Fermentation Efficiency and Profile of Volatile Compounds in Rye Grain Mashes from Crops Fertilised with Agrifood Waste Ashes
Abstract
1. Introduction
2. Results and Discussion
2.1. Raw Material Analysis
2.2. Results of Analysis of Sweet Mashes
2.3. Results of Analysis of Fermented Mashes
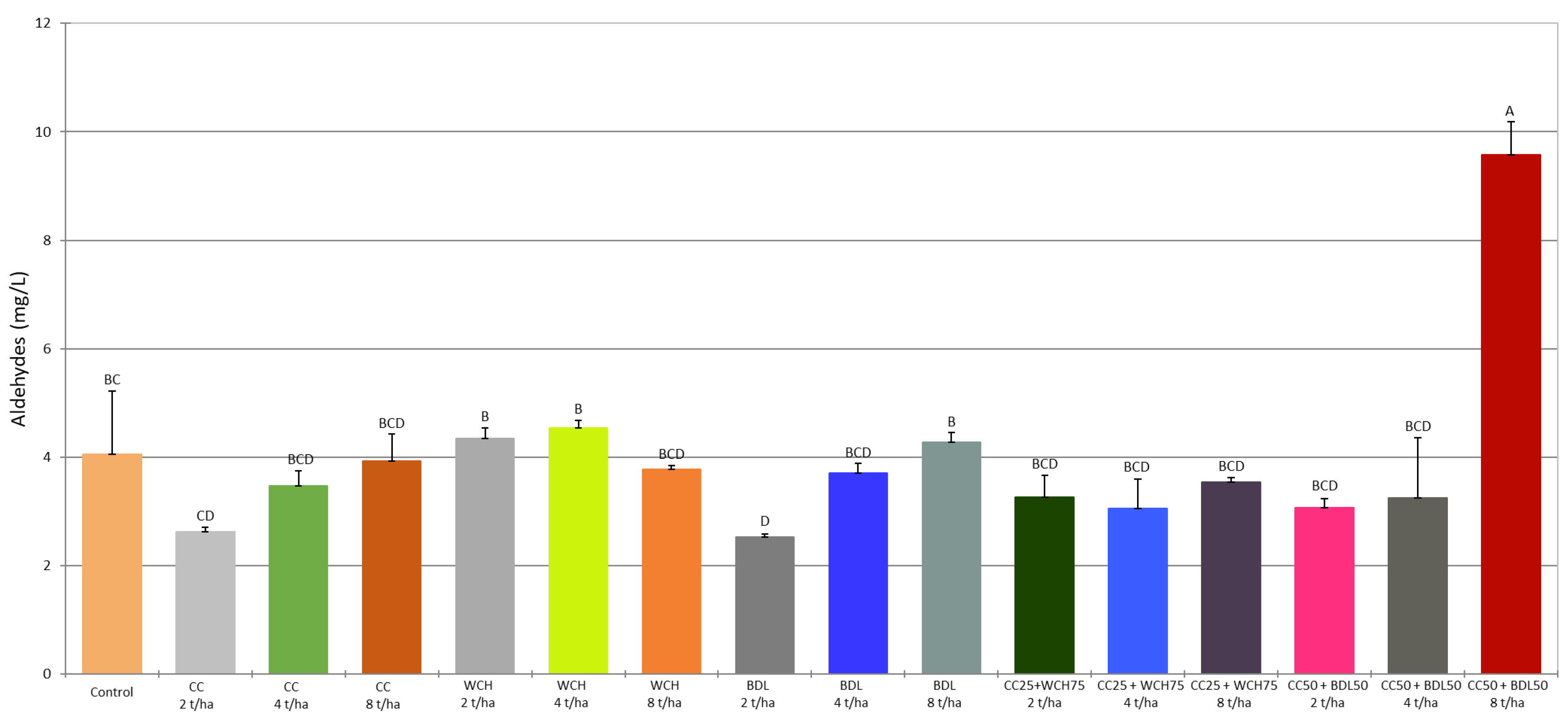
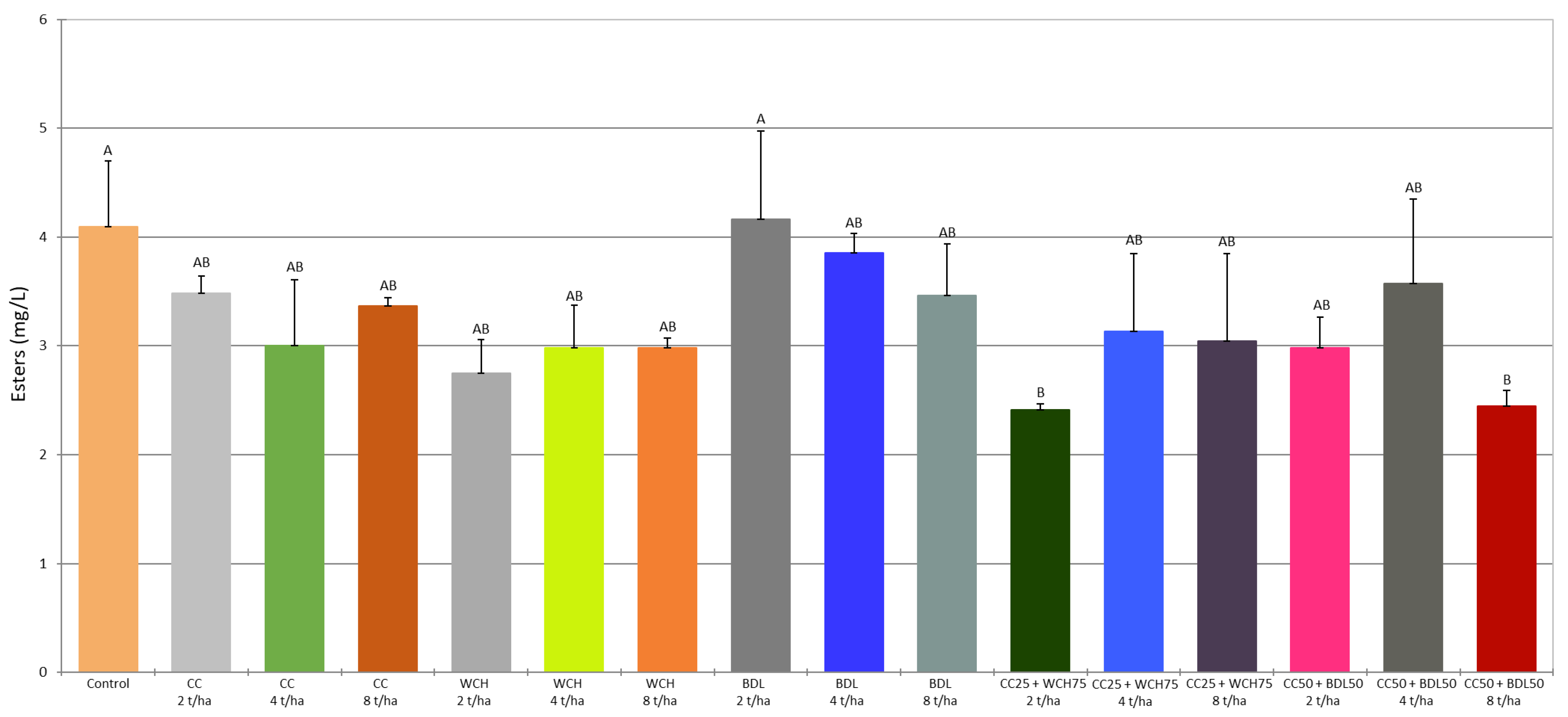
2.4. Volatile Compounds in the Fermented Mashes
3. Materials and Methods
3.1. Materials
- Liquoflow—a liquefying preparation (thermostable α-amylase), applied at 0.2 mL/kg of starch;
- Saczyme® Plus—a saccharifying preparation (amyloglucosidase), applied at 0.6 mL/kg of raw material;
- Viscoferm—a mash viscosity-lowering auxiliary preparation (non-starch polysaccharide hydrolases: xylanase, β-glucanase and cellulase), applied at 0.15 mL/kg of raw material;
- Alphalase AFP—an auxiliary preparation (protease) catalysing the hydrolysis of native proteins present in raw materials, increasing the content of free amino nitrogen assimilable by yeast, applied at 1 mL/kg of raw material.
3.2. Rye Cultivation
3.3. Course of Mashing and Fermentation Experiments
3.4. Methods
- Temperature settings: oven temperature 50 °C, loop temperature 60 °C, and transfer line temperature 70 °C;
- Timing settings: vial equilibration time 20 min, injection duration 0.7 min, and GC cycle time 47 min;
- Vial and loop settings: vial shaking 71 shakes/min, fill pressure 15 psi, and vial pressurisation gas helium.
3.5. Statistical Analysis
- |r| = 0.00—no correlation;
- 0 < |r| < 0.3—very weak correlation;
- 0.3 ≤ |r| < 0.5—weak correlation;
- 0.5 ≤ |r| < 0.7—moderate correlation;
- 0.7 ≤ |r| < 0.9—strong correlation;
- 0.9 ≤ |r| ≤ 1.0—very strong correlation.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CC | Corn Cob |
| WCH | Wood Chips |
| BDL | Biomass with Defecation Lime |
References
- Waste Framework Directive (2008/98/EC) of the European Parliament and of the Council of 19 November 2008 on Waste and Repealing Certain Directives. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:32008L0098:EN:NOT (accessed on 12 June 2025).
- Silva, F.C.; Cruz, N.C.; Tarelho, L.A.C.; Rodrigues, S.M. Use of biomass ash-based materials as soil fertilisers: Critical review of the existing regulatory framework. J. Clean. Prod. 2019, 214, 112–124. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the composition and application of biomass ash. Part 1. Fuel, Phase–mineral and chemical composition and classification. Fuel 2013, 105, 40–76. [Google Scholar] [CrossRef]
- Odzijewicz, J.I.; Wołejko, E.; Wydro, U.; Wasil, M.; Jabłońska-Trypuć, A. Utilization of ashes from biomass combustion. Energies 2022, 15, 9653. [Google Scholar] [CrossRef]
- Cruz, N.C.; Rodrigues, S.M.; Carvalho, L.; Duarte, A.C.; Pereira, E.; Römkens, P.F.A.M.; Tarelho, L.A.C. Ashes from fluidized bed combustion of residual forest biomass: Recycling to soil as a viable management option. Environ. Sci. Pollut. Res. 2017, 24, 14770–14781. [Google Scholar] [CrossRef] [PubMed]
- Szpunar-Krok, E.; Wondołowska-Grabowska, A. Effect of fertilization with ash from biomass combustion on the fatty acid composition of winter rapeseed oil. Agronomy 2025, 15, 231. [Google Scholar] [CrossRef]
- Meller, E.; Bilenda, E. Effects of biomass ash fertilisation on flint maize yield and nutrient uptake. Energy Policy J. 2013, 16, 339–345. (In Polish) [Google Scholar]
- Bunevičienė, K.; Drapanauskaitė, D.; Mažeika, R. Possibilities of using biofuel ash in the cultivation of spring barley. ŽEMĖS ŪKIO Moksl. 2020, 27, 90–99. [Google Scholar] [CrossRef]
- Gupta, Y.; Hossain, M.; Islam, M.R.; Moyeed, M.; Talukder, H.; Atiqur, M.; Khokon, R.; Uddin, M.; Kabir, H.; Carey, M.; et al. Recycled household ash in rice paddies of Bangladesh for sustainable production of rice without altering grain arsenic and cadmium. Expo. Health 2023, 16, 87–99. [Google Scholar] [CrossRef]
- Pycia, K.; Szpunar-Krok, E.; Szostek, M.; Pawlak, R.; Juszczak, L. Selected physicochemical, thermal, and rheological properties of barley starch depending on the type of soil and fertilization with ash from biomass combustion. Foods 2023, 13, 49. [Google Scholar] [CrossRef]
- Czernicka, M.; Puchalski, C.; Pawlak, R.; Szostek, M.; Szpunar-Krok, E. Analysis of the free amino acid profile of barley grain from organic fertilisation with ash from biomass combustion. Molecules 2024, 29, 95. [Google Scholar] [CrossRef]
- Arseniuk, E.; Oleksiak, T. Rye production and breeding in Poland. Plant Breed. Seed Sci. 2003, 47, 7–16. Available online: https://ojs.ihar.edu.pl/index.php/pbss/article/view/749 (accessed on 12 June 2025).
- Buksa, K.; Sułek, A.; Szczypek, M. The influence of integrated and intensive grain production on the content and properties of chemical components in rye grain. Molecules 2025, 30, 1880. [Google Scholar] [CrossRef] [PubMed]
- Balcerek, M.; Pielech-Przybylska, K.; Dziekońska-Kubczak, U.; Patelski, P.; Strąk, E. Fermentation results and chemical composition of agricultural distillates obtained from rye and barley grains and the corresponding malts as a source of amylolytic enzymes and starch. Molecules 2016, 21, 1320. [Google Scholar] [CrossRef] [PubMed]
- Pielech-Przybylska, K.; Balcerek, M.; Ługowoj, S.; Królak, K.; Dziekońska-Kubczak, U.; Kuta, A.; Rozbicki, J.; Studnicki, M. Effects of rye cultivars and management intensity on volatile profile of rye-based spirit distillates. J. Cereal Sci. 2022, 108, 103552. [Google Scholar] [CrossRef]
- Pielech-Przybylska, K.; Balcerek, M.; Klebeko, M.; Dziekońska-Kubczak, U.; Hebdzyński, M. Ethanolic fermentation of rye mashes: Factors influencing the formation of aldehydes and process efficiency. Biomolecules 2022, 12, 1085. [Google Scholar] [CrossRef]
- Strąk, E.; Balcerek, M.; Dziekońska-Kubczak, U. Fermentation efficiency of high-gravity rye mashes using pressureless starch liberation methods. Czech J. Food Sci. 2017, 35, 267–273. [Google Scholar] [CrossRef]
- Balcerek, M.; Pielech-Przybylska, K.; Strąk, E.; Patelski, P.; Dziekońska, U. Comparison of fermentation results and quality of the agricultural distillates obtained by application of commercial amylolytic preparations and cereal malts. Eur. Food Res. Technol. 2016, 242, 321–335. [Google Scholar] [CrossRef]
- Liu, S.; Greenhut, I.; Heist, E.; Heist, M.; Moe, L. Bacterial community dynamics during distilled spirit fermentation: Influence of mash recipes and fermentation processes. Microbiol. Spectr. 2023, 11, e0162423. [Google Scholar] [CrossRef]
- Dongdong, X.; Yingqi, S.; Yanan, L. Effect of glucose levels on carbon flow rate, antioxidant status, and enzyme activity of yeast during fermentation. J. Sci. Food Agric. 2022, 102, 5333–5347. [Google Scholar] [CrossRef]
- Ioannidou, M.D.; Samouris, G.; Achilias, D.S. Acetaldehyde contamination of water, alcoholic, and non-alcoholic beverages stored in glass or plastic bottles. Toxicol. Environ. Chem. 2015, 98, 1183–1190. [Google Scholar] [CrossRef]
- Commission Delegated Regulation (EU) 2022/1303 of 25 April 2022 Amending Regulation (EU) 2019/787 of the European Parliament and of the Council as Regards the Definition of and Requirements for Ethyl Alcohol of Agricultural Origin. Available online: https://eur-lex.europa.eu/eli/reg_del/2022/1303/oj (accessed on 12 June 2025).
- Lytra, G.; Tempere, S.; Le Floch, A.; de Revel, G.; Barbe, J.-C. Study of sensory interactions among red wine fruity esters in a model solution. J. Agric. Food Chem. 2013, 61, 8504–8513. [Google Scholar] [CrossRef]
- de Souza, J.B.; Silva, R.K.; Ferreira, D.S.; de Sá Leitão Paiva Junior, S.; de Barros Pita, W.; de Morais Junior, M.A. Magnesium ions in yeast: Setting free the metabolism from glucose catabolite repression. Metallomics 2016, 8, 1193–1203. [Google Scholar] [CrossRef] [PubMed]
- Birch, R.M.; Walker, G.M. Influence of magnesium ions on heat shock and ethanol stress responses of Saccharomyces cerevisiae. Enzym. Microb. Technol. 2000, 26, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Filho, N.; Linforth, R.; Powell, C.D.; Fisk, I.D. Influence of essential inorganic elements on flavour formation during yeast fermentation. Food Chem. 2021, 361, 130025. [Google Scholar] [CrossRef] [PubMed]
- Soares, E.V.; Hebbelinck, K.; Soares, H.M.V.M. Toxic effects caused by heavy metals in the yeast Saccharomyces cerevisiae: A comparative study. Can. J. Microbiol. 2003, 49, 336–343. [Google Scholar] [CrossRef]
- Tuccillo, F.; Wang, Y.; Edelmann, M.; Lampi, A.-M.; Coda, R.; Katina, K. Fermentation conditions affect the synthesis of volatile compounds, dextran, and organic acids by Weissella confusa A16 in faba bean protein concentrate. Foods 2022, 11, 3579. [Google Scholar] [CrossRef]
- Raj, S.B.; Ramaswamy, S.; Plapp, B.V. Yeast alcohol dehydrogenase structure and catalysis. Biochemistry 2014, 53, 5791–5803. [Google Scholar] [CrossRef]
- Zhao, X.-Q.; Bai, F. Zinc and yeast stress tolerance: Micronutrient plays a big role. J. Biotechnol. 2012, 158, 176–183. [Google Scholar] [CrossRef]
- Klikocka, H.; Podleśna, A.; Podleśny, J.; Narolski, B.; Haneklaus, S.; Bloem, E.; Schnug, E. Improvement of the content and uptake of micronutrients in spring rye grain DM through nitrogen and sulfur supplementation. Agronomy 2020, 10, 35. [Google Scholar] [CrossRef]
- Ikram, A.; Saeed, F.; Afzaal, M.; Abdullah, M.; Niaz, B.; Asif Khan, M.; Hussain, M.; Adnan Nasir, M.; Siddeeg, A. Comparative study of biochemical properties, anti-nutritional profile, and antioxidant activity of newly developed rye variants. Int. J. Food Prop. 2022, 25, 608–616. [Google Scholar] [CrossRef]
- Yenush, L. Potassium and sodium transport in yeast. In Yeast Membrane Transport; Ramos, J., Sychrová, H., Kschischo, M., Eds.; Part Series: Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2016; Volume 892, pp. 187–228. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, H.; Brennan, C.S.; Coldea, T.E.; Zhao, H. Cellular mechanism for the improvement of multiple stress tolerance in brewer’s yeast by potassium ion supplementation. Int. J. Food Sci. Technol. 2020, 55, 2419–2427. [Google Scholar] [CrossRef]
- Silva, R.K.; de Barros Pita, W.; Marcos Antonio de Morais Junior, M.A.; de Souza, R.B. Influence of mineral ions on the growth and fermentation performance of Dekkera bruxellensis GDB248. Lett. Appl. Microbiol. 2023, 76, ovad058. [Google Scholar] [CrossRef]
- Mikulski, D.; Rolbiecka, A.J.; Kłosowski, G.R. Potential influence of compounds released in degradation of phytates on the course of alcoholic fermentation of high gravity mashes—Simulation with analogs of these compounds. Pol. J. Chem. Technol. 2017, 19, 27–34. [Google Scholar] [CrossRef][Green Version]
- Niu, D.; Qi, J.; Lei, H.; Li, F. Ultrasonic-enzymolysis effects on proso millet wine fermentation. LWT 2025, 217, 117383. [Google Scholar] [CrossRef]
- Kleiber, T.; Golcz, A.; Krzesiński, W. Effect of magnesium nutrition of onion (Allium cepa L.). Part I. Yielding and nutrient status. Ecol. Chem. Eng. S 2012, 19, 97–105. [Google Scholar] [CrossRef]
- Dziekońska-Kubczak, U.; Berłowska, J.; Dziugan, P.; Patelski, P.; Pielech-Przybylska, K.; Balcerek, M. Nitric acid pretreatment of Jerusalem artichoke stalks for enzymatic saccharification and bioethanol production. Energies 2018, 11, 2153. [Google Scholar] [CrossRef]
- Hinkle, D.E.; Wiersma, W.; Jurs, S.G. Applied Statistics for the Behavioral Sciences, 5th ed.; Houghton Mifflin Company: Boston, MA, USA, 2003. [Google Scholar]
- Mukaka, M.M. A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar] [PubMed]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation coefficients: Appropriate use and interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef] [PubMed]

| Sample | Total Reducing Sugars g Glucose/100 g | Starch g/100 g | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Control | 1.22 ab | 0.10 | 57.12 abc | 1.52 |
| CC 2 t/ha | 0.87 ef | 0.07 | 55.90 bcd | 1.04 |
| CC 4 t/ha | 1.15 abc | 0.04 | 55.20 bcd | 1.63 |
| CC 8 t/ha | 1.29 a | 0.16 | 56.34 abcd | 3.21 |
| WCH 2 t/ha | 0.96 def | 0.04 | 57.83 ab | 2.46 |
| WCH 4 t/ha | 1.11 bcd | 0.15 | 59.52 a | 0.55 |
| WCH 8 t/ha | 1.02 cde | 0.07 | 58.41 ab | 1.89 |
| BDL 2 t/ha | 0.93 ef | 0.09 | 58.51 ab | 2.09 |
| BDL 4 t/ha | 0.86 ef | 0.01 | 56.90 abc | 1.40 |
| BDL 8 t/ha | 1.01 cde | 0.09 | 57.09 abc | 0.55 |
| CC25 + WCH75 2 t/ha | 0.82 f | 0.07 | 54.12 cd | 3.00 |
| CC25 + WCH75 4 t/ha | 1.11 bcd | 0.09 | 55.57 bcd | 0.44 |
| CC25 + WCH75 8 t/ha | 0.88 ef | 0.02 | 58.01 ab | 1.03 |
| CC50 + BDL50 2 t/ha | 1.11 bcd | 0.03 | 56.81 abc | 0.49 |
| CC50 + BDL50 4 t/ha | 0.94 ef | 0.02 | 55.64 bcd | 1.07 |
| CC50 + BDL50 8 t/ha | 0.96 def | 0.01 | 53.03 d | 1.71 |
| p-values signification codes 1 | *** | *** | ||
| Compound Parameter | Control | CC, t/ha | WCH, t/ha | BDL, t/ha | CC25 + WCH75, t/ha | CC50 + BDL50, t/ha | p-Values Signification Codes 1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 4 | 8 | 2 | 4 | 8 | 2 | 4 | 8 | 2 | 4 | 8 | 2 | 4 | 8 | |||
| Maltotriose, g/L | 15.627 a | 14.836 abc | 12.353 bcd | 15.598 a | 13.611 abc | 16.271 a | 11.808 cd | 16.193 a | 13.761 abc | 14.047 abc | 12.250 bcd | 14.037 abc | 14.980 ab | 10.354 d | 16.408 a | 15.059 ab | *** |
| SD | 1.312 | 0.217 | 0.410 | 1.262 | 0.706 | 1.179 | 1.785 | 1.774 | 2.820 | 0.296 | 2.471 | 1.295 | 1.020 | 2.465 | 0.452 | 0.655 | |
| Maltose, g/L | 42.748 abcd | 42.490 abcd | 46.295 abc | 42.015 abcd | 47.439 a | 41.711 abcd | 34.516 cd | 48.280 a | 50.800 a | 51.103 a | 35.481 bcd | 47.219 ab | 48.414 a | 33.781 d | 44.323 abcd | 43.548 abcd | *** |
| SD | 0.739 | 0.135 | 11.068 | 4.518 | 3.993 | 5.567 | 8.032 | 6.619 | 6.080 | 10.823 | 3.171 | 1.301 | 0.965 | 7.908 | 3.426 | 1.333 | |
| Glucose, g/L | 16.204 de | 12.499 e | 19.603 bcde | 11.749 e | 20.347 bcde | 18.100 cde | 25.701 bc | 19.421 bcde | 35.040 a | 17.097 cde | 21.674 bcd | 14.569 de | 27.954 ab | 15.009 de | 15.100 de | 19.648 bcde | *** |
| SD | 5.356 | 2.496 | 3.346 | 1.691 | 5.350 | 3.436 | 3.714 | 5.376 | 5.579 | 3.728 | 4.702 | 2.833 | 5.347 | 3.959 | 5.369 | 1.951 | |
| Total reducing sugars, g glucose/L | 1.526 cde | 1.462 de | 1.702 bcde | 1.218 e | 1.948 bcd | 1.210 e | 2.120 abc | 1.793 bcde | 2.644 a | 1.737 bcde | 1.827 bcde | 1.416 de | 2.315 ab | 1.278 e | 1.594 cde | 1.841 bcde | *** |
| SD | 0.383 | 0.149 | 0.286 | 0.066 | 0.357 | 0.207 | 0.432 | 0.347 | 0.341 | 0.249 | 0.442 | 0.217 | 0.370 | 0.278 | 0.352 | 0.216 | |
| Total sugars, g glucose/L | 78.005 cd | 73.178 cd | 81.617 bc | 72.747 cd | 84.918 bc | 79.503 bc | 74.730 cd | 87.654 abc | 103.310 a | 85.995 bc | 72.195 cd | 79.367 bc | 95.024 ab | 61.702 d | 79.400 bc | 81.680 bc | *** |
| SD | 6.666 | 2.684 | 13.550 | 6.757 | 7.487 | 4.031 | 11.066 | 7.794 | 6.443 | 9.402 | 10.538 | 2.903 | 5.401 | 13.565 | 6.545 | 2.035 | |
| Dextrins, g/L | 174.835 abc | 170.210 bcd | 168.877 bcd | 174.135 abc | 176.250 ab | 182.500 a | 178.156 ab | 178.240 ab | 173.196 abc | 174.196 abc | 164.746 cd | 169.896 bcd | 176.596 ab | 173.613 abc | 169.613 bcd | 161.858 d | *** |
| SD | 4.805 | 3.047 | 4.933 | 7.386 | 7.283 | 9.209 | 5.840 | 6.380 | 4.247 | 1.787 | 8.825 | 1.177 | 3.156 | 1.409 | 3.174 | 5.143 | |
| Extract, °Blg | 16.87 bcd | 17.33 abc | 16.83 bcd | 17.72 a | 17.37 abc | 17.48 ab | 17.37 abc | 17.37 abc | 16.80 bcd | 17.38 abc | 16.63 cd | 17.03 abcd | 17.08 abcd | 16.53 d | 17.37 abc | 15.68 e | *** |
| SD | 0.79 | 0.25 | 0.51 | 0.41 | 0.38 | 0.58 | 0.33 | 0.15 | 0.20 | 0.12 | 0.34 | 0.15 | 0.04 | 0.14 | 0.29 | 0.50 | |
| Compound | Control | CC, t/ha | WCH, t/ha | BDL, t/ha | CC25 + WCH75, t/ha | CC50 + BDL50, t/ha | p-Values Signification Codes 1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 4 | 8 | 2 | 4 | 8 | 2 | 4 | 8 | 2 | 4 | 8 | 2 | 4 | 8 | |||
| Maltotriose, g/L | 1.163 j | 1.568 abc | 1.546 abcd | 1.628 a | 1.407 defg | 1.524 abcde | 1.464 bcde | 1.262 hij | 1.276 ghij | 1.595 ab | 1.479 bcde | 1.398 efgh | 1.160 j | 1.445 cdef | 1.320 fghi | 1.182 ij | *** |
| SD | 0.005 | 0.013 | 0.007 | 0.007 | 0.004 | 0.001 | 0.002 | 0.005 | 0.007 | 0.005 | 0.004 | 0.004 | 0.003 | 0.004 | 0.003 | 0.000 | |
| Maltose, g/L | 0.875 ef | 1.091 cde | 1.357 bcd | 0.797 ef | 1.458 bc | 0.948 de | 0.989 de | 0.823 ef | 1.028 de | 1.122 cde | 1.103 cde | 2.064 a | 0.955 de | 0.907 e | 0.478 f | 1.569 b | *** |
| SD | 0.026 | 0.114 | 0.053 | 0.004 | 0.076 | 0.012 | 0.012 | 0.010 | 0.027 | 0.003 | 0.002 | 0.001 | 0.000 | 0.045 | 0.107 | 0.165 | |
| Glucose, g/L | 0.021 b | 0.012 b | 0.015 b | 0.008 b | 0.009 b | 0.013 b | 0.022 b | 0.022 b | 0.020 b | 0.014 b | 0.013 b | 0.010 b | 0.020 b | 0.016 b | 0.036 b | 3.074 a | *** |
| SD | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 5.618 | |
| Xylose, g/L | 0.584 b | 0.741 b | 0.564 b | 0.585 b | 0.420 b | 0.557 b | 0.610 b | 0.605 b | 0.584 b | 0.651 b | 0.611 b | 0.689 b | 0.578 b | 0.619 b | 0.435 b | 1.798 a | *** |
| SD | 0.001 | 0.009 | 0.000 | 0.000 | 0.043 | 0.000 | 0.002 | 0.001 | 0.001 | 0.002 | 0.001 | 0.002 | 0.000 | 0.001 | 0.041 | 1.045 | |
| Succinic acid, g/L | 1.129 cd | 1.280 a | 1.141 bcd | 1.193 abc | 1.073 d | 1.187 abc | 1.166 bcd | 1.153 bcd | 1.162 bcd | 1.245 ab | 1.171 bcd | 1.179 abcd | 1.156 bcd | 1.129 cd | 1.130 cd | 1.171 bcd | *** |
| SD | 0.004 | 0.001 | 0.001 | 0.001 | 0.007 | 0.001 | 0.001 | 0.006 | 0.001 | 0.000 | 0.000 | 0.001 | 0.001 | 0.000 | 0.015 | 0.004 | |
| Glycerol, g/L | 6.003 ef | 6.887 a | 6.197 cdef | 6.382 bcde | 6.868 a | 6.334 bcde | 6.344 bcde | 5.784 f | 6.463 abcd | 6.683 ab | 6.293 bcde | 6.485 abc | 6.172 cdef | 6.099 cdef | 6.075 cdef | 6.022 def | *** |
| SD | 0.119 | 0.085 | 0.025 | 0.013 | 0.153 | 0.023 | 0.038 | 0.132 | 0.005 | 0.002 | 0.019 | 0.010 | 0.021 | 0.039 | 0.007 | 0.104 | |
| Acetic acid, g/L | 0.041 ab | 0.021 cde | 0.034 abcde | 0.034 abcde | 0.038 abc | 0.020 de | 0.035 abcd | 0.028 abcde | 0.039 ab | 0.032 abcde | 0.028 abcde | 0.017 e | 0.030 abcde | 0.027 bcde | 0.046 a | 0.032 abcde | *** |
| SD | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | |
| Ethyl alcohol, g/L | 64.287 ab | 66.041 ab | 65.354 ab | 68.122 a | 65.706 ab | 67.065 ab | 67.563 a | 63.327 b | 66.925 ab | 67.720 a | 63.048 b | 64.459 ab | 64.708 ab | 63.091 b | 65.920 ab | 55.549 c | *** |
| SD | 14.376 | 3.643 | 6.429 | 3.082 | 1.969 | 7.235 | 0.464 | 8.960 | 0.879 | 0.738 | 1.564 | 0.647 | 1.281 | 0.800 | 8.463 | 2.171 | |
| Total sugars, g glucose/L | 2.192 c | 2.847 bc | 3.106 bc | 2.597 bc | 3.057 bc | 2.649 bc | 2.637 bc | 2.245 bc | 2.473 bc | 2.911 bc | 2.765 bc | 3.686 b | 2.273 bc | 2.524 bc | 1.958 c | 5.996 a | *** |
| SD | 0.049 | 0.064 | 0.072 | 0.023 | 0.062 | 0.011 | 0.023 | 0.024 | 0.068 | 0.016 | 0.000 | 0.001 | 0.002 | 0.024 | 0.068 | 7.707 | |
| Parameter/Indices | Control | CC, t/ha | WCH, t/ha | BDL, t/ha | CC25 + WCH75, t/ha | CC50 + BDL50, t/ha | p-Values Signification Codes 1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 4 | 8 | 2 | 4 | 2 | 4 | 8 | 2 | 4 | 2 | 4 | 8 | 2 | 4 | |||
| Extract, °Blg | 0.65 b | 0.67 b | 0.82 b | 0.69 b | 0.89 b | 0.78 b | 0.81 b | 0.63 b | 0.66 b | 0.77 b | 0.91 b | 0.81 b | 0.72 b | 0.67 b | 0.81 b | 1.26 a | *** |
| SD | 0.05 | 0.16 | 0.07 | 0.01 | 0.04 | 0.02 | 0.07 | 0.05 | 0.04 | 0.05 | 0.07 | 0.06 | 0.04 | 0.08 | 0.09 | 0.52 | |
| pH | 3.88 gh | 4.14 abcd | 4.13 bcd | 4.06 cdef | 4.30 a | 4.04 cdefg | 4.09 bcde | 3.81 h | 3.92 fgh | 4.18 abc | 4.20 abc | 3.99 defg | 4.10 bcde | 4.11 bcde | 4.25 ab | 3.95 efgh | *** |
| SD | 0.01 | 0.07 | 0.06 | 0.01 | 0.15 | 0.04 | 0.01 | 0.01 | 0.19 | 0.05 | 0.02 | 0.06 | 0.04 | 0.02 | 0.02 | 0.17 | |
| Intake of sugars, % | 98.75 a | 98.33 ab | 98.16 ab | 98.51 ab | 98.27 ab | 98.55 ab | 98.52 ab | 98.74 a | 98.57 ab | 98.33 ab | 98.32 ab | 97.83 b | 98.71 a | 98.55 ab | 98.84 a | 96.34 c | *** |
| SD | 0.02 | 0.02 | 0.05 | 0.00 | 0.01 | 0.00 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.00 | 0.00 | 0.01 | 0.03 | 2.66 | |
| Fermentation yield (% of theoretical) | 71.89 abcd | 75.91 ab | 75.77 ab | 76.59 a | 72.99 abc | 71.93 abcd | 74.26 abc | 69.68 cd | 75.62 ab | 76.07 ab | 74.99 ab | 74.24 abc | 71.69 abcd | 71.10 bcd | 76.01 ab | 67.25 d | *** |
| SD | 5.16 | 1.98 | 13.20 | 3.09 | 2.23 | 0.59 | 5.93 | 32.03 | 0.65 | 2.29 | 6.88 | 1.61 | 0.00 | 0.19 | 3.65 | 15.99 | |
| Compound (mg/L) | Control | CC, t/ha | WCH, t/ha | BDL, t/ha | CC25 + WCH75, t/ha | CC50 + BDL50, t/ha | p-Values Signification Codes 1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 4 | 8 | 2 | 4 | 8 | 2 | 4 | 8 | 2 | 4 | 8 | 2 | 4 | 8 | |||
| Ethanal | 3.28 bc | 1.97 cd | 2.59 bcd | 3.15 bcd | 3.62 b | 3.69 b | 3.03 bcd | 1.78 d | 3.00 bcd | 3.44 b | 2.49 bcd | 2.41 bcd | 2.80 bcd | 2.29 bcd | 2.42 bcd | 8.98 a | *** |
| SD | 1.20 | 0.08 | 0.29 | 0.37 | 0.18 | 0.08 | 0.07 | 0.04 | 0.16 | 0.11 | 0.30 | 0.45 | 0.10 | 0.23 | 0.95 | 0.71 | |
| Propan-2-one | 0.14 a | 0.10 a | 0.14 a | 0.11 a | 0.13 a | 0.14 a | 0.12 a | 0.12 a | 0.13 a | 0.12 a | 0.12 a | 0.12 a | 0.12 a | 0.12 a | 0.11 a | 0.12 a | ° |
| SD | 0.02 | 0.00 | 0.01 | 0.02 | 0.00 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.03 | 0.01 | 0.02 | 0.00 | 0.03 | 0.01 | |
| Methanol | 1.984 bc | 2.27 abc | 2.62 a | 2.11 abc | 2.19 abc | 2.25 abc | 2.03 abc | 2.06 abc | 1.959 bc | 2.33 ab | 2.11 abc | 2.22 abc | 1.70 c | 2.14 abc | 1.99 bc | 1.94 bc | ** |
| SD | 0.04 | 0.13 | 0.20 | 0.31 | 0.04 | 0.20 | 0.04 | 0.11 | 0.18 | 0.09 | 0.33 | 0.34 | 0.16 | 0.07 | 0.32 | 0.15 | |
| Propan-1-ol | 7.72 ab | 6.76 ab | 8.53 a | 8.08 a | 7.87 ab | 8.07 a | 7.49 ab | 8.77 a | 7.47 ab | 8.85 a | 6.80 ab | 6.51 ab | 6.80 ab | 7.87 ab | 8.79 a | 4.97 b | ** |
| SD | 0.48 | 1.63 | 0.23 | 1.32 | 0.14 | 0.19 | 0.57 | 0.30 | 0.58 | 0.84 | 0.86 | 0.87 | 0.51 | 0.65 | 2.41 | 1.26 | |
| Hexanal | 0.70 ab | 0.59 ab | 0.81 a | 0.70 ab | 0.65 ab | 0.76 a | 0.67 ab | 0.70 ab | 0.65 ab | 0.75 a | 0.70 ab | 0.58 ab | 0.67 ab | 0.72 ab | 0.77 a | 0.47 b | * |
| SD | 0.04 | 0.15 | 0.02 | 0.12 | 0.01 | 0.06 | 0.02 | 0.02 | 0.02 | 0.08 | 0.11 | 0.10 | 0.06 | 0.09 | 0.16 | 0.16 | |
| Butan-1-ol | 0.31 a | 0.31 a | 0.32 a | 0.33 a | 0.33 a | 0.35 a | 0.36 a | 0.30 a | 0.26 a | 0.35 a | 0.27 a | 0.29 a | 0.30 a | 0.27 a | 0.27 a | 0.25 a | * |
| SD | 0.06 | 0.02 | 0.06 | 0.05 | 0.04 | 0.07 | 0.01 | 0.01 | 0.01 | 0.01 | 0.03 | 0.04 | 0.03 | 0.01 | 0.01 | 0.03 | |
| Ethyl ethanoate | 3.81 abc | 3.30 abc | 2.76 abc | 3.08 abc | 2.51 bc | 2.69 abc | 2.74 abc | 3.96 a | 3.59 abc | 3.23 abc | 2.22 c | 2.93 abc | 2.83 abc | 2.73 abc | 3.36 abc | 2.31 c | ** |
| SD | 0.54 | 0.15 | 0.58 | 0.02 | 0.29 | 0.34 | 0.07 | 0.79 | 0.18 | 0.45 | 0.05 | 0.72 | 0.80 | 0.31 | 0.72 | 0.16 | |
| 2-Methylpropan-1-ol | 110.16 a | 110.54 a | 116.26 a | 122.85 a | 91.05 ab | 119.64 a | 108.94 a | 123.36 a | 108.35 ab | 114.32 a | 103.55 ab | 83.32 ab | 101.89 ab | 107.35 ab | 125.01 a | 65.02 b | ** |
| SD | 7.71 | 5.00 | 1.66 | 3.61 | 17.37 | 11.11 | 7.27 | 14.49 | 4.81 | 11.41 | 8.73 | 18.83 | 7.80 | 8.20 | 20.25 | 37.91 | |
| 3-Methylbutan-1-ol | 92.69 cde | 112.80 a | 100.74 abcd | 108.62 ab | 95.13 bcde | 101.64 abc | 102.05 abc | 102.49 abc | 86.93 de | 108.29 ab | 93.04 cde | 97.55 bcde | 85.99 e | 92.07 cde | 93.58 cde | 90.85 cde | *** |
| SD | 5.79 | 5.10 | 6.66 | 1.16 | 2.45 | 2.28 | 0.82 | 10.59 | 1.46 | 5.05 | 5.02 | 5.85 | 3.84 | 2.13 | 2.92 | 2.17 | |
| 2-Methylbutan-1-ol | 43.19 ab | 46.91 ab | 45.01 ab | 49.10 a | 38.99 bc | 47.37 ab | 45.45 ab | 49.31 a | 40.71 abc | 47.24 ab | 41.99 abc | 38.87 bc | 40.22 abc | 42.32 abc | 45.95 ab | 33.58 c | *** |
| SD | 1.32 | 2.12 | 2.28 | 0.63 | 3.97 | 1.75 | 1.45 | 4.64 | 0.62 | 1.45 | 2.43 | 3.41 | 3.01 | 1.25 | 3.38 | 7.49 | |
| Compound (μg/L) | Control | CC, t/ha | WCH, t/ha | BDL, t/ha | CC25 + WCH75, t/ha | CC50 + BDL50, t/ha | p-Values Signification Codes 1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 4 | 8 | 2 | 4 | 2 | 4 | 8 | 2 | 4 | 2 | 4 | 8 | 2 | 4 | |||
| Ethyl methanoate | 157.2 a | 72.6 bc | 126.3 abc | 145.4 ab | 148.6 ab | 159.6 a | 136.5 abc | 73.9 bc | 131.0 abc | 108.4 abc | 97.9 abc | 95.9 abc | 106.7 abc | 137.8 abc | 92.6 abc | 58.0 c | *** |
| SD | 39.2 | 8.5 | 12.0 | 57.7 | 6.9 | 35.1 | 15.2 | 5.4 | 23.6 | 16.0 | 11.2 | 31.7 | 21.3 | 35.8 | 34.9 | 4.6 | |
| Ethyl 2-methylbutanoate | 0.7 a | 0.6 a | 0.6 a | 0.6 a | 0.5 a | 0.6 a | 0.4 a | 0.7 a | 0.5 a | 0.5 a | 0.6 a | 0.6 a | 0.5 a | 0.6 a | 0.6 a | 0.4 a | * |
| SD | 0.0 | 0.0 | 0.2 | 0.1 | 0.1 | 0.1 | 0.0 | 0.1 | 0.0 | 0.0 | 0.2 | 0.1 | 0.0 | 0.1 | 0.2 | 0.1 | |
| Ethyl propanoate | 46.8 a | 39.7 ab | 33.9 ab | 41.6 ab | 29.2 b | 38.0 ab | 33.3 ab | 44.5 ab | 41.5 ab | 43.5 ab | 30.8 b | 40.1 ab | 35.5 ab | 38.6 ab | 36.7 ab | 32.3 ab | ** |
| SD | 5.6 | 1.8 | 6.2 | 0.9 | 4.4 | 6.5 | 3.8 | 5.4 | 4.3 | 6.4 | 0.9 | 8.5 | 9.7 | 2.7 | 2.8 | 1.0 | |
| Ethyl 2-methylpropanoate | 6.0 ab | 5.4 abc | 4.6 abcd | 5.5 abc | 4.1 bcd | 4.9 abc | 4.3 bcd | 6.4 a | 6.1 ab | 5.2 abc | 4.1 bcd | 3.8 cd | 4.7 abc | 4.9 abc | 6.4 a | 2.5 d | *** |
| SD | 0.4 | 0.2 | 0.6 | 0.2 | 0.8 | 1.1 | 0.2 | 0.7 | 0.7 | 0.6 | 0.2 | 0.2 | 1.3 | 0.1 | 0.9 | 1.1 | |
| Isobutyl ethanoate | 9.9 ab | 5.5 bc | 8.4 abc | 9.4 ab | 6.1 bc | 9.1 ab | 7.6 abc | 8.6 abc | 11.7 a | 8.3 abc | 6.0 bc | 5.0 bc | 8.3 abc | 7.4 abc | 10.0 ab | 3.6 c | *** |
| SD | 0.7 | 0.2 | 1.2 | 0.1 | 2.0 | 1.8 | 0.7 | 1.1 | 0.8 | 1.8 | 0.6 | 0.9 | 2.5 | 0.6 | 4.1 | 2.8 | |
| Ethyl butanoate | 5.2 ab | 4.1 ab | 4.4 ab | 5.4 a | 3.7 abc | 4.7 ab | 4.1 ab | 5.0 ab | 5.4 a | 4.8 ab | 3.4 bc | 3.5 bc | 3.9 ab | 4.1 ab | 5.0 ab | 2.2 c | *** |
| SD | 0.6 | 0.2 | 0.8 | 0.0 | 0.6 | 0.7 | 0.1 | 0.3 | 0.4 | 0.7 | 0.2 | 0.2 | 0.8 | 0.3 | 1.0 | 1.0 | |
| Ethyl 3-methylbutanoate | 0.2 b | 0.3 a | 0.2 b | 0.3 a | 0.2 b | 0.2 b | 0.2 b | 0.2 b | 0.2 b | 0.2 b | 0.2 b | 0.2 b | 0.2 b | 0.2 b | 0.2 b | 0.1 c | *** |
| SD | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 3-Methylbutyl ethanoate | 41.0 ab | 27.8 bc | 35.5 ab | 41.7 ab | 30.5 abc | 36.9 ab | 32.2 abc | 38.0 ab | 47.7 a | 36.0 ab | 25.2 bc | 27.4 bc | 32.9 abc | 32.8 abc | 41.9 ab | 16.2 c | *** |
| SD | 8.9 | 1.3 | 7.5 | 0.8 | 3.5 | 6.3 | 1.4 | 3.7 | 2.0 | 6.9 | 2.4 | 4.3 | 9.2 | 2.0 | 13.3 | 6.8 | |
| 2-Methylbutyl ethanoate | 9.8 abc | 5.1 cd | 8.2 abcd | 9.5 abc | 6.3 bcd | 9.0 abc | 7.4 abcd | 8.8 abc | 11.5 a | 8.1 abcd | 5.7 bcd | 5.4 bcd | 8.0 abcd | 7.5 abcd | 10.2 ab | 3.3 d | *** |
| SD | 1.8 | 0.2 | 1.5 | 0.2 | 1.6 | 1.7 | 0.7 | 0.8 | 0.7 | 2.0 | 0.5 | 0.8 | 2.3 | 0.5 | 3.9 | 2.5 | |
| Ethyl pentanoate | 1.4 abc | 1.7 ab | 1.3 bcd | 1.7 a | 1.2 cd | 1.5 abc | 1.3 abcd | 1.5 abc | 1.3 bcd | 1.4 abc | 1.1 cde | 1.3 abcd | 0.9 de | 1.3 cd | 1.2 cd | 0.7 e | *** |
| SD | 0.3 | 0.1 | 0.2 | 0.0 | 0.2 | 0.2 | 0.1 | 0.1 | 0.1 | 0.0 | 0.0 | 0.2 | 0.1 | 0.0 | 0.0 | 0.0 | |
| Ethyl hexanoate | 10.2 bc | 11.1 abc | 13.1 abc | 16.9 a | 11.5 abc | 14.2 ab | 15.1 ab | 11.8 abc | 10.8 bc | 14.4 ab | 10.1 bc | 14.6 ab | 7.7 c | 7.6 c | 9.4 bc | 13.4 abc | *** |
| SD | 2.6 | 0.5 | 2.8 | 1.1 | 2.4 | 1.4 | 2.6 | 1.3 | 1.1 | 1.1 | 0.5 | 4.1 | 1.6 | 1.0 | 1.1 | 1.9 | |
| Ethyl octanoate | 1.3 cd | 1.2 cd | 1.9 cd | 4.4 a | 1.6 cd | 3.3 ab | 2.0 bcd | 1.3 cd | 1.3 cd | 2.5 bc | 2.2 bcd | 1.5 cd | 1.1 d | 1.0 d | 1.4 cd | 1.2 cd | *** |
| SD | 0.1 | 0.1 | 0.2 | 0.3 | 0.4 | 0.4 | 0.2 | 0.1 | 0.2 | 1.0 | 0.2 | 0.7 | 0.3 | 0.0 | 0.2 | 0.9 | |
| Ethyl nonanoate | 0.1 e | 0.1 e | 0.1 e | 0.4 a | 0.1 e | 0.3 b | 0.2 c | 0.1 e | 0.1 e | 0.2 c | 0.1 e | 0.1 e | 0.1 e | 0.1 e | 0.1 e | 0.1 e | *** |
| SD | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Ethyl decanoate | 1.0 cd | 1.0 cd | 1.1 cd | 2.6 a | 1.0 cd | 2.0 ab | 1.3 cd | 0.8 d | 0.9 d | 1.6 bc | 1.2 bc | 0.8 d | 0.8 d | 0.8 d | 0.8 d | 0.7 d | *** |
| SD | 0.1 | 0.0 | 0.1 | 0.1 | 0.2 | 0.2 | 0.1 | 0.0 | 0.1 | 0.5 | 0.1 | 0.3 | 0.1 | 0.0 | 0.1 | 0.4 | |
| Ethyl dodecanoate | 0.2 e | 0.4 cd | 0.3 de | 0.8 a | 0.3 de | 0.6 b | 0.4 cd | 0.2 e | 0.2 e | 0.5 bc | 0.3 de | 0.3 de | 0.2 e | 0.2 e | 0.2 e | 0.2 e | *** |
| SD | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | |
| Compound (μg/L) | Control | CC, t/ha | WCH, t/ha | BDL, t/ha | CC25 + WCH75, t/ha | CC50 + BDL50, t/ha | p-Values Signification Codes 1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 4 | 8 | 2 | 4 | 8 | 2 | 4 | 8 | 2 | 4 | 8 | 2 | 4 | 8 | |||
| 2-Methylpropanal | 39.9 bc | 23.7 c | 36.1 bc | 33.0 bc | 36.7 bc | 51.0 ab | 34.8 bc | 22.88 c | 36.6 bc | 45.1 abc | 38.2 bc | 29.6 bc | 40.3 bc | 29.7 bc | 25.5 bc | 70.5 a | *** |
| SD | 3.8 | 1.0 | 1.5 | 7.6 | 2.4 | 1.9 | 2.1 | 1.1 | 0.9 | 4.1 | 1.4 | 9.1 | 2.4 | 3.4 | 1.2 | 2.9 | |
| 2-Methylbutanal | 4.2 b | 2.5 b | 3.6 b | 3.9 b | 4.1 b | 5.8 ab | 3.7 b | 2.7 b | 3.8 b | 4.9 b | 4.0 b | 3.4 b | 3.9 b | 2.9 b | 3.1 b | 10.8 a | *** |
| SD | 0.8 | 0.2 | 0.1 | 0.7 | 0.2 | 0.2 | 0.1 | 0.2 | 0.1 | 0.3 | 0.2 | 0.6 | 0.1 | 0.3 | 0.7 | 6.6 | |
| 3-Methylbutanal | 9.1 b | 6.5 b | 9.2 b | 9.9 b | 9.2 b | 11.2 ab | 8.1 b | 5.5 b | 7.9 b | 11.7 ab | 9.1 b | 7.3 b | 8.0 b | 6.6 b | 5.7 b | 19.6 a | ** |
| SD | 2.1 | 0.7 | 1.1 | 1.6 | 0.3 | 0.2 | 0.3 | 0.6 | 0.3 | 1.0 | 0.6 | 2.6 | 0.2 | 0.4 | 1.1 | 11.5 | |
| Furan-2-carbaldehyde | 16.7 bc | 22.9 abc | 21.6 abc | 27.5 a | 21.3 abc | 26.6 ab | 23.6 abc | 19.5 abc | 16.4 bc | 22.3 abc | 19.6 abc | 23.6 abc | 14.7 c | 17.0 bc | 19.0 abc | 22.8 abc | ** |
| SD | 1.9 | 0.5 | 3.2 | 10.2 | 0.6 | 2.6 | 1.4 | 1.5 | 0.6 | 5.1 | 1.4 | 4.0 | 0.4 | 1.5 | 2.4 | 0.7 | |
| Butane-2,3-dione | 26.6 c | 55.4 c | 44.2 c | 33.9 c | 392.5 a | 36.1 c | 35.0 c | 36.9 c | 23.3 c | 40.3 c | 46.5 c | 52.5 c | 22.7 c | 29.7 c | 74.2 bc | 262.5 ab | *** |
| SD | 1.7 | 6.0 | 7.5 | 2.7 | 3.3 | 2.2 | 3.9 | 1.2 | 0.6 | 1.3 | 9.3 | 9.9 | 0.6 | 5.5 | 4.4 | 16.7 | |
| 1,1-Diethoxyethane | 13.1 abcd | 7.9 cd | 9.1 cd | 21.0 a | 12.1 bcd | 19.0 ab | 13.9 abcd | 6.8 cd | 15.0 abc | 19.1 ab | 6.2 d | 7.2 cd | 11.4 bcd | 7.8 cd | 9.3 cd | 9.1 cd | *** |
| SD | 6.7 | 0.4 | 2.1 | 2.1 | 4.4 | 1.4 | 1.1 | 0.4 | 0.9 | 1.1 | 1.1 | 2.4 | 3.0 | 0.9 | 5.2 | 2.8 | |
| Hexan-1-ol | 80.1 abc | 93.2 abc | 86.5 abc | 107.2 ab | 64.5 c | 121.5 a | 93.5 abc | 71.3 bc | 73.2 bc | 85.4 abc | 89.6 abc | 75.7 bc | 75.2 bc | 110.4 ab | 78.1 bc | 100.5 abc | *** |
| SD | 23.5 | 30.8 | 7.5 | 2.7 | 13.1 | 13.7 | 12.1 | 3.9 | 5.8 | 1.3 | 17.5 | 5.6 | 12.2 | 14.8 | 15.3 | 2.6 | |
| Octan-1-ol | 4.5 a | 4.1 a | 5.8 a | 5.9 a | 4.0 a | 6.1 a | 4.8 a | 5.5 a | 4.8 a | 5.5 a | 4.8 a | 3.7 a | 4.2 a | 4.2 a | 4.7 a | 4.7 a | . |
| SD | 0.3 | 1.1 | 1.5 | 0.1 | 0.7 | 0.5 | 0.7 | 1.4 | 0.3 | 0.7 | 1.5 | 0.3 | 0.4 | 0.8 | 1.6 | 1.3 | |
| No. | Treatment * | Rate of Ash/Ashes ** (Per 7 kg of Soil) | Rate of Ash/Ashes (t/ha) |
|---|---|---|---|
| 1 | Control | - | - |
| 2 | CC 2 t/ha | 8 g CC | 2 |
| 3 | CC 4 t/ha | 16 g CC | 4 |
| 4 | CC 8 t/ha | 32 g CC | 8 |
| 5 | WCH 2 t/ha | 8 g WCH | 2 |
| 6 | WCH 4 t/ha | 16 g WCH | 4 |
| 7 | WCH 8 t/ha | 32 g WCH | 8 |
| 8 | BDL 2 t/ha | 8 g BDL | 2 |
| 9 | BDL 4 t/ha | 16 g BDL | 4 |
| 10 | BDL 8 t/ha | 32 g BDL | 8 |
| 11 | CC25 + WCH75 2 t/ha | 8 g (CC 25% + WCH 75%) | 2 |
| 12 | CC25 + WCH75 4 t/ha | 16 g (CC 25% + WCH 75%) | 4 |
| 13 | CC25 + WCH75 8 t/ha | 32 g (CC 25% + WCH 75%) | 8 |
| 14 | CC50 + BDL50 2 t/ha | 8 g (CC 50% + BDL 50%) | 2 |
| 15 | CC50 + BDL50 4 t/ha | 16 g (CC 50% + BDL 50%) | 4 |
| 16 | CC50 + BDL50 8 t/ha | 32 g (CC 50% + BDL 50%) | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ściubak, Ł.; Baryga, A.; Balcerek, M.; Pielech-Przybylska, K.; Dziekońska-Kubczak, U.; Brzeziński, S. Fermentation Efficiency and Profile of Volatile Compounds in Rye Grain Mashes from Crops Fertilised with Agrifood Waste Ashes. Molecules 2025, 30, 3251. https://doi.org/10.3390/molecules30153251
Ściubak Ł, Baryga A, Balcerek M, Pielech-Przybylska K, Dziekońska-Kubczak U, Brzeziński S. Fermentation Efficiency and Profile of Volatile Compounds in Rye Grain Mashes from Crops Fertilised with Agrifood Waste Ashes. Molecules. 2025; 30(15):3251. https://doi.org/10.3390/molecules30153251
Chicago/Turabian StyleŚciubak, Łukasz, Andrzej Baryga, Maria Balcerek, Katarzyna Pielech-Przybylska, Urszula Dziekońska-Kubczak, and Stanisław Brzeziński. 2025. "Fermentation Efficiency and Profile of Volatile Compounds in Rye Grain Mashes from Crops Fertilised with Agrifood Waste Ashes" Molecules 30, no. 15: 3251. https://doi.org/10.3390/molecules30153251
APA StyleŚciubak, Ł., Baryga, A., Balcerek, M., Pielech-Przybylska, K., Dziekońska-Kubczak, U., & Brzeziński, S. (2025). Fermentation Efficiency and Profile of Volatile Compounds in Rye Grain Mashes from Crops Fertilised with Agrifood Waste Ashes. Molecules, 30(15), 3251. https://doi.org/10.3390/molecules30153251





