Oxygen Reduction by Amide-Ligated Cobalt Complexes: Effect of Hydrogen Bond Acceptor
Abstract
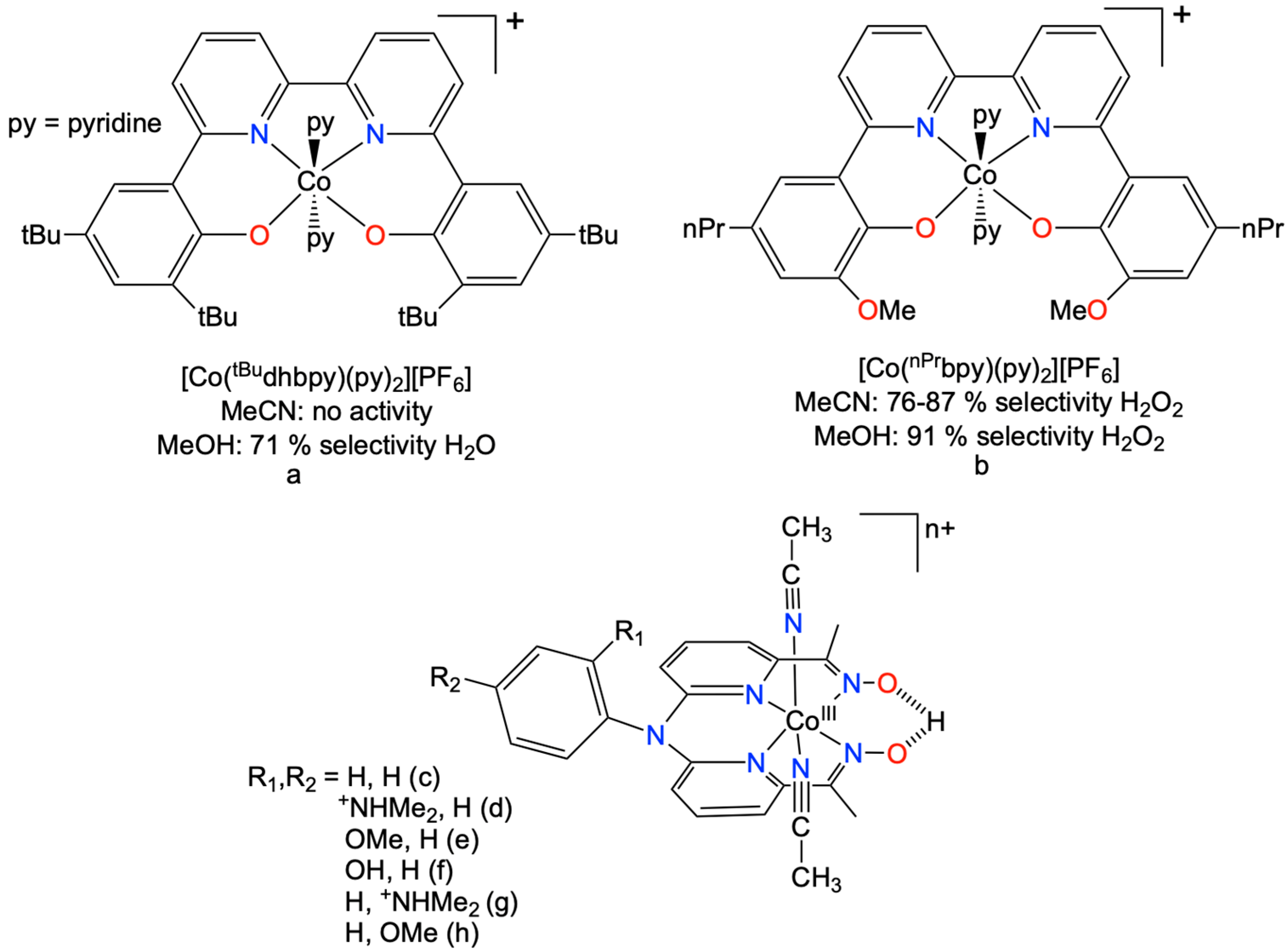
1. Introduction
2. Results
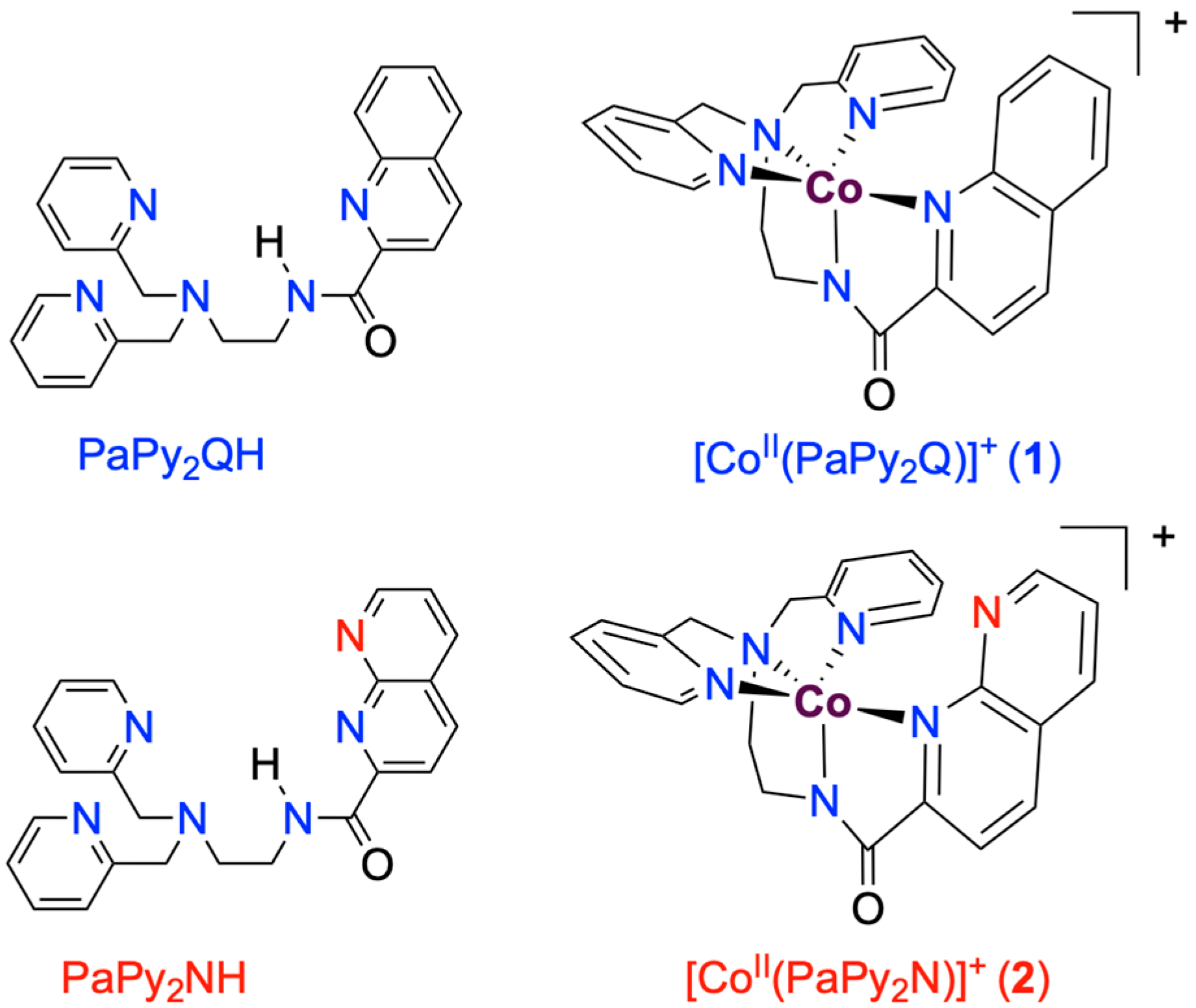
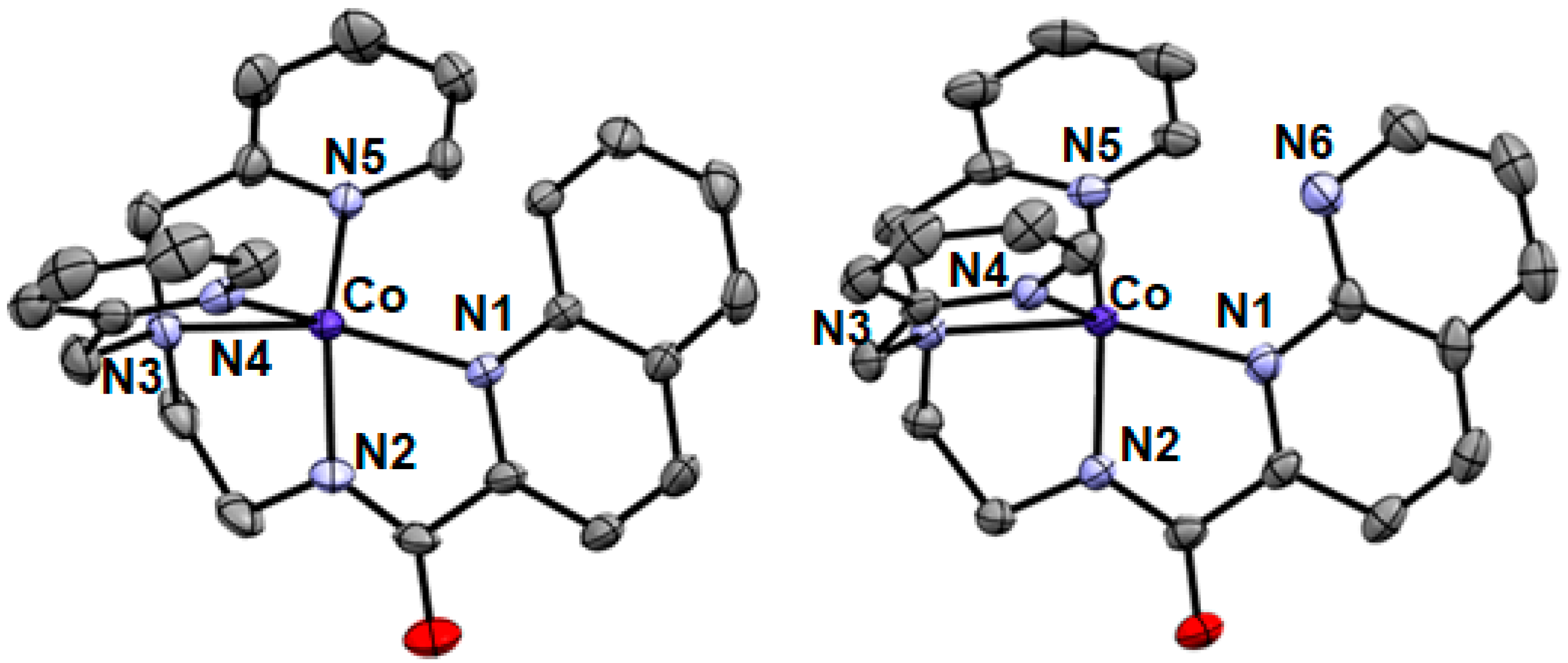
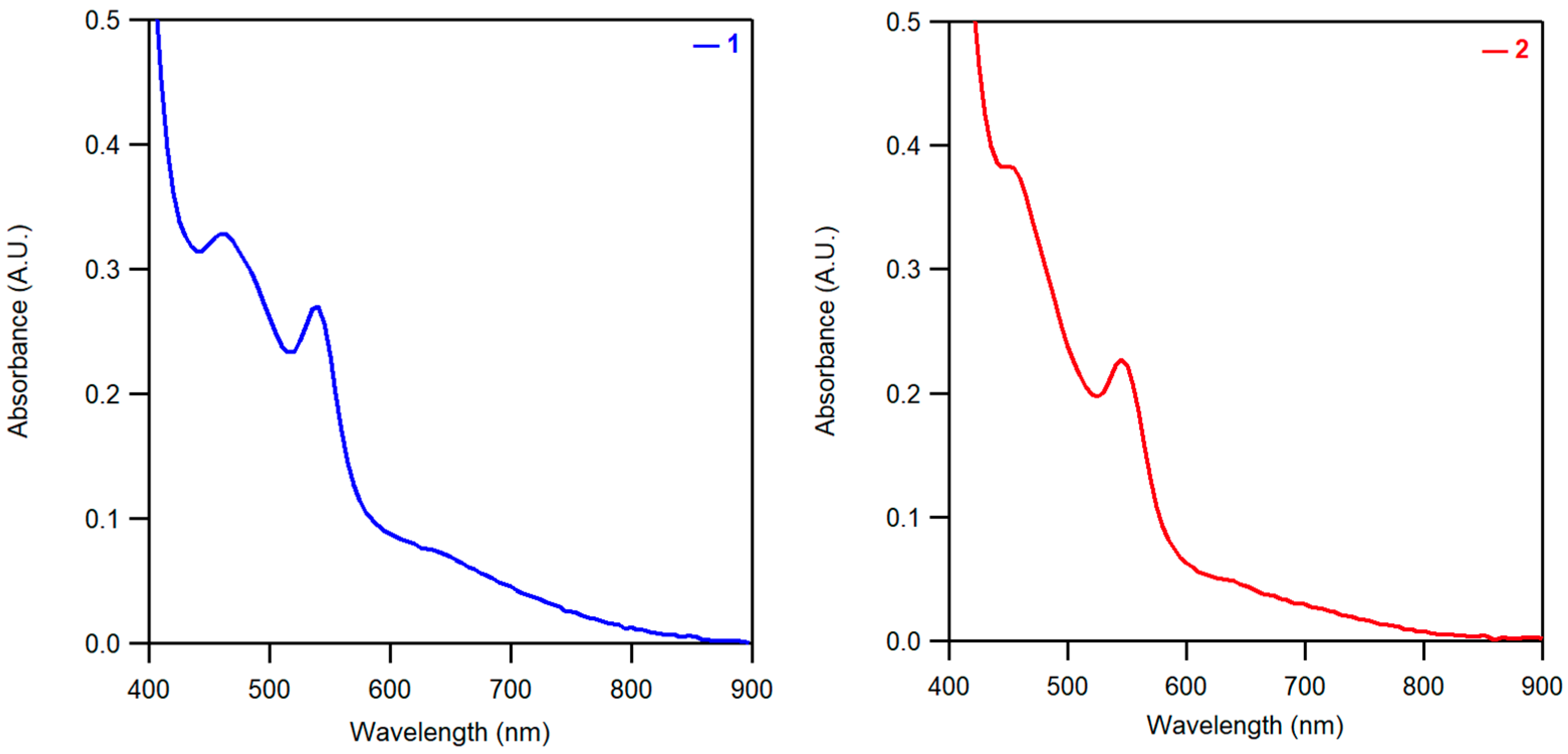
2.1. Formation and Characterization of Complexes [CoII(PaPy2Q)](OTf) (1) and Na[CoII(PaPy2N)](OTf)2 (2)
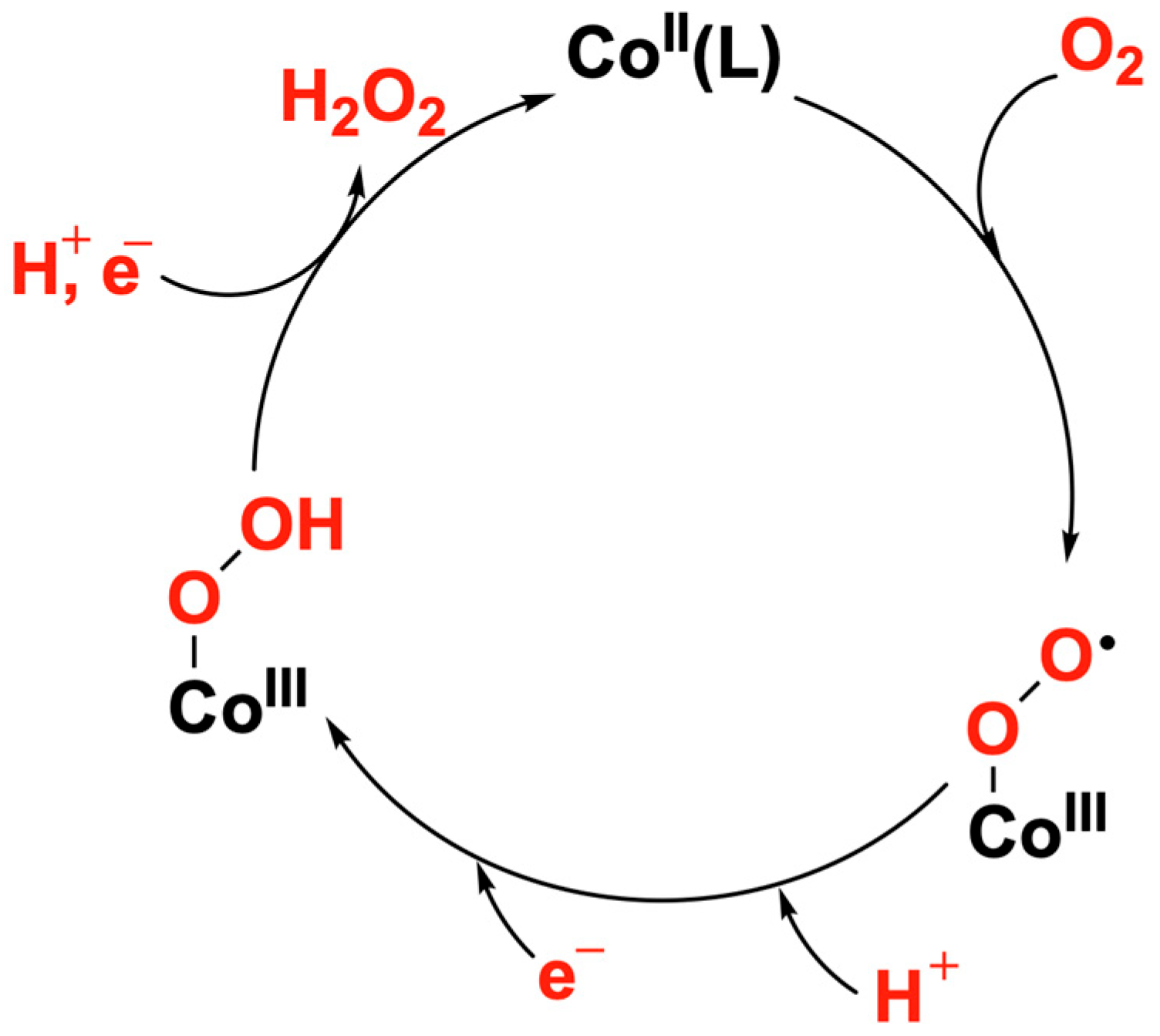
2.2. Reduction of Dioxygen with 1 and 2
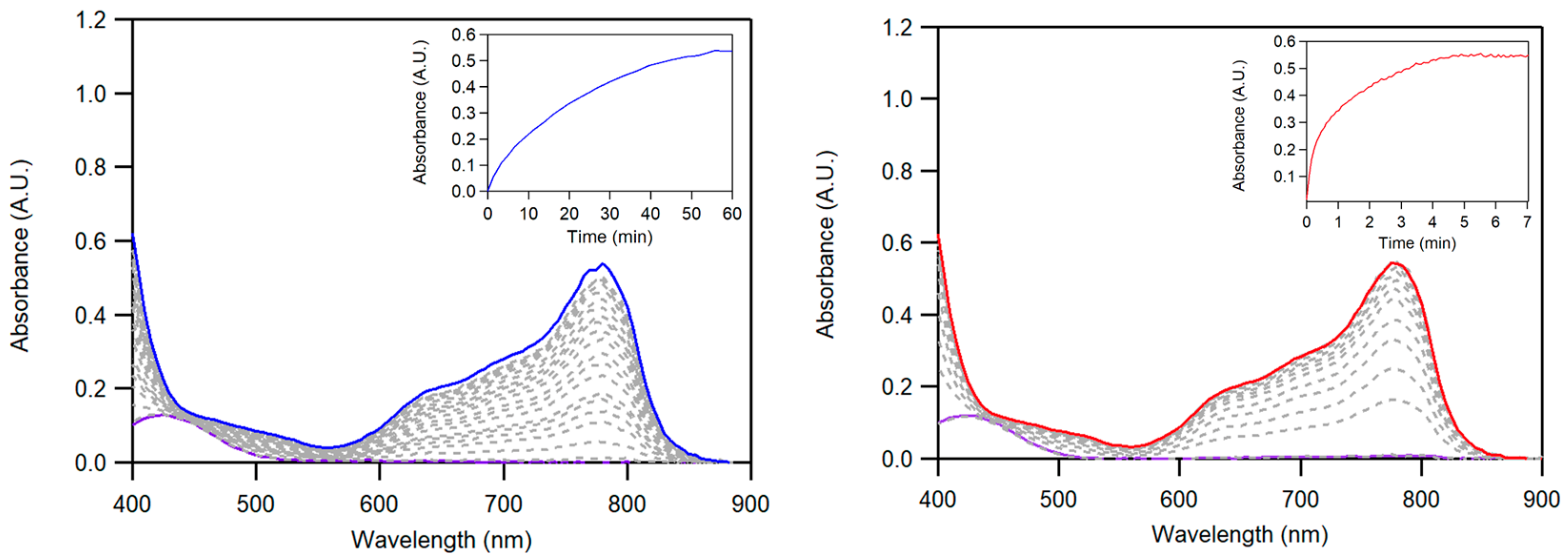
2.2.1. Chemical Reduction of Dioxygen
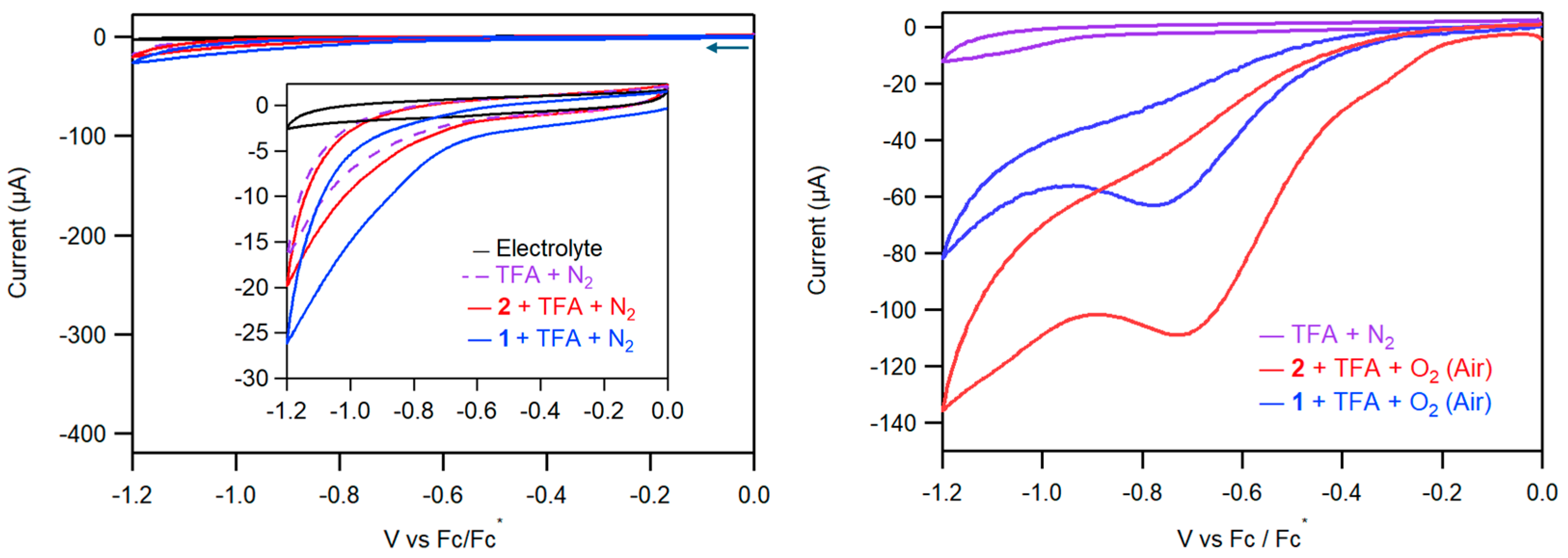
2.2.2. Electrocatalytic ORR by Complexes 1 and 2
3. Discussion
4. Materials and Methods
4.1. General Methods and Instrumentation
4.2. Synthesis of 1 and 2
4.2.1. Synthesis of [CoII(PaPy2Q)](OTf) (1)
4.2.2. Synthesis of Na[CoII(PaPy2N)](OTf)2 (2)
4.3. XRD Characterizations
4.4. Catalytic Reduction of Dioxygen
4.4.1. Chemical Catalytic ORR by UV–Vis Spectroscopy
4.4.2. Electrocatalytic ORR by CV
4.5. Quantification of Produced H2O2 from Chemical Catalytic ORR
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.-L.; Goh, K.; Zhao, L.; Sui, X.-L.; Gong, X.-F.; Cai, J.-J.; Zhou, Q.-Y.; Zhang, H.-D.; Li, L.; Kong, F.-R.; et al. Advanced Non-Noble Materials in Bifunctional Catalysts for ORR and OER toward Aqueous Metal–Air Batteries. Nanoscale 2020, 12, 21534–21559. [Google Scholar] [CrossRef]
- Pegis, M.L.; Wise, C.F.; Martin, D.J.; Mayer, J.M. Oxygen Reduction by Homogeneous Molecular Catalysts and Electrocatalysts. Chem. Rev. 2018, 118, 2340–2391. [Google Scholar] [CrossRef]
- Siahrostami, S.; Verdaguer-Casadevall, A.; Karamad, M.; Deiana, D.; Malacrida, P.; Wickman, B.; Escudero-Escribano, M.; Paoli, E.A.; Frydendal, R.; Hansen, T.W.; et al. Enabling Direct H2O2 Production through Rational Electrocatalyst Design. Nat. Mater. 2013, 12, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Debe, M.K. Electrocatalyst Approaches and Challenges for Automotive Fuel Cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, S.; Ghatak, A.; Dey, A. Second Sphere Effects on Oxygen Reduction and Peroxide Activation by Mononuclear Iron Porphyrins and Related Systems. Chem. Rev. 2022, 122, 12370–12426. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, J.W. Free Radical Catalysis by Galactose Oxidase. Chem. Rev. 2003, 103, 2347–2364. [Google Scholar] [CrossRef] [PubMed]
- Solomon, E.I.; Heppner, D.E.; Johnston, E.M.; Ginsbach, J.W.; Cirera, J.; Qayyum, M.; Kieber-Emmons, M.T.; Kjaergaard, C.H.; Hadt, R.G.; Tian, L. Copper Active Sites in Biology. Chem. Rev. 2014, 114, 3659–3853. [Google Scholar] [CrossRef]
- Das, A.; Ali, A.; Gupta, G.; Santra, A.; Jain, P.; Ingole, P.P.; Paul, S.; Paria, S. Catalytic Four-Electron Reduction of Oxygen to Water by a Molecular Cobalt Complex Consisting of a Proton Exchanging Site at the Secondary Coordination Sphere. ACS Catal. 2023, 13, 5285–5297. [Google Scholar] [CrossRef]
- Luo, J.; Tian, X.; Zeng, J.; Li, Y.; Song, H.; Liao, S. Limitations and Improvement Strategies for Early-Transition-Metal Nitrides as Competitive Catalysts toward the Oxygen Reduction Reaction. ACS Catal. 2016, 6, 6165–6174. [Google Scholar] [CrossRef]
- Gewirth, A.A.; Varnell, J.A.; DiAscro, A.M. Nonprecious Metal Catalysts for Oxygen Reduction in Heterogeneous Aqueous Systems. Chem. Rev. 2018, 118, 2313–2339. [Google Scholar] [CrossRef]
- Sun, L.; Gutierrez, J.; Goff, A.L.; Gatineau, D.; Jackson, T.A.; Gennari, M.; Duboc, C. A Non-Macrocycle Thiolate-Based Cobalt Catalyst for Selective O2 Reduction into H2O. ChemCatChem 2024, 16, e202400270. [Google Scholar] [CrossRef]
- Cook, E.N.; Machan, C.W. Bioinspired Mononuclear Mn Complexes for O2 Activation and Biologically Relevant Reactions. Dalton Trans. 2021, 50, 16871–16886. [Google Scholar] [CrossRef] [PubMed]
- McCormick, M.J.; Machan, C.W. Developing Homogeneous First Row Early Transition Metal Catalysts for the Oxygen Reduction Reaction. Dalton Trans. 2024, 53, 16807–16814. [Google Scholar] [CrossRef]
- Nishiori, D.; Menzel, J.P.; Armada, N.; Reyes Cruz, E.A.; Nannenga, B.L.; Batista, V.S.; Moore, G.F. Breaking a Molecular Scaling Relationship Using an Iron–Iron Fused Porphyrin Electrocatalyst for Oxygen Reduction. J. Am. Chem. Soc. 2024, 146, 11622–11633. [Google Scholar] [CrossRef]
- Bhunia, S.; Ghatak, A.; Rana, A.; Dey, A. Amine Groups in the Second Sphere of Iron Porphyrins Allow for Higher and Selective 4e–/4H+ Oxygen Reduction Rates at Lower Overpotentials. J. Am. Chem. Soc. 2023, 145, 3812–3825. [Google Scholar] [CrossRef]
- Martin, D.J.; Mayer, J.M. Oriented Electrostatic Effects on O2 and CO2 Reduction by a Polycationic Iron Porphyrin. J. Am. Chem. Soc. 2021, 143, 11423–11434. [Google Scholar] [CrossRef]
- Bhunia, S.; Rana, A.; Ghosh Dey, S.; Ivancich, A.; Dey, A. A Designed Second-Sphere Hydrogen-Bond Interaction That Critically Influences the O–O Bond Activation for Heterolytic Cleavage in Ferric Iron–Porphyrin Complexes. Chem. Sci. 2020, 11, 2681–2695. [Google Scholar] [CrossRef] [PubMed]
- Ghatak, A.; Bhakta, S.; Bhunia, S.; Dey, A. Influence of the Distal Guanidine Group on the Rate and Selectivity of O2 Reduction by Iron Porphyrin. Chem. Sci. 2019, 10, 9692–9698. [Google Scholar] [CrossRef]
- Bhunia, S.; Rana, A.; Roy, P.; Martin, D.J.; Pegis, M.L.; Roy, B.; Dey, A. Rational Design of Mononuclear Iron Porphyrins for Facile and Selective 4e–/4H+ O2 Reduction: Activation of O–O Bond by 2nd Sphere Hydrogen Bonding. J. Am. Chem. Soc. 2018, 140, 9444–9457. [Google Scholar] [CrossRef]
- Dinda, S.; Siddiqui, A.I.; Behera, S.; Mondal, B. Demystifying the Role of Covalently Attached Electron–Proton Transfer Mediator in Oxygen Reduction Reaction Catalyst. ACS Catal. 2023, 13, 12643–12647. [Google Scholar] [CrossRef]
- Sinha, S.; Ghosh, M.; Warren, J.J. Changing the Selectivity of O2 Reduction Catalysis with One Ligand Heteroatom. ACS Catal. 2019, 9, 2685–2691. [Google Scholar] [CrossRef]
- Xu, Y.; Jin, X.; Zhang, J.; Qin, H.; Mei, B.; Liu, T.; Lei, S.; Li, S.; Bo, Y.; Li, X.; et al. Regulating Steric Effect of Cobalt Corroles for Promoted Oxygen Electrocatalysis. ACS Catal. 2024, 14, 14350–14355. [Google Scholar] [CrossRef]
- Gao, Y.; Lei, H.; Bao, Z.; Liu, X.; Qin, L.; Yin, Z.; Li, H.; Huang, S.; Zhang, W.; Cao, R. Electrocatalytic Oxygen Reduction with Cobalt Corroles Bearing Cationic Substituents. Phys. Chem. Chem. Phys. 2023, 25, 4604–4610. [Google Scholar] [CrossRef] [PubMed]
- Nichols, A.W.; Cook, E.N.; Gan, Y.J.; Miedaner, P.R.; Dressel, J.M.; Dickie, D.A.; Shafaat, H.S.; Machan, C.W. Pendent Relay Enhances H2O2 Selectivity during Dioxygen Reduction Mediated by Bipyridine-Based Co–N2O2 Complexes. J. Am. Chem. Soc. 2021, 143, 13065–13073. [Google Scholar] [CrossRef]
- Das, A.; Santra, A.; Kumari, A.; Ghosh, D.; Paria, S. Breaking the Scaling Relationship for Oxygen Reduction Reaction Using Molecular Cobalt Complexes. J. Am. Chem. Soc. 2025, 147, 6549–6560. [Google Scholar] [CrossRef]
- Opalade, A.A.; Hessefort, L.; Day, V.W.; Jackson, T.A. Controlling the Reactivity of a Metal-Hydroxo Adduct with a Hydrogen Bond. J. Am. Chem. Soc. 2021, 143, 15159–15175. [Google Scholar] [CrossRef]
- Chang, C.J.; Loh, Z.-H.; Shi, C.; Anson, F.C.; Nocera, D.G. Targeted Proton Delivery in the Catalyzed Reduction of Oxygen to Water by Bimetallic Pacman Porphyrins. J. Am. Chem. Soc. 2004, 126, 10013–10020. [Google Scholar] [CrossRef]
- McGuire, R., Jr.; Dogutan, D.K.; Teets, T.S.; Suntivich, J.; Shao-Horn, Y.; Nocera, D.G. Oxygen Reduction Reactivity of Cobalt(II) Hangman Porphyrins. Chem. Sci. 2010, 1, 411–414. [Google Scholar] [CrossRef]
- Gliemann, G. A. B. P. Lever: Inorganic Electronic Spectroscopy, Vol. 33 Aus: Studies in Physical and Theoretical Chemistry, Elsevier, Amsterdam, Oxford, New York, Tokio 1984. 863 Seiten, Preis: $113, 50. Berichte Bunsenges. Phys. Chem. 1985, 89, 99–100. [Google Scholar] [CrossRef]
- Wang, C.-C.; Chang, H.-C.; Lai, Y.-C.; Fang, H.; Li, C.-C.; Hsu, H.-K.; Li, Z.-Y.; Lin, T.-S.; Kuo, T.-S.; Neese, F.; et al. A Structurally Characterized Nonheme Cobalt–Hydroperoxo Complex Derived from Its Superoxo Intermediate via Hydrogen Atom Abstraction. J. Am. Chem. Soc. 2016, 138, 14186–14189. [Google Scholar] [CrossRef]
- Stoufer, R.C.; Smith, D.W.; Clevenger, E.A.; Norris, T.E. Complexes of Cobalt(II). I. On the Anomalous Magnetic Behavior of Some Six-Coordinate Cobalt(II) Complexes. Inorg. Chem. 1966, 5, 1167–1171. [Google Scholar] [CrossRef]
- Thuery, P.; Zarembowitch, J. Spin State of Cobalt(II) in Five- and Six-Coordinate Lewis Base Adducts of N,N’-Ethylenebis(3-Carboxysalicylaldiminato)Cobalt(II). New Spin-Crossover Complexes. Inorg. Chem. 1986, 25, 2001–2008. [Google Scholar] [CrossRef]
- Wang, L.; Gennari, M.; Cantú Reinhard, F.G.; Gutiérrez, J.; Morozan, A.; Philouze, C.; Demeshko, S.; Artero, V.; Meyer, F.; de Visser, S.P.; et al. A Non-Heme Diiron Complex for (Electro)Catalytic Reduction of Dioxygen: Tuning the Selectivity through Electron Delivery. J. Am. Chem. Soc. 2019, 141, 8244–8253. [Google Scholar] [CrossRef]
- Mangue, J.; Gondre, C.; Pécaut, J.; Duboc, C.; Ménage, S.; Torelli, S. Controlled O2 Reduction at a Mixed-Valent (II,I) Cu2S Core. Chem. Commun. 2020, 56, 9636–9639. [Google Scholar] [CrossRef]
- Lin, J.; Xin, Q.; Gao, X. Real-Time Detection of Hydrogen Peroxide Using Microelectrodes in an Ultrasonic Enhanced Heterogeneous Fenton Process Catalyzed by Ferrocene. Environ. Sci. Pollut. Res. Int. 2015, 22, 11170–11174. [Google Scholar] [CrossRef]
- Lubach, J.; Drenth, W. Enolization and Oxidation: II. Oxidation of Ferrocene by Molecular Oxygen and Hydrogen Peroxide in Acidic Media. Recl. Trav. Chim. Pays-Bas 1973, 92, 586–592. [Google Scholar] [CrossRef]
- Fomin, F.M.; Zaitseva, K.S. Mechanism of the Oxidation of Ferrocene and Its Derivatives with Hydrogen Peroxide: A New Effect. Russ. J. Phys. Chem. 2014, 88, 466–470. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, G.-S.; Liu, H.-C.; Zhu, M.-J.; Xie, M.-Y.; Cen, M.-S.; Li, Q.-J.; Wang, T.-S.; Zhang, H.-X. Bidirectional O2 Reduction/H2O Oxidation Boosted by a Pentadentate Pyridylalkylamine Copper(II) Complex. Electrochimica Acta 2023, 446, 142099. [Google Scholar] [CrossRef]
- Passard, G.; Dogutan, D.K.; Qiu, M.; Costentin, C.; Nocera, D.G. Oxygen Reduction Reaction Promoted by Manganese Porphyrins. ACS Catal. 2018, 8, 8671–8679. [Google Scholar] [CrossRef]
- Yoshikawa, S.; Shimada, A. Reaction Mechanism of Cytochrome c Oxidase. Chem. Rev. 2015, 115, 1936–1989. [Google Scholar] [CrossRef] [PubMed]
- Bullock, R.M.; Chen, J.G.; Gagliardi, L.; Chirik, P.J.; Farha, O.K.; Hendon, C.H.; Jones, C.W.; Keith, J.A.; Klosin, J.; Minteer, S.D.; et al. Using Nature’s Blueprint to Expand Catalysis with Earth-Abundant Metals. Science 2020, 369, eabc3183. [Google Scholar] [CrossRef] [PubMed]
- Cook, E.N.; Machan, C.W. Homogeneous Catalysis of Dioxygen Reduction by Molecular Mn Complexes. Chem. Commun. 2022, 58, 11746–11761. [Google Scholar] [CrossRef]
- Ghosh, A.C.; Duboc, C.; Gennari, M. Synergy between Metals for Small Molecule Activation: Enzymes and Bio-Inspired Complexes. Coord. Chem. Rev. 2021, 428, 213606. [Google Scholar] [CrossRef]
- Passard, G.; Ullman, A.M.; Brodsky, C.N.; Nocera, D.G. Oxygen Reduction Catalysis at a Dicobalt Center: The Relationship of Faradaic Efficiency to Overpotential. J. Am. Chem. Soc. 2016, 138, 2925–2928. [Google Scholar] [CrossRef]
- Monte-Pérez, I.; Kundu, S.; Chandra, A.; Craigo, K.E.; Chernev, P.; Kuhlmann, U.; Dau, H.; Hildebrandt, P.; Greco, C.; Van Stappen, C.; et al. Temperature Dependence of the Catalytic Two- versus Four-Electron Reduction of Dioxygen by a Hexanuclear Cobalt Complex. J. Am. Chem. Soc. 2017, 139, 15033–15042. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Pegis, M.L.; Mayer, J.M.; Stahl, S.S. Molecular Cobalt Catalysts for O2 Reduction: Low-Overpotential Production of H2O2 and Comparison with Iron-Based Catalysts. J. Am. Chem. Soc. 2017, 139, 16458–16461. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Goldsmith, Z.K.; Schneider, P.E.; Anson, C.W.; Gerken, J.B.; Ghosh, S.; Hammes-Schiffer, S.; Stahl, S.S. Kinetic and Mechanistic Characterization of Low-Overpotential, H2O2-Selective Reduction of O2 Catalyzed by N2O2-Ligated Cobalt Complexes. J. Am. Chem. Soc. 2018, 140, 10890–10899. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Mondal, B.; Stahl, S.S. Molecular Cobalt Catalysts for O2 Reduction to H2O2: Benchmarking Catalyst Performance via Rate–Overpotential Correlations. ACS Catal. 2020, 10, 12031–12039. [Google Scholar] [CrossRef]
- Lv, B.; Li, X.; Guo, K.; Ma, J.; Wang, Y.; Lei, H.; Wang, F.; Jin, X.; Zhang, Q.; Zhang, W.; et al. Controlling Oxygen Reduction Selectivity through Steric Effects: Electrocatalytic Two-Electron and Four-Electron Oxygen Reduction with Cobalt Porphyrin Atropisomers. Angew. Chem. Int. Ed. 2021, 60, 12742–12746. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, P.; Li, X.; Xie, L.; Wang, N.; Lei, H.; Zhang, C.; Zhang, W.; Lee, Y.-M.; Zhang, W.; et al. Crucial Roles of a Pendant Imidazole Ligand of a Cobalt Porphyrin Complex in the Stoichiometric and Catalytic Reduction of Dioxygen. Angew. Chem. Int. Ed. 2022, 61, e202208143. [Google Scholar] [CrossRef]
- Eroy-Reveles, A.A.; Leung, Y.; Beavers, C.M.; Olmstead, M.M.; Mascharak, P.K. Near-Infrared Light Activated Release of Nitric Oxide from Designed Photoactive Manganese Nitrosyls: Strategy, Design, and Potential as NO Donors. J. Am. Chem. Soc. 2008, 130, 4447–4458. [Google Scholar] [CrossRef]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of Silver and Molybdenum Microfocus X-Ray Sources for Single-Crystal Structure Determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, C.; Kawamoto, N.; Takamura, K. Oxo[5,10,15,20-Tetra(4-Pyridyl)Porphyrinato]Titanium(IV): An Ultra-High Sensitivity Spectrophotometric Reagent for Hydrogen Peroxide. Analyst 1992, 117, 1781–1784. [Google Scholar] [CrossRef]
- Takamura, K.; Matsubara, C.; Matsumoto, T. Reaction Specificity of a Titanium(IV)-Porphyrin Complex to Hydrogen Peroxide in View of an Ab Initio Study. Anal. Sci. 2008, 24, 401–404. [Google Scholar] [CrossRef]

| Property | 1 | 2 |
|---|---|---|
| L = PaPy2Q | L = PaPy2N | |
| Cobalt–ligand bond lengths | ||
| Co–N1 | 2.107 (3) | 2.0806 (19) |
| Co–N2 | 1.935 (3) | 1.959 (2) |
| Co–N3 | 2.182 (3) | 2.1914 (19) |
| Co–N4 | 2.036 (3) | 2.041 (2) |
| Co–N5 | 2.058 (3) | 2.0503 (19) |
| λmax (ε) | 650 (50) | 650 (20) |
| 540 (180) | 545 (115) | |
| 460 (150) | 450 (190) | |
| 1H NMR | 149.1 | 141.5 |
| 132.6 | 121.2 | |
| 79.9 | 95.7 | |
| 77.4 | 90.4 | |
| 65.6 | 73.0 | |
| 55.5 | 65.9 | |
| 53.9 | 53.8 | |
| 44.3 | 45.8 | |
| −1.5 | −0.4 | |
| −7.4 | −5.9 | |
| g | ~4.4 | ~4.4 |
| ~2.0 | ~1.9 | |
| μeff | 2.6 | 2.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aghaei, Z.; Opalade, A.A.; Day, V.W.; Jackson, T.A. Oxygen Reduction by Amide-Ligated Cobalt Complexes: Effect of Hydrogen Bond Acceptor. Molecules 2025, 30, 3274. https://doi.org/10.3390/molecules30153274
Aghaei Z, Opalade AA, Day VW, Jackson TA. Oxygen Reduction by Amide-Ligated Cobalt Complexes: Effect of Hydrogen Bond Acceptor. Molecules. 2025; 30(15):3274. https://doi.org/10.3390/molecules30153274
Chicago/Turabian StyleAghaei, Zahra, Adedamola A. Opalade, Victor W. Day, and Timothy A. Jackson. 2025. "Oxygen Reduction by Amide-Ligated Cobalt Complexes: Effect of Hydrogen Bond Acceptor" Molecules 30, no. 15: 3274. https://doi.org/10.3390/molecules30153274
APA StyleAghaei, Z., Opalade, A. A., Day, V. W., & Jackson, T. A. (2025). Oxygen Reduction by Amide-Ligated Cobalt Complexes: Effect of Hydrogen Bond Acceptor. Molecules, 30(15), 3274. https://doi.org/10.3390/molecules30153274








