Development of an LC-MS Method for the Analysis of Birch (Betula sp.) Bark Bioactives Extracted with Biosolvents
Abstract
1. Introduction
2. Results and Discussion
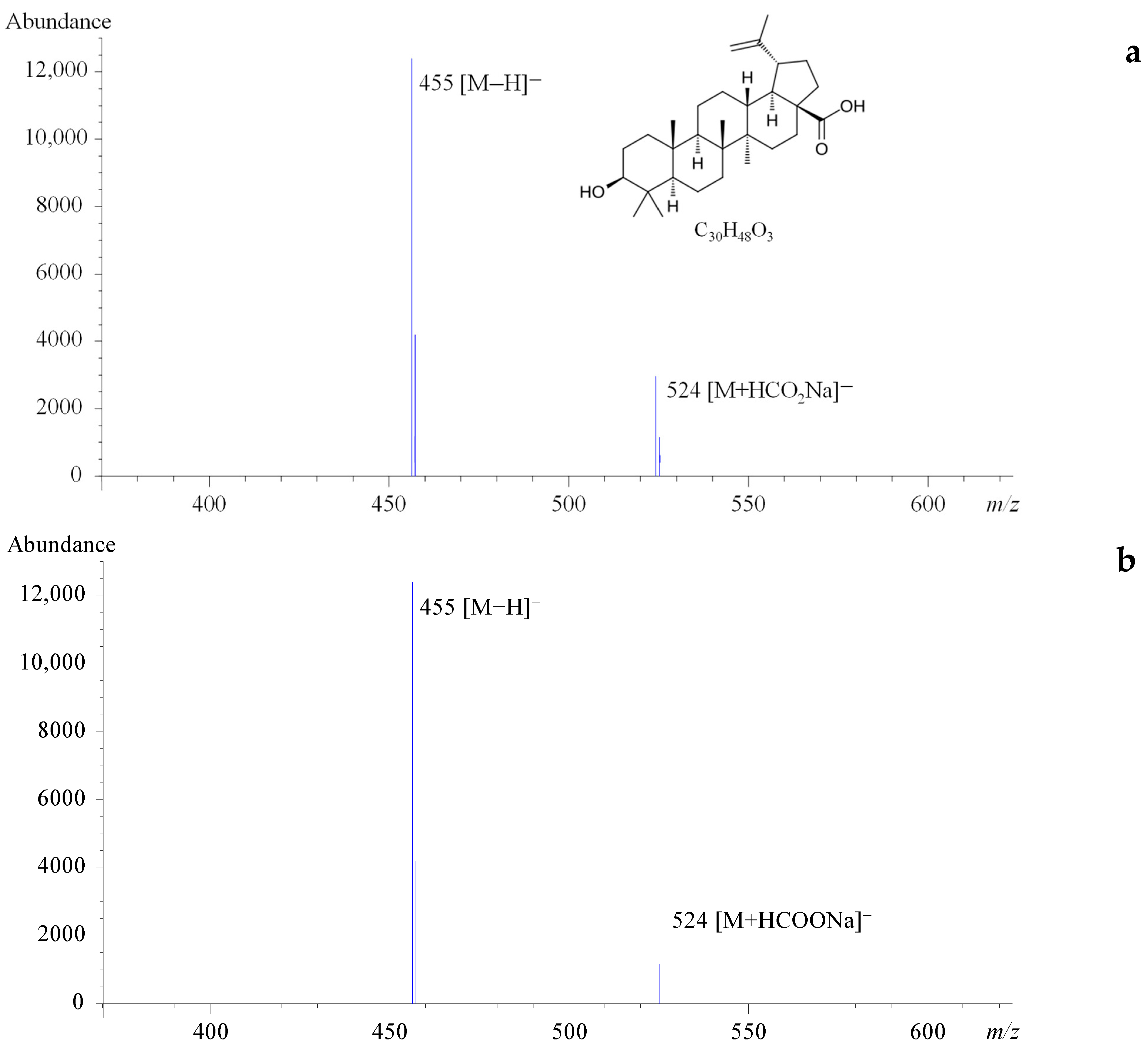
2.1. Development of an LC-MS Method for the Analysis of Bet and BAc
2.2. Analytical Characterization
2.3. Application of the Optimized LC-MS Method to Different BB Extracts
3. Materials and Methods
3.1. Samples and Standards
3.2. NADES Preparation
3.3. Betulin and Betulinic Acid Extraction
3.4. LC-MS Analysis
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hordyjewska, A.; Ostapiuk, A.; Horecka, A.; Kurzepa, J. Betulin and Betulinic Acid: Triterpenoids Derivatives with a Powerful Biological Potential. Phytochem. Rev. 2019, 18, 929–951. [Google Scholar] [CrossRef]
- Kuznetsova, S.A.; Skvortsova, G.P.; Maliar, I.N.; Skurydina, E.S.; Veselova, O.F. Extraction of Betulin from Birch Bark and Study of its Physico-Chemical and Pharmacological Properties. Russ. J. Bioorg. Chem. 2014, 40, 742–747. [Google Scholar] [CrossRef]
- Tuli, H.S.; Sak, K.; Gupta, D.S.; Kaur, G.; Aggarwal, D.; Parashar, N.C.; Choudhary, R.; Yerer, M.B.; Kaur, J.; Kumar, M.; et al. Anti-Inflammatory and Anticancer Properties of Birch Bark-Derived Betulin: Recent Developments. Plants 2021, 10, 2663. [Google Scholar] [CrossRef]
- Lavoie, J.M.; Stevanovic, T. Variation of Chemical Composition of the Lipophilic Extracts from Yellow Birch (Betula alleghaniensis) Foliage. J. Agric. Food Chem. 2005, 53, 4747–4756. [Google Scholar] [CrossRef]
- Zhao, G.; Yan, W.; Cao, D. Simultaneous Determination of Betulin and Betulinic Acid in White Birch Bark Using RP-HPLC. J. Pharm. Biomed. Anal. 2007, 43, 959–962. [Google Scholar] [CrossRef] [PubMed]
- Freysdottir, J.; Sigurpalsson, M.B.; Omarsdottir, S.; Olafsdottir, E.S.; Vikingsson, A.; Hardardottir, I. Ethanol Extract from Birch Bark (Betula pubescens) Suppresses Human Dendritic Cell Mediated Th1 Responses and Directs it Towards a Th17 Regulatory Response in Vitro. Immunol. Lett. 2011, 136, 90–96. [Google Scholar] [CrossRef]
- Koptelova, E.N.; Kutakova, N.A.; Tret’yakov, S.I. Isolation of the Extractives and Betulin from Birch Bark Exposed to a Microwave Field. Russ. J. Bioorg. Chem. 2014, 40, 791–795. [Google Scholar] [CrossRef]
- Ferreira, R.; Garcia, H.; Sousa, A.F.; Freire, C.S.R.; Silvestre, A.J.D.; Kunz, W.; Rebelo, L.P.N.; Silva Pereira, C. Microwave Assisted Extraction of Betulin from Birch Outer Bark. RSC Adv. 2013, 3, 21285–21288. [Google Scholar] [CrossRef]
- Grazhdannikov, A.E.; Kornaukhova, L.M.; Rodionov, V.I.; Pankrushina, N.A.; Shults, E.E.; Fabiano-Tixier, A.S.; Popov, S.A.; Chemat, F. Selecting a Green Strategy on Extraction of Birch Bark and Isolation of Pure Betulin Using Monoterpenes. ACS Sustain. Chem. Eng. 2018, 6, 6281–6288. [Google Scholar] [CrossRef]
- Silva, N.H.C.S.; Morais, E.S.; Freire, C.S.R.; Freire, M.G.; Silvestre, A.J.D. Extraction of High Value Triterpenic Acids from Eucalyptus globulus Biomass Using Hydrophobic Deep Eutectic Solvents. Molecules 2020, 25, 210. [Google Scholar] [CrossRef] [PubMed]
- Olmo-García, L.; Bajoub, A.; Fernández-Gutiérrez, A.; Carrasco-Pancorbo, A. Evaluating the Potential of LC Coupled to Three Alternative Detection Systems (ESI-IT, APCI-TOF and DAD) for the Targeted Determination of Triterpenic Acids and Dialcohols in Olive Tissues. Talanta 2016, 150, 355–366. [Google Scholar] [CrossRef]
- Taralkar, S.V.; Chattopadhyay, S. A HPLC Method for Determination of Ursolic Acid and Betulinic Acids from Their Methanolic Extracts of Vitex negundo Linn. J. Anal. Bioanal. Tech. 2012, 3, 2–6. [Google Scholar] [CrossRef]
- Falev, D.I.; Kosyakov, D.S.; Ul’yanovskii, N.V.; Ovchinnikov, D.V. Rapid Simultaneous Determination of Pentacyclic Triterpenoids by Mixed-Mode Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. A 2020, 1609, 460458. [Google Scholar] [CrossRef]
- Olmo-García, L.; Bajoub, A.; Monasterio, R.P.; Fernández-Gutiérrez, A.; Carrasco-Pancorbo, A. Development and Validation of LC-MS-Based Alternative Methodologies to GC–MS for the Simultaneous Determination of Triterpenic Acids and Dialcohols in Virgin Olive Oil. Food Chem. 2018, 239, 631–639. [Google Scholar] [CrossRef]
- Rhourri-Frih, B.; Chaimbault, P.; Claude, B.; Lamy, C.; André, P.; Lafosse, M. Analysis of Pentacyclic Triterpenes by LC–MS. A Comparative Study between APCI and APPI. J. Mass Spectrom. 2009, 44, 71–80. [Google Scholar] [CrossRef]
- Williams, M.L.; Olomukoro, A.A.; Emmons, R.V.; Godage, N.H.; Gionfriddo, E. Matrix effects demystified: Strategies for Resolving Challenges in Analytical Separations of Complex Samples. J. Sep. Sci. 2023, 46, 2300571. [Google Scholar] [CrossRef] [PubMed]
- Heck, K.L.; Si, L.; Jung, D.J.; Calderón, A.I. Application of Eco-Friendly Natural Deep Eutectic Solvents (NADES) in HPLC for Separation of Complex Natural Products: Current Limitations and Future Directions. J. Pharm. Biomed. Anal. 2024, 244, 116102. [Google Scholar] [CrossRef] [PubMed]
- Sebbah, T.; Yahla, I.; Cunha, E.; Riazi, A.; Amorim, C.G.; Rodriguez-Diaz, J.M.; Montenegro, M.C.B.S.M. Enhanced Extraction and Separation with HPLC-DAD of Phenolic and Flavonoid Antioxidants from Portulaca oleracea L. Leaves Using Tailored Terpenoid-Based NADES: Comparative Assessment of Antiradical and Antimicrobial Activities. Antioxidants 2025, 14, 132. [Google Scholar] [CrossRef]
- Oomen, W.W.; Begines, P.; Mustafa, N.R.; Wilson, E.G.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvent Extraction of Flavonoids of Scutellaria baicalensis as a Replacement for Conventional Organic Solvents. Molecules 2020, 25, 617. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Amezcua, I.; Díez-Municio, M.; Soria, A.C.; Ruiz-Matute, A.I.; Sanz, M.L. Flow Injection Analysis–Mass Spectrometry for the Fast Detection of Frauds in Coleus forskohlii Food Supplements. J. Chromatogr. A 2025, 1740, 465547. [Google Scholar] [CrossRef]
- Craveiro, R.; Aroso, I.; Flammia, V.; Carvalho, T.; Viciosa, M.T.; Dionísio, M.; Barreiros, S.; Reis, R.L.; Duarte, A.R.C.; Paiva, A. Properties and Thermal Behavior of Natural Deep Eutectic Solvents. J. Mol. Liq. 2016, 215, 534–540. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvents as New Potential Media for Green Technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Puerta, A.; Tejedor-Matellanes, P.; Luque-Jurado, I.; Gonzalez-Jimenez, C.; Soria, A.C.; de Frutos, M. Capillary Electrophoresis Instrumentation for Determination of Viscosity of Deep Eutectic Solvents. Adv. Sample Prep. 2023, 7, 100086. [Google Scholar] [CrossRef]


| Bet | BAc | |
|---|---|---|
| Calibration curve | y = 2.88·108 x − 13.754 R2 = 0.994 | y = 2.40·108 x + 26.317 R2 = 0.996 |
| Linear range (µg mL−1) | 0.075–100 | 0.098–2 |
| Intraday precision (RSD%, n = 5) | 0.52 | 0.61 |
| Inter-day precision (RSD%, n = 5) | 3.04 | 3.28 |
| LOD (µg mL−1) | 0.023 (0.0001) * | 0.029 (0.00004) |
| LOQ (µg mL−1) | 0.075 (0.0003) | 0.098 (0.0002) |
| Accuracy (%) | 102.27 (2.10) | 94.76 (2.13) |
| 25 °C | 55 °C | |||
|---|---|---|---|---|
| Extractant | Bet | BAc | Bet | BAc |
| NADES1 (thymol:octanoic acid) | 8.42 (0.73) *d | 0.65 (0.04) c | 14.38 (0.59) b | 0.91 (0.03) a |
| NADES2 (thymol:1-propanol) | 10.84 (0.21) c | 0.75 (0.01) b | 12.66 (0.50) bc | 0.88 (0.06) a |
| NADES3 (thymol:1-octanol) | 9.81 (0.69) c | 0.67 (0.02) c | 17.50 (1.67) a | 0.92 (0.04) a |
| NADES4 (thymol:menthol) | 7.56 (0.05) d | 0.600 (0.004) c | 14.31 (0.51) b | 0.93 (0.02) a |
| Limonene | 4.26 (0.01) e | 0.38 (0.02) d | 11.28 (0.33) c | 0.63 (0.04) b |
| Methanol | 12.75 (0.79) b | 0.86 (0.04) a | 17.66 (0.47) a | 0.48 (0.01) c |
| Acetone | 14.22 (0.21) a | 0.85 (0.05) a | 17.61 (0.21) a | 0.47 (0.01) c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luque-Jurado, I.; Quintanilla-López, J.E.; Lebrón-Aguilar, R.; Soria, A.C.; Sanz, M.L. Development of an LC-MS Method for the Analysis of Birch (Betula sp.) Bark Bioactives Extracted with Biosolvents. Molecules 2025, 30, 3181. https://doi.org/10.3390/molecules30153181
Luque-Jurado I, Quintanilla-López JE, Lebrón-Aguilar R, Soria AC, Sanz ML. Development of an LC-MS Method for the Analysis of Birch (Betula sp.) Bark Bioactives Extracted with Biosolvents. Molecules. 2025; 30(15):3181. https://doi.org/10.3390/molecules30153181
Chicago/Turabian StyleLuque-Jurado, Inmaculada, Jesús E. Quintanilla-López, Rosa Lebrón-Aguilar, Ana Cristina Soria, and María Luz Sanz. 2025. "Development of an LC-MS Method for the Analysis of Birch (Betula sp.) Bark Bioactives Extracted with Biosolvents" Molecules 30, no. 15: 3181. https://doi.org/10.3390/molecules30153181
APA StyleLuque-Jurado, I., Quintanilla-López, J. E., Lebrón-Aguilar, R., Soria, A. C., & Sanz, M. L. (2025). Development of an LC-MS Method for the Analysis of Birch (Betula sp.) Bark Bioactives Extracted with Biosolvents. Molecules, 30(15), 3181. https://doi.org/10.3390/molecules30153181







