes. Appl. Microbiol. Biotechnol. 2008, 81, 399–417. [Google Scholar] [CrossRef]
- Akbar Aly, A.B.; Shanmugaraj, B.; Ramalingam, S. Industrial Applications of Phanerochaete Chrysosporium Lignin-Degrading Enzymes: Current Status, Production Challenges, and Future Directions. World J. Microbiol. Biotechnol. 2025, 41, 171. [Google Scholar] [CrossRef]
- van der Made, J.J.A.; Landis, E.A.; Deans, G.T.; Lai, R.A.; Chandran, K. Synergistic Lignin Degradation between Phanerochaete Chrysosporium and Fenton Chemistry Is Mediated through Iron Cycling and Ligninolytic Enzyme Induction. Sci. Total Environ. 2023, 905, 166767. [Google Scholar] [CrossRef] [PubMed]
- Atiwesh, G.; Parrish, C.C.; Banoub, J.; Le, T.A.T. Lignin Degradation by Microorganisms: A Review. Biotechnol. Prog. 2022, 38, e3226. [Google Scholar] [CrossRef]
- Li, S.F.; Wang, H.; Chen, J.L.; Zhu, H.X.; Yao, R.S.; Wu, H. Degradation and Transformation of Lignin by a Fungus Aspergillus Flavus Strain F-1. Iran. J. Biotechnol. 2020, 18, 62–69. [Google Scholar] [CrossRef]
- Oddou, J.; Stentelaire, C.; Lesage-Meessen, L.; Asther, M.; Colonna Ceccaldi, B. Improvement of Ferulic Acid Bioconversion into Vanillin by Use of High-Density Cultures of Pycnoporus Cinnabarinus. Appl. Microbiol. Biotechnol. 1999, 53, 1–6. [Google Scholar] [CrossRef]
- Falconnier, B.; Lapierre, C.; Lesage-Meessen, L.; Yonnet, G.; Brunerie, P.; Colonna-Ceccaldi, B.; Corrieu, G.; Asther, M. Vanillin as a Product of Ferulic Acid Biotransformation by the White-Rot Fungus Pycnoporus Cinnabarinus I-937: Identification of Metabolic Pathways. J. Biotechnol. 1994, 37, 123–132. [Google Scholar] [CrossRef]
- Tang, P.L.; Hassan, O. Bioconversion of Ferulic Acid Attained from Pineapple Peels and Pineapple Crown Leaves into Vanillic Acid and Vanillin by Aspergillus niger I-1472. BMC Chem. 2020, 14, 7. [Google Scholar] [CrossRef]
- Tilay, A.; Bule, M.; Annapure, U. Production of Biovanillin by One-Step Biotransformation Using Fungus Pycnoporous Cinnabarinus. J. Agric. Food Chem. 2010, 58, 4401–4405. [Google Scholar] [CrossRef]
- dos Santos Barbosa, E.; Perrone, D.; do Amaral Vendramini, A.L.; Ferreira Leite, S.G. Vanillin Production by Phanerochaete Chrysosporium Grown on Green Coconut Agroindustrial Husk in Solid State Fermentation. Bioresources 2008, 3, 1042–1050. [Google Scholar] [CrossRef]
- Moreira, M.T.; Feijoo, G.; Lema, J.M. Fungal Bioreactors: Applications to White-Rot Fungi. Rev. Environ. Sci. Biotechnol. 2003, 2, 247–259. [Google Scholar] [CrossRef]
- Sibhatu, H.K.; Anuradha Jabasingh, S.; Yimam, A.; Ahmed, S. Ferulic Acid Production from Brewery Spent Grains, an Agro-Industrial Waste. LWT 2021, 135, 110009. [Google Scholar] [CrossRef]
- Paul, V.; Agarwal, A.; Dutt Tripathi, A.; Sirohi, R. Valorization of Lignin for the Production of Vanillin by Bacillus Aryabhattai NCIM 5503. Bioresour. Technol. 2023, 385, 129420. [Google Scholar] [CrossRef] [PubMed]
- Nirwana, W.O.C.; Hung, I.H.; Shu, C.H. Enhancing the Bioconversion of Ferulic Acid from Alkaline Hydrolysate of Corn Cobs to Vanillin by Amycolatopsis Thermoflava under Nutrient Limitation and Reducing Sugar Control. J. Chem. Technol. Biotechnol. 2023, 98, 238–246. [Google Scholar] [CrossRef]
- Sujatha, M.; Jaya Madhuri, R.; Thagaraju, K. Antimicrobial Potential of Bio Vanillin an Industrial Product from Bacillus Subtilis Sp., MSJM5. J. Pure Appl. Microbiol. 2022, 16, 1755–1761. [Google Scholar] [CrossRef]
- Nurika, I.; Suhartini, S.; Azizah, N.; Barker, G.C. Extraction of Vanillin Following Bioconversion of Rice Straw and Its Optimization by Response Surface Methodology. Molecules 2020, 25, 6031. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, T.; Saleem, F.; Javed, S.; Nawaz, S.; Sultan, A.; Safdar, A.; Ullah, A.; Waseem, R.; Saeed, S.; Abbas, M.; et al. Biotransformation of Agricultural By-Products into Biovanillin through Solid-State Fermentation (SSF) and Optimization of Different Parameters Using Response Surface Methodology (RSM). Fermentation 2022, 8, 206. [Google Scholar] [CrossRef]
| Run No. | A | B | C | D | Vanillin Content in the Extracts [mg/kg d.m. of Substrate] | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aspergillus sp. AM31 | P. chrysosporium CBS246.84 | P. chrysosporium CBS481.73 | A. flavus KKP3556 | P. cinnabarinus DSM3022 | P. chrysosporium DSM6909 | F. culmorum MUT5855 | |||||
| 2 | 60 | 35 | 0.5 | 0.3 | 70 | 221 | 172 | 137 | 204 | 132 | 134 |
| 5 | 60 | 30 | 0.5 | 0.4 | 95 | 363 | 229 | 123 | 114 | 164 | 186 |
| 6 | 70 | 30 | 0.5 | 0.3 | 121 | 194 | 183 | 218 | 139 | 148 | 103 |
| 23 | 60 | 25 | 0.5 | 0.3 | 176 | 298 | 189 | 129 | 85 | 139 | 203 |
| Run No. | A | B | C | Vanillin Content in the Extracts [mg/kg d.m. of Substrate] |
|---|---|---|---|---|
| 1 | 32 | 1.75 | 4 | 957 |
| 2 | 22 | 1.75 | 8 | 559.7 |
| 3 | 22 | 0.5 | 6 | 346.6 |
| 4 | 27 | 1.75 | 6 | 1321.8 |
| 5 | 27 | 0.5 | 4 | 490.5 |
| 6 | 32 | 1.75 | 8 | 575.2 |
| 7 | 27 | 1.75 | 6 | 1268.9 |
| 8 | 32 | 0.5 | 6 | 1107.9 |
| 9 | 27 | 1.75 | 6 | 1269.6 |
| 10 | 27 | 1.75 | 6 | 1137 |
| 11 | 32 | 3 | 6 | 827 |
| 12 | 27 | 3 | 4 | 704.2 |
| 13 | 27 | 1.75 | 6 | 1306.8 |
| 14 | 27 | 0.5 | 8 | 511 |
| 15 | 22 | 3 | 6 | 686.3 |
| 16 | 27 | 3 | 8 | 862.4 |
| 17 | 22 | 1.75 | 4 | 257.6 |
| Source | Sum of Squares | df 1 | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 0.0015 | 9 | 0.0002 | 80.31 | <0.0001 | significant |
| A—Temperature | 0.0004 | 1 | 0.0004 | 194.06 | <0.0001 | |
| B—Air flow | 0.0001 | 1 | 0.0001 | 47.36 | 0.0002 | |
| C—Time | 0.0000 | 1 | 0.0000 | 13.52 | 0.0079 | |
| AB | 0.0001 | 1 | 0.0001 | 48.03 | 0.0002 | |
| AC | 0.0002 | 1 | 0.0002 | 101.08 | <0.0001 | |
| BC | 1.844 × 10−6 | 1 | 1.844 × 10−6 | 0.8621 | 0.3840 | |
| A2 | 0.0002 | 1 | 0.0002 | 115.98 | <0.0001 | |
| B2 | 0.0000 | 1 | 0.0000 | 21.53 | 0.0024 | |
| C2 | 0.0003 | 1 | 0.0003 | 151.85 | <0.0001 | |
| Residual | 0.0000 | 7 | 2.138 × 10−6 | |||
| Lack of fit | 0.0000 | 3 | 4.015 × 10−6 | 5.49 | 0.0667 | not significant |
| Pure error | 2.925 × 10−6 | 4 | 7.313 × 10−7 | |||
| Cor Total | 0.0016 | 16 |
| Run No. | A | B | C | D |
|---|---|---|---|---|
| 1 | 60 | 35 | 2 | 0.2 |
| 2 | 60 | 35 | 0.5 | 0.3 |
| 3 | 70 | 25 | 2 | 0.3 |
| 4 | 60 | 30 | 2 | 0.3 |
| 5 | 60 | 30 | 0.5 | 0.4 |
| 6 | 70 | 30 | 0.5 | 0.3 |
| 7 | 60 | 30 | 0.5 | 0.2 |
| 8 | 50 | 35 | 2 | 0.3 |
| 9 | 60 | 25 | 2 | 0.4 |
| 10 | 60 | 30 | 2 | 0.3 |
| 11 | 60 | 35 | 3.5 | 0.3 |
| 12 | 60 | 30 | 3.5 | 0.2 |
| 13 | 60 | 25 | 2 | 0.2 |
| 14 | 70 | 35 | 2 | 0.3 |
| 15 | 70 | 30 | 3.5 | 0.3 |
| 16 | 50 | 30 | 2 | 0.4 |
| 17 | 60 | 25 | 3.5 | 0.3 |
| 18 | 50 | 25 | 2 | 0.3 |
| 19 | 70 | 30 | 2 | 0.2 |
| 20 | 70 | 30 | 2 | 0.4 |
| 21 | 60 | 35 | 2 | 0.4 |
| 22 | 50 | 30 | 0.5 | 0.3 |
| 23 | 60 | 25 | 0.5 | 0.3 |
| 24 | 60 | 30 | 2 | 0.3 |
| 25 | 60 | 30 | 3.5 | 0.4 |
| 26 | 60 | 30 | 2 | 0.3 |
| 27 | 50 | 30 | 3.5 | 0.3 |
| 28 | 50 | 30 | 2 | 0.2 |
| 29 | 60 | 30 | 2 | 0.3 |
| Run No. | A | B | C |
|---|---|---|---|
| 1 | 32 | 1.75 | 4 |
| 2 | 22 | 1.75 | 8 |
| 3 | 22 | 0.5 | 6 |
| 4 | 27 | 1.75 | 6 |
| 5 | 27 | 0.5 | 4 |
| 6 | 32 | 1.75 | 8 |
| 7 | 27 | 1.75 | 6 |
| 8 | 32 | 0.5 | 6 |
| 9 | 27 | 1.75 | 6 |
| 10 | 27 | 1.75 | 6 |
| 11 | 32 | 3 | 6 |
| 12 | 27 | 3 | 4 |
| 13 | 27 | 1.75 | 6 |
| 14 | 27 | 0.5 | 8 |
| 15 | 22 | 3 | 6 |
| 16 | 27 | 3 | 8 |
| 17 | 22 | 1.75 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczepańska, E.; Pietrzak, W.; Boratyński, F. High-Yield Vanillin Production Through RSM-Optimized Solid-State Fermentation Process from Brewer’s Spent Grains in a Single-Use Bag Bioreactor. Molecules 2025, 30, 3452. https://doi.org/10.3390/molecules30173452
Szczepańska E, Pietrzak W, Boratyński F. High-Yield Vanillin Production Through RSM-Optimized Solid-State Fermentation Process from Brewer’s Spent Grains in a Single-Use Bag Bioreactor. Molecules. 2025; 30(17):3452. https://doi.org/10.3390/molecules30173452
Chicago/Turabian StyleSzczepańska, Ewa, Witold Pietrzak, and Filip Boratyński. 2025. "High-Yield Vanillin Production Through RSM-Optimized Solid-State Fermentation Process from Brewer’s Spent Grains in a Single-Use Bag Bioreactor" Molecules 30, no. 17: 3452. https://doi.org/10.3390/molecules30173452
APA StyleSzczepańska, E., Pietrzak, W., & Boratyński, F. (2025). High-Yield Vanillin Production Through RSM-Optimized Solid-State Fermentation Process from Brewer’s Spent Grains in a Single-Use Bag Bioreactor. Molecules, 30(17), 3452. https://doi.org/10.3390/molecules30173452





