Tuning the Structure and Photoluminescence of [SbCl5]2−-Based Halides via Modification of Imidazolium-Based Cations
Abstract
1. Introduction
2. Results and Discussion
2.1. Crystal Structure Description
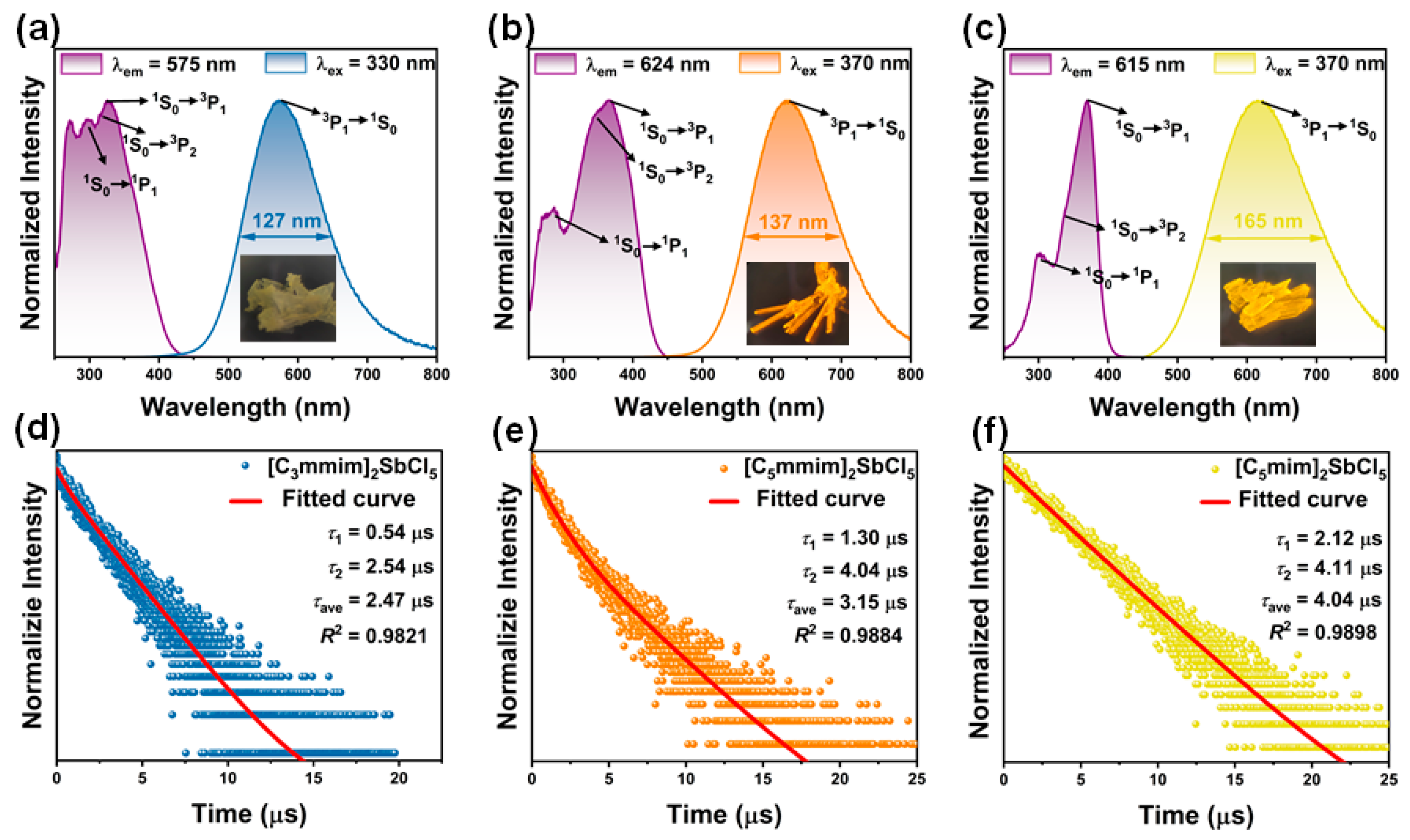
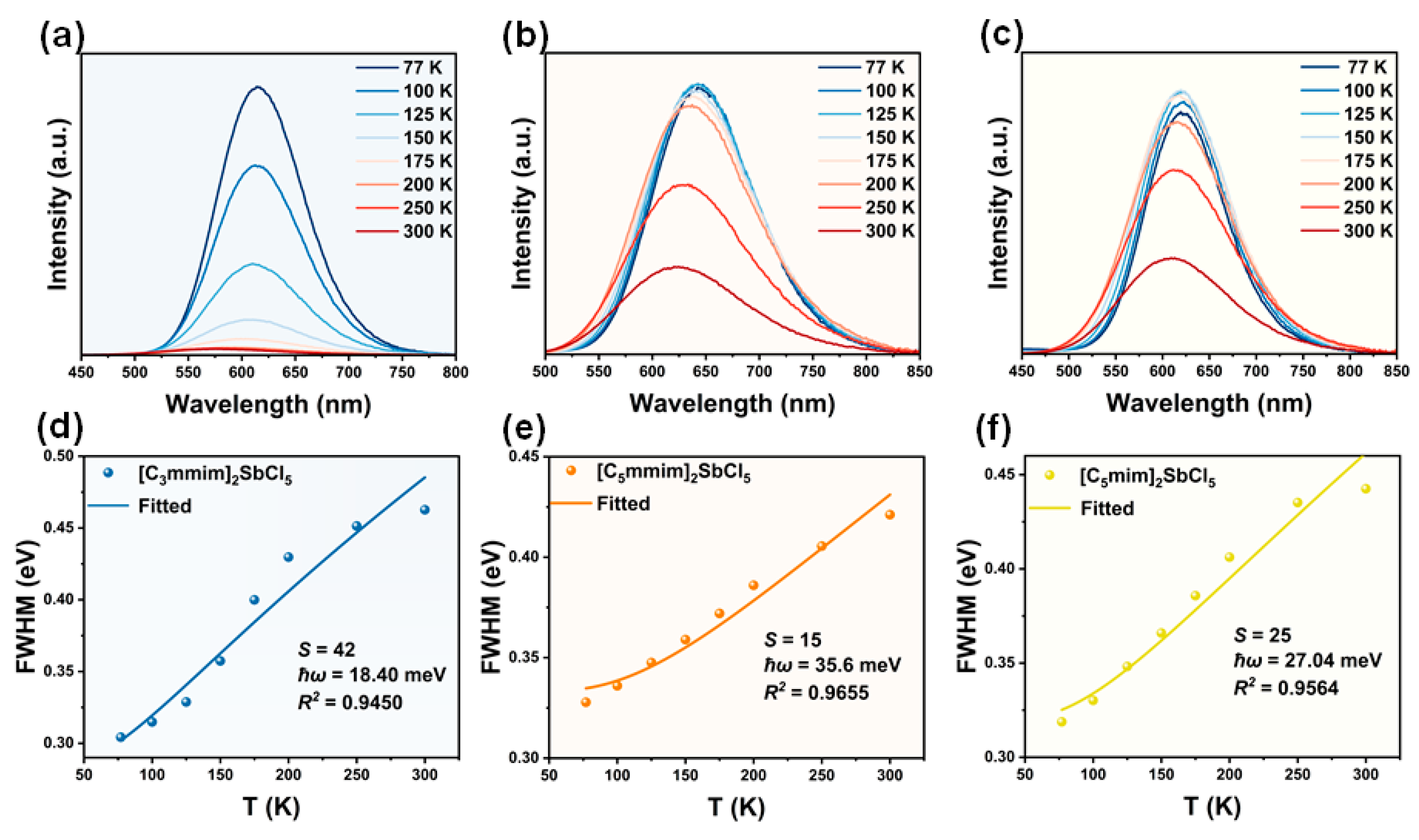
2.2. Optical Properties
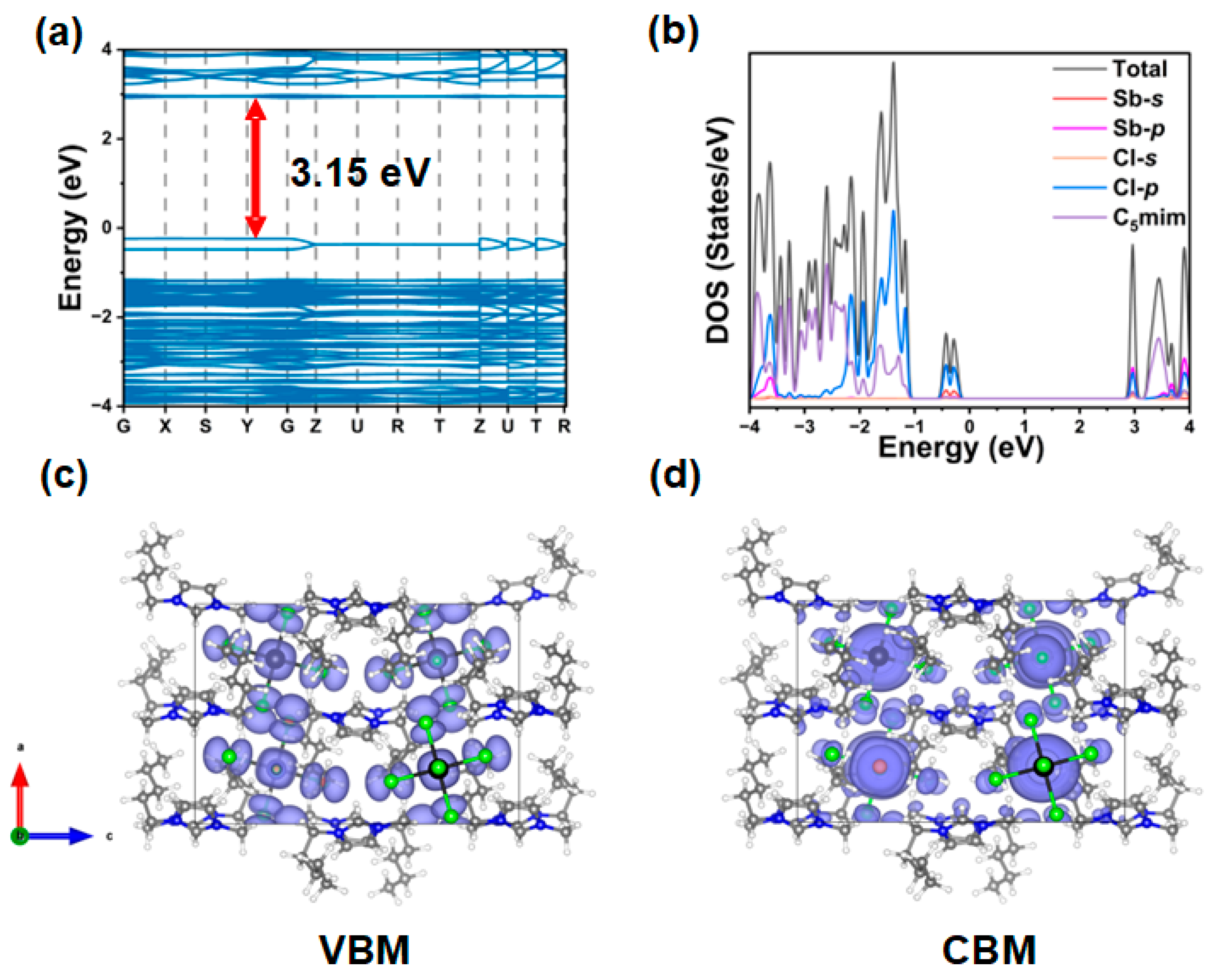
2.3. Density Functional Theory (DFT) Calculations
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meng, H.; Li, Y.; Wang, Y.; Zhu, M.; Xiao, J.; Shen, G. Highly Efficient Flexible Antimony Halide Scintillator Films with In Situ Preparation for High-Resolution X-Ray Imaging. Laser Photonics Rev. 2024, 19, 2401703. [Google Scholar] [CrossRef]
- He, Q.; Zhou, C.; Xu, L.; Lee, S.; Lin, X.; Neu, J.; Worku, M.; Chaaban, M.; Ma, B. Highly Stable Organic Antimony Halide Crystals for X-ray Scintillation. ACS Mater. Lett. 2020, 2, 633–638. [Google Scholar] [CrossRef]
- Zhang, Z.; Lin, Y.; Jin, J.; Gong, L.; Peng, Y.; Song, Y.; Shen, N.; Wang, Z.; Du, K.; Huang, X. Crystalline-Phase-Recognition-Induced Domino Phase Transition and Luminescence Switching for Advanced Information Encryption. Angew. Chem. Int. Ed. 2021, 60, 23373–23379. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Li, W.; Xiao, J.; Li, A.; Han, X. Low-Dimensional Metal Halide for High Performance Scintillators. Adv. Funct. Mater. 2024, 34, 2402902. [Google Scholar] [CrossRef]
- An, B.; Deng, Y.; Jin, Z.; Sun, S. Scintillators for Neutron Detection and Imaging: Advances and Prospects. Adv. Funct. Mater. 2024, 35, 2422522. [Google Scholar] [CrossRef]
- Gong, Z.; Zhang, J.; Deng, X.; Ren, M.P.; Wang, W.Q.; Wang, Y.J.; Cao, H.; Wang, L.; He, Y.C.; Lei, X.W. Near-unity broadband emissive hybrid manganese bromides as highly-efficient radiation scintillators. Aggregate 2024, 5, e574. [Google Scholar] [CrossRef]
- Han, J.H.; Seo, J.M.; Choi, S.H.; Noh, J.G.; Min, J.W.; Kim, Y.R.; Kim, H.W.; Cho, S.B.; Cha, B.K.; Im, W.B. Solvent-Tuned Plasticity for Various Binder-Free Applications of a New Lead-Free Manganese Halide. Adv. Mater. 2024, 37, 2415247. [Google Scholar] [CrossRef]
- Han, K.; Sakhatskyi, K.; Jin, J.; Zhang, Q.; Kovalenko, M.V.; Xia, Z. Seed-Crystal-Induced Cold Sintering Toward Metal Halide Transparent Ceramic Scintillators. Adv. Mater. 2022, 34, 2110420. [Google Scholar] [CrossRef]
- Nelyubina, Y.V.; Shaplov, A.S.; Lozinskaya, E.I.; Buzin, M.I.; Vygodskii, Y.S. A New Volume-Based Approach for Predicting Thermophysical Behavior of Ionic Liquids and Ionic Liquid Crystals. J. Am. Chem. Soc. 2016, 138, 10076–10079. [Google Scholar] [CrossRef]
- Morad, V.; Shynkarenko, Y.; Yakunin, S.; Brumberg, A.; Schaller, R.D.; Kovalenko, M.V. Disphenoidal Zero-Dimensional Lead, Tin, and Germanium Halides: Highly Emissive Singlet and Triplet Self-Trapped Excitons and X-ray Scintillation. J. Am. Chem. Soc. 2019, 141, 9764–9768. [Google Scholar] [CrossRef]
- Li, H.; Teng, Z.; Zhou, M.; Ji, T.; Yue, Y.; Yang, H.Y.; Qiu, J.; Wang, Q.; Xu, X.; Yu, X. A Thermoplastic Organic Metal Halide Scintillator. ACS Mater. Lett. 2023, 5, 2481–2487. [Google Scholar] [CrossRef]
- Elleuch, N.; Lhoste, J.; Boujelbene, M. Characterization, Hirshfeld surface analysis andvibrational properties of 2,6-diaminopurinium chloride tetrachloroantimonates(III) monohydrate (C5H8N6)[SbCl4]Cl·H2O. J. Mol. Struct. 2020, 1217, 128386. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Liang, P.; Zhou, T.; Wang, L.; Xie, R.-J. Dual-Band Luminescent Lead-Free Antimony Chloride Halides with Near-Unity Photoluminescence Quantum Efficiency. Chem. Mater. 2019, 31, 9363–9371. [Google Scholar] [CrossRef]
- Song, G.; Li, M.; Zhang, S.; Wang, N.; Gong, P.; Xia, Z.; Lin, Z. Enhancing Photoluminescence Quantum Yield in 0D Metal Halides by Introducing Water Molecules. Adv. Funct. Mater. 2020, 30, 2002468. [Google Scholar] [CrossRef]
- Zhou, C.; Lin, H.; Tian, Y.; Yuan, Z.; Clark, R.; Chen, B.; van de Burgt, L.J.; Wang, J.C.; Zhou, Y.; Hanson, K.; et al. Luminescent zero-dimensional organic metal halide hybrids with near-unity quantum efficiency. Chem. Sci. 2018, 9, 586–593. [Google Scholar] [CrossRef]
- Zaffalon, M.L.; Wu, Y.; Cova, F.; Gironi, L.; Li, X.; Pinchetti, V.; Liu, Y.; Imran, M.; Cemmi, A.; Di Sarcina, I.; et al. Zero-Dimensional Gua3SbCl6 Crystals as Intrinsically Reabsorption-Free Scintillators for Radiation Detection. Adv. Funct. Mater. 2023, 33, 2305564. [Google Scholar] [CrossRef]
- Peng, Y.-C.; Jin, J.-C.; Zhou, S.-H.; Lin, H.-W.; Huang, D.-D.; Deng, Z.-H.; Dong, Y.; Xu, H.-J.; Du, K.-Z.; Wang, Z.-P.; et al. Regulating photoluminescence through single-crystal-to-single-crystal transformation of solvent-containing zero-dimensional hybrid metal halide isomers. Chem. Eng. J. 2024, 488, 151026. [Google Scholar] [CrossRef]
- Chen, F.; Wang, S.; Li, Y.-H.; Huang, W. Effects of Anionic Geometries on Hydrogen-Bonding Networks of 1-(4-pyridyl) Piperazine. J. Chem. Crystallogr. 2016, 46, 309–323. [Google Scholar] [CrossRef]
- Wojciechowska, M.; Szklarz, P.; Białońska, A.; Baran, J.; Janicki, R.; Medycki, W.; Durlak, P.; Piecha-Bisiorek, A.; Jakubas, R. Enormous lattice distortion through an isomorphous phase transition in an organic-inorganic hybrid based on haloantimonate(III). CrystEngComm 2016, 18, 6184–6194. [Google Scholar] [CrossRef]
- Parmar, S.; Pal, S.; Biswas, A.; Gosavi, S.; Chakraborty, S.; Reddy, M.C.; Ogale, S. Designing a new family of oxonium-cation based structurally diverse organic-inorganic hybrid iodoantimonate crystals. Chem. Commun. 2019, 55, 7562–7565. [Google Scholar] [CrossRef]
- Wojtaś, M.; Jakubas, R.; Ciunik, Z.; Medycki, W. Structure and phase transitions in [(CH3)4P]3[Sb2Br9] and [(CH3)4P]3[Bi2Br9]. J. Solid State Chem. 2004, 177, 1575–1584. [Google Scholar] [CrossRef]
- Benin, B.M.; McCall, K.M.; Wörle, M.; Morad, V.; Aebli, M.; Yakunin, S.; Shynkarenko, Y.; Kovalenko, M.V. The Rb7Bi3-3xSb3xCl16 Family: A Fully Inorganic Solid Solution with Room-Temperature Luminescent Members. Angew. Chem. Int. Ed. 2020, 59, 14490–14497. [Google Scholar] [CrossRef]
- Piecha, A.; Pietraszko, A.; Bator, G.; Jakubas, R. Structural characterization and ferroelectric ordering in (C3N2H5)5Sb2Br11. J. Solid State Chem. 2008, 181, 1155–1166. [Google Scholar] [CrossRef]
- Saidaminov, M.I.; Mohammed, O.F.; Bakr, O.M. Low-Dimensional-Networked Metal Halide Perovskites: The Next Big Thing. ACS Mater. Lett. 2017, 2, 889–896. [Google Scholar] [CrossRef]
- Lin, H.; Zhou, C.; Tian, Y.; Siegrist, T.; Ma, B. Low-Dimensional Organometal Halide Perovskites. ACS Mater. Lett. 2017, 3, 54–62. [Google Scholar] [CrossRef]
- Li, M.; Xia, Z. Recent progress of zero-dimensional luminescent metal halides. Chem. Soc. Rev. 2021, 50, 2626–2662. [Google Scholar] [CrossRef]
- Jing, Y.; Liu, Y.; Li, M.; Xia, Z. Photoluminescence of Singlet/Triplet Self-Trapped Excitons in Sb3+-Based Metal Halides. Adv. Opt. Mater. 2021, 9, 2002213. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, X. Luminescent Organic-Inorganic Hybrid Metal Halides: An Emerging Class of Stimuli-Responsive Materials. Chem. Eur. J. 2022, 28, e202200609. [Google Scholar] [CrossRef]
- Ma, Z.; Yu, J.; Dai, S. Preparation of Inorganic Materials Using Ionic Liquids. Adv. Mater. 2009, 22, 261–285. [Google Scholar] [CrossRef]
- Nakamura, D.; Gunjishima, I.; Yamaguchi, S.; Ito, T.; Okamoto, A.; Kondo, H.; Onda, S.; Takatori, K. Ultrahigh-quality silicon carbide single crystals. Nature 2004, 430, 1009–1012. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Z.; Tao, L.; Shen, N.; Hu, B.; Gong, L.; Li, J.; Chen, X.; Huang, X. Hybrid Chloroantimonates(III): Thermally Induced Triple-Mode Reversible Luminescent Switching and Laser-Printable Rewritable Luminescent Paper. Angew. Chem. Int. Ed. 2019, 58, 9974–9978. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Tong, H.; Lin, H.; Liu, W. Manipulating the inorganic motif by kinetic control of antimony halide organic-inorganic hybrid materials for larger Stokes shift and significantly enhanced quantum efficiency. Chem. Commun. 2022, 58, 12596–12599. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Guo, Z.; Sun, N.; Liu, K.; He, S.; Chen, X.; Zhao, J.; Liu, Q.; Yuan, W. Improving the Chemical Stability of Narrow-Band Green-Emitting Manganese(II) Hybrid by Zn-Doping. Inorg. Chem. 2022, 61, 15266–15272. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Zhang, Y.; Suescun, L.; Chen, M.; Song, Z.; Liu, Q. Linking the pyramid distortion to luminescence in Sb-based metal halide by best fitted ideal polyhedron characterization. J. Mater. Chem. C 2025, 13, 5988–5992. [Google Scholar] [CrossRef]
- Biswas, A.; Bakthavatsalam, R.; Mali, B.P.; Bahadur, V.; Biswas, C.; Raavi, S.S.K.; Gonnade, R.G.; Kundu, J. The metal halide structure and the extent of distortion control the photo-physical properties of luminescent zero dimensional organic-antimony(III) halide hybrids. J. Mater. Chem. C 2021, 9, 348–358. [Google Scholar] [CrossRef]
- Sedakova, T.V.; Mirochnik, A.G.; Karasev, V.E. Structure and luminescence properties of antimony(III) complex compounds. Opt. Spectrosc. 2008, 105, 517–523. [Google Scholar] [CrossRef]
- Petrochenkova, N.V.; Storozhuk, T.V.; Mirochnik, A.G.; Karasev, V.E. Antimony(III) Complexes with Quaternary Ammonium Bases: Synthesis, Spectral, and Luminescent Properties. Russ. J. Coord. Chem. 2002, 28, 468–472. [Google Scholar] [CrossRef]
- Storozhuk, T.V.; Mirochnik, A.G.; Petrochenkova, N.V.; Karasev, V.E. Sensitization of Luminescence of Antimony(III) in Complexes with 6-Methylquinoline in the Spectral Region of the A Band. Opt. Spectrosc. 2003, 94, 920–923. [Google Scholar] [CrossRef]
- Molokeev, M.S.; Su, B.; Aleksandrovsky, A.S.; Golovnev, N.N.; Plyaskin, M.E.; Xia, Z. Machine Learning Analysis and Discovery of Zero-Dimensional ns2 Metal Halides toward Enhanced Photoluminescence Quantum Yield. Chem. Mater. 2022, 34, 537–546. [Google Scholar] [CrossRef]
- Huang, H.; Yang, Y.; Qiao, S.; Wu, X.; Chen, Z.; Chao, Y.; Yang, K.; Guo, W.; Luo, Z.; Song, X.; et al. Accommodative Organoammonium Cations in A-Sites of Sb-In Halide Perovskite Derivatives for Tailoring BroadBand Photoluminescence with X-Ray Scintillation and White-Light Emission. Adv. Funct. Mater. 2023, 34, 2309112. [Google Scholar] [CrossRef]
- Wei, Q.; Chang, T.; Zeng, R.; Cao, S.; Zhao, J.; Han, X.; Wang, L.; Zou, B. Self-Trapped Exciton Emission in a Zero-Dimensional (TMA)2SbCl5·DMF Single Crystal and Molecular Dynamics Simulation of Structural Stability. J. Phys. Chem. Lett. 2021, 12, 7091–7099. [Google Scholar] [CrossRef]
- Huang, T.; Li, K.; Lei, J.; Niu, Q.; Peng, H.; Zou, B. Origin of singlet self-trapped exciton and enhancement of photoluminescence quantum yield of organic-inorganic hybrid antimony(III) chlorides with the [SbCl5]2− units. Nano Res. 2023, 16, 12680–12688. [Google Scholar] [CrossRef]
- Wang, Z.-P.; Wang, J.-Y.; Li, J.-R.; Feng, M.-L.; Zou, G.-D.; Huang, X.-Y. [Bmim]2SbCl5: A main group metal-containing ionic liquid exhibiting tunable photoluminescence and white-light emission. Chem. Commun. 2015, 51, 3094–3097. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.-C.; Zhang, Z.-Z.; Lin, Y.-P.; Jin, J.-C.; Zhuang, T.-H.; Gong, L.-K.; Wang, Z.-P.; Du, K.-Z.; Huang, X.-Y. A deep-red-emission antimony(III) chloride with dual-cations: Extremely large Stokes shift due to high [SbCl6] distortion. Chem. Commun. 2021, 57, 13784–13787. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Guo, P.; Wang, S.; Cheetham, A.K.; Seshadri, R. Design Principles for Enhancing Photoluminescence Quantum Yield in Hybrid Manganese Bromides. J. Am. Chem. Soc. 2020, 142, 13582–13589. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, Z.; Huang, J.; Molokeev, M.S.; Xiao, Z.; Ma, C.; Xia, Z. Unraveling the Near-Unity Narrow-Band Green Emission in Zero-Dimensional Mn2+-Based Metal Halides: A Case Study of (C10H16N)2Zn1–xMnxBr4 Solid Solutions. J. Phys. Chem. Lett. 2020, 11, 5956–5962. [Google Scholar] [CrossRef]
- Vogler, A.; Nikol, H. The Structures of s2 Metal Complexes in the Ground and sp Excited States. Comments Inorg. Chem. 1993, 14, 245–261. [Google Scholar] [CrossRef]
- Nikol, H.; Vogler, A. Photoluminescence of Antimony(III) and Bismuth(III) Chloride Complexes in Solution. J. Am. Chem. Soc. 1991, 113, 8988–8990. [Google Scholar] [CrossRef]
- Boens, N.; Ameloot, M.; Yamazaki, I.; Deschryver, F.C. On the use and the perfornance of the delta-function convolution method for the estimation of fluorescence decay parameters. Chem. Phys. 1988, 121, 73–86. [Google Scholar] [CrossRef]
- GZatryb; Klak, M.M. On the choice of proper average lifetime formula for an ensemble of emitters showing non-single exponential photoluminescence decay. J. Phys. Condens. Matter 2020, 32, 415902. [Google Scholar] [CrossRef]
- Wang, S.; Xu, Y.; Chen, T.; Jiang, W.; Liu, J.; Zhang, X.; Jiang, W.; Wang, L. A red phosphor LaSc3(BO3)4:Eu3+ with zero-thermal-quenching and high quantum efficiency for LEDs. Chem. Eng. J. 2021, 404, 125912. [Google Scholar] [CrossRef]
- Wei, Y.; Yang, H.; Gao, Z.; Liu, Y.; Xing, G.; Dang, P.; Kheraif, A.A.A.; Li, G.; Lin, J.; Liu, R.S. Strategies for Designing Antithermal-Quenching Red Phosphors. Adv. Sci. 2020, 7, 1903060. [Google Scholar] [CrossRef] [PubMed]
- Song, K.S.; Williams, R.T. Temperature-dependent self-trapped exciton relaxation in alkali halides: Molecular dynamics study. Phys. Status Solidi B 2006, 243, 3782–3794. [Google Scholar] [CrossRef]
- Wu, L.-K.; Sun, H.-Y.; Li, L.-H.; Li, R.-F.; Ye, H.-Y.; Li, J.-R. Te4+-Doping Rubidium Scandium Halide Perovskite Single Crystals Enabling Optical Thermometry. J. Phys. Chem. C 2022, 126, 21689–21698. [Google Scholar] [CrossRef]
- Peng, H.; Zou, B. Effects of Electron-Phonon Coupling and Spin-Spin Coupling on the Photoluminescence of Low-Dimensional Metal Halides. J. Phys. Chem. Lett. 2022, 13, 1752–1764. [Google Scholar] [CrossRef]
- Luo, H.; Guo, S.; Zhang, Y.; Bu, K.; Lin, H.; Wang, Y.; Yin, Y.; Zhang, D.; Jin, S.; Zhang, W.; et al. Regulating Exciton-Phonon Coupling to Achieve a Near-Unity Photoluminescence Quantum Yield in One-Dimensional Hybrid Metal Halides. Adv. Sci. 2021, 8, 2100786. [Google Scholar] [CrossRef]
- Hou, J.; Liu, R.; Han, P.; Luo, C.; Ding, Z.; Zhou, W.; Li, C.; Li, J.; Zhao, Y.; Chen, J.; et al. Unveiling the Localized Exciton-Based Photoluminescence of Manganese Doped Cesium Zinc Halide Nanocrystals. Nano Lett. 2023, 23, 3762–3768. [Google Scholar] [CrossRef]
- Peng, H.; Tian, Y.; Yu, Z.; Wang, X.; Ke, B.; Zhao, Y.; Dong, T.; Wang, J.; Zou, B. (C16H28N)2SbCl5: A new lead-free zero-dimensional metal-halide hybrid with bright orange emission. Sci. China Mater. 2022, 65, 1594–1600. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for open-shell transition metals. Phys. Rev. B 1993, 48, 13115–13118. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]

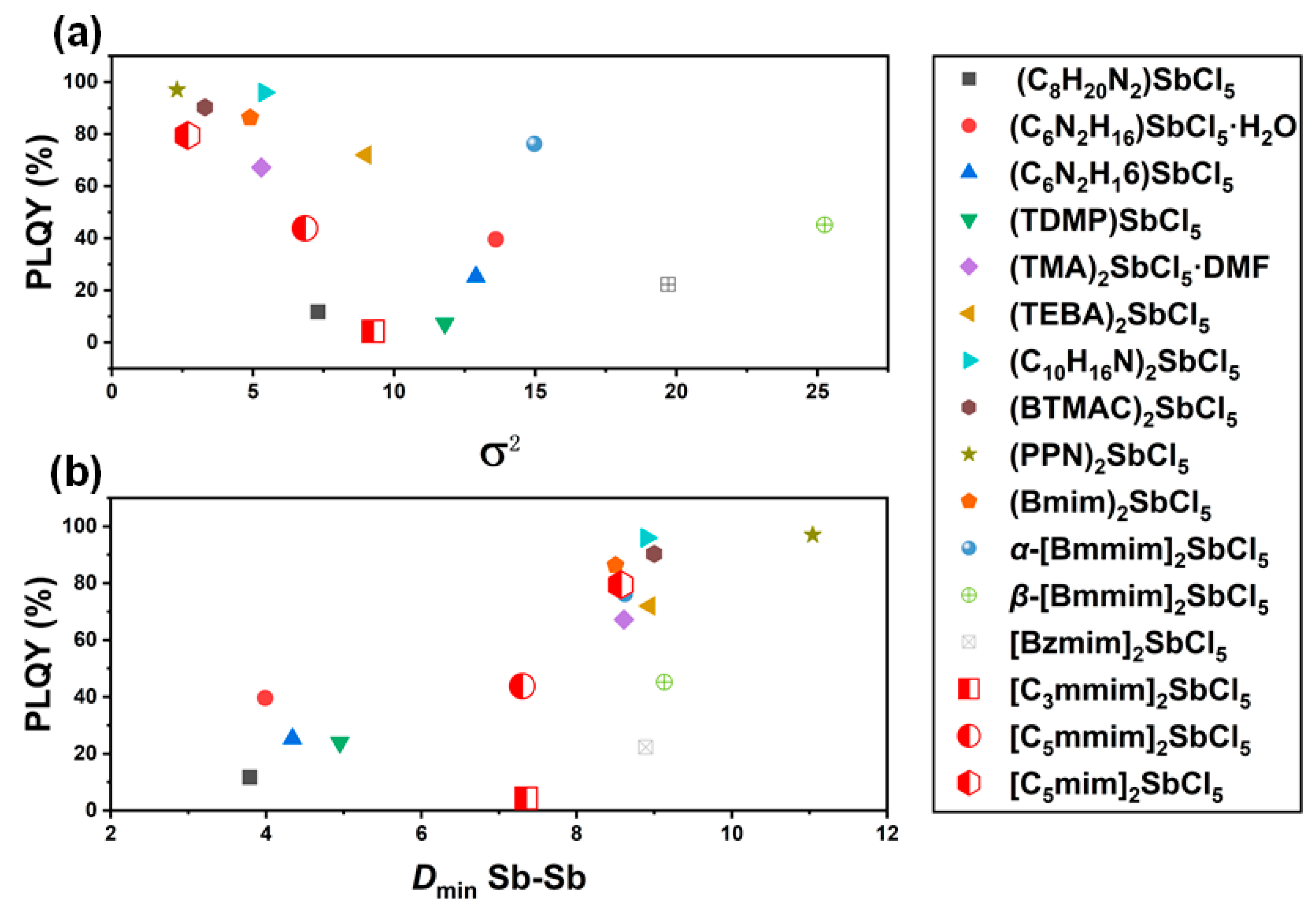
| Compound | Dmin Sb–Sb (Å) | dn (10−4) | θn | PLQY (%) | Ref. |
|---|---|---|---|---|---|
| (C8H20N2)SbCl5 | 3.79 | 5.7 | 7.3 | 11.8 | [39] |
| (C6N2H16)SbCl5·H2O | 3.99 | 9.1 | 13.6 | 39.6 | [14] |
| (C6N2H16)SbCl5 | 4.34 | 4.8 | 12.9 | 25.3 | [14] |
| (TDMP)SbCl5 | 4.95 | 8.3 | 11.8 | 24.0 | [40] |
| (TMA)2SbCl5·DMF | 8.61 | 1.5 | 5.3 | 67.2 | [41] |
| (TEBA)2SbCl5 | 8.94 | 1.6 | 9.0 | 72.0 | [13] |
| (C10H16N)2SbCl5 | 8.90 | 1.5 | 5.4 | 96.0 | [39] |
| (BTMAC)2SbCl5 | 9.00 | 1.4 | 3.3 | 90.3 | [42] |
| (PPN)2SbCl5 | 11.04 | 13.7 | 2.3 | 97.0 | [2] |
| (Bmim)2SbCl5 | 8.50 | 3.3 | 4.9 | 86.3 | [43] |
| α-[Bmmim]2SbCl5 | 8.62 | 2.3 | 15.0 | 76.2 | [3] |
| β-[Bmmim]2SbCl5 | 9.13 | 2.7 | 25.3 | 45.2 | [3] |
| [Bzmim]2SbCl5 | 8.89 | 4.9 | 19.7 | 22.3 | [31] |
| [C3mmim]2SbCl5 | 7.35 | 1.5 | 9.3 | 4.3 | This work |
| [C5mmim]2SbCl5 | 7.30 | 1.3 | 6.8 | 43.8 | This work |
| [C5mim]2SbCl5 | 8.57 | 1.5 | 2.7 | 79.5 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.; Guo, X.; Lin, H.; Zhang, Z.; Ablez, A.; Ren, Y.; Du, K.; Huang, X. Tuning the Structure and Photoluminescence of [SbCl5]2−-Based Halides via Modification of Imidazolium-Based Cations. Molecules 2025, 30, 3431. https://doi.org/10.3390/molecules30163431
Chen G, Guo X, Lin H, Zhang Z, Ablez A, Ren Y, Du K, Huang X. Tuning the Structure and Photoluminescence of [SbCl5]2−-Based Halides via Modification of Imidazolium-Based Cations. Molecules. 2025; 30(16):3431. https://doi.org/10.3390/molecules30163431
Chicago/Turabian StyleChen, Guoyang, Xinping Guo, Haowei Lin, Zhizhuan Zhang, Abdusalam Ablez, Yuwei Ren, Kezhao Du, and Xiaoying Huang. 2025. "Tuning the Structure and Photoluminescence of [SbCl5]2−-Based Halides via Modification of Imidazolium-Based Cations" Molecules 30, no. 16: 3431. https://doi.org/10.3390/molecules30163431
APA StyleChen, G., Guo, X., Lin, H., Zhang, Z., Ablez, A., Ren, Y., Du, K., & Huang, X. (2025). Tuning the Structure and Photoluminescence of [SbCl5]2−-Based Halides via Modification of Imidazolium-Based Cations. Molecules, 30(16), 3431. https://doi.org/10.3390/molecules30163431








