Synthesis of Carboranyl-Containing β-Arylaliphatic Acids for Potential Application in BNCT
Abstract
1. Introduction
2. Results and Discussion
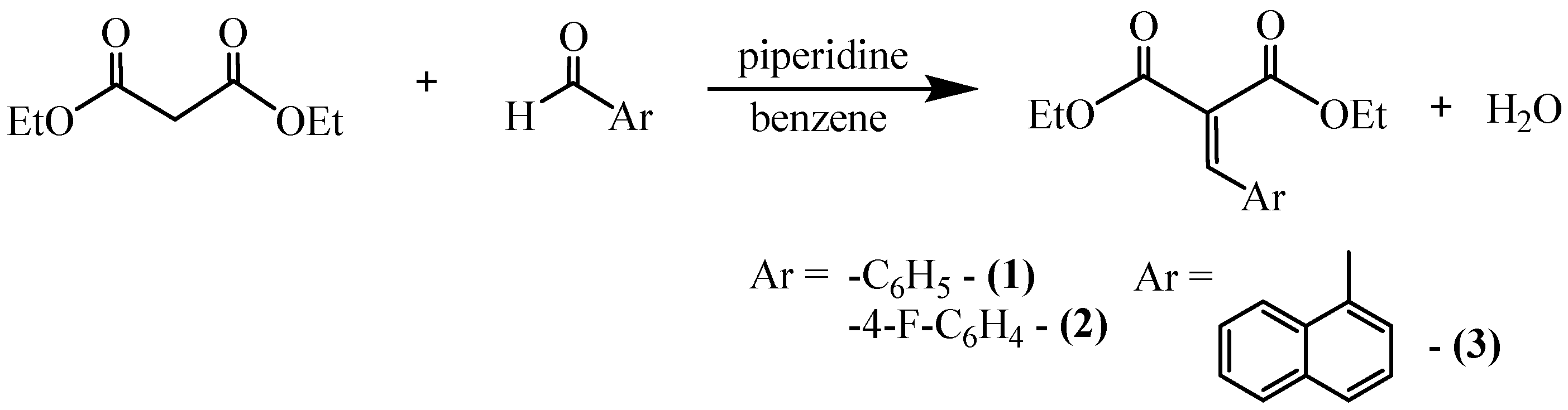
2.1. Synthesis of Arylidenemalonates
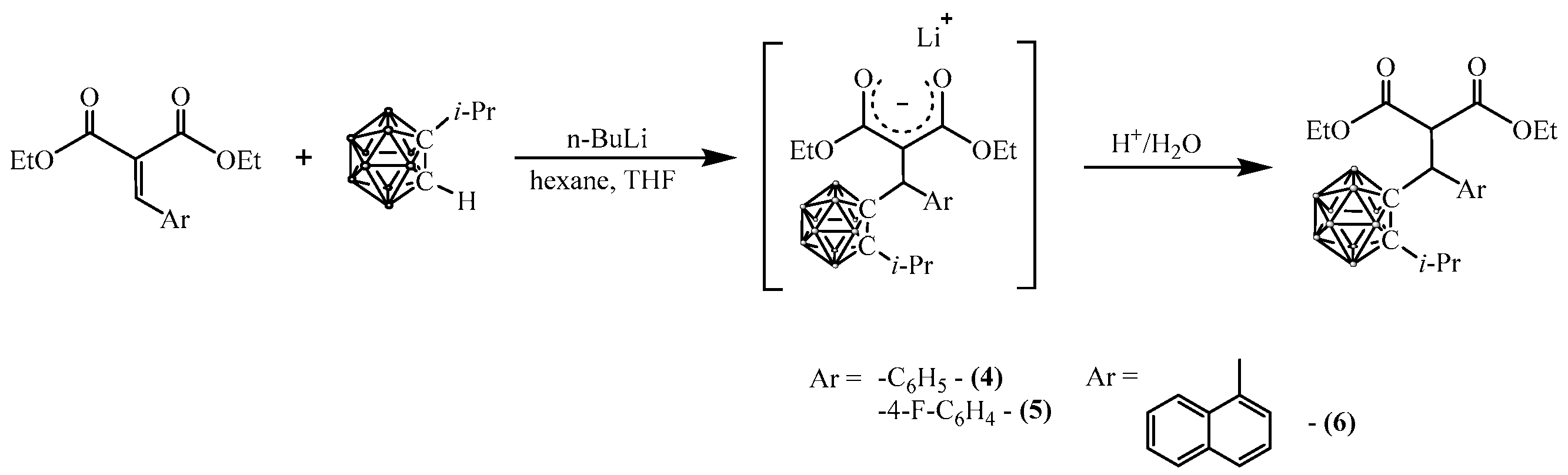
2.2. Synthesis of Carboranyl-Containing Derivatives of Arylidene Malonates
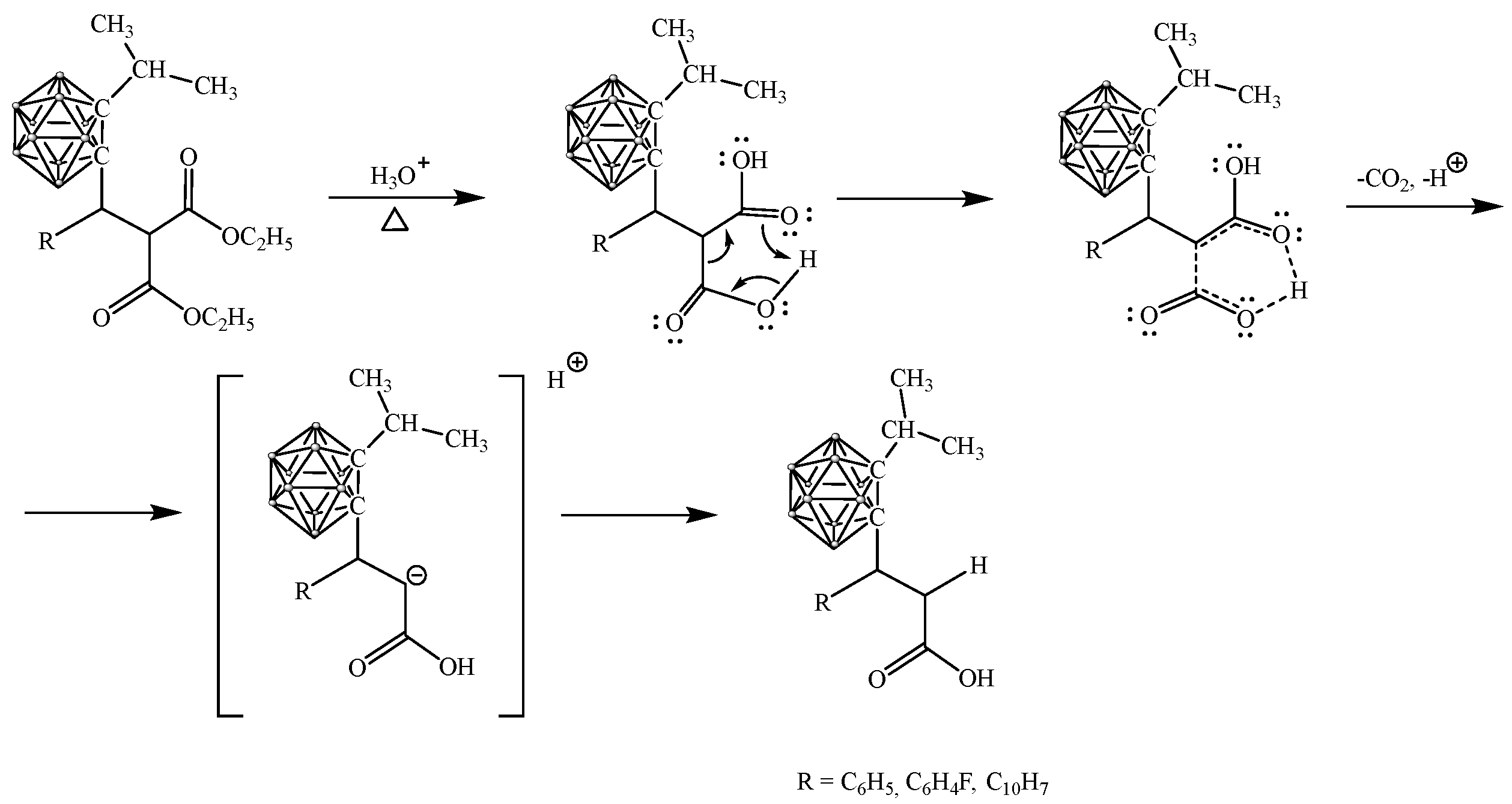
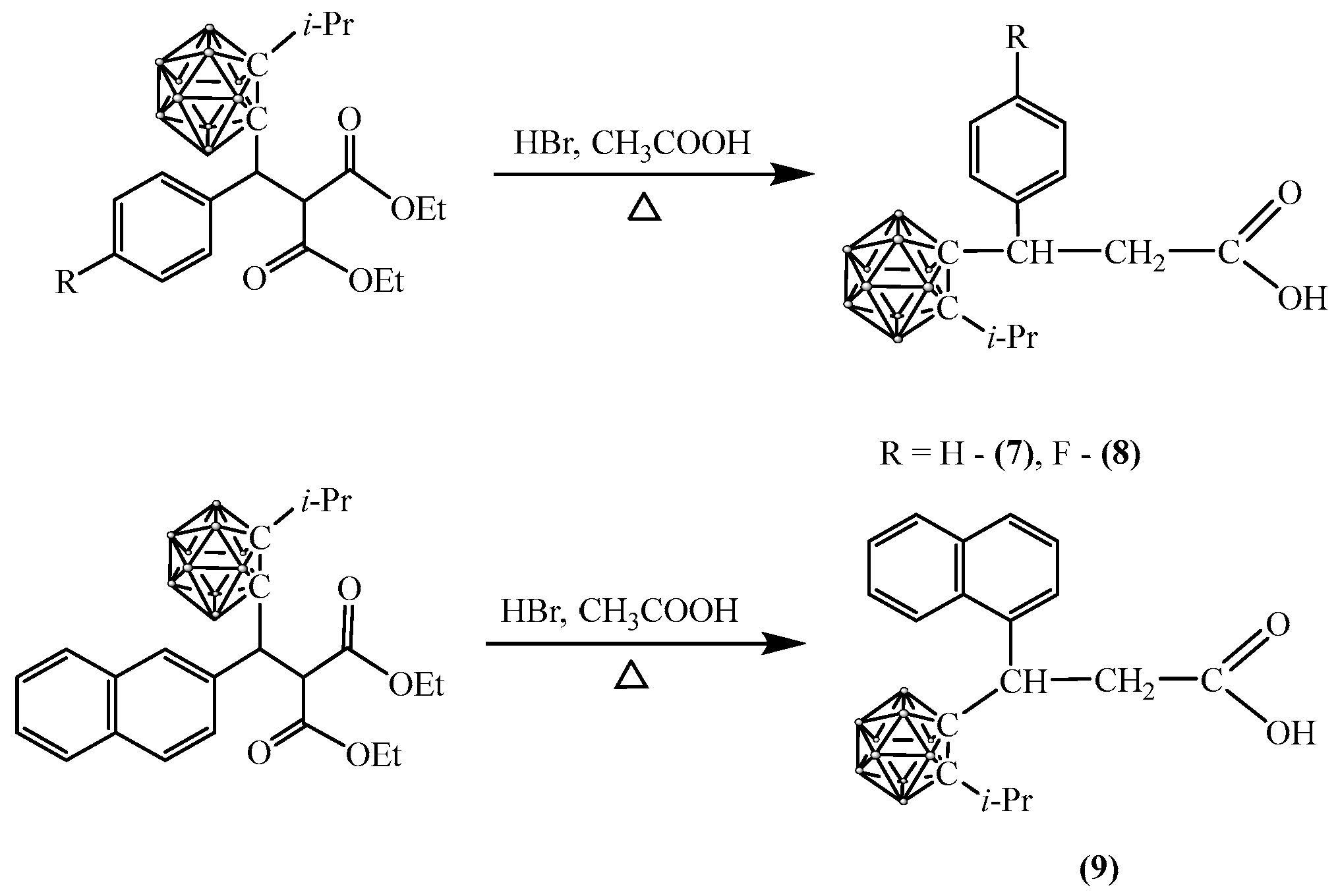
2.3. Synthesis of Carboranyl-Containing Derivatives of 3-Arylpropanoic Acid
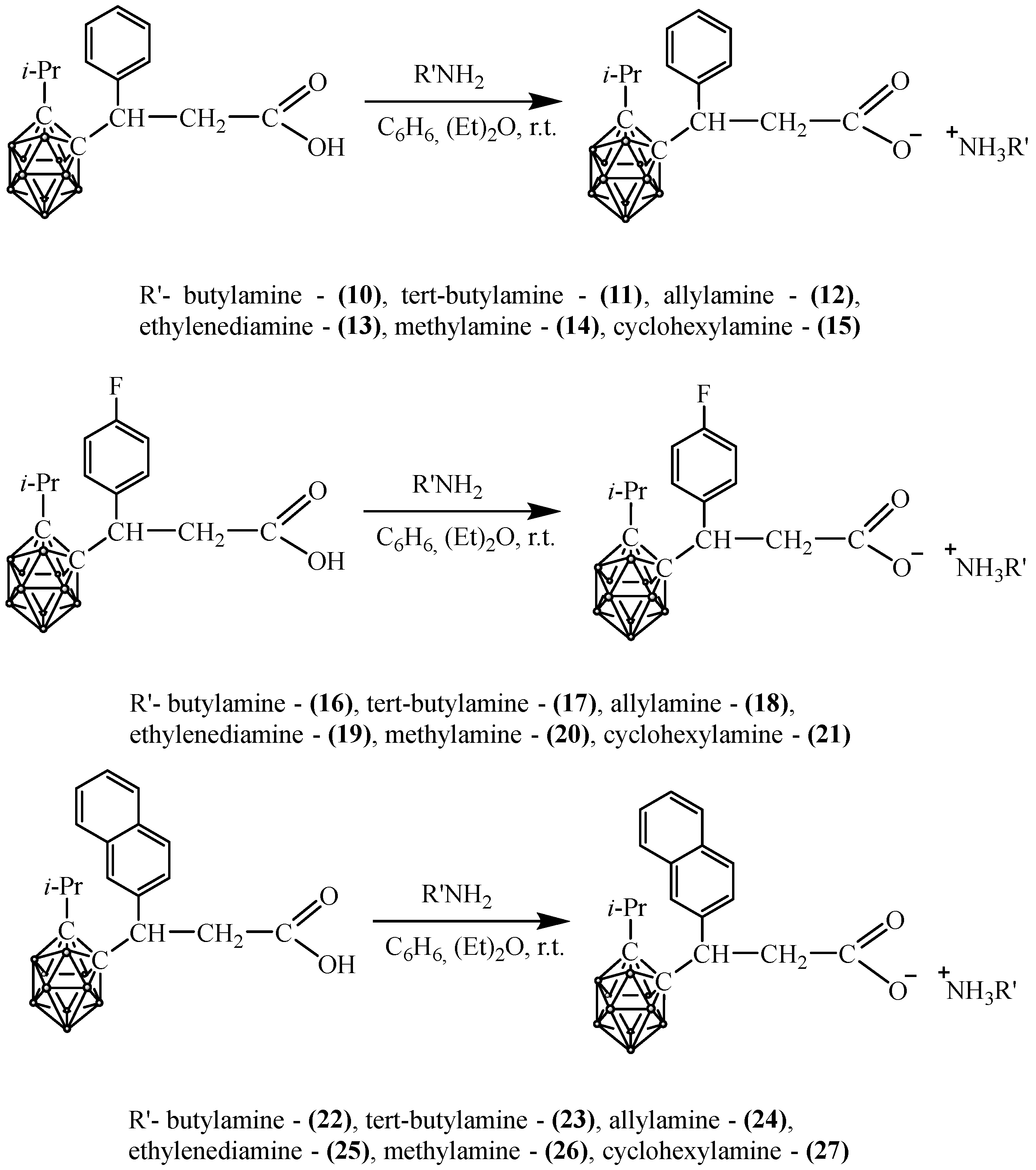
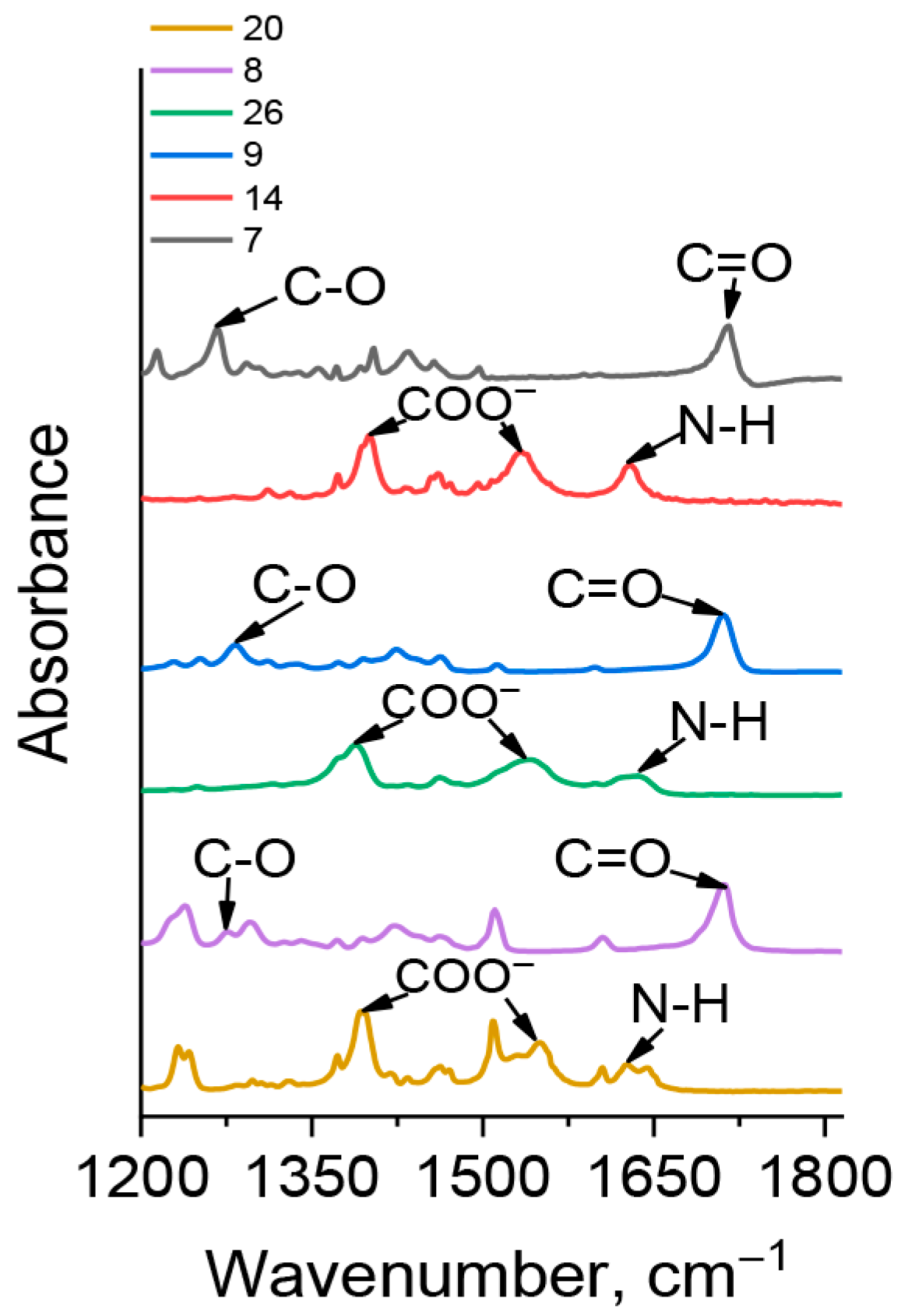
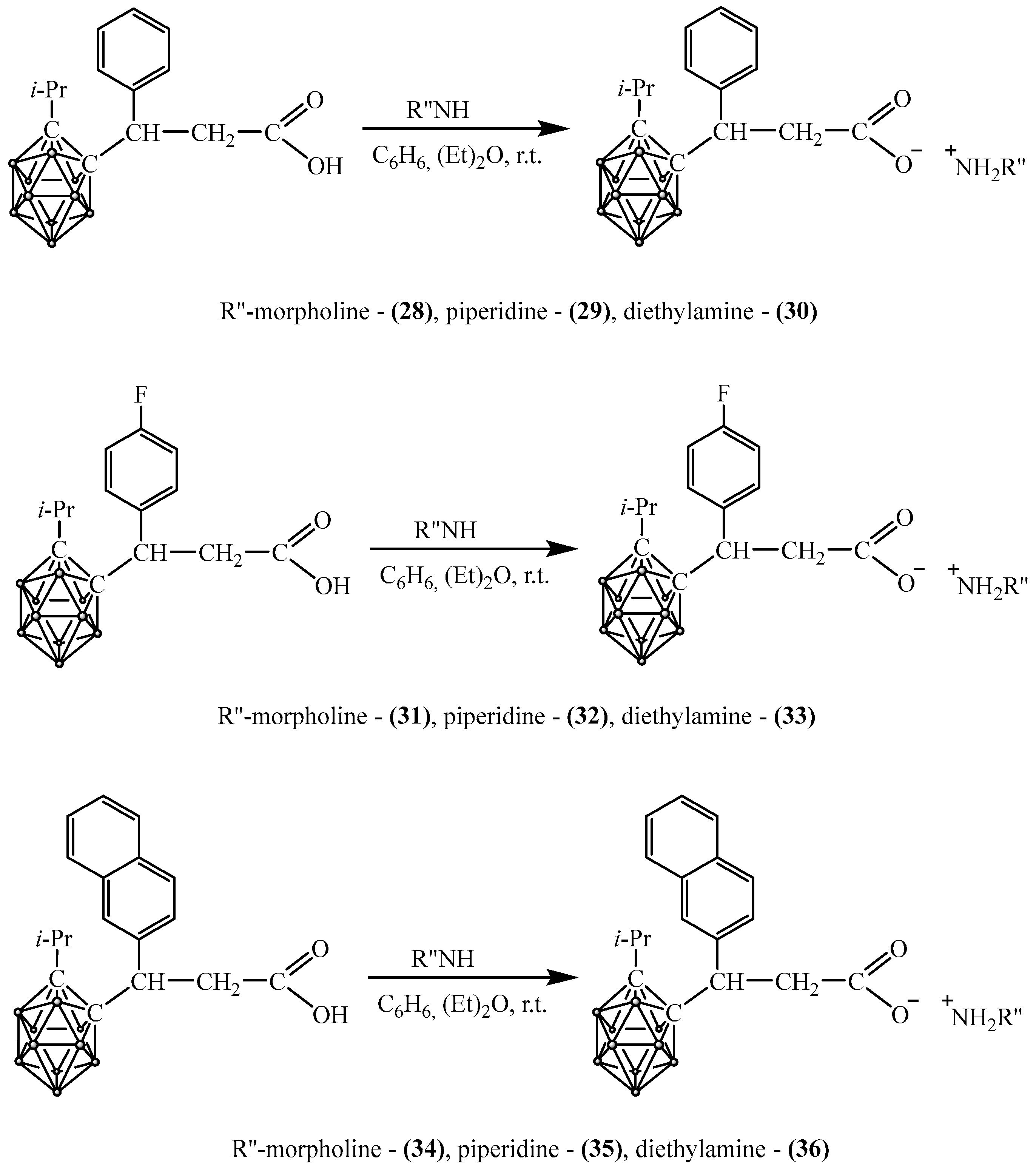
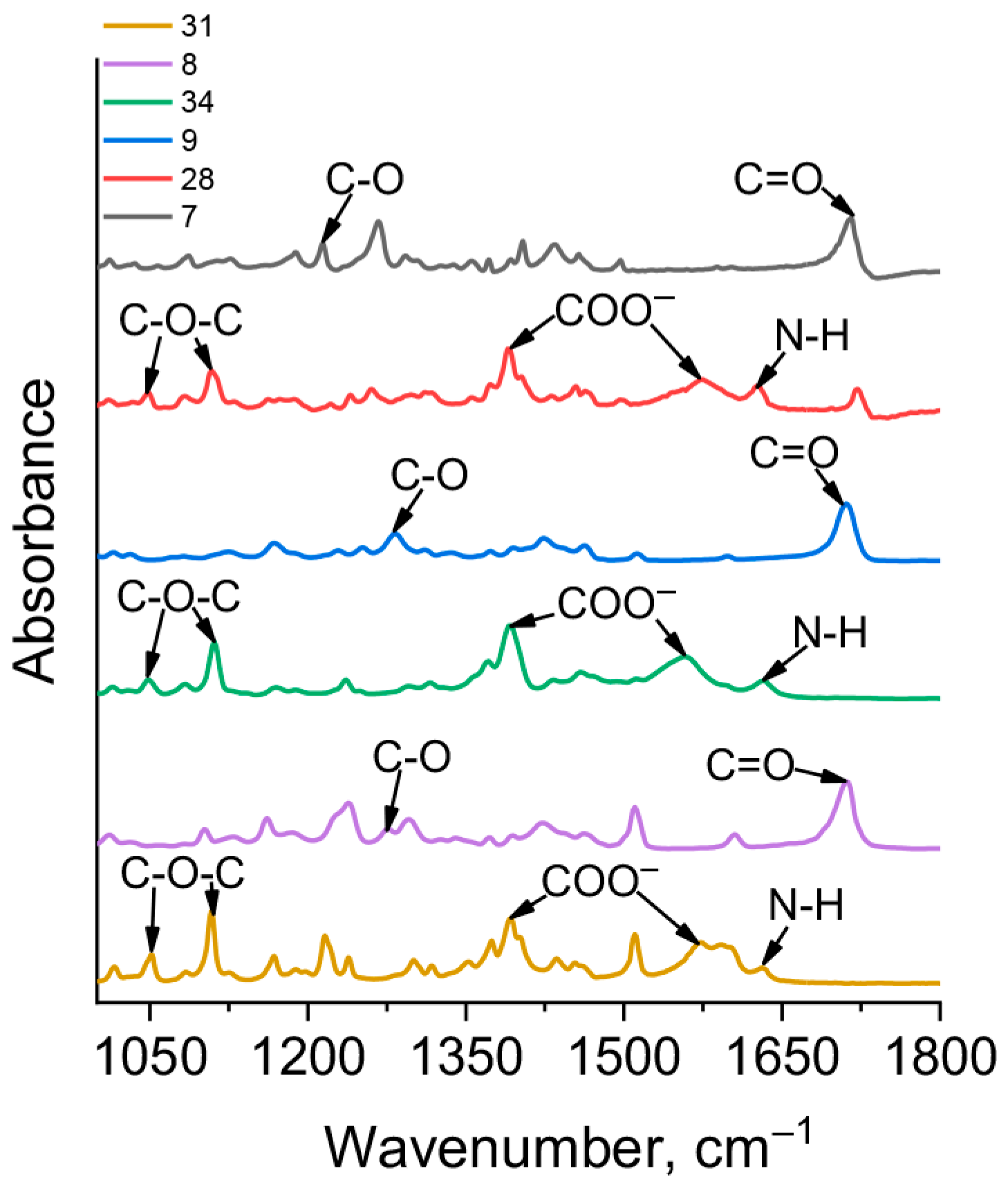
2.4. Synthesis of Carboranyl-Containing Ammonium Carboxylate Salts
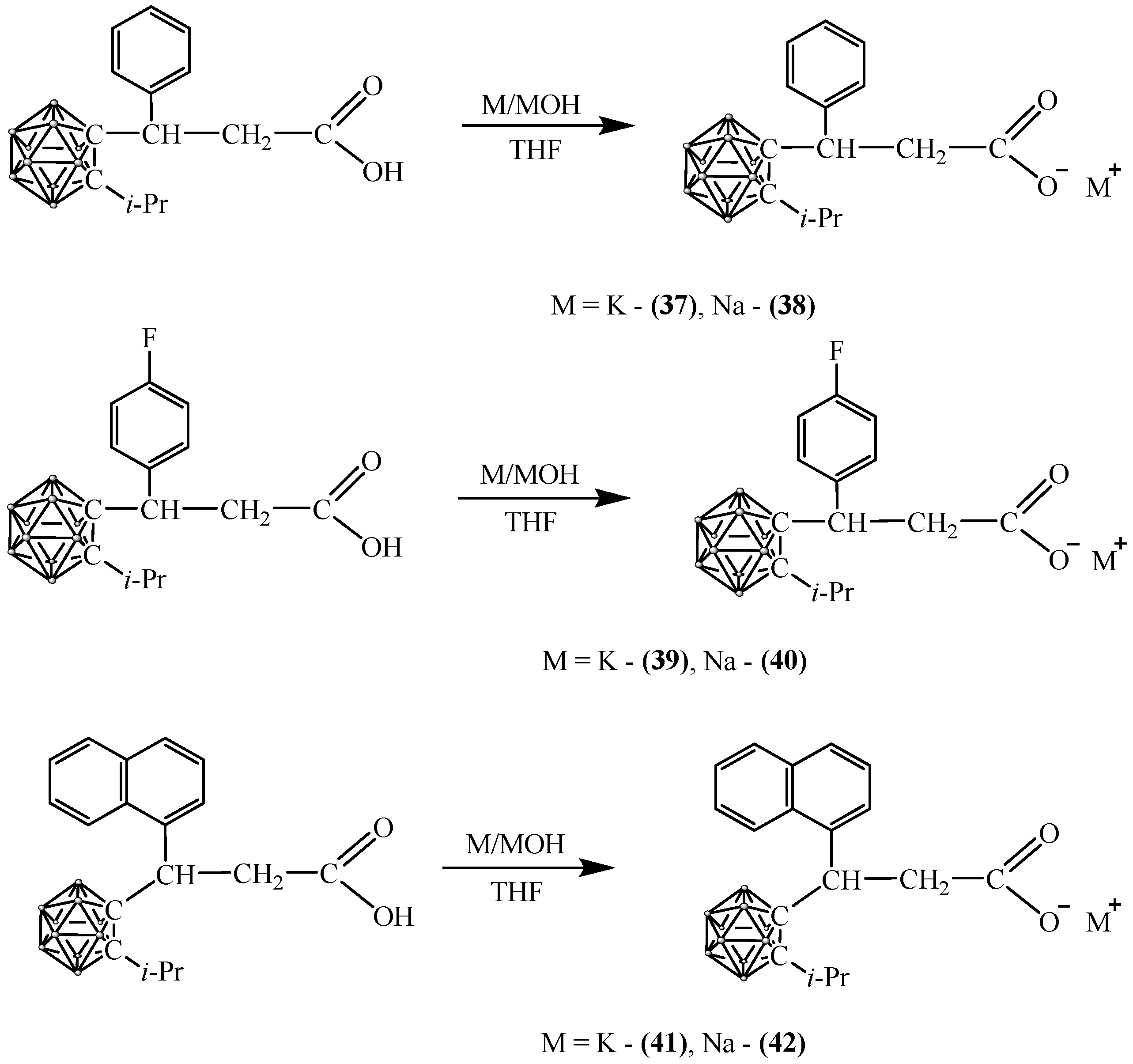
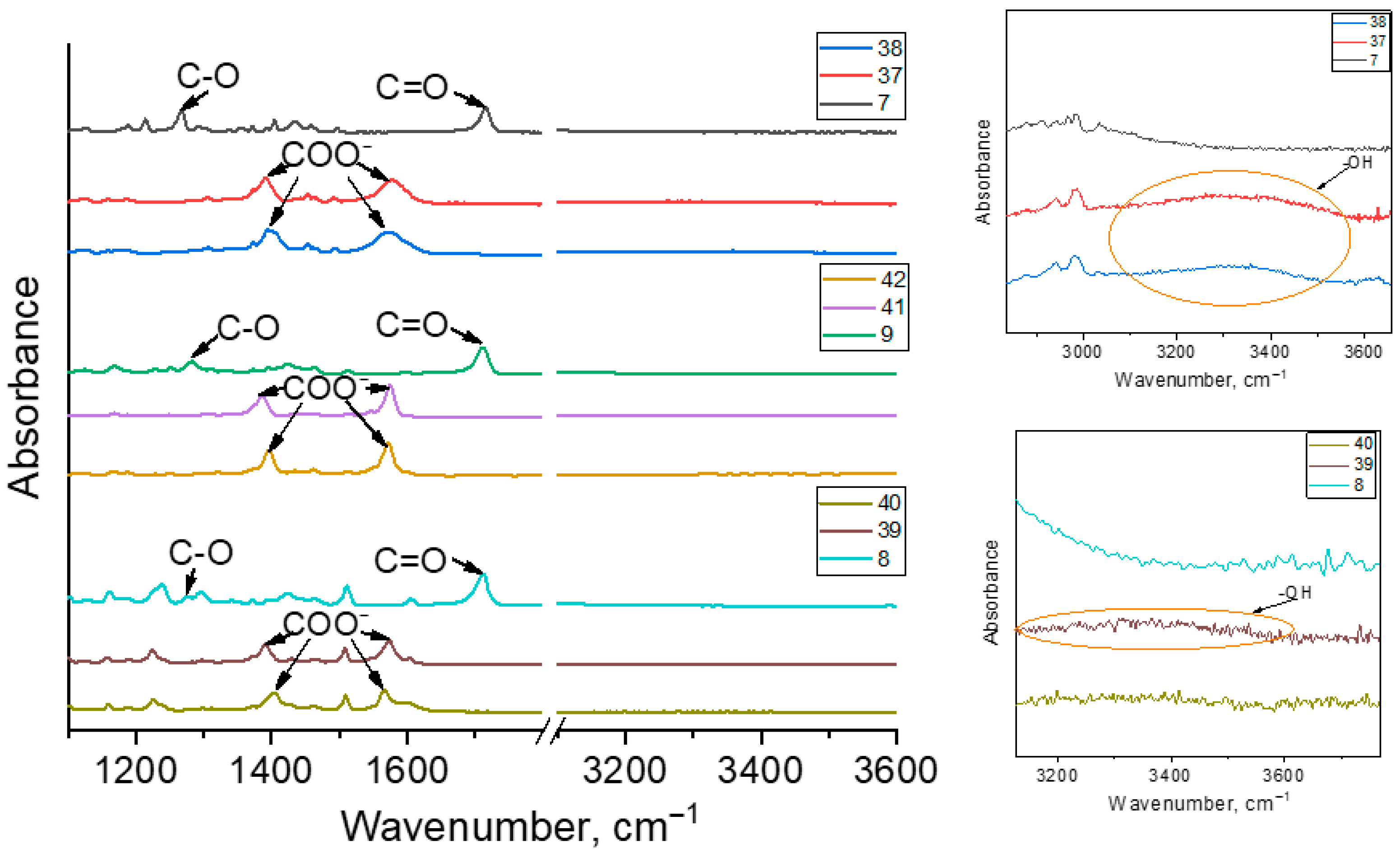
2.5. Synthesis of Carboranyl-Containing Carboxylate Salts Using Metals and Their Hydroxides
2.6. In Vitro Cytotoxicity Testing
3. Materials and Methods
3.1. Materials and Chemicals
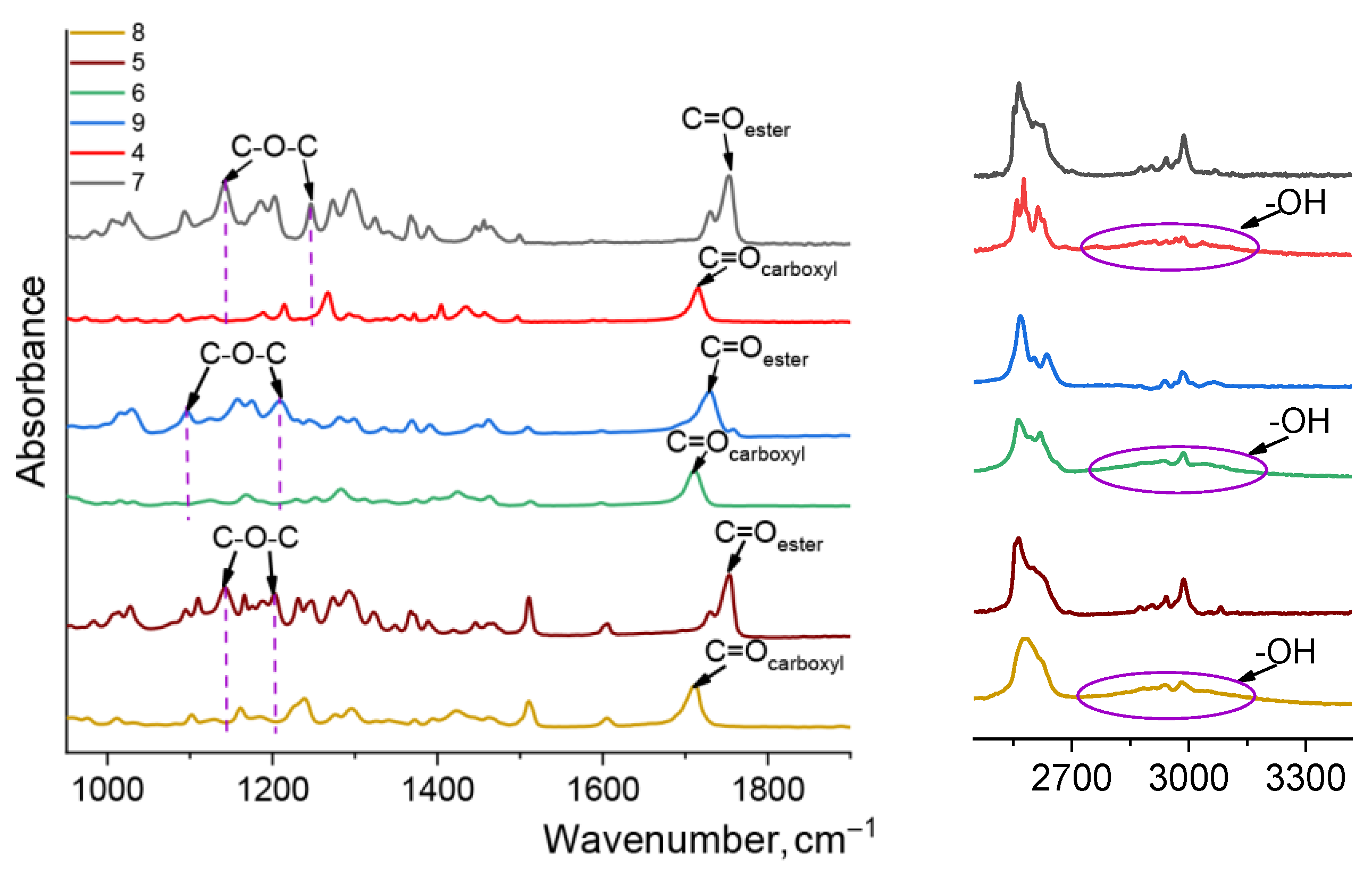
3.2. Methods of Characterization
3.3. Synthesis of Diethyl Arylidenemalonates: General Procedure
3.4. Synthesis of Diethyl Arylidenemalonates with Isopropyl-o-carborane: General Procedure
3.5. Synthesis of Isopropyl-o-carboranyl Containing Derivatives of 3-Arylpropanoic Acid: General Procedure
3.6. Synthesis of Isopropyl-o-carboranyl Containing 3-Arylpropanoic Acid with Amines: General Procedure
3.6.1. Synthesis of Isopropyl-o-carboranyl Containing 3-Arylpropanoic Acid with Primary Amines
3.6.2. Synthesis of Isopropyl-o-carboranyl Containing 3-Arylpropanoic Acid with Secondary Amines
3.7. Synthesis of Carboranyl-Containing 3-Arylpropanoic Acid with Potassium and Sodium Metallic/Hydroxides
3.8. Cytotoxic Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Begum, H.; Sheikh, K.; Kumar, M.; Masood, I.; Khokhar, A. International Cancer Burden Analysis 2020–2024: GLOBOCAN-Derived Estimates of Incidence and Mortality for 30 Malignancies in 190 Geographic Regions. Am. J. Biomed. 2025, 13, 1–18. [Google Scholar] [CrossRef]
- Liao, L. Inequality in Breast Cancer: Global Statistics from 2022 to 2050. Breast 2025, 79, 103851. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Hulvat, M.C. Cancer Incidence and Trends. Surg. Clin. N. Am. 2020, 100, 469–481. [Google Scholar] [CrossRef]
- Murilla, R.M.; Edilo, G.G.; Budlayan, M.L.M.; Auxtero, E.S. Boron Delivery Agents in BNCT: A Mini Review of Current Developments and Emerging Trends. Nano TransMed 2025, 4, 100081. [Google Scholar] [CrossRef]
- Jin, W.H.; Seldon, C.; Butkus, M.; Sauerwein, W.; Giap, H.B. A Review of Boron Neutron Capture Therapy: Its History and Current Challenges. Int. J. Part. Ther. 2022, 9, 71–82. [Google Scholar] [CrossRef]
- He, H.; Li, J.; Jiang, P.; Tian, S.; Wang, H.; Fan, R.; Liu, J.; Yang, Y.; Liu, Z.; Wang, J. The Basis and Advances in Clinical Application of Boron Neutron Capture Therapy. Radiat. Oncol. 2021, 16, 216. [Google Scholar] [CrossRef] [PubMed]
- Barth, R.F.; Mi, P.; Yang, W. Boron Delivery Agents for Neutron Capture Therapy of Cancer. Cancer Commun. 2018, 38, 35. [Google Scholar] [CrossRef] [PubMed]
- Vos, M.J.; Turowski, B.; Zanella, F.E.; Paquis, P.; Siefert, A.; Hideghéty, K.; Haselsberger, K.; Grochulla, F.; Postma, T.J.; Wittig, A.; et al. Radiologic Findings in Patients Treated with Boron Neutron Capture Therapy for Glioblastoma Multiforme within EORTC Trial 11961. Int. J. Radiat. Oncol. Biol. Phys. 2005, 61, 392–399. [Google Scholar] [CrossRef]
- Coderre, J.A.; Elowitz, E.H.; Chadha, M.; Bergland, R.; Capala, J.; Joel, D.D.; Liu, H.B.; Slatkin, D.N.; Chanana, A.D. Boron neutron capture therapy for glioblastoma multiforme using p-boronophenylalanine and epithermal neutrons: Trial design and early clinical results. J. Neurooncol. 1997, 33, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Sköld, K.; H-Stenstam, B.; Diaz, A.Z.; Giusti, V.; Pellettieri, L.; Hopewell, J.W. Boron Neutron Capture Therapy for Glioblastoma Multiforme: Advantage of Prolonged Infusion of BPA-f. Acta Neurol. Scand. 2010, 122, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Kankaanranta, L.; Seppälä, T.; Koivunoro, H.; Välimäki, P.; Beule, A.; Collan, J.; Kortesniemi, M.; Uusi-Simola, J.; Kotiluoto, P.; Auterinen, I.; et al. L-Boronophenylalanine-Mediated Boron Neutron Capture Therapy for Malignant Glioma Progressing after External Beam Radiation Therapy: A Phase i Study. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Kyonghon, P.I.; Kobayashi, T.; Kageji, T.; Uyama, S.; Matsumura, A.; Kumada, H. Clinical Review of the Japanese Experience with Boron Neutron Capture Therapy and a Proposed Strategy Using Epithermal Neutron Beams; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003; Volume 62. [Google Scholar]
- Lamba, M.; Goswami, A.; Bandyopadhyay, A. A Periodic Development of BPA and BSH Based Derivatives in Boron Neutron Capture Therapy (BNCT). Chem. Commun. 2021, 57, 827–839. [Google Scholar] [CrossRef]
- Chen, Y.; Du, F.; Tang, L.; Xu, J.; Zhao, Y.; Wu, X.; Li, M.; Shen, J.; Wen, Q.; Cho, C.H.; et al. Carboranes as Unique Pharmacophores in Antitumor Medicinal Chemistry. Mol. Ther. Oncolytics 2022, 24, 400–416. [Google Scholar] [CrossRef]
- Gruzdev, D.A.; Levit, G.L.; Krasnov, V.P.; Charushin, V.N. Carborane-Containing Amino Acids and Peptides: Synthesis, Properties and Applications. Coord. Chem. Rev. 2021, 433, 213753. [Google Scholar] [CrossRef]
- Hoppenz, P.; Els-Heindl, S.; Kellert, M.; Kuhnert, R.; Saretz, S.; Lerchen, H.G.; Köbberling, J.; Riedl, B.; Hey-Hawkins, E.; Beck-Sickinger, A.G. A Selective Carborane-Functionalized Gastrin-Releasing Peptide Receptor Agonist as Boron Delivery Agent for Boron Neutron Capture Therapy. J. Org. Chem. 2020, 85, 1446–1457. [Google Scholar] [CrossRef]
- Calabrese, G.; Daou, A.; Barbu, E.; Tsibouklis, J. Towards Carborane-Functionalised Structures for the Treatment of Brain Cancer. Drug Discov. Today 2018, 23, 63–75. [Google Scholar] [CrossRef]
- Kreimann, E.L.; Miura, M.; Itoiz, M.E.; Heber, E.; Garavaglia, R.N.; Batistoni, D.; Jiménez Rebagliati, R.; Roberti, M.J.; Micca, P.L.; Coderre, J.A.; et al. Biodistribution of a Carborane-Containing Porphyrin as a Targeting Agent for Boron Neutron Capture Therapy of Oral Cancer in the Hamster Cheek Pouch. Arch. Oral Biol. 2003, 48, 223–232. [Google Scholar] [CrossRef]
- Soloway, A.H.; Zhuo, J.-C.; Rong, F.-G.; Lunato, A.J.; Ives, D.H.; Barth, R.F.; Anisuzzaman, A.K.M.; Barth, C.D.; Barnum, B.A. Identification, Development, Synthesis and Evaluation of Boron-Containing Nucleosides for Neutron Capture Therapy. J. Organomet. Chem. 1999, 581, 150–155. [Google Scholar] [CrossRef]
- Pitto-Barry, A. Polymers and Boron Neutron Capture Therapy (BNCT): A Potent Combination. Polym. Chem. 2021, 12, 2035–2044. [Google Scholar] [CrossRef]
- Zhang, X.; Rendina, L.M.; Müllner, M. Carborane-Containing Polymers: Synthesis, Properties, and Applications. ACS Polym. Au 2024, 4, 7–33. [Google Scholar] [CrossRef]
- Imperio, D.; Panza, L. Sweet Boron: Boron-Containing Sugar Derivatives as Potential Agents for Boron Neutron Capture Therapy. Symmetry 2022, 14, 182. [Google Scholar] [CrossRef]
- Coghi, P.; Hosmane, N.; Zhu, Y. Next Generation of Boron Neutron Capturetherapy (BNCT) Agents for Cancer Treatment. Med. Res. Rev. 2023, 43, 1809–1830. [Google Scholar] [CrossRef]
- Hu, K.; Yang, Z.; Zhang, L.; Xie, L.; Wang, L.; Xu, H.; Josephson, L.; Liang, S.H.; Zhang, M.R. Boron Agents for Neutron Capture Therapy. Coord. Chem. Rev. 2020, 405, 213139. [Google Scholar] [CrossRef]
- Oloo, S.O.; Smith, K.M.; Vicente, M.d.G.H. Multi-Functional Boron-Delivery Agents for Boron Neutron Capture Therapy of Cancers. Cancers 2023, 15, 3277. [Google Scholar] [CrossRef] [PubMed]
- Kasar, R.A.; Knudsen, G.M.; Kahl, S.B. Synthesis of 3-Amino-1-Carboxy-o-Carborane and an Improved, General Method for the Synthesis of All Three C-Amino-C-Carboxycarboranes. Inorg. Chem. 1999, 38, 2936–2940. [Google Scholar] [CrossRef] [PubMed]
- Ujváry, I.; Nachman, R.J. Synthesis of 3-(12-Hydroxy-p-Carboranyl)Propionic Acid, a Hydrophobic, N-Terminal Tyrosine-Mimetic for Peptides. Peptides 2001, 22, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Ujváry, I.; Nachman, R.J. Synthesis of Heterobifunctional P-Carborane Derivatives. 3-[ 12-(Mercaptomethyl)-1,12-Dicarba-Closo-Dodecaboran(12)-1-Yl]Propionic Acid. Tetrahedron Lett. 1999, 40, 5147–5149. [Google Scholar] [CrossRef]
- Huang, S.; Liu, W.; Li, Y.; Zhang, K.; Zheng, X.; Wu, H.; Tang, G. Design, Synthesis, and Activity Study of Cinnamic Acid Derivatives as Potent Antineuroinflammatory Agents. ACS Chem. Neurosci. 2021, 12, 419–429. [Google Scholar] [CrossRef]
- Abd El-Raouf, O.M.; El-Sayed, E.S.M.; Manie, M.F. Cinnamic Acid and Cinnamaldehyde Ameliorate Cisplatin-Induced Splenotoxicity in Rats. J. Biochem. Mol. Toxicol. 2015, 29, 426–431. [Google Scholar] [CrossRef]
- Sova, M. Antioxidant and Antimicrobial Activities of Cinnamic Acid Derivatives. Mini Rev. Med. Chem. 2012, 12, 749–767. [Google Scholar] [CrossRef]
- De, P.; Baltas, M.; Bedos-Belval, F. Cinnamic Acid Derivatives as Anticancer Agents-A Review. Curr. Med. Chem. 2011, 18, 1672–1703. [Google Scholar] [CrossRef]
- Ruwizhi, N.; Aderibigbe, B.A. Cinnamic Acid Derivatives and Their Biological Efficacy. Int. J. Mol. Sci. 2020, 21, 5712. [Google Scholar] [CrossRef] [PubMed]
- Korošec, B.; Sova, M.; Turk, S.; Kraševec, N.; Novak, M.; Lah, L.; Stojan, J.; Podobnik, B.; Berne, S.; Zupanec, N.; et al. Antifungal Activity of Cinnamic Acid Derivatives Involves Inhibition of Benzoate 4-Hydroxylase (CYP53). J. Appl. Microbiol. 2014, 116, 955–966. [Google Scholar] [CrossRef]
- Mielecki, M.; Lesyng, B. Cinnamic Acid Derivatives as Inhibitors of Oncogenic Protein Kinases—Structure, Mechanisms and Biomedical Effects#. Curr.Med. Chem. 2016, 23, 954–982. [Google Scholar] [CrossRef]
- Korneev, S.M. Hydrocinnamic Acids: Application and Strategy of Synthesis. Synthesis 2013, 45, 1000–1015. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, L.; Liu, S.; Cai, Y. Synthesis and Performances of Hydrocinnamic Acid Derivatives Containing Alkyl Dihydrazide. In Proceedings of the E3S Web of Conferences, Dali, China, 21 May 2021; EDP Sciences: Paris, France, 2021; Volume 261. [Google Scholar]
- Nomura, M.; Tanase, T.; Ide, T.; Tsunoda, M.; Suzuki, M.; Uchiki, H.; Murakami, K.; Miyachi, H. Design, Synthesis, and Evaluation of Substituted Phenylpropanoic Acid Derivatives as Human Peroxisome Proliferator Activated Receptor Activators. Discovery of Potent and Human Peroxisome Proliferator Activated Receptor α Subtype-Selective Activators. J. Med. Chem. 2003, 46, 3581–3599. [Google Scholar] [CrossRef]
- Yang, J.; Gu, E.; Yan, T.; Shen, D.; Feng, B.; Tang, C. Design, Synthesis, and Evaluation of a Series of Novel Phenylpropanoic Acid Derivatives Agonists for the FFA1. Chem. Biol. Drug Des. 2019, 93, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Shaala, L.A.; Youssef, D.T.A.; Alzughaibi, T.A.; Elhady, S.S. Antimicrobial Chlorinated 3-Phenylpropanoic Acid Derivatives from the Red Sea Marine Actinomycete Streptomyces Coelicolor LY001. Mar. Drugs 2020, 18, 450. [Google Scholar] [CrossRef]
- Grigoryan, S.H.; Zhamharyan, A.G.; Saghyan, A.S.; Chitchiyan, A.A.; Balyan, L.S.; Poghosyan, A.S.; Topchyan, H.V.; Balasanyan, M.G. Synthesis and Pharmacological Activity of S(-)-2-Amino-2-Methyl-3-Phenylpropanoic Acid. Pharm. Chem. J. 2019, 53, 620–623. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Cai, K.; Cai, L.; Lu, N.; Shi, J. Identification of Benzoic Acid and 3-Phenylpropanoic Acid in Tobacco Root Exudates and Their Role in the Growth of Rhizosphere Microorganisms. Appl. Soil Ecol. 2015, 93, 78–87. [Google Scholar] [CrossRef]
- Fracchiolla, G.; Lavecchia, A.; Laghezza, A.; Piemontese, L.; Trisolini, R.; Carbonara, G.; Tortorella, P.; Novellino, E.; Loiodice, F. Synthesis, Biological Evaluation, and Molecular Modeling Investigation of Chiral 2-(4-Chloro-Phenoxy)-3-Phenyl-Propanoic Acid Derivatives with PPARα and PPARγ Agonist Activity. Bioorg. Med. Chem. 2008, 16, 9498–9510. [Google Scholar] [CrossRef]
- Li, F.; Zhao, D.; Ren, J.; Hao, F.; Liu, G.; Jin, S.; Jing, Y.; Cheng, M. 2-(2-Methylfuran-3-Carboxamido)-3-Phenylpropanoic Acid, a Potential CYP26A1 Inhibitor to Enhance All-Trans Retinoic Acid-Induced Leukemia Cell Differentiation Based on Virtual Screening and Biological Evaluation. Bioorg. Med. Chem. 2013, 21, 3256–3261. [Google Scholar] [CrossRef]
- Guckenbiehl, Y.; Ortner, E.; Rothkopf, I.; Buettner, A. Refining and Conching Alter the Volatile Composition of Dark Chocolate —Revealing Profile Changes in Aroma-Active Volatiles and Volatile Organic Compounds. J. Agric. Food Res. 2025, 19, 101664. [Google Scholar] [CrossRef]
- Badawi, H.M.; Khan, I. A Comparative Study of the Vibrational Spectra of the Anticancer Drug Melphalan and Its Fundamental Molecules 3-Phenylpropionic Acid and l-Phenylalanine. J. Mol. Struct. 2016, 1109, 171–178. [Google Scholar] [CrossRef]
- Hemaiswarya, S.; Doble, M. Combination of Phenylpropanoids with 5-Fluorouracil as Anti-Cancer Agents against Human Cervical Cancer (HeLa) Cell Line. Phytomedicine 2013, 20, 151–158. [Google Scholar] [CrossRef]
- Mahmood, S.; Khan, S.G.; Rasul, A.; Christensen, J.B.; Abourehab, M.A.S. Ultrasound Assisted Synthesis and In Silico Modelling of 1,2,4-Triazole Coupled Acetamide Derivatives of 2-(4-Isobutyl Phenyl)Propanoic Acid as Potential Anticancer Agents. Molecules 2022, 27, 7984. [Google Scholar] [CrossRef] [PubMed]
- Kavaliauskas, P.; Grybaitė, B.; Sapijanskaite-Banevič, B.; Anusevičius, K.; Jonuškienė, I.; Stankevičienė, R.; Petraitienė, R.; Petraitis, V.; Grigalevičiūtė, R.; Meškinytė, E.; et al. Identification of 3-((4-Hydroxyphenyl)Amino)Propanoic Acid Derivatives as Anticancer Candidates with Promising Antioxidant Properties. Molecules 2024, 29, 3125. [Google Scholar] [CrossRef] [PubMed]
- Kavaliauskas, P.; Grybaitė, B.; Sapijanskaitė-Banevič, B.; Vaickelionienė, R.; Petraitis, V.; Petraitienė, R.; Naing, E.; Garcia, A.; Grigalevičiūtė, R.; Mickevičius, V. Synthesis of 3-((4-Hydroxyphenyl)Amino)Propanoic Acid Derivatives as Promising Scaffolds for the Development of Antimicrobial Candidates Targeting Multidrug-Resistant Bacterial and Fungal Pathogens. Antibiotics 2024, 13, 193. [Google Scholar] [CrossRef]
- He, T.; Musah, R.A. Evaluation of the Potential of 2-Amino-3-(1,7-Dicarba- Closo-Dodecaboranyl-1-Thio)Propanoic Acid as a Boron Neutron Capture Therapy Agent. ACS Omega 2019, 4, 3820–3826. [Google Scholar] [CrossRef]
- Colín-Lozano, B.; Estrada-Soto, S.; Chávez-Silva, F.; Gutiérrez-Hernández, A.; Cerón-Romero, L.; Giacoman-Martínez, A.; Almanza-Pérez, J.C.; Hernández-Núñez, E.; Wang, Z.; Xie, X.; et al. Design, Synthesis and in Combo Antidiabetic Bioevaluation of Multitarget Phenylpropanoic Acids. Molecules 2018, 23, 340. [Google Scholar] [CrossRef]
- Kuranov, S.; Luzina, O.; Khvostov, M.; Baev, D.; Kuznetsova, D.; Zhukova, N.; Vassiliev, P.; Kochetkov, A.; Tolstikova, T.; Salakhutdinov, N. Bornyl Derivatives of P-(Benzyloxy)Phenylpropionic Acid: In Vivo Evaluation of Antidiabetic Activity. Pharmaceuticals 2020, 13, 104. [Google Scholar] [CrossRef]
- Kaumanns, O.; Lucius, R.; Mayr, H. Determination of the Electrophilicity Parameters of Diethyl Benzylidenemalonates in Dimethyl Sulfoxide: Reference Electrophiles for Characterizing Strong Nucleophiles. Chem.—Eur. J. 2008, 14, 9675–9682. [Google Scholar] [CrossRef]
- Wu, D.P.; Ou, W.; Huang, P.Q. Ir-Catalyzed Chemoselective Reductive Condensation Reactions of Tertiary Amides with Active Methylene Compounds. Org. Lett. 2022, 24, 5366–5371. [Google Scholar] [CrossRef]
- Chauhan, S.; Verma, P.; Kandasamy, J.; Srivastava, V. A Practical Synthesis of 3-Functionalized Coumarins from o-Cresols and Active Methylene Compounds under Metal and Catalyst-Free Conditions Using Tert-Butyl Hydrogen Peroxide. ChemistrySelect 2020, 5, 9030–9033. [Google Scholar] [CrossRef]
- Ghosh, S.; Jana, C.K. Metal Free Biomimetic Deaminative Direct C–C Coupling of Unprotected Primary Amines with Active Methylene Compounds. Org. Biomol. Chem. 2019, 17, 10153–10157. [Google Scholar] [CrossRef] [PubMed]
- Kazantsev, A.V.; Otrashchenkov, E.A.; Aksartov, M.M. Some Specific Features of Conjugate Addition Reactions with Lithium and Magnesium O-Carborane Derivatives. Russ. J. Org. Chem. 2004, 40, 364–367. [Google Scholar] [CrossRef]
- Kurti, L. Strategic Applications of Named Reactions in Organic Synthesis; Background and Detailed Mechanisms By László Kürti and Barbara Czakó (University of Pennsylvania); Elsevier Academic Press: San Diego, CA, USA, 2005; Volume 128, p. 1032. ISBN 0-12-429785-4. [Google Scholar] [CrossRef]
- Sasakura, N.; Yamauchi, T.; Nakano, K.; Ichikawa, Y.; Kotsuki, H. Efficient and Mild Procedure for the Decarboxylative Cyanomethyl Esterification of Arylmalonic Acids Using Clch2cn/1,8-Diazabicyclo [5. 4.0]Undec-7-Ene. Heterocycles 2011, 83, 2773–2778. [Google Scholar] [CrossRef]
- Lafrance, D.; Bowles, P.; Leeman, K.; Rafka, R. Mild Decarboxylative Activation of Malonic Acid Derivatives by 1,1′-Carbonyldiimidazole. Org. Lett. 2011, 13, 2322–2325. [Google Scholar] [CrossRef]
- Tellitu, I.; Beitia, I.; Díaz, M.; Alonso, A.; Moreno, I.; Domínguez, E. An Improved Solvent-Free System for the Microwave-Assisted Decarboxylation of Malonate Derivatives Based on the Use of Imidazole. Tetrahedron 2015, 71, 8251–8255. [Google Scholar] [CrossRef]
- Cabrera-Rivera, F.A.; Hernández-Vázquez, L.G.; Flores-Sánchez, P.; Durán-Galván, M.; Escalante, J. Solvent- and Catalyst-Free Microwave-Assisted Decarboxylation of Malonic Acid Derivatives. Green Sustain. Chem. 2017, 7, 270–280. [Google Scholar] [CrossRef][Green Version]
- Lanigan, R.M.; Sheppard, T.D. Recent Developments in Amide Synthesis: Direct Amidation of Carboxylic Acids and Transamidation Reactions. Eur. J. Org. Chem. 2013, 2013, 7453–7465. [Google Scholar] [CrossRef]
- Leggio, A.; Bagalà, J.; Belsito, E.L.; Comandè, A.; Greco, M.; Liguori, A. Formation of Amides: One-Pot Condensation of Carboxylic Acids and Amines Mediated by TiCl4. Chem. Cent. J. 2017, 11, 87. [Google Scholar] [CrossRef]
- Lundberg, H.; Tinnis, F.; Selander, N.; Adolfsson, H. Catalytic Amide Formation from Non-Activated Carboxylic Acids and Amines. Chem. Soc. Rev. 2014, 43, 2714–2742. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Huang, D.M.; Sun, H.T.; Song, S.N.; Su, X. Bin Heterocyclic Boron Acid Catalyzed Dehydrative Amidation of Aliphatic/Aromatic Carboxylic Acids with Amines. J. Org. Chem. 2023, 88, 2832–2840. [Google Scholar] [CrossRef]
- Odendal, J.A.; Bruce, J.C.; Koch, K.R.; Haynes, D.A. Packing Motifs in Organic Ammonium Carboxylate Salts: Extension of the Ring-Stacking and Ring-Laddering Concepts. Cryst. Eng. Commun. 2010, 12, 2398–2408. [Google Scholar] [CrossRef]
- Haynes, D.A.; Pietersen, L.K. Hydrogen Bonding Networks in Ammonium Carboxylate Salts: The Crystal Structures of Phenylethylammonium Fumarate-Fumaric Acid, Phenylethylammonium Succinate-Succinic Acid and Anilinium Fumarate-Fumaric Acid. Cryst. Eng. Commun. 2008, 10, 518–524. [Google Scholar] [CrossRef]
- Lemmerer, A. Six Two-and Three-Component Ammonium Carboxylate Salt Structures with a Ladder-Type Hydrogen-Bonding Motif, Three Incorporating Neutral Carboxylic Acid Molecules. Acta Crystallogr. C 2011, 67, o92–o99. [Google Scholar] [CrossRef]
- Shmal’ko, A.V.; Anufriev, S.A.; Suponitsky, K.Y.; Sivaev, I.B. How to Protect Ortho-Carborane from Decapitation—Practical Synthesis of 3,6-Dihalogen Derivatives 3,6-X2-1,2-C2B10H10 (X = Cl, Br, I). Inorganics 2022, 10, 207. [Google Scholar] [CrossRef]
- Fox, M.A.; Goeta, A.E.; Hughes, A.K.; Johnson, A.L. Crystal and Molecular Structures of the Nido-Carborane Anions, 7,9-and 2,9-C2B9H12−. J. Chem. Soc. Dalton Trans. 2002, 10, 2132–2141. [Google Scholar] [CrossRef]
- Fox, M.A.; Wade, K. Deboronation of 9-Substituted-Ortho- and -Meta-Carboranes. J. Organomet. Chem. 1999, 573, 279–291. [Google Scholar] [CrossRef]
- Yoo, J.; Hwang, J.W.; Do, Y. Facile and Mild Deboronation of O-Carboranes Using Cesium Fluoride. Inorg. Chem. 2001, 40, 568–570. [Google Scholar] [CrossRef] [PubMed]
- Ogunlakin, A.D.; Ojo, O.A.; Iyobhebhe, M.; Ajisafe, T.L.; Adeoye, E.O.; Ayokunle, D.L.; Sonibare, M.A.; Ambali, O.A.; Adebodun, G.O.; Ajayi-Odoko, O.A.; et al. Sodium 3-Phenylpropanoate Alleviate Oxidative Stress and Iron-Induced Testicular Toxicity in Wistar Rats. J. Appl. Pharm. Sci. 2024, 14, 88–94. [Google Scholar] [CrossRef]
- Thomas, N.C.; Beaumont, O.A.; Deacon, G.B.; Gaertner, C.; Forsyth, C.M.; Somers, A.E.; Junk, P.C. Preparation and Structures of Rare Earth 3-Benzoylpropanoates and 3-Phenylpropanoates. Aust. J. Chem. 2020, 73, 1250–1259. [Google Scholar] [CrossRef]
- Korolkov, I.V.; Zaboronok, A.; Izbasar, K.A.; Bekbol, Z.A.; Lissovskaya, L.I.; Zibert, A.V.; Shakirzyanov, R.I.; Korganbayeva, L.N.; Yang, H.; Ishikawa, E.; et al. Synthesis of Gd-DTPA Carborane-Containing Compound and Its Immobilization on Iron Oxide Nanoparticles for Potential Application in Neutron Capture Therapy. Pharmaceutics 2024, 16, 797. [Google Scholar] [CrossRef]

| pKa of Primary Amine | Primary Amine |  Yield, % | 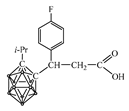 Yield, % | 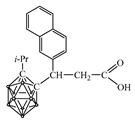 Yield, % |
|---|---|---|---|---|
| 10.6 | Methylamine | 82 | 66 | 86 |
| 10.6 | Butylamine | 90 | 75 | 88 |
| 10.6 | Cyclohexylamine | 75 | 80 | 83 |
| 10.4 | Tert-butylamine | 66 | 88 | 68 |
| 10.1 | Ethylenediamine | 54 | 44 | 93 |
| 9.5 | Allylamine | 14 | 78 | 75 |
| pKa of Secondary Amine | Secondary Amine | 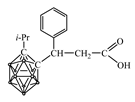 Yield, % | 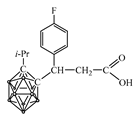 Yield, % | 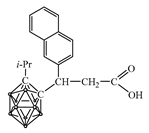 Yield, % |
|---|---|---|---|---|
| 11.2 | Piperidine | 78 | 85 | 85 |
| 10.98 | Diethylamine | 54 | 62 | 68 |
| 8.36 | Morpholine | 90 | 85 | 63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lissovskaya, L.I.; Korolkov, I.V. Synthesis of Carboranyl-Containing β-Arylaliphatic Acids for Potential Application in BNCT. Molecules 2025, 30, 3250. https://doi.org/10.3390/molecules30153250
Lissovskaya LI, Korolkov IV. Synthesis of Carboranyl-Containing β-Arylaliphatic Acids for Potential Application in BNCT. Molecules. 2025; 30(15):3250. https://doi.org/10.3390/molecules30153250
Chicago/Turabian StyleLissovskaya, Lana I., and Ilya V. Korolkov. 2025. "Synthesis of Carboranyl-Containing β-Arylaliphatic Acids for Potential Application in BNCT" Molecules 30, no. 15: 3250. https://doi.org/10.3390/molecules30153250
APA StyleLissovskaya, L. I., & Korolkov, I. V. (2025). Synthesis of Carboranyl-Containing β-Arylaliphatic Acids for Potential Application in BNCT. Molecules, 30(15), 3250. https://doi.org/10.3390/molecules30153250







