From Waste to Biocatalyst: Cocoa Bean Shells as Immobilization Support and Substrate Source in Lipase-Catalyzed Hydrolysis
Abstract
1. Introduction
2. Results and Discussion
2.1. Evaluation and Selection of the Optimal Immobilized Lipase System
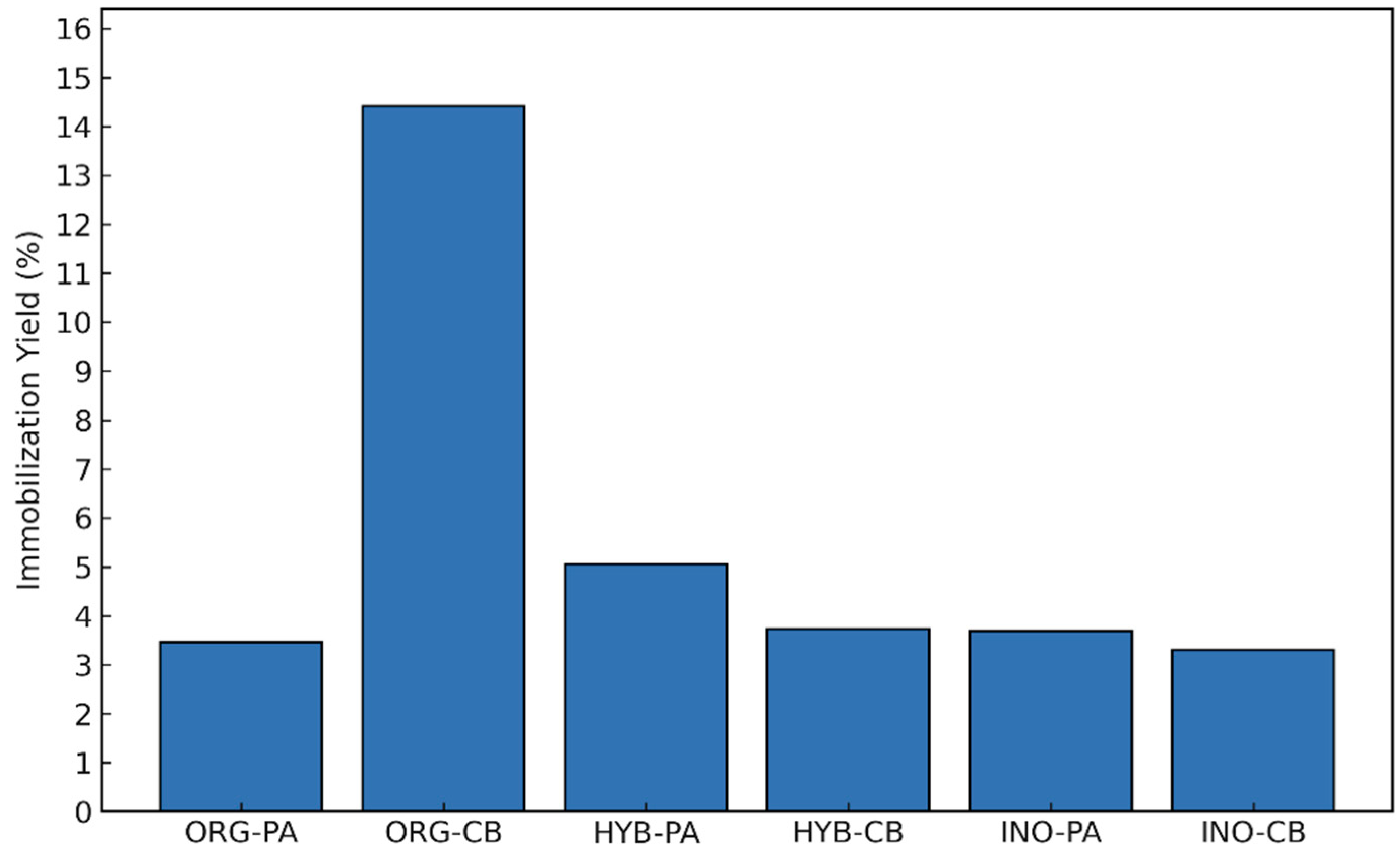
2.1.1. Immobilization Yield
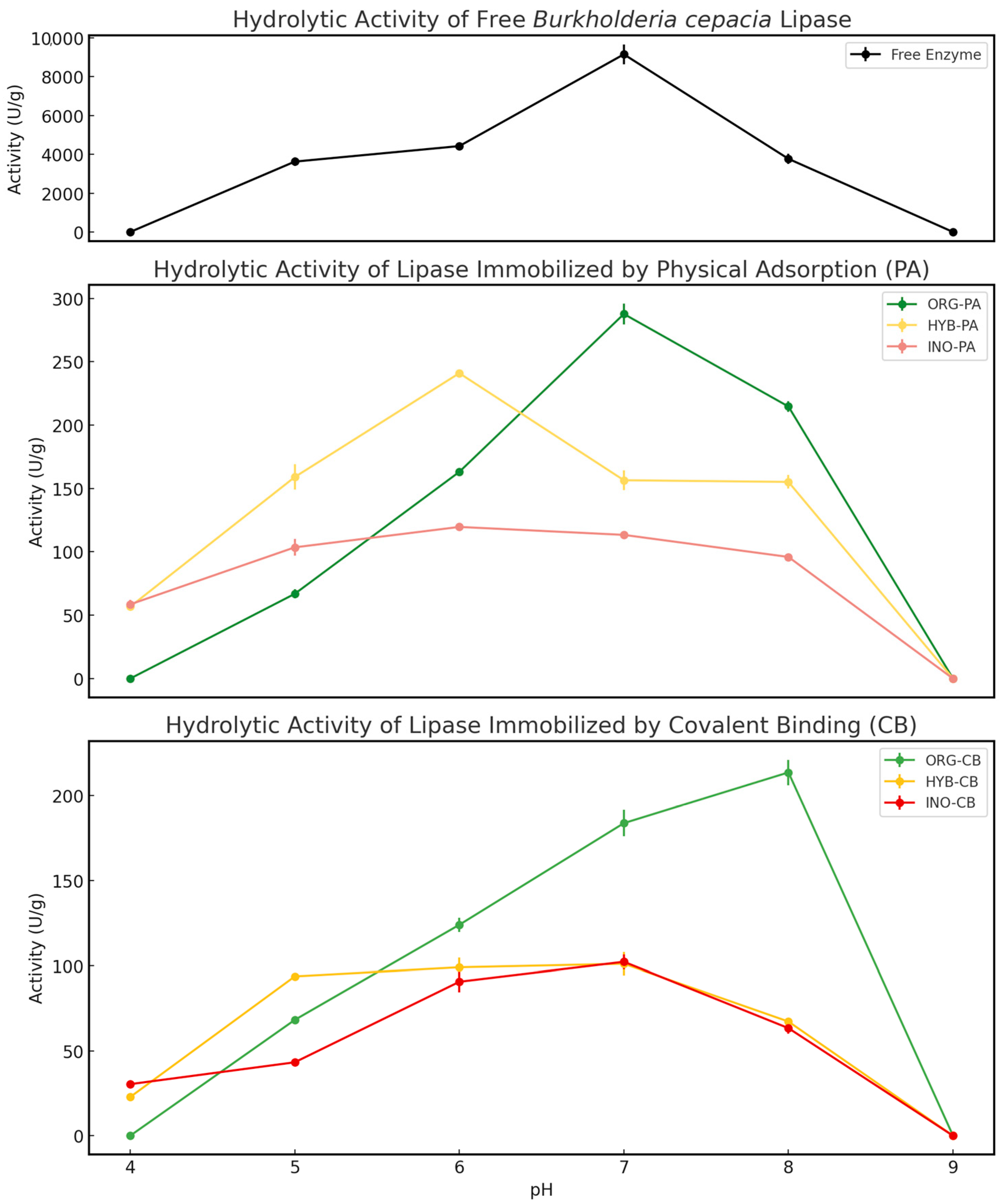
2.1.2. Effect of pH on Hydrolytic Activity
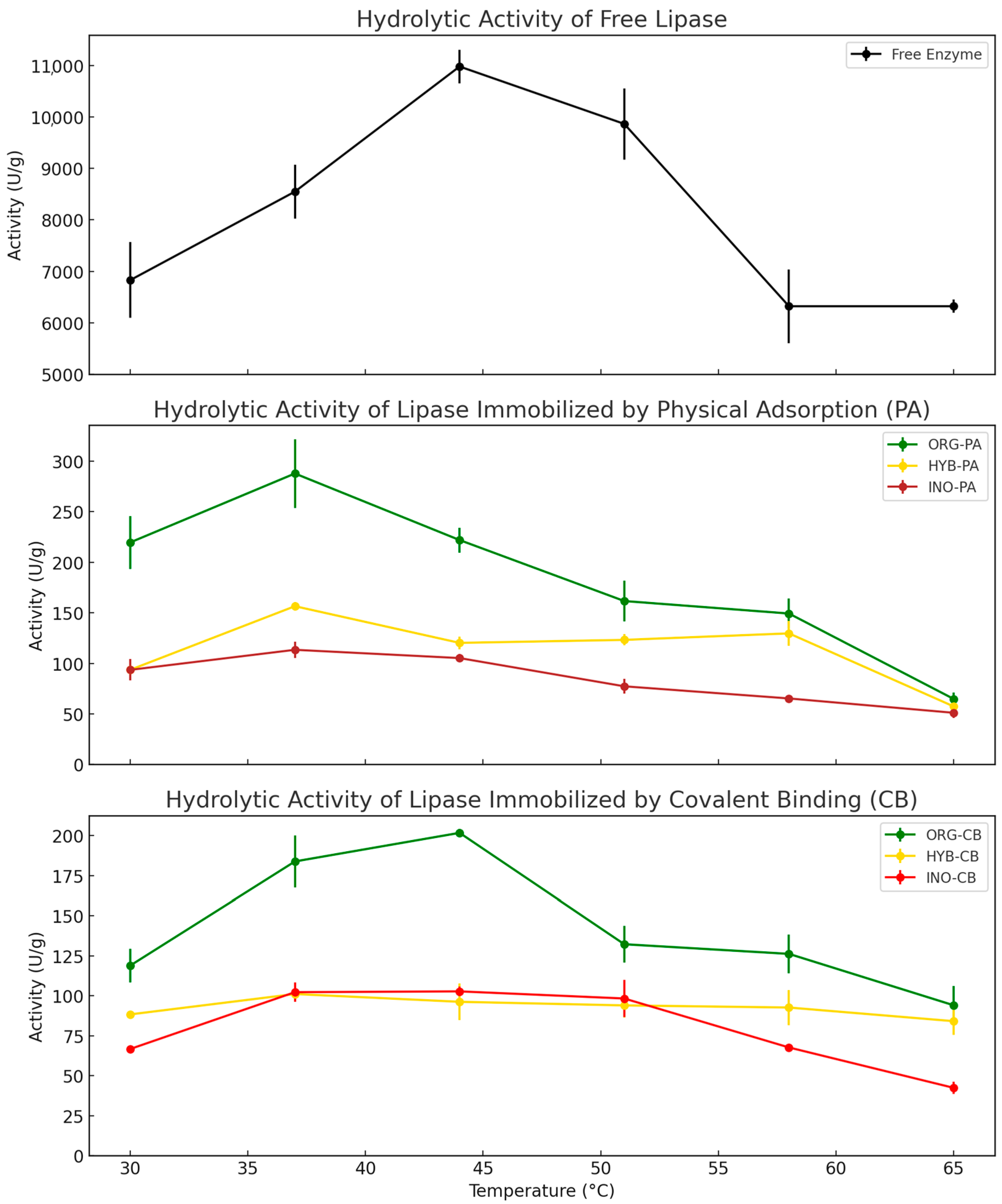
2.1.3. Effect of Temperature on Hydrolytic Activity
2.1.4. Catalytic Performance
2.1.5. Operational Stability (Reuse Cycles)
2.1.6. Integrated Evaluation and Selection of the Optimal Biocatalyst
2.2. Evaluation of Selected Biocatalyst
2.2.1. Thermal Stability of Selected Biocatalyst
2.2.2. Application: Hydrolysis of Cocoa Bean Shell Oil
2.3. Morphological and Physicochemical Characterization of Selected Biocatalyst
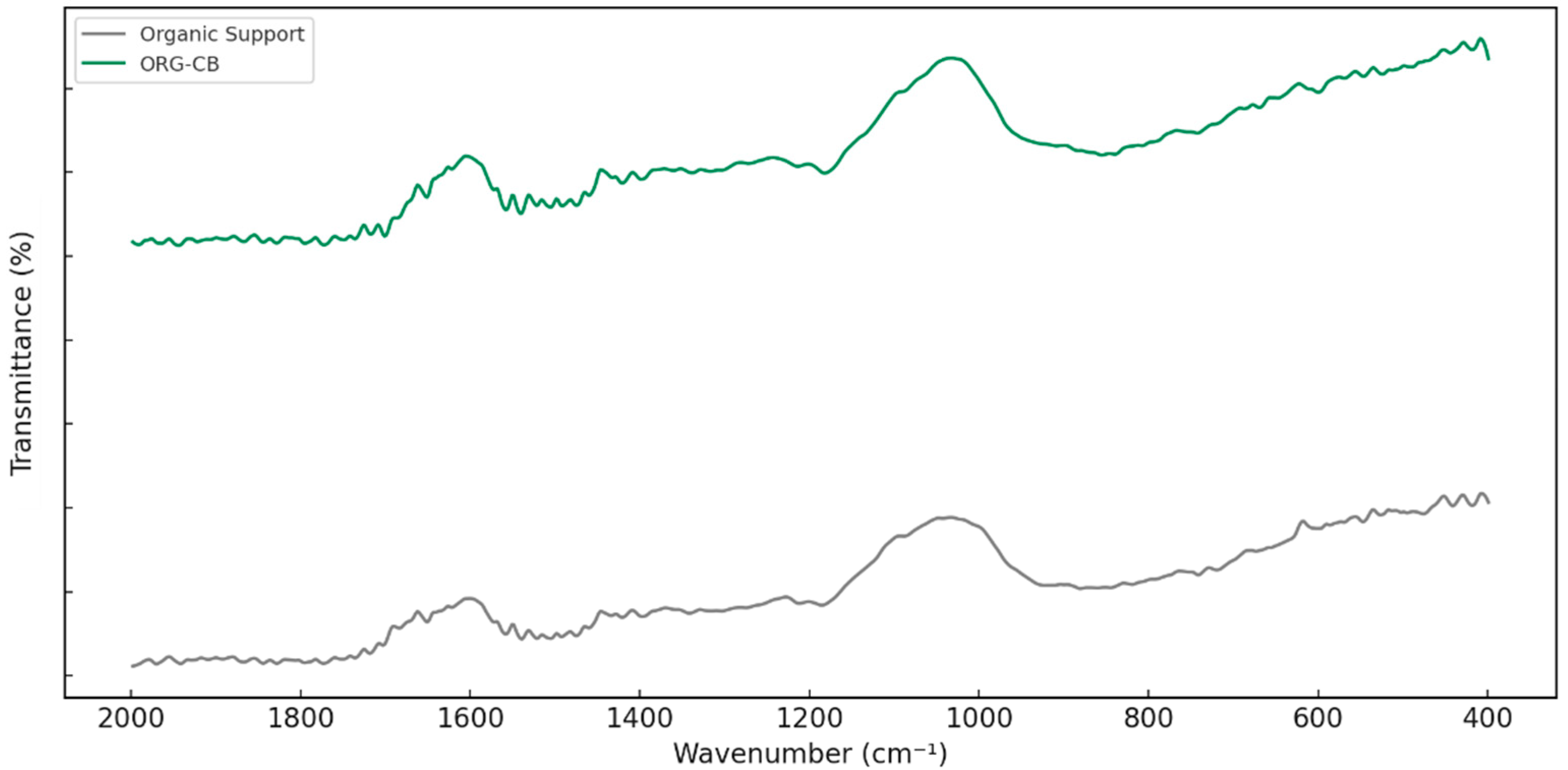
2.3.1. FTIR Analysis
2.3.2. Morphology Study of Support and ORG-CB Biocatalyst by SEM
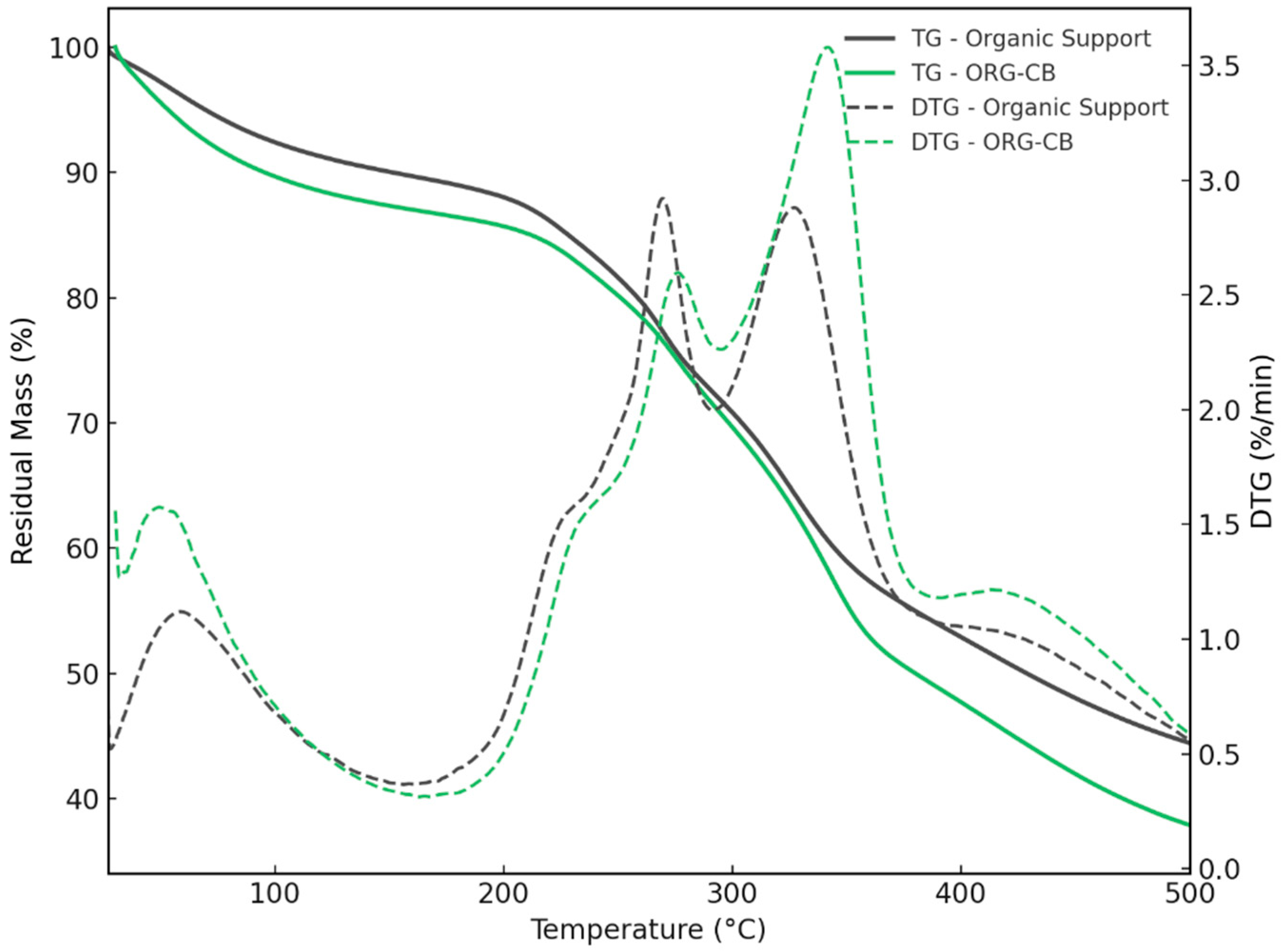
2.3.3. Thermogravimetric Analysis (TGA) and Derivative Thermogravimetry (DTG)
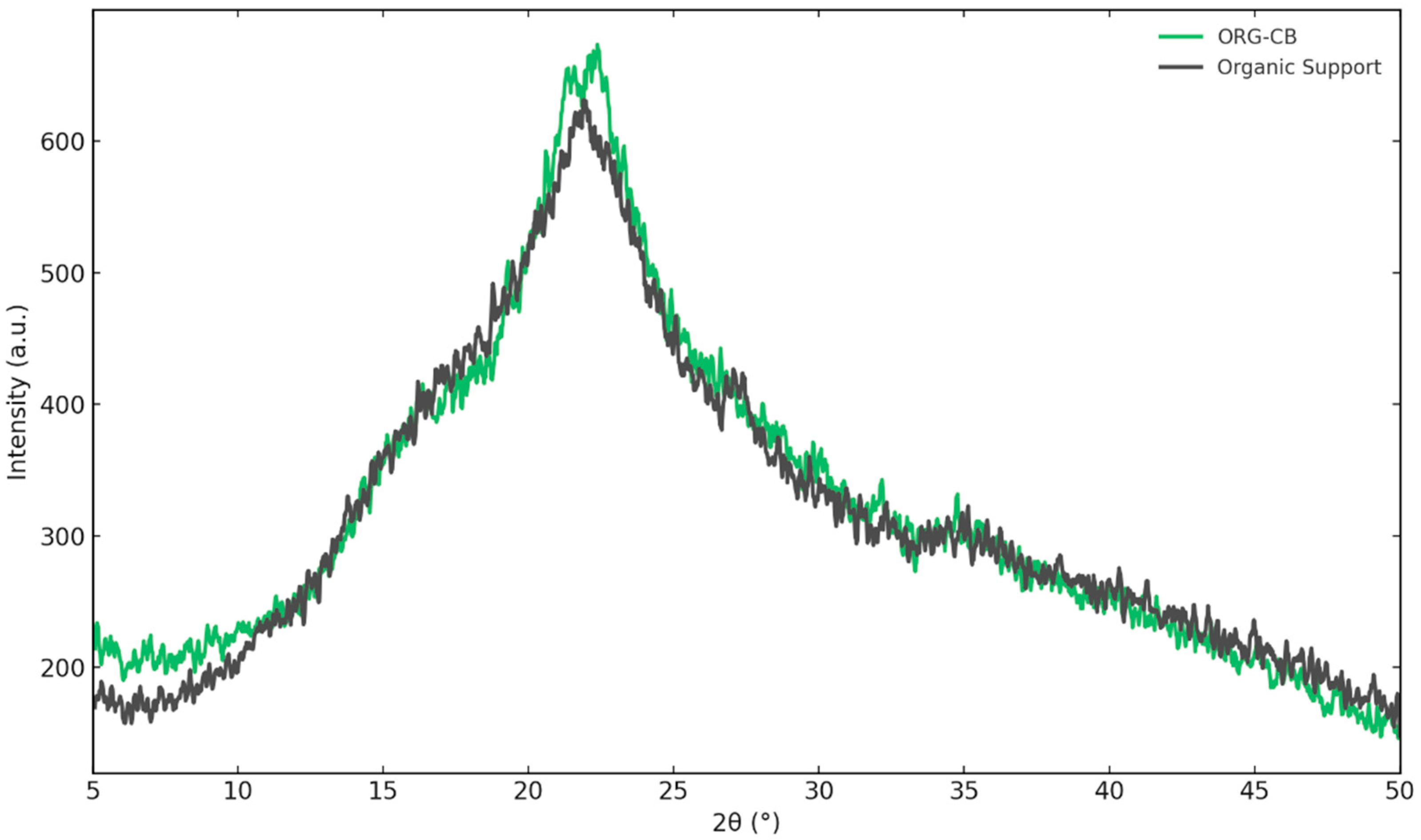
2.3.4. X-Ray Diffraction (XRD)
3. Materials and Methods
3.1. Materials
3.2. Cocoa Bean Shell Oil Extraction and Characterization
3.2.1. Oil Extraction from Cocoa Bean Shells
3.2.2. Fatty Acid Composition of Cocoa Bean Shells
3.3. Support Preparation
3.3.1. Organic Support
3.3.2. Inorganic Support
3.3.3. Hybrid Support
3.4. Lipase Immobilization
3.4.1. Immobilization by Physical Adsorption (PA)
3.4.2. Immobilization by Covalent Binding (CB)
3.5. Biochemical Characterization of Free and Immobilized Lipase Systems
3.5.1. Determination of Hydrolytic Activity and Immobilization Yield
3.5.2. Effect of pH and Temperature on Activity
3.5.3. Determination of Kinetic Constants
3.5.4. Operational Stability (Reuse Cycles)
3.6. Evaluation of Selected Biocatalyst
3.6.1. Thermal Stability Assessment
3.6.2. Application: Hydrolysis of Cocoa Bean Shell Oil
3.6.3. Physicochemical and Morphological Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TAG | Triacylglycerol |
| FFA | Free fatty acids |
| CBSs | Cocoa bean shells |
| BCL | Burkholderia cepacia Lipase |
| ORG | Organic support (Cocoa bean shell-based) |
| HYB | Hybrid support (Cocoa shells silica composite) |
| INO | Inorganic support (Silica) |
| PA | Physical adsorption |
| CB | Covalent binding |
| Vmax | Maximum reaction rate |
| Km | Michaelis–Menten constant |
| Ke | Catalytic efficiency |
| Kα | Diffusion constant |
| Kd | Thermal deactivation constant |
| Ed | Deactivation activation energy |
| ΔH | Enthalpy of activation for thermal deactivation |
| ΔG | Gibbs free energy of activation for thermal deactivation |
| ΔS | Entropy of activation for thermal deactivation |
| U | Enzyme activity units |
| SEM | Scanning electron microscopy |
| FTIR | Fourier-transform infrared spectroscopy |
| TGA | Thermogravimetric analysis |
| XRD | X-ray diffraction |
References
- Girelli, A.M.; Chiappini, V. Renewable, Sustainable, and Natural Lignocellulosic Carriers for Lipase Immobilization: A Review. J. Biotechnol. 2023, 365, 29–47. [Google Scholar] [CrossRef]
- Kawamura, M.; da Silva Brito, G.F.; Araruna, T.; Alves Martins, P.; Fabiana Chan Salum, T.; Machado, F. Novel Polymeric Supports for Lipase Immobilization and Their Application in the Transesterification of Soybean Oil for Biodiesel Production. Fuel 2025, 388, 134547. [Google Scholar] [CrossRef]
- Shu, Z.; Lin, R.; Jiang, H.; Zhang, Y.; Wang, M.; Huang, J. A Rapid and Efficient Method for Directed Screening of Lipase-Producing Burkholderia cepacia Complex Strains with Organic Solvent Tolerance from Rhizosphere. J. Biosci. Bioeng. 2009, 107, 658–661. [Google Scholar] [CrossRef]
- de Almeida, L.C.; de Jesus, F.A.; Wiltshire, F.M.S.; Santos, R.M.; Fricks, A.T.; Freitas, L.d.S.; Pereira, M.M.; Lima, Á.S.; Soares, C.M.F. Development of Carbon-Based Support Using Biochar from Guava Seeds for Lipase Immobilization. C 2022, 8, 64. [Google Scholar] [CrossRef]
- Zieniuk, B.; Małajowicz, J.; Jasińska, K.; Wierzchowska, K.; Uğur, Ş.; Fabiszewska, A. Agri-Food and Food Waste Lignocellulosic Materials for Lipase Immobilization as a Sustainable Source of Enzyme Support—A Comparative Study. Foods 2024, 13, 3759. [Google Scholar] [CrossRef]
- Abdulla, R.; Sanny, S.A.; Derman, E. Stability Studies of Immobilized Lipase on Rice Husk and Eggshell Membrane. IOP Conf. Ser. Mater. Sci. Eng. 2017, 206, 012032. [Google Scholar] [CrossRef]
- Sánchez, M.; Laca, A.; Laca, A.; Díaz, M. Cocoa Bean Shell: A By-Product with High Potential for Nutritional and Biotechnological Applications. Antioxidants 2023, 12, 1028. [Google Scholar] [CrossRef] [PubMed]
- Quarterly Bulletin of Cocoa Statistics. Available online: https://www.icco.org/wp-content/uploads/Grindings_QBCS-LI-No.-2.pdf (accessed on 10 February 2025).
- Lordêlo Nascimento, L.; dos Santos, P.N.; Granja, H.S.; da Silveira Ferreira, L.; Ferreira Lima, J.V.; de Moura Pita, B.L.; dos Santos Polidoro, A.; dos Santos Freitas, L.; Caramão, E.B.; de Souza Dias, F.; et al. Mixture Design and Doehlert Matrix for Optimization of Energized Dispersive Guided Extraction (EDGE) of Theobromine and Caffeine from Cocoa Bean Shells. Foods 2025, 14, 740. [Google Scholar] [CrossRef]
- Melo, M.N.; Pereira, F.M.; Rocha, M.A.; Ribeiro, J.G.; Diz, F.M.; Monteiro, W.F.; Ligabue, R.A.; Severino, P.; Fricks, A.T. Immobilization and Characterization of Horseradish Peroxidase into Chitosan and Chitosan/PEG Nanoparticles: A Comparative Study. Process Biochem. 2020, 98, 160–171. [Google Scholar] [CrossRef]
- Panjaitan, W.; Prihandini, G.; Restiawaty, E.; RendraGraha, H.P.; Miyamoto, M.; Uemiya, S.; Akhmaloka, A.; Budhi, Y.W. Enhanced Fatty Acid Production Using Recombinant Lipase in a Rotating Bed Reactor (RBR). Case Stud. Chem. Environ. Eng. 2024, 10, 101017. [Google Scholar] [CrossRef]
- Singh, K.; Muttathukattil, A.N.; Singh, P.C.; Reddy, G. PH Regulates Ligand Binding to an Enzyme Active Site by Modulating Intermediate Populations. J. Phys. Chem. B 2022, 126, 9759–9770. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, M.; Li, J.; Li, Z.; He, P.; Liu, H.; Li, J. Highly Active Horseradish Peroxidase Immobilized in 1-Butyl-3-Methylimidazolium Tetrafluoroborate Room-Temperature Ionic Liquid Based Sol–Gel Host Materials. Chem. Commun. 2005, 13, 1778–1780. [Google Scholar] [CrossRef]
- Eisenthal, R.; Danson, M.J.; Hough, D.W. Catalytic Efficiency and kcat/KM: A Useful Comparator? Trends Biotechnol. 2007, 25, 247–249. [Google Scholar] [CrossRef]
- Prabhakar, T.; Giaretta, J.; Zulli, R.; Rath, R.J.; Farajikhah, S.; Talebian, S.; Dehghani, F. Covalent Immobilization: A Review from an Enzyme Perspective. Chem. Eng. J. 2025, 503, 158054. [Google Scholar] [CrossRef]
- Sheldon, R.A.; van Pelt, S. Enzyme Immobilisation in Biocatalysis: Why, What and How. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Christena, L.R.; Rajaram, Y.R.S. Enzyme Immobilization: An Overview on Techniques and Support Materials. 3 Biotech 2013, 3, 1–9. [Google Scholar] [CrossRef]
- Jacob, A.G.; Wahab, R.A.; Misson, M. Operational Stability, Regenerability, and Thermodynamics Studies on Biogenic Silica/Magnetite/Graphene Oxide Nanocomposite-Activated Candida Rugosa Lipase. Polymers 2021, 13, 3854. [Google Scholar] [CrossRef]
- Elias, N.; Wahab, R.A.; Chandren, S.; Lau, W.J. Performance of Candida Rugosa Lipase Supported on Nanocellulose-Silica-Reinforced Polyethersulfone Membrane for the Synthesis of Pentyl Valerate: Kinetic, Thermodynamic and Regenerability Studies. Mol. Catal. 2021, 514, 111852. [Google Scholar] [CrossRef]
- Marangoni, A.G. Characterization of Enzyme Stability. In Enzyme Kinetics; Wiley online library: Hoboken, NJ, USA, 2002; pp. 140–157. ISBN 9780471267294. [Google Scholar]
- Soares, T.F.; Oliveira, M.B.P.P. Cocoa By-Products: Characterization of Bioactive Compounds and Beneficial Health Effects. Molecules 2022, 27, 1625. [Google Scholar] [CrossRef] [PubMed]
- Martins, P.A.; Trobo-Maseda, L.; Lima, F.A.; de Morais Júnior, W.G.; De Marco, J.L.; Salum, T.F.C.; Guisán, J.M. Omega-3 Production by Fish Oil Hydrolysis Using a Lipase from Burkholderia Gladioli BRM58833 Immobilized and Stabilized by Post-Immobilization Techniques. Biochem. Biophys. Rep. 2022, 29, 101193. [Google Scholar] [CrossRef]
- Liu, R.; Li, P.; Li, S.; Zhuang, Y.; Chen, S.; Huang, Y.; Zheng, Y.; Xiong, Y.; Wang, D.; He, M.; et al. Immobilized Burkholderia Cepacia Lipase and Its Application in Selective Enrichment of EPA by Hydrolysis of Fish Oil. LWT 2024, 206, 116598. [Google Scholar] [CrossRef]
- Willerding, A.L.; Neto, F.G.M.d.R.C.; da Gama, A.M.; Carioca, C.R.F.; de Oliveira, L.A. Hydrolytic Activity of Bacterial Lipases in Amazonian Vegetable Oils. Quim. Nova 2012, 35, 1782–1786. [Google Scholar] [CrossRef]
- Santos, R.P.S.; Araujo, L.L.; Oliveira, A.A.; da Silva, T.F.; Rocha, T.G.; Fernandez-Lafuente, R.; Monteiro, R.R.C.; Vieira, R.S. Optimization of the Full Hydrolysis of Babassu Oil by Combi-Lipases. Catalysts 2025, 15, 209. [Google Scholar] [CrossRef]
- Miller, L.M.; Bourassa, M.W.; Smith, R.J. FTIR Spectroscopic Imaging of Protein Aggregation in Living Cells. Biochim. Biophys. Acta (BBA)–Biomembr. 2013, 1828, 2339–2346. [Google Scholar] [CrossRef]
- Paul, C.; Borza, P.; Marcu, A.; Rusu, G.; Bîrdeanu, M.; Zarcula, S.M.; Péter, F. Influence of the Physico-Chemical Characteristics of the Hybrid Matrix on the Catalytic Properties of Sol-Gel Entrapped Pseudomonas Fluorescens Lipase. Nanomater. Nanotechnol. 2016, 6, 3. [Google Scholar] [CrossRef]
- Barreto, P.L.M.; Pires, A.T.N.; Soldi, V. Thermal Degradation of Edible Films Based on Milk Proteins and Gelatin in Inert Atmosphere. Polym. Degrad. Stab. 2003, 79, 147–152. [Google Scholar] [CrossRef]
- Gentili, A.; Curini, R.; Cernia, E.; D’Ascenzo, G. Thermal Stability and Activity of Candida Cylindracea Lipase. J. Mol. Catal. B Enzym. 1997, 3, 43–49. [Google Scholar] [CrossRef]
- Joseph, J.D.; Ackman, R.G. Capillary Column Gas Chromatographic Method for Analysis of Encapsulated Fish Oils and Fish Oil Ethyl Esters: Collaborative Study. J. AOAC Int. 1992, 75, 488–506. [Google Scholar] [CrossRef]
- Mota, D.A.; Santos, J.C.B.; Faria, D.; Lima, Á.S.; Krause, L.C.; Soares, C.M.F.; Ferreira-Dias, S. Synthesis of Dietetic Structured Lipids from Spent Coffee Grounds Crude Oil Catalyzed by Commercial Immobilized Lipases and Immobilized Rhizopus Oryzae Lipase on Biochar and Hybrid Support. Processes 2020, 8, 1542. [Google Scholar] [CrossRef]
- Cabrera-Padilla, R.Y.; Lisboa, M.C.; Fricks, A.T.; Franceschi, E.; Lima, A.S.; Silva, D.P.; Soares, C.M.F. Immobilization of Candida Rugosa Lipase on Poly(3-Hydroxybutyrate-Co-Hydroxyvalerate): A New Eco-Friendly Support. J. Ind. Microbiol. Biotechnol. 2012, 39, 289–298. [Google Scholar] [CrossRef]


| System | Vmax (µmol·min−1) | Km (mM) | Kcat (min−1) | Kα (min−1) | Ke (mM−1·min−1) | Relative Ke (%) |
|---|---|---|---|---|---|---|
| Free Enzyme | 21.7 | 599.0 | 202.5 | 36.2 | 0.338 | 100.0 |
| ORG-PA | 752.1 | 72,848.1 | 702.0 | 10.3 | 0.010 | 2.9 |
| ORG-CB | 5.6 | 83.4 | 5.2 | 67.4 | 0.063 | 18.6 |
| HYB-PA | 35.9 | 5303.9 | 33.5 | 6.8 | 0.006 | 1.9 |
| HYB-LC | 2.3 | 287.4 | 2.2 | 8.1 | 0.008 | 2.2 |
| INO-PA | 8.1 | 1320.0 | 7.6 | 6.2 | 0.006 | 1.7 |
| INO-LC | 8.9 | 151.5 | 8.3 | 58.5 | 0.055 | 16.2 |
| System | Temperature (°C) | kd (h−1) | t1/2 (h) | ΔH‡ (kJ mol−1) | ΔG‡ (kJ mol−1·K−1) | ΔS‡ (kJ mol−1) | Ed (kJ mol−1) |
|---|---|---|---|---|---|---|---|
| 40 | 0.091 | 7.63 | 33.30 | 6.24 | 74.80 | ||
| ORG-CB | 50 | 0.125 | 5.54 | 33.22 | 5.58 | 74.27 | 35.90 |
| 60 | 0.192 | 3.62 | 33.14 | 4.57 | 74.82 | ||
| 40 | 0.100 | 6.92 | 28.05 | 5.79 | 76.23 | ||
| Free lipase | 50 | 0.125 | 5.53 | 27.96 | 5.22 | 75.38 | 60.65 |
| 60 | 0.166 | 4.17 | 27.88 | 4.51 | 75.00 |
| Fatty Acid | Cx:y a | MM (g.mol−1) | % mass b | % mmol |
|---|---|---|---|---|
| Palmitic | C16:0 | 256.43 | 28 ± 1 | 30.2 ± 1.08 |
| Stearic | C18:0 | 284.49 | 23.2 ± 0.8 | 22.6 ± 0.78 |
| Oleic | C18:1 | 282.47 | 35.7 ± 1 | 35.0 ± 0.98 |
| Linoleic | C18:2 | 282.47 | 11.8 ± 0.04 | 11.6 ± 0.04 |
| Linolenic | C18:3 | 280.45 | 0.7 ± 0.1 | 0.7 ± 0.10 |
| Average molar mass MM (g.mol−1) | 275.59 | |||
| Saturated/Unsaturated Ratio (by mass) | 1.06 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lordelo Nascimento, L.; Pita, B.L.d.M.; Rodrigues, C.d.A.; dos Santos, P.N.A.; Almeida, Y.A.d.; Ferreira, L.d.S.; de Oliveira, M.L.; de Almeida, L.S.; Soares, C.M.F.; de Souza Dias, F.; et al. From Waste to Biocatalyst: Cocoa Bean Shells as Immobilization Support and Substrate Source in Lipase-Catalyzed Hydrolysis. Molecules 2025, 30, 3207. https://doi.org/10.3390/molecules30153207
Lordelo Nascimento L, Pita BLdM, Rodrigues CdA, dos Santos PNA, Almeida YAd, Ferreira LdS, de Oliveira ML, de Almeida LS, Soares CMF, de Souza Dias F, et al. From Waste to Biocatalyst: Cocoa Bean Shells as Immobilization Support and Substrate Source in Lipase-Catalyzed Hydrolysis. Molecules. 2025; 30(15):3207. https://doi.org/10.3390/molecules30153207
Chicago/Turabian StyleLordelo Nascimento, Luciana, Bruna Louise de Moura Pita, César de Almeida Rodrigues, Paulo Natan Alves dos Santos, Yslaine Andrade de Almeida, Larissa da Silveira Ferreira, Maira Lima de Oliveira, Lorena Santos de Almeida, Cleide Maria Faria Soares, Fabio de Souza Dias, and et al. 2025. "From Waste to Biocatalyst: Cocoa Bean Shells as Immobilization Support and Substrate Source in Lipase-Catalyzed Hydrolysis" Molecules 30, no. 15: 3207. https://doi.org/10.3390/molecules30153207
APA StyleLordelo Nascimento, L., Pita, B. L. d. M., Rodrigues, C. d. A., dos Santos, P. N. A., Almeida, Y. A. d., Ferreira, L. d. S., de Oliveira, M. L., de Almeida, L. S., Soares, C. M. F., de Souza Dias, F., & Fricks, A. T. (2025). From Waste to Biocatalyst: Cocoa Bean Shells as Immobilization Support and Substrate Source in Lipase-Catalyzed Hydrolysis. Molecules, 30(15), 3207. https://doi.org/10.3390/molecules30153207







