Mono-(Ni, Au) and Bimetallic (Ni-Au) Nanoparticles-Loaded ZnAlO Mixed Oxides as Sunlight-Driven Photocatalysts for Environmental Remediation
Abstract
1. Introduction
2. Results and Discussion
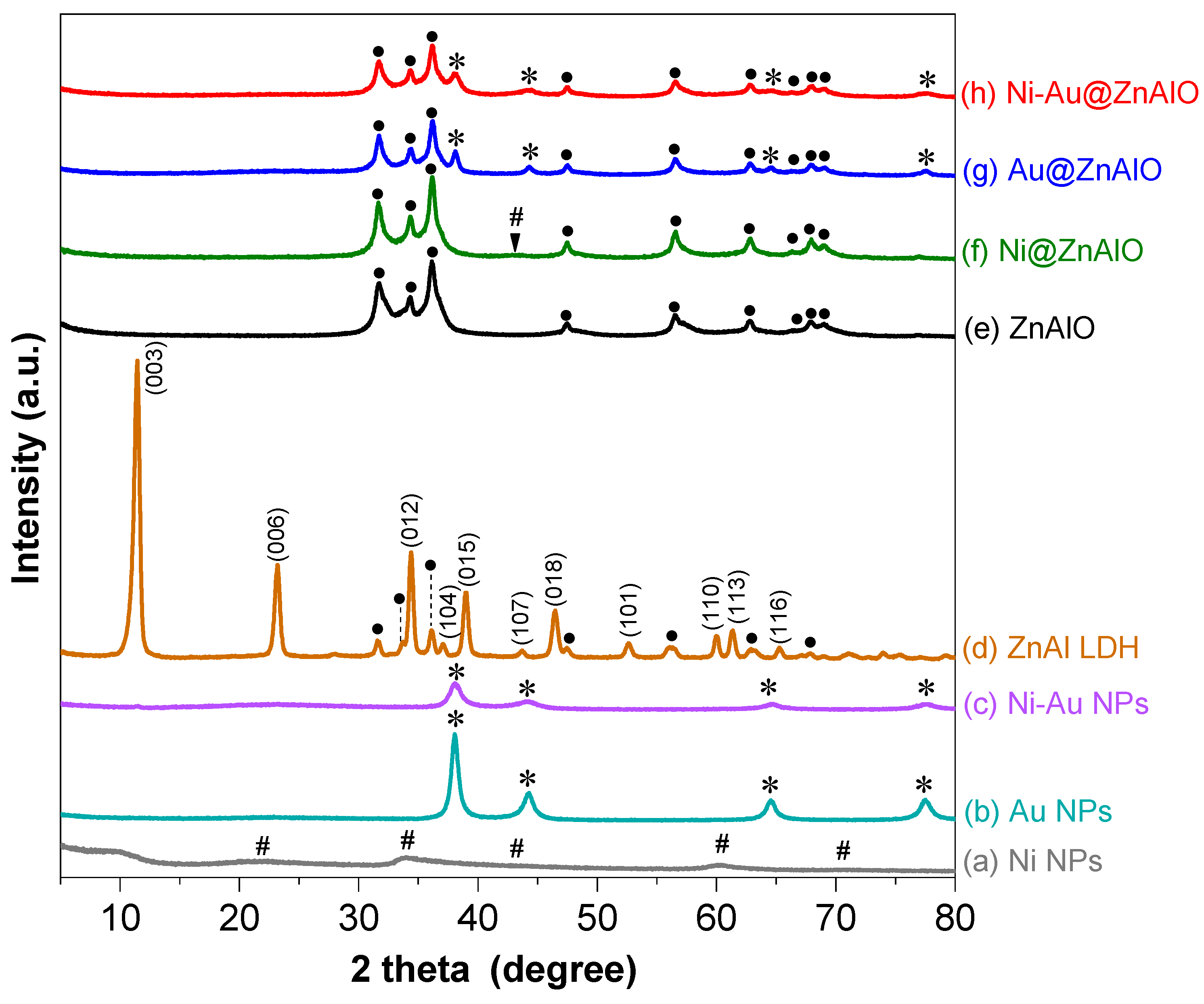
2.1. Structural Analyses
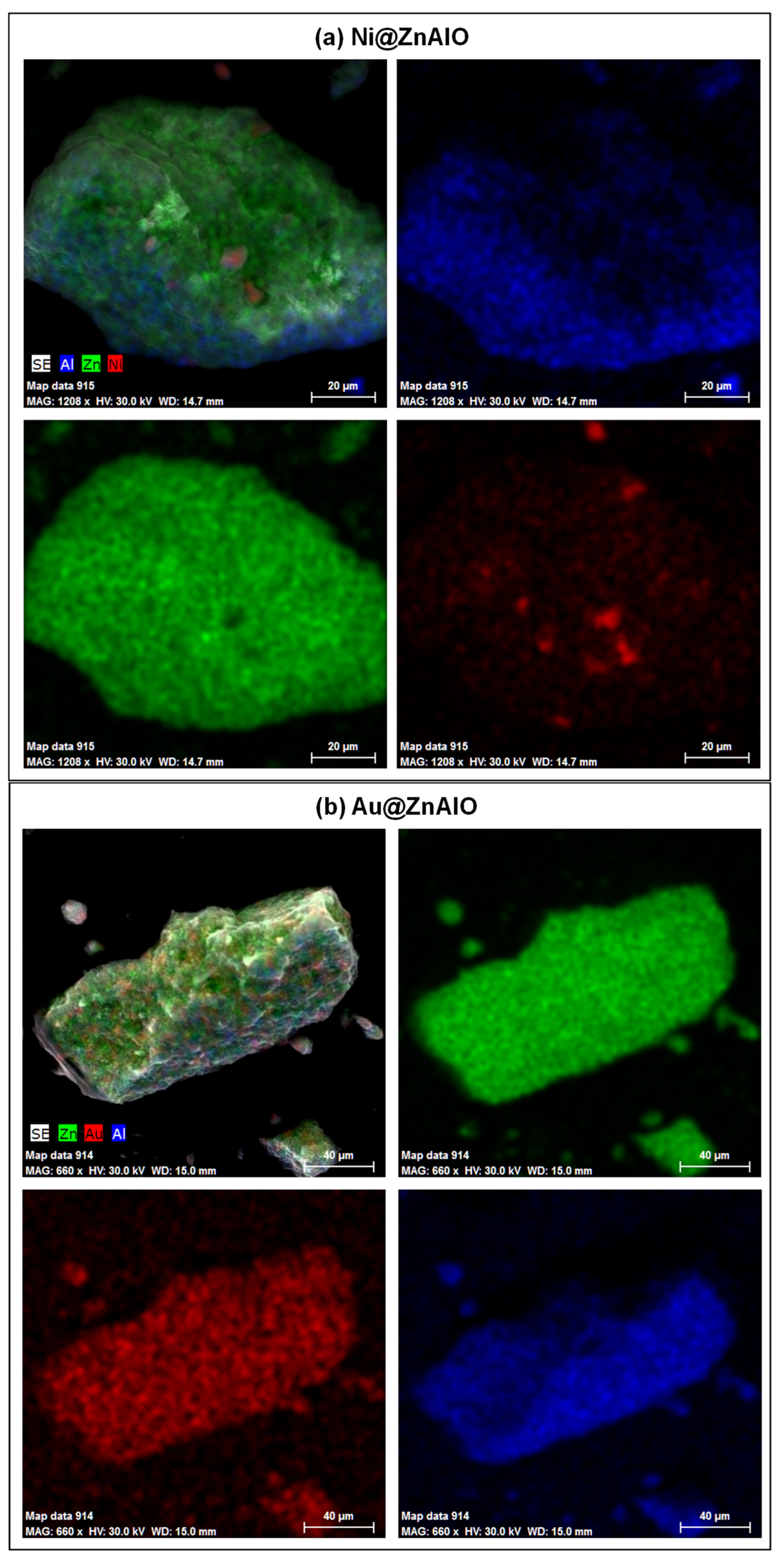
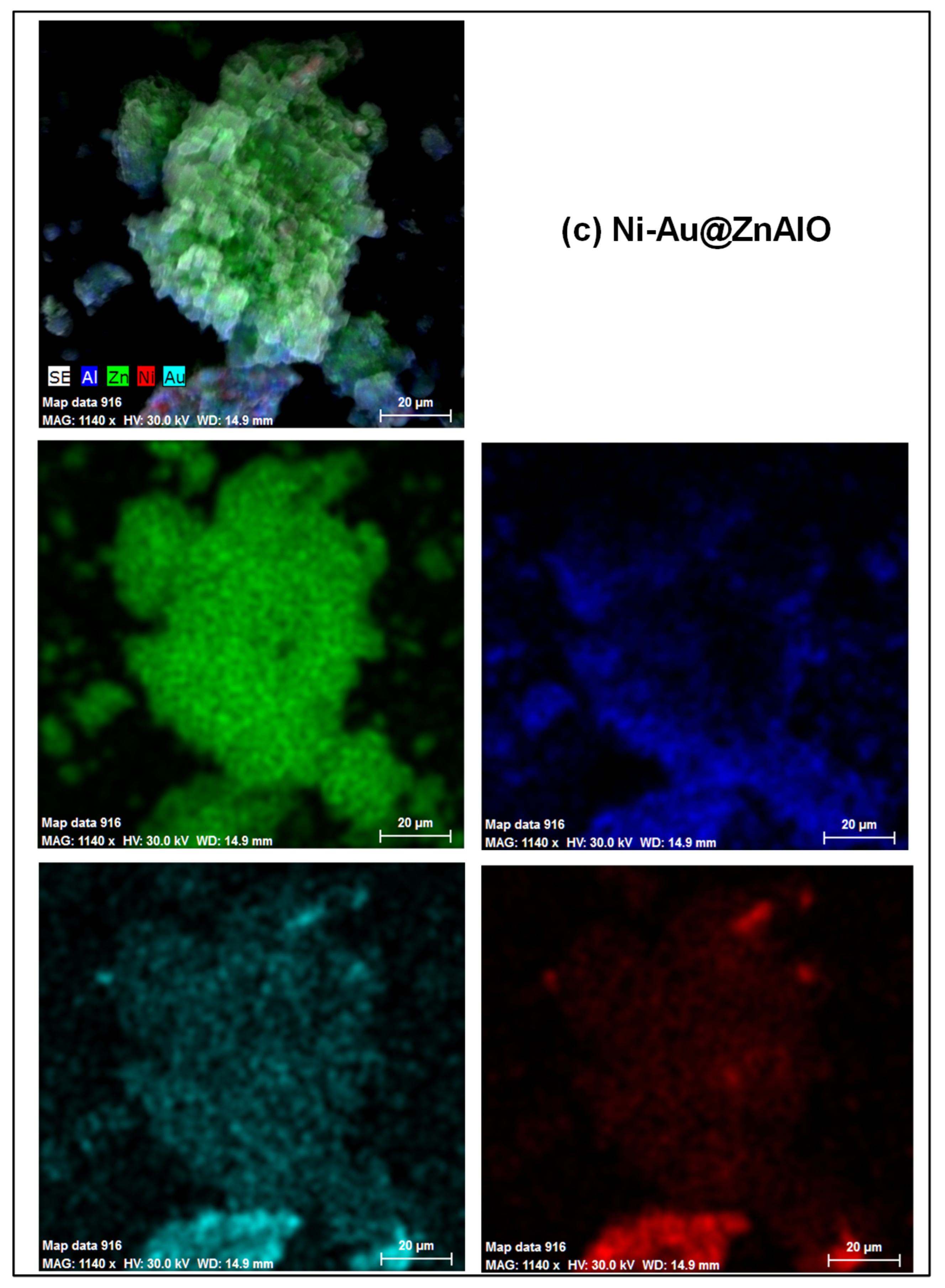
2.2. Scanning Electron Microscopy (SEM)
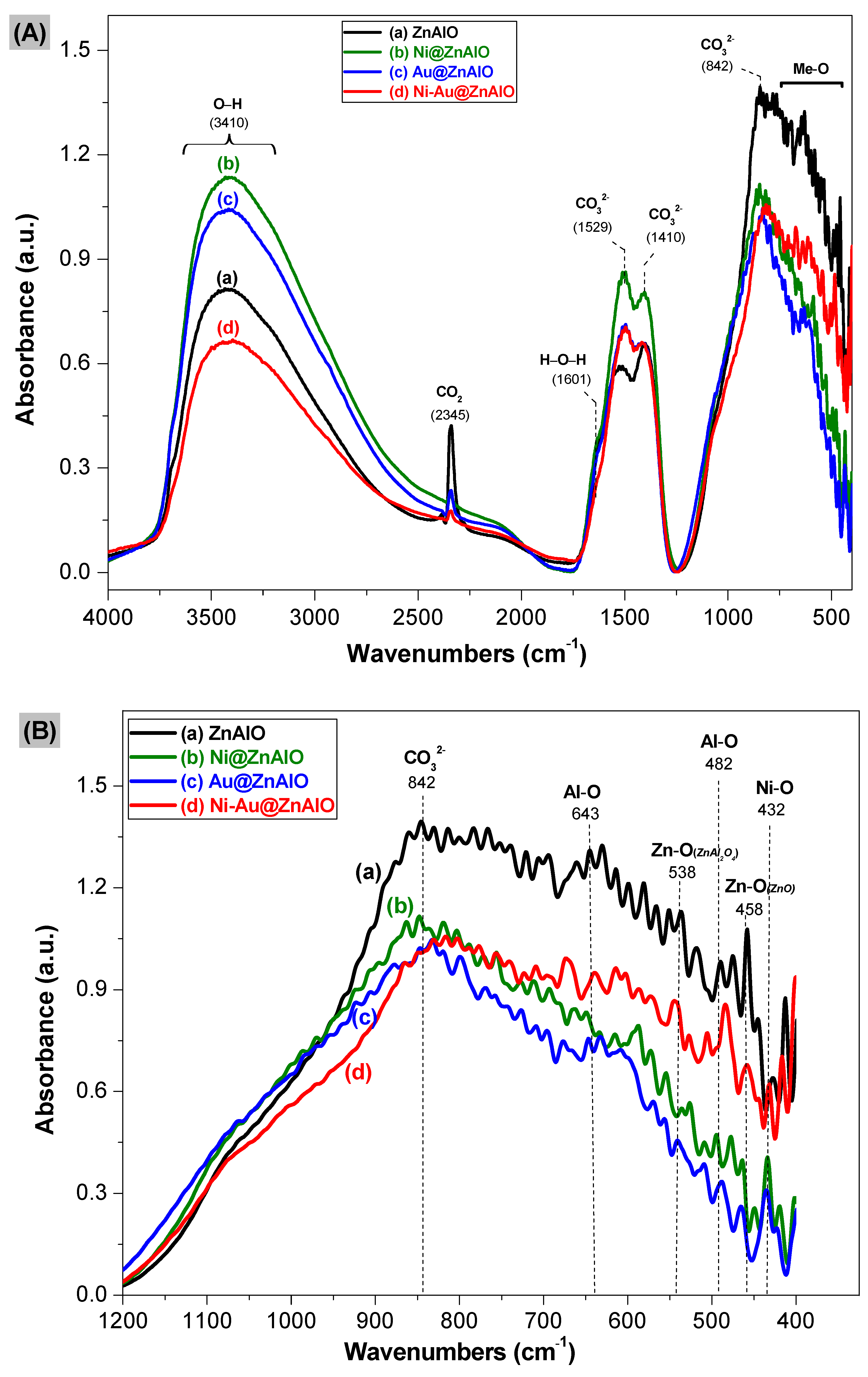
2.3. Infrared Spectroscopy
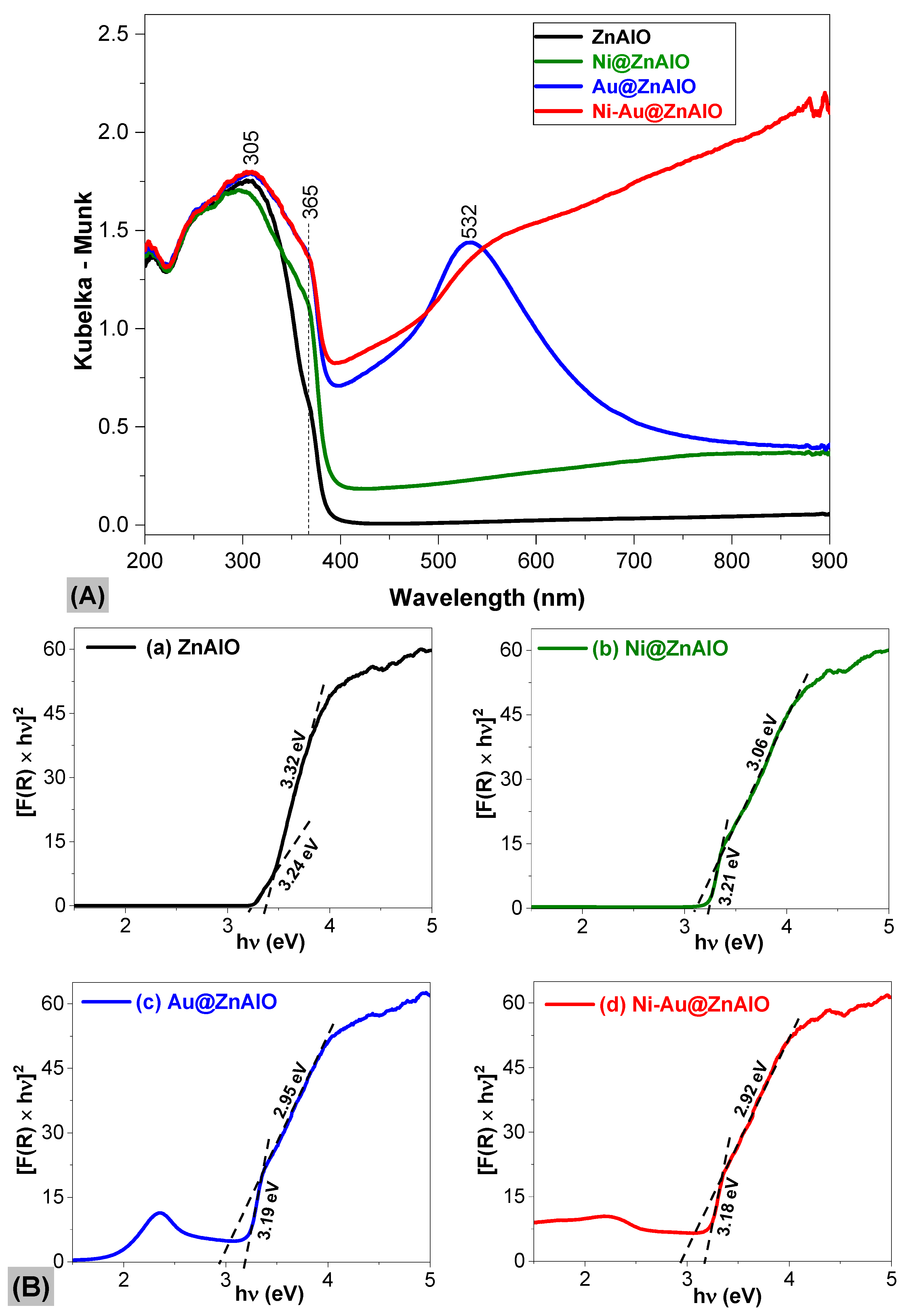
2.4. UV-Vis Spectroscopy
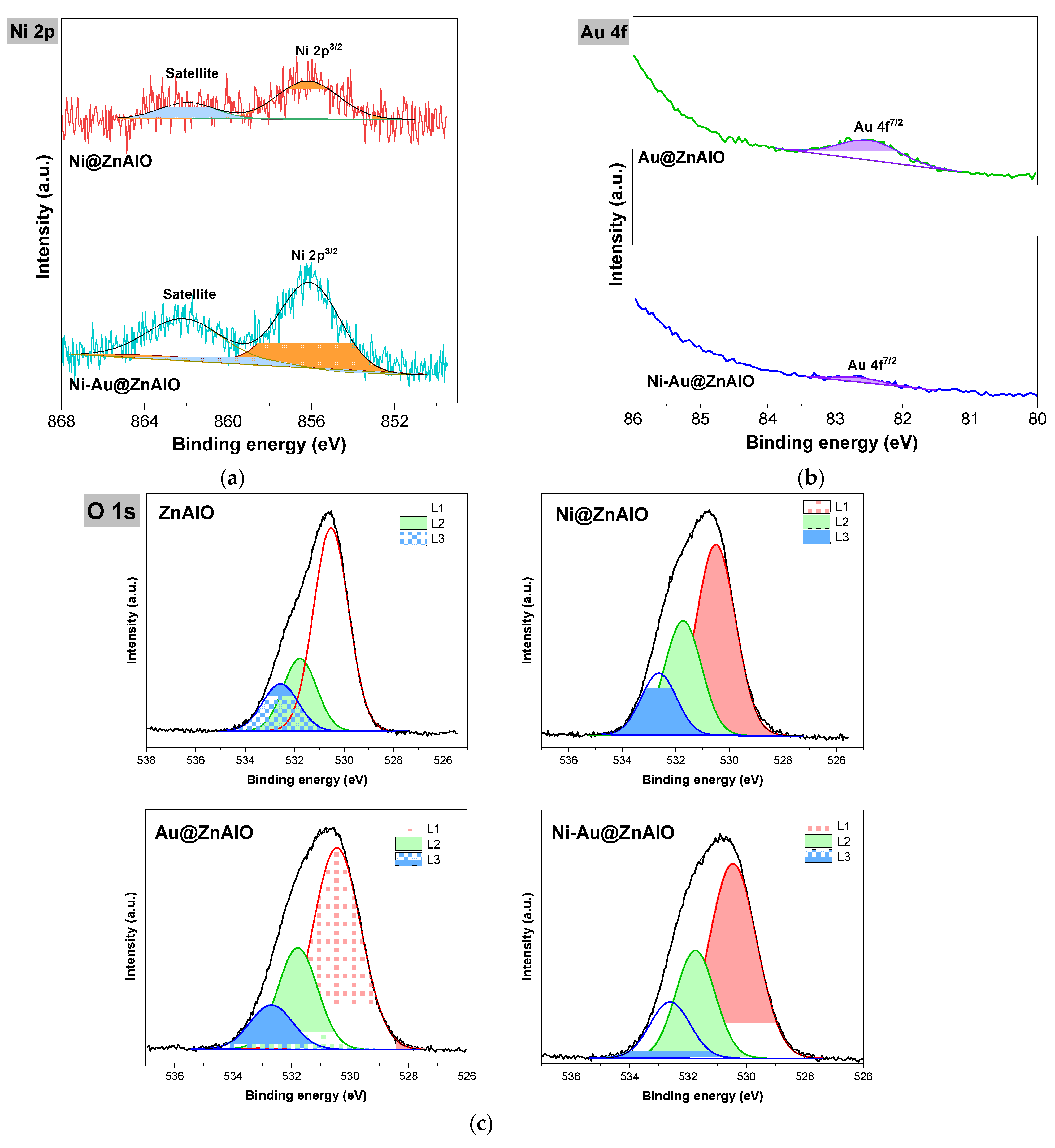
2.5. X-Ray Photoelectron Spectroscopy (XPS) Studies
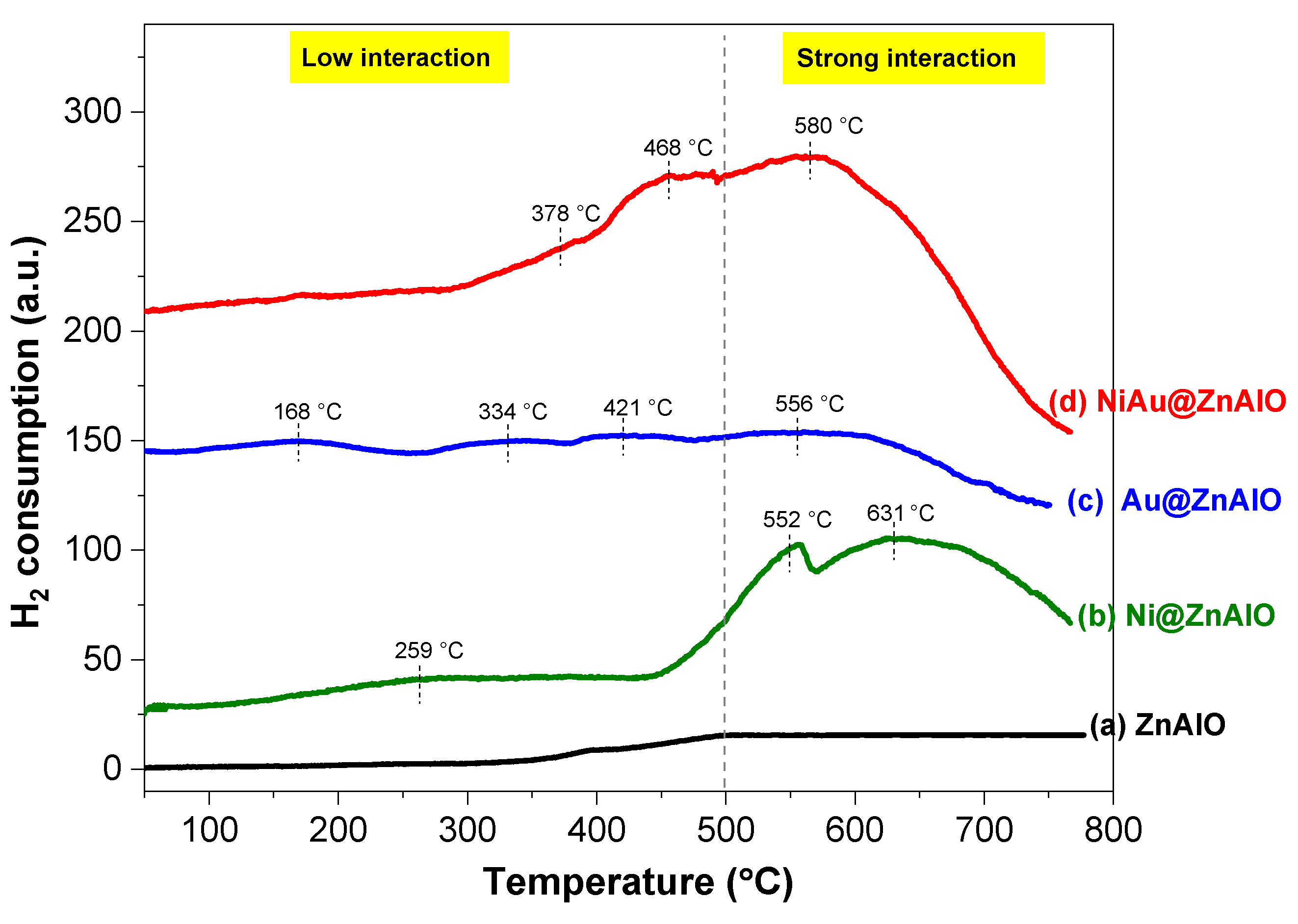
2.6. H2-TPR Measurements
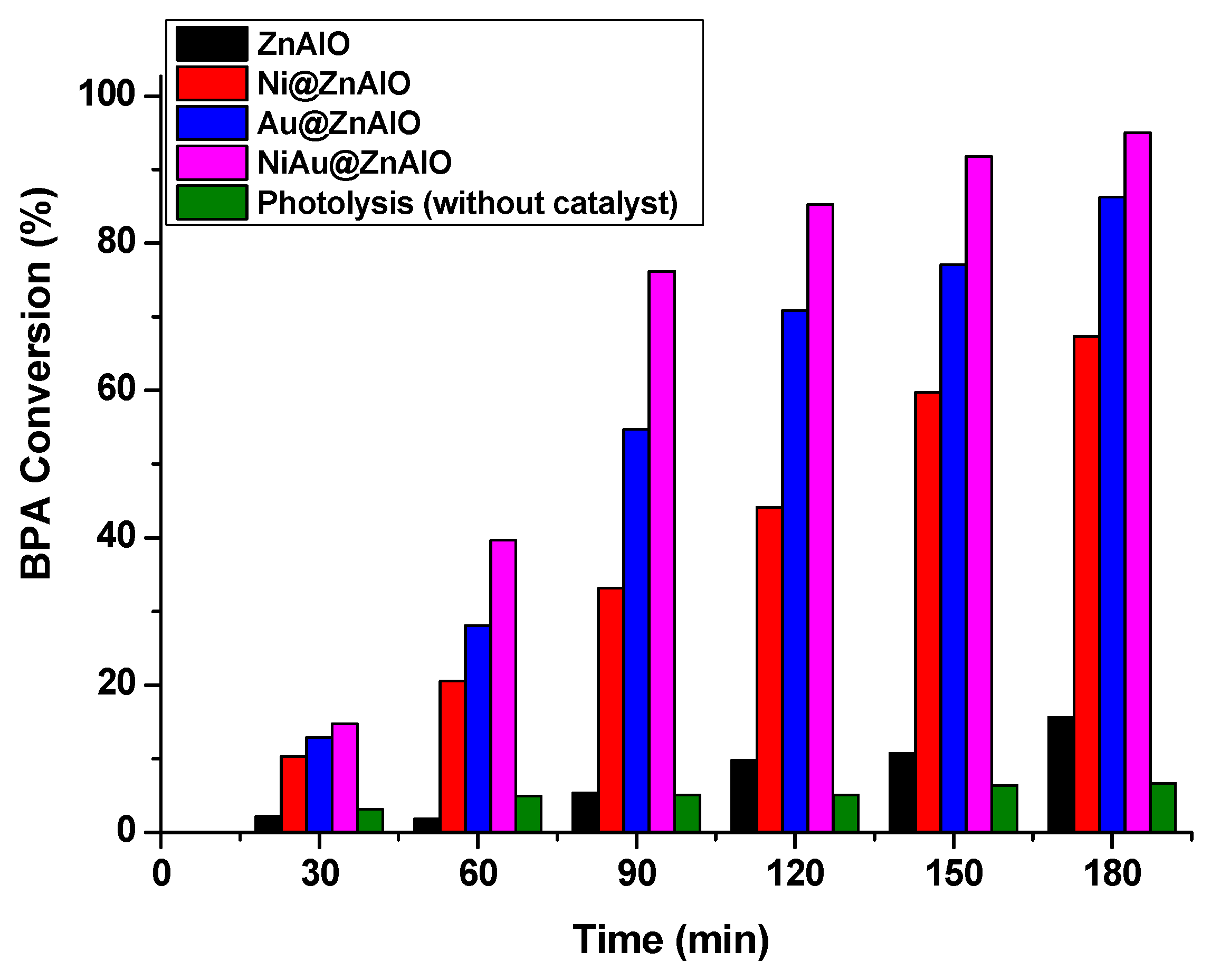
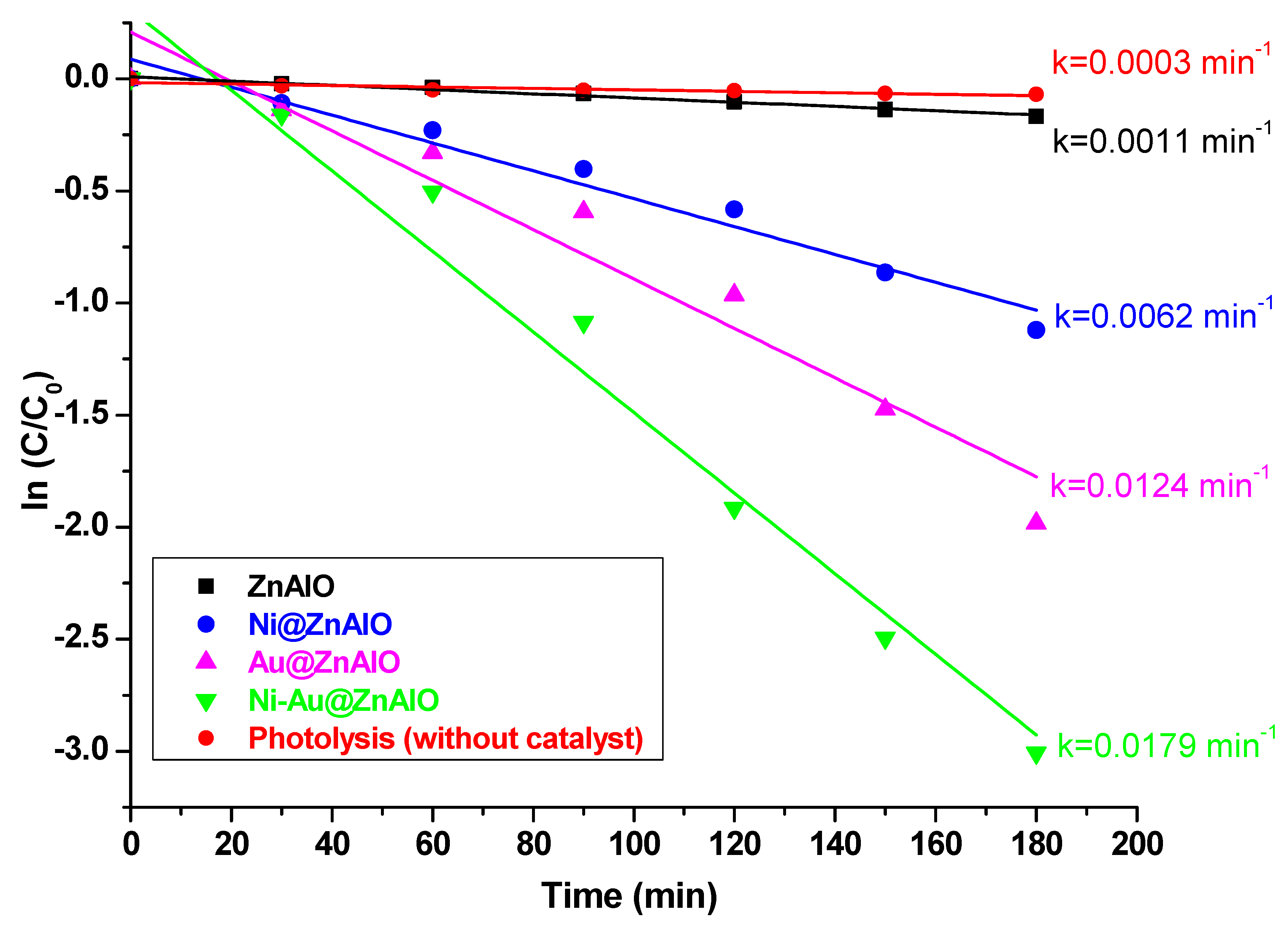
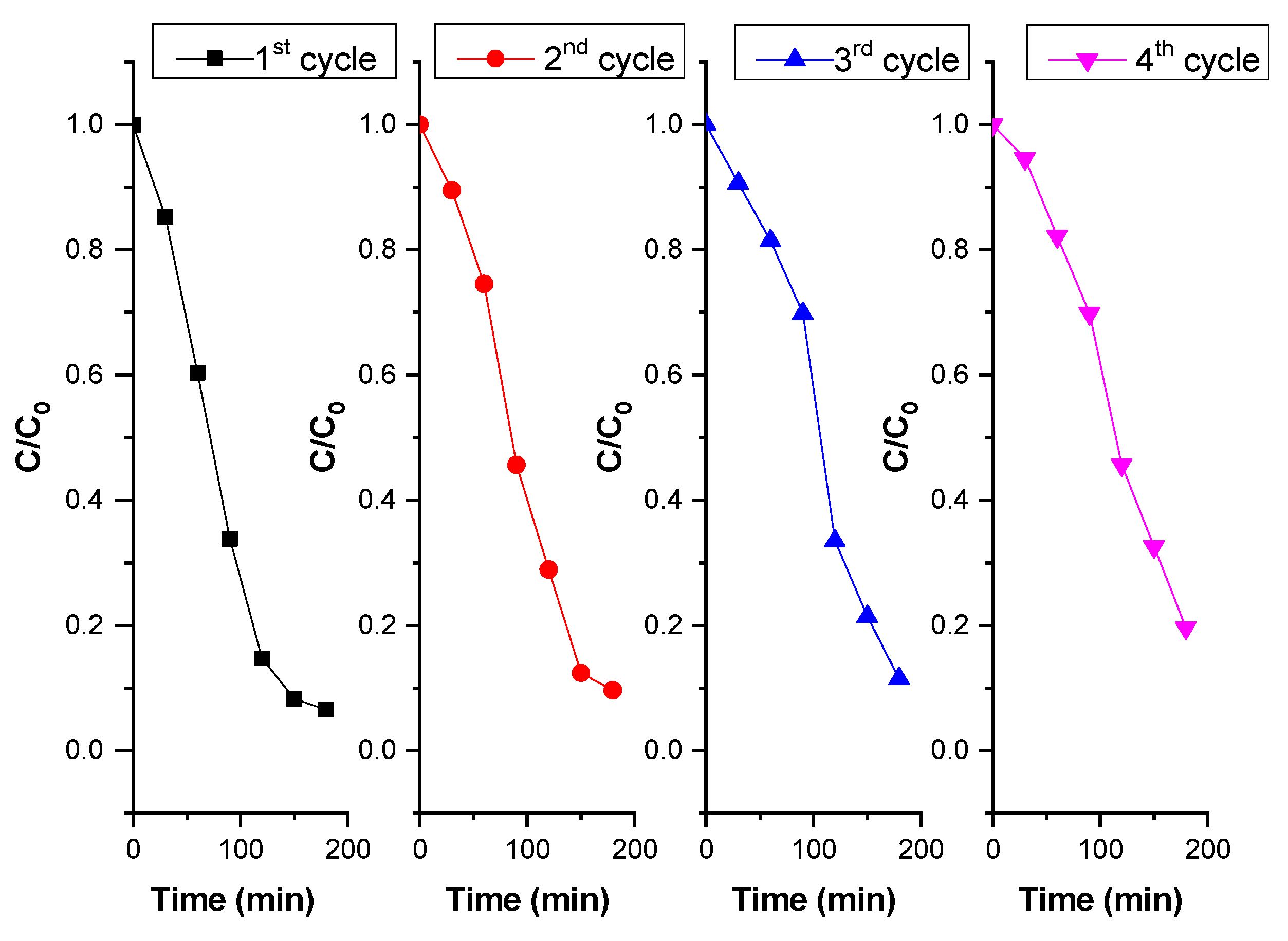
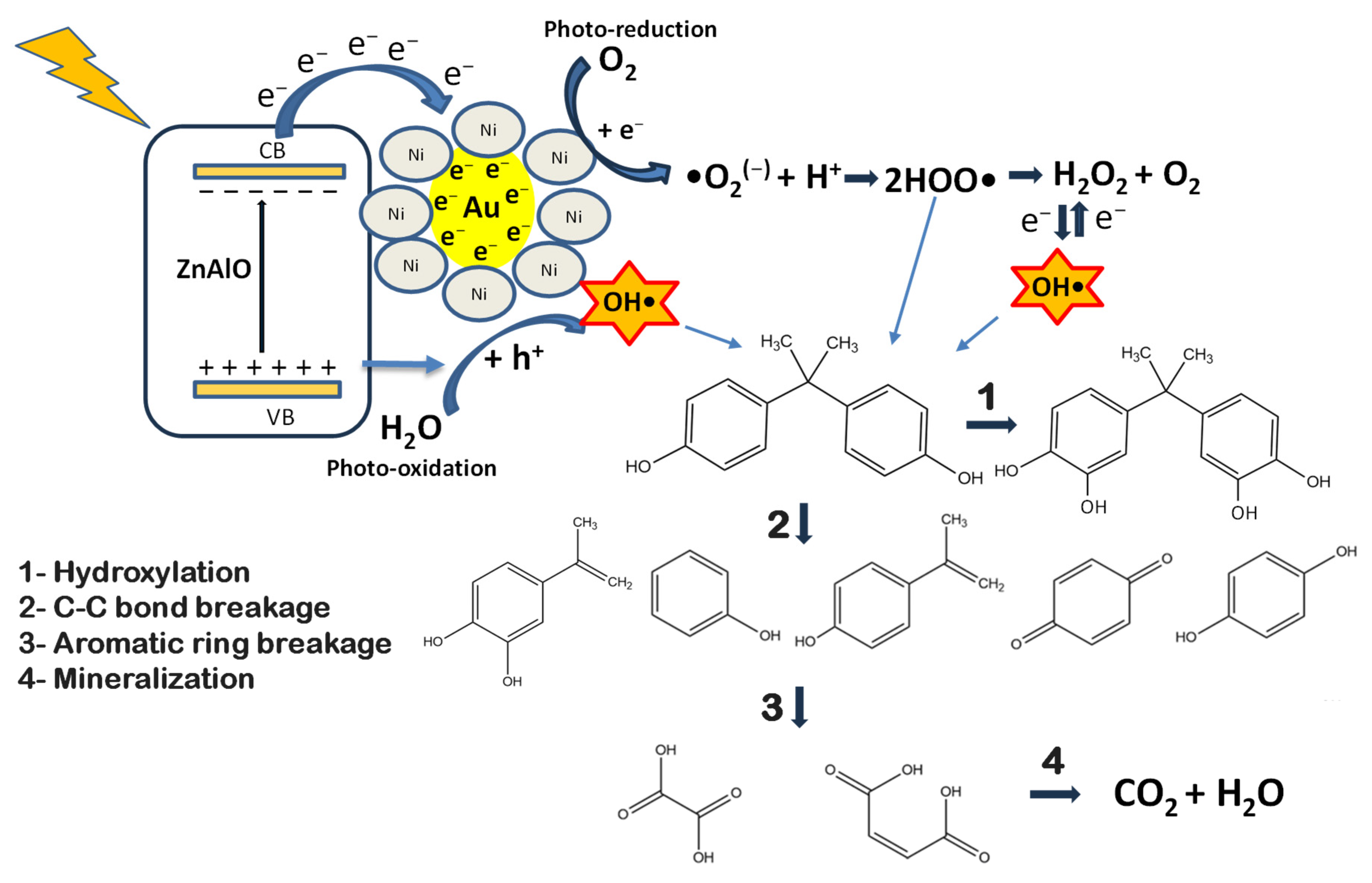
2.7. Photocatalytic Degradation of Bisphenol A (BPA) Under Simulated Solar Irradiation
3. Materials and Methods
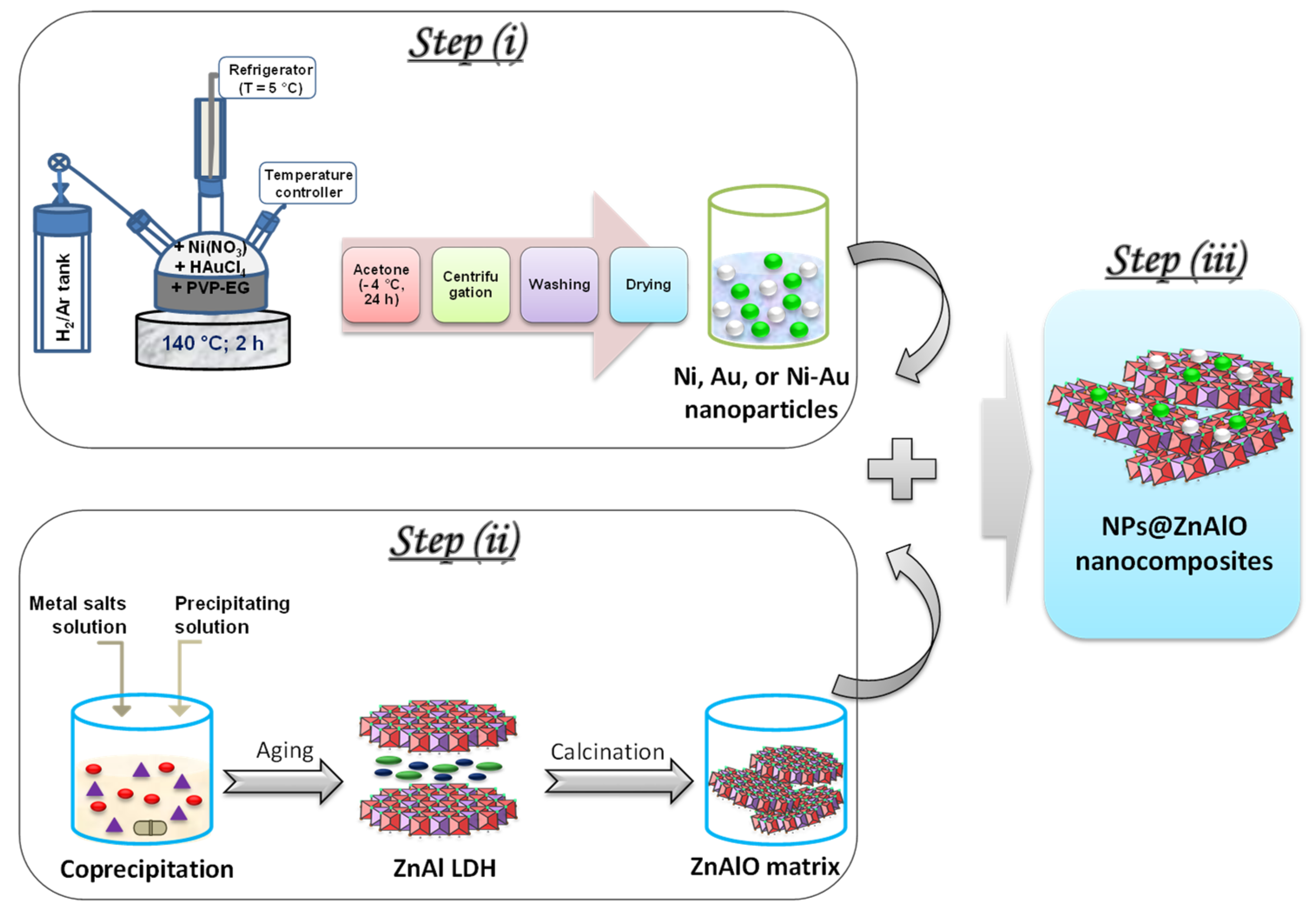
3.1. Synthesis of Materials
3.2. Characterization of the Photocatalysts
3.3. Photocatalytic Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Râpă, M.; Cârstea, E.M.; Șăulean, A.A.; Popa, C.L.; Matei, E.; Predescu, A.M.; Predescu, C.; Donțu, S.I.; Dincă, A.G. An overview of the current trends in marine plastic litter management for a sustainable development. Recycling 2024, 9, 30. [Google Scholar] [CrossRef]
- OECD. Global Plastics Outlook: Economic Drivers, Environmental Impacts and Policy Options; OECD Publishing: Paris, France, 2022. [Google Scholar] [CrossRef]
- Mortula, M.M.; Atabay, S.; Fattah, K.P.; Madbuly, A. Leachability of microplastic from different plastic materials. J. Environ. Manag. 2021, 294, 112995. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liu, H.; Wu, J.; Yuan, L.; Wang, Y.; Du, X.; Wang, R.; Marwa, P.W.; Petlulu, P.; Chen, X.; et al. The adverse health effects of bisphenol A and related toxicity mechanisms. Environ. Res. 2019, 176, 108575. [Google Scholar] [CrossRef]
- Wu, N.C.; Seebacher, F. Effect of the plastic pollutant bisphenol A on the biology of aquatic organisms: A meta-analysis. Glob Chang Biol. 2020, 26, 3821–3833. [Google Scholar] [CrossRef]
- Available online: https://food.ec.europa.eu/food-safety-news-0/commission-adopts-ban-bisphenol-food-contact-materials-2024-12-19_en (accessed on 14 May 2025).
- Zheng, S.; Sun, Z.; Park, Y.; Ayoko, G.A.; Frost, R.L. Removal of bisphenol A from wastewater by Ca-montmorillonite modified with selected surfactants. Chem. Eng. J. 2013, 234, 416–422. [Google Scholar] [CrossRef]
- Wang, L.; Yun, J.; Zhang, H.; Si, J.; Fang, X.; Shao, L. Degradation of Bisphenol A by ozonation in rotating packed bed: Effects of operational parameters and co-existing chemicals. Chemosphere 2021, 274, 129769. [Google Scholar] [CrossRef]
- Godiya, C.B.; Park, B.J. Removal of bisphenol A from wastewater by physical, chemical and biological remediation techniques. A review. Environ. Chem. Lett. 2022, 20, 1801–1837. [Google Scholar] [CrossRef]
- Cheng, F.; Wang, J. Removal of bisphenol A from wastewater by adsorption and membrane separation: Performances and mechanisms. Chem. Eng. J. 2024, 484, 149414. [Google Scholar] [CrossRef]
- Kim, B.; Jang, J.; Lee, D.S. Enhanced photocatalytic degradation of bisphenol A by magnetically separable bismuth oxyiodide magnetite nanocomposites under solar light irradiation. Chemosphere 2022, 289, 133040. [Google Scholar] [CrossRef]
- Vergara–Arenas, B.I.; Nicholls, R.L.; Negron–Silva, G.E.; Lomas–Romero, L.; Morales–Sern, J.A.; Nguyen, B.N. Effect of mixed oxide catalysts on the synthesis of cyclic carbonates from epoxides under atmospheric CO2 pressure. ACS Omega 2025, 10, 673–682. [Google Scholar] [CrossRef]
- Liu, L.; Wang, S.; Zhang, B.; Jiang, G.; Yang, J. Supercritical hydrothermal synthesis of nano-ZrO2: Influence of technological parameters and mechanism. J. Alloys Comp. 2022, 898, 162878. [Google Scholar] [CrossRef]
- Raciulete, M.; Kachina, A.; Puzenat, E.; Afanasiev, P. Preparation of nanodispersed titania using stabilized ammonium nitrate melts. J. Solid State Chem. 2010, 183, 2438–2444. [Google Scholar] [CrossRef]
- Poolakkandya, R.R.; Menamparambath, M.M. Soft-template-assisted synthesis: A promising approach for the fabrication of transition metal oxides. Nanoscale Adv. 2020, 2, 5015–5045. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Song, H.; Lin, S.; Zhou, Y.; Zhan, X.; Hu, Z.; Zhang, Q.; Sun, J.; Yang, B.; Li, T.; et al. Scalable salt-templated synthesis of two-dimensional transition metal oxides. Nat. Commun. 2016, 7, 11296. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumari, S.; Sharma, S.; Singh, T.; Kumar, S.; Thakur, A.; Bhatia, S.K.; Sharma, A.K. Layered double hydroxides: An insight into the role of hydrotalcite-type anionic clays in energy and environmental applications with current progress and recent prospects. Mater. Tod. Sustain. 2023, 22, 100399. [Google Scholar] [CrossRef]
- Yan, Q.; Hou, X.; Liu, G.; Li, Y.; Zhu, T.; Xin, Y.; Wang, Q. Recent advances in layered double hydroxides (LDHs) derived catalysts for selective catalytic reduction of NOx with NH3. J. Hazard. Mater. 2020, 400, 123260. [Google Scholar] [CrossRef]
- Joudeh, N.; Linke, D. Nanoparticle classification, physicochemical properties, characterization, and applications: A comprehensive review for biologists. J. Nanobiotechnol. 2022, 20, 262. [Google Scholar] [CrossRef]
- Fu, Y.; Yin, Z.; Qin, L.; Huang, D.; Yi, H.; Liu, X.; Liu, S.; Zhang, M.; Li, B.; Li, L.; et al. Recent progress of noble metals with tailored features in catalytic oxidation for organic pollutants degradation. J. Hazard. Mater. 2022, 422, 126950. [Google Scholar] [CrossRef]
- Papa, F.; Negrila, C.; Miyazaki, A.; Balint, I. Morphology and chemical state of PVP-protected Pt, Pt–Cu, and Pt–Ag nanoparticles prepared by alkaline polyol method. J. Nanopart. Res. 2011, 13, 5057–5064. [Google Scholar] [CrossRef]
- Papa, F.; Balint, I.; Negrila, C.; Olaru, E.-A.; Zgura, I.; Bradu, C. Supported Pd–Cu Nanoparticles for Water Phase Reduction of Nitrates. Influence of the Support and of the pH Conditions. Ind. Eng. Chem. Res. 2014, 53, 19094–19103. [Google Scholar] [CrossRef]
- Miyazaki, A.; Matsuda, K.; Papa, F.; Scurtu, M.; Negrila, C.; Dobrescu, G.; Balint, I. Impact of particle size and metal-support interaction on denitration behavior of welldefined Pt-Cu nanoparticles. Catal. Sci. Technol. 2015, 5, 492–503. [Google Scholar] [CrossRef]
- State, R.; Scurtu, M.; Miyazaki, A.; Papa, F.; Atkinson, I.; Munteanu, C.; Balint, I. Influence of metal-support interaction on nitrate hydrogenation over Rh and Rh-Cu nanoparticles dispersed on Al2O3 and TiO2 supports. Arab. J. Chem. 2017, 10, 975–984. [Google Scholar] [CrossRef]
- Sandulescu, A.; Anastasescu, C.; Papa, F.; Raciulete, M.; Vasile, A.; Spataru, T.; Scarisoreanu, M.; Fleaca, C.; Mihailescu, C.N.; Teodorescu, V.S.; et al. Advancements on basic working principles of photo-driven oxidative degradation of organic substrates over pristine and noble metal-modified TiO2. Model case of phenol photo oxidation. Catalysts 2021, 11, 487. [Google Scholar] [CrossRef]
- Jaji, N.D.; Lee, H.L.; Hussin, M.H.; Akil, H.M.; Zakaria, M.R.; Othman, M.B.H. Advanced nickel nanoparticles technology: From synthesis to applications. Nanotechnol. Rev. 2020, 9, 1456–1480. [Google Scholar] [CrossRef]
- Thompson, D.T. Using gold nanoparticles for catalysis. Nanotoday 2007, 2, 40–43. [Google Scholar] [CrossRef]
- Lin, H.; Deng, J.; Jing, L.; Wang, Z.; Wei, L.; Wei, Z.; Hou, Z.; Tao, J.; Dai, H. Bimetallic nanoparticles: Advances in fundamental investigations and catalytic applications. Environ. Sci. Adv. 2025, 4, 33–56. [Google Scholar] [CrossRef]
- Sun, Y.; Zhuang, L.; Lu, J.; Hong, X.; Liu, P. Collapse in crystalline structure and decline in catalytic activity of Pt nanoparticles on reducing particle size to 1 nm. J. Am. Chem. Soc. 2007, 129, 15465–15467. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, D.P.; Patnaik, S.; Ratha, D.; Parida, K.M. Synergistic effects of plasmon induced Ag@Ag3VO4/ZnCr LDH ternary heterostructures towards visible light responsive O2 evolution and phenol oxidation reactions. Inorg. Chem. Front. 2018, 5, 879–896. [Google Scholar] [CrossRef]
- Benito, P.; Guinea, I.; Labajos, F.M.; Rocha, J.; Rives, V. Microwave-hydrothermally aged Zn,Al hydrotalcite-like compounds: Influence of the composition and the irradiation conditions. Micropor. Mesopor. Mater. 2008, 110, 292–302. [Google Scholar] [CrossRef]
- Raciulete, M.; Layrac, G.; Tichit, D.; Marcu, I.C. Comparison of CuxZnAlO mixed oxide catalysts derived from multicationic and hybrid LDH precursors for methane total oxidation. Appl. Catal. A Gen. 2014, 477, 195–204. [Google Scholar] [CrossRef]
- Starukh, G.; Rozovik, O.; Oranska, O. Organo/Zn-Al LDH Nanocomposites for Cationic Dye Removal from Aqueous Media. Nanoscale Res. Lett. 2016, 11, 228. [Google Scholar] [CrossRef]
- Dinh, T.D.; Zhang, D.; Tuan, V.N. High iodine adsorption performances under off-gasconditions by bismuth-modified ZnAl-LDH layereddouble hydroxide. RSC. Adv. 2020, 10, 14360–14367. [Google Scholar] [CrossRef] [PubMed]
- Antoniak–Jurak, A.; Kowalik, P.; Próchniak, W.; Bicki, R.; Michalska, K.; Słowik, G. Ecofriendly K-decorated ZnO/Zn(Al,La)2O4 catalyst for hydrogen production—Effect of heterostructure on catalyst activity at steam–lean process gas. Fuel 2021, 302, 121067. [Google Scholar] [CrossRef]
- Abbas, H.; Nadeem, K.; Hassan, A.; Rahman, S.; Krenn, H. Enhanced photocatalytic activity of ferromagnetic Fe–doped NiO nanoparticles. Optik 2020, 202, 163637. [Google Scholar] [CrossRef]
- Balamurugan, K.; Karthik, R.; Chen, S.M.; Sukanya, R.; Subramanian, B.T.; Biju, V.M.N.; Shim, J.J.; Breslin, C.B. Heterostructures of mixed metal oxides (ZnMnO3/ZnO) synthesized by a wet-chemical approach and their application for the electrochemical detection of the drug chlorpromazine. Compos. Part B Eng. 2022, 236, 109822. [Google Scholar] [CrossRef]
- Davar, F.; Niasari, S.M. Synthesis and characterization of spynel-type zinc aluminate nanoparticles by a modified sol-gel methob using new precursor. J. Alloys Comp. 2011, 509, 2487–2492. [Google Scholar] [CrossRef]
- Mihaylov, M.; Knözinger, H.; Hadjiivanov, K.; Gates, B.C. Characterization of the oxidation states of supported gold species by IR spectroscopy of adsorbed CO. Chem. Ing. Tech. 2007, 79, 795–806. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How to correctly determine the band gap energy of modified semiconductor photocatalysts based on UV–Vis spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Zak, A.K.; Abrishami, M.E.; Majid, W.H.A.; Yousefi, R.; Hosseini, S.M. Effects of annealing temperature on somestructural and optical properties of ZnO nanoparticles prepared by a modified sol–gel combustion method. Ceram. Internat. 2011, 37, 393–398. [Google Scholar] [CrossRef]
- Barnawi, N.; Allehyani, S.; Seoudi, R. Biosynthesis and characterization of gold nanoparticles and its application in eliminating nickel from water. J. Mater. Res. Techn. 2022, 17, 537–545. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, F.; Xu, S.; Evans, D.G.; Duan, X. From layered double hydroxides to ZnO–based mixed metal oxides by thermal decomposition: Transformation mechanism and UV-blocking properties of the product. Chem. Mater. 2010, 22, 3933–3942. [Google Scholar] [CrossRef]
- Azar, B.E.; Ramazani, A.; Fardood, S.T.; Morsali, A. Green synthesis and characterization of ZnAl2O4@ZnO nanocomposite and its environmental applications in rapid dye degradation. Optik 2020, 208, 164129. [Google Scholar] [CrossRef]
- Jain, M.; Manju; Kumar, R.M.; Won, S.O.; Chae, K.H.; Vij, A.; Thakur, A. Defect states and kinetic parameter analysis of ZnAl2O4 nanocrystals by X–ray photoelectron spectroscopy and thermoluminescence. Sci. Rep. 2020, 10, 385. [Google Scholar] [CrossRef]
- Davis, K.; Yarbrough, R.; Froeschle, M.; White, J.; Rathnayake, H. Band gap engineered zinc oxide nanostructures via a sol–gel synthesis of solvent driven shape-controlled crystal growth. RSC. Adv. 2019, 9, 14638–14648. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Xiao, H.; Liu, D.; Qin, Y.; Wu, H.; Li, H.; Du, N.; Hou, W. Preparation and properties of mixed metal oxides based layered double hydroxide as anode materials for dye-sensitized solar cell. Chem. Eng. J. 2014, 250, 1–5. [Google Scholar] [CrossRef]
- Modwi, A.; Ghanem, M.A.; Al-Mayouf, A.M.; Houas, A. Lowering energy band gap and enhancing photocatalytic properties of Cu/ZnO composite decorated by transition metals. J. Molec. Struct. 2018, 1173, 1–6. [Google Scholar] [CrossRef]
- Uribe López, M.C.; Alvarez Lemus, M.A.; Hidalgo, M.C.; López González, R.; Quintana Owen, P.; Oros–Ruiz, S.; Uribe López, S.A.; Acosta, J. Synthesis and Characterization of ZnO–ZrO2 Nanocomposites for photocatalytic degradation and mineralization of phenol. J. Nanomater. 2019, 1, 1015876. [Google Scholar] [CrossRef]
- Idriss, H. On the wrong assignment of the XPS O 1s signal at 531–532 eV attributed to oxygen vacancies in photo– and electro-catalysts for water splitting and other materials applications. Surf. Sci. 2021, 712, 121894. [Google Scholar] [CrossRef]
- Frankcombe, T.J.; Liu, Y. Interpretation of oxygen 1s X–ray photoelectron spectroscopy of ZnO. Chem. Mater. 2023, 35, 5468–5474. [Google Scholar] [CrossRef]
- Ren, J.; Ma, Q.; Sun, X.; Ma, J.; Liu, G.; Yang, H. In3+–doping and oxygen vacancies co–engineering active sites of Bi2WO6 hollow nanospheres to achieve efficient photoreduction of CO2 to CO with nearly 100% selectivity. Fuel 2025, 397, 135454. [Google Scholar] [CrossRef]
- Gholami, P.; Khataee, A.; Ritala, M. Template–free hierarchical trimetallic oxide photocatalyst derived from organically modified ZnCuCo layered double hydroxide. J. Clean. Prod. 2022, 366, 132761. [Google Scholar] [CrossRef]
- Tang, C.W.; Chen, Y.J.; Yeh, C.T.; Wu, R.C.; Wang, C.C.; Wang, C.B. Reforming of methanol to produce hydrogen over the Au/ZnO catalyst. Int. J. Hydrogen Energy 2021, 46, 80–88. [Google Scholar] [CrossRef]
- Pan, Y.; Wu, G.; He, Y.; Feng, J.; Li, D. Identification of the Au/ZnO interface as the specific active site for the selective oxidation of the secondary alcohol group in glycerol. J. Catal. 2019, 369, 222–232. [Google Scholar] [CrossRef]
- Perez-Lopez, O.W.; Senger, A.; Marcilio, N.R.; Lansarin, M.A. Effect of composition and thermal pretreatment on properties of Ni–Mg–Al catalysts for CO2 reforming of methane. Appl. Catal. A Gen. 2006, 303, 234–244. [Google Scholar] [CrossRef]
- Wang, W.; Li, X.; Zhang, Y.; Zhang, R.; Ge, H.; Bi, J.; Tang, M. Strong metal–support interactions between Ni and ZnO particles and their effect on the methanation performance of Ni/ZnO. Catal. Sci. Technol. 2017, 7, 4413–4421. [Google Scholar] [CrossRef]
- Tabakova, T.; Gabrovska, M.; Nikolova, D.; Ivanov, I.; Venezia, A.M.; Tenchev, K. Exploring the role of promoters (Au, Cu and Re) in the performance of Ni–Al layered double hydroxides for water-gas shift reaction. Int. J. Hydrogen Energy 2023, 48, 11998–12014. [Google Scholar] [CrossRef]
- Hansen, T.W.; DeLaRiva, A.T.; Challa, S.R.; Datye, A.K. Sintering of catalytic nanoparticles: Particle migration or Ostwald ripening? Acc. Chem. Res. 2013, 46, 1720–1730. [Google Scholar] [CrossRef]
- Gatin, A.K.; Grishin, M.V.; Dokhlikova, N.V.; Sarvadii, S.Y.; Shub, B.R. Hydrogenation of HOPG–supported gold nanoparticles: Features of initial stages. Crystals 2019, 9, 350. [Google Scholar] [CrossRef]
- Liu, Q.; Yu, Z.; Liang, D.; Xiong, J.; Gan, T.; Hu, H.; Huang, Z.; Zhang, Y. Elemental imprinting–induced interfacialgrowth strategy to bridge g–C3N4 and Bi2MoO6 with enigineering rapid electron transfer pathway for efficient visible light-diven photocatalysis. Chem. Eng. J. 2024, 496, 153961. [Google Scholar] [CrossRef]
- Velumani, M.; Rajamohan, S.; Pandeyc, A.; Pham, N.D.K.; Nguyen, V.G.; Hoang, A.T. Nanocomposite from tannery sludge-derived biochar and zinc oxide nanoparticles for photocatalytic degradation of Bisphenol A toward dual environmental benefits. Sci. Total Environ. 2024, 907, 167896. [Google Scholar] [CrossRef]
- Mahmoudian, M.H.; Mesdaghinia, A.; Mahvi, A.H.; Nasseri, S.; Nabizadeh, R.; Dehghani, M.H. Photocatalytic degradation of bisphenol a from aqueous solution using bismuth ferric magnetic nanoparticle: Synthesis, characterization and response surface methodology-central composite design modeling. J. Environ. Health Sci. Eng. 2022, 20, 617–628. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Huang, C.P.; Doong, R. Photocatalytic degradation of bisphenol A over a ZnFe2O4/TiO2 nanocomposite under visible light. Sci. Total Environ. 2019, 646, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.V.L.; Kim, K.H.; Kavitha, B.; Kumar, V.; Raza, N.; Kalagara, S. Photocatalytic degradation of bisphenol A in aqueous media: A review. J. Environ. Manag. 2018, 213, 189–205. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.M.; Barrocas, B.T.; Nardeli, J.V.; Montemor, M.F.; Maçoas, E.; Conceição Oliveira, M.; de Carvalho, C.C.C.R.; Lauria, A.; Niederberger, M.; Marques, A.C. Maximizing photocatalytic efficiency with minimal amount of gold: Solar–driven TiO2 photocatalysis supported by MICROSCAFS® for facile catalyst recovery. J. Environ. Chem. Eng. 2024, 12, 112043. [Google Scholar] [CrossRef]
- Shen, J.; Shi, A.; Wu, M.; Zhang, H.; Jiang, Z. Efficient degradation of bisphenol A over facilely optimized ternary Ag/ZnO/ZnAl—LDH composite with enhanced photocatalytic performance under visible light irradiation. Solid State Sci. 2022, 132, 106992. [Google Scholar] [CrossRef]
- Ali, W.; Ullah, H.; Zada, A.; Muhammad, W.; Ali, S.; Shaheen, S.; Alamgir, M.K.; Ansar, M.Z.; Khan, Z.U.; Bilal, H.; et al. Synthesis of TiO2 modified self-assembled honeycomb ZnO/SnO2 nanocomposites for exceptional photocatalytic degradation of 2,4-dichlorophenol and bisphenol A. Sci. Total Environ. 2020, 746, 141291. [Google Scholar] [CrossRef]
- Ma, T.; Mao, Y.; Liu, C.; Sun, M.; Li, Z.; Chen, M.; Zheng, R.; Dai, S.; Guo, X.; Zhao, T. Mechanochemical establishment of CuBi2O4/Zn-Al LDH p-n heterogeneous junction for effectively promoted bisphenol A photodegradation under visible-light driven. J. Alloys Comp. 2023, 941, 169032. [Google Scholar] [CrossRef]
- Vadivel, S.; Madkour, M.; Rajendran, S.; Sengottaiyan, C. Effective degradation of aqueous bisphenol-A using novel Ag2C2O4/Ag@GNS photocatalyst under visible light. Int. J. Hydrogen Energy 2023, 48, 6510–6520. [Google Scholar] [CrossRef]
- Chang, C.; Fu, Y.; Hu, M.; Wang, C.; Shan, G.; Zhu, L. Photodegradation of bisphenol A by highly stable palladium-dopedmesoporous graphite carbon nitride (Pd/mpg-C3N4) under simulatedsolar light irradiation. Appl. Catal. B Environ. 2013, 142, 553–560. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X. Solvent and Simple Ion-Stabilized Metal Nanoclusters: Chemical Synthesis and Application. In Metal Nanoclusters in Catalysis and Materials Science; Elsevier: Amsterdam, The Netherlands, 2008; pp. 327–340. [Google Scholar] [CrossRef]

| Catalyst | SSA * (m2·g−1) | Zn (at.%) | Al (at.%) | Ni (at.%) | Au (at.%) | O (at.%) | Zn2+/Al3+ Atomic Ratio |
|---|---|---|---|---|---|---|---|
| ZnAlO | 86 | 36.4 | 11.0 | - | - | 52.6 | 3.3 |
| Ni@ZnAlO | 81 | 37.2 | 9.7 | 0.4 (1.36 **) | - | 52.8 | 3.8 |
| Au@ZnAlO | 78 | 33.6 | 11.4 | - | 0.12 (1.87 **) | 55.0 | 2.9 |
| Ni-Au@ZnAlO | 74 | 33.1 | 10.2 | 1.2 | 0.10 (0.96 **) | 55.4 | 3.2 |
| Catalyst | Metallic Surface Area (m2·g−1) | Particle Size (nm) | Dispersion (%) | Total H2 Consumption (µmol·g−1) |
|---|---|---|---|---|
| ZnAlO | - | - | - | - |
| Ni@ZnAlO | 2.03 | 2.73 | 41.40 | 171 |
| Au@ZnAlO | - | - | - | 5 |
| Ni-Au@ZnAlO | 2.46 | 1.97 | 52.21 | 146 |
| Photocatalyst | Rate Constant of BPA Degradation | Conversion of BPA (%) |
|---|---|---|
| k (min−1) | ||
| Photolysis (without catalyst) | 0.0003 | 6.64 |
| ZnAlO | 0.0011 | 15.67 |
| Ni@ZnAlO | 0.0062 | 67.38 |
| Au@ZnAlO | 0.0124 | 87.22 |
| Ni-Au@ZnAlO | 0.0179 | 95.05 |
| Photocatalyst | Reaction Conditions | Light Source | Irradiation Time | Removal Efficiency (%) | Ref. |
|---|---|---|---|---|---|
| Ag/ZnO/ZnAl-LDH | Conc. of BPA = 10 mg·L−1 0.50 g L−1 of cat. | 120 W LED lamp, λ = 380–780 nm | 360 min | 80 | [67] |
| TiO2@ZnO/SnO2 | Conc. of BPA = 20 mg·L−1 0.10 g of cat. | 300 W Xe lamp UV light | 120 min | 58 | [68] |
| 10 wt% CuBi2O4/ZnAl-LDH | Conc. of BPA = 20 mg·L−1 0.20 g of cat. | 500 W Xe lamp (λcut∼420 nm) visible light | 240 min | 84 | [69] |
| Ag2C2O4/Ag@GNS/PMS | Conc. of BPA = 10 mg·L−1 0.06 g of cat. | Visible light (λ > 420 nm) | 180 min | 93 | [70] |
| 1.5 wt% Pd/mpg-C3N4 | Conc. of BPA = 20 mg·L−1 1.00 g/L of cat. | 350 W Xe lamp Simulated solar light | 180 min | 93.9 | [71] |
| Ni-Au@ZnAlO | Conc. of BPA = 25 mg·L−1 0.05 g of cat. | 150 W Xe short-arc lamp Simulated solar light | 180 min | 95 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavel, M.; Cretu, L.; Negrila, C.; Culita, D.C.; Vasile, A.; State, R.; Balint, I.; Papa, F. Mono-(Ni, Au) and Bimetallic (Ni-Au) Nanoparticles-Loaded ZnAlO Mixed Oxides as Sunlight-Driven Photocatalysts for Environmental Remediation. Molecules 2025, 30, 3249. https://doi.org/10.3390/molecules30153249
Pavel M, Cretu L, Negrila C, Culita DC, Vasile A, State R, Balint I, Papa F. Mono-(Ni, Au) and Bimetallic (Ni-Au) Nanoparticles-Loaded ZnAlO Mixed Oxides as Sunlight-Driven Photocatalysts for Environmental Remediation. Molecules. 2025; 30(15):3249. https://doi.org/10.3390/molecules30153249
Chicago/Turabian StylePavel, Monica, Liubovi Cretu, Catalin Negrila, Daniela C. Culita, Anca Vasile, Razvan State, Ioan Balint, and Florica Papa. 2025. "Mono-(Ni, Au) and Bimetallic (Ni-Au) Nanoparticles-Loaded ZnAlO Mixed Oxides as Sunlight-Driven Photocatalysts for Environmental Remediation" Molecules 30, no. 15: 3249. https://doi.org/10.3390/molecules30153249
APA StylePavel, M., Cretu, L., Negrila, C., Culita, D. C., Vasile, A., State, R., Balint, I., & Papa, F. (2025). Mono-(Ni, Au) and Bimetallic (Ni-Au) Nanoparticles-Loaded ZnAlO Mixed Oxides as Sunlight-Driven Photocatalysts for Environmental Remediation. Molecules, 30(15), 3249. https://doi.org/10.3390/molecules30153249












