Innovative Integrated Model of Industrial Wastewater Treatment with the Circular Use of Cerium Compounds as Multifunctional Coagulants: Comprehensive Assessment of the Process and Environmental and Economic Aspects
Abstract
1. Introduction
2. Results and Discussion
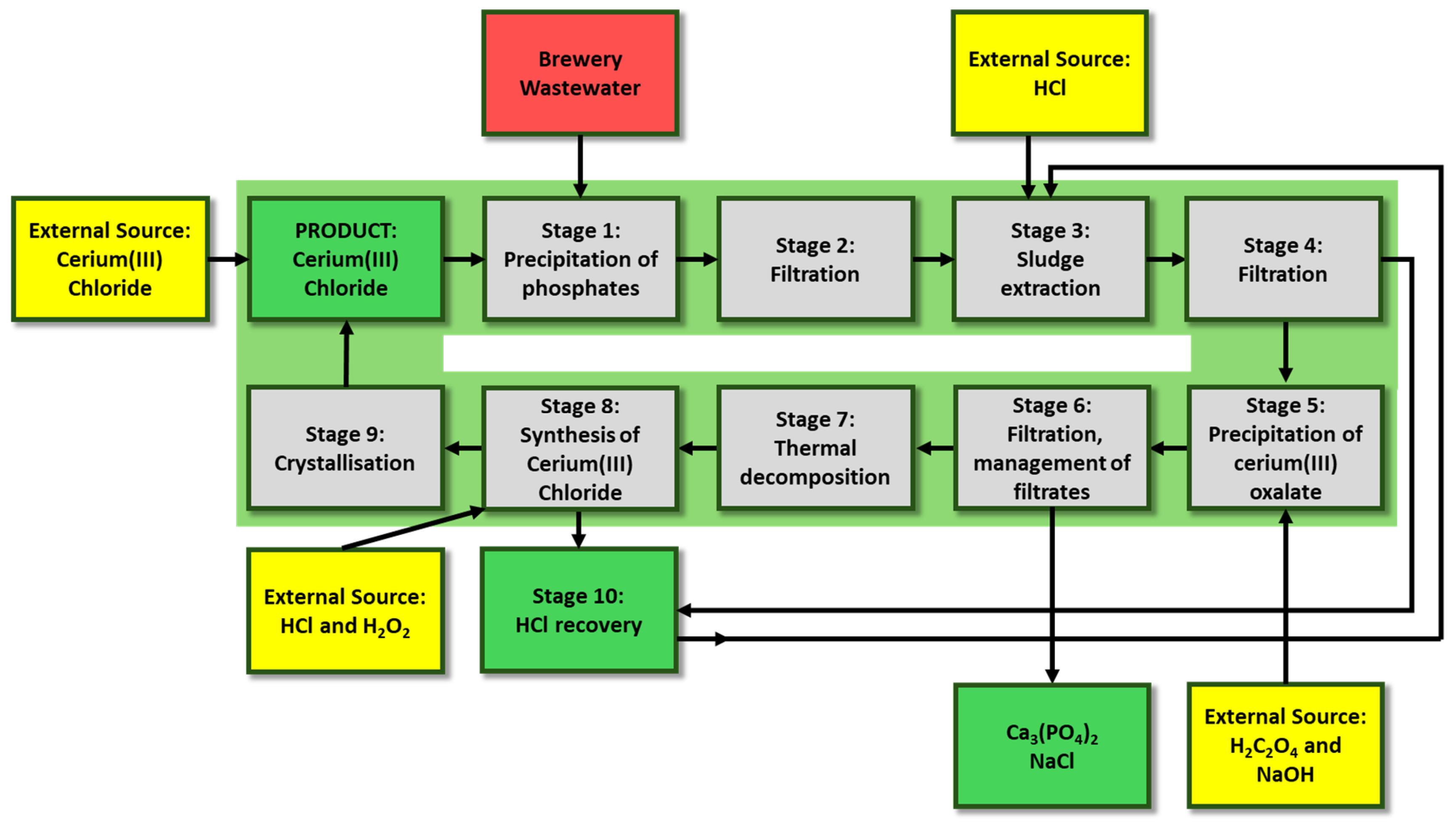
2.1. Process Analysis (Operating Conditions and Initial Assumptions)
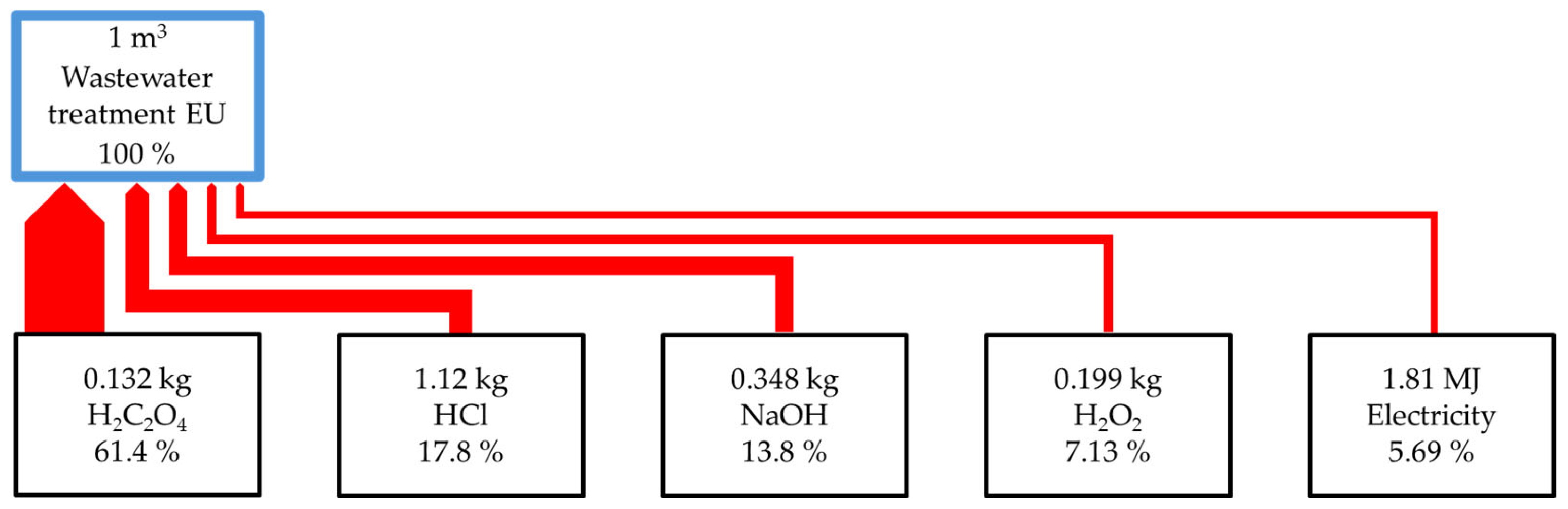
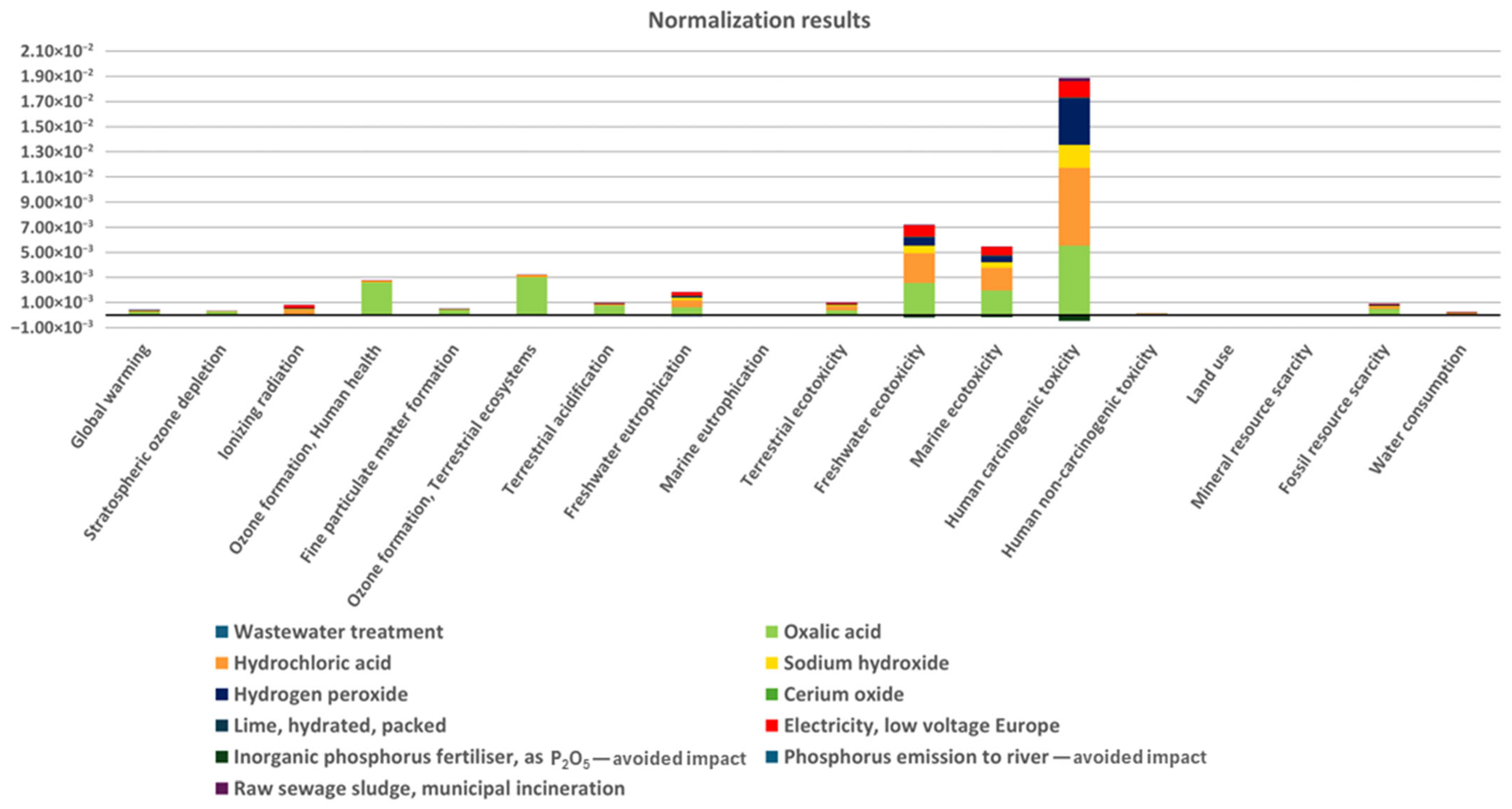
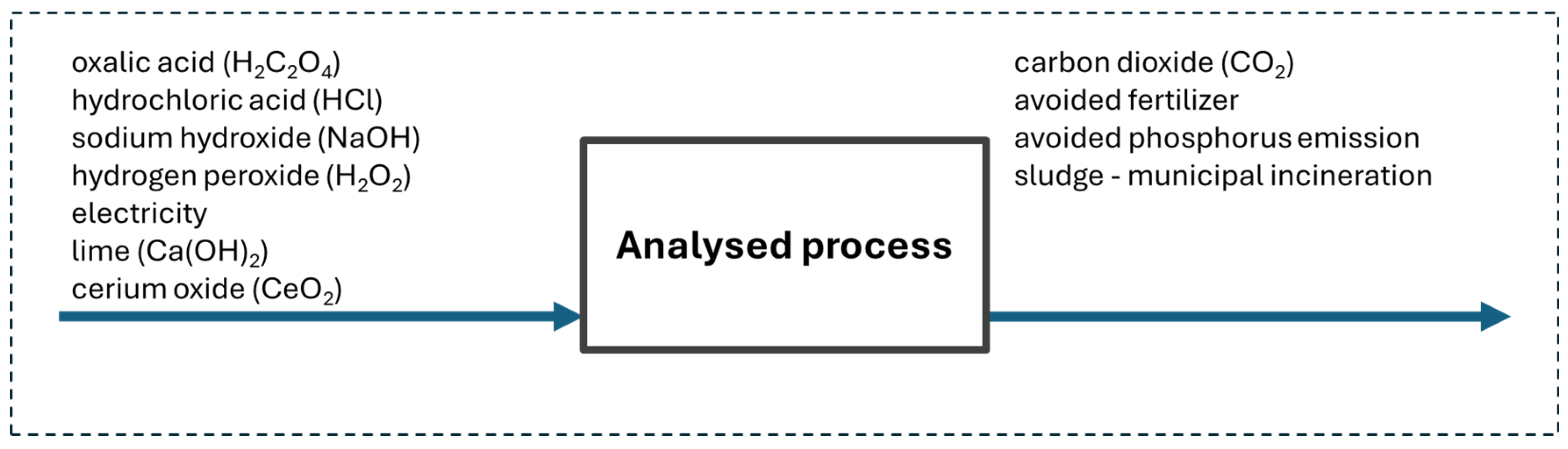
2.2. Environmental Assessment
2.3. Economic Assessment
3. Materials and Methods
3.1. Assumptions Underlying the Design of a Cerium-Based Wastewater Treatment Process
3.2. Process Analysis (Operating Conditions and Initial Assumptions)
3.3. Environmental Assessment
3.4. Economic Assessment Methodology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 1.4-DCB-eq | 1,4-dichlorobenzene equivalent (used in toxicity assessment) |
| CAPEX | Capital Expenditures |
| DGC | Dynamic Generation Cost |
| LCA | Life Cycle Assessment |
| LCI | Life Cycle Inventory |
| LCIA | Life Cycle Impact Assessment |
| OPEX | Operational Expenditures |
| REE | Rare Earth Elements |
References
- Kundzewicz, Z.W. Hydrosphere. In Encyclopedia of Ecology; Elsevier: Amsterdam, The Netherlands, 2008; pp. 1923–1930. [Google Scholar]
- Boretti, A.; Rosa, L. Reassessing the Projections of the World Water Development Report. npj Clean Water 2019, 2, 15. [Google Scholar] [CrossRef]
- Navarro, I.; de la Torre, A.; Sanz, P.; Abrantes, N.; Campos, I.; Alaoui, A.; Christ, F.; Alcon, F.; Contreras, J.; Glavan, M.; et al. Assessing Pesticide Residues Occurrence and Risks in Water Systems: A Pan-European and Argentina Perspective. Water Res. 2024, 254, 121419. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Asad, U.; Maryam, L.; Masood, M.; Saeed, M.F.; Jamal, A.; Mubeen, M. Treatment Methods for Lead Removal from Wastewater. In Lead Toxicity: Challenges and Solution; Springer Nature: Cham, Switzerland, 2023; pp. 197–226. [Google Scholar]
- Lee, Y.; Lee, C.; Min, K.J.; Park, S. Efficient Cadmium Removal from Industrial Wastewater Generated from Smelter Using Chemical Precipitation and Oxidation Assistance. Water Environ. Res. 2024, 96, e11059. [Google Scholar] [CrossRef]
- Kazmi, S.A.R.; Husnain, S.M.; Khan, A.R.; Qureshi, T.M.; Lemaoui, T.; AlNashef, I.M.; Arafat, H.A.; Shahzad, F. Removal of Nickel Ions from Industrial Wastewater Using Tms-EDTA-Functionalized Ti3C2Tx: Experimental and Statistical Physics Modeling. J. Hazard. Mater. 2025, 490, 137667. [Google Scholar] [CrossRef]
- Suess, E.; Berg, M.; Bouchet, S.; Cayo, L.; Hug, S.J.; Kaegi, R.; Voegelin, A.; Winkel, L.H.E.; Tessier, E.; Amouroux, D.; et al. Mercury Loads and Fluxes from Wastewater: A Nationwide Survey in Switzerland. Water Res. 2020, 175, 115708. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Cui, Y.; Chen, N. Removal of Copper Ions from Wastewater: A Review. Int. J. Environ. Res. Public. Health 2023, 20, 3885. [Google Scholar] [CrossRef]
- Yuan, B.; Yang, Z.; Wu, P.; Yin, X.; Liu, C.; Sun, F.; He, J.; Jiang, W. Efficient Treatment of Chromium-Containing Wastewater Based on Auxiliary Intelligent Model with Rapid-Response Adsorbents. Sep. Purif. Technol. 2025, 363, 132037. [Google Scholar] [CrossRef]
- Khanzada, A.K.; Rizwan, M.; Al-Hazmi, H.E.; Majtacz, J.; Kurniawan, T.A.; Mąkinia, J. Removal of Arsenic from Wastewater Using Hydrochar Prepared from Red Macroalgae: Investigating Its Adsorption Efficiency and Mechanism. Water 2023, 15, 3866. [Google Scholar] [CrossRef]
- Yang, L.; Kang, S.; Luo, X.; Wang, Z. Microplastics in Drinking Water: A Review on Methods, Occurrence, Sources, and Potential Risks Assessment. Environ. Pollut. 2024, 348, 123857. [Google Scholar] [CrossRef]
- Komorowska-Kaufman, M.; Zembrzuska, J. Application of Oxidation Processes in Wastewater Quaternary Treatment for Organic Compounds, Antibiotics and Nonsteroidal Anti-Inflammatory Drugs Removal and Disinfection. Desalination Water Treat. 2025, 321, 101059. [Google Scholar] [CrossRef]
- Verma, V.; Chaudhari, P.K. Optimization of Multiple Parameters for Treatment of Coking Wastewater Using Fenton Oxidation. Arab. J. Chem. 2020, 13, 5084–5095. [Google Scholar] [CrossRef]
- Zhang, Q.; Meng, J.; Su, G.; Liu, Z.; Shi, B.; Wang, T. Source Apportionment and Risk Assessment for Polycyclic Aromatic Hydrocarbons in Soils at a Typical Coking Plant. Ecotoxicol. Environ. Saf. 2021, 222, 112509. [Google Scholar] [CrossRef] [PubMed]
- Lejwoda, P.; Białecka, B.; Thomas, M. Holistic Insight into the Viability of Employing Cerium(IV) Sulphate to Oxidise Toxic Contaminants in Effluent from the Coal Gasification Process: Optimisation Studies. J. Water Process Eng. 2024, 67, 106243. [Google Scholar] [CrossRef]
- Charazińska, S.; Lochyński, P.; Burszta-Adamiak, E. Removal of Heavy Metal Ions Form Acidic Electrolyte for Stainless Steel Electropolishing via Adsorption Using Polish Peats. J. Water Process Eng. 2021, 42, 102169. [Google Scholar] [CrossRef]
- Rao, D.P.; Krishnasamy, V.D.; Selvaraju, M.; Sundramurthy, V.P.; Kandavalli, S.R.; Chitra, M.S.; Sivasamy, N.; Thirumoorthy, P. Adsorptive Removal of Cadmium from Electroplating Wastewater Using Hybrid Composite of Thiol-Grafted Seed Gum of Tamarindus indica and Teff Hay Biocarbon. Z. Phys. Chem. 2025, 239, 633–654. [Google Scholar] [CrossRef]
- Pohl, A. Removal of Heavy Metal Ions from Water and Wastewaters by Sulfur-Containing Precipitation Agents. Water Air Soil Pollut. 2020, 231, 503. [Google Scholar] [CrossRef]
- Zhang, W.; Rezaee, M.; Bhagavatula, A.; Li, Y.; Groppo, J.; Honaker, R. A Review of the Occurrence and Promising Recovery Methods of Rare Earth Elements from Coal and Coal By-Products. Int. J. Coal Prep. Util. 2015, 35, 295–330. [Google Scholar] [CrossRef]
- Yang, X.; Hu, X.; Kong, L.; Peng, X. Selective Recovery of Cu(II) from Strongly Acidic Wastewater by Zinc Dimethyldithiocarbamate: Affecting Factors, Efficiency and Mechanism. J. Environ. Sci. 2023, 129, 115–127. [Google Scholar] [CrossRef]
- Thomas, M.; Białecka, B.; Zdebik, D. Removal of Copper, Nickel and Tin from Model and Real Industrial Wastewater Using Sodium Trithiocarbonate. The Negative Impact of Complexing Compounds. Arch. Environ. Prot. 2018, 44, 33–47. [Google Scholar]
- Wang, Q.; Zheng, C.; Zhang, J.; He, F.; Yao, Y.; Zhang, T.C.; He, C. Insights into the Adsorption of Pb(II) over Trimercapto-s-Triazine Trisodium Salt-Modified Lignin in a Wide PH Range. Chem. Eng. J. Adv. 2020, 1, 100002. [Google Scholar] [CrossRef]
- Matlock, M.M.; Howerton, B.S.; Atwood, D.A. Chemical Precipitation of Heavy Metals from Acid Mine Drainage. Water Res. 2002, 36, 4757–4764. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.; Takács, I.; Murthy, S.; Daigger, G.T.; Szabó, A. Phosphate Complexation Model and Its Implications for Chemical Phosphorus Removal. Water Environ. Res. 2008, 80, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.A.M.; Abbas Shanshool, H. Phosphorus Removal from Water and Waste Water by Chemical Precipitation Using Alum and Calcium Chloride. Iraqi J. Chem. Pet. Eng. 2009, 10, 47–52. [Google Scholar] [CrossRef]
- Szabó, A.; Takács, I.; Murthy, S.; Daigger, G.T.; Licskó, I.; Smith, S. Significance of Design and Operational Variables in Chemical Phosphorus Removal. Water Environ. Res. 2008, 80, 407–416. [Google Scholar] [CrossRef]
- Wang, J.; Song, J.; Lu, J.; Zhao, X. Comparison of Three Aluminum Coagulants for Phosphorus Removal. J. Water Resour. Prot. 2014, 6, 902–908. [Google Scholar] [CrossRef]
- Akinnawo, S.O. Eutrophication: Causes, Consequences, Physical, Chemical and Biological Techniques for Mitigation Strategies. Environ. Chall. 2023, 12, 100733. [Google Scholar] [CrossRef]
- Owodunni, A.A.; Ismail, S.; Kurniawan, S.B.; Ahmad, A.; Imron, M.F.; Abdullah, S.R.S. A Review on Revolutionary Technique for Phosphate Removal in Wastewater Using Green Coagulant. J. Water Process Eng. 2023, 52, 103573. [Google Scholar] [CrossRef]
- Huang, S.; Fu, Y.; Zhang, H.; Wang, C.; Zou, C.; Lu, X. Research Progress of Novel Bio-Denitrification Technology in Deep Wastewater Treatment. Front. Microbiol. 2023, 14, 1284369. [Google Scholar] [CrossRef]
- Kim, D.W.; Yu, S.I.; Im, K.; Shin, J.; Shin, S.G. Responses of Coagulant Type, Dosage and Process Conditions to Phosphate Removal Efficiency from Anaerobic Sludge. Int. J. Environ. Res. Public. Health 2022, 19, 1693. [Google Scholar] [CrossRef]
- Sengupta, S.; Nawaz, T.; Beaudry, J. Nitrogen and Phosphorus Recovery from Wastewater. Curr. Pollut. Rep. 2015, 1, 155–166. [Google Scholar] [CrossRef]
- Zhou, Q.; Sun, H.; Jia, L.; Wu, W.; Wang, J. Simultaneous Biological Removal of Nitrogen and Phosphorus from Secondary Effluent of Wastewater Treatment Plants by Advanced Treatment: A Review. Chemosphere 2022, 296, 134054. [Google Scholar] [CrossRef]
- Kajjumba, G.W.; Fischer, D.; Risso, L.A.; Koury, D.; Marti, E.J. Application of Cerium and Lanthanum Coagulants in Wastewater Treatment—A Comparative Assessment to Magnesium, Aluminum, and Iron Coagulants. Chem. Eng. J. 2021, 426, 131268. [Google Scholar] [CrossRef]
- Lejwoda, P.; Białecka, B.; Thomas, M. Removal of Phosphate from Brewery Wastewater by Cerium(III) Chloride Originating from Spent Polishing Agent: Recovery and Optimization Studies. Sci. Total Environ. 2023, 875, 162643. [Google Scholar] [CrossRef] [PubMed]
- U.S. Geological Survey. Mineral Commodity Summaries 2025; U.S. Geological Survey: Reston, VA, USA, 2025.
- European Commission Directorate–General for Internal Market, Industry; Entrepreneurship and SMEs; Grohol, M.; Veeh, C. Study on the Critical Raw Materials for the EU 2023—Final Report; Publications Office of the European Union: Luxembourg, 2023; Available online: https://op.europa.eu/en/publication-detail/-/publication/57318397-fdd4-11ed-a05c-01aa75ed71a1/language-en (accessed on 5 April 2025).
- Lejwoda, P.; Białecka, B.; Barbusiński, K.; Thomas, M. Recovery of Cerium Salts from Sewage Sludge Resulting from the Coagulation of Brewery Wastewater with Recycled Cerium Coagulant. Materials 2024, 17, 938. [Google Scholar] [CrossRef] [PubMed]
- Śpiewak, K. Gasification of Sewage Sludge—A Review. Energies 2024, 17, 4476. [Google Scholar] [CrossRef]
- Rodríguez, F.A.; Santiago, D.E.; Franquiz Suárez, N.; Ortega Méndez, J.A.; Veza, J.M. Comparison of Evaporation Rates for Seawater and Brine from Reverse Osmosis in Traditional Salt Works: Empirical Correlations. Water Supply 2012, 12, 234–240. [Google Scholar] [CrossRef]
- Huijbregts, M.A.J.; Steinmann, Z.J.N.; Elshout, P.M.F.; Stam, G.; Verones, F.; Vieira, M.; Zijp, M.; Hollander, A.; van Zelm, R. ReCiPe2016: A Harmonised Life Cycle Impact Assessment Method at Midpoint and Endpoint Level. Int. J. Life Cycle Assess. 2017, 22, 138–147. [Google Scholar] [CrossRef]
- CENY WODY 2025 W POLSCE MIASTA ALFABETYCZNIE I MALEJĄCO. Available online: http://www.cena-pradu.pl/woda.html (accessed on 1 April 2025).
- Kajjumba, G.W.; Marti, E.J. A Review of the Application of Cerium and Lanthanum in Phosphorus Removal during Wastewater Treatment: Characteristics, Mechanism, and Recovery. Chemosphere 2022, 309, 136462. [Google Scholar] [CrossRef]
- Dahle, J.; Arai, Y. Environmental Geochemistry of Cerium: Applications and Toxicology of Cerium Oxide Nanoparticles. Int. J. Environ. Res. Public. Health 2015, 12, 1253–1278. [Google Scholar] [CrossRef]
- Byrne, R.H.; Kim, K.-H. Rare Earth Precipitation and Coprecipitation Behavior: The Limiting Role of PO43− on Dissolved Rare Earth Concentrations in Seawater. Geochim. Cosmochim. Acta 1993, 57, 519–526. [Google Scholar] [CrossRef]
- Liu, X.; Byrne, R.H. Rare Earth and Yttrium Phosphate Solubilities in Aqueous Solution. Geochim. Cosmochim. Acta 1997, 61, 1625–1633. [Google Scholar] [CrossRef]
- Rodríguez-Ruiz, I.; Teychené, S.; Vitry, Y.; Biscans, B.; Charton, S. Thermodynamic Modeling of Neodymium and Cerium Oxalates Reactive Precipitation in Concentrated Nitric Acid Media. Chem. Eng. Sci. 2018, 183, 20–25. [Google Scholar] [CrossRef]
- Crouthamel, C.E.; Martin, D.S. Solubility of the Rare Earth Oxalates and Complex Ion Formation in Oxalate Solution. II. Neodymium and Cerium(III) 1. J. Am. Chem. Soc. 1951, 73, 569–573. [Google Scholar] [CrossRef]
- Żelazny, S.; Świnder, H.; Białecka, B.; Jarosiński, A. Odzysk Pierwiastków Ziem Rzadkich z Popiołów Lotnych. Cz. II. Wytrącanie z Roztworu. PRZEMYSŁ Chem. 2017, 1, 102–108. [Google Scholar] [CrossRef]
- Lopez, H.F.; Mendoza, H. Temperature Effects on the Crystallization and Coarsening of Nano-CeO 2 Powders. ISRN Nanomater. 2013, 2013, 208614. [Google Scholar] [CrossRef]
- Chen, F.; Liu, F.; Wang, L.; Wang, J. Comparison of the Preparation Process of Rare Earth Oxides from the Water Leaching Solution of Waste Nd-Fe-B Magnets’ Sulfate Roasting Products. Processes 2022, 10, 2310. [Google Scholar] [CrossRef]
- Poscher, A.; Luidold, S.; Schnideritsch, H.; Antrekowitsch, H. Extraction of Lanthanides from Spent Polishing Agent. In Proceedings of the ERES2014-1st European Rare Earth Resources Conference, Milos, Greece, 4–7 September 2014; pp. 209–222. [Google Scholar]
- Skudaev, V.I.; Solomonov, A.B.; Morozovskii, A.I.; Isakov, N.A. Oxidation of Hydrogen Chloride with Hydrogen Peroxide in Aqueous Solution. Russ. J. Appl. Chem. 2008, 81, 14–16. [Google Scholar] [CrossRef]
- ISO 14040; Environmental Management—Life Cycle Assessment—Principles and Framework. International Standard Organization: Geneva, Switzerland, 2006.
- ISO 14044; Environmental Management—Life Cycle Assessment—Requirements and Guidelines. International Standard Organization: Geneva, Switzerland, 2006.
- Kowalski, Z.; Kulczycka, J.; Góralczyk, M. Ekologiczna Ocena Cyklu Życia Procesów Wytwórczych (LCA); PWN: Warszawa, Poland, 2007. [Google Scholar]
- Burchart-Korol, D. Zastosowanie Oceny Cyklu Życia (LCA) w Analizie Procesów Przemysłowych. Probl. Ekol. 2009, 13, 300–305. [Google Scholar]
- Kurczewski, P.; Lewandowska, A. Zasady Projektowania Prośrodowiskowego Obiektów Technicznych Dla Potrzeb Zarządzania Ich Cyklem Życia: Praca Zbiorowa; Politechnika Poznańska: Poznań, Poland, 2008; ISBN 9788361352204. [Google Scholar]
- Rączka, J. Cost Effectiveness Analysis Based on Dynamic Generation Cost Index. Training Materials Developed Under the TRANSFORM ADVICE PROGRAMME—Investment in Environmental Infrastructure in Poland; (In Polish: Analiza Efektywności Kosztowej w Oparciu o Wskaźnik Dynamicznego Kosztu Jednostkowego). Available online: https://www.gov.pl/attachment/ae722d67-2dae-4af0-b269-35fee9faea87 (accessed on 3 April 2025).
- Advancement of Cost Engineering (AACE) International. Cost Estimate Classification System—As Applied in Engineering, Procurement, and Construction for the Process Industries; TCM Framework 7.3—Cost Estimating and Budgeting, AACE International Recommended Practice No. 18R-97; AACE International: Denver, CO, USA, 2005; Revised 29 November 2011; Available online: https://aheinc.ca/wp-content/uploads/2018/12/AACE-Cost-Estimate-Classification-System.pdf (accessed on 3 April 2025).
- Minister Funduszy i Polityki Regionalnej. Wytyczne Dotyczące Zagadnień Związanych z Przygotowaniem Projektów Inwestycyjnych, w Tym Hybrydowych Na Lata 2021–2027; Minister Funduszy i Polityki Regionalnej: Warszawa, Poland, 2023.
- Minister Finansów. Guidelines on the Use of Uniform Macroeconomic Indicators Constituting the Basis for Estimating the Financial Impact of Draft Laws; (In Polish: Wytyczne Dotyczące Stosowania Jednolitych Wskaźników Makroekonomicznych Będących Podstawą Oszacowania Skutków Finansowych Projektowanych Ustaw); Minister Finansów: Warszawa, Poland, 2024.
- Pyrka, M.; Jeszke, R.; Boratyński, J.; Witajewski-Baltvilks, J.; Antosiewicz, M.; Tatarewicz, I.; Rabiega, W.; Wąs, A.; Tobiasz, I.; Lewarski, M.; et al. VIIEW on EU ETS 2050: Changing the Scope of the EU Emissions Trading System; Krajowy Ośrodek Bilansowania i Zarządzania Emisjami/Instytut Ochrony Środowiska (KOBIZE/IOŚ): Warszawa, Poland, 2023. [Google Scholar]
| Country | REEs Production in 2024 (Tonnes) | REEs Reserves (Tonnes) |
|---|---|---|
| China | 270,000 | 44,000,000 |
| USA | 45,000 | 1,900,000 |
| Myanmar | 31,000 | Not available |
| Thailand | 13,000 | 4500 |
| Nigeria | 13,000 | Not available |
| Australia | 13,000 | 5,700,000 |
| Russia | 2500 | 3,800,000 |
| India | 2900 | 6,900,000 |
| Madagascar | 2000 | Not available |
| Vietnam | 300 | 3,500,000 |
| Brazil | 20 | 21,000,000 |
| Malaysia | 130 | Not available |
| Canada | Not available | 830,000 |
| South Africa | Not available | 860,000 |
| Greenland | Not available | 1,500,000 |
| Tanzania | Not available | 890,000 |
| Other countries | 1100 | Not available |
| Stage No. | Stage |
|---|---|
| I | Precipitation of phosphates(V) (a) |
| II | Filtration (a) |
| III | Sludge extraction (a) |
| IV | Filtration (a) |
| V | Precipitation of cerium(III) oxalate (a) |
| VI | Filtration, management of filtrates (a,b) |
| VII | Thermal decomposition (a) |
| VIII | Synthesis of cerium(III) chloride (a,b) |
| IX | Crystallisation (a) |
| X | HCl recovery (b) |
| Impact Category | Unit | Total | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Global warming | kg CO2 eq | 3.30 | 2.08 × 10−4 | 2.04 | 5.80 × 10−1 | 2.84 × 10−1 | 2.35 × 10−1 | 6.85 × 10−3 | 3.91 × 10−2 | 1.88 × 10−1 | −7.86 × 10−2 | 0.00 | 7.67 × 10−3 |
| Stratospheric ozone depletion | kg CFC11 eq | 1.92 × 10−5 | 0.00 | 1.80 × 10−5 | 7.94 × 10−7 | 2.86 × 10−7 | 5.75 × 10−8 | 3.83 × 10−9 | 1.32 × 10−9 | 8.65 × 10−8 | −1.02 × 10−7 | 0.00 | 7.56 × 10−8 |
| Ionising radiation | kBq Co60 eq | 3.75 × 10−1 | 0.00 | 3.93 × 10−2 | 1.68 × 10−1 | 3.16 × 10−2 | 3.28 × 10−2 | 3.42 × 10−4 | 3.89 × 10−4 | 1.08 × 10−1 | −6.13 × 10−3 | 0.00 | 7.91 × 10−4 |
| Ozone formation, Human health | kg NOx eq | 5.64 × 10−2 | 0.00 | 5.36 × 10−2 | 1.30 × 10−3 | 7.58 × 10−4 | 3.84 × 10−4 | 2.71 × 10−5 | 2.69 × 10−5 | 3.48 × 10−4 | −1.54 × 10−4 | 0.00 | 9.61 × 10−5 |
| Fine particulate matter formation | kg PM2.5 eq | 1.25 × 10−2 | 0.00 | 1.02 × 10−2 | 1.39 × 10−3 | 5.80 × 10−4 | 2.12 × 10−4 | 1.35 × 10−5 | 8.42 × 10−6 | 2.88 × 10−4 | −1.94 × 10−4 | 0.00 | 1.67 × 10−5 |
| Ozone formation, Terrestrial ecosystems | kg NOx eq | 5.67 × 10−2 | 0.00 | 5.38 × 10−2 | 1.36 × 10−3 | 7.75 × 10−4 | 4.22 × 10−4 | 2.76 × 10−5 | 3.02 × 10−5 | 3.60 × 10−4 | −1.64 × 10−4 | 0.00 | 9.75 × 10−5 |
| Terrestrial acidification | kg SO2 eq | 3.77 × 10−2 | 0.00 | 3.21 × 10−2 | 3.89 × 10−3 | 1.04 × 10−3 | 5.59 × 10−4 | 2.83 × 10−5 | 2.28 × 10−5 | 7.32 × 10−4 | −6.67 × 10−4 | 0.00 | 4.88 × 10−5 |
| Freshwater eutrophication | kg P eq | 1.15 × 10−3 | 0.00 | 3.94 × 10−4 | 3.79 × 10−4 | 1.33 × 10−4 | 8.81 × 10−5 | 3.22 × 10−6 | 6.27 × 10−7 | 1.75 × 10−4 | −2.78 × 10−5 | −3.31 × 10−5 | 3.57 × 10−5 |
| Marine eutrophication | kg N eq | 2.48 × 10−4 | 0.00 | 2.74 × 10−5 | 5.53 × 10−5 | 1.19 × 10−5 | 1.54 × 10−5 | 2.29 × 10−5 | 3.29 × 10−7 | 1.25 × 10−5 | −3.30 × 10−6 | 0.00 | 1.05 × 10−4 |
| Terrestrial ecotoxicity | kg 1,4-DCB | 1.42 × 101 | 0.00 | 5.96 | 5.41 | 1.25 | 9.31 × 10−1 | 4.96 × 10−2 | 1.45 × 10−2 | 1.09 | −5.30 × 10−1 | 0.00 | 2.40 × 10−2 |
| Freshwater ecotoxicity | kg 1,4-DCB | 1.77 × 10−1 | 0.00 | 6.43 × 10−2 | 6.00 × 10−2 | 1.49 × 10−2 | 1.65 × 10−2 | 1.18 × 10−3 | 6.60 × 10−5 | 2.32 × 10−2 | −4.79 × 10−3 | 0.00 | 1.65 × 10−3 |
| Marine ecotoxicity | kg 1,4-DCB | 2.32 × 10−1 | 0.00 | 8.50 × 10−2 | 7.85 × 10−2 | 1.96 × 10−2 | 2.18 × 10−2 | 1.52 × 10−3 | 1.00 × 10−4 | 2.94 × 10−2 | −6.32 × 10−3 | 0.00 | 2.03 × 10−3 |
| Human carcinogenic toxicity | kg 1,4-DCB | 1.90 × 10−1 | 0.00 | 5.68 × 10−2 | 6.40 × 10−2 | 1.87 × 10−2 | 3.82 × 10−2 | 4.92 × 10−4 | 1.70 × 10−4 | 1.33 × 10−2 | −4.70 × 10−3 | 0.00 | 2.72 × 10−3 |
| Human noncarcinogenic toxicity | kg 1,4-DCB | 3.65 | 0.00 | 1.49 | 1.18 | 3.31 × 10−1 | 3.30 × 10−1 | 4.05 × 10−2 | 1.70 × 10−3 | 3.54 × 10−1 | −9.10 × 10−2 | 0.00 | 1.44 × 10−2 |
| Land use | m2a crop eq | 5.22 × 10−2 | 0.00 | 1.82 × 10−2 | 2.11 × 10−2 | 6.82 × 10−3 | 4.41 × 10−3 | 6.49 × 10−4 | 5.39 × 10−4 | 6.17 × 10−3 | −5.83 × 10−3 | 0.00 | 1.10 × 10−4 |
| Mineral resource scarcity | kg Cu eq | 1.01 × 10−2 | 0.00 | 4.16 × 10−3 | 4.32 × 10−3 | 9.88 × 10−4 | 9.33 × 10−4 | 1.08 × 10−3 | 7.84 × 10−6 | 7.31 × 10−4 | −2.15 × 10−3 | 0.00 | 3.61 × 10−5 |
| Fossil resource scarcity | kg oil eq | 8.40 × 10−1 | 0.00 | 4.79 × 10−1 | 1.79 × 10−1 | 6.90 × 10−2 | 7.92 × 10−2 | 1.55 × 10−3 | 3.55 × 10−3 | 5.09 × 10−2 | −2.28 × 10−2 | 0.00 | 1.20 × 10−3 |
| Water consumption | m3 | 5.87 × 10−2 | 0.00 | 1.55 × 10−2 | 1.92 × 10−2 | 6.76 × 10−3 | 1.51 × 10−2 | −8.24 × 10−6 | 5.30 × 10−5 | 3.29 × 10−3 | −1.38 × 10−3 | 0.00 | 1.40 × 10−4 |
| Item | Unit | Construction Period | Plant Operation Period | |||||
|---|---|---|---|---|---|---|---|---|
| 2025 | 2026 | 2027 | 2030 | 2035 | 2040 | 2046 | ||
| Investment expenditures | USD | 1,202,530 | 1,202,530 | 0 | 0 | 0 | 0 | 0 |
| Raw materials, chemicals | USD | 0 | 0 | 1,700,228 | 1,846,185 | 1,916,928 | 1,935,208 | 1,952,690 |
| Maintenance and repairs | USD | 0 | 0 | 16,941 | 18,396 | 19,101 | 19,283 | 19,457 |
| Electricity | USD | 0 | 0 | 263,372 | 287,793 | 260,142 | 235,148 | 208,304 |
| Employee salaries | USD | 0 | 0 | 114,133 | 123,268 | 139,331 | 156,566 | 179,454 |
| Monitoring | USD | 0 | 0 | 19,324 | 20,983 | 21,787 | 21,995 | 22,193 |
| Total—Costs | USD | 1,202,530 | 1,202,530 | 2,113,997 | 2,296,625 | 2,357,288 | 2,368,200 | 2,382,098 |
| Volume of treated wastewater | m3/year | 0 | 0 | 1,825,000 | 1,825,000 | 1,825,000 | 1,825,000 | 1,825,000 |
| Discount factor | - | 1.00000 | 0.96154 | 0.92456 | 0.82193 | 0.67556 | 0.55526 | 0.43883 |
| Discounted costs | USD | 1,202,530 | 1,156,279 | 1,954,509 | 1,887,658 | 1,592,500 | 1,314,977 | 1,045,345 |
| Discounted volume of wastewater | m3/year | 0 | 0 | 1,687,315 | 1,500,017 | 1,232,905 | 1,013,358 | 800,871 |
| Total discounted costs | USD | 32,670,246 | ||||||
| Total discounted volume of wastewater | m3 | 23,848,409 | ||||||
| Item | Cost [USD] |
|---|---|
| Stage I. Phosphate(V) precipitation | |
| Coagulant preparation tank with mixing | 5000 |
| Coagulant dosing pump | 4000 |
| Wastewater tank for phosphate(V) removal | 50,000 |
| Pump for wastewater discharge into the nitrification process (87% of wastewater) | 2000 |
| Pump for sludge discharge to filter press (650 m3) | 2100 |
| Stage II. Filtration | |
| Filter press × 2 | 12,000 |
| Pump for filtrate discharge into the nitrification process | 2100 |
| Belt conveyor to reactors × 2 | 2000 |
| Stage III. Sludge extraction | |
| Acid-resistant reactor for extraction × 2 | 76,000 |
| 30% HCl storage tank for extraction | 5000 |
| Acid-resistant pump for HCl | 4000 |
| Water storage tank | 5000 |
| Water pump | 1500 |
| Acid-resistant pump for acidic extract | 8000 |
| Stage IV. Filtration | |
| Acid-resistant filter press | 12,000 |
| Belt conveyor for sludge transport for agricultural use | 1000 |
| Acid-resistant pump for acidic extract | 4000 |
| Acid-resistant reactor for cerium(III) oxalate precipitation × 2 | 76,000 |
| Stage V. Cerium(III) oxalate precipitation | |
| NaOH preparation tank with mixing | 8000 |
| Alkali-resistant pump | 4000 |
| Oxalic acid preparation tank | 8000 |
| Acid-resistant pump × 2 | 8000 |
| Stage VI. Filtration and filtrate management | |
| Acid-resistant filter press | 6000 |
| Belt conveyor for cerium oxalate transport | 2000 |
| Acid-resistant pump | 8000 |
| Reaction tank with agitator | 130,000 |
| Sedimentation tank | 70,000 |
| Filter press | 75,000 |
| Lime dosing system | 30,000 |
| Land preparation, membrane, drainage systems, and barriers | 600,000 |
| Stage VII. Thermal decomposition | |
| Furnace for cerium(III) oxalate decomposition into cerium(IV) oxide at 350 °C | 5500 |
| Belt conveyor for cerium(IV) oxide to CeCl3 synthesis reactor | 1000 |
| Stage VIII. CeCl3·7H2O synthesis | |
| Acid-resistant reactor | 20,000 |
| Acid-resistant pump for HCl | 4000 |
| H2O2 storage tank | 5400 |
| High-resistance pump for H2O2 | 4000 |
| Acid-resistant pump | 4000 |
| Stage IX. Crystallisation | |
| Evaporator for concentration | 350,000 |
| Coagulant dosing pump—recirculation after recovery | 4000 |
| Stage X. Hydrochloric acid recovery | |
| Gasification reactor, control system, and pumps | 60,000 |
| Membrane module | 15,000 |
| High-pressure tanks, compressor | 12,260 |
| Tubular reactor, cooling, and control system | 82,500 |
| Absorption tower and pumps | 55,000 |
| Membrane module for Cl2 separation | 5700 |
| Pressure tank for Cl2 | 12,000 |
| Pumps, pipelines, and process control system | 25,000 |
| Total—Stages I to X | 1,886,060 |
| Additional equipment + piping | 200,000 |
| Automation and control system | 150,000 |
| Buildings | 75,000 |
| Project documentation, administrative decisions, and permits | 94,000 |
| Total—CAPEX | 2,405,060 |
| Item | Unit | Amount | Cost [USD/Year] |
|---|---|---|---|
| Electricity | kWh | 993,006 | 248,253 |
| Monitoring | - | - | 18,250 |
| Labour | - | - | 108,000 |
| Maintenance and repairs | - | - | 16,000 |
| CeCl3·7H2O | kg/year | 11,315 | 16,973 |
| 30% HCl solution | kg/year | 2,693,956 | 1,131,461 |
| 30% H2O2 solution | kg/year | 362,555 | 141,396 |
| Oxalic acid | kg/year | 240,535 | 127,965 |
| NaOH | kg/year | 393,105 | 157,242 |
| Calcium hydroxide | kg/year | 76,767 | 30,707 |
| Total—OPEX | - | - | 1,996,247 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lejwoda, P.; Białecka, B.; Śliwińska, A.; Krawczyk, P.; Thomas, M. Innovative Integrated Model of Industrial Wastewater Treatment with the Circular Use of Cerium Compounds as Multifunctional Coagulants: Comprehensive Assessment of the Process and Environmental and Economic Aspects. Molecules 2025, 30, 3428. https://doi.org/10.3390/molecules30163428
Lejwoda P, Białecka B, Śliwińska A, Krawczyk P, Thomas M. Innovative Integrated Model of Industrial Wastewater Treatment with the Circular Use of Cerium Compounds as Multifunctional Coagulants: Comprehensive Assessment of the Process and Environmental and Economic Aspects. Molecules. 2025; 30(16):3428. https://doi.org/10.3390/molecules30163428
Chicago/Turabian StyleLejwoda, Paweł, Barbara Białecka, Anna Śliwińska, Piotr Krawczyk, and Maciej Thomas. 2025. "Innovative Integrated Model of Industrial Wastewater Treatment with the Circular Use of Cerium Compounds as Multifunctional Coagulants: Comprehensive Assessment of the Process and Environmental and Economic Aspects" Molecules 30, no. 16: 3428. https://doi.org/10.3390/molecules30163428
APA StyleLejwoda, P., Białecka, B., Śliwińska, A., Krawczyk, P., & Thomas, M. (2025). Innovative Integrated Model of Industrial Wastewater Treatment with the Circular Use of Cerium Compounds as Multifunctional Coagulants: Comprehensive Assessment of the Process and Environmental and Economic Aspects. Molecules, 30(16), 3428. https://doi.org/10.3390/molecules30163428








