Herbal and Spice Additives in Functional Confectionery Products: A Review
Abstract
1. Introduction
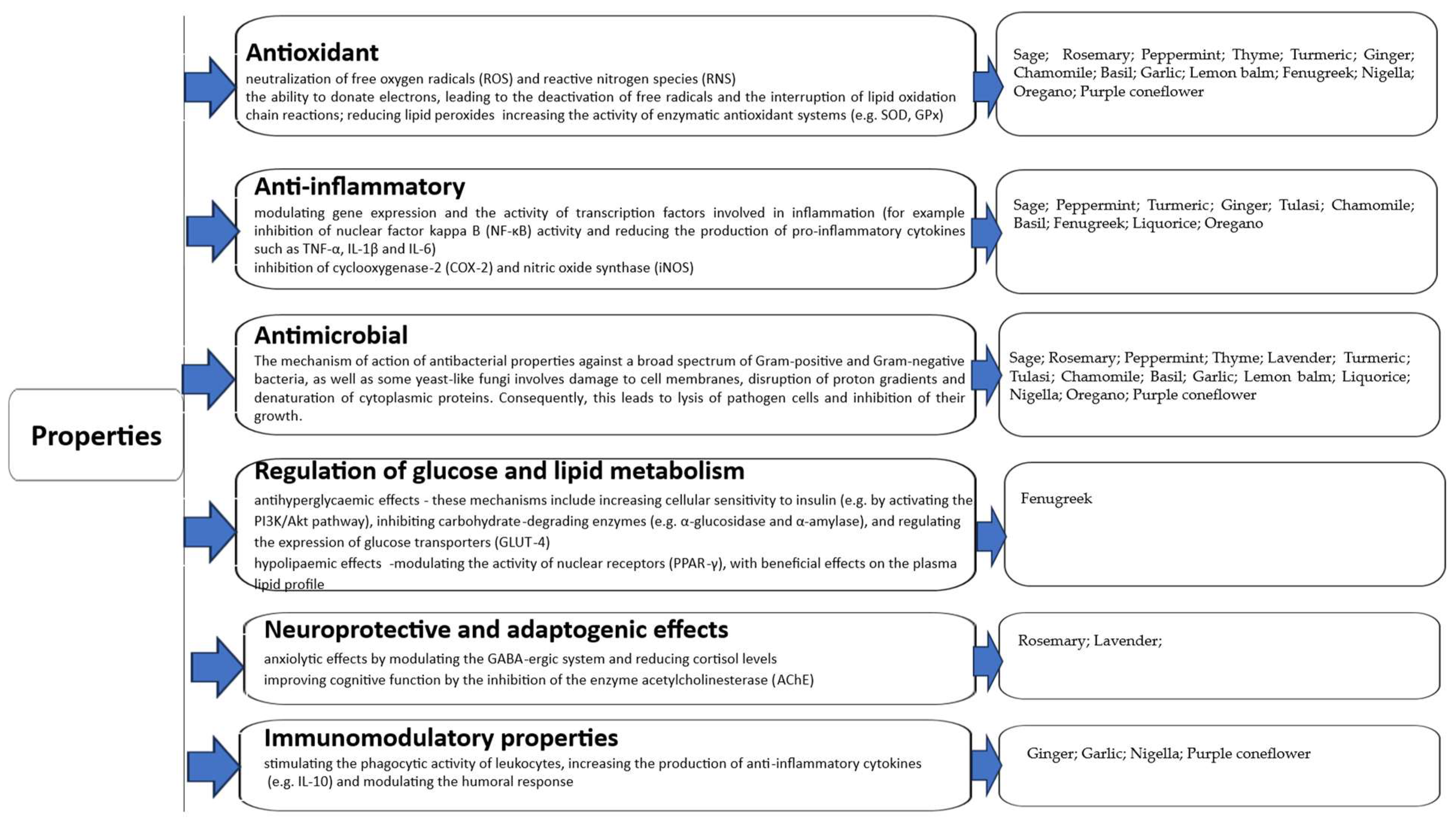
2. Bioactive Properties of Selected Herbs and Spices
Mechanism of Action, Synergistic Potential and Standardisation of Constituents
3. Functional Food Applications
3.1. Herbs in Dairy and Fermented Products
3.2. Herbs in Bakery and Cereal Products
3.3. Herbs in Meat Products and Plant Alternatives
3.4. Herbs in Functional Drinks
3.5. Technological Aspects and Challenges
4. Functional Confectionery Products
4.1. Flour-Based Functional Confectionery
4.2. Dairy Functional Confectionery
4.3. Functional Chocolate and Chocolate Products
4.4. Sugar-Based Functional Confectionery
5. Confectionery Products Applications
| Material Used for Enrichment | Product | Size and Form of the Addition | Impact on Composition and Health-Promoting Effect | Impact on Technological and Sensory Quality | References |
|---|---|---|---|---|---|
| Cinnamon | Butter biscuits | Powder (2, 4 and 6%) | Increase protein, ash and dietary fibre contents | Increment of firmness reduction of crispiness Sensory evaluation: 2%—not different 4%—lower scores for aroma and appearance 6%—lower scores for all parameters (aroma, colour, appearance, crispiness, flavour and overall acceptance) | [192] |
| Rosemary | Shortcrust cookies | Freeze-dried aqueous extracts (0.1, 0.2 and 0.5%) | Increase of total polyphenol content and DPPH radical scavenging capacity (0.5%), Reduction of acrylamide formation | Increment of stickiness (all sizes of addition) and firmness (0.2 and 0.5%) Slight decrease in overall sensory quality | [196] |
| Thyme | Cake | Free essential oil carried by cereal alcohol (0.125 mg/mL), Essential oil in micro particles carried by cereal alcohol (0.125 mg/mL and 0.600 mg/mL) | Antibacterial activity | Preserving cakes (microencapsulation thyme oil acts 10 times better) | [203] |
| Clove bud | Cake | Hot plate-roasted, Microwave- roasted and Unroasted clove bud powder (0.4 g, 0.6 g and 0.8 g) | Increase in total polyphenol content increase in antioxidant activity: DPPH and ABTS radical scavenging capacity (all supplemented samples), Reducing power (samples with roasted clove bud addition) antimicrobial properties (increasing in all supplemented cakes) | The penetration value of all clove powder-added cakes decreased during the storage period that suggests a softer, more tender cake Sensory quality: decrease in overall acceptability (the largest for the sample with 0.8 g addition of clove) increasing oxidative stability of cakes | [197] |
| Clove | Cookies | Clove powder (0.5, 1, 1.5, 2%) | Increment of minerals (K, Na, Mg, Fe, P, Zn, Ca) and ash content Increase of total polyphenol content and DPPH radical scavenging capacity | Improving pasting properties (peak viscosity, breakdown, final viscosity, and setback) improving the texture properties (hardness, cohesiveness, springiness, adhesiveness, and chewiness) Change in colour (decrease of the lightness (L*) and yellowness (b*) values, but increase of the redness (a*) slight decrease in sensory quality (especially of shape, crunchiness, and overall acceptability) Improving the storage stability (the oxidative stability and antimicrobial properties) | [193] |
| Lemon balm | Chocolate muffins | Extract (2 mg/g) | Increase in antioxidant activity | Inhibition of fungal and bacterial growth. No change in physical parameters, except increased springiness, and decrease in lightness. Preserving quality similar to sorbate | [200] |
| Oregano | Chocolate muffins | Extract (2 mg/g) | Increase in antioxidant activity | Inhibition of fungal and bacterial growth. No change in physical parameters, except increased springiness, and decrease in lightness. Preserving quality similar to sorbate | [200] |
| Rosemary | Chocolate muffins | Extract (2 mg/g) | Significant increase in antioxidant activity | Inhibition of fungal and bacterial growth. No change in physical parameters, except increased springiness, and decrease in lightness | [200] |
| Fennel | Cocoa based bar | essential oil (1%) | Physicochemical and microbiological evaluations demonstrated that the cacao bar enriched with fennel essential oil complies with quality standards | Sensory quality: differences in acceptability depending on the amount of addition: the most acceptable samples with 1% addition | [202] |
| Sakura green tea leaves | Dark chocolate | Leaves powder (2%) | Increase of total polyphenol content (additionally, a change in proportions of individual classes of phenolic compounds) Increase in antioxidant activity: DPPH and RP | Not analysed | [198] |
| Turmeric | Dark chocolate | Turmeric powder (8%) | Increase of total polyphenol content (additionally, a change in proportions of individual classes of phenolic compounds) Increase in antioxidant activity: DPPH and RP | Not analysed | [198] |
| Cinnamon | Dark chocolate | Powder (4.2%) | Enrichment in bioactive compounds, i.e., copaene, cinnamaldehyde, 4-methylene-cyclohexene, bicyclo (3.1.1.heptane, 6-methyl) and bicyclo (3.1. hexan-2-ol, methyl) | Sensory quality: decreasing the evaluation of bitterness, chocolate, coarseness, thickness, and hardness; no effect on sweetness, cinnamon odour, chocolate odour, and overall acceptability | [199] |
| Cinnamon bark | Dark chocolate | Cinnamon bark oleoresin microcapsules (4, 6 and 8%) | Increase of total phenolic compounds and tocopherols content and DPPH radical scavenging capacity | Sensory quality—without changes Colour: Change in colour (increase of the lightness (L*), yellowness (b*) values, and redness (a*) Texture properties: increasing of hardness | [169] |
| Cinnamon | Dark chocolate | Essential oil (0.25, 0.50 and 0.75%) | Not analysed | Sensory quality: differences in acceptability depending on the amount of addition: the most acceptable samples with 0.25% addition, the least acceptable with 0.75% addition. Colour: Change in colour (increase of the lightness (L*), but no statistically significant differences in yellowness (b*) values, and redness (a*) | [168] |
| Lemongrass | Ice cream | Distillate (2.50% and 3.50%) and Leaf powder (0.70 and 0.75%) | Ice creams prepared using lemongrass powder had significantly higher fat, protein, carbohydrates and total solids content when compared to ice creams prepared using lemongrass distillates (the samples were not compared with ice cream without additives) | Sensory quality: ice creams prepared using lemongrass distillates were rated higher than those with added powder (the samples were not compared with ice cream without additives) | [207] |
| Curry leaf | Ice cream | Distillate (2.50% and 3.50%) and Leaf powder (0.70 and 0.75%) | Ice creams prepared using curry leaf powder had significantly higher fat, protein, carbohydrate and total solids content when compared to ice creams prepared using curry leaf distillates (the samples were not compared with ice cream without additives) | Sensory quality: ice creams prepared using curry leaf distillates were rated higher than those with added powder (the samples were not compared with ice cream without additives) | [207] |
| Ginger | Ice cream | Ginger juice, ginger paste and ginger syrup (5%) | Decrease in total solids, total soluble solids, fat content, and an increase in ash content increase in antioxidant activity (RP) antimicrobial properties | Increased the first dripping time Textural Properties: higher values for all textural properties (hardness, springiness, cohesiveness, gumminess and chewiness) Sensory quality: Ice creams with the addition of different forms of ginger were rated higher in taste, texture, aroma and overall acceptability (most preferred sample with syrup) | [194] |
| Tulsi, Ginger, Clove | Ice cream | Tulsi paste (2.5%), Ginger juice (2.0%) and Clove extract (4.0%) | Decrease in total solids, carbohydrate content, and an increase in fat, protein, and ash content | Reduction of meltdown time Specific gravity—no changes | [195] |
| Psydrax umbellata, | Herbal candy | Leaf extract (5 g) | Minor increases in protein, ash, fibre, vitamin C, total minerals, total phenolics content Increase in antioxidant activity (DPPH radical scavenging capacity) | Sensory quality—without changes in appearance, taste, texture, overall acceptance, but slightly lower in odour score | [182] |
6. Technological Aspects, Quality Control and Safety of Functional Confectionery Products
7. Conclusions and Directions for Further Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Drewnowski, A.; Rehm, C.D. Consumption of added sugars among US children and adults by food purchase location and food source. Am. J. Clin. Nutr. 2014, 100, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Gunes, R.; Palabiyik, I.; Konar, N.; Toker, O.S. Soft confectionery products: Quality parameters, interactions with processing and ingredients. Food Chem. 2022, 385, 132735. [Google Scholar] [CrossRef] [PubMed]
- Arshad, S.; Rehman, T.; Saif, S.; Rajoka, M.S.R.; Ranjha, M.M.A.N.; Hassoun, A.; Cropotova, J.; Trif, M.; Younas, A.; Aadil, R.M. Replacement of refined sugar by natural sweeteners: Focus on potential health benefits. Heliyon 2022, 8, e10711. [Google Scholar] [CrossRef] [PubMed]
- Essa, M.M.; Bishir, M.; Bhat, A.; Chidambaram, S.B.; Al-Balushi, B.; Hamdan, H.; Govindarajan, N.; Freidland, R.P.; Qoronfleh, M.W. Functional foods and their impact on health. J. Food Sci. Technol. 2023, 60, 820–834. [Google Scholar] [CrossRef]
- Žuljević, S.O.; Akagić, A. Flour-Based Confectionery as Functional Food. In Functional Foods: Phytochemicals and Health Promoting Potential; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Das, M.; Tiwari, P.; Mondal, M.; Khalua, R.K. A review on milk cake: A traditional dairy product. Int. J. Food Sci. Nutr. 2023, 8, 77–79. [Google Scholar]
- Hasler, C.M. Functional foods: Benefits, concerns and challenges—A position paper from the American Council on Science and Health. J. Nutr. 2002, 132, 3772–3781. [Google Scholar] [CrossRef]
- Thiviya, P.; Gamage, A.; Piumali, D.; Merah, O.; Madhujith, T. Apiaceae as an important source of antioxidants and their applications. Cosmetics 2021, 8, 111. [Google Scholar] [CrossRef]
- Yu, X.; Xia, K.; Wu, S.; Wang, Q.; Cheng, W.; Ji, C.; Yang, W.; Kang, C.; Yuan, Z.; Li, Y. Simultaneous determination and pharmacokinetic study of six components in beagle dog plasma by UPLC–MS/MS after oral administration of Astragalus Membranaceus aqueous extract. Biomed. Chromatogr. 2022, 36, e5488. [Google Scholar] [CrossRef]
- Oroian, M.; Escriche, I. Antioxidants: Characterization, natural sources, extraction and analysis. Food Res. Int. 2015, 74, 10–36. [Google Scholar] [CrossRef]
- Tapsell, L.C.; Hemphill, I.; Cobiac, L.; Sullivan, D.R.; Fenech, M.; Patch, C.S.; Roodenrys, S.; Keogh, J.B.; Clifton, P.M.; Williams, P.G.; et al. Health benefits of herbs and spices: The past, the present, the future. Med. J. Aust. 2006, 185, S1–S24. [Google Scholar] [CrossRef]
- Kailey, R.; Rasane, P.; Singh, J.; Kaur, S.; Gunjal, M.; Kaur, J.; Bhadariya, V.; Avinashe, H. Herbal Candies: A Potential Source of Health Benefits. Curr. Nutr. Food Sci. 2024, 20, 1039–1048. [Google Scholar] [CrossRef]
- Calderón-Oliver, M.; Ponce-Alquicira, E. The role of microencapsulation in food application. Molecules 2022, 27, 1499. [Google Scholar] [CrossRef] [PubMed]
- Tatasciore, S.; Santarelli, V.; Neri, L.; Ortega, R.G.; Faieta, M.; Di Mattia, C.D.; Di Michele, A.; Pittia, P. Freeze-drying microencapsulation of Hop extract: Effect of carrier composition on physical, techno-functional, and stability properties. Antioxidants 2023, 12, 442. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects–A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Wink, M. Modes of action of herbal medicines and plant secondary metabolites. Medicines 2015, 2, 251–286. [Google Scholar] [CrossRef]
- Grigore-Gurgu, L.; Dumitrașcu, L.; Aprodu, I. Aromatic herbs as a source of bioactive compounds: An overview of their antioxidant capacity, antimicrobial activity, and major applications. Molecules 2025, 30, 1304. [Google Scholar] [CrossRef]
- Riaz, M.; Khalid, R.; Afzal, M.; Anjum, F.; Fatima, H.; Zia, S.; Rasool, G.; Egbuna, C.; Mtewa, A.G.; Uche, C.Z.; et al. Phytobioactive compounds as therapeutic agents for human diseases: A review. Food Sci. Nutr. 2023, 11, 2500–2529. [Google Scholar] [CrossRef]
- Kintzios, S.E. The Genus Salvia. Medicinal and Aromatic Plants: Industrial Profiles; CRC Press: Boca Raton, FL, USA, 2000; p. 14. [Google Scholar]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med. 2017, 7, 433–440. [Google Scholar] [CrossRef]
- Zdolec, N.; Franičević, M.; Klanac, L.; Kavain, I.; Batinić, J.; Zadravec, M.; Pleadin, J.; Čobanov, D.; Kiš, M. Antimicrobial Properties of Basil (Ocimum basilicum L.), Sage (Salvia officinalis L.), Lavender (Lavandula officinalis L.), Immortelle (Helichrysum italicum (Roth) G. Don), and Savory (Satureja montana L.) and Their Application in Hard Cheese Production. Hygiene 2024, 4, 135–145. [Google Scholar] [CrossRef]
- Brindisi, M.; Bouzidi, C.; Frattaruolo, L.; Loizzo, M.R.; Cappello, M.S.; Dugay, A.; Deguin, B.; Lauria, G.; Cappello, A.R.; Tundis, R. New insights into the antioxidant and anti-inflammatory effects of Italian Salvia officinalis leaf and flower extracts in lipopolysaccharide and tumor-mediated inflammation models. Antioxidants 2021, 10, 311. [Google Scholar] [CrossRef]
- Benyaich, A.; Aksissou, M. The Pharmacological and Nutritional Properties of Rosmarinus officinalis: A Comprehensive Review. Trop. J. Nat. Prod. Res. 2024, 8, 8945–8954. [Google Scholar]
- Zhang, Y.; Smuts, J.P.; Dodbiba, E.; Rangarajan, R.; Lang, J.C.; Armstrong, D.W. Degradation study of carnosic acid, carnosol, rosmarinic acid, and rosemary extract (Rosmarinus officinalis L.) assessed using HPLC. J. Agric. Food Chem. 2012, 60, 9305–9314. [Google Scholar] [CrossRef] [PubMed]
- Celiktas, O.Y.; Kocabas, E.H.; Bedir, E.; Sukan, F.V.; Ozek, T.; Baser, K.H.C. Antimicrobial activities of methanol extracts and essential oils of Rosmarinus officinalis, depending on location and seasonal variations. Food Chem. 2007, 100, 553–559. [Google Scholar] [CrossRef]
- Satoh, T.; McKercher, S.R.; Lipton, S.A. Nrf2/ARE-mediated antioxidant actions of pro-electrophilic drugs. Free. Radic. Biol. Med. 2013, 65, 645–657. [Google Scholar] [CrossRef]
- The Editors of Encyclopaedia Britannica. Peppermint. Encyclopedia Britannica. Available online: https://www.britannica.com/plant/peppermint (accessed on 13 March 2025).
- Machewar, K.; Kakde, R.; Sabale, P. Evaluation of Topical Anti-Inflammatory Potential of Mentha piperita L. Extract by Formulation of Microemulgel. J. Young Pharm. 2024, 16, 488–497. [Google Scholar] [CrossRef]
- Lim, H.-W.; Kim, D.-H.; Kim, S.-H.; Lee, J.-M.; Chon, J.-W.; Song, K.-Y.; Bae, D.; Kim, J.; Kim, H.; Seo, K.-H. Antimicrobial effect of Mentha piperita (Peppermint) oil against Bacillus cereus, Staphylococcus aureus, Cronobacter sakazakii, and Salmonella Enteritidis in various dairy foods: Preliminary study. J. Dairy Sci. Biotechnol. 2018, 36, 146–154. [Google Scholar] [CrossRef]
- Hanif, M.A.; Nisar, S.; Khan, G.S.; Mushtaq, Z.; Zubair, M. Essential oils. In Essential Oil Research; Trends in Biosynthesis, Analytics, Industrial Applications and Biotechnological Production; Springer: Cham, Switzerland, 2019; pp. 3–17. [Google Scholar]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Afonso, A.F.; Pereira, O.R.; Cardoso, S.M. Health-promoting effects of Thymus phenolic-rich extracts: Antioxidant, anti-inflammatory and antitumoral properties. Antioxidants 2020, 9, 814. [Google Scholar] [CrossRef]
- Soković, M.; Glamočlija, J.; Marin, P.D.; Brkić, D.; Van Griensven, L.J. Antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model. Molecules 2010, 15, 7532–7546. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Koulivand, P.H.; Khaleghi Ghadiri, M.; Gorji, A. Lavender and the nervous system. Evid.-Based Complement. Altern. Med. 2013, 2013, 681304. [Google Scholar] [CrossRef] [PubMed]
- Malcolm, B.J.; Tallian, K. Essential oil of lavender in anxiety disorders: Ready for prime time? Ment. Health Clin. 2017, 7, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Lis-Balchin, M.; Hart, S. Studies on the mode of action of the essential oil of Lavender Lavandula angustifolia P. Miller. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 1999, 13, 540–542. [Google Scholar]
- Sandur, S.K.; Pandey, M.K.; Sung, B.; Ahn, K.S.; Murakami, A.; Sethi, G.; Limtrakul, P.; Badmaev, V.; Aggarwal, B.B. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis 2007, 28, 1765–1773. [Google Scholar] [CrossRef]
- Nisar, T.; Iqbal, M.; Raza, A.; Safdar, M.; Iftikhar, F.; Waheed, M. Estimation of total phenolics and free radical scavenging of turmeric (Curcuma longa). Environ. Sci. 2015, 15, 1272–1277. [Google Scholar]
- Guimarães, A.F.; Vinhas, A.C.A.; Gomes, A.F.; Souza, L.H.; Krepsky, P.B. Essential oil of Curcuma longa L. rhizomes chemical composition, yield variation and stability. Química Nova 2020, 43, 909–913. [Google Scholar] [CrossRef]
- Gutierres, V.O.; Pinheiro, C.M.; Assis, R.P.; Vendramini, R.C.; Pepato, M.T.; Brunetti, I.L. Curcumin-supplemented yoghurt improves physiological and biochemical markers of experimental diabetes. Br. J. Nutr. 2012, 108, 440–448. [Google Scholar] [CrossRef]
- Khan, M.K.; Khan, I.A.; Iqbal, M.O.; Eed, E.M.; Ahmad, A.; Naeem, M.; Ashiq, H.T.; Munawar, N. The antimicrobial effect of Curcuma longa and Allium sativum decoction in rats explains its utility in wound care. Am. J. Transl. Res. 2024, 16, 6159. [Google Scholar] [CrossRef]
- Hussain, Y.; Alam, W.; Ullah, H.; Dacrema, M.; Daglia, M.; Khan, H.; Arciola, C.R. Antimicrobial potential of curcumin: Therapeutic potential and challenges to clinical applications. Antibiotics 2022, 11, 322. [Google Scholar] [CrossRef]
- Nicoliche, T.; Bartolomeo, C.S.; Lemes, R.M.R.; Pereira, G.C.; Nunes, T.A.; Oliveira, R.B.; Nicastro, A.L.M.; Soares, É.N.; Lima, B.F.d.C.; Rodrigues, B.M.; et al. Antiviral, anti-inflammatory and antioxidant effects of curcumin and curcuminoids in SH-SY5Y cells infected by SARS-CoV-2. Sci. Rep. 2024, 14, 10696. [Google Scholar] [CrossRef]
- Han, Y.A.; Song, C.W.; Koh, W.S.; Yon, G.H.; Kim, Y.S.; Ryu, S.Y.; Kwon, H.J.; Lee, K.H. Anti-inflammatory effects of the Zingiber officinale roscoe constituent 12-dehydrogingerdione in lipopolysaccharide-stimulated Raw 264.7 cells. Phytother. Res. 2013, 27, 1200–1205. [Google Scholar] [CrossRef]
- Stoner, G.D. Ginger: Is it ready for prime time? Cancer Prev. Res. 2013, 6, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Turgeon, D.K.; Wright, B.D.; Sidahmed, E.; Ruffin, M.T.; Brenner, D.E.; Sen, A.; Zick, S.M. Effect of ginger root on cyclooxygenase-1 and 15-hydroxyprostaglandin dehydrogenase expression in colonic mucosa of humans at normal and increased risk for colorectal cancer. Eur. J. Cancer Prev. 2013, 22, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Hsiang, C.Y.; Lo, H.Y.; Huang, H.C.; Li, C.C.; Wu, S.L.; Ho, T.Y. Ginger extract and zingerone ameliorated trinitrobenzene sulphonic acid-induced colitis in mice via modulation of nuclear factor-κB activity and interleukin-1β signalling pathway. Food Chem. 2013, 136, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Nile, S.H.; Park, S.W. Chromatographic analysis, antioxidant, anti-inflammatory, and xanthine oxidase inhibitory activities of ginger extracts and its reference compounds. Ind. Crops Prod. 2015, 70, 238–244. [Google Scholar] [CrossRef]
- Tjendraputra, E.; Tran, V.H.; Liu-Brennan, D.; Roufogalis, B.D.; Duke, C.C. Effect of ginger constituents and synthetic analogues on cyclooxygenase-2 enzyme in intact cells. Bioorganic Chem. 2001, 29, 156–163. [Google Scholar] [CrossRef]
- Mallikarjun, S.; Rao, A.; Rajesh, G.; Shenoy, R.; Pai, M. Antimicrobial efficacy of Tulsi leaf (Ocimum sanctum) extract on periodontal pathogens: An: In vitro: Study. J. Indian Soc. Periodontol. 2016, 20, 145–150. [Google Scholar] [CrossRef]
- Shafi, T.A.; Bansal, B.K.; Gupta, D.K. In vitro antibacterial activity and minimum inhibitory concentration of Ocimum sanctum leaves against common bovine mastitis pathogens. J. Dairy Vet. Anim. Res. 2018, 7, 322–324. [Google Scholar] [CrossRef]
- Srichok, J.; Yingbun, N.; Kowawisetsut, T.; Kornmatitsuk, S.; Suttisansanee, U.; Temviriyanukul, P.; Chantong, B. Synergistic antibacterial and anti-inflammatory activities of ocimum tenuiflorum ethanolic extract against major bacterial mastitis pathogens. Antibiotics 2022, 11, 510. [Google Scholar] [CrossRef] [PubMed]
- Eshraghian, A. Anti-Inflammatory, gastrointestinal and hepatoprotective effects of Ocimum sanctum Linn: An ancient remedy with new application. Inflamm. Allergy-Drug Targets 2013, 12, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Torres, M.P.; Chakraborty, S.; Souchek, J.J.; Rachagani, S.; Kaur, S.; Macha, M.; Ganti, A.K.; Hauke, R.J.; Batra, S.K. Holy Basil leaf extract decreases tumorigenicity and metastasis of aggressive human pancreatic cancer cells in vitro and in vivo: Potential role in therapy. Cancer Lett. 2013, 336, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Munir, N.; Iqbal, A.S.; Altaf, I.; Bashir, R.; Sharif, N.; Saleem, F.; Naz, S. Evaluation of antioxidant and antimicrobial potential of two endangered plant species Atropa belladonna and Matricaria chamomilla. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 111–117. [Google Scholar] [CrossRef]
- El Mihyaoui, A.; Esteves da Silva, J.C.; Charfi, S.; Candela Castillo, M.E.; Lamarti, A.; Arnao, M.B. Chamomile (Matricaria chamomilla L.): A review of ethnomedicinal use, phytochemistry and pharmacological uses. Life 2022, 12, 479. [Google Scholar] [CrossRef]
- De Cicco, P.; Ercolano, G.; Sirignano, C.; Rubino, V.; Rigano, D.; Ianaro, A.; Formisano, C. Chamomile essential oils exert anti-inflammatory effects involving human and murine macrophages: Evidence to support a therapeutic action. J. Ethnopharmacol. 2023, 311, 116391. [Google Scholar] [CrossRef]
- Lairikyengbam, D.; Wetterauer, B.; Schmiech, M.; Jahraus, B.; Kirchgessner, H.; Wetterauer, P.; Berschneider, K.; Beier, V.; Niesler, B.; Balta, E.; et al. Comparative analysis of whole plant, flower and root extracts of Chamomilla recutita L. and characteristic pure compounds reveals differential anti-inflammatory effects on human T cells. Front. Immunol. 2024, 15, 1388962. [Google Scholar] [CrossRef]
- Stanojevic, L.P.; Marjanovic-Balaban, Z.R.; Kalaba, V.D.; Stanojevic, J.S.; Cvetkovic, D.J. Chemical composition, antioxidant and antimicrobial activity of chamomile flowers essential oil (Matricaria chamomilla L.). J. Essent. Oil Bear. Plants 2016, 19, 2017–2028. [Google Scholar] [CrossRef]
- Ahmed, A.F.; Attia, F.A.; Liu, Z.; Li, C.; Wei, J.; Kang, W. Antioxidant activity and total phenolic content of essential oils and extracts of sweet basil (Ocimum basilicum L.) plants. Food Sci. Hum. Wellness 2019, 8, 299–305. [Google Scholar] [CrossRef]
- Ababutain, I.M. Antimicrobial Activity and Gas Chromatography-Mass Spectrometry (GC-MS) Analysis of Saudi Arabian Ocimum basilicum Leaves Extracts. J. Pure Appl. Microbiol. 2019, 13, 823–833. [Google Scholar] [CrossRef]
- Takeuchi, H.; Takahashi-Muto, C.; Nagase, M.; Kassai, M.; Tanaka-Yachi, R.; Kiyose, C. Anti-inflammatory effects of extracts of sweet basil (Ocimum basilicum L.) on a co-culture of 3T3-L1 adipocytes and RAW264. 7 macrophages. J. Oleo Sci. 2020, 69, 487–493. [Google Scholar] [CrossRef]
- Omar, S.H.; Al-Wabel, N.A. Organosulfur compounds and possible mechanism of garlic in cancer. Saudi Pharm. J. 2010, 18, 51–58. [Google Scholar] [CrossRef]
- Yin, M.C.; Hwang, S.W.; Chan, K.C. Nonenzymatic antioxidant activity of four organosulfur compounds derived from garlic. J. Agric. Food Chem. 2002, 50, 6143–6147. [Google Scholar] [CrossRef] [PubMed]
- Badr, G.M.; Al-Mulhim, J.A. The protective effect of aged garlic extract on nonsteroidal anti-inflammatory drug-induced gastric inflammations in male albino rats. Evid. -Based Complement. Altern. Med. 2014, 2014, 759642. [Google Scholar] [CrossRef] [PubMed]
- Gharavi, M.J.; Nobakht, M.; Khademvatan, S.H.; Bandani, E.; Bakhshayesh, M.; Roozbehani, M. The effect of garlic extract on expression of INFγ and inos genes in macrophages infected with Leishmania major. Iran. J. Parasitol. 2011, 6, 74. [Google Scholar] [PubMed]
- Liu, C.T.; Su, H.M.; Lii, C.K.; Sheen, L.Y. Effect of supplementation with garlic oil on activity of Th1 and Th2 lymphocytes from rats. Planta Medica 2009, 75, 205–210. [Google Scholar] [CrossRef]
- Yoshida, H.; Katsuzaki, H.; Ohta, R.; Ishikawa, K.; Fukuda, H.; Fujino, T.; Suzuki, A. Antimicrobial activity of the thiosulfinates isolated from oil-macerated garlic extract. Biosci. Biotechnol. Biochem. 1999, 63, 591–594. [Google Scholar] [CrossRef]
- Draginic, N.; Andjic, M.; Jeremic, J.; Zivkovic, V.; Kocovic, A.; Tomovic, M.; Bozin, B.; Kladar, N.; Bolevich, S.; Jakovljevic, V.; et al. Anti-inflammatory and antioxidant effects of Melissa officinalis extracts: A comparative study. Iran. J. Pharm. Res. IJPR 2022, 21, e126561. [Google Scholar] [CrossRef]
- Abbasnia, V.; Esfahani, D.E.; Khazdair, M.R.; Oryan, S.; Foadoddini, M. The therapeutic potential of Melissa officinalis L. hydroalcoholic extract and rosmarinic acid in a rat asthmatic model: A study on anti-inflammatory and antioxidant effects. Avicenna J. Phytomedicine 2024, 14, 252. [Google Scholar] [CrossRef]
- Yu, H.; Pei, J.; Qiu, W.; Mei, J.; Xie, J. The antimicrobial effect of Melissa officinalis L. essential oil on Vibrio parahaemolyticus: Insights based on the cell membrane and external structure. Front. Microbiol. 2022, 13, 812792. [Google Scholar] [CrossRef]
- Carvalho, F.; Coimbra, A.T.; Silva, L.; Duarte, A.P.; Ferreira, S. Melissa officinalis essential oil as an antimicrobial agent against Listeria monocytogenes in watermelon juice. Food Microbiol. 2023, 109, 104105. [Google Scholar] [CrossRef] [PubMed]
- Juee, L.Y.; Sofi, S.H.; Adham, A.N. Melissa officinalis gastroprotective and antioxidant efficacy. J. Funct. Foods 2023, 105, 105550. [Google Scholar] [CrossRef]
- Robert, S.D.; Ismail, A.A.S.; Wan Rosli, W.I. Trigonella Foenum-Graecum Seeds Lowers Postprandial Blood Glucose in Overweight and Obese Individuals. J. Nutr. Metab. 2014, 2014, 964873. [Google Scholar] [CrossRef] [PubMed]
- Pundarikakshudu, K.; Shah, D.H.; Panchal, A.H.; Bhavsar, G.C. Anti-inflammatory activity of fenugreek (Trigonella foenum-graecum Linn) seed petroleum ether extract. Indian J. Pharmacol. 2016, 48, 441–444. [Google Scholar] [CrossRef]
- Fatima, H.; Shahid, M.; Pruitt, C.; Pung, M.A.; Mills, P.J.; Riaz, M.; Ashraf, R. Chemical fingerprinting, antioxidant, and anti-inflammatory potential of hydroethanolic extract of Trigonella foenum-graecum. Antioxidants 2022, 11, 364. [Google Scholar] [CrossRef]
- Prema, A.; Thenmozhi, A.J.; Manivasagam, T.; Essa, M.M.; Akbar, M.D.; Akbar, M. Fenugreek seed powder nullified aluminium chloride induced memory loss, biochemical changes, Aβ burden and apoptosis via regulating Akt/GSK3β signaling pathway. PLoS ONE 2016, 11, e0165955. [Google Scholar] [CrossRef]
- Bin-Hafeez, B.; Haque, R.; Parvez, S.; Pandey, S.; Sayeed, I.; Raisuddin, S. Immunomodulatory effects of fenugreek (Trigonella foenum graecum L.) extract in mice. Int. Immunopharmacol. 2003, 3, 257–265. [Google Scholar] [CrossRef]
- Leite, C.D.S.; Bonafé, G.A.; Carvalho Santos, J.; Martinez, C.A.R.; Ortega, M.M.; Ribeiro, M.L. The anti-inflammatory properties of licorice (Glycyrrhiza glabra)-derived compounds in intestinal disorders. Int. J. Mol. Sci. 2022, 23, 4121. [Google Scholar] [CrossRef]
- Yu, J.Y.; Ha, J.Y.; Kim, K.M.; Jung, Y.S.; Jung, J.C.; Oh, S. Anti-inflammatory activities of licorice extract and its active compounds, glycyrrhizic acid, liquiritin and liquiritigenin, in BV2 cells and mice liver. Molecules 2015, 20, 13041–13054. [Google Scholar] [CrossRef]
- Chopra, P.K.P.G.; Saraf, B.D.; Inam, F.A.R.H.I.N.; Deo, S.S. Antimicrobial and antioxidant activities of methanol extract roots of Glycyrrhiza glabra and HPLC analysis. Int. J. Pharm. Pharmacol. Sci. 2013, 5, 157–160. [Google Scholar]
- Zhurinov, M.Z.; Miftakhova, A.F.; Keyer, V.; Shulgau, Z.T.; Solodova, E.V.; Kalykberdiyev, M.K.; Abilmagzhanov, A.Z.; Talgatov, E.T.; Ait, S.; Shustov, A.V. Glycyrrhiza glabra L. extracts and other therapeutics against SARS-CoV-2 in central eurasia: Available but overlooked. Molecules 2023, 28, 6142. [Google Scholar] [CrossRef]
- Zuo, J.; Meng, T.; Wang, Y.; Tang, W. A review of the antiviral activities of glycyrrhizic acid, glycyrrhetinic acid and glycyrrhetinic acid monoglucuronide. Pharmaceuticals 2023, 16, 641. [Google Scholar] [CrossRef] [PubMed]
- Jayasekara, K.G.; Suresh, S.; Goonasekara, C.; Soyza, P.; Perera, N.; Gunasekera, K. Anti-dengue viral activity of Glycyrrhiza glabra roots in Vero cells. Sci. Rep. 2024, 14, 25922. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, S.; Asano, R.; Iwahori, Y.; Narui, T.; Okada, Y.; Singab, A.N.B.; Okuyama, T. Hematological studies on black cumin oil from the seeds of Nigella sativa L. Biol. Pharm. Bull. 2001, 24, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, L.; Fedeli, D.; Nasuti, C.; Maggi, F.; Papa, F.; Wabitsch, M.; De Caterina, R.; Gabbianelli, R. Antioxidant and anti-inflammatory properties of Nigella sativa oil in human pre-adipocytes. Antioxidants 2019, 8, 51. [Google Scholar] [CrossRef]
- Pop, R.M.; Sabin, O.; Suciu, Ș.; Vesa, S.C.; Socaci, S.A.; Chedea, V.S.; Bocsan, I.C.; Buzoianu, A.D. Nigella sativa’s anti-inflammatory and antioxidative effects in experimental inflammation. Antioxidants 2020, 9, 921. [Google Scholar] [CrossRef]
- Chaieb, K.; Kouidhi, B.; Jrah, H.; Mahdouani, K.; Bakhrouf, A. Antibacterial activity of Thymoquinone, an active principle of Nigella sativa and its potency to prevent bacterial biofilm formation. BMC Complement. Altern. Med. 2011, 11, 1–6. [Google Scholar] [CrossRef]
- Khan, M.A.U.; Ashfaq, M.K.; Zuberi, H.S.; Mahmood, M.S.; Gilani, A.H. The in vivo antifungal activity of the aqueous extract from Nigella sativa seeds. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2003, 17, 183–186. [Google Scholar] [CrossRef]
- Hikmah, Z.; Endaryanto, A.; Ugrasena, I.D.G.; Rahaju, A.S.; Arifin, S. Nigella sativa L. as immunomodulator and preventive effect on renal tissue damage of lupus mice induced by pristane. Heliyon 2022, 8, e09242. [Google Scholar] [CrossRef]
- Mihaylova, A.; Doncheva, N.; Vlasheva, M.; Katsarova, M.; Gardjeva, P.; Dimitrova, S.; Kostadinov, I. Investigation of the Immunomodulatory and Neuroprotective Properties of Nigella sativa Oil in Experimental Systemic and Neuroinflammation. Int. J. Mol. Sci. 2025, 26, 2235. [Google Scholar] [CrossRef]
- Gnanasekaran, P.; Roy, A.; Natesh, N.S.; Raman, V.; Ganapathy, P.; Arumugam, M.K. Removal of microbial pathogens and anticancer activity of synthesized nano-thymoquinone from Nigella sativa seeds. Environ. Technol. Innov. 2021, 24, 102068. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Burton, D.; Parra, F.; López, J.; Muñoz, P.; Escobar, H.; Parra, C. Antioxidant and antibacterial capacities of Origanum vulgare L. essential oil from the arid Andean Region of Chile and its chemical characterization by GC-MS. Metabolites 2020, 10, 414. [Google Scholar] [CrossRef]
- Jafari Khorsand, G.; Morshedloo, M.R.; Mumivand, H.; Emami Bistgani, Z.; Maggi, F.; Khademi, A. Natural diversity in phenolic components and antioxidant properties of oregano (Origanum vulgare L.) accessions, grown under the same conditions. Sci. Rep. 2022, 12, 5813. [Google Scholar] [CrossRef]
- Walasek-Janusz, M.; Grzegorczyk, A.; Malm, A.; Nurzyńska-Wierdak, R.; Zalewski, D. Chemical composition, and antioxidant and antimicrobial activity of oregano essential oil. Molecules 2024, 29, 435. [Google Scholar] [CrossRef]
- Stojanović, N.M.; Mitić, K.V.; Nešić, M.; Stanković, M.; Petrović, V.; Baralić, M.; Randjelović, P.J.; Sokolović, D.; Radulović, N. Oregano (Origanum vulgare) essential oil and its constituents prevent rat kidney tissue injury and inflammation induced by a high dose of l-arginine. Int. J. Mol. Sci. 2024, 25, 941. [Google Scholar] [CrossRef] [PubMed]
- Manayi, A.; Vazirian, M.; Saeidnia, S. Echinacea purpurea: Pharmacology, phytochemistry and analysis methods. Pharmacogn. Rev. 2015, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.; Huang, Y.-C.; Mao, C.-F.; Chen, C.-K.; Thomas, S.; Kuo, H.-P.; Miao, S.; Kong, Z.-L. Protective effect of ethanolic extract of Echinacea purpurea contained nanoparticles on meniscal/ligamentous injury induced osteoarthritis in obese male rats. Sci. Rep. 2022, 12, 5354. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahhab, K.G.; Elqattan, G.M.; El-Sahra, D.G.; Hassan, L.K.; Sayed, R.S.; Mannaa, F.A. Immuno-antioxidative reno-modulatory effectiveness of Echinacea purpurea extract against bifenthrin-induced renal poisoning. Sci. Rep. 2024, 14, 5892. [Google Scholar] [CrossRef]
- De Oliveira, B.G.; Santos, L.F.F.; da Costa, M.C.; Bastos, R.W.; Carmo, P.H.F.D.; Santos, D.d.A.; Pianetti, G.A.; César, I.C. Antimicrobial and immunomodulatory activities of dried extracts of Echinacea Purpurea. Braz. J. Pharm. Sci. 2022, 58, e21026. [Google Scholar] [CrossRef]
- Burlou-Nagy, C.; Bănică, F.; Jurca, T.; Vicaș, L.G.; Marian, E.; Muresan, M.E.; Bácskay, I.; Kiss, R.; Fehér, P.; Pallag, A. Echinacea purpurea (L.) Moench: Biological and pharmacological properties. A review. Plants 2022, 11, 1244. [Google Scholar] [CrossRef]
- Benamar-Aissa, B.; Gourine, N.; Ouinten, M.; Yousfi, M. Synergistic effects of essential oils and phenolic extracts on antimicrobial activities using blends of Artemisia campestris, Artemisia herba alba, and Citrus aurantium. Biomol. Concepts 2024, 15, 20220040. [Google Scholar] [CrossRef] [PubMed]
- Carrubba, A.; Lazzara, S.; Giovino, A.; Ruberto, G.; Napoli, E. Content variability of bioactive secondary metabolites in Hypericum perforatum L. Phytochem. Lett. 2021, 46, 71–78. [Google Scholar]
- Kunle, O.F.; Egharevba, H.O.; Ahmadu, P.O. Standardization of herbal medicines—A review. Int. J. Biodivers. Conserv. 2012, 4, 101–112. [Google Scholar] [CrossRef]
- Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Nur, H.; Ismail, A.F.; Sharif, S.; RamaKrishna, S.; Berto, F. Antioxidant, antimicrobial and antiviral properties of herbal materials. Antioxidants 2020, 9, 1309. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Z.; Li, S.; Ye, X.; Li, X.; He, K. Synergy effects of herb extracts: Pharmacokinetics and pharmacodynamic basis. Fitoterapia 2014, 92, 133–147. [Google Scholar] [CrossRef]
- Roşian, Ş.H.; Boarescu, I.; Boarescu, P.M. Antioxidant and Anti-Inflammatory Effects of Bioactive Compounds in Atherosclerosis. Int. J. Mol. Sci. 2025, 26, 1379. [Google Scholar] [CrossRef]
- Zhao, Q.; Luan, X.; Zheng, M.; Tian, X.H.; Zhao, J.; Zhang, W.D.; Ma, B.L. Synergistic mechanisms of constituents in herbal extracts during intestinal absorption: Focus on natural occurring nanoparticles. Pharmaceutics 2020, 12, 128. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Tuberoso, C.I.G.; Jerković, I. The Role of Rosmarinic Acid in Cancer Prevention and Therapy: Mechanisms of Antioxidant and Anticancer Activity. Antioxidants 2024, 13, 1313. [Google Scholar] [CrossRef]
- Poulios, E.; Giaginis, C.; Vasios, G.K. Current state of the art on the antioxidant activity of sage (Salvia spp.) and its bioactive components. Planta Medica 2020, 86, 224–238. [Google Scholar] [CrossRef]
- Roby, M.H.H.; Sarhan, M.A.; Selim, K.A.H.; Khalel, K.I. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts. Ind. Crops Prod. 2013, 43, 827–831. [Google Scholar] [CrossRef]
- Habtemariam, S. Anti-inflammatory therapeutic mechanisms of natural products: Insight from rosemary diterpenes, carnosic acid and carnosol. Biomedicines 2023, 11, 545. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Li, N.; Li, D.; Chen, L.; Deng, H.; Wang, S.; Tang, J.; Ouyang, W. Carnosic acid inhibits NLRP3 inflammasome activation by targeting both priming and assembly steps. Int. Immunopharmacol. 2023, 116, 109819. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-C.; Yeh, C.-H.; Yang, M.-L.; Kuan, Y.-H. Luteolin Suppresses Inflammatory Mediator Expression by Blocking the Akt/NF κ B Pathway in Acute Lung Injury Induced by Lipopolysaccharide in Mice. Evid.-Based Complement. Altern. Med. 2011, 2012, 1–8. [Google Scholar]
- Chen, C.Y.; Peng, W.H.; Tsai, K.D.; Hsu, S.L. Luteolin suppresses inflammation-associated gene expression by blocking NF-κB and AP-1 activation pathway in mouse alveolar macrophages. Life Sci. 2007, 81, 1602–1614. [Google Scholar] [CrossRef]
- Zengin, H.; Baysal, A.H. Antibacterial and antioxidant activity of essential oil terpenes against pathogenic and spoilage-forming bacteria and cell structure-activity relationships evaluated by SEM microscopy. Molecules 2014, 19, 17773–17798. [Google Scholar] [CrossRef]
- Guo, F.; Chen, Q.; Liang, Q.; Zhang, M.; Chen, W.; Chen, H.; Yun, Y.; Zhong, Q.; Chen, W. Antimicrobial activity and proposed action mechanism of linalool against Pseudomonas fluorescens. Front. Microbiol. 2021, 12, 562094. [Google Scholar] [CrossRef]
- Khwaza, V.; Aderibigbe, B.A. Antibacterial Activity of Selected Essential Oil Components and Their Derivatives: A Review. Antibiotics 2025, 14, 68. [Google Scholar] [CrossRef]
- Home, T.I.J.; Edition, S. Effect of Natural Flavonoid Apigenin in Lowering High Glucose-Induced Insulin Resistance via Targeting PI3K/AKT Pathway in 3T3-L1 Adipocytes–Evidence Through an In-vitro and In-silico Approach. Texila Int. J. Public Health 2025, 17–31. [Google Scholar] [CrossRef]
- Wen, D.; Li, M. The Emerging Role of Flavonoids in the Treatment of Type 2 Diabetes Mellitus: Regulating the Enteroendocrine System. Explor. Res. Hypothesis Med. 2025, 10, 56–68. [Google Scholar] [CrossRef]
- Feng, X.; Weng, D.; Zhou, F.; Owen, Y.D.; Qin, H.; Zhao, J.; Yu, W.; Huang, Y.; Chen, J.; Fu, H.; et al. Activation of PPARγ by a natural flavonoid modulator, apigenin ameliorates obesity-related inflammation via regulation of macrophage polarization. EBioMedicine 2016, 9, 61–76. [Google Scholar] [CrossRef]
- Martín, M.Á.; Ramos, S. Dietary flavonoids and insulin signaling in diabetes and obesity. Cells 2021, 10, 1474. [Google Scholar] [CrossRef]
- Yi, X.; Dong, M.; Guo, N.; Tian, J.; Lei, P.; Wang, S.; Yang, Y.; Shi, Y. Flavonoids improve type 2 diabetes mellitus and its complications: A review. Front. Nutr. 2023, 10, 1192131. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Nam, E.S.; Lee, Y.; Kim, M. How strong is the evidence for the anxiolytic efficacy of lavender?: Systematic review and meta-analysis of randomized controlled trials. Asian Nurs. Res. 2019, 13, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, É.R.; Maia, J.G.S.; Fontes-Júnior, E.A.; do Socorro Ferraz Maia, C. Linalool as a therapeutic and medicinal tool in depression treatment: A review. Curr. Neuropharmacol. 2022, 20, 1073–1092. [Google Scholar] [CrossRef] [PubMed]
- Faridzadeh, A.; Salimi, Y.; Ghasemirad, H.; Kargar, M.; Rashtchian, A.; Mahmoudvand, G.; Karimi, M.A.; Zerangian, N.; Jahani, N.; Masoudi, A.; et al. Neuroprotective potential of aromatic herbs: Rosemary, sage, and lavender. Front. Neurosci. 2022, 16, 909833. [Google Scholar] [CrossRef]
- Pengelly, A.; Snow, J.; Mills, S.Y.; Scholey, A.; Wesnes, K.; Butler, L.R. Short-term study on the effects of rosemary on cognitive function in an elderly population. J. Med. Food 2012, 15, 10–17. [Google Scholar] [CrossRef]
- Alanazi, H.H.; Elasbali, A.M.; Alanazi, M.K.; El Azab, E.F. Medicinal herbs: Promising immunomodulators for the treatment of infectious diseases. Molecules 2023, 28, 8045. [Google Scholar] [CrossRef]
- Mahmoodi, M.; Ayoobi, F.; Aghaei, A.; Rahmani, M.; Taghipour, Z.; Hosseini, A.; Jafarzadeh, A.; Sankian, M. Beneficial effects of Thymus vulgaris extract in experimental autoimmune encephalomyelitis: Clinical, histological and cytokine alterations. Biomed. Pharmacother. 2019, 109, 2100–2108. [Google Scholar] [CrossRef]
- Pelvan, E.; Karaoğlu, Ö.; Fırat, E.Ö.; Kalyon, K.B.; Ros, E.; Alasalvar, C. Immunomodulatory effects of selected medicinal herbs and their essential oils: A comprehensive review. J. Funct. Foods 2022, 94, 105108. [Google Scholar] [CrossRef]
- Tullio, V.; Roana, J.; Cavallo, L.; Mandras, N. Immune defences: A view from the side of the essential oils. Molecules 2023, 28, 435. [Google Scholar] [CrossRef]
- Stobiecka, M.; Król, J.; Brodziak, A. Antioxidant activity of milk and dairy products. Animals 2022, 12, 245. [Google Scholar] [CrossRef]
- Granato, D.; Santos, J.S.; Salem, R.D.; Mortazavian, A.M.; Rocha, R.S.; Cruz, A.G. Effects of herbal extracts on quality traits of yogurts, cheeses, fermented milks, and ice creams: A technological perspective. Curr. Opin. Food Sci. 2018, 19, 1–7. [Google Scholar] [CrossRef]
- Azizkhani, M.; Tooryan, F. Antimicrobial activities of probiotic yogurts flavored with peppermint, basil, and zataria against Escherichia coli and Listeria monocytogenes. J. Food Qual. Hazards Control. 2016, 3, 79–86. [Google Scholar]
- Iorgachova, K.; Makarova, O.; Sokolova, N.; Khvostenko, K. Herbs as a source of natural preservatives against rancidity in the low-moisture bakery products. Rural. Dev. 2020, 2019, 28–33. [Google Scholar] [CrossRef]
- Kulbat-Warycha, K.; Stoińska, K.; Żyżelewicz, D. Aromatic Herbs of the Lamiaceae Family as Functional Ingredients in Wheat Tortilla. Appl. Sci. 2024, 14, 7584. [Google Scholar] [CrossRef]
- Medagama, A.B. The glycaemic outcomes of Cinnamon, a review of the experimental evidence and clinical trials. Nutr. J. 2015, 14, 108. [Google Scholar] [CrossRef]
- Gavahian, M.; Chu, Y.H.; Lorenzo, J.M.; Mousavi Khaneghah, A.; Barba, F.J. Essential oils as natural preservatives for bakery products: Understanding the mechanisms of action, recent findings, and applications. Crit. Rev. Food Sci. Nutr. 2020, 60, 310–321. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Santos, M.J.M.C.; Silva, L.K.R.; Pereira, L.C.L.; Santos, I.A.; da Silva Lannes, S.C.; da Silva, M.V. Natural antioxidants used in meat products: A brief review. Meat Sci. 2019, 148, 181–188. [Google Scholar] [CrossRef]
- Fasseas, M.K.; Mountzouris, K.C.; Tarantilis, P.A.; Polissiou, M.; Zervas, G. Antioxidant activity in meat treated with oregano and sage essential oils. Food Chem. 2008, 106, 1188–1194. [Google Scholar] [CrossRef]
- Reddy, D.M.; Reddy, G.V.B.; Mandal, P.K. Application of natural antioxidants in meat and meat products—A review. Food Nutr. J. 2018, 10, 2575–7091. [Google Scholar] [CrossRef]
- Jang, J.; Lee, D.W. Advancements in plant based meat analogs enhancing sensory and nutritional attributes. Npj Sci. Food 2024, 8, 50. [Google Scholar] [CrossRef]
- Sadowska, U.; Armenta Villavicencio, R.; Dziadek, K.; Skoczylas, J.; Sadowski, S.K.; Kopeć, A. The identification of polyphenolic compounds and the determination of antioxidant activity in extracts and infusions of peppermint, lemon balm and lavender. Appl. Sci. 2024, 14, 699. [Google Scholar] [CrossRef]
- Kosmopoulou, D.; Lafara, M.P.; Adamantidi, T.; Ofrydopoulou, A.; Grabrucker, A.M.; Tsoupras, A. Neuroprotective Benefits of Rosmarinus officinalis and Its Bioactives against Alzheimer’s and Parkinson’s Diseases. Appl. Sci. 2024, 14, 6417. [Google Scholar] [CrossRef]
- Ke, X.; Ma, H.; Yang, J.; Qiu, M.; Wang, J.; Han, L.; Zhang, D. New strategies for identifying and masking the bitter taste in traditional herbal medicines: The example of Huanglian Jiedu Decoction. Front. Pharmacol. 2022, 13, 843821. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Guan, Y.; Zeng, M.; Wang, Z.; Qin, F.; Chen, J.; He, Z. Effect of whey protein isolate and phenolic copigments in the thermal stability of mulberry anthocyanin extract at an acidic pH. Food Chem. 2022, 377, 132005. [Google Scholar] [CrossRef] [PubMed]
- Lang, Y.; Gao, H.; Tian, J.; Shu, C.; Sun, R.; Li, B.; Meng, X. Protective effects of α-casein or β-casein on the stability and antioxidant capacity of blueberry anthocyanins and their interaction mechanism. Lwt 2019, 115, 108434. [Google Scholar] [CrossRef]
- Zabot, G.L.; Rodrigues, F.S.; Ody, L.P.; Tres, M.V.; Herrera, E.; Palacin, H.; Córdova-Ramos, J.S.; Best, I.; Olivera-Montenegro, L. Encapsulation of bioactive compounds for food and agricultural applications. Polymers 2022, 14, 4194. [Google Scholar] [CrossRef]
- Ingale, O.S.; Pravin, B.P.; Pawase, P.A.; Shams, R.; Dash, K.K.; Bashir, O.; Roy, S. Enhancing bioactive stability and applications: Microencapsulation in fruit and vegetable waste valorization. Discov. Food 2025, 5, 1–32. [Google Scholar] [CrossRef]
- Zaikina, M.; Chebotareva, K.; Gurenko, A. Innovative technology of flour confectionery products for therapeutic and preventive nutrition of patients with diabetes mellitus. BIO Web Conf. 2021, 32, 03010. [Google Scholar] [CrossRef]
- Bomba, M.; Pandiak, I.; Fedyna, L.; Krektun, B. Application of functional ingredients in confectionery manufacturing technol-ogy. Sci. Messenger LNU Vet. Med. Biotechnologies. Ser. Food Technol. 2025, 27, 10–14. [Google Scholar] [CrossRef]
- Schmidt, C.; Geweke, I.; Struck, S.; Zahn, S.; Rohm, H. Blackcurrant pomace from juice processing as partial flour substitute in savoury crackers: Dough characteristics and product properties. Int. J. Food Sci. Technol. 2018, 53, 237–245. [Google Scholar] [CrossRef]
- Antoniewska, A.; Rutkowska, J.; Pineda, M.M. Antioxidative, sensory and volatile profiles of cookies enriched with freeze- dried Japanese quince (Chaenomeles japonica) fruits. Food Chem. 2019, 286, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Pasqualone, A.; Bianco, A.M.; Paradiso, V.M.; Summo, C.; Gambacorta, G.; Caponio, F. Physico-chemical, sensory and volatile profiles of biscuits enriched with grape marc extract. Food Res. Int. 2014, 65, 385–393. [Google Scholar] [CrossRef]
- Poiana, M.-A.; Alexa, E.; Radulov, I.; Raba, D.-N.; Cocan, I.; Negrea, M.; Misca, C.D.; Dragomir, C.; Dossa, S.; Suster, G. Strategies to Formulate Value-Added Pastry Products from Composite Flours Based on Spelt Flour and Grape Pomace Powder. Foods 2023, 12, 3239. [Google Scholar] [CrossRef]
- Szymanowska, U.; Karaś, M.; Bochnak-Niedźwiecka, J. Antioxidant and anti-inflammatory potential and consumer acceptance of wafers enriched with Freeze-Dried Raspberry Pomace. Appl. Sci. 2021, 11, 6807. [Google Scholar] [CrossRef]
- Anastasova, L.; Ivanovska, T.P.; Petkovska, R.; Petrusevska-Tozi, L. Concepts, benefits and perspectives of functional dairy food products. Maced. Pharm. Bull. 2018, 64, 73–83. [Google Scholar] [CrossRef]
- Elkot, W.F. Functional dairy foods. A review. J. Agroaliment. Process. Technol. 2022, 28, 223–225. [Google Scholar]
- Samanta, S.; Sarkar, T.; Chakraborty, R.; Rebezov, M.; Shariati, M.A.; Thiruvengadam, M.; Rengasamy, K.R. Dark chocolate: An overview of its biological activity, processing, and fortification approaches. Curr. Res. Food Sci. 2022, 5, 1916–1943. [Google Scholar] [CrossRef]
- Zugravu, C.; Otelea, M.R. Dark chocolate: To eat or not to eat? A review. J. AOAC Int. 2019, 102, 1388–1396. [Google Scholar] [CrossRef]
- Didar, Z. Enrichment of dark chocolate with vitamin D3 (free or liposome) and assessment quality parameters. J. Food Sci. Technol. 2021, 58, 3065–3072. [Google Scholar] [CrossRef]
- Mattia, C.D.D.; Sacchetti, G.; Mastrocola, D.; Serafini, M. From cocoa to chocolate: The impact of processing on in vitro antioxidant activity and the effects of chocolate on antioxidant markers in vivo. Front. Immunol. 2017, 8, 1207. [Google Scholar] [CrossRef]
- Godočiková, L.; Ivanišová, E.; Kačániová, M. The influence of fortification of dark chocolate with Sea buckthorn and mulberry on the content of biologically active substances. Adv. Res. Life Sci. 2017, 1, 26–31. [Google Scholar] [CrossRef]
- Lorenzo, N.D.; Dos Santos, O.V.; Lannes, S.C.d.S. Structure and nutrition of dark chocolate with pequi mesocarp (Caryocar villosum (Alb. ) Pers.) Food Sci. Technol. 2022, 42, e88021. [Google Scholar] [CrossRef]
- Dwijatmokoa, M.I.; Praseptiangga, D.; Muhammad, D.R.A. Effect of cinnamon essential oils addition in the sensory attributes of dark chocolate. Nusant. Biosci. 2016, 8, 301–305. [Google Scholar] [CrossRef]
- Praseptiangga, D.; Invicta, S.E.; Khasanah, L.U. Sensory and physicochemical characteristics of dark chocolate bar with addition of cinnamon (Cinnamomum burmannii) bark oleoresin microcapsule. J. Food Sci. Technol. 2019, 56, 4323–4332. [Google Scholar] [CrossRef]
- Norhayati, H.; Suzielawanis, I.R.; Khan, A.M. Effect of storage conditions on quality of prebiotic dark chocolate. Malays. J. Nutr. 2013, 19, 111–120. [Google Scholar] [PubMed]
- Fernández, A.R.G.; Beltran, P.F.; Sanchez, N.E.O.; Carrillo, E.P.; Santacruz, A.A.J. Physicochemical properties and sensory acceptability of sugar-free dark chocolate formulations added with probiotics. Rev. Mex. Ing. Quim. 2021, 20, 697–709. [Google Scholar] [CrossRef]
- Nastaj, M.; Sołowiej, B.G.; Stasiak, D.M.; Mleko, S.; Terpiłowski, K.; Łyszczek, R.J.; Tomasevic, I.B.; Tomczyńska-Mleko, M. Development and physicochemical properties of reformulated, high-protein, untempered sugar-free dark chocolates with addition of whey protein isolate and erythritol. Int. Dairy J. 2022, 134, 105450. [Google Scholar] [CrossRef]
- Toker, O.S.; Konar, N.; Pirouzian, H.R.; Oba, S.; Polat, D.G.; Palabiyik, I.; Poyrazoglu, E.S.; Sagdic, O. Developing functional white chocolate by incorporating different forms of EPA and DHA-Effects on product quality. LWT 2018, 87, 177–185. [Google Scholar] [CrossRef]
- Konar, N.; Toker, O.S.; Rasouli Pirouzian, H.; Oba, S.; Genc Polat, D.; Palabiyik, I.; Poyrazoglu, E.S.; Sagdic, O. Enrichment of milk chocolate by using EPA and DHA originated from various origins: Effects on product quality. Sugar Tech. 2018, 20, 745–755. [Google Scholar] [CrossRef]
- Ostertag, L.M.; Philo, M.; Colquhoun, I.J.; Tapp, H.S.; Saha, S.; Duthie, G.G.; Kemsley, E.K.; De Roos, B.; Kroon, P.A.; Le Gall, G. Acute consumption of flavan-3-ol-enriched dark chocolate affects human endogenous metabolism. J. Proteome Res. 2017, 16, 2516–2526. [Google Scholar] [CrossRef]
- Ngamdee, P.; Jamkrajang, S.; Yankin, S. Development and study on physical and sensory properties of dark chocolates fortified with anthocyanin from broken. RMUTP Res. J. 2020, 14, 45–56. [Google Scholar]
- Wolf, B. Confectionery and sugar-based foods. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Konar, N.; Gunes, R.; Palabiyik, I.; Toker, O.S. Health conscious consumers and sugar confectionery: Present aspects and projections. Trends Food Sci. Technol. 2022, 123, 57–68. [Google Scholar] [CrossRef]
- Šeremet, D.; Mandura, A.; Cebin, A.V.; Martinić, A.; Galić, K.; Komes, D. Challenges in confectionery industry: Development and storage stability of innovative white tea-based candies. J. Food Sci. 2020, 85, 2060–2068. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Kumari, A.; Chauhan, A.K.; Verma, T. Development of Functional Candy with Banana, Ginger and Skim Milk Powder as a source of Phenolics and Antioxidants. Curr. Res. Nutr. Food Sci. 2021, 9, 855–865. [Google Scholar] [CrossRef]
- Nagar, V.; Rastogi, M. Preparation and Nutritional Quality Evaluation of Fruit Peel Candies. J. Res. Appl. Sci. Biotechnol. 2022, 1, 57–64. [Google Scholar] [CrossRef]
- Manoj, S.P.; Neelagund, S.E.; Kumar, M.V.C.; Chirag, H.M.; Kumar, K.V.K.; Narayan, D.H.V. Promoting the Nutrition and Sensory Properties of Herbal Candy Using Psydrax Umbellata Leaf Extract. J. Chem. Health Risks 2024, 14, 1566–1573. [Google Scholar]
- Dhawan, K.; Rasane, P.; Singh, J.; Kaur, S.; Kaur, D.; Avinashe, H.; Mahato, D.K.; Kumar, P.; Gunjal, M.; Capanoglu, E.; et al. Effect of Spice Incorporation on Sensory and Physico-chemical Properties of Matcha-Based Hard Candy. ACS Omega 2023, 8, 29247–29252. [Google Scholar] [CrossRef]
- Kaur, R.; Kumar, V.; Aggarwal, P.; Singh, G. Valorization of citrus residue for the development of phytochemical enriched candy:Textural, bioactive, molecular, and structural characterization. Biomass Convers. Biorefin. 2023, 15, 2805–2816. [Google Scholar] [CrossRef]
- Kamboj, S.; Bandral, J.D.; Sood, M.; Gupta, N. Utilization of waste unripe mango for preparation of candy with enhanced bioactive and mineral composition. Indian J. Ecol. 2023, 50, 1569–1574. [Google Scholar]
- Cappa, C.; Lavelli, V.; Mariotti, M. Fruit candies enriched with grape skin powders: Physicochemical properties. LWT Food Sci. Technol. 2015, 62, 569–575. [Google Scholar] [CrossRef]
- Ciurlă, L.; Enache, I.M.; Buterchi, I.; Mihalache, G.; Lipșa, F.D.; Patra, A. A New Approach to Recover Bioactive Compounds from Apple Pomace: Healthy Jelly Candies. Foods 2024, 14, 39. [Google Scholar] [CrossRef]
- Lele, V.; Ruzauskas, M.; Zavistanaviciute, P.; Laurusiene, R.; Rimene, G.; Kiudulaite, D.; Tomkeviciute, J.; Nemeikstyte, J.; Stankevicius, R.; Bartkiene, E. Development and characterization of the gummy–supplements, enriched with probiotics and prebiotics. CyTA-J. Food 2018, 16, 580–587. [Google Scholar] [CrossRef]
- Kamil, R.Z.; Fadhila, F.H.; Rachmasari, A.D.; Murdiati, A.; Juffrie, M.; Rahayu, E.S. Development of probiotic gummy candy using the indigenous lactobacillus plantarum dad-13 strain; evaluation of its gastrointestinal resistance and shelf-life prediction. Food Res. 2021, 5, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Rayan, A.M.; Ebrahem, H.N.; El-Shamei, Z.A.; Omran, H.T.; Youssef, K.M. Omega-3 Rich Jelly Candy Fortified with Portulaca oleracea Seeds Oil: Physicochemical Properties, Fatty Acids Composition and Acceptability. Suez Canal Univ. J. Food Sci. 2022, 9, 1–8. [Google Scholar] [CrossRef]
- Ryveka, A.; Lestari, L.A.; Pratiwi, D.; Sundjaya, T. The Development of Multivitamin Mineral Jelly Candy “Previmin” for Stunting Prevention. Amerta Nutr. 2023, 7, 10. [Google Scholar] [CrossRef]
- Sze, H.; Ng, W.I.; Wan, R. Effect of Cinnamon Powder as Sucrose Replacer on Nutritional Compositions, Physical Properties and Sensory Acceptability of Butter Biscuit. Malays. J. Nutr. 2014, 20, 245–253. [Google Scholar]
- Aljobair, M.O. Physicochemical, nutritional, and sensory quality and storage stability of cookies: Effect of clove powder. Int. J. Food Prop. 2022, 25, 1009–1020. [Google Scholar] [CrossRef]
- Pagthinathan, M. Characterization and evaluation of physicochemical and sensory acceptability of ice creams incorporated with processed ginger. Eur. J. Food Sci. Technol. 2020, 8, 32–45. [Google Scholar]
- Solanki, K.; Rani, R.; Gaur, G.K. The Development and Characterization of Herbal Kulfi (Ice Cream) Using Tulsi, Ginger, and Clove: Development Kulfi (Ice Cream) using herb and spices. Indian J. Dairy Sci. 2023, 76, 448–457. [Google Scholar] [CrossRef]
- Miśkiewicz, K.; Nebesny, E.; Rosicka-Kaczmarek, J.; Żyżelewicz, D.; Budryn, G. The effects of baking conditions on acrylamide content in shortcrust cookies with added freeze-dried aqueous rosemary extract. J. Food Sci. Technol. 2018, 55, 4184–4196. [Google Scholar] [CrossRef]
- Dev, M.; Ghosh, M.; Bhattacharyya, D.K. Physico-chemical, antimicrobial, and organoleptic properties of roasted aromatic spice (clove bud) in baked product. Appl. Biochem. Biotechnol. 2021, 193, 1813–1835. [Google Scholar] [CrossRef] [PubMed]
- Martini, S.; Conte, A.; Tagliazucchi, D. Comprehensive evaluation of phenolic profile in dark chocolate and dark chocolate enriched with Sakura green tea leaves or turmeric powder. Food Res. Int. 2018, 112, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Albak, F.; Tekin, A.R. Effect of cinnamon powder addition during conching on the flavor of dark chocolate mass. J. Food Sci. Technol. 2015, 52, 1960–1970. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, M.C.; Ueda, J.M.; Melgar, B.; Dias, M.I.; Pinela, J.; Calhelha, R.C.; Ivanov, M.; Soković, M.; Heleno, S.; da Silva, A.B.; et al. Preservation of chocolate muffins with lemon balm, oregano, and rosemary extracts. Foods 2021, 10, 165. [Google Scholar] [CrossRef]
- Masutti, M.F.; Patrignani, M.; Conforti, P.A. Development and characterization of cracker fillings with natural antioxidants. J. Food Meas. Charact. 2020, 14, 446–454. [Google Scholar] [CrossRef]
- Salazar Cerón, J.; Paz Ruiz, N.; Ramos Velasco, J.C.; Ramos Cabrera, E.V.; Delgado Espinosa, Z.Y. Formulation and Characterization of a Theobroma cacao—Based Bar with the Addition of Foeniculum vulgare Essential Oil. Processes 2025, 13, 1648. [Google Scholar] [CrossRef]
- Gonçalves, N.D.; de Lima Pena, F.; Sartoratto, A.; Derlamelina, C.; Duarte, M.C.T.; Antunes, A.E.C.; Prata, A.S. Encapsulated thyme (Thymus vulgaris) essential oil used as a natural preservative in bakery product. Food Res. Int. 2017, 96, 154–160. [Google Scholar] [CrossRef]
- Caleja, C.; Barros, L.; Barreira, J.C.; Ciric, A.; Sokovic, M.; Calhelha, R.C.; Beatriz, M.; Oliveira, P.; Ferreira, I.C. Suitability of lemon balm (Melissa officinalis L.) extract rich in rosmarinic acid as a potential enhancer of functional properties in cupcakes. Food Chem. 2018, 250, 67–74. [Google Scholar] [CrossRef]
- Starowicz, M.; Lelujka, E.; Ciska, E.; Lamparski, G.; Sawicki, T.; Wronkowska, M. The application of Lamiaceae Lindl. promotes aroma compounds formation, sensory properties, and antioxidant activity of oat and buckwheat-based cookies. Molecules 2020, 25, 5626. [Google Scholar] [CrossRef]
- Chochkov, R.; Gergana, G. Sensory evaluation of pastry biscuits with thyme, oregano and sage. Int. J. Sci. Eng. Res. 2017, 8, 430–433. [Google Scholar]
- Kumar, R.; Atanu, J.; Ankit, D.; Satish, P. Suitability of type of herb and its form as flavoring in herbal ice cream. Int. J. Chem. Stud. 2018, 6, 1562–1567. [Google Scholar]
- Sahoo, M.R.; Marakanam, S.U.; Varier, R.R. Development and Evaluation of Essential Oil-based Lozenges using Menthol and Eucalyptus and in vitro Evaluation of their Antimicrobial activity in S. aureus and E. coli. Res. J. Pharm. Technol. 2022, 15, 5283–5288. [Google Scholar] [CrossRef]
- Souiy, Z.; Amri, Z.; Sharif, H.; Souiy, A.; Cheraief, I.; Hamden, K.; Hammami, M. The Use of D-Optimal Mixture Design in Optimizing Formulation of a Nutraceutical Hard Candy. Int. J. Food Sci. 2023, 2023, 7510452. [Google Scholar] [CrossRef] [PubMed]
- Szanto, L.G.; Marc, R.A.; Mureşan, A.E.; Mureșan, C.C.; Puşcaş, A.; Ranga, F.; Fetea, F.; Moraru, P.I.; Filip, M.; Muste, S. Improving Jelly Nutrient Profile with Bioactive Compounds from Pine (Pinus sylvestris L.) Extracts. Forests 2024, 16, 11. [Google Scholar] [CrossRef]
- Avelar, M.H.M.; Lima, L.C.D.; Efraim, P. Maintenance of fruit bioactive compounds in jelly candy manufacturing by alginate/pectin cold-set gelation. J. Food Process. Technol. 2020, 11, 1–8. [Google Scholar]
- De Avelar, M.H.M.; de Castilho Queiroz, G.; Efraim, P. Sustainable performance of cold-set gelation in the confectionery manufacturing and its effects on perception of sensory quality of jelly candies. Clean. Eng. Technol. 2020, 1, 100005. [Google Scholar] [CrossRef]
- Blanch, G.P.; Ruiz Del Castillo, M.L. Effect of Baking Temperature on the Phenolic Content and Antioxidant Activity of Black Corn (Zea mays L.) Bread. Foods 2021, 10, 1202. [Google Scholar] [CrossRef]
- Song, X.; Sui, X.; Jiang, L. Protection Function and Mechanism of Rosemary (Rosmarinus officinalis L.) Extract on the Thermal Oxidative Stability of Vegetable Oils. Foods 2023, 12, 2177. [Google Scholar] [CrossRef]
- Harlina, P.W.; Ma, M.; Shahzad, R.; Khalifa, I. Effect of rosemary extract on lipid oxidation, fatty acid composition, antioxidant capacity, and volatile compounds of salted duck eggs. Food Sci. Anim. Resour. 2022, 42, 689. [Google Scholar] [CrossRef]
- Intrasook, J.; Tsusaka, T.W.; Anal, A.K. Trends and current food safety regulations and policies for functional foods and beverages containing botanicals. J. Food Drug Anal. 2024, 32, 112. [Google Scholar] [CrossRef]
- Umamaheswari, D.; Muthuraja, R.; Kumar, M.; Venkateswarlu, B.S. Standardization of herbal drugs—A overview. Int. J. Pharm. Sci. Rev. Res. 2021, 68, 10–47583. [Google Scholar] [CrossRef]
- Opuni, K.F.M.; Kretchy, J.P.; Agyabeng, K.; Boadu, J.A.; Adanu, T.; Ankamah, S.; Appiah, A.; Amoah, G.B.; Baidoo, M.; Kretchy, I.A. Contamination of herbal medicinal products in low-and-middle-income countries: A systematic review. Heliyon 2023, 9, e19370. [Google Scholar] [CrossRef]
- Kowalska, G. The Safety Assessment of Toxic Metals in Commonly Used Herbs, Spices, Tea, and Coffee in Poland. Int. J. Environ. Res. Public Health 2021, 18, 5779. [Google Scholar] [CrossRef]

| Herb/Spice | Main Bioactive Compounds | Main Health-Promoting Properties | References |
|---|---|---|---|
| Sage (Salvia officinalis L.) | Rosmarinic acid, carnosol, luteolin, thujone | Antioxidant, Antimicrobial, Anti-inflammatory | [20,21,22,23] |
| Rosemary (Rosmarinus officinalis L.) | Carnosic acid, Rosmarinic acid, Eugenol | Antioxidant, Antimicrobial, Neuroprotective | [24,25,26,27] |
| Peppermint (Mentha piperita L.) | Menthol, Menthone, Hesperidin | Antimicrobial, Anti-inflammatory, Digestive | [28,29,30] |
| Thyme (Thymus vulgaris L.) | Thymol, Carvacrol, Flavonoids | Antimicrobial, Anti-inflammatory, Antioxidant | [31,32,33,34,35,36] |
| Lavender (Lavandula angustifolia L.) | Linalool, Linalyl acetate | Sedative, Anxiolytic, Antimicrobial | [37,38,39] |
| Turmeric (Curcuma longa L.) | Curcumin, Demethoxycurcumin | Anti-inflammatory, Antioxidant, Antimicrobial | [40,41,42,43,44,45,46] |
| Ginger (Zingiber officinale L.) | Gingerol, Shogaol, Paradol | Anti-inflammatory, Antioxidant, Immunomodulatory | [47,48,49,50,51,52] |
| Tulasi (Ocimum tenuiflorum L.) | Eugenol, Ursolic acid, Rosmarinic acid | Antimicrobial, Anti-inflammatory, Anticancer | [53,54,55,56,57] |
| Chamomile (Matricaria recutita L.) | Apigenin, Chamazulene, α-Bisabolol | Antioxidant, Antimicrobial, Anti-inflammatory | [58,59,60,61,62] |
| Common basil (Ocimum basilicum L.) | Linalool, Eugenol, Methyl chavicol | Antioxidant, Antimicrobial, Anti-inflammatory | [63,64,65] |
| Garlic (Allium sativum L.) | Allicin, Ajoene, S-allylcysteine | Antimicrobial, Antioxidant, Immunomodulatory | [66,67,68,69,70,71] |
| Lemon balm (Melissa officinalis L.) | Rosmarinic acid, Citral, Flavonoids | Antioxidant, Antimicrobial, Gastroprotective | [72,73,74,75,76] |
| Fenugreek (Trigonella foenum-graecum L.) | Trigonelline, Saponins, Flavonoids | Anti-inflammatory, Antioxidant, Hypoglycemic | [77,78,79,80,81] |
| Liquorice (Glycyrrhiza glabra L.) | Glycyrrhizin, Glabridin, Liquiritin | Anti-inflammatory, Antiviral, Hepatoprotective | [82,83,84,85,86,87] |
| Nigella (Nigella sativa L.) | Thymoquinone, Nigellone | Antioxidant, Antimicrobial, Immunomodulatory | [88,89,90,91,92,93,94,95] |
| Oregano (Origanum vulgare L.) | Carvacrol, Thymol, Rosmarinic acid | Antioxidant, Antimicrobial, Anti-inflammatory | [96,97,98,99] |
| Purple Coneflower (Echinacea purpurea L.) | Cichoric acid, Alkamides, Polysaccharides | Immunomodulatory, Antioxidant, Antiviral | [100,101,102,103,104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishchenko, S.; Złotek, U. Herbal and Spice Additives in Functional Confectionery Products: A Review. Molecules 2025, 30, 3449. https://doi.org/10.3390/molecules30163449
Ishchenko S, Złotek U. Herbal and Spice Additives in Functional Confectionery Products: A Review. Molecules. 2025; 30(16):3449. https://doi.org/10.3390/molecules30163449
Chicago/Turabian StyleIshchenko, Savelii, and Urszula Złotek. 2025. "Herbal and Spice Additives in Functional Confectionery Products: A Review" Molecules 30, no. 16: 3449. https://doi.org/10.3390/molecules30163449
APA StyleIshchenko, S., & Złotek, U. (2025). Herbal and Spice Additives in Functional Confectionery Products: A Review. Molecules, 30(16), 3449. https://doi.org/10.3390/molecules30163449