Preparation and Characterization of Highland Barley Distillers’ Grains Gliadin–Chitosan Nanoparticles and Composite Properties
Abstract
1. Introduction
2. Results and Discussion
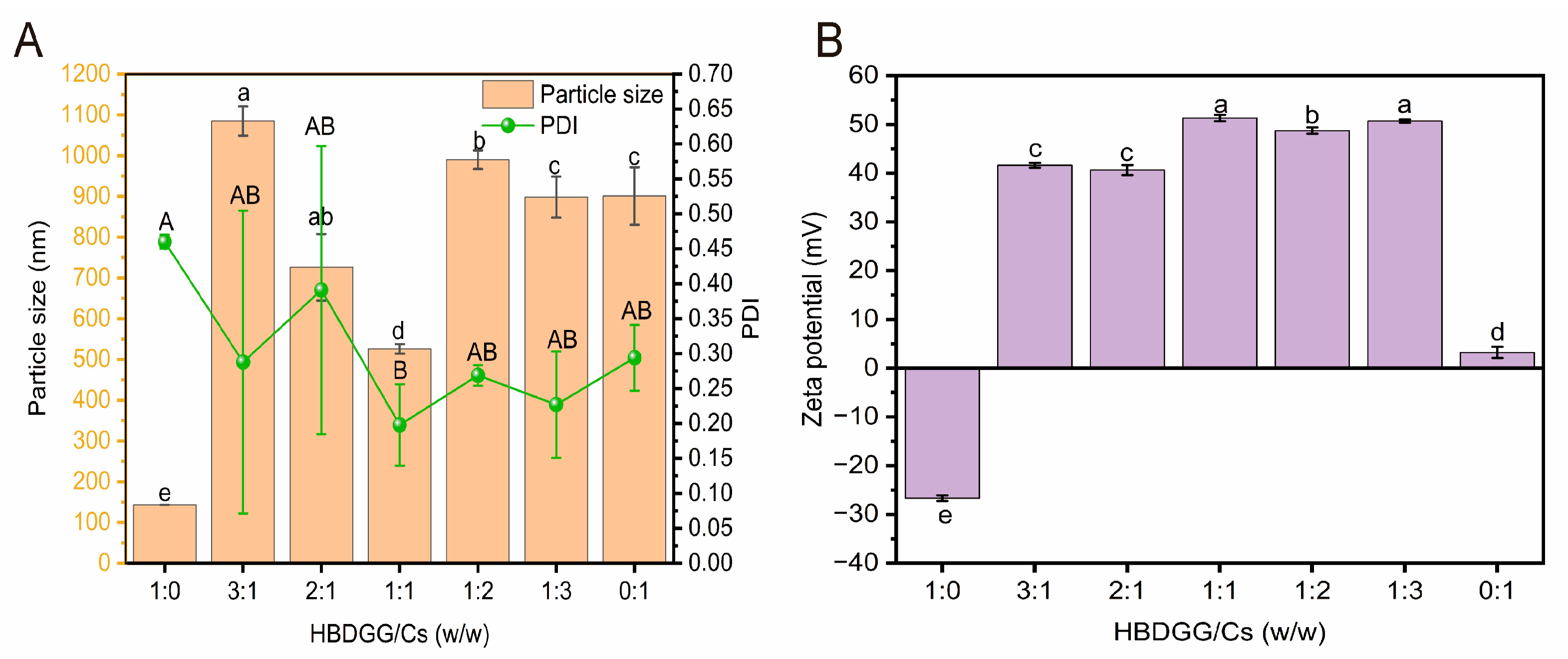
2.1. Effect of HBDGG/Cs Ratios on Properties of HBDGG-Cs Composite Nanoparticles
2.2. Structural Properties
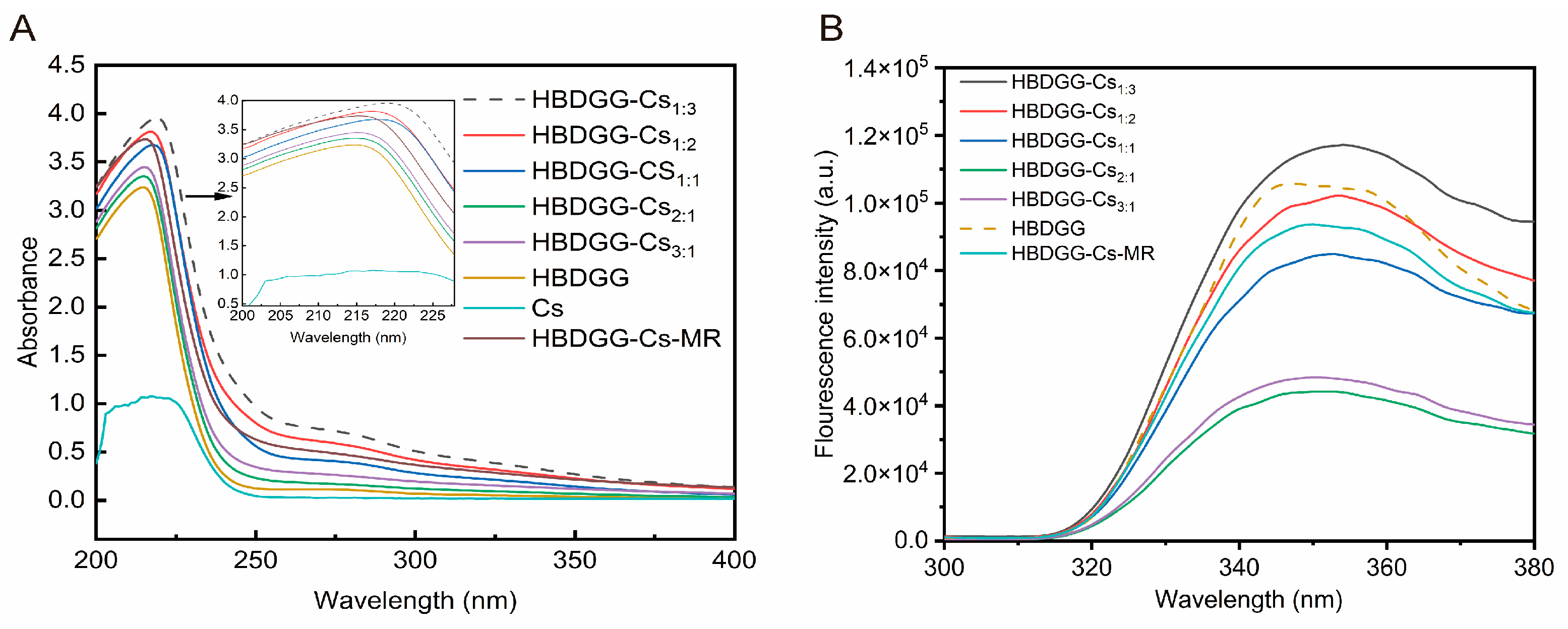
2.2.1. Ultraviolet–Visible Spectrum
2.2.2. Fluorescence Spectroscopy
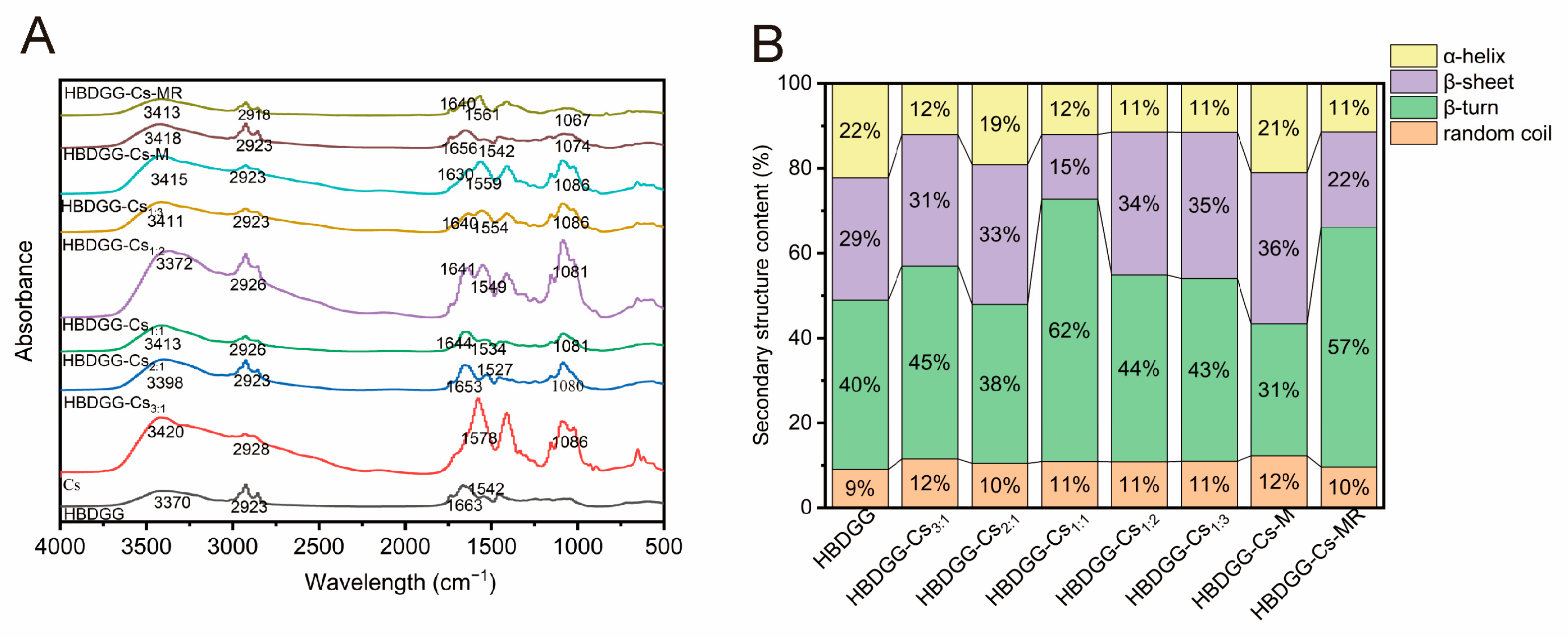
2.2.3. Infrared Absorption Intensity
2.2.4. Protein Secondary Structure Content
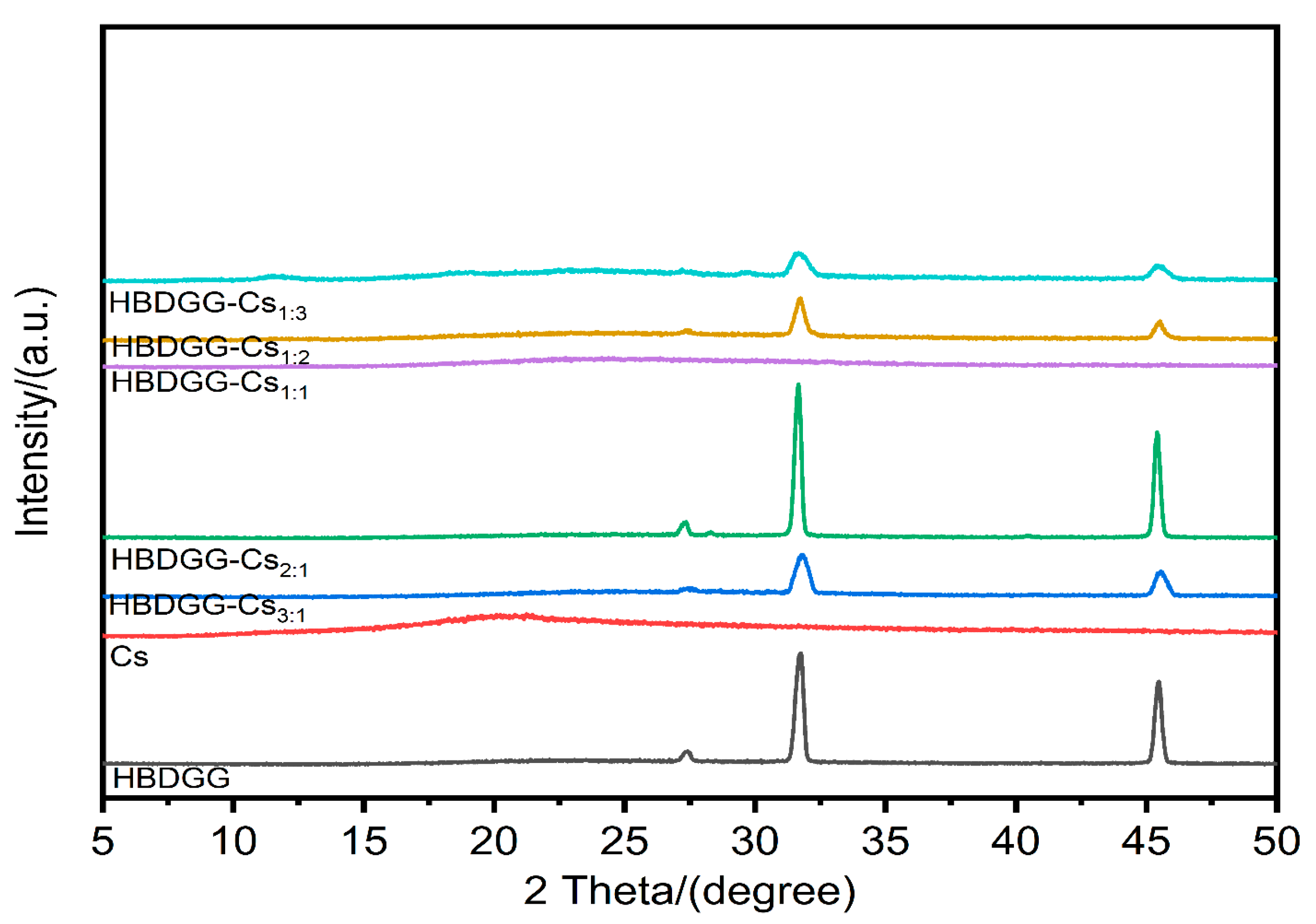
2.2.5. Analysis of X-Ray Diffraction (XRD)
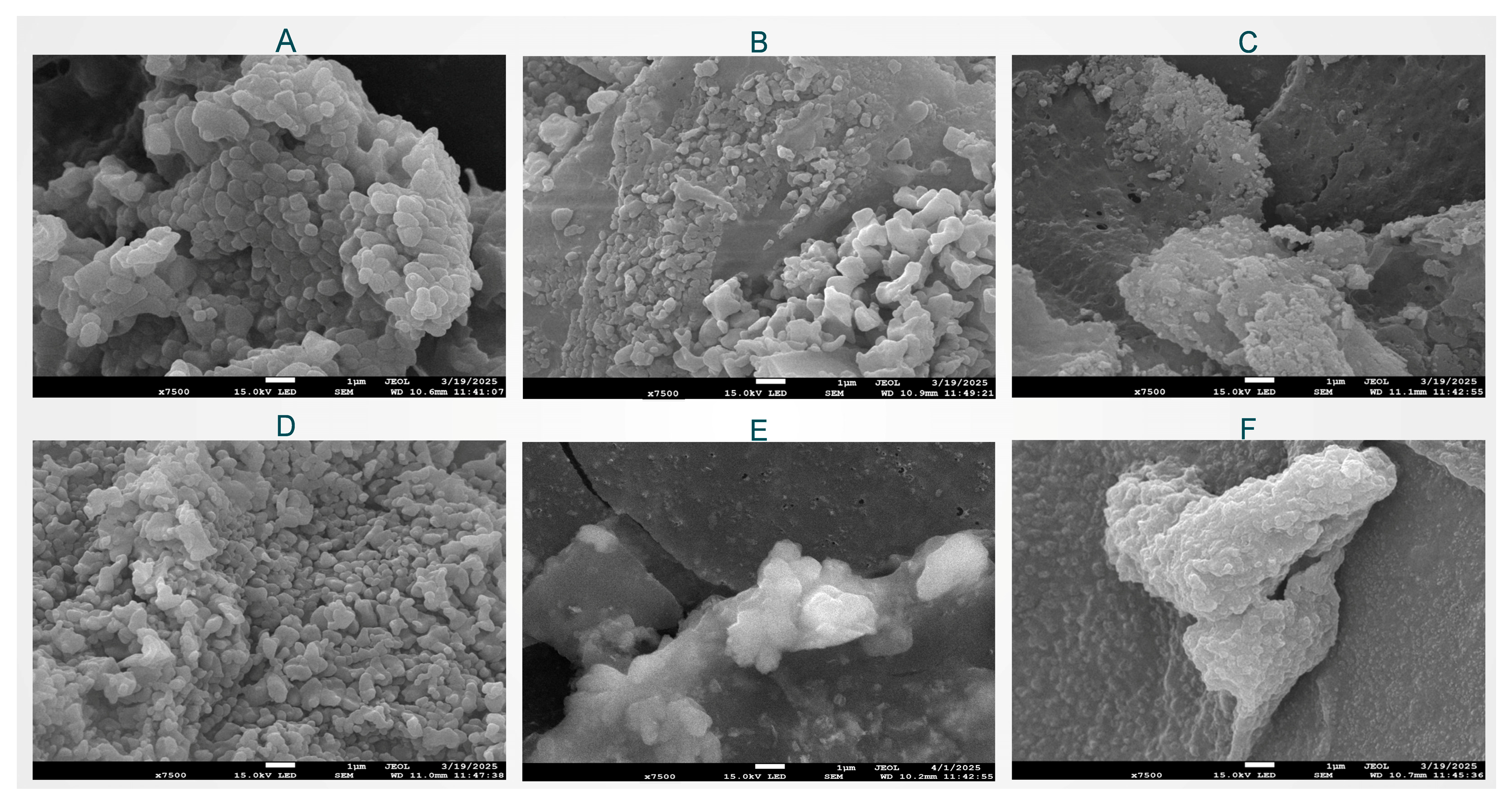
2.2.6. Microstructure Analysis
2.3. Stability Analysis of HBDGG-Cs Nanoparticles
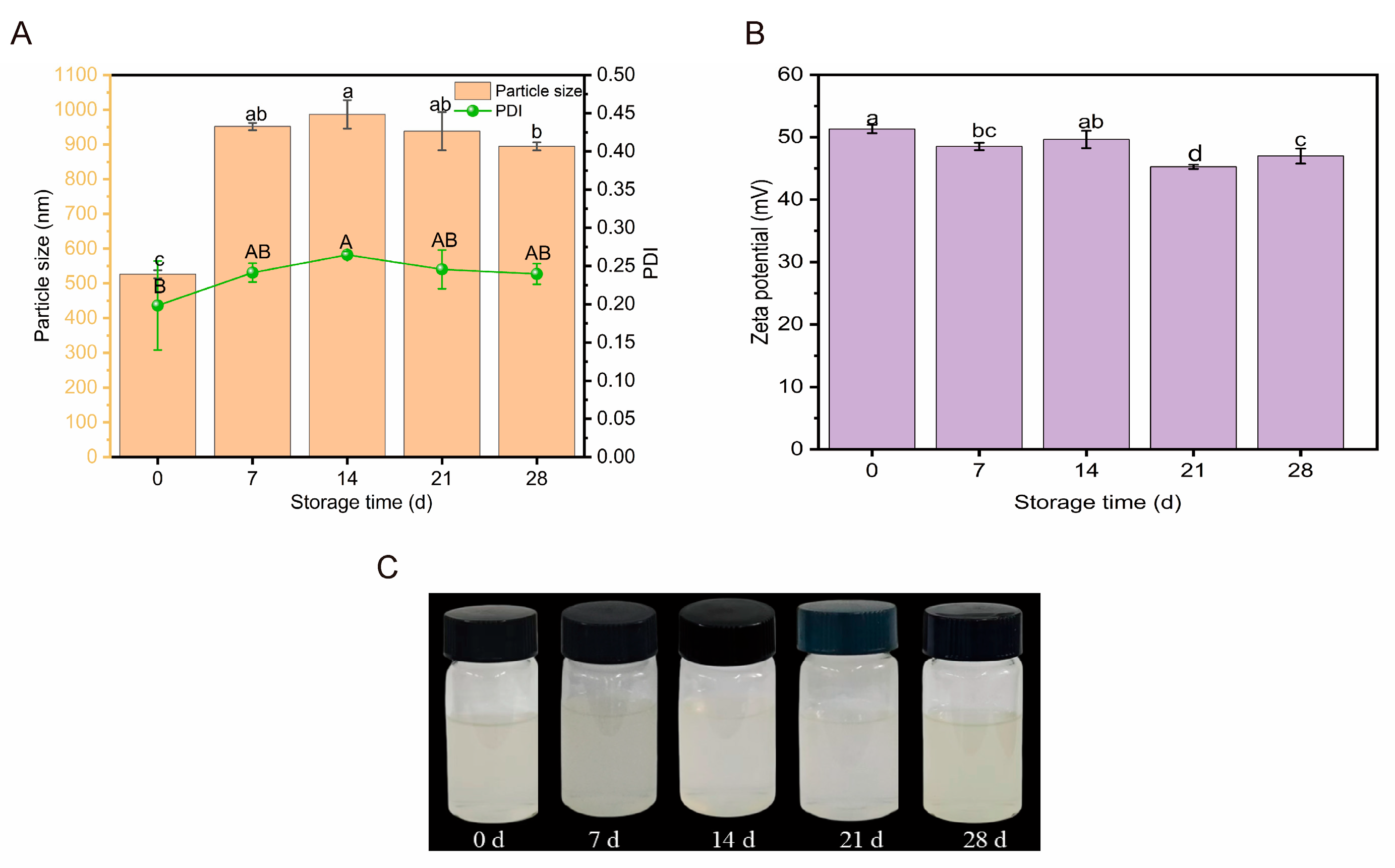
2.3.1. Storage Stability
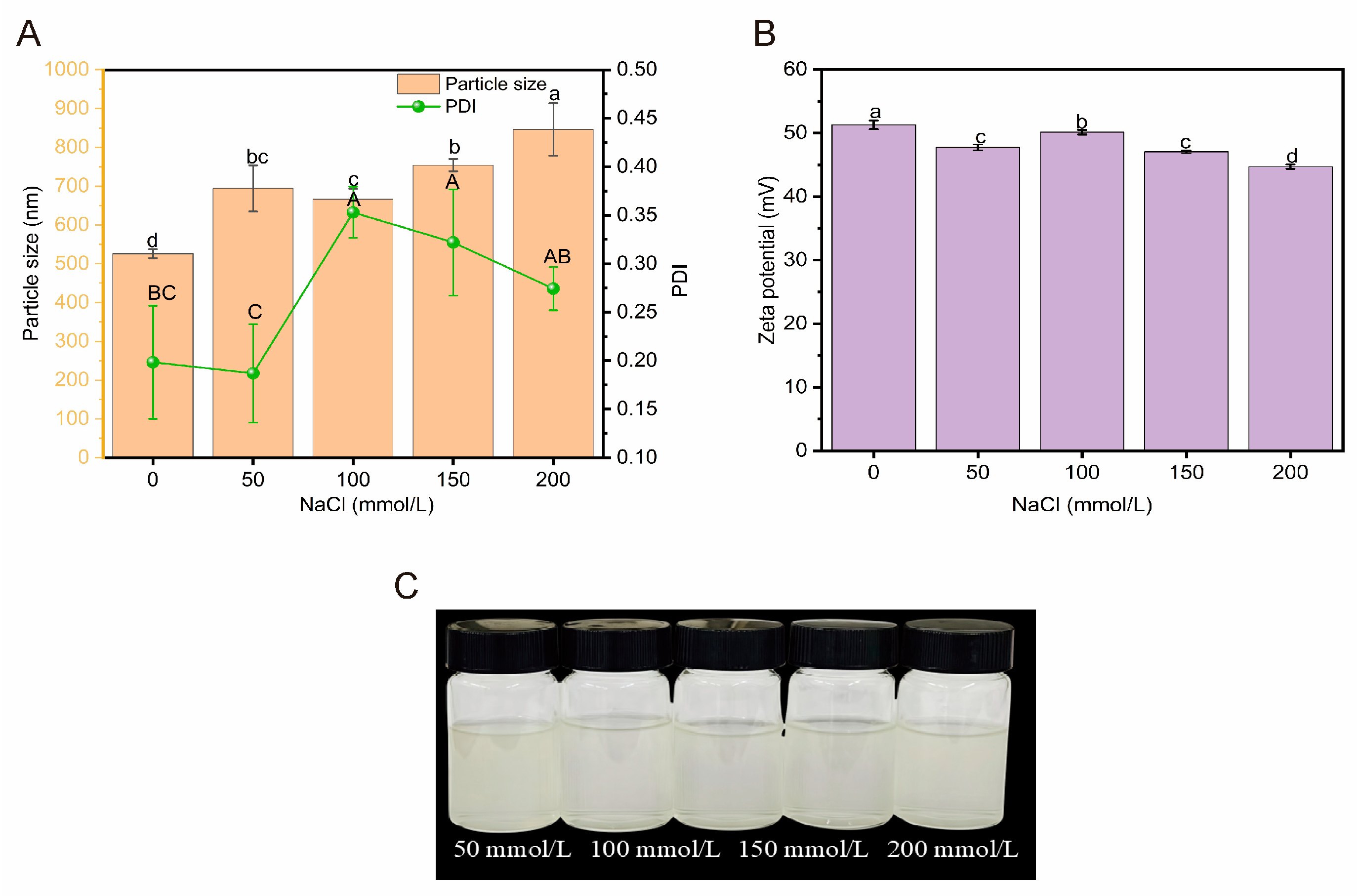
2.3.2. Ionic Stability
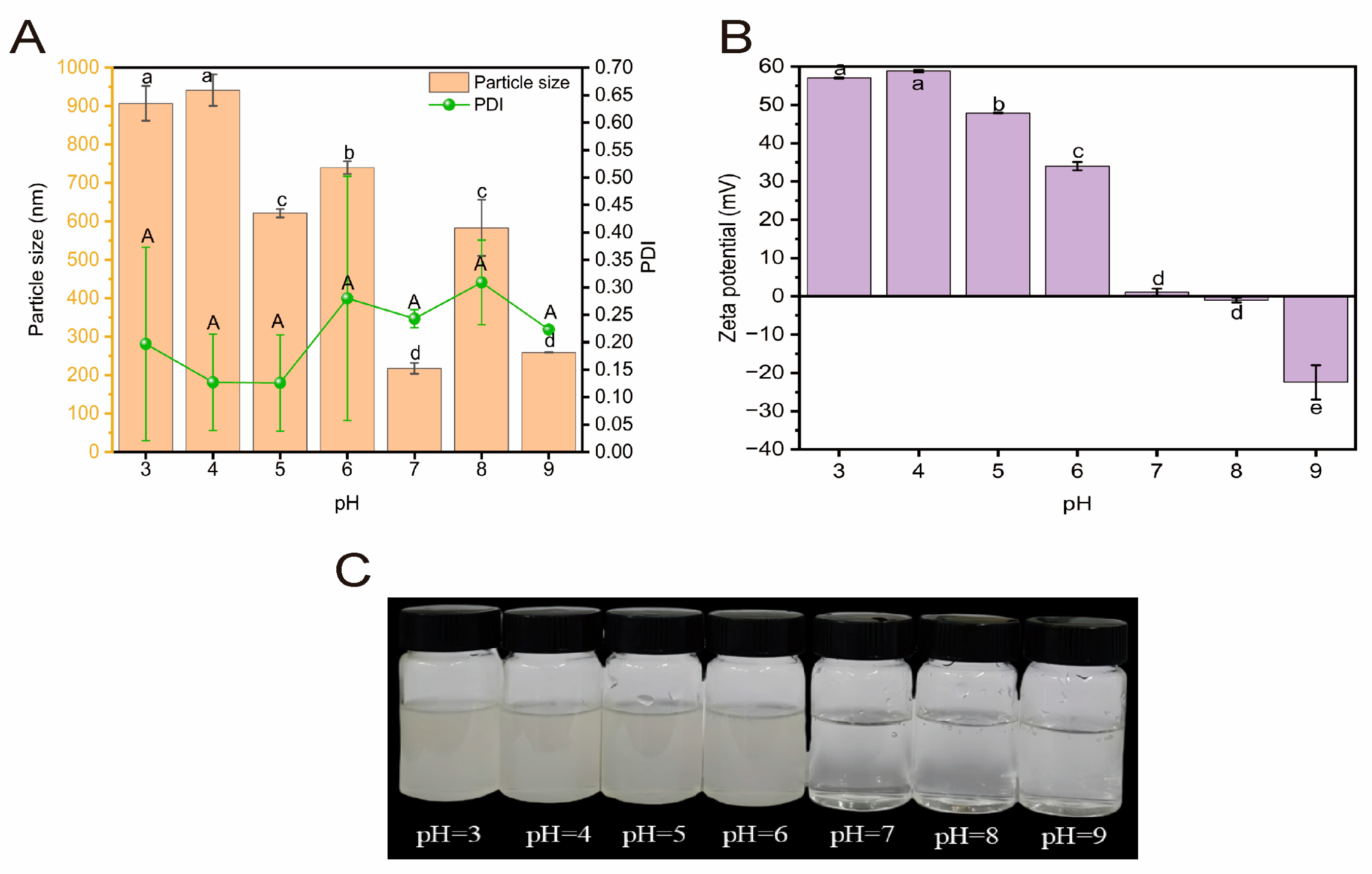
2.3.3. Acid-Base Stability
3. Materials and Methods
3.1. Materials
3.2. Extraction of HBDGG
3.3. Preparation of HBDGG-Cs Nanoparticles
3.4. Preparation of the HBDGG-Cs Conjugate (HBDGG-Cs-MR)
3.5. Characterization of HBDGG, Cs, and HBDGG-Cs Nanocomposite Particles
3.5.1. Particle Size, PDI, and Zeta Potential
3.5.2. UV-Absorbed Intensity
3.5.3. Endogenous Fluorescence Spectroscopy
3.5.4. Fourier Transform Infrared (FT-IR) Spectroscopy
3.5.5. Analysis of the Secondary Structure
3.5.6. X-Ray Diffraction (XRD)
3.5.7. Microstructure
3.6. Stability Analysis of HBDGG-Cs Complexes
3.6.1. Analysis of Storage Stability
3.6.2. Analysis of Ionic Stability
3.6.3. Analysis of Acid-Base Stability
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xia, T.L. Chitosan-Highland Barley Gliadin Interaction Mechanism and Application in Pickering Emulsion. Ph.D. Thesis, Jiangnan University, Wuxi, China, 2024. [Google Scholar]
- Rico, D.; Peñas, E.; García, M.d.C.; Martínez-Villaluenga, C.; Rai, D.K.; Birsan, R.I.; Frias, J.; Martín-Diana, A.B. Sprouted Barley Flour as a Nutritious and Functional Ingredient. Foods 2020, 9, 296. [Google Scholar] [CrossRef]
- Liu, X.H. Analysis of quality characteristics and processing utilization status of highland barley. Agric. Mach. 2013, 14, 49–53. [Google Scholar]
- Wang, J.J.; Wang, Y.; Wang, Q.; Yang, J.; Hu, S.-Q.; Chen, L. Mechanically Strong and Highly Tough Prolamin Protein Hydrogels Designed from Double-Cross-Linked Assembled Networks. ACS Appl. Polym. Mater. 2019, 1, 1272–1279. [Google Scholar] [CrossRef]
- Zeng, X.; Guo, Y.; Xu, Q.; Mascher, M.; Guo, G.; Li, S.; Mao, L.; Liu, Q.; Xia, Z.; Zhou, J.; et al. Origin and evolution of qingke barley in Tibet. Nat. Commun. 2018, 9, 5433. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.M. Extraction technology of protein from highland barley distiller’s grains. Hubei Agric. Sci. 2022, 61, 169–172. [Google Scholar]
- Huang, D.D. Extraction of Distilled Spirit Spent Grain Prolamin and Preliminary Investigation of Curcumin-Loaded Nanoparticles. Ph.D. Thesis, Guizhou University, Guiyang, China, 2024. [Google Scholar]
- Wouters, A.G.; A Delcour, J. Cereal protein-based nanoparticles as agents stabilizing air–water and oil–water interfaces in food systems. Curr. Opin. Food Sci. 2019, 25, 19–27. [Google Scholar] [CrossRef]
- Zhong, Q.; Jin, M. Zein nanoparticles produced by liquid–liquid dispersion. Food Hydrocoll. 2009, 23, 2380–2387. [Google Scholar] [CrossRef]
- Chang, C.; Wang, T.; Hu, Q.; Luo, Y. Zein/caseinate/pectin complex nanoparticles: Formation; characterization. Int. J. Biol. Macromol. 2017, 104, 117–124. [Google Scholar] [CrossRef]
- Irache, J.M.; González-Navarro, C.J. Zein Nanoparticles as Vehicles for Oral Delivery Purposes. Nanomedicine 2017, 12, 1209–1211. [Google Scholar] [CrossRef]
- Han, Q.Y.; Shuai, Y.; Gan, Z.L.; Zhang, G.Q.; Tian, Z.; Bai, T.H.; Sun, A.D. Preparation and Characterization of Zein-Peach Gum Polysaccharide Nanoparticles and Composite Properties. Sci. Technol. Food Ind. 2025, 1–17. [Google Scholar] [CrossRef]
- Harugade, A.; Sherje, A.P.; Pethe, A. Chitosan: A review on properties, biological activities and recent progress in biomedical applications. React. Funct. Polym. 2023, 191, 105634. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, Z.; Li, J.; Li, S.; Kong, L.; Li, P.; Wang, Q. Development and evaluation of novel flavour microcapsules containing vanilla oil using complex coacervation approach. Food Chem. 2014, 145, 272–277. [Google Scholar] [CrossRef]
- Hu, Q.; Luo, Y. Chitosan-based nanocarriers for encapsulation and delivery of curcumin: A review. Int. J. Biol. Macromol. 2021, 179, 125–135. [Google Scholar] [CrossRef]
- Sharkawy, A.; Silva, A.M.; Rodrigues, F.; Barreiro, F.; Rodrigues, A. Pickering emulsions stabilized with chitosan/collagen peptides nanoparticles as green topical delivery vehicles for cannabidiol (CBD). Colloids Surfaces A Physicochem. Eng. Asp. 2021, 631, 127677. [Google Scholar] [CrossRef]
- Nitta, S.K.; Numata, K. Biopolymer-Based Nanoparticles for Drug/Gene Delivery and Tissue Engineering. Int. J. Mol. Sci. 2013, 14, 1629–1654. [Google Scholar] [CrossRef]
- Gentile, L. Protein–polysaccharide interactions and aggregates in food formulations. Curr. Opin. Colloid Interface Sci. 2020, 48, 18–27. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, J.; Chen, J.; Wang, Z.; Wang, X.; Zhong, J. Protein nanoparticles for Pickering emulsions: A comprehensive review on their shapes, preparation methods, and modification methods. Trends Food Sci. Technol. 2021, 113, 26–41. [Google Scholar] [CrossRef]
- Baars, R.J.; van Leeuwen, Y.M.; Hendrix, Y.; Velikov, K.P.; Kegel, W.K.; Philipse, A.P. Morphology-controlled functional colloids by heterocoagulation of zein and nanoparticles. Colloids Surfaces A Physicochem. Eng. Asp. 2015, 483, 209–215. [Google Scholar] [CrossRef]
- Chen, S.; Han, Y.; Jian, L.; Liao, W.; Zhang, Y.; Gao, Y. Fabrication, characterization, physicochemical stability of zein-chitosan nanocomplex for co-encapsulating curcumin and resveratrol. Carbohydr. Polym. 2020, 236, 116090. [Google Scholar] [CrossRef]
- Chen, G.; Dong, S.; Chen, Y.; Gao, Y.; Zhang, Z.; Li, S.; Chen, Y. Complex coacervation of zein-chitosan via atmospheric cold plasma treatment: Improvement of encapsulation efficiency and dispersion stability. Food Hydrocoll. 2020, 107, 105943. [Google Scholar] [CrossRef]
- Sha, L.; Raza, H.; Jia, C.; Khan, I.M.; Yang, H.; Chen, G. Genipin-enriched chitosan-Zein nanoparticles for improved curcumin encapsulation. Int. J. Biol. Macromol. 2025, 288, 138555. [Google Scholar] [CrossRef]
- Pauluk, D.; Padilha, A.K.; Khalil, N.M.; Mainardes, R.M. Chitosan-coated zein nanoparticles for oral delivery of resveratrol: Formation, characterization, stability, mucoadhesive properties and antioxidant activity. Food Hydrocoll. 2019, 94, 411–417. [Google Scholar] [CrossRef]
- Li, M.-F.; Chen, L.; Xu, M.-Z.; Zhang, J.-L.; Wang, Q.; Zeng, Q.-Z.; Wei, X.-C.; Yuan, Y. The formation of zein-chitosan complex coacervated particles: Relationship to encapsulation and controlled release properties. Int. J. Biol. Macromol. 2018, 116, 1232–1239. [Google Scholar] [CrossRef]
- Song, J.; Sun, C.; Gul, K.; Mata, A.; Fang, Y. Prolamin-based complexes: Structure design and food-related applications. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1120–1149. [Google Scholar] [CrossRef]
- Hu, K.; McClements, D.J. Fabrication of biopolymer nanoparticles by antisolvent precipitation and electrostatic deposition: Zein-alginate core/shell nanoparticles. Food Hydrocoll. 2015, 44, 101–108. [Google Scholar] [CrossRef]
- Li, H.; Yuan, Y.; Zhu, J.; Wang, T.; Wang, D.; Xu, Y. Zein/soluble soybean polysaccharide composite nanoparticles for encapsulation and oral delivery of lutein. Food Hydrocoll. 2020, 103, 105715. [Google Scholar] [CrossRef]
- Liu, Q.; Qin, Y.; Jiang, B.; Chen, J.; Zhang, T. Development of self-assembled zein-fucoidan complex nanoparticles as a delivery system for resveratrol. Colloids Surfaces B Biointerfaces 2022, 216, 112529. [Google Scholar] [CrossRef] [PubMed]
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems—A review (Part 1). Trop. J. Pharm. Res. 2013, 12, 255–264. [Google Scholar]
- Sun, C.X. Research progress on preparation methods, structural characterization, and functional properties of corn soluble protein polysaccharide nanocomposites. Food Sci. 2020, 41, 323–331. [Google Scholar]
- Wang, L.; Zhang, H.; Li, H.; Zhang, H.; Chi, Y.; Xia, N.; Li, Z.; Jiang, L.; Zhang, X.; Rayan, A.M. Fabrication and digestive characteristics of high internal phase Pickering emulsions stabilized by ovalbumin-pectin complexes for improving the stability and bioaccessibility of curcumin. Food Chem. 2022, 389, 133055. [Google Scholar] [CrossRef]
- Sun, C.; Dai, L.; Liu, F.; Gao, Y. Simultaneous treatment of heat and high pressure homogenization of zein in ethanol–water solution: Physical, structural, thermal and morphological characteristics. Innov. Food Sci. Emerg. Technol. 2016, 34, 161–170. [Google Scholar] [CrossRef]
- Antosiewicz, J.M.; Shugar, D. UV–Vis spectroscopy of tyrosine side-groups in studies of protein structure. Part 1: Basic principles and properties of tyrosine chromophore. Biophys. Rev. 2016, 8, 151–161. [Google Scholar] [CrossRef]
- Huangfu, Y.P.; Bao, Y.H.; Zhao, X.L.; Luo, J.Y.; Jiang, Z.H. Functional properties and structural characterization of soybean protein isolate-tannic acid-polysaccharide ternary conjugates. J. Food Sci. Technol. 2022, 40, 37–51. [Google Scholar]
- Ma, Y.; Qiao, Z.; Zeng, Q.; Yang, P.; Li, L.; Chen, L.; Feng, X. Effects of different thermal sterilization conditions on structural and functional properties of whey and casein proteins in goat milk. Food Sci. 2024, 45, 67–76. [Google Scholar]
- He, W.; Tian, L.; Zhang, S.; Pan, S. A novel method to prepare protein-polysaccharide conjugates with high grafting and low browning: Application in encapsulating curcumin. LWT 2021, 145, 111349. [Google Scholar] [CrossRef]
- Zhou, X.; Guo, N.; Zhang, F.; Zhuo, K.; Zhu, G. Improving stability and bioavailability of ACNs based on Gellan gum-whey protein isolate nanocomplexes. Food Chem. X 2024, 24, 102050. [Google Scholar] [CrossRef]
- Chen, S.R. Functional Properties and Application of Xylo-Oligosaccharide Modified Casein. Ph.D. Thesis, Jilin University, Changchun, China, 2022. [Google Scholar]
- Xue, F.; Li, C.; Zhu, X.; Wang, L.; Pan, S. Comparative studies on the physicochemical properties of soy protein isolate-maltodextrin and soy protein isolate-gum acacia conjugate prepared through Maillard reaction. Food Res. Int. 2013, 51, 490–495. [Google Scholar] [CrossRef]
- Nooshkam, M.; Madadlou, A. Microwave-assisted isomerisation of lactose to lactulose and Maillard conjugation of lactulose and lactose with whey proteins and peptides. Food Chem. 2016, 200, 1–9. [Google Scholar] [CrossRef]
- Özbaş, F.; Tüzün, E.; Yıldız, A.; Karakuş, S. Sonosynthesis and characterization of konjac gum/xanthan gum supported iron oxide nanoparticles. Int. J. Biol. Macromol. 2021, 183, 1047–1057. [Google Scholar] [CrossRef]
- Li, J.; Xu, X.; Chen, Z.; Wang, T.; Wang, L.; Zhong, Q. Biological macromolecule delivery system fabricated using zein and gum arabic to control the release rate of encapsulated tocopherol during in vitro digestion. Food Res. Int. 2018, 114, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Qiu, X.; Hou, X.; Li, M.; Zhou, M.; Liu, X.; Chen, A.; Zhang, Z. Development of Zanthoxylum bungeanum essential oil Pickering emulsions using potato protein-chitosan nanoparticles and its application in mandarin preservation. Int. J. Biol. Macromol. 2024, 277, 134100. [Google Scholar] [CrossRef]
- Chen, S.; Li, Q.; McClements, D.J.; Han, Y.; Dai, L.; Mao, L.; Gao, Y. Co-delivery of curcumin and piperine in zein-carrageenan core-shell nanoparticles: Formation, structure, stability and in vitro gastrointestinal digestion. Food Hydrocoll. 2020, 99, 105334. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, S.; Jiang, X.; Hou, P.; Xin, Y.; Zhang, L.; Zhang, J.; Zhou, D. Impact of weakly charged insoluble karaya gum on zein nanoparticle and mechanism for stabilizing Pickering emulsions. Int. J. Biol. Macromol. 2022, 222, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wang, J.; Zhao, P.; McClements, D.J.; Liu, X.; Liu, F. Effect of ultrasound-assisted Maillard reaction on glycosylation of goat whey protein: Structure and functional properties. Food Chem. 2024, 441, 138292. [Google Scholar] [CrossRef]
- Que, F.; Xia, Y.T.; Gao, T.Q.; Wang, C.; Wang, L.; Xiong, G.Q.; Shi, L.; Ding, A.Z.; Wu, W.J. Preparation and structural characterization of β-dextranase hydrolytic products of konjac glucomannan. Food Sci. 2023, 44, 9–15. [Google Scholar]
- Shi, Y.; Li, R.-Y.; Tu, Z.-C.; Ma, D.; Wang, H.; Huang, X.-Q.; He, N. Effect of γ-irradiation on the physicochemical properties and structure of fish myofibrillar proteins. Radiat. Phys. Chem. 2015, 109, 70–72. [Google Scholar] [CrossRef]
- Liu, L.; Li, X.; Du, L.; Zhang, X.; Yang, W.; Zhang, H. Effect of ultrasound assisted heating on structure and antioxidant activity of whey protein peptide grafted with galactose. LWT 2019, 109, 130–136. [Google Scholar] [CrossRef]
- Ye, Z.; Gan, S.; Wang, J.; Yang, F.; Dong, G. Conjugation of flaxseed protein and plant polysaccharides: Process optimization, structural characterization and technical-function evaluation. Food Hydrocoll. 2025, 166, 111371. [Google Scholar] [CrossRef]
- Zhao, L.; Gao, H.; Xiao, Y.; Sun, L.; Li, S.; Zhu, Y.; Lee, J.-H.; Zhao, G.; Wang, Y.; Wang, Y.; et al. Preparation and characteristics of tea water-insoluble protein/chitosan composite particles. LWT 2024, 212, 117009. [Google Scholar] [CrossRef]
- Xiong, W.; Ren, C.; Tian, M.; Yang, X.; Li, J.; Li, B. Emulsion stability and dilatational viscoelasticity of ovalbumin/chitosan complexes at the oil-in-water interface. Food Chem. 2018, 252, 181–188. [Google Scholar] [CrossRef]
- Wang, W.-Q.; Bao, Y.-H.; Chen, Y. Characteristics and antioxidant activity of water-soluble Maillard reaction products from interactions in a whey protein isolate and sugars system. Food Chem. 2013, 139, 355–361. [Google Scholar] [CrossRef]
- Ke, C.; Li, L. Influence mechanism of polysaccharides induced Maillard reaction on plant proteins structure and functional properties: A review. Carbohydr. Polym. 2023, 302, 120430. [Google Scholar] [CrossRef]
- Ren, X.; Hou, T.; Liang, Q.; Zhang, X.; Hu, D.; Xu, B.; Chen, X.; Chalamaiah, M.; Ma, H. Effects of frequency ultrasound on the properties of zein-chitosan complex coacervation for resveratrol encapsulation. Food Chem. 2019, 279, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Zhou, B.; He, L.; An, Y.; Lin, L.; Li, Y.; Liu, S.; Chen, Y.; Li, B. Fabrication of zein/quaternized chitosan nanoparticles for the encapsulation and protection of curcumin. RSC Adv. 2015, 5, 13891–13900. [Google Scholar] [CrossRef]
- Hu, K.; McClements, D.J. Fabrication of surfactant-stabilized zein nanoparticles: A pH modulated antisolvent precipitation method. Food Res. Int. 2014, 64, 329–335. [Google Scholar] [CrossRef]
- Melander, W.; Horváth, C. Salt effects on hydrophobic interactions in precipitation and chromatography of proteins: An interpretation of the lyotropic series. Arch. Biochem. Biophys. 1977, 183, 200–215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Fu, Y.; Xu, Y.; Niu, F.; Li, Z.; Ba, C.; Jin, B.; Chen, G.; Li, X. One-step assembly of zein/caseinate/alginate nanoparticles for encapsulation and improved bioaccessibility of propolis. Food Funct. 2019, 10, 635–645. [Google Scholar] [CrossRef]
- Zhang, Y.Q. Preparation, Characterization of Zein-Based Composite Particles with High Physical Stability and Its Application in Yogurt. Ph.D. Thesis, Gansu Agricultural University, Lanzhou, China, 2021. [Google Scholar]
- Guo, L. Preparation and properties of zein from corn gluten meal. Food Sci. Technol. 2021, 46, 234–241. [Google Scholar]
- Yang, S.; Zhang, G.; Chu, H.; Du, P.; Li, A.; Liu, L.; Li, C. Changes in the functional properties of casein conjugates prepared by Maillard reaction with pectin or arabinogalactan. Food Res. Int. 2023, 165, 112510. [Google Scholar] [CrossRef]
- Dai, L.; Wei, Y.; Sun, C.; Mao, L.; McClements, D.J.; Gao, Y. Development of protein-polysaccharide-surfactant ternary complex particles as delivery vehicles for curcumin. Food Hydrocoll. 2018, 85, 75–85. [Google Scholar] [CrossRef]
- Feng, S.; Sun, Y.; Wang, D.; Sun, P.; Shao, P. Effect of adjusting pH and chondroitin sulfate on the formation of curcumin-zein nanoparticles: Synthesis, characterization and morphology. Carbohydr. Polym. 2020, 250, 116970. [Google Scholar] [CrossRef]
- Dong, Z.; Du, Z.; Wu, X.; Zhai, K.; Wei, Z.; Rashed, M.M. Fabrication and characterization of ZnO nanofilms using extracted pectin of Premna microphylla Turcz leaves and carboxymethyl cellulose. Int. J. Biol. Macromol. 2022, 209, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Hasankhan, S.; Tabibiazar, M.; Hosseini, S.M.; Ehsani, A.; Ghorbani, M. Fabrication of curcumin-zein-ethyl cellulose composite nanoparticles using antisolvent co-precipitation method. Int. J. Biol. Macromol. 2020, 163, 1538–1545. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Wu, Y.; Zhang, L.; Li, Y.; Liu, S.; Wang, H.; Li, C. Enhanced chemical stability; intestinal absorption, and intracellular antioxidant activity of cyanidin-3-O-glucoside by composite nanogel encapsulation. J. Agric. Food Chem. 2019, 67, 10432–10447. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, Q.; Zhang, Y. Preparation and Characterization of Highland Barley Distillers’ Grains Gliadin–Chitosan Nanoparticles and Composite Properties. Molecules 2025, 30, 3390. https://doi.org/10.3390/molecules30163390
Lv Q, Zhang Y. Preparation and Characterization of Highland Barley Distillers’ Grains Gliadin–Chitosan Nanoparticles and Composite Properties. Molecules. 2025; 30(16):3390. https://doi.org/10.3390/molecules30163390
Chicago/Turabian StyleLv, Qian, and Yiquan Zhang. 2025. "Preparation and Characterization of Highland Barley Distillers’ Grains Gliadin–Chitosan Nanoparticles and Composite Properties" Molecules 30, no. 16: 3390. https://doi.org/10.3390/molecules30163390
APA StyleLv, Q., & Zhang, Y. (2025). Preparation and Characterization of Highland Barley Distillers’ Grains Gliadin–Chitosan Nanoparticles and Composite Properties. Molecules, 30(16), 3390. https://doi.org/10.3390/molecules30163390





