Study on the Electro-Optical Properties of Polymer-Dispersed Liquid Crystals Doped with Cellulose Nanocrystals
Abstract
1. Introduction
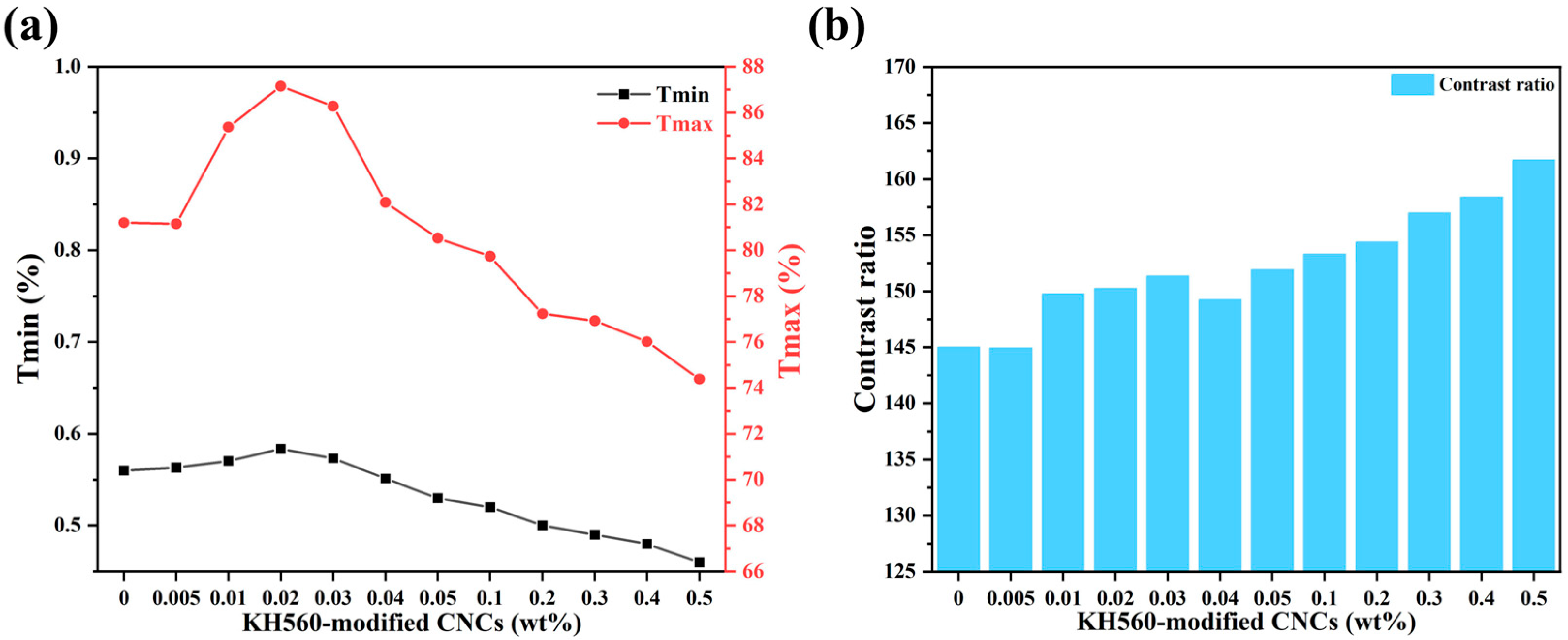
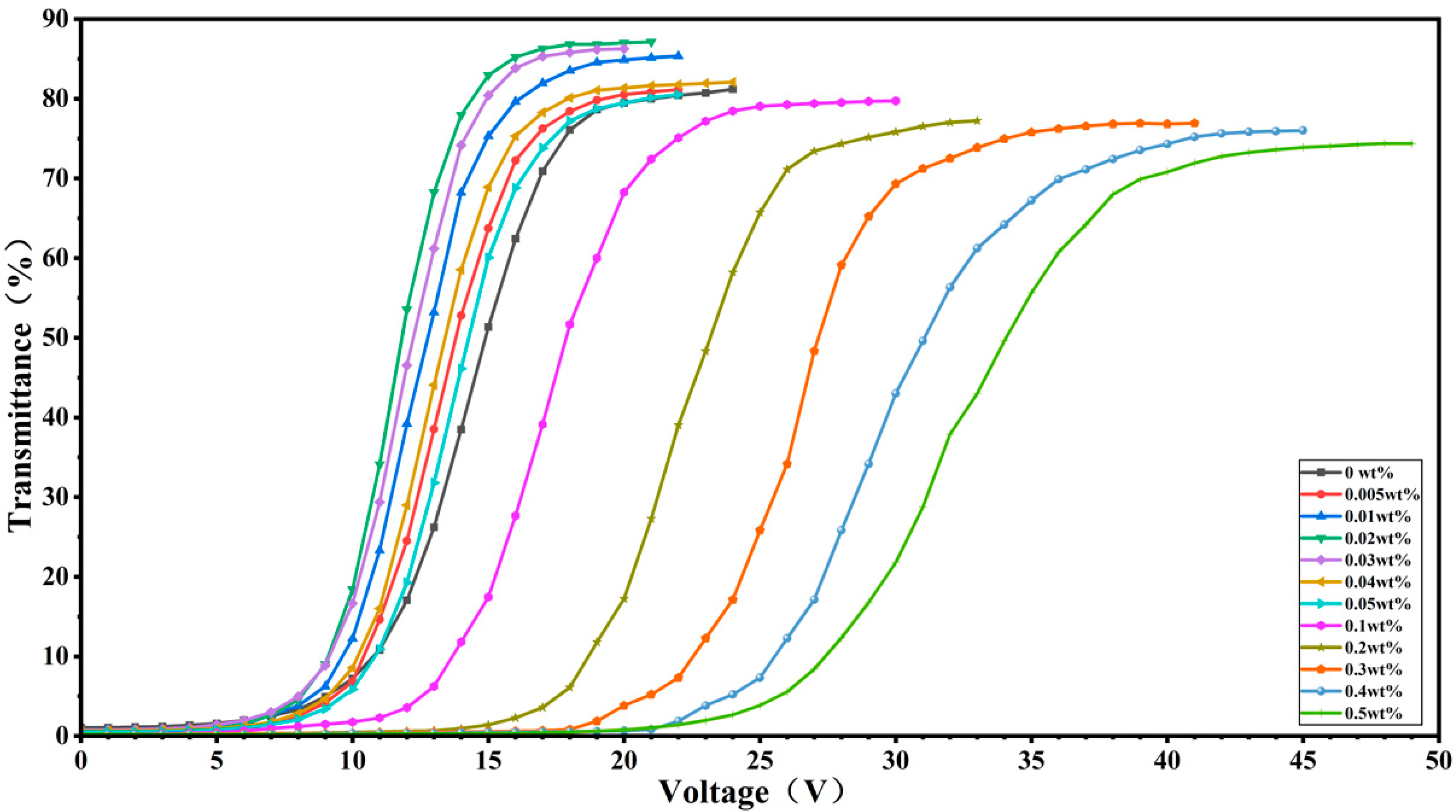
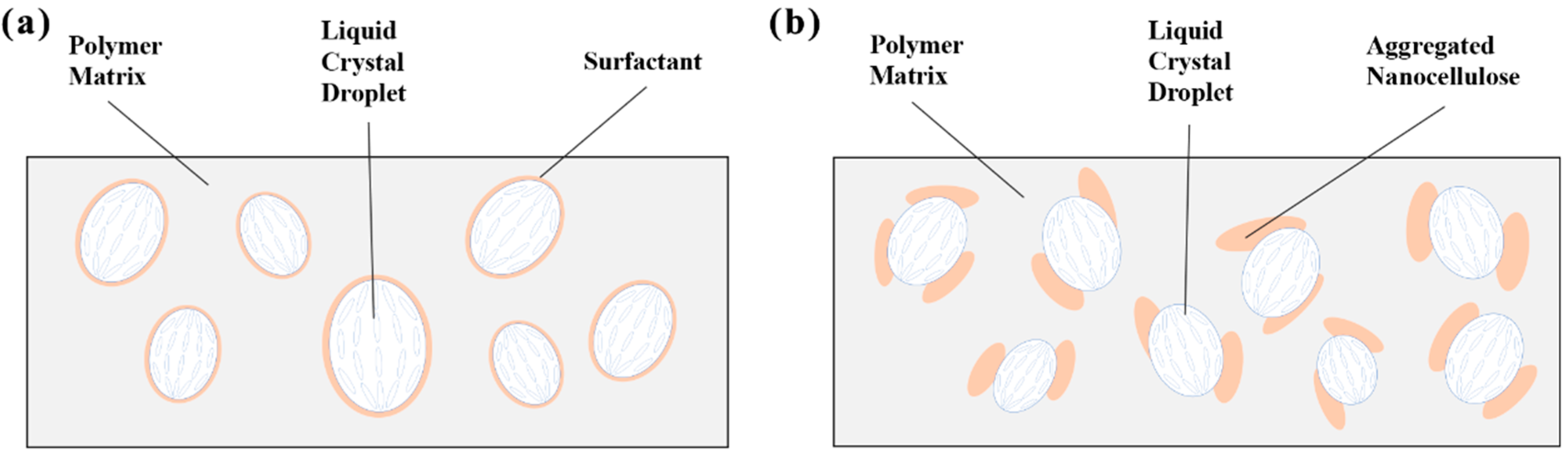
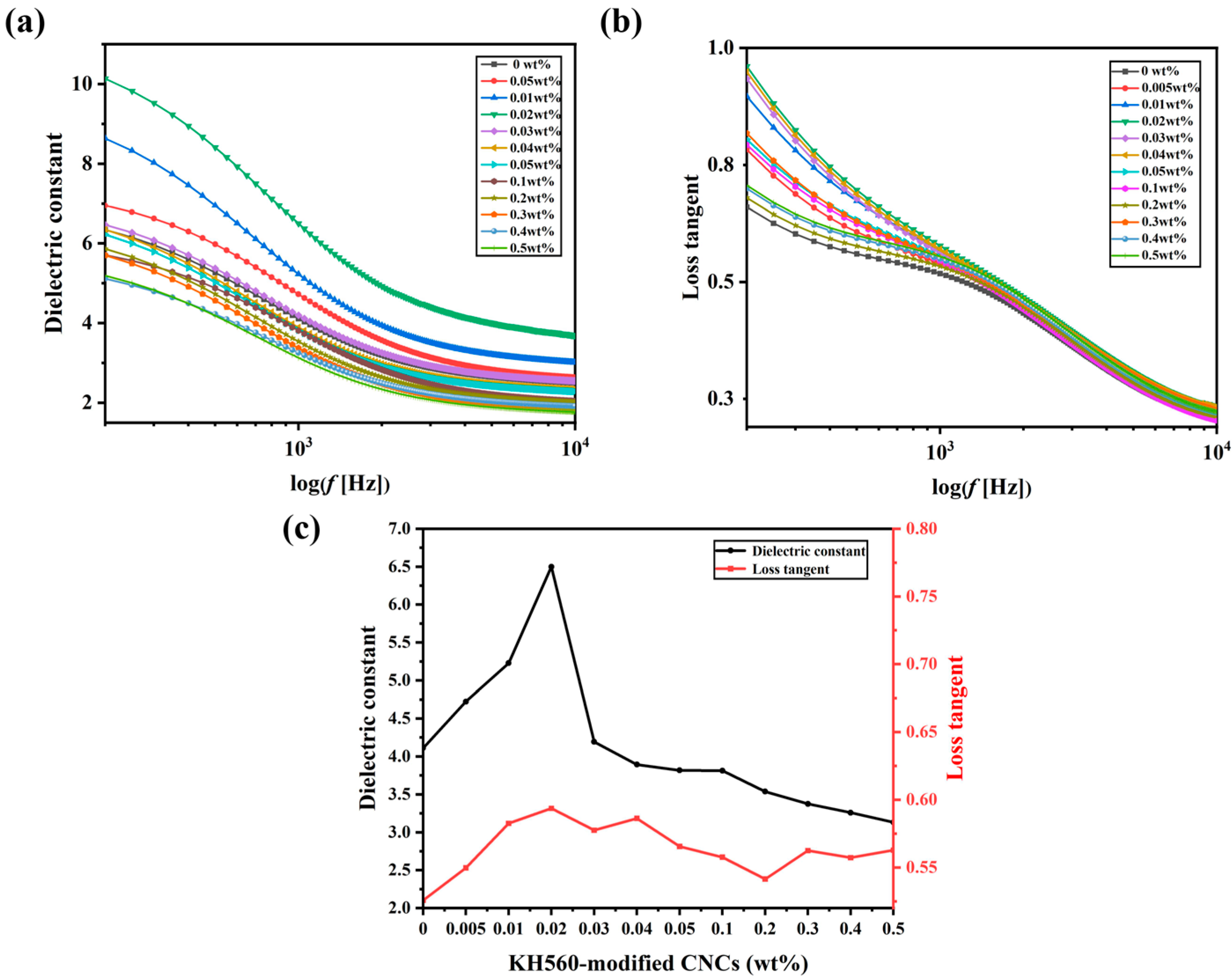
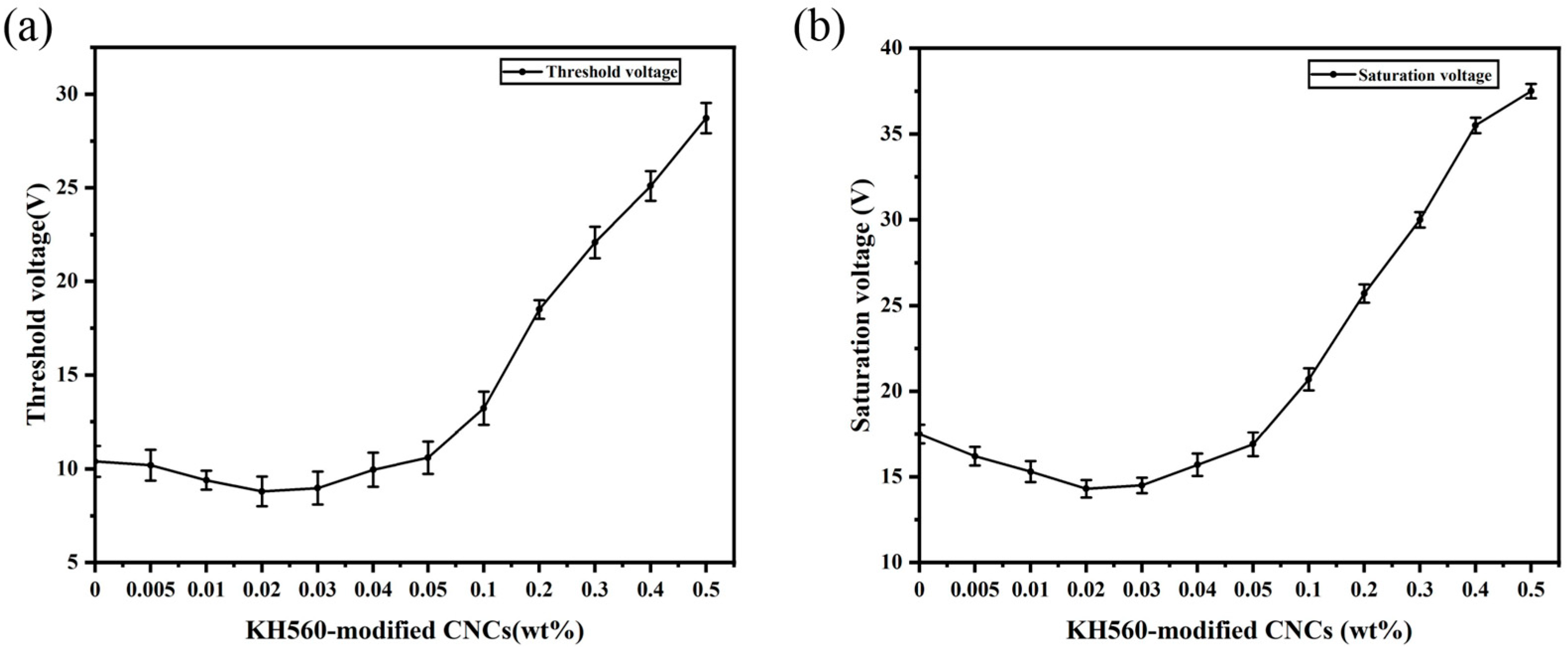
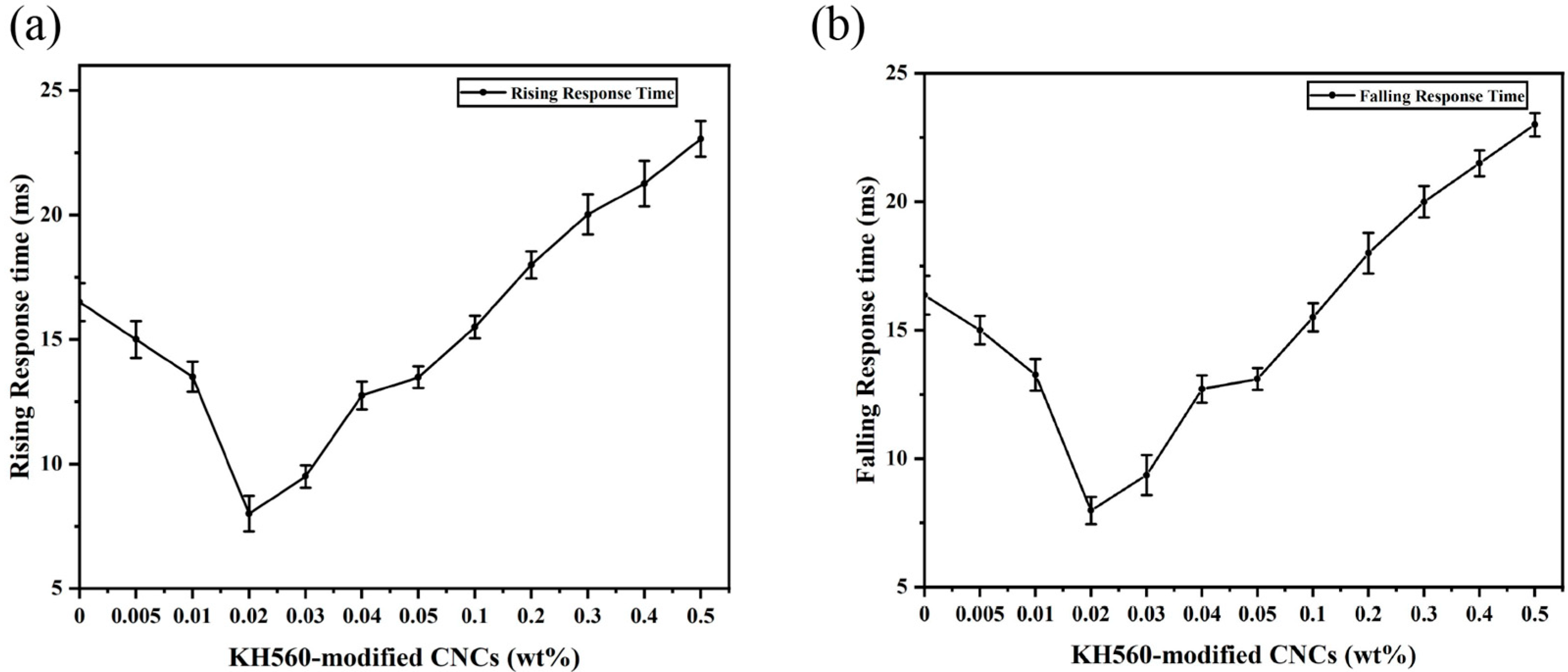
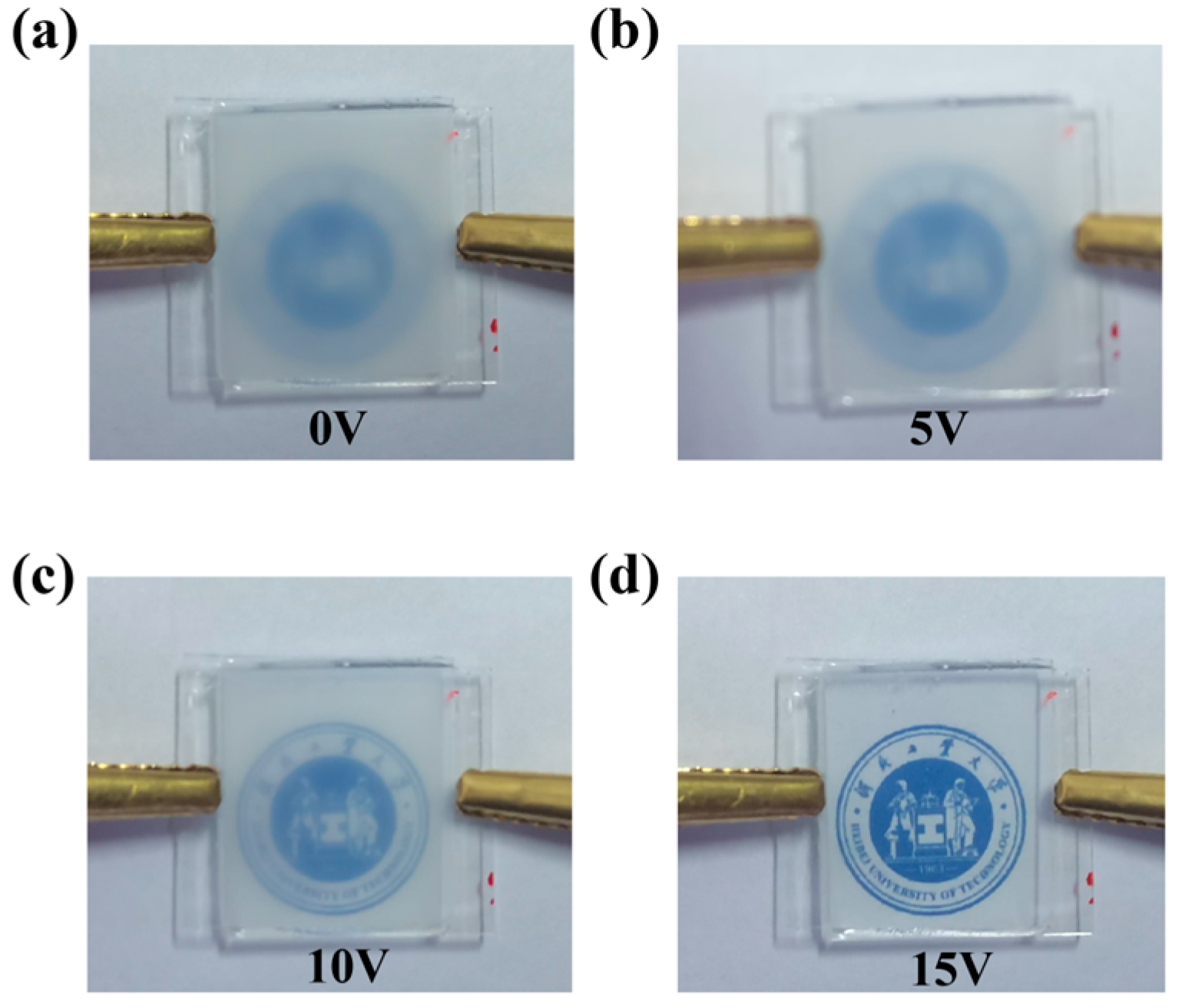
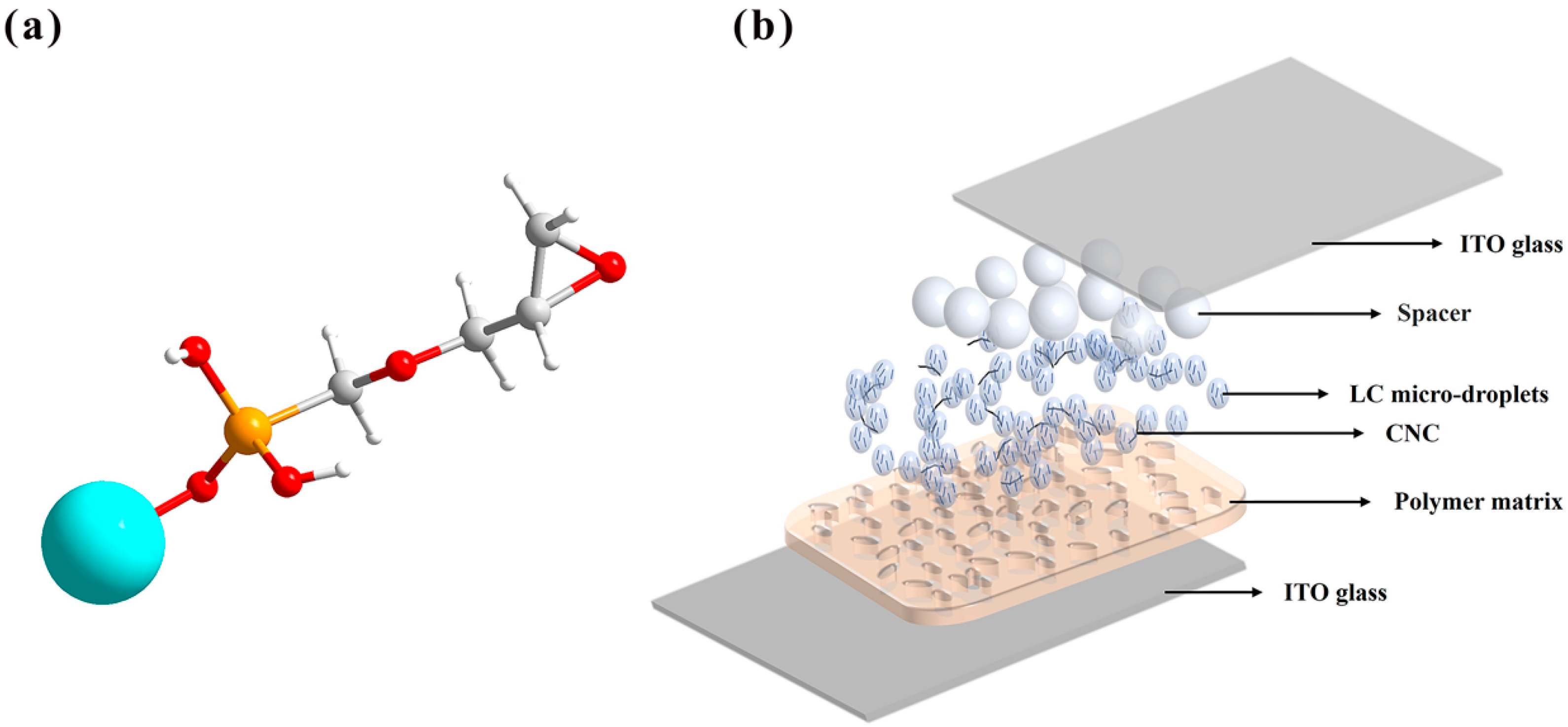
2. Results and Discussion
3. Experiments
3.1. Materials
3.2. Functionalization of KH560-Modified CNCs
3.3. Preparation of the PDLC Films
3.4. Characterization
3.5. Definition of Electro-Optical Performance Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hassanein, G.N.; Kattan, N.; Ellabban, M.A. Electro-optic properties of aligned and non-aligned polymer dispersed liquid crystals driven by an amplitude-modulated electric signal. Optik 2019, 186, 137–146. [Google Scholar] [CrossRef]
- Khandelwal, H.; Schenning, A.P.H.J.; Debije, M.G. Infrared Regulating Smart Window Based on Organic Materials. Adv. Energy Mater. 2017, 7, 1602209. [Google Scholar] [CrossRef]
- Hemaida, A.; Ghosh, A.; Sundaram, S.; Mallick, T.K. Simulation study for a switchable adaptive polymer dispersed liquid crystal smart window for two climate zones (Riyadh and London). Energy Build. 2021, 251, 111381. [Google Scholar] [CrossRef]
- Mondal, I.; Kiruthika, S.; Ganesha, M.K.; Baral, M.; Kumar, A.; Vimala, S.; Lakshmi Madhuri, P.; Nair, G.G.; Prasad, S.K.; Singh, A.K. ITO-free large area PDLC smart windows: A cost-effective fabrication using spray coated SnO2 on an invisible Al mesh. J. Mater. Chem. A 2021, 9, 23157–23168. [Google Scholar] [CrossRef]
- Ramanitra, H.; Chanclou, P.; Dupont, L.; Vinouze, B. Polymer-dispersed liquid crystal structure for variable optical attenuator application. Opt. Eng. 2004, 43, 1445–1453. [Google Scholar] [CrossRef]
- Nabil, G.; Ho, W.F.; Chan, H.P. Experimental study on the performance of a variable optical attenuator using polymer dispersed liquid crystal. Appl. Opt. 2013, 52, E15–E21. [Google Scholar] [CrossRef]
- She, J.; Xu, S.; Tao, T.; Wang, Q.; Sheng, Y.; Hsu, D.; Yu, C.; Lee, B. Design and fabrication of a variable optical attenuator based on polymer-dispersed liquid crystal. Proc. SPIE 2004, 5636, 621–629. [Google Scholar]
- Edwards, R.S.; Ward, J.; Zhou, L.Q.; Trushkevych, O. The interaction of polymer dispersed liquid crystal sensors with ultrasound. Appl. Phys. Lett. 2020, 116, 044104. [Google Scholar] [CrossRef]
- Lai, Y.T.; Kuo, J.C.; Yang, Y.J. Polymer-dispersed liquid crystal doped with carbon nanotubes for dimethyl methylphosphonate vapor-sensing application. Appl. Phys. Lett. 2013, 102, 191912. [Google Scholar] [CrossRef]
- Manzoor, Z. Polarisation detection sensor with EO polymer-dispersed liquid crystals. Electron. Lett. 2020, 56, 9–11. [Google Scholar] [CrossRef]
- De Sio, L.; Lloyd, P.F.; Tabiryan, N.V.; Placido, T.; Comparelli, R.; Curri, M.L.; Bunning, T.J. Thermoplasmonic Activated Reverse-Mode Liquid Crystal Gratings. ACS Appl. Nano Mater. 2019, 2, 3315–3322. [Google Scholar] [CrossRef]
- Zheng, Z.; Ma, J.; Li, W.; Song, J.; Liu, Y.; Xuan, L. Improvements in morphological and electro-optical properties of polymer-dispersed liquid crystal grating using a highly fluorine-substituted acrylate monomer. Liq. Cryst. 2008, 35, 885–893. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, J.; Shen, T.; Zheng, J.; Zhuang, S. Electro-optical properties and frequency response of polymer-dispersed liquid crystal gratings doped with multi-walled carbon nanotubes. J. Mater. Sci. 2021, 56, 12660–12670. [Google Scholar] [CrossRef]
- Zhang, H.-L.; Deng, H.; Li, J.-J.; He, M.-Y.; Li, D.-H.; Wang, Q.-H. Integral imaging-based 2D/3D convertible display system by using holographic optical element and polymer dispersed liquid crystal. Opt. Lett. 2019, 44, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lo, W.-L.; Lee, S.-Y.; Lin, Y.-M. Controlling sintering temperature for biphasic calcium phosphate scaffolds using submicron hydroxyapatite slurries for LCD 3D printing. Ceram. Int. 2024, 50, 11060–11074. [Google Scholar] [CrossRef]
- Belyaev, V.V. PDLC shutters for 3D imaging. Proc. SPIE 2005, 5821, 117–124. [Google Scholar]
- Li, W.; Zhu, M.; Ding, X.; Li, B.; Huang, W.; Cao, H.; Yang, Z.; Yang, H. Studies on electro-optical properties of polymer matrix/LC/SiO2nanoparticles composites. J. Appl. Polym. Sci. 2009, 111, 1449–1453. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Zhou, L.; Gao, Y.; Hai, M.; Zhang, L.; Li, F.; Zhou, G.; Li, X.; Zhang, C.; et al. Preparation of polymer-dispersed liquid crystal doped with indium tin oxide nanoparticles. Liq. Cryst. 2018, 45, 1068–1077. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, J.; Yu, Y.; Zhao, Y.; Guo, Z.; Yao, R.; Gao, J.; Zhang, Y.; Wang, D. Electro-optical characteristics of polymer dispersed liquid crystal doped with MgO nanoparticles. Molecules 2022, 27, 7265. [Google Scholar] [CrossRef]
- Ahmad, F.; Luqman, M.; Jamil, M. Advances in the metal nanoparticles (MNPs) doped liquid crystals and polymer dispersed liquid crystal (PDLC) composites and their applications—A review. Mol. Cryst. Liq. Cryst. 2021, 731, 1–33. [Google Scholar] [CrossRef]
- Shriyan, S.K.; Fontecchio, A.K. Improved Electro-Optic Response of Polymer Dispersed Liquid Crystals Doped with Oxidized Multiwalled Carbon Nanotubes. Mol. Cryst. Liq. Cryst. 2010, 525, 158–166. [Google Scholar] [CrossRef]
- Kumar, P.; Sharma, V.; Raina, K.K. Studies on inter-dependency of electrooptic characteristics of orange azo and blue anthraquinone dichroic dye doped polymer dispersed liquid crystals. J. Mol. Liq. 2018, 251, 407–416. [Google Scholar] [CrossRef]
- Katariya-Jain, A.; Deshmukh, R.R. Effects of dye doping on electro-optical, thermo-electro-optical and dielectric properties of polymer dispersed liquid crystal films. J. Phys. Chem. Solids 2022, 160, 110363. [Google Scholar] [CrossRef]
- Huang, J.; Hu, W.; Yu, M.; Ren, Y.; Zhang, L.; Yang, H. Effects of terpene alcohol dopant on the morphology and electro-optical properties of polymer-dispersed liquid-crystal composite films. Polym. Adv. Technol. 2021, 32, 4153–4161. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, C.; Zhou, L.; Fang, H.; Huang, J.; Ma, H.; Zhang, Y.; Yang, J.; Zhang, L.-Y.; Song, P.; et al. Effect of a polymercaptan material on the electro-optical properties of polymer-dispersed liquid crystal films. Molecules 2016, 22, 43. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Bai, J.; Tian, P.; Xie, R.; Duan, Z.; Lv, Q.; Tao, Y. The Application Status of Nanoscale Cellulose-Based Hydrogels in Tissue Engineering and Regenerative Biomedicine. Front. Bioeng. Biotech. 2021, 9, 732513. [Google Scholar] [CrossRef] [PubMed]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From fundamentals to advanced applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Zhang, H.; Yu, H.-Y.; Tam, K.C. Electroconductive cellulose nanocrystals—Synthesis, properties and applications: A review. Carbohydr. Polym. 2022, 289, 119419. [Google Scholar] [CrossRef]
- Mariano, M.; El Kissi, N.; Dufresne, A. Cellulose nanocrystals and related nanocomposites: Review of some properties and challenges. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 791–806. [Google Scholar] [CrossRef]
- Mugo, S.M.; Lu, W.; Mundle, T.; Berg, D. Thin Film Composite Conductive Polymers Chemiresistive Sensor and Sample Holder for Methanol Detection in Adulterated Beverages. IEEE Sens. J. 2020, 20, 656–663. [Google Scholar] [CrossRef]
- Ikram, M.; Asghar, R.; Imran, M.; Naz, M.; Haider, A.; Ul-Hamid, A.; Haider, J.; Shahzadi, A.; Nabgan, W.; Goumri-Said, S.; et al. Experimental and Computational Study of Zr and CNC-Doped MnO2 Nanorods for Photocatalytic and Antibacterial Activity. ACS Omega 2022, 7, 14045–14056. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.; Zhao, Y.; Zhang, H.; Huang, H.; Zhou, C.; Cong, T.; Muhammad, J.; Yang, X.; Zhang, Y.; Fan, Z.; et al. Surface modification of helical carbon nanocoil (CNC) with N-doped and Co-anchored carbon layer for efficient microwave absorption. J. Colloid Interface Sci. 2022, 608, 1894–1906. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Q.; Fan, W.; Lin, W. Doped CNC-Na2CO3 reinforcing alginate bio-based fiber as fire-safety composite applied in the household textile. Compos. Commun. 2023, 37, 101435. [Google Scholar] [CrossRef]
- Miao, Z.; Jia, M.; Wang, D. Exploration of nanofibre/nanoparticle/PDLC composite system. Liq. Cryst. 2022, 49, 1942–1952. [Google Scholar] [CrossRef]
- Deng, X.; Zhao, Y.; Gao, H.; Wang, D.; Miao, Z.; Cao, H.; Yang, Z.; He, W. Studies on electro-optical properties of polymer dispersed liquid crystals doped with reticular nanofiber films prepared by electrospinning. Liq. Cryst. 2021, 48, 1850–1858. [Google Scholar] [CrossRef]
- Satapathy, P.; Parthasarathi, S.; Rao, D.S.S.; Bano, S.; Negi, Y.S.; Prasad, S.K. Switchable smart windows using a biopolymer network of cellulose nanocrystals imposed on a nematic liquid crystal. Appl. Phys. Lett. 2020, 117, 103701. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, L.; He, X.; Zhang, Z.; Zhou, F.; Zou, C.; Gao, Y.; Yu, M.; Yang, H. Regulation of refractive index of polymer matrix and its effect on properties of polymer-dispersed liquid crystal films. J. Mol. Liq. 2024, 413, 125916. [Google Scholar] [CrossRef]
- Ma, J.; Zheng, Z.-G.; Liu, Y.-G.; Xuan, L. Electro-optical properties of polymer stabilized cholesteric liquid crystal film. Chin. Phys. B. 2011, 20, 024212. [Google Scholar] [CrossRef]
- He, Z.; Yin, K.; Hsiang, E.-L.; Wu, S.-T. Volumetric light-shaping polymer-dispersed liquid crystal films for mini-LED backlights. Liq. Cryst. 2020, 47, 1458–1463. [Google Scholar] [CrossRef]
- Li, J.; Baird, G.; Lin, Y.; Ren, H.; Wu, S. Refractive-index matching between liquid crystals and photopolymers. J. Soc. Inf. Display 2005, 13, 1035–1040. [Google Scholar] [CrossRef]
- Doane, J.W.; Vaz, N.A.; Wu, B.-G.; Žumer, S. Field controlled light scattering from nematic microdroplets. Appl. Phys. Lett. 1986, 48, 269–271. [Google Scholar] [CrossRef]
- Gai, W.; Han, Y.; Zhang, M.; Liu, R.; Li, Z.; Zheng, G.; Fan, Z.; Zhang, Y.; Zhang, H. Study on polymer dispersed liquid crystal films with wide viewing angle. Liq. Cryst. 2024, 51, 841–851. [Google Scholar] [CrossRef]
- Wang, L.; Meng, F.; Sun, Y.; Yang, H. Effect of surfactant-modified ZnS:Mn nanoparticles on the electro-optical properties of composite polymer-dispersed liquid crystal films. Compos. Part B Eng. 2013, 45, 780–784. [Google Scholar] [CrossRef]
- Patnaik, S.S.; Pachter, R. Anchoring characteristics and interfacial interactions in a polymer dispersed liquid crystal: A molecular dynamics study. Polymer 1999, 40, 6507–6519. [Google Scholar] [CrossRef]
- Katariya-Jain, A.; Mhatre, M.; Dierking, I.; Deshmukh, R. Enhanced thermo-electro-optical and dielectric properties of carbon nanoparticle-doped polymer dispersed liquid crystal based switchable windows. J. Mol. Liq. 2024, 393, 123575. [Google Scholar] [CrossRef]
- Wu, W.; Sun, X.; Wang, D.; Qian, H.; Wang, B.; Wang, X.; Rong, X.; Zhang, X.; Wu, G. The effect of high dielectric BaTiO3 nanoparticle dimensions on the dielectric properties and electro-optical performance of polymer dispersed liquid crystal films. J. Mater. Chem. C 2024, 12, 7386–7397. [Google Scholar] [CrossRef]
- Mhatre, M.M.; Katariya-Jain, A.; Saeed, M.H.; Deshmukh, R. Effects of multifunctional thiol monomers and BaTiO3 nanoparticles on electro-optical and dielectric properties of polymer dispersed liquid crystal films. J. Mol. Liq. 2024, 413, 125945. [Google Scholar] [CrossRef]
- Zobov, K.V.; Zharkova, G.M.; Syzrantsev, V.V. Effect of dopant nanoparticles on reorientation process in polymer-dispersed liquid crystals. EPL 2016, 113, 24001. [Google Scholar] [CrossRef]
- Tran, A.; Boott, C.E.; MacLachlan, M.J. Understanding the Self-Assembly of Cellulose Nanocrystals—Toward Chiral Photonic Materials. Adv. Mater. 2020, 32, e1905876. [Google Scholar] [CrossRef]
- Mhatre, M.M.; Katariya-Jain, A.; Deshmukh, R.R. Enhancing morphological, electro-optical and dielectric properties of polymer-dispersed liquid crystal by doping of disperse Orange 25 dye in LC E7. Liq. Cryst. 2021, 49, 790–803. [Google Scholar] [CrossRef]
- Wu, B.G.; Erdmann, J.H.; Doane, J.W. Erdmann and Doane. Liq. Cryst. 1989, 5, 1453. [Google Scholar] [CrossRef]
- Mhatre, M.M.; Katariya-Jain, A.; Deshmukh, R.R. Improved electro-optical and dielectric properties of polymer dispersed liquid crystal doped with disperse dye red 1 and carbon nanoparticles. Liq. Cryst. 2023, 50, 957–976. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Ulman, A. Polymer surfaces and interfaces. Vol. II Polym. Int. 1994, 33, 438. [Google Scholar]
- Wines, T.H. Polymer interfaces and emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2000, 166, 269–270. [Google Scholar] [CrossRef]
- Allen, K.W. Acid-base interactions: Relevance to adhesion science and technology. Int. J. Adhes. 1992, 12, 123–124. [Google Scholar] [CrossRef]
- Perez, E.; Proust, J.E.; Ter-Minassian-Saraga, L. Interfacial origin of liquid crystal anchorage. Mol. Cryst. Liq. Cryst. 1977, 42, 167–174. [Google Scholar] [CrossRef]
- Ma, L.; Shi, T.; Zhang, Z.; Liu, X.; Wang, H. Wettability of HPMC/PEG/CS thermosensitive porous hydrogels. Gels 2023, 9, 667. [Google Scholar] [CrossRef]
- Woo, J.Y.; Kim, B.K. Surfactant effects on morphology and switching of holographic PDLCs based on polyurethane acrylates. ChemPhysChem 2007, 8, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Wang, H.; Bai, H.; Wang, Y.; Qiu, L.; Zhu, J.; Xu, M. Polymer-dispersed liquid crystal with low driving voltage utilising reaction selectivity of two-stage polymerisation. Liq. Cryst. 2024, 51, 761–772. [Google Scholar] [CrossRef]
- Gai, W.; An, R.; Han, Y.; Zhang, J.; Zhang, H.; Zheng, G.; Zhang, Y.; Li, Z. Analysis of electro-optical properties of surfactant doped polymer dispersed liquid crystal. Colloid Polym. Sci. 2025, 303, 123–135. [Google Scholar] [CrossRef]
- Lin, H.; Zhao, Y.; He, Z.; Gao, H.; Du, Z.; Zhang, H.; Li, C.; Wang, D.; Luan, Y. Dye-assisted bicomponent spun nanofibers toward PDLC electro-optical properties modulation and controlled patterning display. Appl. Mater. Today 2024, 38, 102260. [Google Scholar] [CrossRef]
- Wang, L.; Luo, S.; Liu, S.; He, Z.; Miao, Z. Mechanism of the effect of gel doping on the electro-optical properties of PDLC. Liq. Cryst. 2024, 51, 1980–1994. [Google Scholar] [CrossRef]
- Ahmad, F.; Jeon, A.-R.; Jeon, Y.J.; Jamil, M. A novel technique of fabrication of nanoparticle acrylate doped polymer dispersed liquid crystal (PDLC) film. J. Disper. Sci. Technol. 2022, 43, 1506–1512. [Google Scholar] [CrossRef]
- He, Z.; Yu, P.; Guo, Z.; Zhang, Y.; Feng, X.; Zhang, H.; Zhao, Y.; Miao, Z. Effects of thiol-ene click reaction on morphology and electro-optical properties of polyhedral oligomeric silsesquioxane nanostructure-based polymer dispersed liquid crystal film. Polym. Adv. Technol. 2023, 34, 79–88. [Google Scholar] [CrossRef]
- Meng, X.; Li, J.; Lin, Y.; Liu, X.; Zhao, J.; Li, G.; Li, D.; He, Z. Effect of the magnetic nanoparticle concentration on the electro-optical properties of the polymer-dispersed liquid crystals. Liq. Cryst. 2022, 49, 1612–1622. [Google Scholar] [CrossRef]
- Sharma, V.; Kumar, P. Electro-optically oriented Kerr and orientational phase study of normal mode polymer dispersed liquid crystals - Effect of dispersion of nanoparticles. J. Mol. Liq. 2022, 348, 118030. [Google Scholar] [CrossRef]
- Wang, X.; Hu, W.; Chen, H.; Saeed, M.H.; Huang, J.; Hu, J.; Ren, Y.; Xu, J.; Zhang, L.; Yu, M.; et al. Effects of chemically functionalized TiO2 nanoparticles on the UV-shielding characteristics of polymer-dispersed liquid crystals. Polym. Adv. Technol. 2022, 33, 1561–1568. [Google Scholar] [CrossRef]
- Wang, X.; Lin, H.; Zhao, Y.; He, Z.; Gao, H.; Gao, J.; Wang, D.; Luan, Y. The role of sub-regional electrostatic spinning nanofiber loaded with WO3 nanoparticle in modulating electro-optic performance and stepwise display of polymer dispersed liquid crystal. Liq. Cryst. 2024, 51, 2435–2451. [Google Scholar] [CrossRef]
- Meng, X.; Li, J.; Lin, Y.; Liu, X.; Zhao, J.; Li, D.; He, Z. Optimization approach for the dilute magnetic polymer-dispersed liquid crystal. Opt. Mater. 2022, 131, 112670. [Google Scholar] [CrossRef]
- Singh, A.K.; Malik, P. Textural, electro-optical, dielectric and fluorescence studies of citrate buffer stabilized gold nanoparticles doped in polymer-dispersed liquid crystals composites. Liq. Cryst. 2022, 49, 864–874. [Google Scholar] [CrossRef]


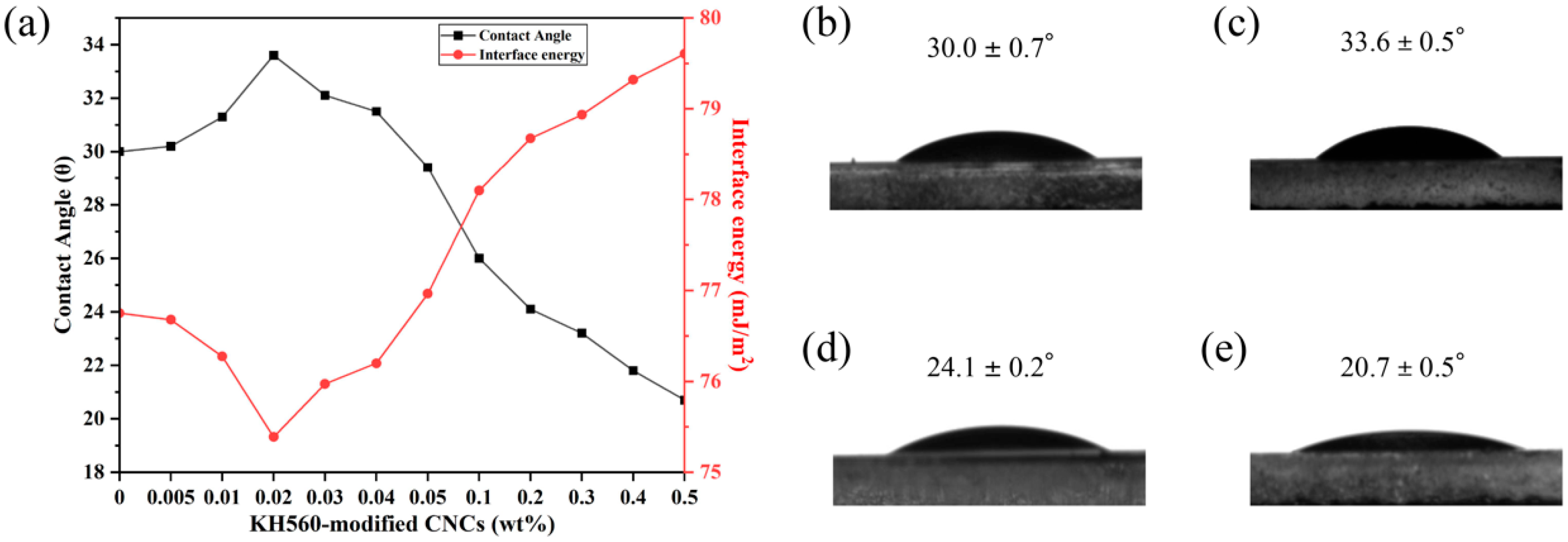
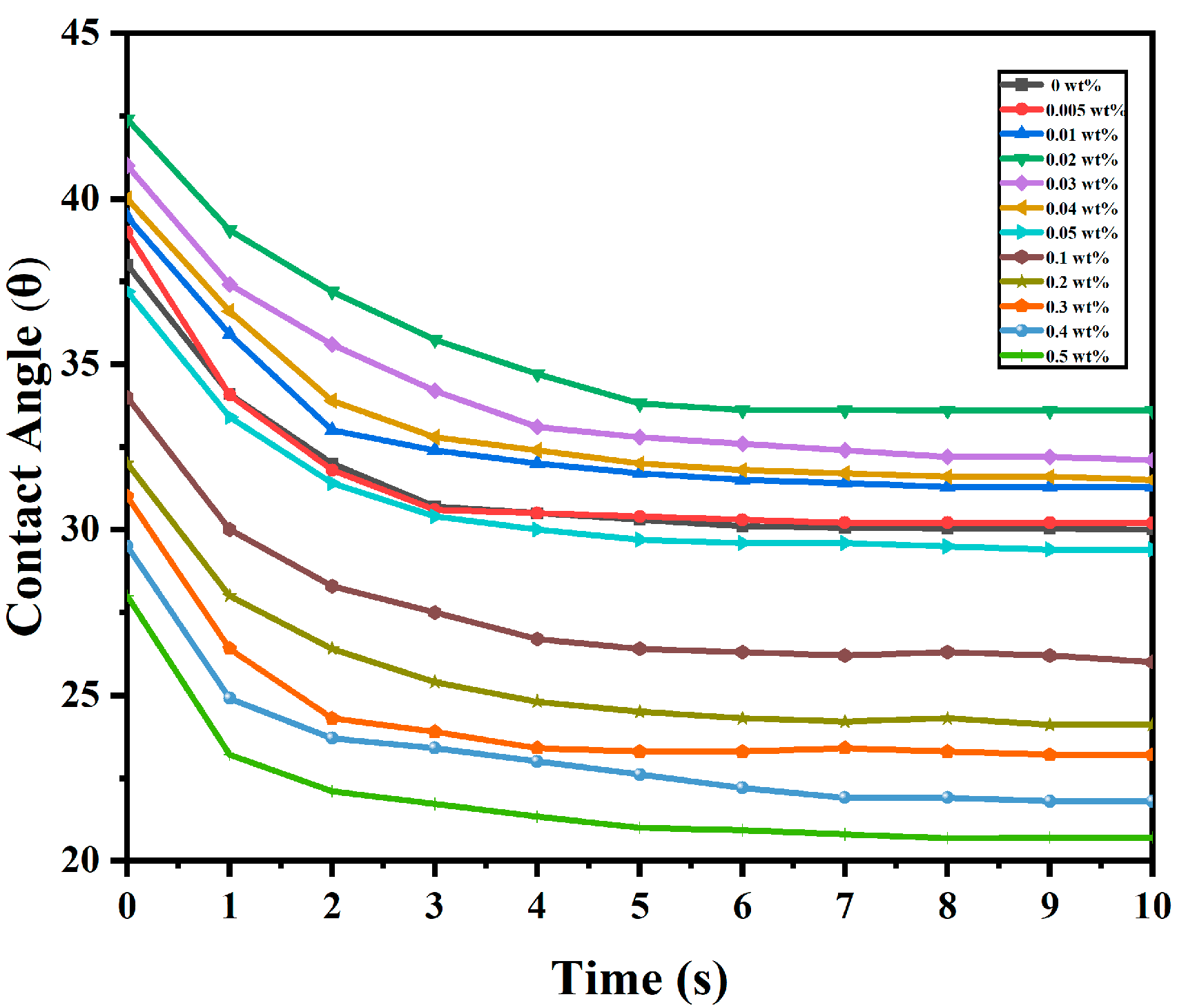

| Liquid | () | () | () | |
|---|---|---|---|---|
| Water | 72.8 | 21.8 | 51.0 | 74.1 |
| Ethylene glycol | 48.2 | 29.0 | 19.2 | 40.2 |
| Diiodomethane | 50.8 | 48.5 | 2.3 | 39.2 |
| Materials | (V) | (V) | (V) | (V) | CR | (ms) | (ms) | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| Span-80 | 5 | decrease 33% | 12 | decrease 29% | - | - | - | - | [61] |
| ZrO2 | 20 | decrease 20% | 39 | decrease 18% | 73 | increase 40% | 280 | increase 115% | [62] |
| DBS | 16 | decrease 35% | 21 | decrease 46% | 205 | increase 25% | 488.3 | increase 163% | [63] |
| CNC | 8.8 | decrease 15% | 14.4 | decrease 19% | 152 | increase 7% | 8 | decrease 52% | This work |
| ZnA | 10 | decrease 50% | 20 | decrease 33% | 7 | increase 27% | 8.1 | decrease 8% | [64] |
| KH570-SiO2 | 5 | decrease 81% | 21 | decrease 63% | 159 | increase 33% | 180 | increase 92% | [65] |
| MNPs | 3.53 | decrease 58% | 14.6 | decrease 19% | 14.3 | increase 48% | - | - | [66] |
| silica NPs | 3.7 | decrease 62% | 8 | decrease 73% | - | - | - | - | [67] |
| TiO2/ MPTS | 16 | increase 77% | 30 | increase 25% | 97 | increase 177% | 10 | decrease 82% | [68] |
| WO3 NPs | 13.6 | decrease 33% | 20 | decrease 32% | 194 | increase 84% | - | - | [69] |
| γ-Fe2O3 MNPs | 9.5 | decrease 33% | 22 | decrease 19% | - | - | - | - | [70] |
| AuNPs | 6.5 | decrease 13% | 34 | decrease 5.5% | 3 | decrease 2% | - | - | [71] |
| Polymer Composition (wt%) | E7 (wt%) | |
|---|---|---|
| INAA | 6.8 | 60 |
| IBOA | 9.6 | |
| IBOMA | 9.6 | |
| CN131 | 12.8 | |
| PI 184 | 1.2 | |
| Polymer: LC (wt%) | CNC (wt%) | UV-A Curing | Ultrasonic Dispersion | Magnetic Stirring Time (h) | |||
|---|---|---|---|---|---|---|---|
| Intensity (mW/cm2) | Time (min) | Time (h) | |||||
| 40:60 | 0 | 12 | 1.5 | 4 | 60 | 2 | 25 |
| 0.005 | |||||||
| 0.01 | |||||||
| 0.02 | |||||||
| 0.03 | |||||||
| 0.04 | |||||||
| 0.05 | |||||||
| 0.1 | |||||||
| 0.2 | |||||||
| 0.3 | |||||||
| 0.4 | |||||||
| 0.5 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Qiao, Y.; Yang, Z.; Han, Y.; Zhang, H.; Li, Z.; Zheng, G.; Zhang, Y.; Zhu, L. Study on the Electro-Optical Properties of Polymer-Dispersed Liquid Crystals Doped with Cellulose Nanocrystals. Molecules 2025, 30, 3273. https://doi.org/10.3390/molecules30153273
Wang J, Qiao Y, Yang Z, Han Y, Zhang H, Li Z, Zheng G, Zhang Y, Zhu L. Study on the Electro-Optical Properties of Polymer-Dispersed Liquid Crystals Doped with Cellulose Nanocrystals. Molecules. 2025; 30(15):3273. https://doi.org/10.3390/molecules30153273
Chicago/Turabian StyleWang, Jiayan, Yan Qiao, Ziyi Yang, Yue Han, Hui Zhang, Zhiguang Li, Guili Zheng, Yanjun Zhang, and Lizhi Zhu. 2025. "Study on the Electro-Optical Properties of Polymer-Dispersed Liquid Crystals Doped with Cellulose Nanocrystals" Molecules 30, no. 15: 3273. https://doi.org/10.3390/molecules30153273
APA StyleWang, J., Qiao, Y., Yang, Z., Han, Y., Zhang, H., Li, Z., Zheng, G., Zhang, Y., & Zhu, L. (2025). Study on the Electro-Optical Properties of Polymer-Dispersed Liquid Crystals Doped with Cellulose Nanocrystals. Molecules, 30(15), 3273. https://doi.org/10.3390/molecules30153273





