Microwave-Enhanced Catalytic Performance of Benzene Oxidation on MOF-Derived Mn/Ce-Co Oxides
Abstract
1. Introduction
2. Results and Discussion
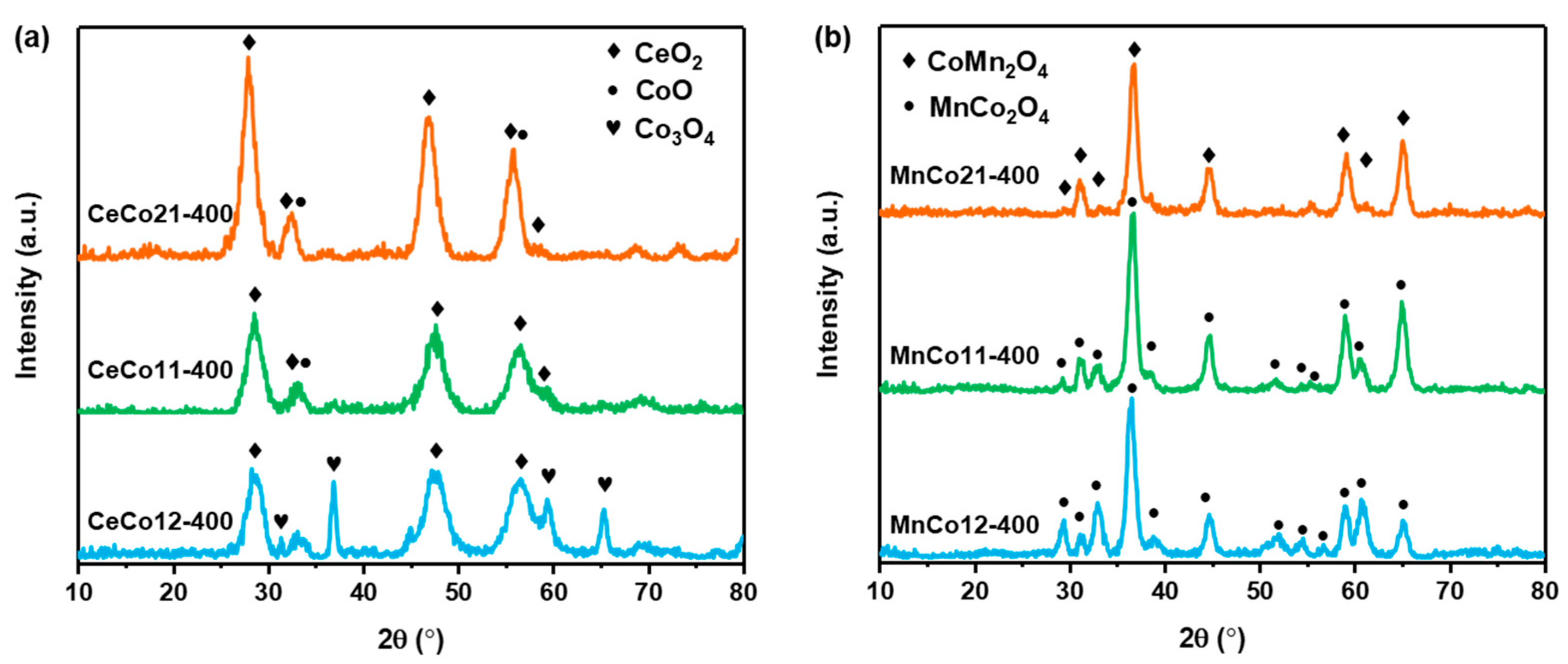
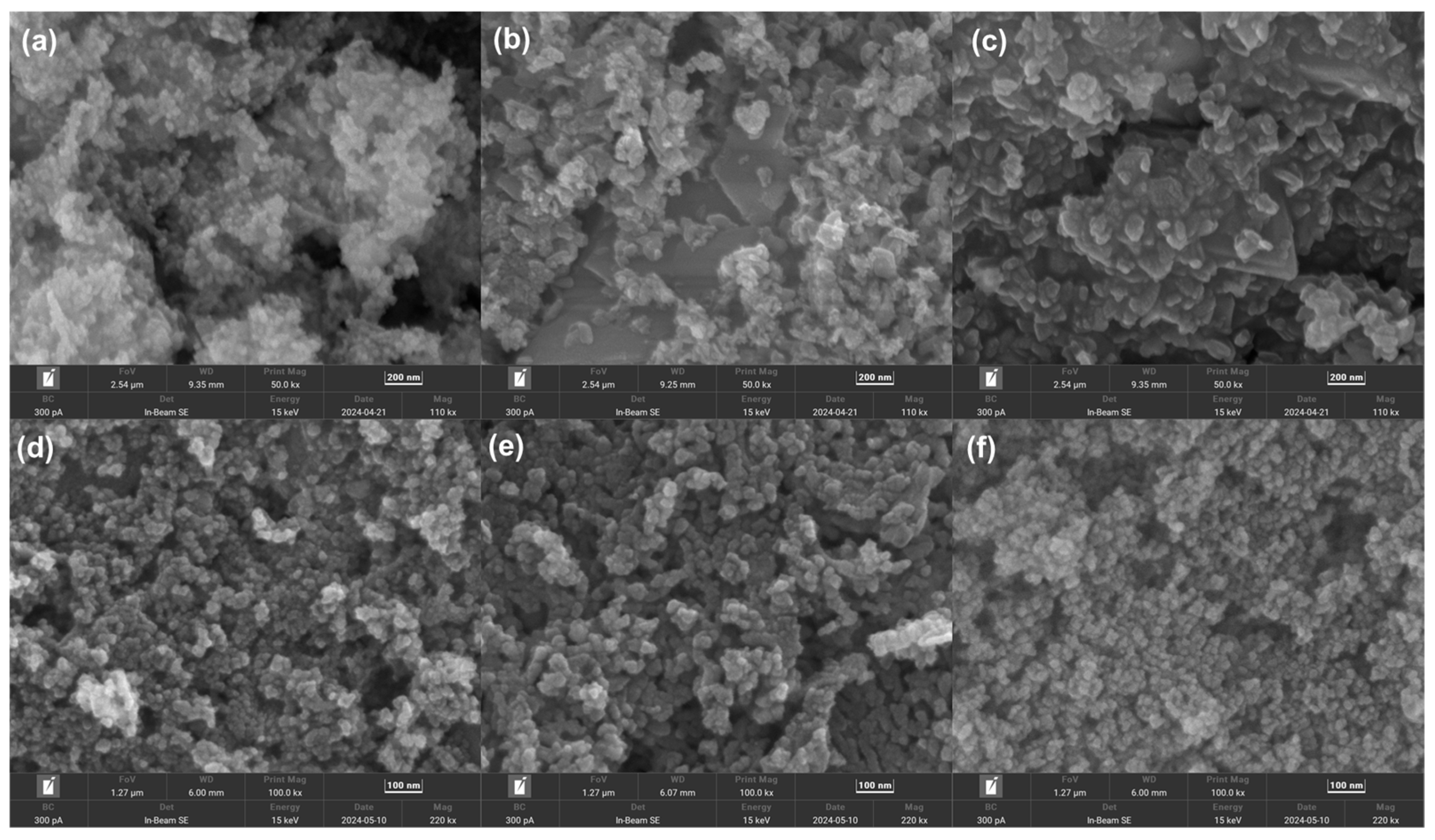
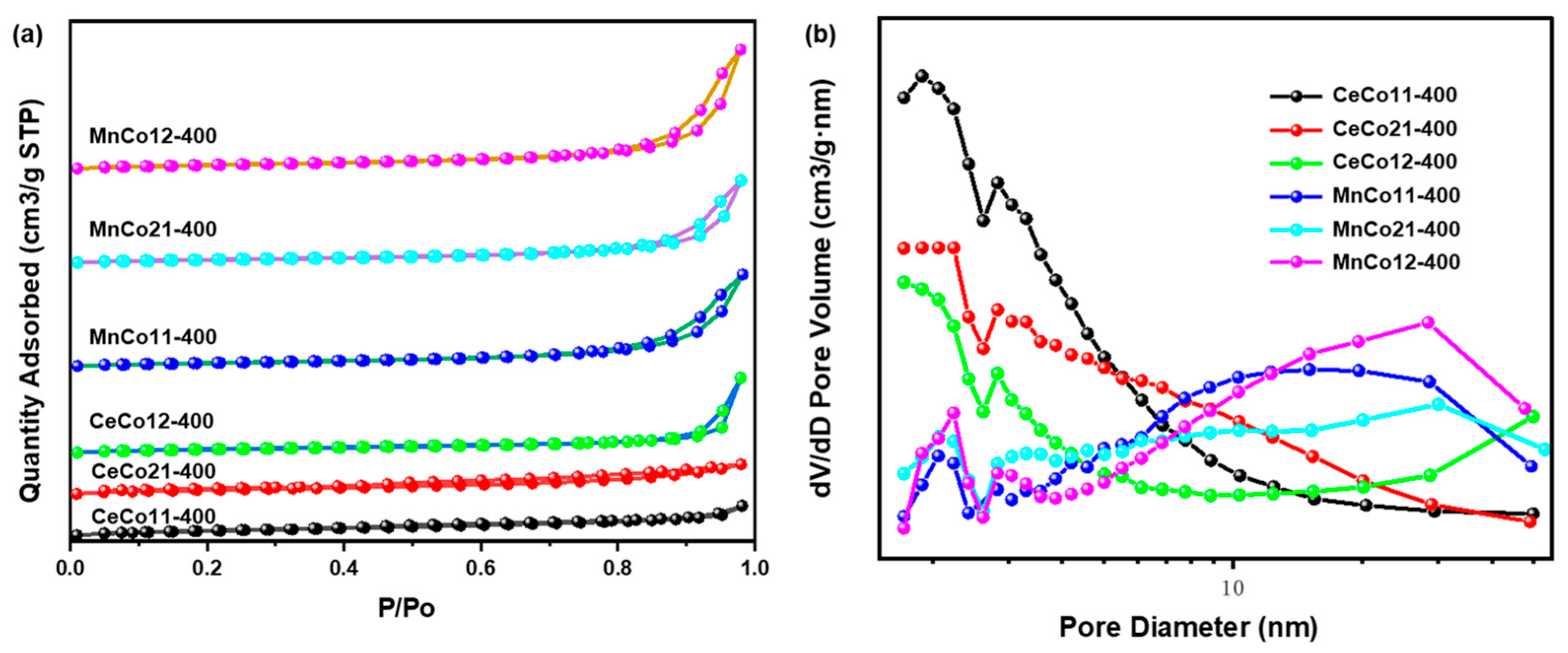
2.1. Structural and Morphology Characterization
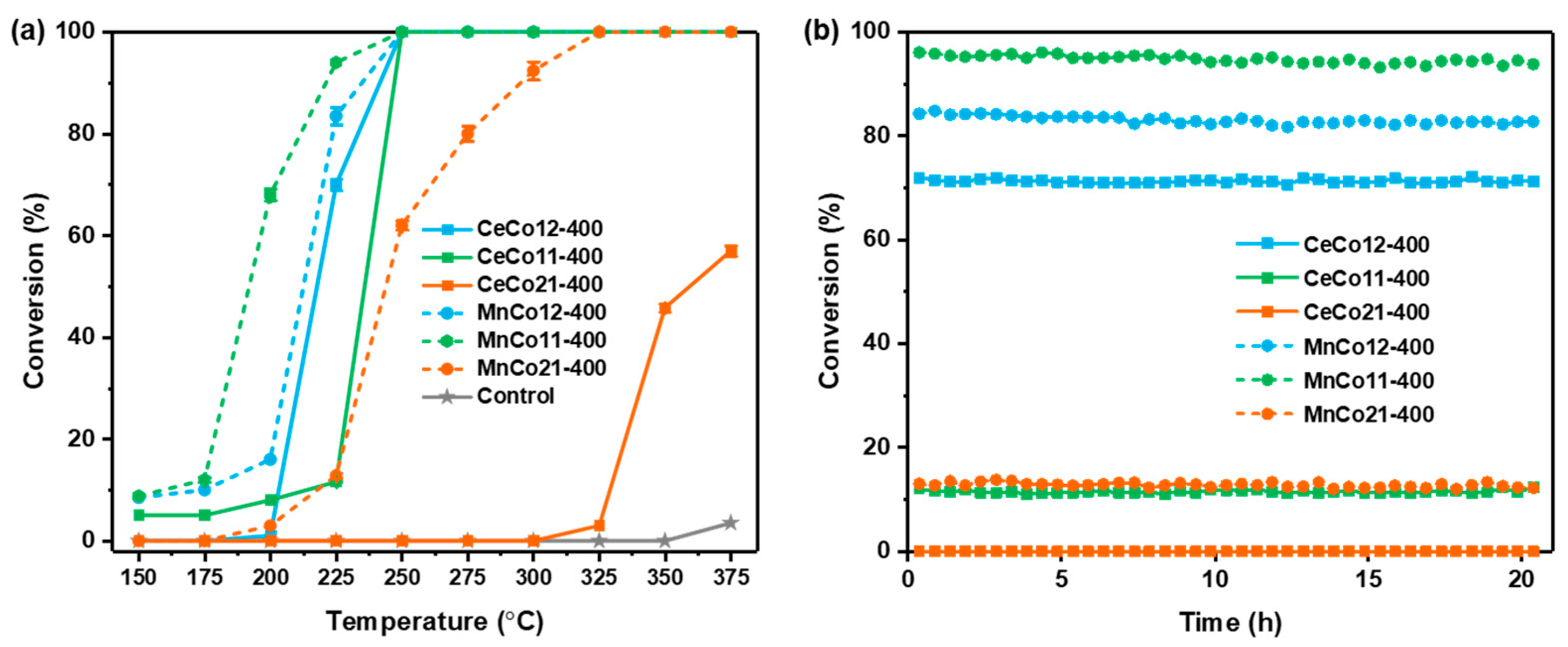
2.2. Catalytic Performance of Benzene Oxidation
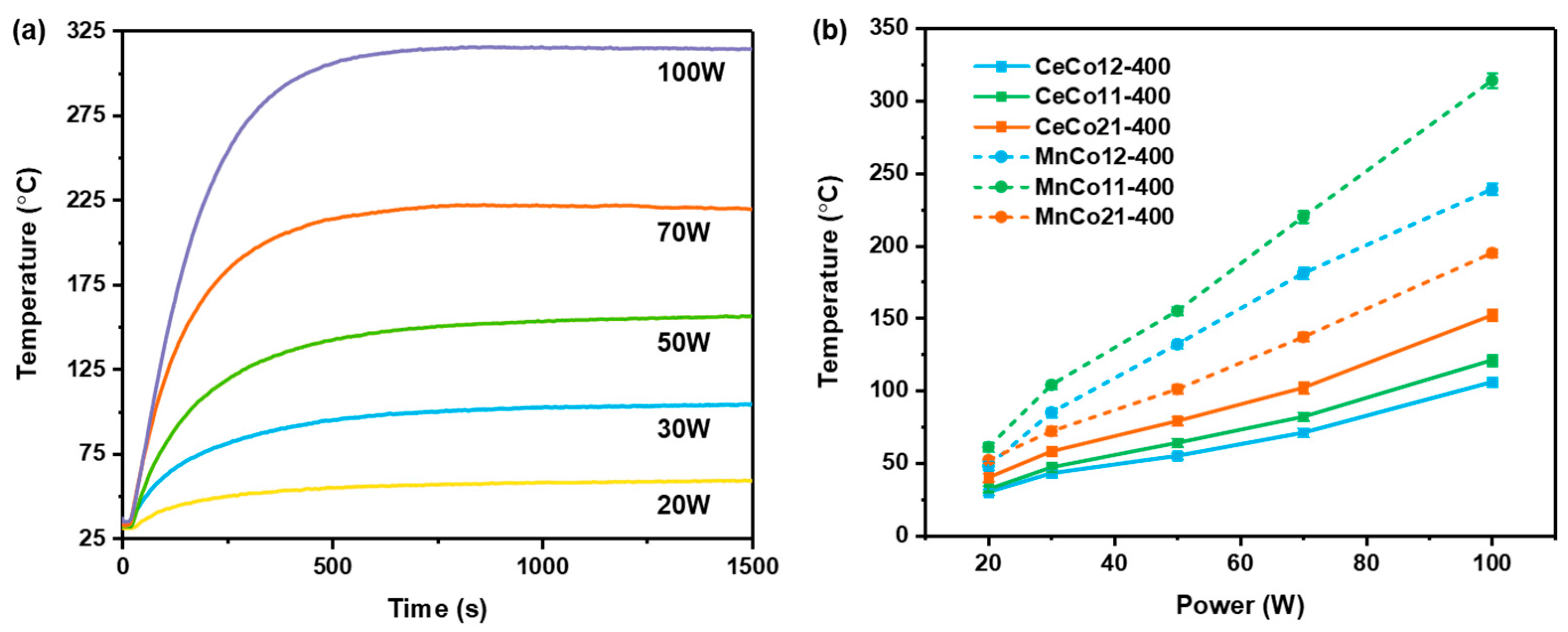
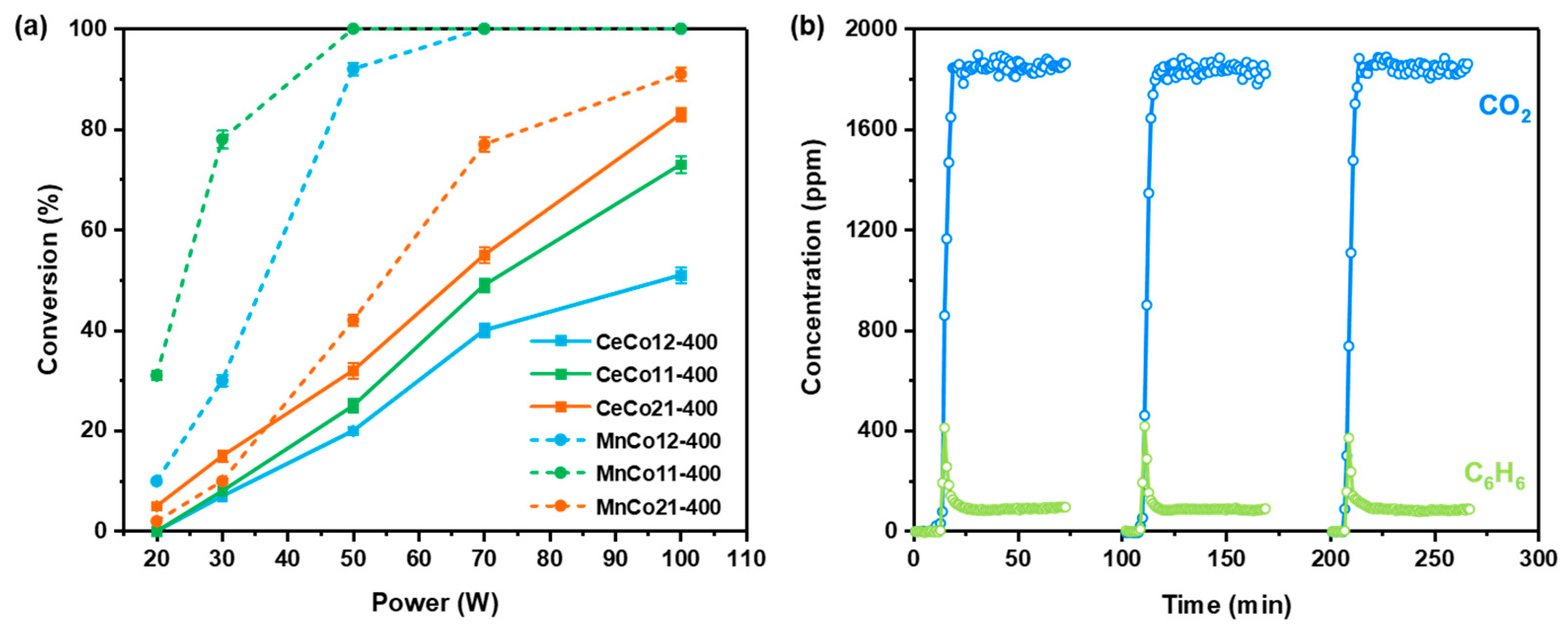
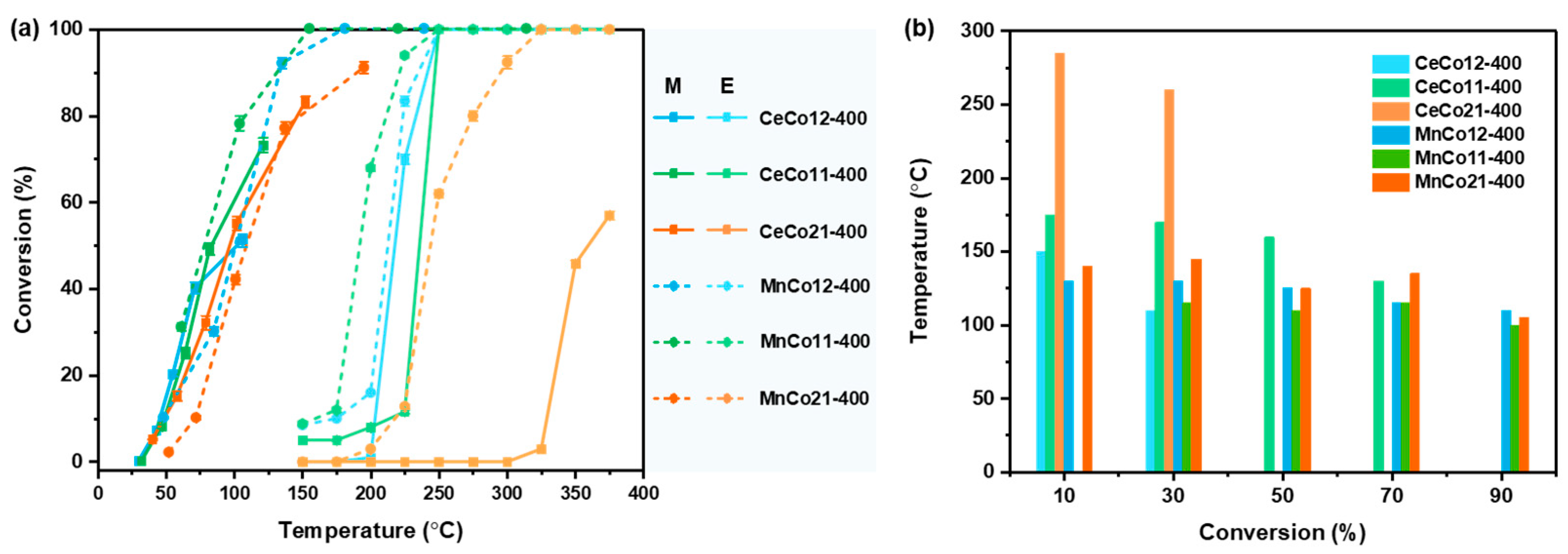
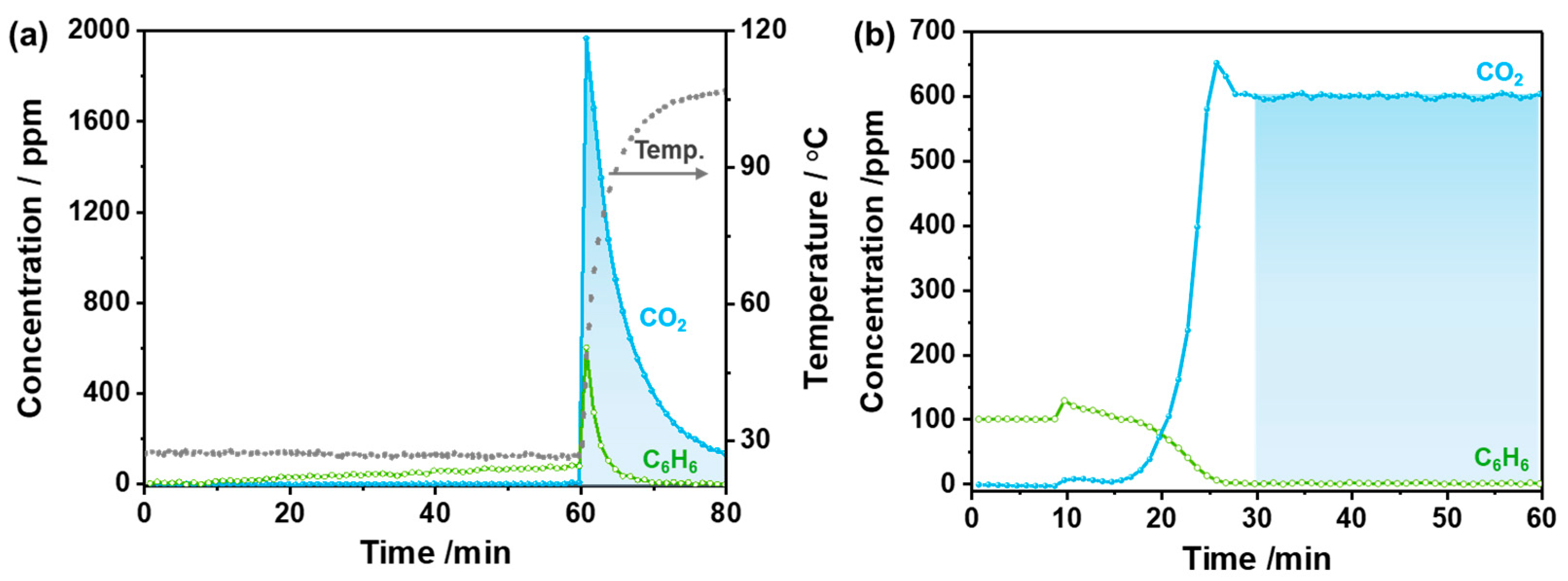
2.3. Microwave-Enhanced Catalytic Performance of Benzene Oxidation
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Characterization
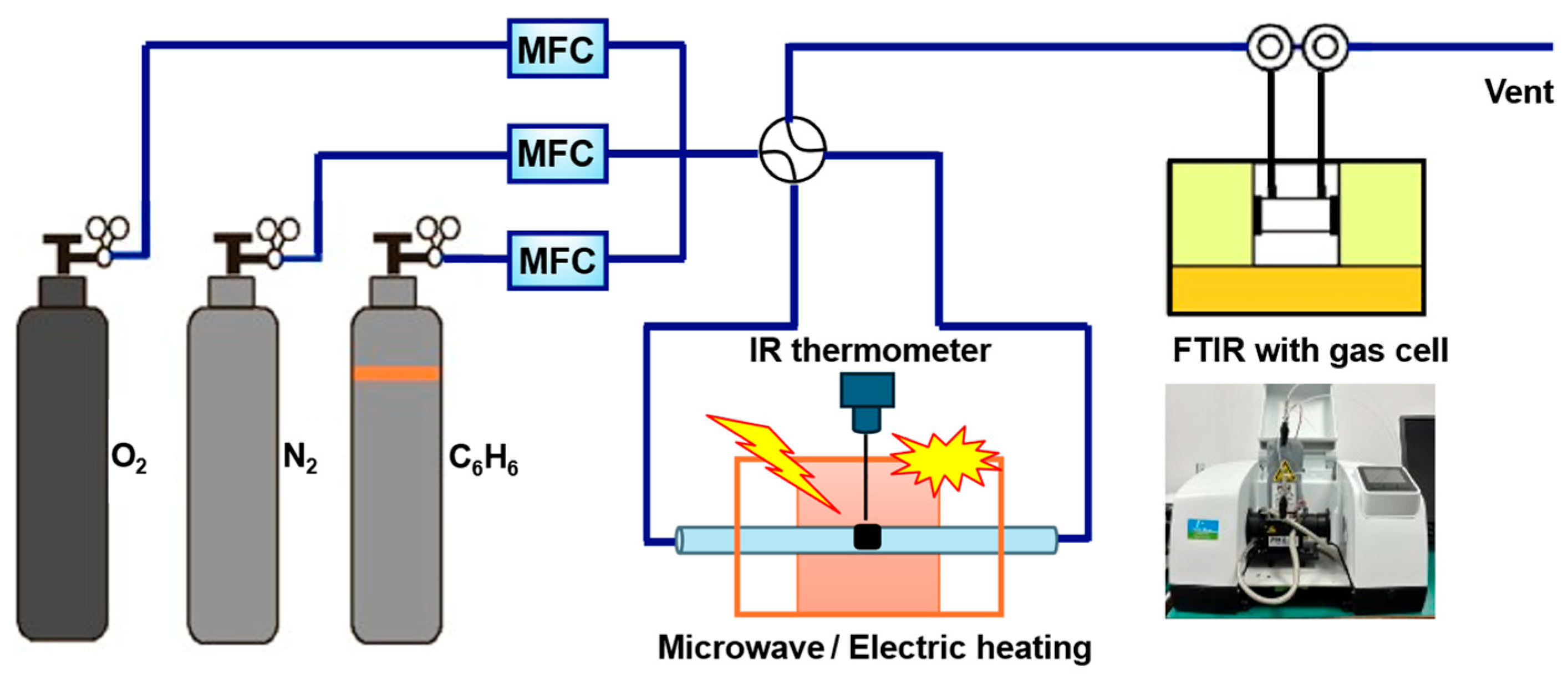
3.3. Catalytic Reactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, D.; Chen, X.; Shu, C.; Huang, Y.; Zhang, P.; Xiao, B.; Zhou, D. Ca-decorated MoBOH as a promising adsorbent for CH2O, C6H6, C3H6O, and C2HCl3 removal at room temperature: A first-principle study. Appl. Surf. Sci. 2021, 563, 150233. [Google Scholar] [CrossRef]
- Azalim, S.; Brahmi, R.; Agunaou, M. Wash coating of cordierite honeycomb with Ce–Zr–Mn mixed oxides for VOC catalytic oxidation. Chem. Eng. J. 2013, 223, 536–546. [Google Scholar] [CrossRef]
- Mazzuca, G.; Ren, X.; Loughner, C.; Estes, M.; Crawford, J.; Pickering, K.; Weinheimer, A.; Dickerson, R. Ozone production and its sensitivity to NOx and VOCs: Results from the DISCOVER-AQ field experiment. Atmos. Chem. Phys. 2016, 16, 14463–14474. [Google Scholar] [CrossRef]
- Chang, Y. Neurotoxic effects of n-hexane on the human central nervous system: Evoked potential abnormalities in n-hexane polyneuropathy. J. Neurol. Neurosurg. Psychiatry 1987, 50, 269–274. [Google Scholar] [CrossRef]
- Kim, R.; Kim, K.; Lee, H. Gene expression profiles of human lung epithelial cells exposed to toluene. J. Toxicol. Environ. Health 2011, 4, 269–276. [Google Scholar] [CrossRef]
- Dumont, E.; Darracq, G.; Couvert, A.; Couriol, C.; Amrane, A.; Thomas, D.; Andrès, Y.; Cloirec, P. Volumetric mass transfer coefficients characterising VOC absorption in water/silicone oil mixtures. Chem. Eng. J. 2013, 221, 308–314. [Google Scholar] [CrossRef]
- Dunn, R.; El-Halwagi, M. Selection of optimal VOC-condensation systems. Waste Manag. 1994, 14, 103–113. [Google Scholar] [CrossRef]
- Liu, X.; Deng, J.; Xie, H. Catalytic removal of volatile organic compounds using ordered porous transition metal oxide and supported noble metal catalysts. Chin. J. Catal. 2016, 37, 1193–1205. [Google Scholar] [CrossRef]
- Tasbihi, M.; Calin, I.; Šuligoj, A. Photocatalytic degradation of gaseous toluene by using TiO2 nanoparticles immobilized on fiberglass cloth. J. Photochem. Photobiol. A 2017, 336, 89–97. [Google Scholar] [CrossRef]
- Guieysse, B.; Hort, C.; Platel, V.; Munoz, R.; Ondarts, M.; Revah, S. Biological treatment of indoor air for VOC removal: Potential and challenges. Biotechnol. Adv. 2008, 26, 398–410. [Google Scholar] [CrossRef]
- Einaga, H.; Nasu, Y.; Oda, M.; Saito, H. Catalytic performances of perovskite oxides for CO oxidation under microwave irradiation. Chem. Eng. J. 2016, 283, 97–104. [Google Scholar] [CrossRef]
- Varisli, D.; Korkusuz, C.; Dogu, T. Microwave-assisted ammonia decomposition reaction over iron incorporated mesoporous carbon catalysts. Appl. Catal. B Environ. 2017, 201, 370–380. [Google Scholar] [CrossRef]
- Bolotov, V.; Chesnokov, V.; Tanashev, Y.; Parmon, V. The oxidative dehydrogenation of ethane: Convectional vs. microwave heating of Ba-containing catalysts. Chem. Eng. Process 2018, 129, 103–108. [Google Scholar] [CrossRef]
- Chen, J.; Xu, W.; Zhu, J.; Wang, X.; Zhou, J. Highly effective direct decomposition of H2S by microwave catalysis on core-shell Mo2N-MoC@SiO2 microwave catalyst. Appl. Catal. B Environ. 2020, 268, 118454. [Google Scholar] [CrossRef]
- Zhang, X.; Hayward, D.O.; Mingos, D.M.P. Apparent equilibrium shifts and hot-spot formation for catalytic reactions induced by microwave dielectric heating. Chem. Commun. 1999, 11, 975–976. [Google Scholar] [CrossRef]
- Zhang, X.; Hayward, D.O.; Lee, C.; Mingos, D.M.P. Microwave assisted catalytic reduction of sulfur dioxide with methane over MoS2 catalysts. Appl. Catal. B Environ. 2001, 33, 137–148. [Google Scholar] [CrossRef]
- Durka, T.; Gerven, T.V.; Stankiewicz, A. Microwaves in heterogeneous gas-phase catalysis: Experimental and numerical approaches. Chem. Eng. Technol. 2009, 32, 1301–1312. [Google Scholar] [CrossRef]
- Gangurde, L.S.; Sturm, G.S.J.; Valero-Romero, M.J.; Mallada, R.; Santamaria, J.; Stankiewicz, A.I.; Stefanidis, G.D. Synthesis, characterization, and application of ruthenium-doped SrTiO3 perovskite catalysts for microwave-assisted methane dry reforming. Chem. Eng. Process 2018, 127, 178–190. [Google Scholar] [CrossRef]
- Dobosz, J.; Zawadzki, M. Total oxidation of lean propane over α-Fe2O3 using microwaves as an energy source. React. Kinet. Mech. Catal. 2015, 114, 157–172. [Google Scholar] [CrossRef]
- Nigar, H.; Julián, I.; Mallada, R. Microwave-assisted catalytic combustion for the efficient continuous cleaning of VOC-containing air streams. Environ. Sci. Techno. 2018, 52, 5892–5901. [Google Scholar] [CrossRef]
- Ding, S.; Zhu, C.; Hojo, H.; Einaga, H. Enhanced Catalytic Performance of spinel-type Cu-Mn Oxides for benzene oxidation under microwave irradiation. J. Hazard. Mater. 2022, 424, 127523. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Hojo, H.; Einaga, H. Microwave-assisted removal of benzene with high efficiency on cobalt modified copper-manganese spinel oxides. J. Environ. Chem. Eng. 2022, 10, 108212. [Google Scholar] [CrossRef]
- Li, S.; Tu, L.; Li, X.; Chen, P.; Zhang, Y.; Ding, S. Investigation into the effect of MnO2 structure toward thermal/microwave-assisted catalytic oxidation of benzene. Appl. Surf. Sci. 2025, 707, 163649. [Google Scholar] [CrossRef]
- Pang, Y.; Lei, H. Degradation of p-nitrophenol through microwave-assisted heterogeneous activation of peroxymonosulfate by manganese ferrite. Chem. Eng. J. 2016, 287, 585–592. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, X.; Wei, J.; Zhao, S.; Wang, L.; Feng, B. Morphology controlled synthesis and novel microwave absorption properties of hollow urchinlike α-MnO2 nanostructures. J. Phys. Chem. C 2011, 115, 1398–1402. [Google Scholar] [CrossRef]
- Duan, Y.; Ma, H.; Li, X.; Liu, S.; Ji, Z. The microwave electromagnetic characteristics of manganese dioxide with different crystallographic structures. Physica B 2010, 405, 1826–1831. [Google Scholar]
- Chen, X.; Chen, X.; Yu, E.; Cai, S.; Jia, H.; Chen, J.; Liang, P. In situ pyrolysis of Ce-MOF to prepare CeO2 catalyst with obviously improved catalytic performance for toluene combustion. Chem. Eng. J. 2018, 344, 469–479. [Google Scholar] [CrossRef]
- Zhang, X.; Hou, F.; Yang, Y.; Wang, Y.; Liu, N.; Chen, D.; Yang, Y. A facile synthesis for cauliflower like CeO2 catalysts from Ce-BTC precursor and their catalytic performance for CO oxidation. Appl. Surf. Sci. 2017, 423, 771–779. [Google Scholar] [CrossRef]
- Zhao, P.; Qin, F.; Huang, Z.; Sun, C.; Shen, W.; Xu, H. MOF-derived hollow porous Ni/CeO2 octahedron with high efficiency for N2O decomposition. Chem. Eng. J. 2018, 349, 72–81. [Google Scholar] [CrossRef]
- Yao, H.; Cai, S.; Yang, B.; Han, L.; Wang, P.; Li, H.; Yan, T.; Shib, L.; Zhang, D. In situ decorated MOF-derived Mn–Fe oxides on Fe mesh as novel monolithic catalysts for NOx reduction. New J. Chem. 2020, 44, 2357–2366. [Google Scholar] [CrossRef]
- Wang, Q.; Astruc, D. State of the art and prospects in metal–organic framework (MOF)-based and MOF-derived nanocatalysis. Chem. Rev. 2020, 120, 1438–1511. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, Z.; Bañares, M.A.; Weng, L.; Gu, Q.; Price, J.; Han, W.; Yeung, K. A novel approach to high-performance aliovalent-substituted catalysts-2D bimetallic MOF-derived CeCuOx microsheets. Small 2019, 15, 1903525. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.; Sun, X.; Li, S.; Chen, L.; Fu, M.; Wu, J.; Ye, D. Shape effect of Pt/CeO2 catalysts on the catalytic oxidation of toluene. Chem. Eng. J. 2016, 306, 1234–1246. [Google Scholar] [CrossRef]
- Yuan, X.; Ge, H.; Wang, X.; Dong, C.; Dong, W.; Riaz, M.S.; Huang, F. Controlled phase evolution from Co nanochains to CoO nanocubes and their application as OER catalysts. ACS Energy Lett. 2017, 2, 1208–1213. [Google Scholar] [CrossRef]
- Gan, G.; Yang, Z.; Li, Y.; Zhang, G. Efficient photothermal mineralization of toluene over MnCo2O4 with different exposed facets: Revealing the role of oxygen vacancy and photo-/thermo-synergistic mechanism. Appl. Catal. B Environ. 2024, 357, 124308. [Google Scholar] [CrossRef]
- Liotta, L.F.; Di Carlo, G.; Pantaleo, G.; Venezia, A.M.; Deganello, G. Co3O4/CeO2 composite oxides for methane emissions abatement: Relationship between Co3O4-CeO2 interaction and catalytic activity. Appl. Catal. B Environ. 2006, 66, 217–227. [Google Scholar] [CrossRef]
- Yang, R.; Guo, Z.; Cai, L. Investigation into the phase–activity relationship of MnO2 nanomaterials toward ozone-assisted catalytic oxidation of toluene. Small 2021, 17, 2103052. [Google Scholar] [CrossRef]
- Sadeghi, M.A.; Aghighi, M.; Barralet, J.; Gostick, J.T. Pore network modeling of reaction-diffusion in hierarchical porous particles: The effects of microstructure. Chem. Eng. J. 2017, 330, 1002–1011. [Google Scholar] [CrossRef]
- Yin, Z.H.; Zhang, W.P.; Luo, W.; Liu, H.; Wang, J.J. Oxygen spillover: Revolutionizing catalytic performance and disrupting mechanistic paradigms in sustainable chemistry. Adv. Mater. 2025, e11007. [Google Scholar] [CrossRef]
- Sani, Y.; Azis, R.A.S.; Ismail, I.; Yaakob, Y.; Mohammed, J. Enhanced electromagnetic microwave absorbing performance of carbon nanostructures for RAMs: A review. Appl. Surf. Sci. 2023, 18, 100455. [Google Scholar] [CrossRef]
- Li, L.; Liu, X.L.; Geng, H.Y.; Hu, B.; Song, G.W.; Xu, Z.S. A MOF/graphite oxide hybrid (MOF: HKUST-1) material for the adsorption of methylene blue from aqueous solution. J. Mater. Chem. A 2013, 1, 10292–10299. [Google Scholar] [CrossRef]
- Teixeira, R.S.; Schmidt, D.V.; dos Santos, F.S.; Cipriano, D.F.; Faria, D.N.; Freitas, J.C.; Pietre, M.K. Nanostructured faujasites with different structural and textural properties for adsorption of cobalt and nickel. Braz. J. Chem. Eng. 2024, 41, 1271–1283. [Google Scholar] [CrossRef]
- Song, L.; Zhang, H.; Nie, Z.; Tian, J.; Liu, Y.; Ma, C.; Ye, D. Ni Doping Promotes C–H bond activation and conversion of key intermediates for total oxidation of methane over Co3O4 catalysts. ACS Catal. 2023, 13, 15779–15793. [Google Scholar] [CrossRef]
- Meng, J. Understanding and Design of Interstitial Oxygen Conductors. Comput. Mater. Sci. 2025, 259, 114110. [Google Scholar] [CrossRef]
- Xing, Y.; Kim, I.; Kang, K.T.; Park, B.; Wang, Z.; Kim, J.C.; Oh, S.H. Atomic-scale operando observation of oxygen diffusion during topotactic phase transition of a perovskite oxide. Matter 2022, 5, 3009–3022. [Google Scholar] [CrossRef]
- Tan, S.; Zhang, Y.; Zhou, Z.; Guan, X.; Liao, Y.; Li, C.; Feng, G. Construction of Co2NiO4@MnCo2O4.5 Nanocomposites with multiple hetero-interfaces for enhanced electromagnetic wave absorption. Particuology 2023, 81, 86–97. [Google Scholar] [CrossRef]
- Wang, S.; Hou, Y.; Wang, X. Development of a stable MnCo2O4 cocatalyst for photocatalytic CO2 reduction with visible light. ACS Appl. Mater. Inter. 2015, 7, 4327–4335. [Google Scholar] [CrossRef]
- Baral, A.K.; Dasari, H.P.; Kim, B.K.; Lee, J.H. Effect of sintering aid (CoO) on transport properties of nanocrystalline Gd doped ceria (GDC) materials prepared by co-precipitation method. J. Alloys Compd. 2013, 575, 455–460. [Google Scholar] [CrossRef]
- Charles Vincent, V.; Elaiyaraja, P.; Senthil, S.; Antony Prabhu, A.; Ratchagar, V.; Saravanan, G.; Srinivasan, S. Enhanced dielectric stability and electromagnetic shielding efficiency of CdO/CeO2 nanocomposites for high-performance electronic applications. J. Mater. Sci. Mater. Electron. 2015, 36, 1287. [Google Scholar] [CrossRef]
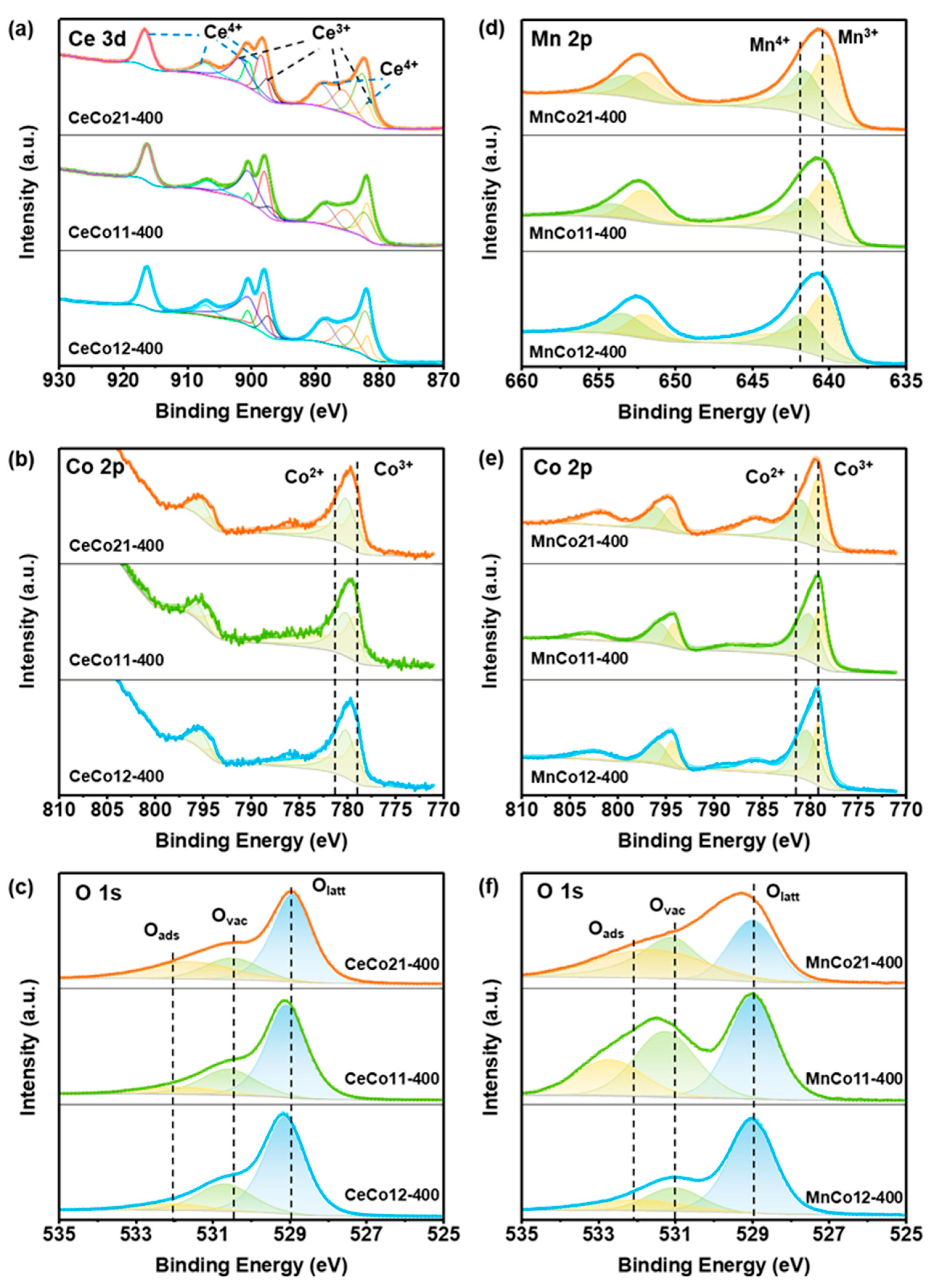
| Samples | Ce3+/Ce4+ | Mn3+/Mn4+ | Oads/Olatt | a Ovac% |
|---|---|---|---|---|
| CeCo12-400 | 0.15 | - | 0.26 | 20.0 |
| CeCo11-400 | 0.22 | - | 0.07 | 21.7 |
| CeCo21-400 | 0.20 | - | 0.05 | 21.1 |
| MnCo12-400 | - | 1.17 | 0.20 | 28.5 |
| MnCo11-400 | - | 1.20 | 0.87 | 33.2 |
| MnCo21-400 | - | 0.91 | 0.19 | 24.4 |
| Samples | SBET/m2·g−1 | Pore Size Distribution/nm | Pore Volume/cm3·g−1 |
|---|---|---|---|
| CeCo12-400 | 72 | 21 | 0.10 |
| CeCo11-400 | 95 | 7 | 0.07 |
| CeCo21-400 | 102 | 7 | 0.05 |
| MnCo12-400 | 81 | 23 | 0.27 |
| MnCo11-400 | 65 | 20 | 0.20 |
| MnCo21-400 | 62 | 21 | 0.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Zhao, P.; Liu, Z.; Wang, C.; Wang, L.; Ding, S. Microwave-Enhanced Catalytic Performance of Benzene Oxidation on MOF-Derived Mn/Ce-Co Oxides. Molecules 2025, 30, 3388. https://doi.org/10.3390/molecules30163388
Li S, Zhao P, Liu Z, Wang C, Wang L, Ding S. Microwave-Enhanced Catalytic Performance of Benzene Oxidation on MOF-Derived Mn/Ce-Co Oxides. Molecules. 2025; 30(16):3388. https://doi.org/10.3390/molecules30163388
Chicago/Turabian StyleLi, Shefeng, Pengyi Zhao, Ziyang Liu, Chang Wang, Linling Wang, and Siyu Ding. 2025. "Microwave-Enhanced Catalytic Performance of Benzene Oxidation on MOF-Derived Mn/Ce-Co Oxides" Molecules 30, no. 16: 3388. https://doi.org/10.3390/molecules30163388
APA StyleLi, S., Zhao, P., Liu, Z., Wang, C., Wang, L., & Ding, S. (2025). Microwave-Enhanced Catalytic Performance of Benzene Oxidation on MOF-Derived Mn/Ce-Co Oxides. Molecules, 30(16), 3388. https://doi.org/10.3390/molecules30163388





