Protein Adsorption on a Multimodal Cation Exchanger: Effect of pH, Salt Type and Concentration, and Elution Conditions
Abstract
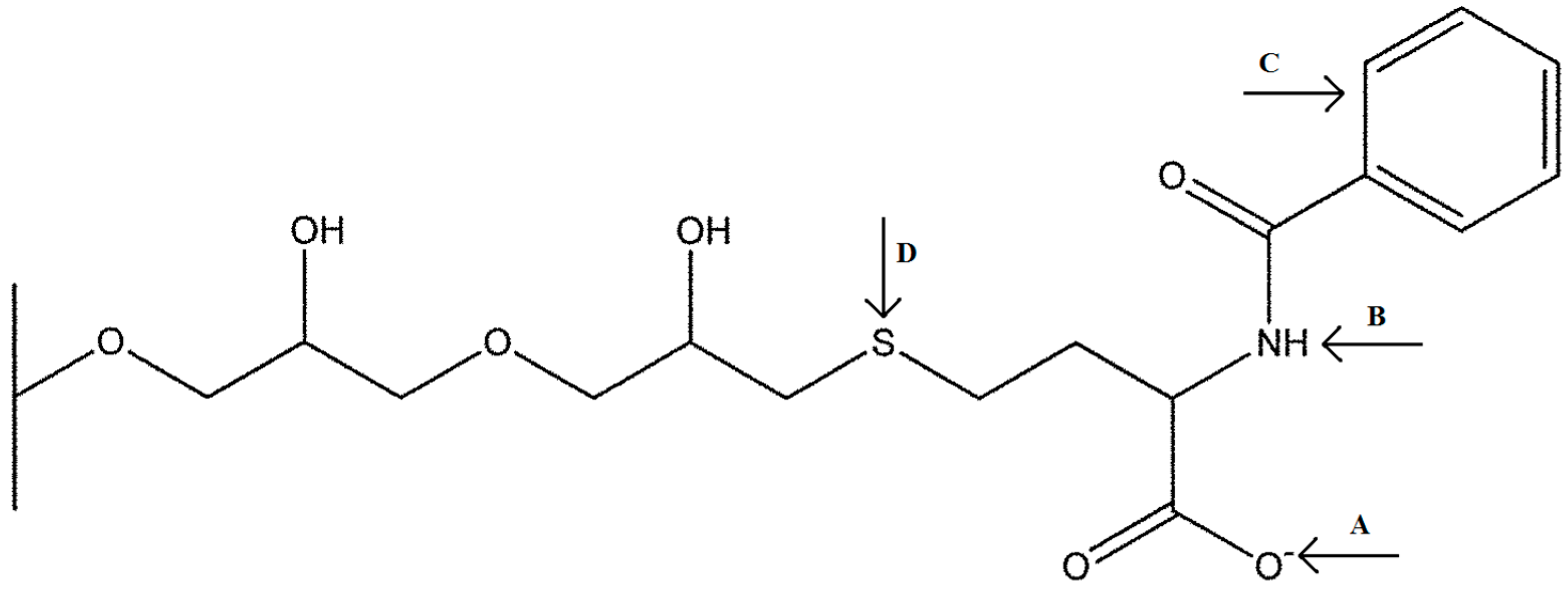
1. Introduction
2. Results and Discussion
2.1. Batch Equilibrium Experiments
2.1.1. Effect of pH and NaCl Concentration
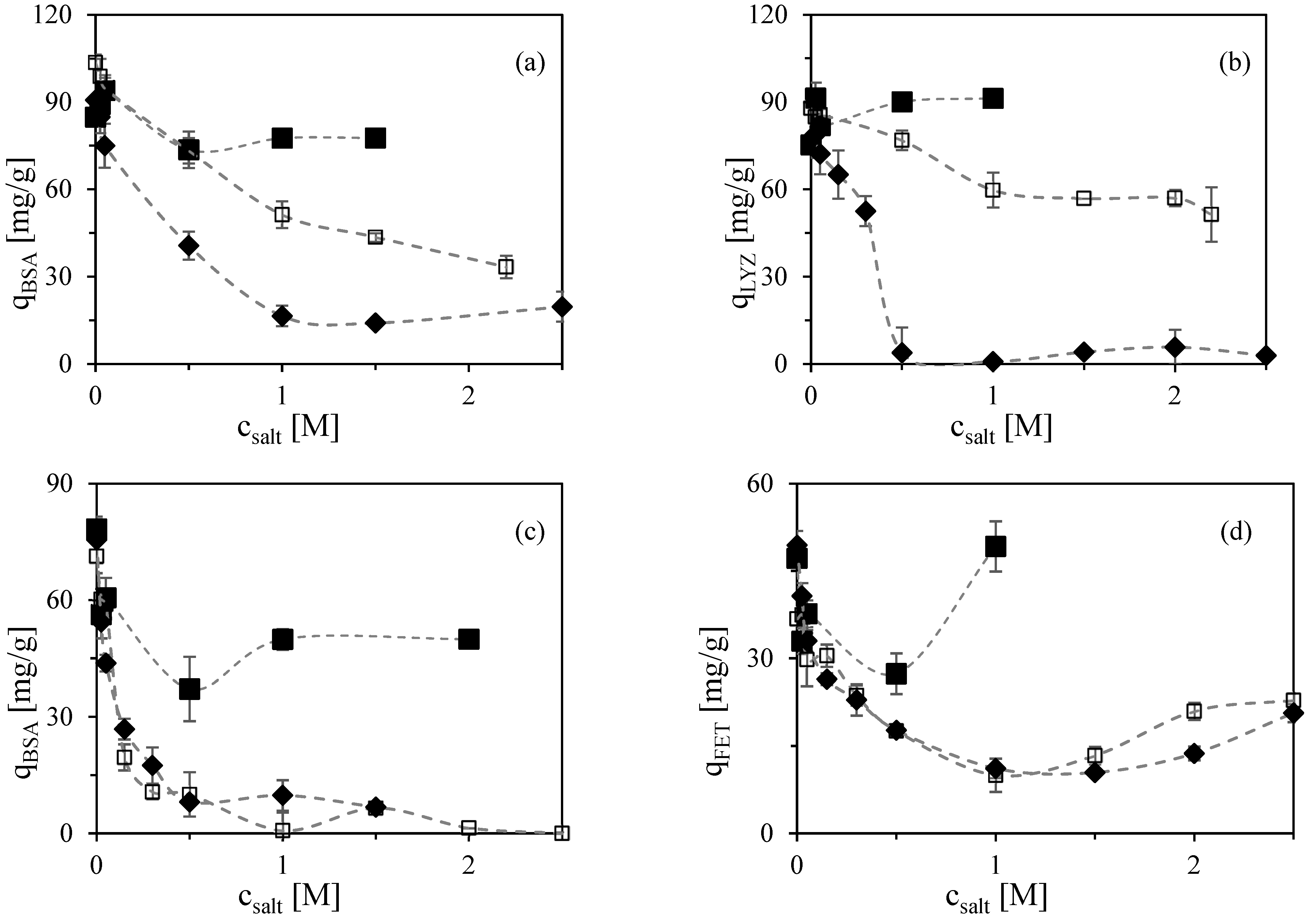
2.1.2. Effect of Salt Type and Concentration
2.2. Chromatographic Column Experiments
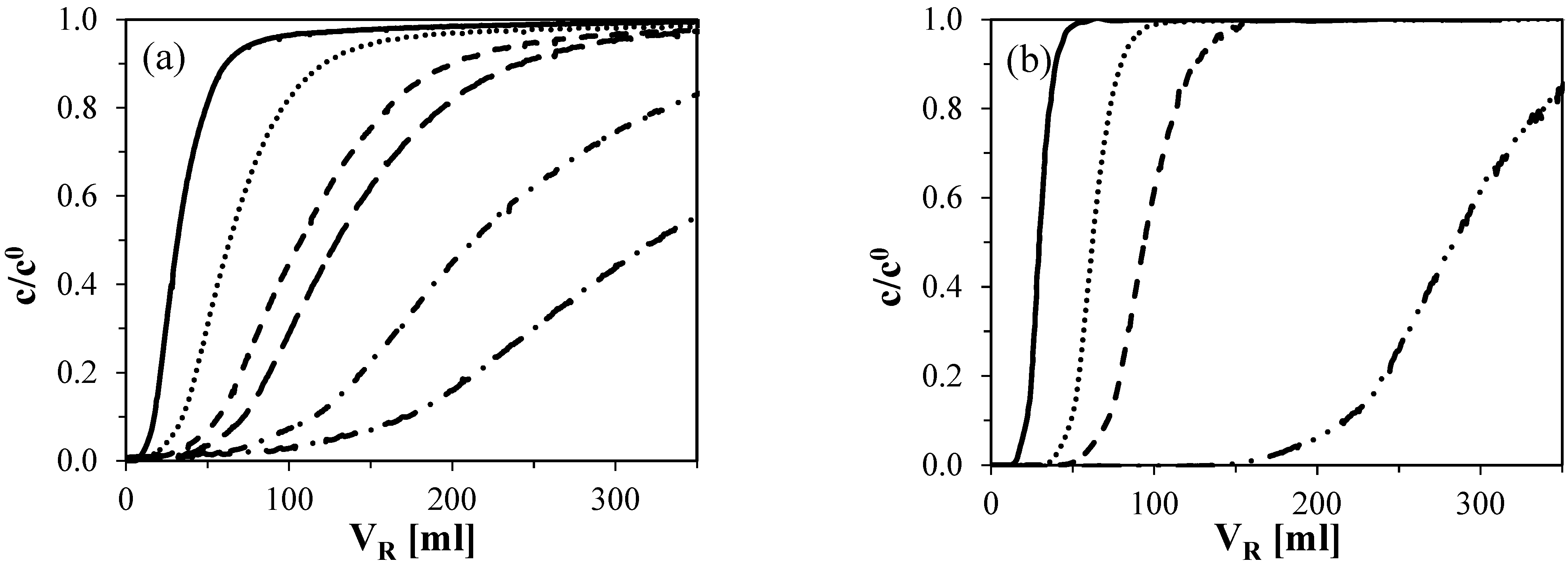
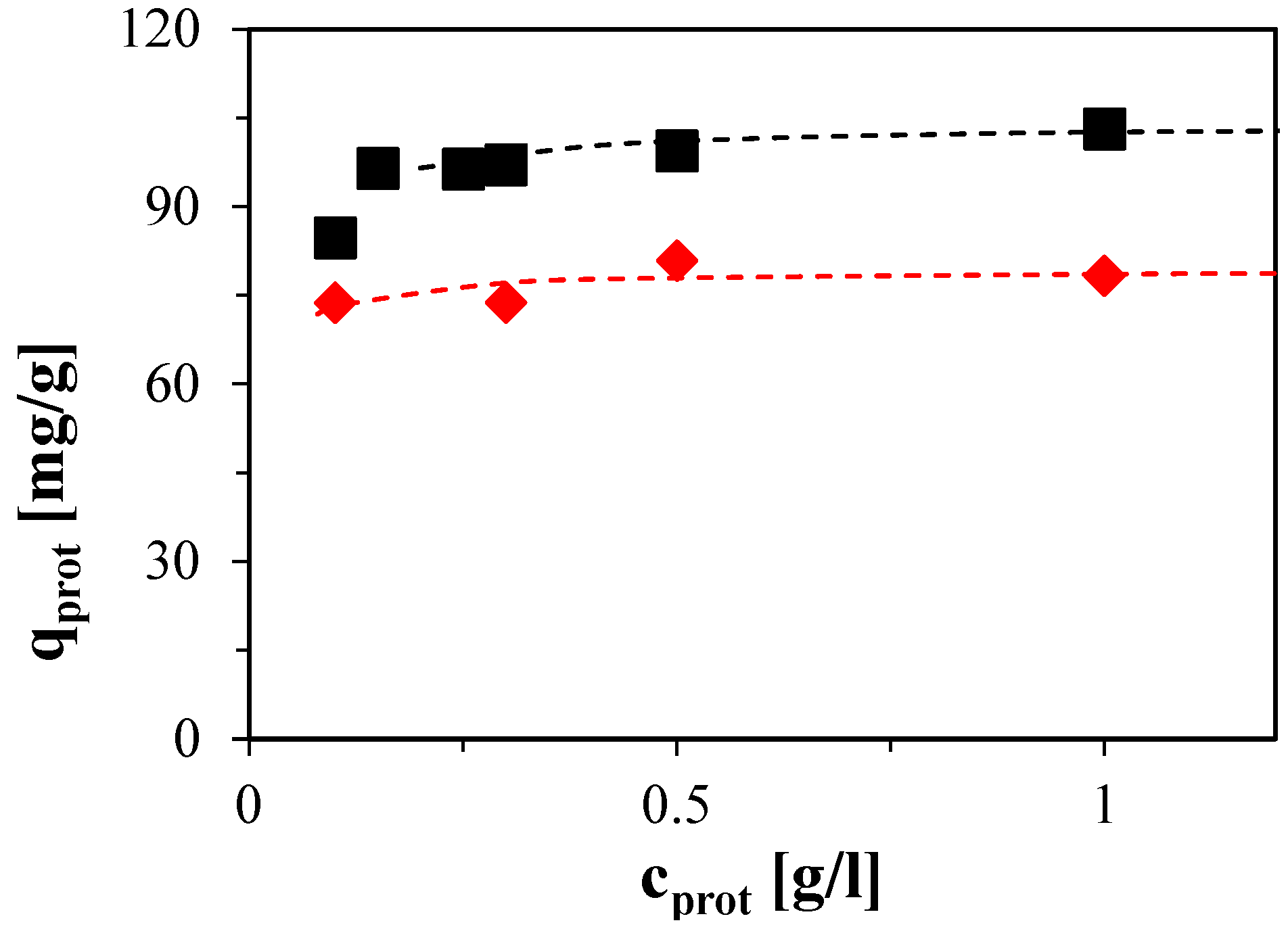
2.2.1. Frontal Experiments
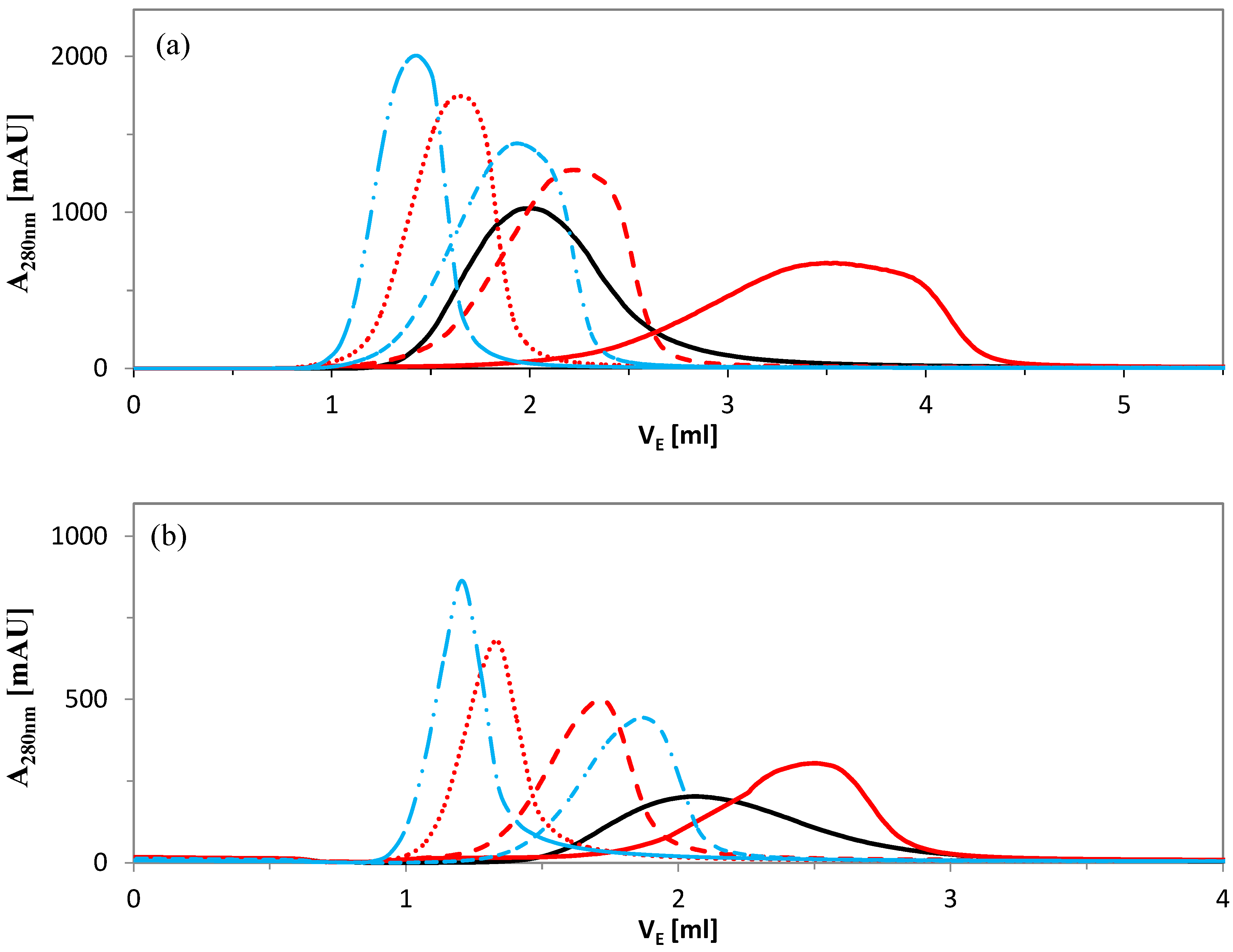
2.2.2. Elution Experiments
3. Materials and Methods
3.1. Materials
3.2. Static Batch Equilibrium Experiments
3.3. Dynamic Column Chromatographic Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schmidt, C.A.; Codevilla, C.F.; Fronza, M.; Casali, R.G.; Dalmora, S.L. Physico-Chemical Characterization and Biological Evaluation of Recombinant Human Erythropoietin in Pharmaceutical Products. Lat. Am. J. Pharm. 2003, 22, 343–350. [Google Scholar]
- Adamíková, J.; Antošová, M.; Polakovič, M. Chromatographic Purification of Recombinant Human Erythropoietin. Biotechnol. Lett. 2019, 41, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cai, L.; Wang, Y.; Li, Y. Processing of High-Salt-Containing Protein A Eluate Using Mixed-Mode Chromatography in Purifying an Aggregation-Prone Antibody. Protein Expr. Purif. 2019, 164, 105458. [Google Scholar] [CrossRef]
- Molnár, T.; Bartošová, M.; Antošová, M.; Škultéty, Ľ.; Polakovič, M. Design of a Three-Step Chromatographic Process of Recombinant Human Erythropoietin Purification. Sep. Purif. Technol. 2021, 267, 118673. [Google Scholar] [CrossRef]
- Matos, T.; Queiroz, J.A.; Bülow, L. Plasmid DNA Purification Using a Multimodal Chromatography Resin. J. Mol. Recognit. 2014, 27, 184–189. [Google Scholar] [CrossRef]
- Kurák, T.; Polakovič, M. Adsorption Performance of a Multimodal Anion-Exchange Chromatography Membrane: Effect of Liquid Phase Composition and Separation Mode. Membranes 2022, 12, 1173. [Google Scholar] [CrossRef]
- Lima, M.D.A.; Freitas, M.D.F.M.D.; Gonçalves, L.R.B.; Silva Junior, I.J.D. Recovery and Purification of a Kluyvermyces Lactis β -Galactosidase by Mixed Mode Chromatography. J. Chromatogr. B 2016, 1015–1016, 181–191. [Google Scholar] [CrossRef]
- Sejergaard, L.; Karkov, H.S.; Krarup, J.K.; Hagel, A.B.B.; Cramer, S.M. Model-based Process Development for the Purification of a Modified Human Growth Hormone Using Multimodal Chromatography. Biotechnol. Progr. 2014, 30, 1057–1064. [Google Scholar] [CrossRef]
- Hirano, A.; Shiraki, K.; Kameda, T. Effects of Arginine on Multimodal Chromatography: Experiments and Simulations. Curr. Protein Pept. Sci. 2019, 20, 40–48. [Google Scholar] [CrossRef]
- Granovski, V.; Abreu-Neto, M.S.; Covas, D.T. Purification Methods for Recombinant Factor VIII Expressed in Human Liver SK-Hep Cells. In Recombinant Glycoprotein Production; Picanço-Castro, V., Swiech, K., Eds.; Springer: New York, NY, USA, 2018; Volume 1674, pp. 195–202. [Google Scholar]
- Toyoda, T.; Arakawa, T.; Yamaguchi, H. N-Glycans Stabilize Human Erythropoietin through Hydrophobic Interactions with the Hydrophobic Protein Surface: Studies by Surface Plasmon Resonance Analysis. J. Biochem. 2002, 131, 511–515. [Google Scholar] [CrossRef]
- Konstantinidis, S.; Poplyk, M.R.; Ma, W.; Reilly, D.; Zhang, Y.; Wang, J.; Thompson, R.; Stiving, A.; Winters, M.A.; Wang, S.-C.; et al. Purification Processes of Live Virus Vaccine Candidates Expressed in Adherent Vero Cell Lines via Multimodal Chromatography in Flowthrough Mode. Biotechnol. Bioeng. 2024, 121, 2482–2499. [Google Scholar] [CrossRef]
- Chen, T.; Guo, G.; Tan, G.; Wang, Y.; Li, Y. Antibody Aggregate Removal Using a Mixed-Mode Chromatography Resin. In Protein Downstream Processing: Design, Development, and Application of High and Low-Resolution Methods; Labrou, N.E., Ed.; Springer: New York, NY, USA, 2021; pp. 345–354. [Google Scholar]
- Wan, Y.; Zhang, T.; Wang, Y.; Wang, Y.; Li, Y. Removing Light Chain-Missing Byproducts and Aggregates by Capto MMC ImpRes Mixed-Mode Chromatography during the Purification of Two WuXiBody-Based Bispecific Antibodies. Protein Expr. Purif. 2020, 175, 105712. [Google Scholar] [CrossRef]
- Rupčíková, V.; Molnár, T.; Kurák, T.; Polakovič, M. Antibody Aggregate Removal by Multimodal Chromatography. Molecules 2025, 30, 2363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wan, Y.; Duan, J.; Li, Y. Removing a Light-Chain Missing Byproduct by MMC ImpRes Mixed-Mode Chromatography under Weak Partitioning Mode in Purifying a WuXiBody-Based Bispecific Antibody. Protein Expr. Purif. 2021, 186, 105927. [Google Scholar] [CrossRef]
- Nitika, N.; Thakur, G.; Rathore, A.S. Continuous Manufacturing of Monoclonal Antibodies: Dynamic Control of Multiple Integrated Polishing Chromatography Steps Using BioSMB. J. Chromatogr. A 2023, 1690, 463784. [Google Scholar] [CrossRef] [PubMed]
- Gospodarek, A.M.; Hiser, D.E.; O’Connell, J.P.; Fernandez, E.J. Unfolding of a Model Protein on Ion Exchange and Mixed Mode Chromatography Surfaces. J. Chromatogr. A 2014, 1355, 238–252. [Google Scholar] [CrossRef] [PubMed]
- Nfor, B.K.; Noverraz, M.; Chilamkurthi, S.; Verhaert, P.D.E.M.; van der Wielen, L.A.M.; Ottens, M. High-Throughput Isotherm Determination and Thermodynamic Modeling of Protein Adsorption on Mixed Mode Adsorbents. J. Chromatogr. A 2010, 1217, 6829–6850. [Google Scholar] [CrossRef]
- Hou, Y.; Cramer, S.M. Evaluation of Selectivity in Multimodal Anion Exchange Systems: A Priori Prediction of Protein Retention and Examination of Mobile Phase Modifier Effects. J. Chromatogr. A 2011, 1218, 7813–7820. [Google Scholar] [CrossRef]
- Wolfe, L.S.; Barringer, C.P.; Mostafa, S.S.; Shukla, A.A. Multimodal Chromatography: Characterization of Protein Binding and Selectivity Enhancement through Mobile Phase Modulators. J. Chromatogr. A 2014, 1340, 151–156. [Google Scholar] [CrossRef]
- Gudhka, R.B.; Roush, D.J.; Cramer, S.M. A Thermodynamic Evaluation of Antibody-Surface Interactions in Multimodal Cation Exchange Chromatography. J. Chromatogr. A 2020, 1628, 461479. [Google Scholar] [CrossRef]
- Woo, J.; Parimal, S.; Brown, M.R.; Heden, R.; Cramer, S.M. The Effect of Geometrical Presentation of Multimodal Cation-Exchange Ligands on Selective Recognition of Hydrophobic Regions on Protein Surfaces. J. Chromatogr. A 2015, 1412, 33–42. [Google Scholar] [CrossRef]
- Woo, J.A.; Chen, H.; Snyder, M.A.; Chai, Y.; Frost, R.G.; Cramer, S.M. Defining the Property Space for Chromatographic Ligands from a Homologous Series of Mixed-Mode Ligands. J. Chromatogr. A 2015, 1407, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Carta, G. Protein Adsorption Equilibrium and Kinetics in Multimodal Cation Exchange Resins. Adsorption 2016, 22, 165–179. [Google Scholar] [CrossRef]
- Yang, T.; Malmquist, G.; Johansson, B.-L.; Maloisel, J.-L.; Cramer, S. Evaluation of Multi-Modal High Salt Binding Ion Exchange Materials. J. Chromatogr. A 2007, 1157, 171–177. [Google Scholar] [CrossRef]
- Kallberg, K.; Becker, K.; Bülow, L. Application of a pH Responsive Multimodal Hydrophobic Interaction Chromatography Medium for the Analysis of Glycosylated Proteins. J. Chromatogr. A 2011, 1218, 678–683. [Google Scholar] [CrossRef]
- Kreusser, J.; Jirasek, F.; Hasse, H. Influence of Salts on the Adsorption of Lysozyme on a Mixed-Mode Resin. Adsorpt. Sci. Technol. 2021, 2021, 6681348. [Google Scholar] [CrossRef]
- Altern, S.H.; Welsh, J.P.; Lyall, J.Y.; Kocot, A.J.; Burgess, S.; Kumar, V.; Williams, C.; Lenhoff, A.M.; Cramer, S.M. Isotherm Model Discrimination for Multimodal Chromatography Using Mechanistic Models Derived from High-Throughput Batch Isotherm Data. J. Chromatogr. A 2023, 1693, 463878. [Google Scholar] [CrossRef]
- Chu, W.-N.; Wu, Q.-C.; Yao, S.-J.; Lin, D.-Q. High-Throughput Screening and Optimization of Mixed-Mode Resins for Human Serum Albumin Separation with Microtiter Filter Plate. Biochem. Eng. J. 2018, 131, 47–57. [Google Scholar] [CrossRef]
- Kopaciewicz, W.; Rounds, M.A.; Fausnaugh, J.; Regnier, F.E. Retention Model for High-Performance Ion-Exchange Chromatography. J. Chromatogr. A 1983, 266, 3–21. [Google Scholar] [CrossRef]
- Wrzosek, K.; Polakovič, M. Effect of pH on Protein Adsorption Capacity of Strong Cation Exchangers with Grafted Layer. J. Chromatogr. A 2011, 1218, 6987–6994. [Google Scholar] [CrossRef] [PubMed]
- Lenhoff, A.M. Ion-Exchange Chromatography of Proteins: The inside Story. Mater. Today Proc. 2016, 3, 3559–3567. [Google Scholar] [CrossRef]
- Teepakorn, C.; Fiaty, K.; Charcosset, C. Effect of Geometry and Scale for Axial and Radial Flow Membrane Chromatography—Experimental Study of Bovin Serum Albumin Adsorption. J. Chromatogr. A 2015, 1403, 45–53. [Google Scholar] [CrossRef]
- Gao, D.; Lin, D.-Q.; Yao, S.-J. Mechanistic Analysis on the Effects of Salt Concentration and pH on Protein Adsorption onto a Mixed-Mode Adsorbent with Cation Ligand. J. Chromatogr. B 2007, 859, 16–23. [Google Scholar] [CrossRef]
- Haskard, C.A.; Li-Chan, E.C.Y. Hydrophobicity of Bovine Serum Albumin and Ovalbumin Determined Using Uncharged (PRODAN) and Anionic (ANS- ) Fluorescent Probes. J. Agric. Food Chem. 1998, 46, 2671–2677. [Google Scholar] [CrossRef]
- Jonas, A.; Weber, G. Presence of Arginine Residues at the Strong, Hydrophobic Anion Binding Sites of Bovine Serum Albumin. Biochemistry 1971, 10, 1335–1339. [Google Scholar] [CrossRef]
- Kosior, A.; Antošová, M.; Faber, R.; Villain, L.; Polakovič, M. Single-Component Adsorption of Proteins on a Cellulose Membrane with the Phenyl Ligand for Hydrophobic Interaction Chromatography. J. Membr. Sci. 2013, 442, 216–224. [Google Scholar] [CrossRef]
- Wang, J.Y.; Yu, A.Y.; Cho, B.G.; Mechref, Y. Assessing the Hydrophobicity of Glycopeptides Using Reversed-Phase Liquid Chromatography and Tandem Mass Spectrometry. J. Chromatogr. A 2023, 1706, 464237. [Google Scholar] [CrossRef] [PubMed]
- Fuqua, J.L.; Wanga, V.; Palmer, K.E. Improving the Large Scale Purification of the HIV Microbicide, Griffithsin. BMC Biotechnol. 2015, 15, 12. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, T.; Bhat, R.; Timasheff, S.N. Preferential Interactions Determine Protein Solubility in 3-Component Solutions: The MgCl2 System. Biochemistry 1990, 29, 1914–1923. [Google Scholar] [CrossRef] [PubMed]
- Creasy, A.; Lomino, J.; Barker, G.; Khetan, A.; Carta, G. Gradient Elution Behavior of Proteins in Hydrophobic Interaction Chromatography with U-Shaped Retention Factor Curves. J. Chromatogr. A 2018, 1547, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Creasy, A.; Lomino, J.; Carta, G. Gradient Elution Behavior of Proteins in Hydrophobic Interaction Chromatography with a U-Shaped Retention Factor Curve under Overloaded Conditions. J. Chromatogr. A 2018, 1578, 28–34. [Google Scholar] [CrossRef]
- Hunter, A.K.; Carta, G. Effects of Bovine Serum Albumin Heterogeneity on Frontal Analysis with Anion-Exchange Media. J. Chromatogr. A 2001, 937, 13–19. [Google Scholar] [CrossRef]
- Arakawa, T.; Ponce, S.; Young, G. Isoform Separation of Proteins by Mixed-Mode Chromatography. Protein Expr. Purif. 2015, 116, 144–151. [Google Scholar] [CrossRef]
- Jungbauer, A.; Machold, C.; Hahn, R. Hydrophobic Interaction Chromatography of Proteins. J. Chromatogr. A 2005, 1079, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Haimer, E.; Tscheliessnig, A.; Hahn, R.; Jungbauer, A. Hydrophobic Interaction Chromatography of Proteins IV. Kinetics of Protein Spreading. J. Chromatogr. A 2007, 1139, 84–94. [Google Scholar] [CrossRef]
- Ueberbacher, R.; Haimer, E.; Hahn, R.; Jungbauer, A. Hydrophobic Interaction Chromatography of Proteins: V. Quantitative Assessment of Conformational Changes. J. Chromatogr. A 2008, 1198–1199, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Sakiyama, T.; Imamura, K. On the Adsorption of Proteins on Solid Surfaces, a Common but Very Complicated Phenomenon. J. Biosci. Bioeng. 2001, 91, 233–244. [Google Scholar] [CrossRef]
- Roberts, J.A.; Carta, G. Protein Adsorption and Separation with Monomodal and Multimodal Anion Exchange Chromatography Resins. Part, I. Multicomponent Adsorption Properties and Frontal Chromatography. J. Chem. Technol. Biotechnol. 2022, 97, 3292–3305. [Google Scholar] [CrossRef]
- Roberts, J.A.; Carta, G. Protein Adsorption and Separation with Monomodal and Multimodal Anion Exchange Chromatography Resins. Part II. Mechanisms of Protein Aggregation on the Chromatographic Surface. J. Chem. Technol. Biotechnol. 2022, 98, 357–368. [Google Scholar] [CrossRef]
- Harvey, D.J.; Wing, D.R.; Küster, B.; Wilson, I.B.H. Composition of N-Linked Carbohydrates from Ovalbumin and Co-Purified Glycoproteins. J. Am. Soc. Mass Spectrom. 2000, 11, 564–571. [Google Scholar] [CrossRef]
- Nilsson, B.; Nordén, N.E.; Svensson, S. Structural Studies on the Carbohydrate Portion of Fetuin. J. Biol. Chem. 1979, 254, 4545–4553. [Google Scholar] [CrossRef] [PubMed]

| Eluent | Elution Conditions | Yield * [%] | |||
|---|---|---|---|---|---|
| No. | Buffer Type | pH | cNaCl [M] | BSA | Fetuin |
| 1 | Acetate | 5 | 2 | 15 | |
| 2 | Acetate | 6 | 2 | 90 | 80 |
| 3 | Bis–Tris | 6 | 2 | 98 | 78 |
| 4 | Bis–Tris | 6.5 | 2 | 99 | 82 |
| 5 | Bis–Tris | 7 | 2 | 96 | 82 |
| 6 | Tris–HCl | 8 | 2 | 100 | 85 |
| 7 | Tris–HCl | 9 | 2 | 96 | 88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krázel Adamíková, J.; Antošová, M.; Kurák, T.; Polakovič, M. Protein Adsorption on a Multimodal Cation Exchanger: Effect of pH, Salt Type and Concentration, and Elution Conditions. Molecules 2025, 30, 3389. https://doi.org/10.3390/molecules30163389
Krázel Adamíková J, Antošová M, Kurák T, Polakovič M. Protein Adsorption on a Multimodal Cation Exchanger: Effect of pH, Salt Type and Concentration, and Elution Conditions. Molecules. 2025; 30(16):3389. https://doi.org/10.3390/molecules30163389
Chicago/Turabian StyleKrázel Adamíková, Jana, Monika Antošová, Tomáš Kurák, and Milan Polakovič. 2025. "Protein Adsorption on a Multimodal Cation Exchanger: Effect of pH, Salt Type and Concentration, and Elution Conditions" Molecules 30, no. 16: 3389. https://doi.org/10.3390/molecules30163389
APA StyleKrázel Adamíková, J., Antošová, M., Kurák, T., & Polakovič, M. (2025). Protein Adsorption on a Multimodal Cation Exchanger: Effect of pH, Salt Type and Concentration, and Elution Conditions. Molecules, 30(16), 3389. https://doi.org/10.3390/molecules30163389









