Separation of the Biofuel Methyl Ethyl Ketone from Aqueous Solutions Using Avocado-Based Activated Carbons: Synthesis Conditions and Multilayer Adsorption Properties
Abstract
1. Introduction
2. Results and Discussion
2.1. Identification of the Best Avocado-Based Activated Carbon for MEK Separation
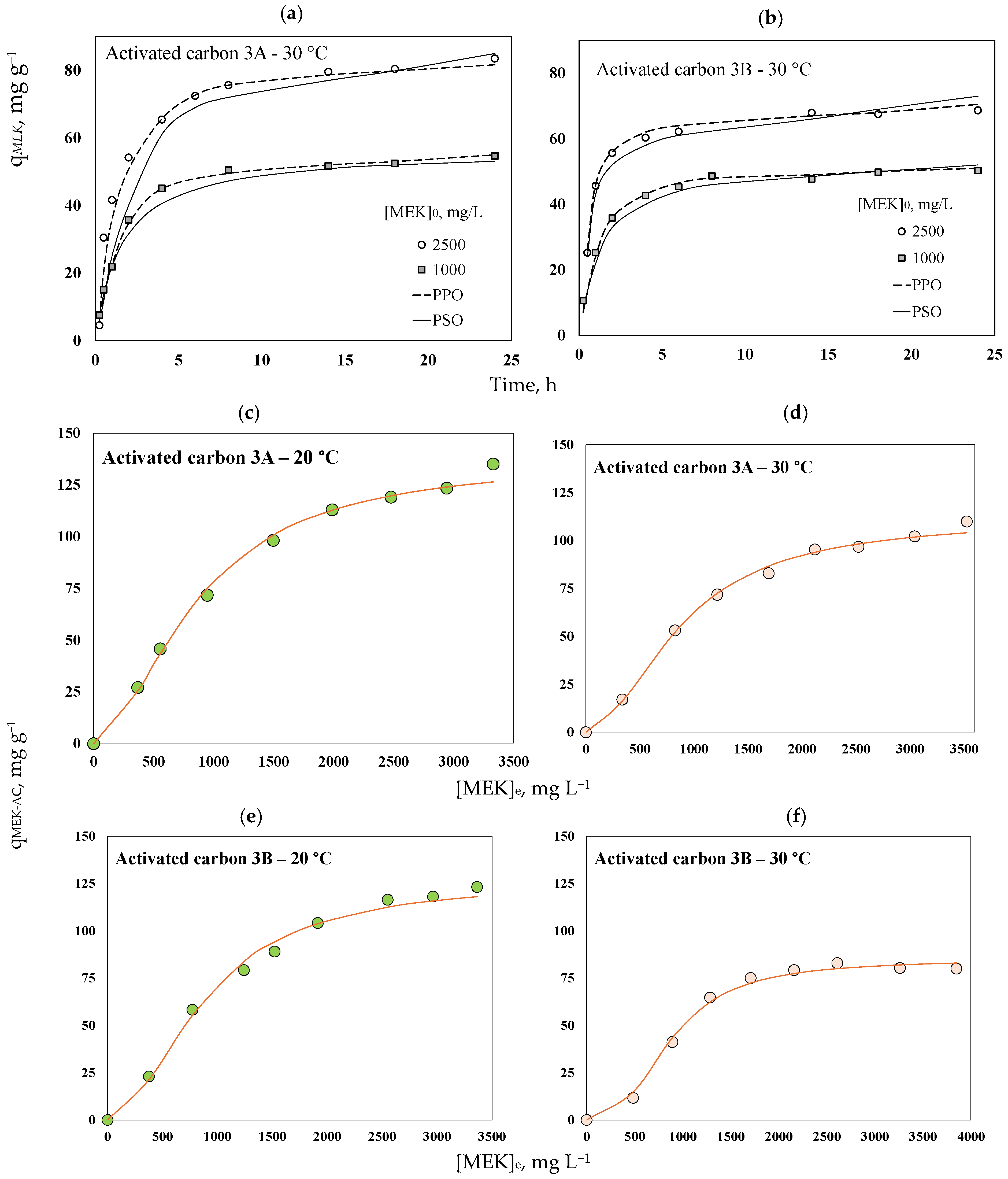
2.2. Thermodynamics of MEK Separation Using Avocado-Based Activated Carbons
| Activated Carbon | |||||
|---|---|---|---|---|---|
| 3A | 3B | ||||
| [MEK]0, mg L−1 | |||||
| Kinetic Model | Parameters | 1000 | 2500 | 1000 | 2500 |
| Pseudo-first order | k1, min−1 | 0.432 | 0.706 | 0.477 | 0.89 |
| qMEKe, mg g−1 | 49.30 | 81.16 | 63.54 | 80.31 | |
| R2 | 0.993 | 0.985 | 0.998 | 0.995 | |
| Pseudo-second order | k2, g mg−1 h−1 | 0.011 | 0.003 | 0.007 | 0.012 |
| qMEKe, mg g−1 | 56.56 | 96.05 | 72.28 | 89.30 | |
| R2 | 0.942 | 0.901 | 0.951 | 0.965 | |
| Separation Temperature, °C | |||||
| Isotherm Model | Parameters | 20 | 30 | 20 | 30 |
Langmuir [91] | KL, L mg−1 | 3.9 × 10−4 | 3.2 × 10−4 | 2.9 × 10−4 | 1.0 × 10−4 |
| qMEKm, mg g−1 | 244.89 | 210.43 | 267.74 | 351.44 | |
| R2 | 0.954 | 0.911 | 0.892 | 0.758 | |
Freundlich [92] | KF, mg1-/n L1/n g−1 | 0.51 | 0.27 | 0.31 | 0.05 |
| nF | 1.44 | 1.34 | 1.33 | 1.09 | |
| R2 | 0.912 | 0.857 | 0.851 | 0.713 | |
Sips [93] | Ks, Ln (mgn)−1 | 6.5 × 10−5 | 1.3 × 10−4 | 3.3 × 10−5 | 1.1 × 10−8 |
| nS | 0.73 | 0.83 | 0.68 | 0.37 | |
| qMEKm, mg g−1 | 138.87 | 115.03 | 112.84 | 86.08 | |
| R2 | 0.992 | 0.998 | 0.996 | 0.991 | |
3. Materials and Methods
3.1. Preparation and Tailoring of Avocado-Based Activated Carbons
3.2. Kinetics and Isotherms for MEK Separation Using Avocado-Based Activated Carbon
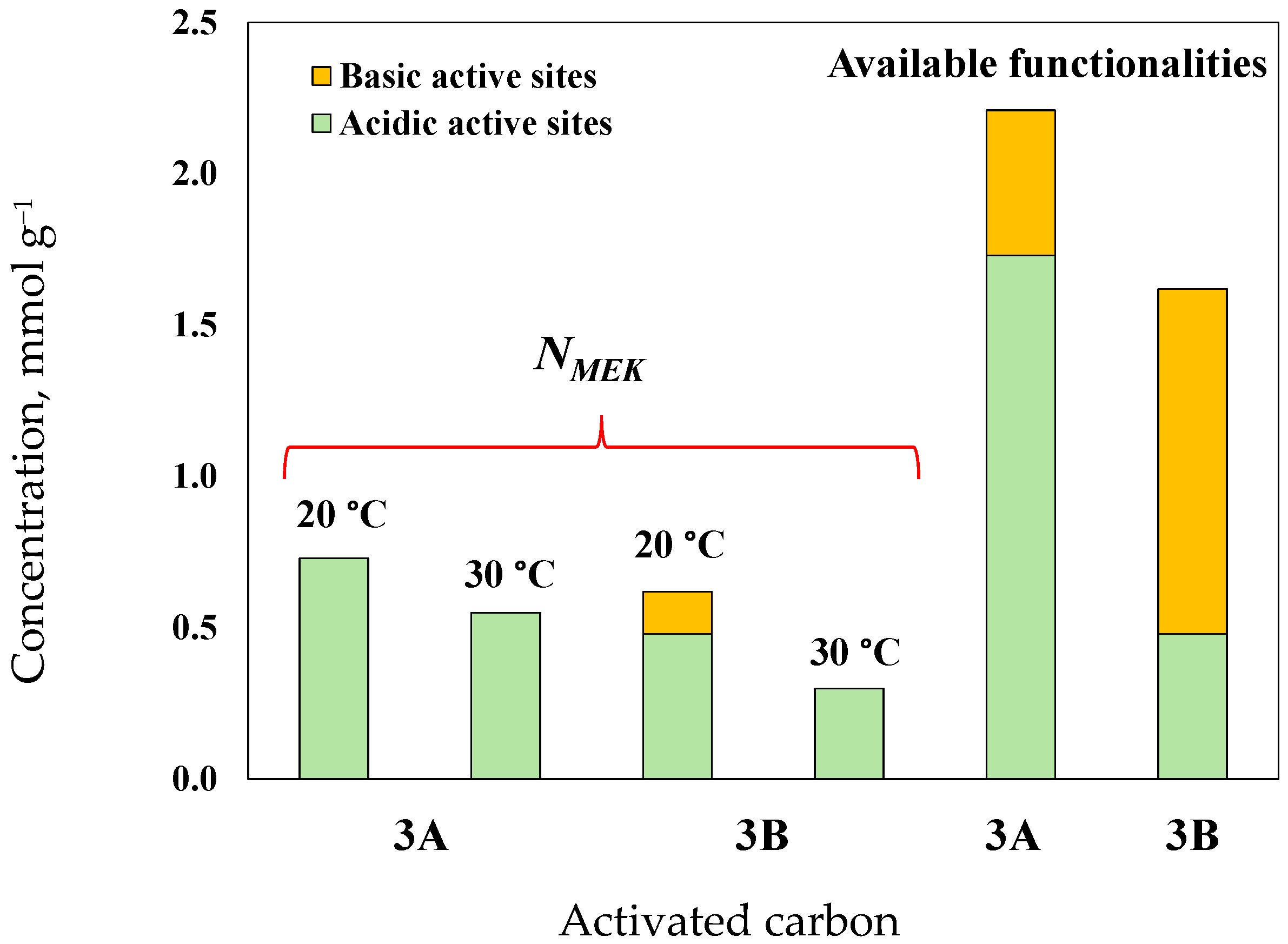
3.3. Surface Chemistry Characterization of Avocado-Based Char and Activated Carbon
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MEK | Methyl ethyl ketone |
References
- Burke, U.; Beeckmann, J.; Kopp, W.A.; Uygun, Y.; Olivier, H.; Leonhard, K.; Pitsch, H.; Heufer, K.A. A comprehensive experimental and kinetic modeling study of butanone. Combust. Flame 2016, 168, 296–309. [Google Scholar] [CrossRef]
- Padhi, U.P.; Agarwal, A.A.; Kumar, S. Effect of 2-butanone addition on laminar burning velocity of gasoline XP95 at higher mixture temperatures. Combust. Flame 2023, 255, 112924. [Google Scholar] [CrossRef]
- Zhang, F.; Sun, D.; Li, Y.; Shan, B.; Ma, Y.; Wang, Y.; Li, X.; Shan, B.; Ma, Y.; Wang, Y.; et al. Heat integration and dynamic control for separating the ternary azeotrope of butanone/isopropanol/n-heptane via pressure-swing distillation. Chem. Eng. Process—Proc. Intens. 2022, 170, 108657. [Google Scholar] [CrossRef]
- National Library of Medicine US. Toxicological Profile for 2-Butanone; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK590510/ (accessed on 28 February 2025).
- Lee, J.; Bhagwat, S.; Kuanyshev, N.; Cho, Y.; Sun, L.; Lee, Y.; Cortés-Peña, Y.; Li, Y.; Rao, C.; Guest, J.; et al. Rewiring yeast metabolism for producing 2,3-butanediol and two downstream applications: Techno-economic analysis and life cycle assessment of methyl ethyl ketone (MEK) and agricultural biostimulant production. Chem. Eng. J. 2023, 451, 138886. [Google Scholar] [CrossRef]
- Penner, D.; Redepenning, C.; Mitsos, A.; Viell, J. Conceptual Design of Methyl Ethyl Ketone Production via 2,3-Butanediol for Fuels and Chemicals. Ind. Eng. Chem. Res. 2017, 56, 3947–3957. [Google Scholar] [CrossRef]
- Ma, X.; Li, L.; Chen, R.; Wang, C.; Zhou, K.; Li, H. Porous carbon materials based on biomass for acetone adsorption: Effect of surface chemistry and porous structure. Appl. Surf. Sci. 2018, 459, 657–664. [Google Scholar] [CrossRef]
- Silva, B.; Rocha, V.; Lago, A.; Costa, F.; Tavares, T. Rehabilitation of a complex industrial wastewater containing heavy metals and organic solvents using low cost permeable bio-barriers—From lab-scale to pilot-scale. Sep. Purif. Technol. 2021, 263, 118381. [Google Scholar] [CrossRef]
- Gar, M.; Nasr, M. Artificial intelligence, regression model, and cost estimation for removal of chlorothalonil pesticide by activated carbon prepared from casuarina charcoal. Sustain. Environ. Res. 2018, 28, 101–110. [Google Scholar] [CrossRef]
- Mishra, R.; Singh, B.; Acharya, B.; Acharya, B. A comprehensive review on activated carbon from pyrolysis of lignocellulosic biomass: An application for energy and the environment. Carbon. Resour. Convers. 2024, 7, 100228. [Google Scholar]
- Bai, Y.; Huang, Z.H.; Kang, F. Surface oxidation of activated electrospun carbon nanofibers and their adsorption performance for benzene, butanone and ethanol. Colloids Surf. A Physicochem. Eng. Asp. 2014, 443, 66–71. [Google Scholar] [CrossRef]
- Janus, R.; Wądrzyk, M.; Natkański, P.; Cool, P.; Kuśtrowski, P. Dynamic adsorption–desorption of methyl ethyl ketone on MCM-41 and SBA-15 decorated with thermally activated polymers. J. Ind. Eng. Chem. 2019, 71, 465–480. [Google Scholar] [CrossRef]
- Vikrant, K.; Kim, K.H.; Peng, W.; Ge, S.; Sik Ok, Y. Adsorption performance of standard biochar materials against volatile organic compounds in air: A case study using benzene and methyl ethyl ketone. Chem. Eng. J. 2020, 387, 123943. [Google Scholar] [CrossRef]
- Tahara, Y.; Azim, M.; Takishima, S.; Ushiki, I. Measurement and modeling of adsorption equilibria of ketone VOCs on activated carbon in supercritical CO2. J. Supercrit. Fluids 2023, 203, 106079. [Google Scholar] [CrossRef]
- Sotelo, J.L.; Uguina, M.A.; Delgado, J.A.; Celemin, L.I. Adsorption of methyl ethyl ketone and trichloroethene from aqueous solutions onto activated carbon fixed-bed adsorbers. Sep. Purif. Technol. 2004, 37, 149–160. [Google Scholar] [CrossRef]
- Uguina, M.A.; Sotelo, J.L.; Delgado, J.A.; Gómez, J.M.; Celemín, L.I. Adsorption of methyl ethyl ketone and trichloroethene from aqueous solutions onto silicalite fixed-bed adsorbers. Sep. Purif. Technol. 2005, 42, 91–99. [Google Scholar] [CrossRef]
- Ali, R.H.; Mohammed, A.J. A study of Adsorption of Acetone and 2-Butanone on Iraqi Siliceouns Rocks Powder. J. Kufa Chem. Sci. 2012, 6, 121–129. [Google Scholar]
- Zhou, Y.; Zhang, L.; Cheng, Z. Removal of organic pollutants from aqueous solution using agricultural wastes: A review. J. Mol. Liq. 2015, 212, 739–762. [Google Scholar] [CrossRef]
- Yang, W.; Fortunati, E.; Luzi, F.; Kenny, J.M.; Torre, L.; Puglia, D. Lignocellulosic Based Bionanocomposites for Different Industrial Applications. Curr. Org. Chem. 2018, 22, 1205–1221. [Google Scholar] [CrossRef]
- Rangabhashiyam, S.; Balasubramanian, P. The potential of lignocellulosic biomass precursors for biochar production: Performance, mechanism and wastewater application—A review. Ind. Crops Prod. 2019, 128, 405–423. [Google Scholar]
- Cheu, S.C.; Kong, H.; Song, S.T.; Johari, K.; Saman, N.; Che, M.A.; Mat, H. Separation of dissolved oil from aqueous solution by sorption onto acetylated lignocellulosic biomass—Equilibrium, kinetics and mechanism studies. J. Environ. 2016, 4, 864–881. [Google Scholar] [CrossRef]
- Neris, J.B.; Luzardo, F.H.M.; Silva, E.G.P.; Velasco, F.G. Evaluation of adsorption processes of metal ions in multi-element aqueous systems by lignocellulosic adsorbents applying different isotherms: A critical review. Chem. Eng. J. 2019, 357, 404–420. [Google Scholar] [CrossRef]
- Mohammad, S.G.; Ahmed, S.M.; Amr, A.E.-G.E.; Kamel, A.H. Porous Activated Carbon from Lignocellulosic Agricultural Waste for the Removal of Acetamiprid Pesticide from Aqueous Solutions. Molecules 2020, 25, 2339. [Google Scholar] [CrossRef]
- Uysal, T.; Duman, G.; Onal, Y.; Yasa, I.; Yanik, J. Production of activated carbon and fungicidal oil from peach stone by two-stage process. J. Anal. Appl. Pyrolysis 2014, 108, 47–55. [Google Scholar] [CrossRef]
- Yanan, C.; Yang, B.; Song, Z.; Wang, H.; He, F.; Han, X. Wheat straw biochar amendments on the removal of polycyclic aromatic hydrocarbons (PAHs) in contaminated soil. Ecotoxicol. Environ. Saf. 2016, 130, 248–255. [Google Scholar] [CrossRef]
- Zhu, Y.; Kolar, P.; Shah, S.B.; Cheng, J.J.; Lim, P.K. Avocado seed-derived activated carbon for mitigation of aqueous ammonium. Ind. Crops Prod. 2016, 92, 34–41. [Google Scholar] [CrossRef]
- Salomón-Negrete, M.Á.; Reynel-Ávila, H.E.; Mendoza-Castillo, D.I.; Bonilla-Petriciolet, A.; Duran-Valle, C.J. Water defluoridation with avocado-based adsorbents: Synthesis, physicochemical characterization and thermodynamic studies. J. Mol. Liq. 2018, 254, 188–197. [Google Scholar] [CrossRef]
- Kutluay, S.; Baytar, O.; Şahin, Ö. Adsorption kinetics, equilibrium and thermodynamics of gas-phase toluene onto char produced from almond shells. Res. Eng. Struct. Mater. 2019, 5, 279–298. [Google Scholar] [CrossRef]
- Aranguri-Llerena, G.; Reyes-Lázaro, W. Adsorption of cyanide contained in aqueous solution using activated carbon obtained from coffee residue: Absorption efficiency, equilibrium and kinetic model. Sci. Agropecu 2019, 10, 315–325. [Google Scholar] [CrossRef]
- Elizalde-González, M.P.; Mattusch, J.; Peláez-Cid, A.A.; Wennrich, R. Characterization of adsorbent materials prepared from avocado kernel seeds: Natural, activated and carbonized forms. J. Anal. Appl. Pyrolysis 2007, 78, 185–193. [Google Scholar] [CrossRef]
- Bhaumik, M.; Choi, H.J.; Seopela, M.P.; McCrindle, R.I.; Maity, A. Highly Effective Removal of Toxic Cr(VI) from Wastewater Using Sulfuric Acid-Modified Avocado Seed. Ind. Eng. Chem. Res. 2014, 53, 1214–1224. [Google Scholar] [CrossRef]
- Kudo, M.; Oliveira, L.; Suquila, F.; de Almeida, F.; Segatelli, M.; Lima, É.; Dias, S.; Tarley, C. Performance of Avocado Seed Activated Carbon as Adsorbent for Highly Sensitive Determination of Cd Using a Flow Injection System Online Coupled to TS-FF-AAS. J. Braz. Chem. Soc. 2020, 31, 100–108. [Google Scholar] [CrossRef]
- Leite, A.J.B.; Thue, P.S.; Reis, G.S.; Dias, S.L.; Lima, E.C.; Vaghetti, J.C.P.; Pavan, F.A.; Alencar, W.S. Activated carbon from avocado seeds for the removal of phenolic compounds from aqueous solutions. Desalination Water Treat. 2017, 71, 168–181. [Google Scholar] [CrossRef]
- Mqehe-Nedzivhe, K.C.; Makhado, K.; Olorundare, O.F.; Arotiba, O.A.; Makhatha, E.; Nomngongo, P.N.; Mabuba, N. Bio-adsorbents for the Removal of Heavy Metals from Water. In Arsenic—Analitcal and Toxicologic Studies, 1st ed.; Stoytcheva, M., Zlatev, R., Eds.; IntechOpen: Winchester, UK, 2018; Volume 1, pp. 26–37. [Google Scholar]
- Leite, A.B.; Saucier, C.; Lima, E.C.; Reis, G.S.; Umpierres, C.S.; Mello, B.L.; Shirmardi, M.; Dias, S.L.; Sampaio, C.H. Activated carbons from avocado seed: Optimisation and application for removal of several emerging organic compounds. Environ. Sci. Pollut. Res. Int. 2018, 25, 7647–7661. [Google Scholar] [CrossRef]
- Chen, Y.T.; Huang, Y.P.; Wang, C.; Deng, J.G.; Hsi, H.C. Comprehending adsorption of methylethylketone and toluene and microwave regeneration effectiveness for beaded activated carbon derived from recycled waste bamboo tar. JA&WMA 2020, 70, 616–628. [Google Scholar]
- Czerwinska, N.; Giosuè, C.; Matos, I.; Sabbatini, S.; Ruello, M.L.; Bernardo, M. Development of activated carbons derived from wastes: Coffee grounds and olive stones as potential porous materials for air depollution. Sci. Total Environ. 2024, 914, 169898. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Li, L.; Chen, R.; Wang, C.; Ma, W.; Ma, X. Adsorption of acetone and isopropanol on organic acid modified activated carbons. J. Environ. 2016, 4, 2045–2051. [Google Scholar] [CrossRef]
- Qiu, C.; Jiang, L.; Gao, Y.; Sheng, L. Effects of oxygen-containing functional groups on carbon materials in supercapacitors: A review. Mater. Des. 2023, 230, 111952. [Google Scholar] [CrossRef]
- Sahira, J.; Mandira, A.; Prasad, P.B.; Ram, P.R. Effects of Activating Agents on the Activated Carbons Prepared from Lapsi Seed Stone. Res. J. Chem. Sci. 2013, 3, 19–24. [Google Scholar]
- Quadros, D.; Oliveira, V.; Freitas, F.C.; Raulino, G.S.C.; Vidal, C.B.; Nascimento, R.F. Chemical modifications of lignocellulosic materials and their application for removal of cations and anions from aqueous solutions. J. Appl. Polym. Sci. 2016, 133, 43286. [Google Scholar] [CrossRef]
- Ekawati, Y.; Wardana, I.N.; Novareza, O.; Setyarini, P.; Alfanaar, R. The role of lime juice in improving the performance of basic sites on activated carbon surfaces in sulfate ion adsorption in seawater. J. King Saud. Univ. Sci. 2023, 35, 102788. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, Q.; Hao, M.; Zhang, Y.; Ren, X.; Cao, F.; Zhang, L.; Sun, Q.; Wennersten, R. Effect of functional groups on VOCs adsorption by activated carbon: DFT study. Surf. Sci. 2023, 736, 122352. [Google Scholar] [CrossRef]
- Durak, H.; Aysu, T. Effect of pyrolysis temperature and catalyst on production of bio-oil and bio-char from avocado seeds. Res. Chem. Intermed. 2015, 41, 8067–8097. [Google Scholar] [CrossRef]
- Rodriguez-Reinoso, F.; Silvestre-Albero, J. Activated Carbon and Adsorption. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2016; Volume 1, pp. 1–14. [Google Scholar]
- Sánchez, F.; Araus, K.; Domínguez, M.P.; Miguel, G.S. Thermochemical Transformation of Residual Avocado Seeds: Torrefaction and Carbonization. Waste Biomass Valori 2017, 8, 2495–2510. [Google Scholar] [CrossRef]
- Palma, C.; Lloret, L.; Puen, A.; Tobar, M.; Contreras, E. Production of carbonaceous material from avocado peel for its application as alternative adsorbent for dyes removal. Chin. J. Chem. Eng. 2016, 24, 521–528. [Google Scholar] [CrossRef]
- Trakal, L.; Sigut, R.; Sillerová, H.; Faturíková, D.; Komárek, M. Copper removal from aqueous solution using biochar: Effect of chemical activation. Arab. J. Chem. 2014, 7, 43–52. [Google Scholar] [CrossRef]
- Bazzo, A.; Adebayo, M.A.; Dias, S.L.; Lima, E.C.; Vaghetti, J.C.; Oliveira, E.R.; Leite, A.J.; Pavan, F.A. Avocado seed powder: Characterization and its application for crystal violet dye removal from aqueous solutions. Desalination Water Treat. 2016, 57, 15873–15888. [Google Scholar] [CrossRef]
- Ogungbenro, A.E.; Quang, D.V.; Al-Ali, K.A.; Vega, L.F.; Abu-Zahra, M.R. Synthesis and characterization of activated carbon from biomass date seeds for carbon dioxide adsorption. J. Environ. 2020, 8, 104257. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, X.; Deng, J.; Li, C.; Sun, K.; Luo, X.; Yuan, S. Adsorption and mechanism study for phenol removal by 10% CO2 activated bio-char after acid or alkali pretreatment. J. Environ. Manag. 2023, 348, 119317. [Google Scholar] [CrossRef]
- Qurat-ul-Ain, M.; Shafiq, S.C.; Capareda, F. Effect of different temperatures on the properties of pyrolysis products of Parthenium hysterophorus. J. Saudi Chem. Soc. 2021, 25, 101197. [Google Scholar] [CrossRef]
- Karume, I.; Bbumba, S.; Tewolde, S.; Mukasa, I.Z.T.; Ntale, M. Impact of carbonization conditions and adsorbate nature on the performance of activated carbon in water treatment. BMC Chem. 2023, 17, 162. [Google Scholar] [CrossRef]
- Zakaria, R.; Jamalluddin, N.A.; Bakar, M.Z. Effect of impregnation ratio and activation temperature on the yield and adsorption performance of mangrove based activated carbon for methylene blue removal. Results Mater. 2021, 10, 100183. [Google Scholar] [CrossRef]
- Tetteh, I.K.; Issahaku, I.; Tetteh, A.Y. Recent advances in synthesis, characterization, and environmental applications of activated carbons and other carbon derivatives. Carbon. Trends 2024, 14, 100328. [Google Scholar] [CrossRef]
- Chellappan, S.; Nair, V.; Sajith, V.; Aparna, K. Synthesis, optimization and characterization of biochar based catalyst from sawdust for simultaneous esterification and transesterification. Chin. J. Chem. Eng. 2018, 26, 2654–2663. [Google Scholar] [CrossRef]
- Budai, A.; Calucci, L.; Rasse, D.; Strand, L.; Pengerud, A.; Wiedemeier, D.; Abiven, S.; Forte, C. Effects of pyrolysis conditions on Miscanthus and corncob chars: Characterization by IR, solid state NMR and BPCA analysis. J. Anal. Appl. Pyrolysis 2017, 128, 335–345. [Google Scholar] [CrossRef]
- He, M.; Zhao, H.; Yang, X.; Jia, J.; Liu, X.; Qu, Z.; Zhou, W.; Sun, F.; Wang, Z. Reconsideration about the competitive adsorption of H2O and CO2 on carbon surfaces: The influence of oxygen functional groups. J. Environ. Chem. Eng. 2023, 11, 111288. [Google Scholar] [CrossRef]
- Axet, M.; Dechy-Cabaret, O.; Durand, J.; Gouygou, M.; Serp, P. Coordination chemistry on carbon surfaces. Coordination Chem. Rev. 2016, 308, 236–345. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, Y.; Ma, Q.; Zhou, H.; Luo, X.; Liu, X.; Wang, S. Evolution of the chemical composition, functional group, pore structure and crystallographic structure of bio-char from palm kernel shell pyrolysis under different temperatures. J. Anal. Appl. Pyrolysis 2017, 127, 350–359. [Google Scholar] [CrossRef]
- di Bitonto, L.; Reynel-Ávila, H.E.; Mendoza-Castillo, D.I.; Bonilla-Petriciolet, A.; Durán-Valle, C.J.; Pastore, C. Synthesis and characterization of nanostructured calcium oxides supported onto biochar and their application as catalysts for biodiesel production. Renew. Energy 2020, 160, 52–66. [Google Scholar] [CrossRef]
- Franco, D.; Silva, L.F.; Boit, K.; Diel, J.C.; Georgin, J.; Netto, M.S.; Pereira, H.A.; Lima, E.C.; Dotto, G.L. Transforming agricultural waste into adsorbent: Application of Fagopyrum esculentum wheat husks treated with H2SO4 to adsorption of the 2, 4-D herbicide. J. Environ. 2021, 9, 106872. [Google Scholar] [CrossRef]
- Fregue, T.T.; Ionel, I.; Gabche, A.S.; Mihaiuti, A.-C. Optimization of the Activated Carbon Preparation from Avocado Seeds, using the Response Surface Methodology. Rev. Chim. 2019, 70, 410–416. [Google Scholar] [CrossRef]
- Subramanian, K.; Kumar, P.; Jeyapal, P.; Venkatesh, N. Characterization of ligno-cellulosic seed fibre from Wrightia tinctoria plant for textile applications—An exploratory investigation. Eur. Polym. J. 2005, 41, 853–861. [Google Scholar] [CrossRef]
- Zou, X.; Zhai, M.; Wang, B.; Guo, L.; Zhang, Y. Molecular-scale elucidating of lignocellulose biomass char steam gasification for ultimately converting to syngas. Fuel Process Technol. 2022, 236, 107430. [Google Scholar] [CrossRef]
- Asadieraghi, M.; Daud, W.M.A. Characterization of lignocellulosic biomass thermal degradation and physiochemical structure: Effects of demineralization by diverse acid solutions. Energy Convers. Manag. 2014, 82, 71–82. [Google Scholar] [CrossRef]
- Abdolali, H.H.; Guo, W.; Zhou, J.L.; Du, B.; Wei, Q.; Wang, X.C.; Nguyen, P.D. Characterization of a multi-metal binding biosorbent: Chemical modification and desorption studies. Bioresour. Technol. 2015, 193, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Pathania, D.; Sharma, S.; Agarwal, S.; Singh, P. Remediation of noxious chromium (VI) utilizing acrylic acid grafted lignocellulosic adsorbent. J. Mol. Liq. 2013, 177, 343–352. [Google Scholar] [CrossRef]
- Mustapha, S.; Shuaib, D.T.; Ndamitso, M.M.; Etsuyankpa, M.B.; Sumaila, A.; Mohammed, U.M.; Nasirudeen, M.B. Adsorption isotherm, kinetic and thermodynamic studies for the removal of Pb(II), Cd(II), Zn(II) and Cu(II) ions from aqueous solutions using Albizia lebbeck pods. Appl. Water Sci. 2019, 9, 142. [Google Scholar] [CrossRef]
- Mopoung, S.; Moonsri, P.; Palas, W.; Khumpai, S. Characterization and Properties of Activated Carbon Prepared from Tamarind Seeds by KOH Activation for Fe(III) Adsorption from Aqueous Solution. Sci. World J. 2015, 2015, 415961. [Google Scholar] [CrossRef]
- Yakout, S.M.; Sharaf, G. Characterization of activated carbon prepared by phosphoric acid activation of olive stones. Arab. J. Chem. 2016, 9, S1155–S1162. [Google Scholar] [CrossRef]
- Machrouhi, A.; Elhalil, A.; Farnane, M.; Mahjoubi, F.Z.; Tounsadi, H.; Abdennouri, M.; Barka, N. Adsorption behavior of methylene blue onto powdered Ziziphus lotus fruit peels and Avocado kernels seeds. Surf. Interfaces 2017, 1, 49–56. [Google Scholar]
- Lim, W.-S.; Kim, J.-Y.; Kim, H.-Y.; Choi, J.-W.; Choi, I.-G.; Lee, J.-W. Structural properties of pretreated biomass from different acid pretreatments and their effects on simultaneous saccharification and ethanol fermentation. Bioresour. Technol. 2013, 139, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Merkel, K.; Rydarowski, H.; Kazimierczak, J.; Bloda, A. Processing and characterization of reinforced polyethylene composites made with lignocellulosic fibres isolated from waste plant biomass such as hemp. Compos. B Eng. 2014, 67, 138–144. [Google Scholar] [CrossRef]
- Shi-Xiang, Z.; Na, T.; Xu-Dong, W. Effect of Temperature on the Structural and Physicochemical Properties of Biochar with Apple Tree Branches as Feedstock Material. Energies 2017, 10, 1293. [Google Scholar] [CrossRef]
- Pellenz, L.; Oliveira, C.; Silva, A.; Silva, L.; Silva, L.; Souza, A.; Souza, S.; Borba, F.; Silva, A. A comprehensive guide for characterization of adsorbent materials. Sep. Purif. Technol. 2023, 305, 122435. [Google Scholar] [CrossRef]
- Omri, A.; Benzina, M. Characterization of activated carbon prepared from a new raw lignocellulosic material: Ziziphus spina-christi seeds. LCT 2015, 14, 175–183. [Google Scholar]
- Takahata, T.; Toda, I.; Ono, H.; Ohshio, S.; Akasaka, H.; Himeno, S.; Kokubu, T.; Saitoh, H. Detailed Structural Analyses of KOH Activated Carbon from Waste Coffee Beans. Jpn. J. Appl. Phys. 2009, 48, 117001. [Google Scholar] [CrossRef]
- Ramesh, T.; Rajalakshmi, N.; Dhathathreyan, K.S. Synthesis and characterization of activated carbon from jute fibers for hydrogen storage. Renew. Sust. Energy Rev. 2017, 2, 4. [Google Scholar] [CrossRef]
- Weissberger, A.; Riddick, J.A.; Bunger, W.B.; Sakano, T.K. Techniques of chemistry.—2: Organic solvents. In Physical Properties and Methods of Purification, 4th ed.; Wiley: New York, NY, USA, 1986; Volume 1, p. 1325. [Google Scholar]
- Becerra-Pérez, O.; Georgopoulos, S.; Lanara, M.; Reynel-Ávila, H.E.; Papadaki, M.; Bonilla-Petriciolet, A.; Mendoza-Castillo, D.I. Energy-saving and sustainable separation of bioalcohols by adsorption on bone char. Adsorption Sci. Technol. 2021, 2021, 1–16. [Google Scholar] [CrossRef]
- Ho, Y.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Dulla, J.; King, P. Kinetic and thermodynamic studies for cadmium (II) biosorption from aqueous solutions using sea urchin test. Int. J. Chemtech Res. 2014, 6, 5535–5543. [Google Scholar]
- Giles, C.; Smith, D.; Huitson, A. A general treatment and classification of the solute adsorption isotherm. I. Theoretical. J. Colloid. Interface Sci. 1974, 47, 755–765. [Google Scholar] [CrossRef]
- Giles, C.; Smith, D.; Huitson, A. A general treatment and classification of the solute adsorption isotherm part. II. Experimental interpretation. J. Colloid. Interface Sci. 1974, 47, 766–778. [Google Scholar] [CrossRef]
- Giraudet, S.; Pré, P.; Cloirec, P.L. Modeling the Temperature Dependence of Adsorption Equilibriums of VOC(s) onto Activated Carbons. J. Environ. Eng. 2010, 136, 1. [Google Scholar] [CrossRef]
- Chiang, Y.; Chiang, P.-C.; Huang, C.-P. Effects of pore structure and temperature on VOC adsorption on activated carbon. Carbon 2001, 39, 523–534. [Google Scholar] [CrossRef]
- Singh, V.K.; Kumar, E. Measurement and analysis of adsorption isotherms of CO2 on activated carbon. Appl. Therm. Eng. 2016, 97, 77–86. [Google Scholar] [CrossRef]
- Singh, J.; Bhunia, H.; Basu, S. Adsorption of CO2 on KOH activated carbon adsorbents: Effect of different mass ratios. J. Environ. Manag. 2019, 250, 109457. [Google Scholar] [CrossRef]
- Hong, T.; Wei, L.; Cui, K.; Dong, Y.; Li, R.L.; Zhang, T.; Zhao, Y.; Luo, L. Adsorption performance of volatile organic compounds on activated carbon fibers in a fixed bed column. J. Environ. Chem. Eng. 2021, 9, 106347. [Google Scholar] [CrossRef]
- Manna, M.; Schmaltz, B.; Bouaziz, N.; Berton, N.; Van, F.; Lamine, A. Adsorption isotherms of N3 dye on TiO2 mesoporous for dye sensitized solar cells: Their realization, their modeling and consequent interpretations using a statistical physics treatment. J. Alloys Compd. 2018, 765, 385–395. [Google Scholar] [CrossRef]
- Fan, G.; Zhang, C.; Wang, T.; Deng, J.; Cao, Y.; Chang, L.; Zhou, G.; Wu, Y.; Li, P. New insight into surface adsorption thermodynamic, kinetic properties and adsorption mechanisms of sodium oleate on ilmenite and titanaugite. Adv. Powder Technol. 2020, 31, 3628–3639. [Google Scholar] [CrossRef]
- Guo, J.; Xu, W.S.; Chen, Y.L.; Lua, A.C. Adsorption of NH3 onto activated carbon prepared from palm shells impregnated with H2SO4. J. Colloid. Interface Sci. 2005, 281, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.-C.; Lai, Y.-C. Adsorption of methyl ethyl ketone onto poly(vinyl alcohol)(PVA)/peat/organoclay composite beads in aqueous solution. J. Polym. Res. 2012, 19, 9816. [Google Scholar] [CrossRef]
- Hashim, M.A.; Kundu, A.; Mukherjee, S.; Ng, Y.S.; Mukhopadhyay, S.; Redzwan, G.; Gupta, B. Arsenic removal by adsorption on activated carbon in a rotating packed bed. J. Water Process Eng. 2019, 30, 100591. [Google Scholar] [CrossRef]
- Kostoglou, M.; Karapantsios, T.D. Why is the linearized form of pseudo-second order adsorption kinetic model so successful in fitting batch adsorption experimental data? Colloids Interfaces 2022, 6, 55. [Google Scholar] [CrossRef]
- Ezzati, R.; Ezzati, S.; Azizi, M. Exact solution of the Langmuir rate equation: New Insights into pseudo-first-order and pseudo-second-order kinetics models for adsorption. Vacuum 2024, 220, 112790. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Kapillarchemie: Eine Darstellung der Chemie der Kolloide und verwandter Gebiete. Akad. Verlagsgesell 1909, 1, 599. [Google Scholar]
- Sips, R. On the Structure of a Catalyst Surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Amrhar, O.; Gana, L.; Mobarak, M. Calculation of adsorption isotherms by statistical physics models: A review. Environ. Chem. Lett. 2021, 19, 4519–4547. [Google Scholar] [CrossRef]
- Tran, H.; You, S.; Hosseini-Bandegharaei, A.; Chao, H. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef]
- Faria, P.C.C.; Órfão, J.J.M.; Pereira, M.F.R. Adsorption of anionic and cationic dyes on activated carbons with different surface chemistries. Water Res. 2004, 38, 2043–2052. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system—Version 5.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Kruse, H.; Grimme, S. A geometrical correction for the inter-and intra-molecular basis set superposition error in Hartree-Fock and density functional theory calculations for large systems. J. Chem. Phys. 2012, 136, 04B613. [Google Scholar] [CrossRef] [PubMed]
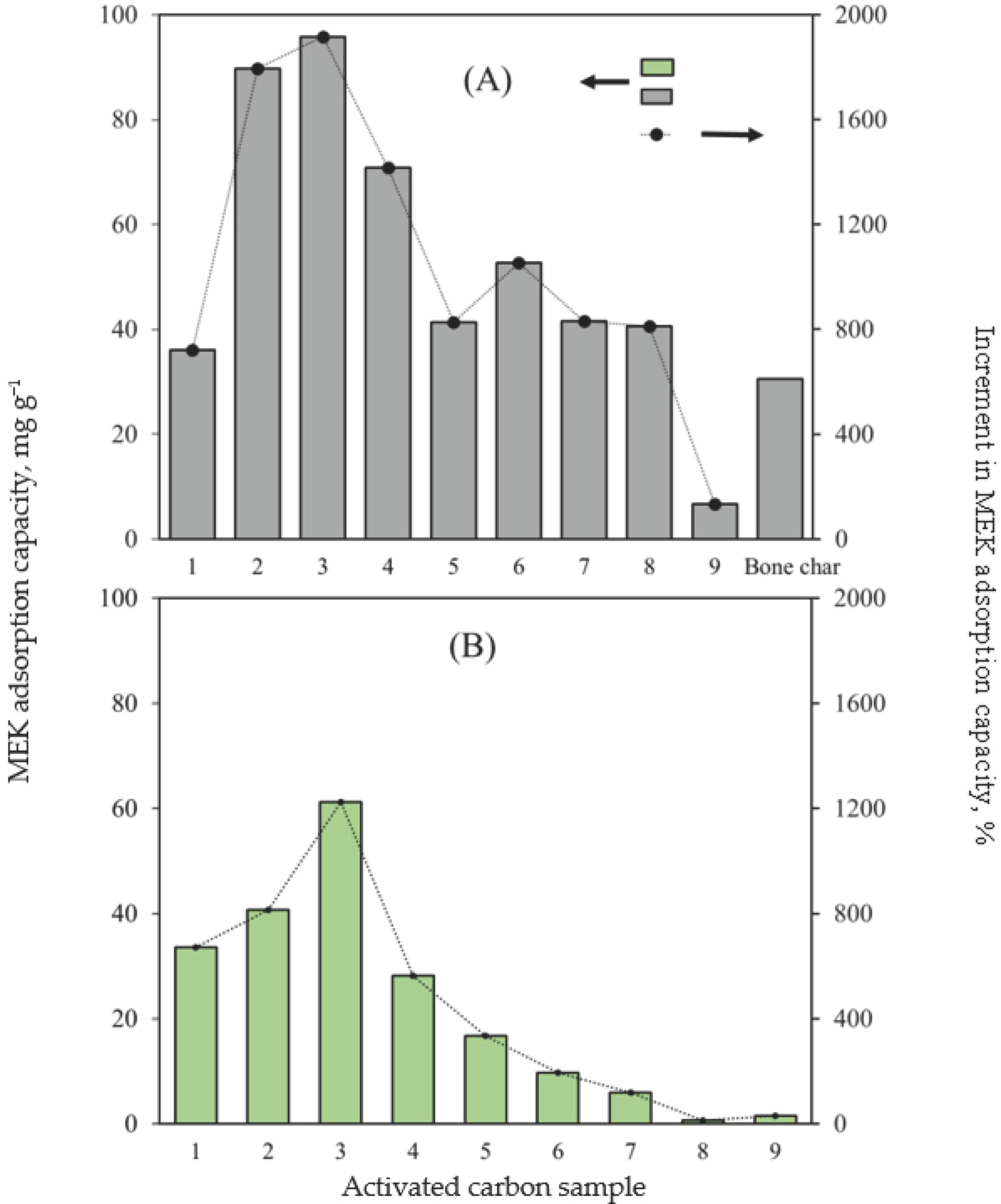
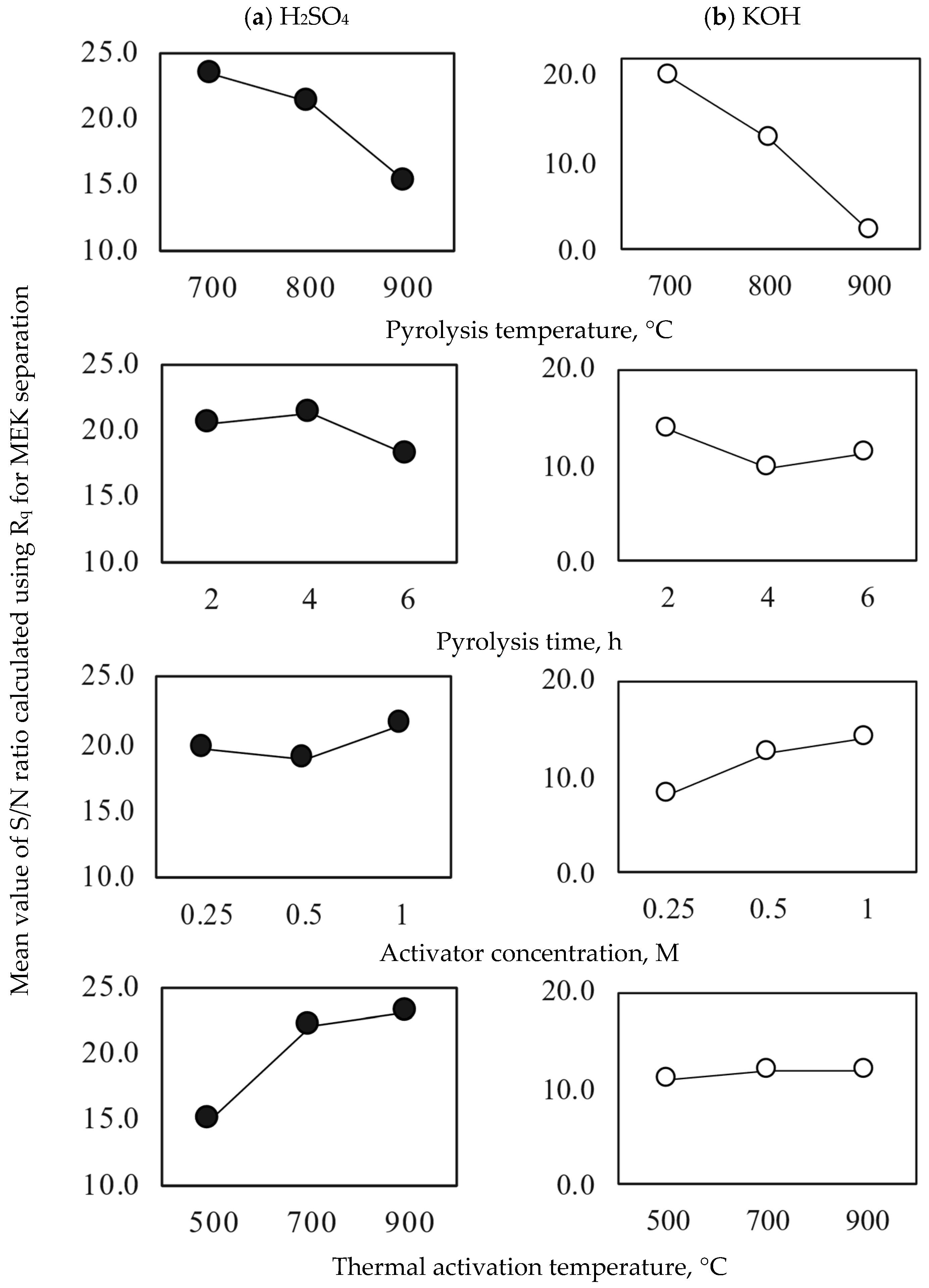
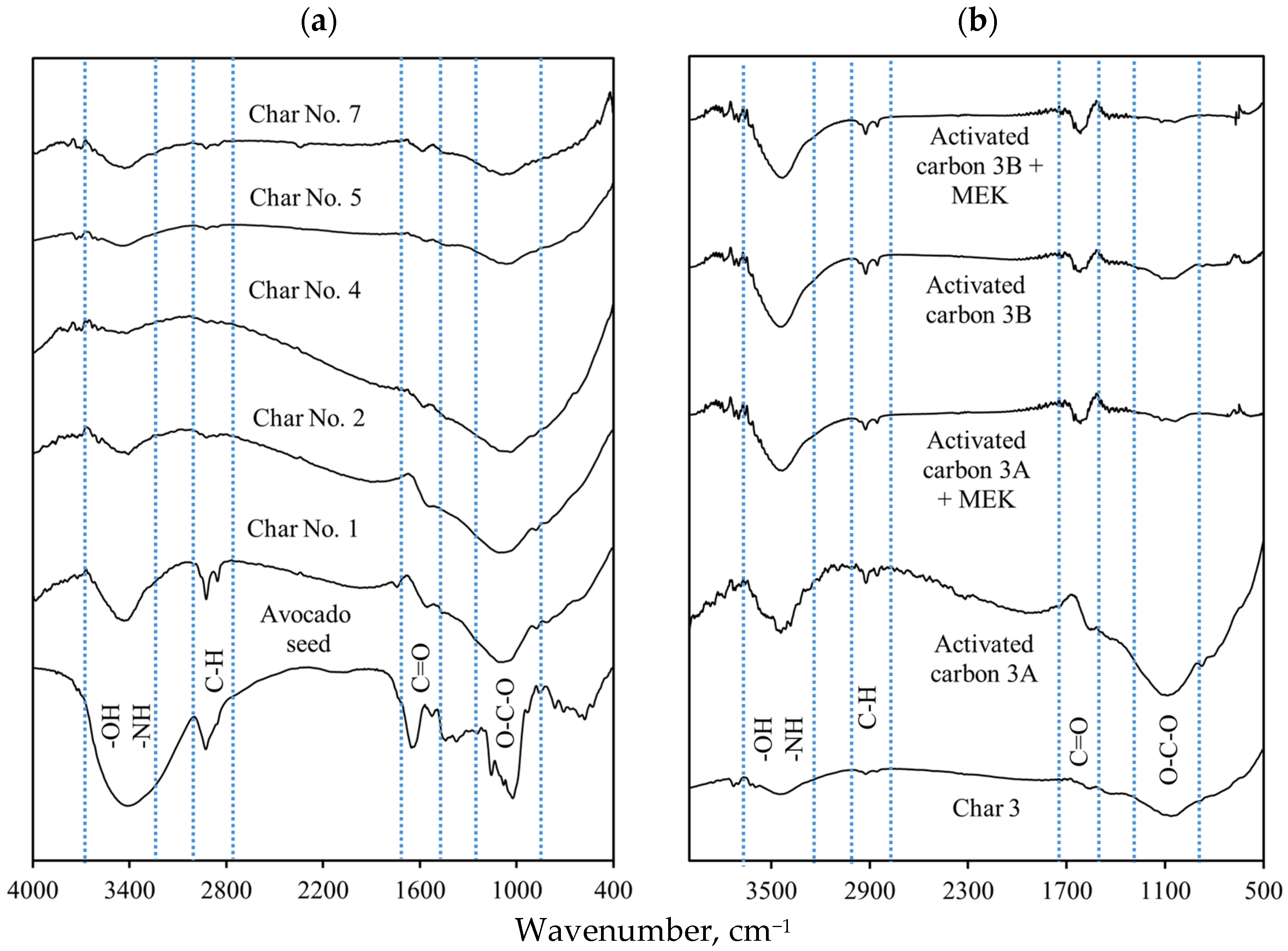
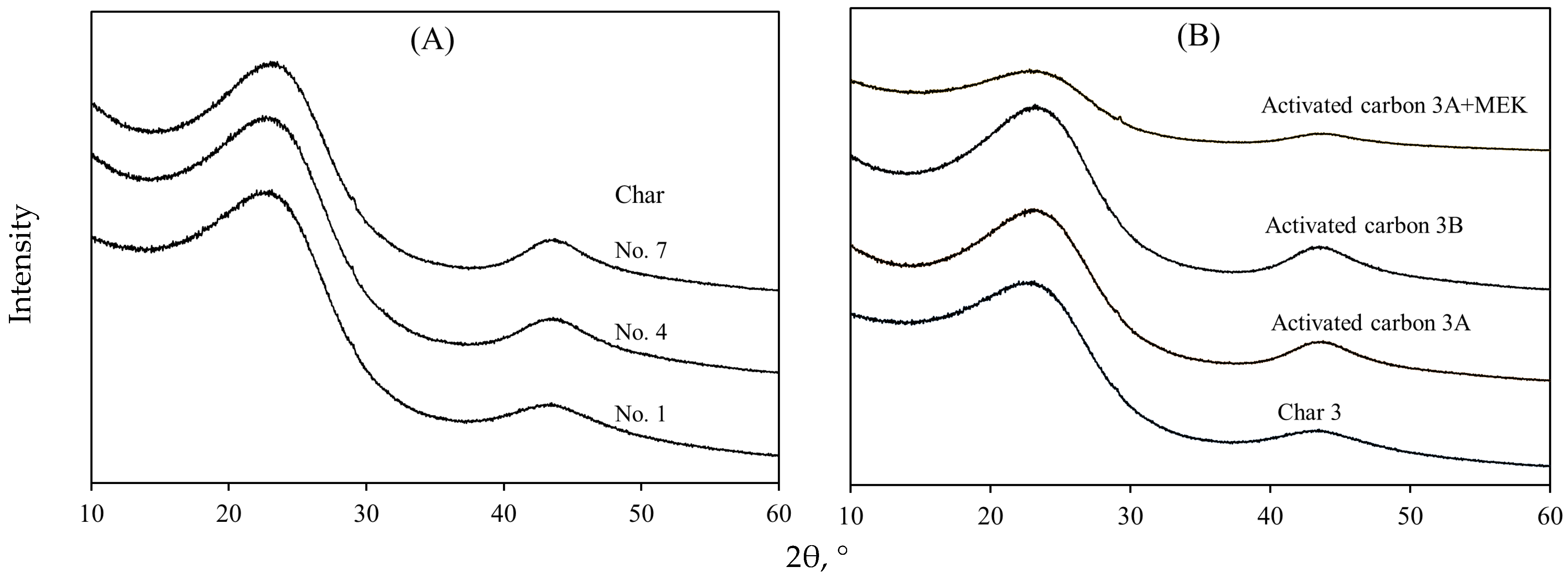
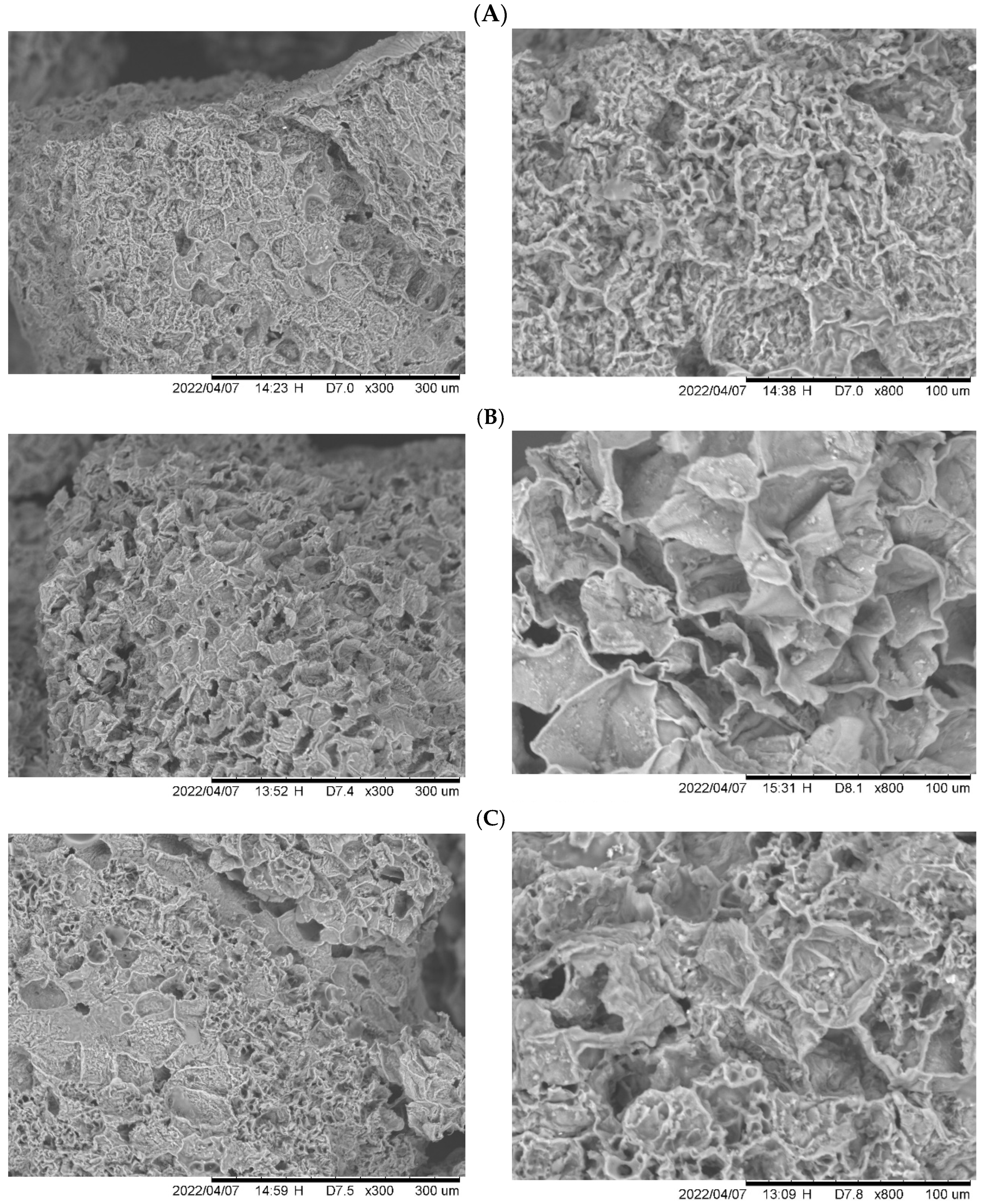


| Pyrolysis Conditions to Obtain Avocado Seed Char | ||||
|---|---|---|---|---|
| Char Sample | Temperature, °C | Dwell Time, h | Yield, % | pHpzc |
| 1 | 700 | 2 | 26.20 | 6.76 |
| 2 | 700 | 4 | 25.37 | 6.75 |
| 3 | 700 | 6 | 25.12 | 6.78 |
| 4 | 800 | 2 | 24.84 | 6.79 |
| 5 | 800 | 4 | 24.52 | 6.77 |
| 6 | 800 | 6 | 23.35 | 6.85 |
| 7 | 900 | 2 | 24.27 | 6.84 |
| 8 | 900 | 4 | 23.05 | 6.94 |
| 9 | 900 | 6 | 22.25 | 7.19 |
| Adsorbent | Element, % (by Mass) | ||||||
|---|---|---|---|---|---|---|---|
| CHON | K | Ca | P | Fe | Mg | S | |
| Avocado seed | 97.12 | 2.59 | 1.23 | 0.37 | ND | 0.21 | 0.22 |
| Char 3 | 96.51 | ND | 1.63 | 0.84 | 0.40 | 0.28 | 0.26 |
| Activated carbon 3A | 97.06 | ND | 1.06 | 0.87 | ND | 0.36 | 0.65 |
| Activated carbon 3B | 93.42 | 3.15 | 1.62 | 0.83 | 0.39 | 0.32 | 0.27 |
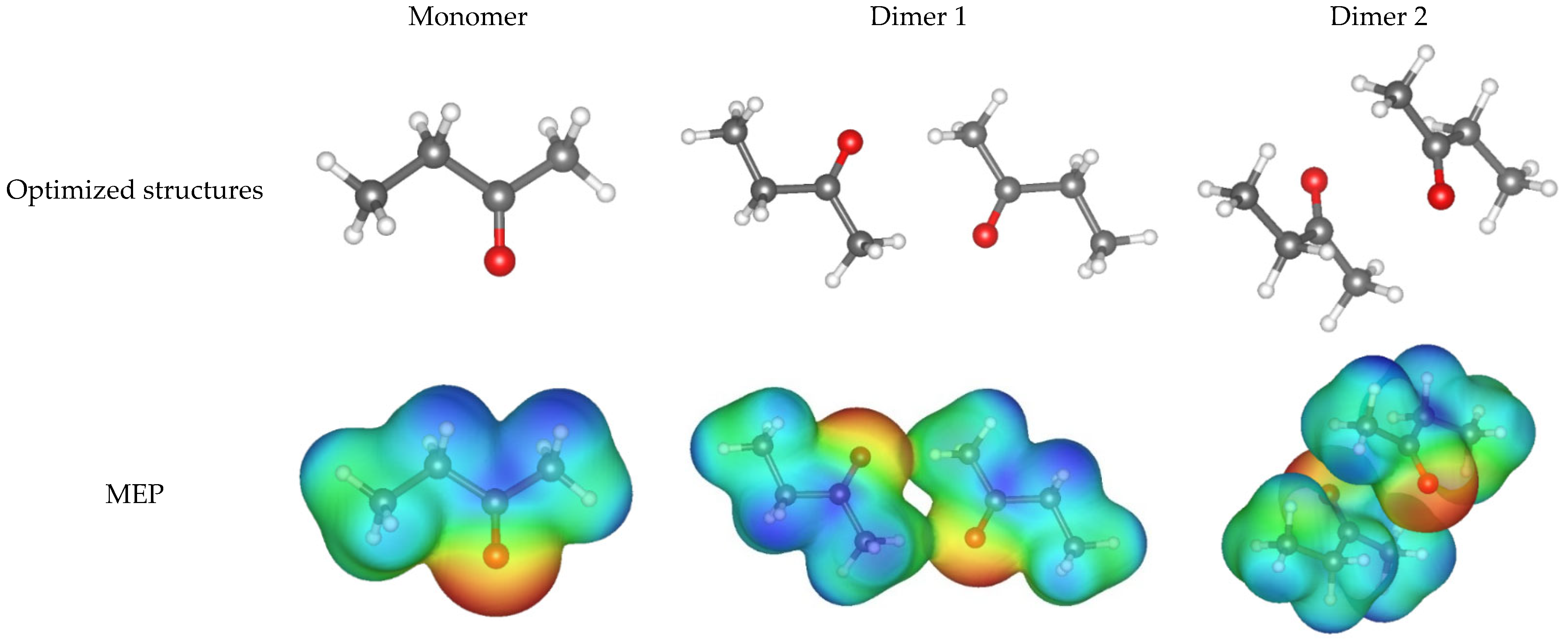
| Structure | Dimensions, Å | Volumes, Å3 | Interaction Energy, kJ mol−1 | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| MEK | 5.6 | 3.1 | 1.7 | 83.9 | - |
| Dimer 1 | 11.4 | 9.2 | 6.8 | 174.0 | –16.7 |
| Dimer 2 | 8.1 | 7.5 | 6.7 | 176.2 | –30.2 |
| Adsorbent | Operational Conditions | MEK Adsorption Capacity, mg g−1 | Reference |
|---|---|---|---|
| Commercial granulated activated carbon | 25 °C, 15 h, batch system | 60 | Sotelo et al. [15] |
| siliceous rocks | 25 °C, pH 7, 0.1 g, batch systems | 470 | Ali et al. [17] |
| Poly(vinyl alcohol) (PVA)/peat/organoclay composite beads | 1 g, pH 6, 35 °C, 35 h, [MEK]0 = 500 mg L−1, batch system | 275 | Chan et al. [94] |
| HNO3-modified Sepiolite with Streptococcus equisimilis biofilm | Packed-bed columns with 90 g, binary solutions containing MEK and DEK at 100 mg L−1 each one, flow of 1 mL min−1 for 120 h | 15 | Silva et al. [8] |
| Avocado seed pyrolyzed and modified with H2SO4 | 20 °C, pH 4, 24 h, [MEK]0 = 3000 mg L−1, 2 g L−1 of adsorbent | 142 | This study |
| Avocado seed pyrolyzed and modified with KOH | 131 | This study |
| Pyrolysis Conditions to Obtain Avocado Char | Activation Conditions of Avocado Char | |||
|---|---|---|---|---|
| Sample | Temperature, °C | Dwell Time, h | Activator Concentration, M | Thermal Activation Temperature, °C |
| 1 | 700 | 2 | 0.25 | 500 |
| 2 | 700 | 4 | 0.5 | 700 |
| 3 | 700 | 6 | 1.0 | 900 |
| 4 | 800 | 2 | 0.5 | 900 |
| 5 | 800 | 4 | 1.0 | 500 |
| 6 | 800 | 6 | 0.25 | 700 |
| 7 | 900 | 2 | 1.0 | 700 |
| 8 | 900 | 4 | 0.25 | 900 |
| 9 | 900 | 6 | 0.5 | 500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reynel-Avila, H.E.; Ledea-Figueredo, E.; Díaz-Muñoz, L.L.; Bonilla-Petriciolet, A.; Aguayo-Villarreal, I.A.; Elvir-Padilla, L.G.; Durán-Valle, C.J. Separation of the Biofuel Methyl Ethyl Ketone from Aqueous Solutions Using Avocado-Based Activated Carbons: Synthesis Conditions and Multilayer Adsorption Properties. Molecules 2025, 30, 3426. https://doi.org/10.3390/molecules30163426
Reynel-Avila HE, Ledea-Figueredo E, Díaz-Muñoz LL, Bonilla-Petriciolet A, Aguayo-Villarreal IA, Elvir-Padilla LG, Durán-Valle CJ. Separation of the Biofuel Methyl Ethyl Ketone from Aqueous Solutions Using Avocado-Based Activated Carbons: Synthesis Conditions and Multilayer Adsorption Properties. Molecules. 2025; 30(16):3426. https://doi.org/10.3390/molecules30163426
Chicago/Turabian StyleReynel-Avila, Hilda Elizabeth, Eduardo Ledea-Figueredo, Lizbeth Liliana Díaz-Muñoz, Adrián Bonilla-Petriciolet, Ismael Alejandro Aguayo-Villarreal, Laura Gabriela Elvir-Padilla, and Carlos Javier Durán-Valle. 2025. "Separation of the Biofuel Methyl Ethyl Ketone from Aqueous Solutions Using Avocado-Based Activated Carbons: Synthesis Conditions and Multilayer Adsorption Properties" Molecules 30, no. 16: 3426. https://doi.org/10.3390/molecules30163426
APA StyleReynel-Avila, H. E., Ledea-Figueredo, E., Díaz-Muñoz, L. L., Bonilla-Petriciolet, A., Aguayo-Villarreal, I. A., Elvir-Padilla, L. G., & Durán-Valle, C. J. (2025). Separation of the Biofuel Methyl Ethyl Ketone from Aqueous Solutions Using Avocado-Based Activated Carbons: Synthesis Conditions and Multilayer Adsorption Properties. Molecules, 30(16), 3426. https://doi.org/10.3390/molecules30163426










