A Novel HDAC6 Inhibitor Ameliorates Imiquimod-Induced Psoriasis-Like Inflammation in Mice
Abstract
1. Introduction
2. Results
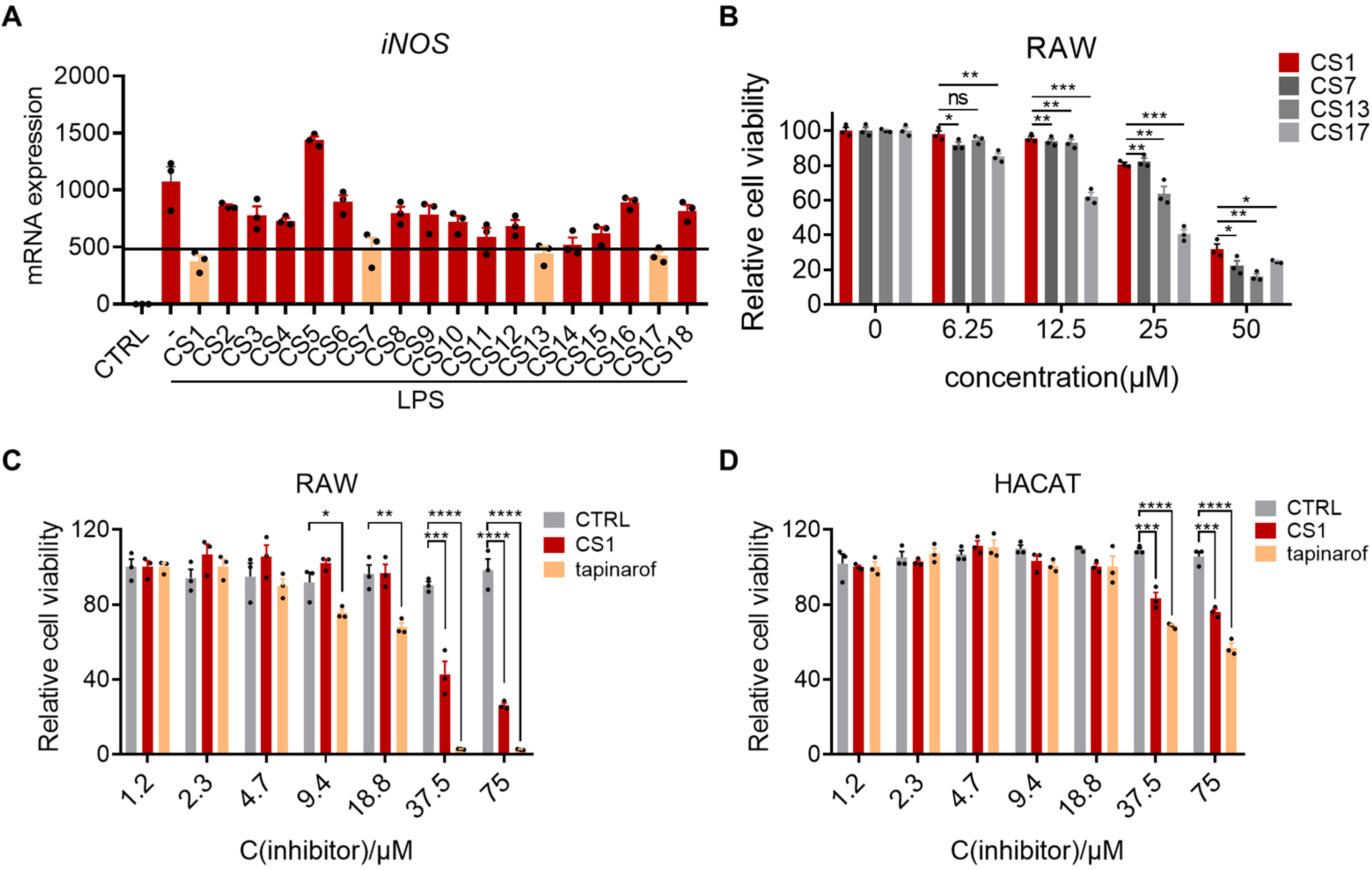
2.1. Cystamine-Based Compounds Effectively Inhibit LPS-Induced Inflammation
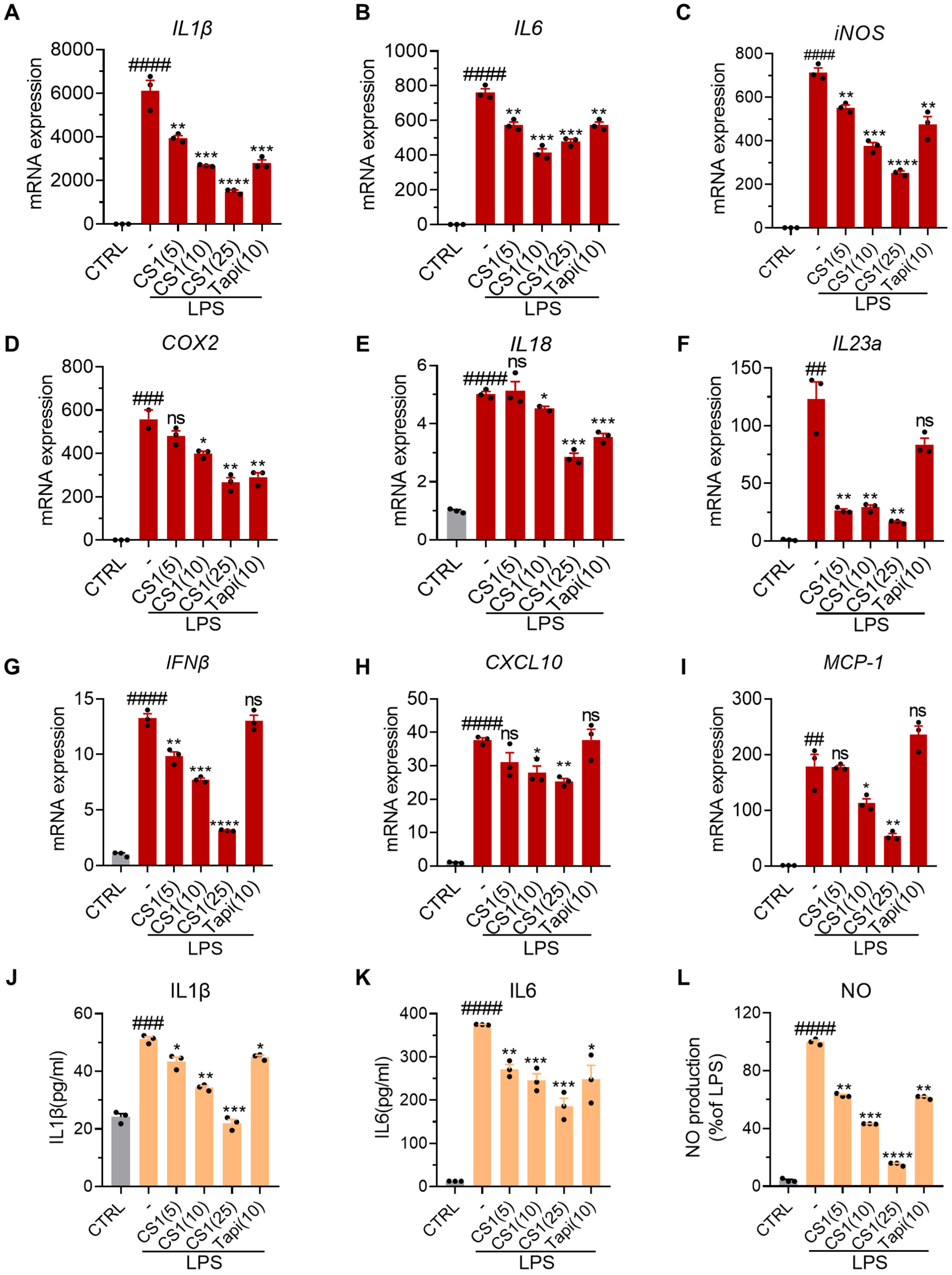
2.2. CS1 Effectively Inhibits LPS-Induced Inflammation in RAW264.7 Cells
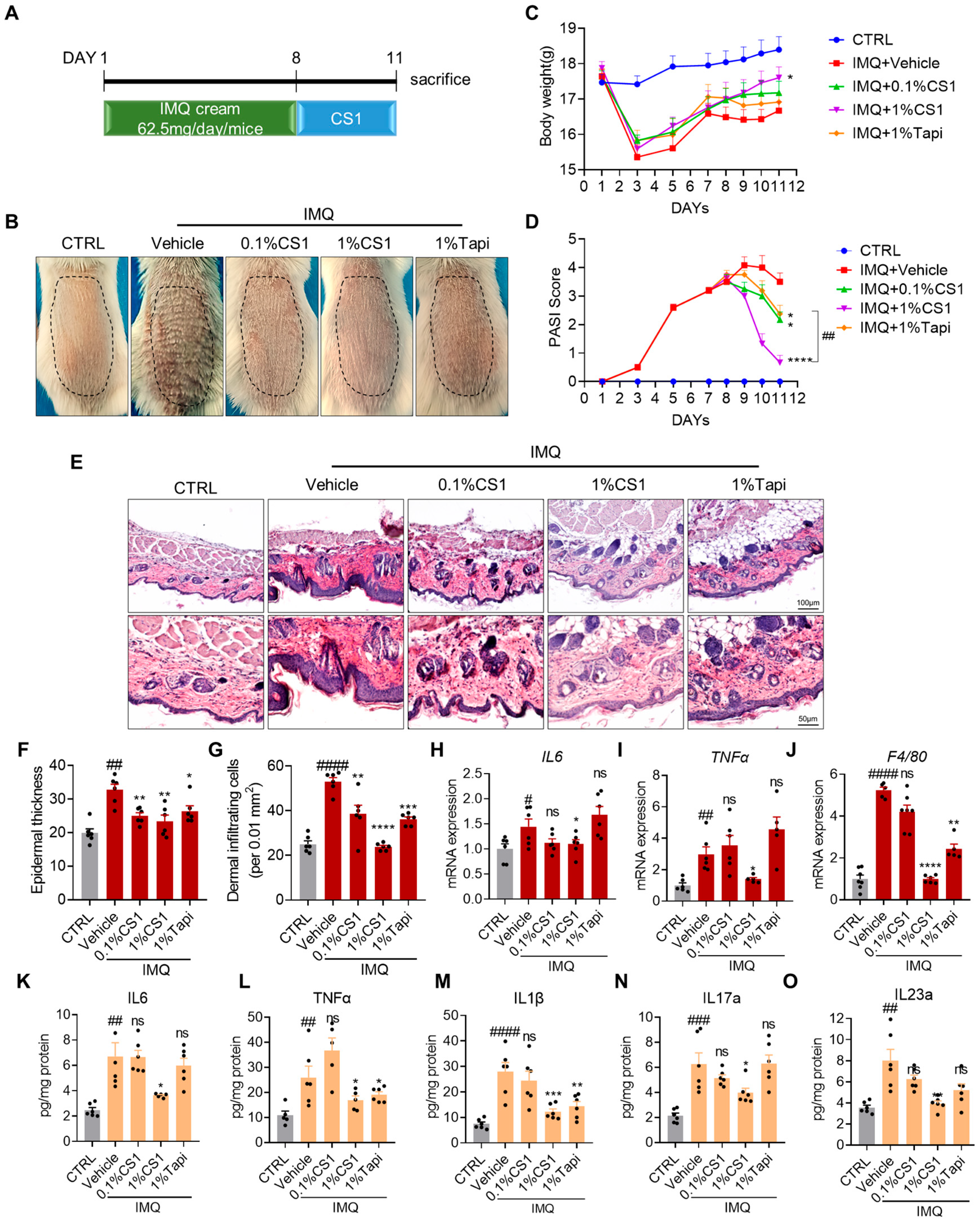
2.3. CS1 Attenuates IMQ-Induced Psoriasis-Like Phenotype in Mice
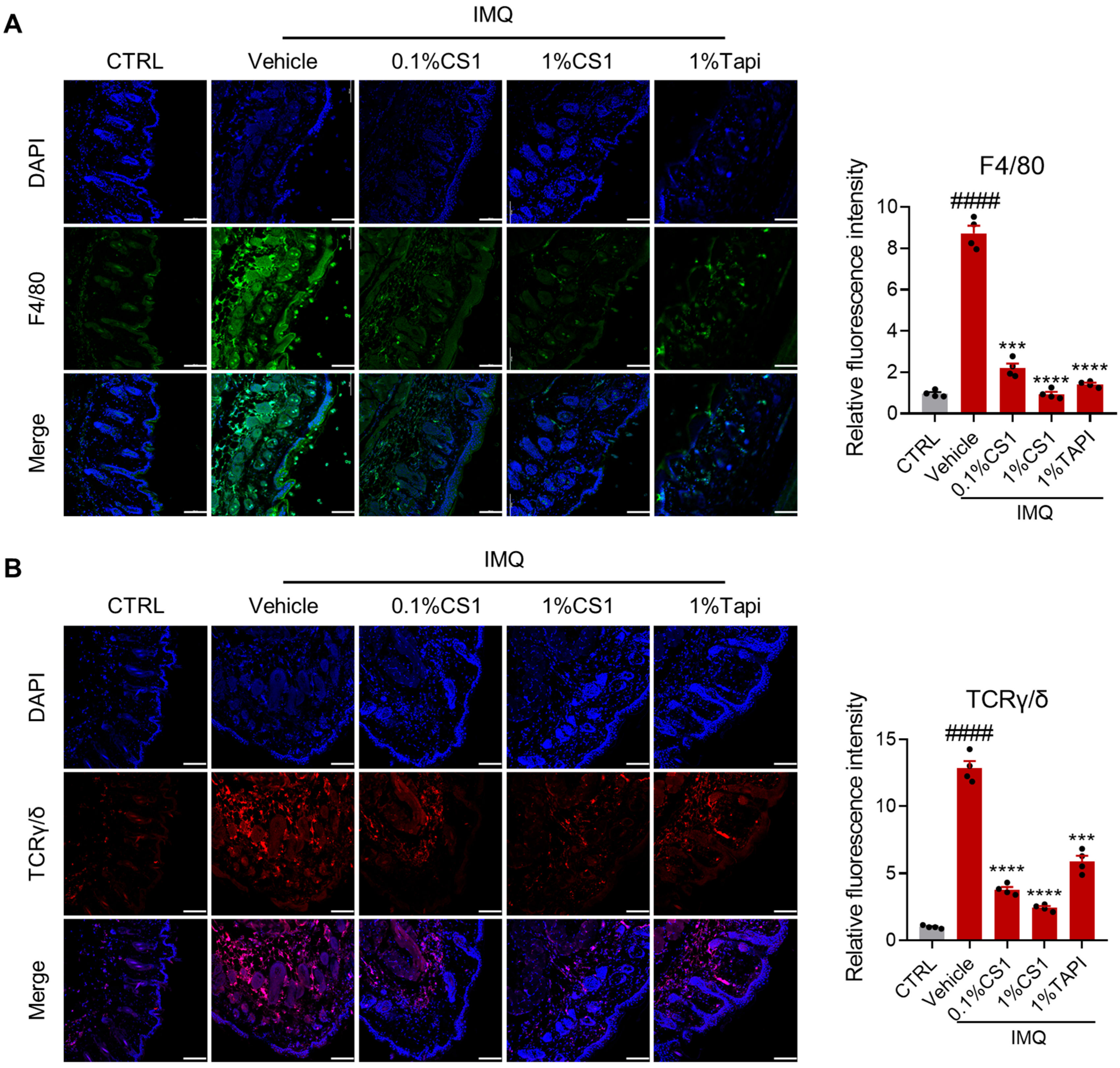
2.4. CS1 Inhibits the Infiltration of Immune Cells
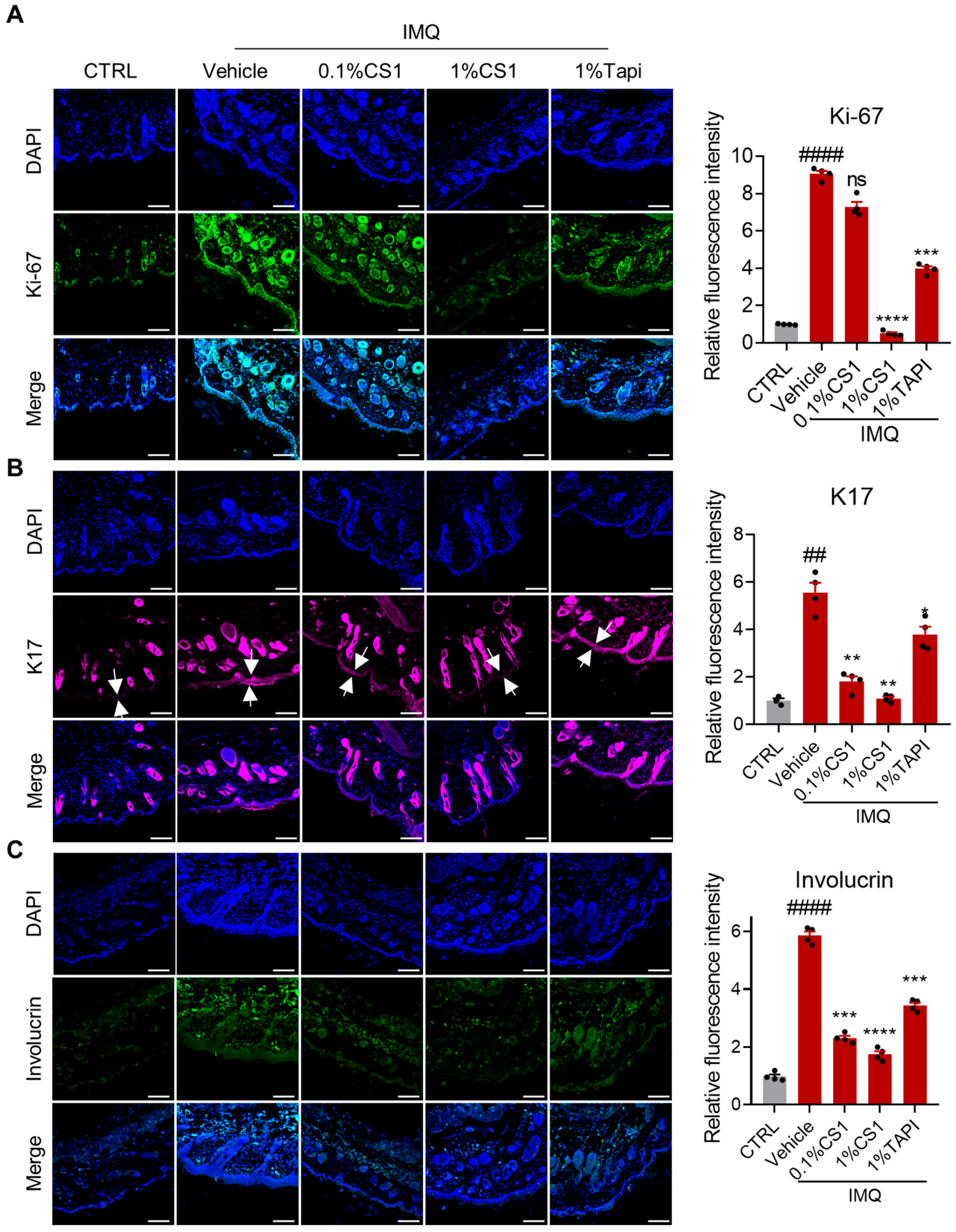
2.5. CS1 Inhibits the Hyperproliferation and Altered Differentiation of Keratinocytes
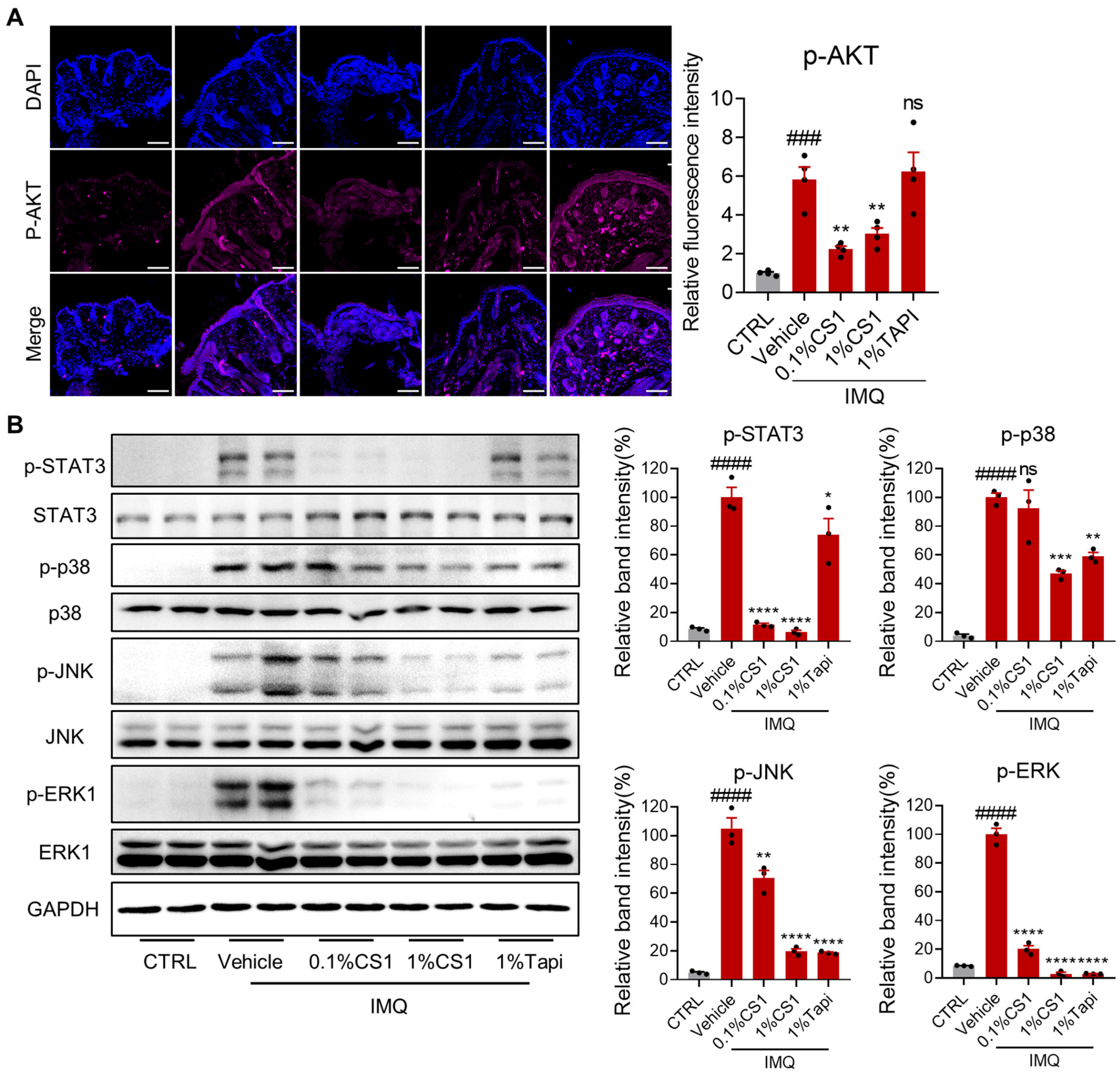
2.6. CS1 Effectively Inhibits the AKT and MAPK Pathways
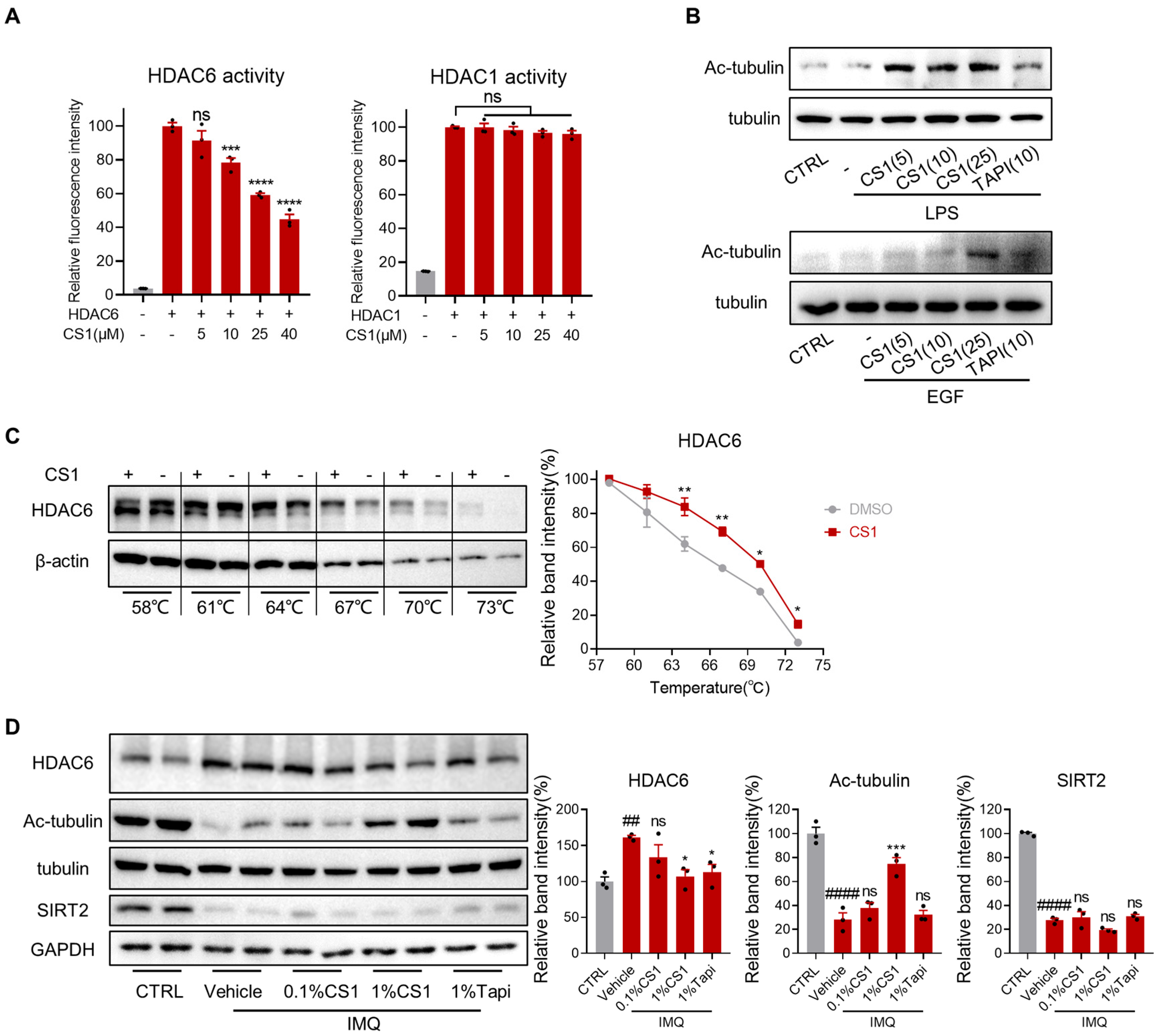
2.7. CS1 Is a Novel HDAC6 Inhibitor
3. Materials and Methods
3.1. Cell Culture
3.2. Cell Viability
3.3. Real-Time Quantitative PCR
3.4. Enzyme-Linked Immunosorbent Assay
3.5. IMQ-Induced Psoriasis-Like Skin Inflammation Model
3.6. Histological Analysis
3.7. Antibodies
3.8. Immunofluorescence (IF)
3.9. Western Blot Analysis
3.10. HDAC6 Activity Assay
3.11. HDAC1 Activity Assay
3.12. Cellular Thermal Shift Assay
3.13. Wound Healing Assay
3.14. Statistical Analysis
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Griffiths, C.E.M.; Barker, J.N.W.N. Pathogenesis and clinical features of psoriasis. Lancet 2007, 370, 263–271. [Google Scholar] [CrossRef]
- Reich, K. The concept of psoriasis as a systemic inflammation: Implications for disease management. J. Eur. Acad. Dermatol. Venereol. 2012, 26 (Suppl. S2), 3–11. [Google Scholar] [CrossRef]
- Buchanan, M.M.; Hutchinson, M.; Watkins, L.R.; Yin, H. Toll-like receptor 4 in CNS pathologies. J. Neurochem. 2010, 114, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Segaert, S.; Calzavara-Pinton, P.; de la Cueva, P.; Jalili, A.; Lons Danic, D.; Pink, A.E.; Thaci, D.; Gooderham, M. Long-term topical management of psoriasis: The road ahead. J. Dermatolog Treat. 2022, 33, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Kimball, A.B.; Jacobson, C.; Weiss, S.; Vreeland, M.G.; Wu, Y. The psychosocial burden of psoriasis. Am. J. Clin. Dermatol. 2005, 6, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kim, M. Challenges and Future Trends in the Treatment of Psoriasis. Int. J. Mol. Sci. 2023, 24, 3313. [Google Scholar] [CrossRef]
- Lowes, M.A.; Bowcock, A.M.; Krueger, J.G. Pathogenesis and therapy of psoriasis. Nature 2007, 445, 866–873. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef]
- Kaushik, S.B.; Lebwohl, M.G. Review of safety and efficacy of approved systemic psoriasis therapies. Int. J. Dermatol. 2019, 58, 649–658. [Google Scholar] [CrossRef]
- Bartucci, R.; Salvati, A.; Olinga, P.; Boersma, Y.L. Vanin 1: Its Physiological Function and Role in Diseases. Int. J. Mol. Sci. 2019, 20, 3891. [Google Scholar] [CrossRef]
- Fu, J.; Liu, S.; Hu, M.; Liao, X.; Wang, X.; Xu, Z.; Li, Q.; Quan, J. Biguanide MC001, a Dual Inhibitor of OXPHOS and Glycolysis, Shows Enhanced Antitumor Activity Without Increasing Lactate Production. ChemMedChem 2022, 17, e202100674. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Wang, X.; Xu, Z.; Li, Q.; Quan, J. N-cystaminylbiguanide MC001 prevents neuron cell death and alleviates motor deficits in the MPTP-model of Parkinson’s disease. Neurosci. Lett. 2022, 784, 136751. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.A.; Kupper, T.S. Misbehaving macrophages in the pathogenesis of psoriasis. J. Clin. Investig. 2006, 116, 2084–2087. [Google Scholar] [CrossRef]
- Gillitzer, R.; Wolff, K.; Tong, D.; Muller, C.; Yoshimura, T.; Hartmann, A.A.; Stingl, G.; Berger, R. MCP-1 mRNA expression in basal keratinocytes of psoriatic lesions. J. Investig. Dermatol. 1993, 101, 127–131. [Google Scholar] [CrossRef]
- Marble, D.J.; Gordon, K.B.; Nickoloff, B.J. Targeting TNFalpha rapidly reduces density of dendritic cells and macrophages in psoriatic plaques with restoration of epidermal keratinocyte differentiation. J. Dermatol. Sci. 2007, 48, 87–101. [Google Scholar] [CrossRef]
- Fuentes-Duculan, J.; Suarez-Farinas, M.; Zaba, L.C.; Nograles, K.E.; Pierson, K.C.; Mitsui, H.; Pensabene, C.A.; Kzhyshkowska, J.; Krueger, J.G.; Lowes, M.A. A subpopulation of CD163-positive macrophages is classically activated in psoriasis. J. Investig. Dermatol. 2010, 130, 2412–2422. [Google Scholar] [CrossRef]
- Prieto, K.; Duong, J.Q.; Feldman, S.R. Tapinarof cream for the topical treatment of plaque psoriasis in adults. Expert. Rev. Clin. Immunol. 2024, 20, 327–337. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Boguniewicz, M.; Quintana, F.J.; Clark, R.A.; Gross, L.; Hirano, I.; Tallman, A.M.; Brown, P.M.; Fredericks, D.; Rubenstein, D.S.; et al. Tapinarof validates the aryl hydrocarbon receptor as a therapeutic target: A clinical review. J. Allergy Clin. Immunol. 2024, 154, 1–10. [Google Scholar] [CrossRef]
- Ogawa, E.; Sato, Y.; Minagawa, A.; Okuyama, R. Pathogenesis of psoriasis and development of treatment. J. Dermatol. 2018, 45, 264–272. [Google Scholar] [CrossRef]
- Rizzo, H.L.; Kagami, S.; Phillips, K.G.; Kurtz, S.E.; Jacques, S.L.; Blauvelt, A. IL-23-mediated psoriasis-like epidermal hyperplasia is dependent on IL-17A. J. Immunol. 2011, 186, 1495–1502. [Google Scholar] [CrossRef]
- Cai, Y.; Shen, X.; Ding, C.; Qi, C.; Li, K.; Li, X.; Jala, V.R.; Zhang, H.G.; Wang, T.; Zheng, J.; et al. Pivotal role of dermal IL-17-producing gammadelta T cells in skin inflammation. Immunity 2011, 35, 596–610. [Google Scholar] [CrossRef] [PubMed]
- Pantelyushin, S.; Haak, S.; Ingold, B.; Kulig, P.; Heppner, F.L.; Navarini, A.A.; Becher, B. Rorgammat+ innate lymphocytes and gammadelta T cells initiate psoriasiform plaque formation in mice. J. Clin. Investig. 2012, 122, 2252–2256. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhang, W.; Li, B.; Wang, G. Keratin 17 in psoriasis: Current understanding and future perspectives. Semin. Cell Dev. Biol. 2022, 128, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Q.; Man, X.Y.; Li, W.; Zhou, J.; Landeck, L.; Cai, S.Q.; Zheng, M. Regulation of involucrin in psoriatic epidermal keratinocytes: The roles of ERK1/2 and GSK-3beta. Cell Biochem. Biophys. 2013, 66, 523–528. [Google Scholar] [CrossRef]
- Simon, M.; Rabeony, H.; Petit-Paris, I.; Garnier, J.; Barrault, C.; Pedretti, N.; Guilloteau, K.; Jegou, J.-F.; Guillet, G.; Huguier, V.; et al. Inhibition of Keratinocyte Differentiation by the Synergistic Effect of IL-17A, IL-22, IL-1α, TNFα and Oncostatin M. PLoS ONE 2014, 9, e101937. [Google Scholar] [CrossRef]
- Gnanaraj, P.; Dayalan, H.; Elango, T.; Malligarjunan, H.; Raghavan, V.; Rao, R. Downregulation of involucrin in psoriatic lesions following therapy with propylthiouracil, an anti-thyroid thioureylene: Immunohistochemistry and gene expression analysis. Int. J. Dermatol. 2014, 54, 302–306. [Google Scholar] [CrossRef]
- Morgner, B.; Tittelbach, J.; Wiegand, C. Induction of psoriasis- and atopic dermatitis-like phenotypes in 3D skin equivalents with a fibroblast-derived matrix. Sci. Rep. 2023, 13, 1807. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, X. The role of PI3K/AKT/FOXO signaling in psoriasis. Arch. Dermatol. Res. 2019, 311, 83–91. [Google Scholar] [CrossRef]
- Johansen, C.; Kragballe, K.; Westergaard, M.; Henningsen, J.; Kristiansen, K.; Iversen, L. The mitogen-activated protein kinases p38 and ERK1/2 are increased in lesional psoriatic skin. Br. J. Dermatol. 2005, 152, 37–42. [Google Scholar] [CrossRef]
- Andrés, R.M.; Hald, A.; Johansen, C.; Kragballe, K.; Iversen, L. Studies of Jak/STAT3 expression and signalling in psoriasis identifies STAT3-Ser727 phosphorylation as a modulator of transcriptional activity. Exp. Dermatol. 2013, 22, 323–328. [Google Scholar] [CrossRef]
- Johansen, C.; Vinter, H.; Soegaard-Madsen, L.; Olsen, L.R.; Steiniche, T.; Iversen, L.; Kragballe, K. Preferential inhibition of the mRNA expression of p38 mitogen-activated protein kinase regulated cytokines in psoriatic skin by anti-TNFα therapy. Br. J. Dermatol. 2010, 163, 1194–1204. [Google Scholar] [CrossRef]
- Calautti, E.; Avalle, L.; Poli, V. Psoriasis: A STAT3-Centric View. Int. J. Mol. Sci. 2018, 19, 171. [Google Scholar] [CrossRef]
- Yang, L.; Li, B.; Dang, E.; Jin, L.; Fan, X.; Wang, G. Impaired function of regulatory T cells in patients with psoriasis is mediated by phosphorylation of STAT3. J. Dermatol. Sci. 2016, 81, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, K.; Fu, J. HDAC6 Mediates Poly (I:C)-Induced TBK1 and Akt Phosphorylation in Macrophages. Front. Immunol. 2020, 11, 1776. [Google Scholar] [CrossRef] [PubMed]
- Youn, G.S.; Lee, K.W.; Choi, S.Y.; Park, J. Overexpression of HDAC6 induces pro-inflammatory responses by regulating ROS-MAPK-NF-kappaB/AP-1 signaling pathways in macrophages. Free Radic. Biol. Med. 2016, 97, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.B.; Yang, F.; Wang, Y.; Jiao, F.Z.; Zhang, H.Y.; Wang, L.W.; Gong, Z.J. Inhibition of HDAC6 attenuates LPS-induced inflammation in macrophages by regulating oxidative stress and suppressing the TLR4-MAPK/NF-kappaB pathways. Biomed. Pharmacother. 2019, 117, 109166. [Google Scholar] [CrossRef]
- Thatikonda, S.; Pooladanda, V.; Sigalapalli, D.K.; Godugu, C. Piperlongumine regulates epigenetic modulation and alleviates psoriasis-like skin inflammation via inhibition of hyperproliferation and inflammation. Cell Death Dis. 2020, 11, 21. [Google Scholar] [CrossRef]
- Song, Y.; Qin, L.; Yang, R.; Yang, F.; Kenechukwu, N.A.; Zhao, X.; Zhou, X.; Wen, X.; Li, L. Inhibition of HDAC6 alleviating lipopolysaccharide-induced p38MAPK phosphorylation and neuroinflammation in mice. Pharm. Biol. 2019, 57, 263–268. [Google Scholar] [CrossRef]
- Kwon, Y.; Choi, Y.; Kim, M.; Jeong, M.S.; Jung, H.S.; Jeoung, D. HDAC6 and CXCL13 Mediate Atopic Dermatitis by Regulating Cellular Interactions and Expression Levels of miR-9 and SIRT1. Front. Pharmacol. 2021, 12, 691279. [Google Scholar] [CrossRef]
- Wei, W.; Huang, C.; Zhang, J.; Chen, Q.; Liu, Z.; Ren, X.; Gan, S.; Wu, P.; Wang, D.; Tang, B.Z.; et al. HDAC6-Activatable Multifunctional Near-Infrared Probe for Glioma Cell Detection and Elimination. Anal. Chem. 2024, 96, 2406–2414. [Google Scholar] [CrossRef]
- Martinez Molina, D.; Jafari, R.; Ignatushchenko, M.; Seki, T.; Larsson, E.A.; Dan, C.; Sreekumar, L.; Cao, Y.; Nordlund, P. Monitoring drug target engagement in cells and tissues using the cellular thermal shift assay. Science 2013, 341, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Jafari, R.; Almqvist, H.; Axelsson, H.; Ignatushchenko, M.; Lundbäck, T.; Nordlund, P.; Molina, D.M. The cellular thermal shift assay for evaluating drug target interactions in cells. Nat. Protoc. 2014, 9, 2100–2122. [Google Scholar] [CrossRef] [PubMed]
- Martinez Molina, D.; Nordlund, P. The Cellular Thermal Shift Assay: A Novel Biophysical Assay for In Situ Drug Target Engagement and Mechanistic Biomarker Studies. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 141–161. [Google Scholar] [CrossRef]
- Hao, L.; Park, J.; Jang, H.Y.; Bae, E.J.; Park, B.H. Inhibiting Protein Kinase Activity of Pyruvate Kinase M2 by SIRT2 Deacetylase Attenuates Psoriasis. J. Investig. Dermatol. 2021, 141, 355–363.e356. [Google Scholar] [CrossRef]
- Rendon, A.; Schakel, K. Psoriasis Pathogenesis and Treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef]
- Tokuyama, M.; Mabuchi, T. New Treatment Addressing the Pathogenesis of Psoriasis. Int. J. Mol. Sci. 2020, 21, 7488. [Google Scholar] [CrossRef]
- Nogueira, S.; Rodrigues, M.A.; Vender, R.; Torres, T. Tapinarof for the treatment of psoriasis. Dermatol. Ther. 2022, 35, e15931. [Google Scholar] [CrossRef]
- Bissonnette, R.; Saint-Cyr Proulx, E.; Jack, C.; Maari, C. Tapinarof for psoriasis and atopic dermatitis: 15 years of clinical research. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 1168–1174. [Google Scholar] [CrossRef]
- Keam, S.J. Tapinarof Cream 1%: First Approval. Drugs 2022, 82, 1221–1228. [Google Scholar] [CrossRef]
- Ran, J.; Zhou, J. Targeted inhibition of histone deacetylase 6 in inflammatory diseases. Thorac. Cancer 2019, 10, 405–412. [Google Scholar] [CrossRef]
- Vishwakarma, S.; Iyer, L.R.; Muley, M.; Singh, P.K.; Shastry, A.; Saxena, A.; Kulathingal, J.; Vijaykanth, G.; Raghul, J.; Rajesh, N.; et al. Tubastatin, a selective histone deacetylase 6 inhibitor shows anti-inflammatory and anti-rheumatic effects. Int. Immunopharmacol. 2013, 16, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Shon, S.; Yoo, H.J.; Suh, D.H.; Bae, D.; Shin, J.; Jun, J.H.; Ha, N.; Song, H.; Choi, Y.I.; et al. Inhibition of histone deacetylase 6 suppresses inflammatory responses and invasiveness of fibroblast-like-synoviocytes in inflammatory arthritis. Arthritis Res. Ther. 2021, 23, 177. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, G.; Okiyama, N.; Villarroel, V.A.; Katz, S.I. Histone deacetylase 6 inhibition impairs effector CD8 T-cell functions during skin inflammation. J. Allergy Clin. Immunol. 2015, 135, 1228–1239. [Google Scholar] [CrossRef]
- Falkenberg, K.J.; Johnstone, R.W. Histone deacetylases and their inhibitors in cancer, neurological diseases and immune disorders. Nat. Rev. Drug Discov. 2014, 13, 673–691. [Google Scholar] [CrossRef] [PubMed]
- Nazri, J.M.; Oikonomopoulou, K.; de Araujo, E.D.; Kraskouskaya, D.; Gunning, P.T.; Chandran, V. Histone deacetylase inhibitors as a potential new treatment for psoriatic disease and other inflammatory conditions. Crit. Rev. Clin. Lab. Sci. 2023, 60, 300–320. [Google Scholar] [CrossRef]
- Tovar-Castillo, L.E.; Cancino-Diaz, J.C.; Garcia-Vazquez, F.; Cancino-Gomez, F.G.; Leon-Dorantes, G.; Blancas-Gonzalez, F.; Jimenez-Zamudio, L.; Garcia-Latorre, E.; Cancino-Diaz, M.E. Under-expression of VHL and over-expression of HDAC-1, HIF-1alpha, LL-37, and IAP-2 in affected skin biopsies of patients with psoriasis. Int. J. Dermatol. 2007, 46, 239–246. [Google Scholar] [CrossRef]
- Hwang, Y.J.; Na, J.I.; Byun, S.Y.; Kwon, S.H.; Yang, S.H.; Lee, H.S.; Choi, H.R.; Cho, S.; Youn, S.W.; Park, K.C. Histone Deacetylase 1 and Sirtuin 1 Expression in Psoriatic Skin: A Comparison between Guttate and Plaque Psoriasis. Life 2020, 10, 157. [Google Scholar] [CrossRef]
- Bondarev, A.D.; Attwood, M.M.; Jonsson, J.; Chubarev, V.N.; Tarasov, V.V.; Schioth, H.B. Recent developments of HDAC inhibitors: Emerging indications and novel molecules. Br. J. Clin. Pharmacol. 2021, 87, 4577–4597. [Google Scholar] [CrossRef]
- Subramanian, S.; Bates, S.E.; Wright, J.J.; Espinoza-Delgado, I.; Piekarz, R.L. Clinical Toxicities of Histone Deacetylase Inhibitors. Pharmaceuticals 2010, 3, 2751–2767. [Google Scholar] [CrossRef]
- Govindarajan, N.; Rao, P.; Burkhardt, S.; Sananbenesi, F.; Schluter, O.M.; Bradke, F.; Lu, J.; Fischer, A. Reducing HDAC6 ameliorates cognitive deficits in a mouse model for Alzheimer’s disease. EMBO Mol. Med. 2013, 5, 52–63. [Google Scholar] [CrossRef]
- Pulya, S.; Amin, S.A.; Adhikari, N.; Biswas, S.; Jha, T.; Ghosh, B. HDAC6 as privileged target in drug discovery: A perspective. Pharmacol. Res. 2021, 163, 105274. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, A.; Li, Y.; Feng, Y.; Wang, X.; Wei, W.; Sun, H.; Quan, J. A Novel HDAC6 Inhibitor Ameliorates Imiquimod-Induced Psoriasis-Like Inflammation in Mice. Molecules 2025, 30, 3224. https://doi.org/10.3390/molecules30153224
Cao A, Li Y, Feng Y, Wang X, Wei W, Sun H, Quan J. A Novel HDAC6 Inhibitor Ameliorates Imiquimod-Induced Psoriasis-Like Inflammation in Mice. Molecules. 2025; 30(15):3224. https://doi.org/10.3390/molecules30153224
Chicago/Turabian StyleCao, Anqi, Yurong Li, Yanqiao Feng, Xiaoquan Wang, Wenyu Wei, Hongyan Sun, and Junmin Quan. 2025. "A Novel HDAC6 Inhibitor Ameliorates Imiquimod-Induced Psoriasis-Like Inflammation in Mice" Molecules 30, no. 15: 3224. https://doi.org/10.3390/molecules30153224
APA StyleCao, A., Li, Y., Feng, Y., Wang, X., Wei, W., Sun, H., & Quan, J. (2025). A Novel HDAC6 Inhibitor Ameliorates Imiquimod-Induced Psoriasis-Like Inflammation in Mice. Molecules, 30(15), 3224. https://doi.org/10.3390/molecules30153224






