Physico-Chemical Characterization of Amino Acid-Based Deep Eutectic Solvents
Abstract
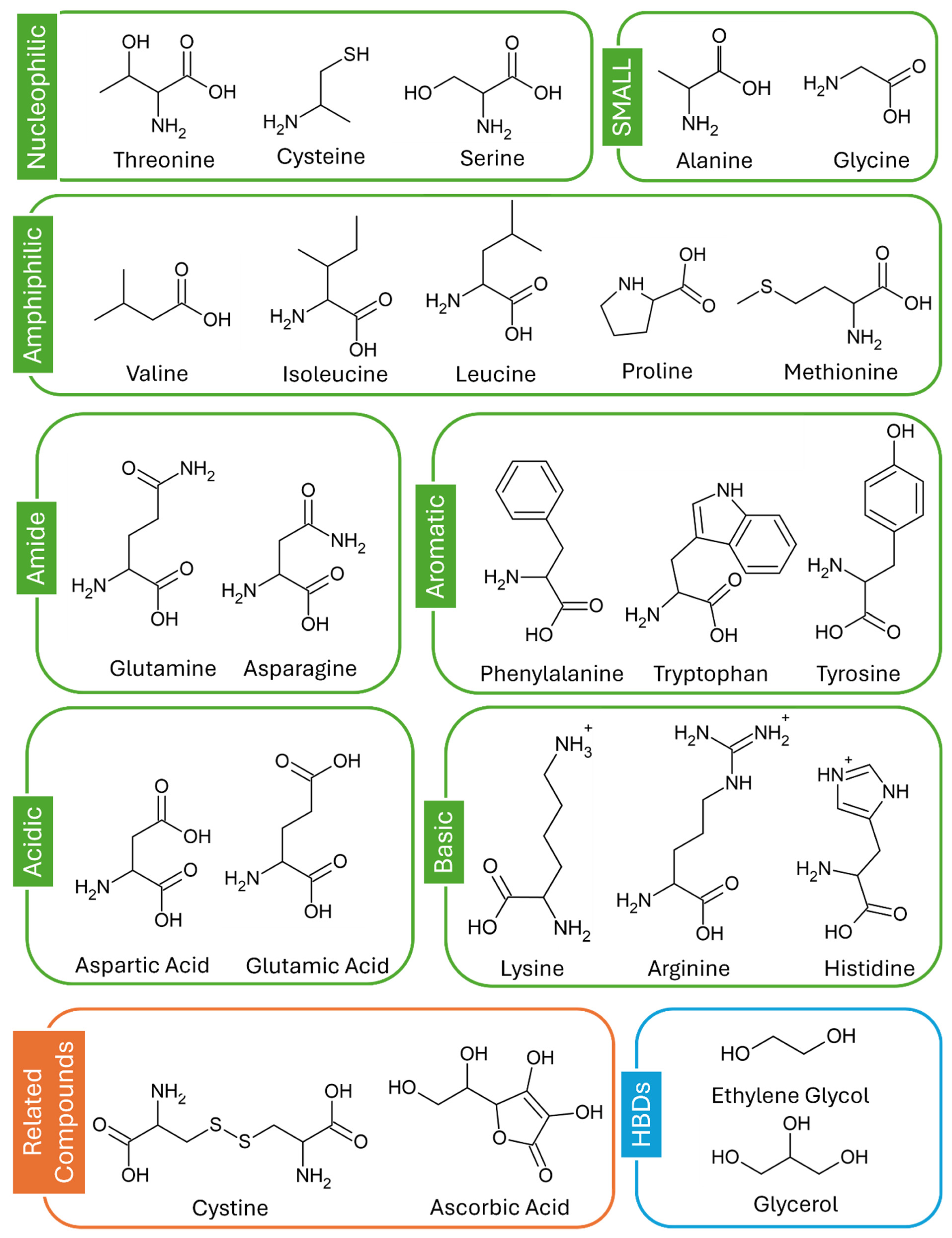
1. Introduction
2. Results and Discussion
2.1. Formation of Homogenous Liquids
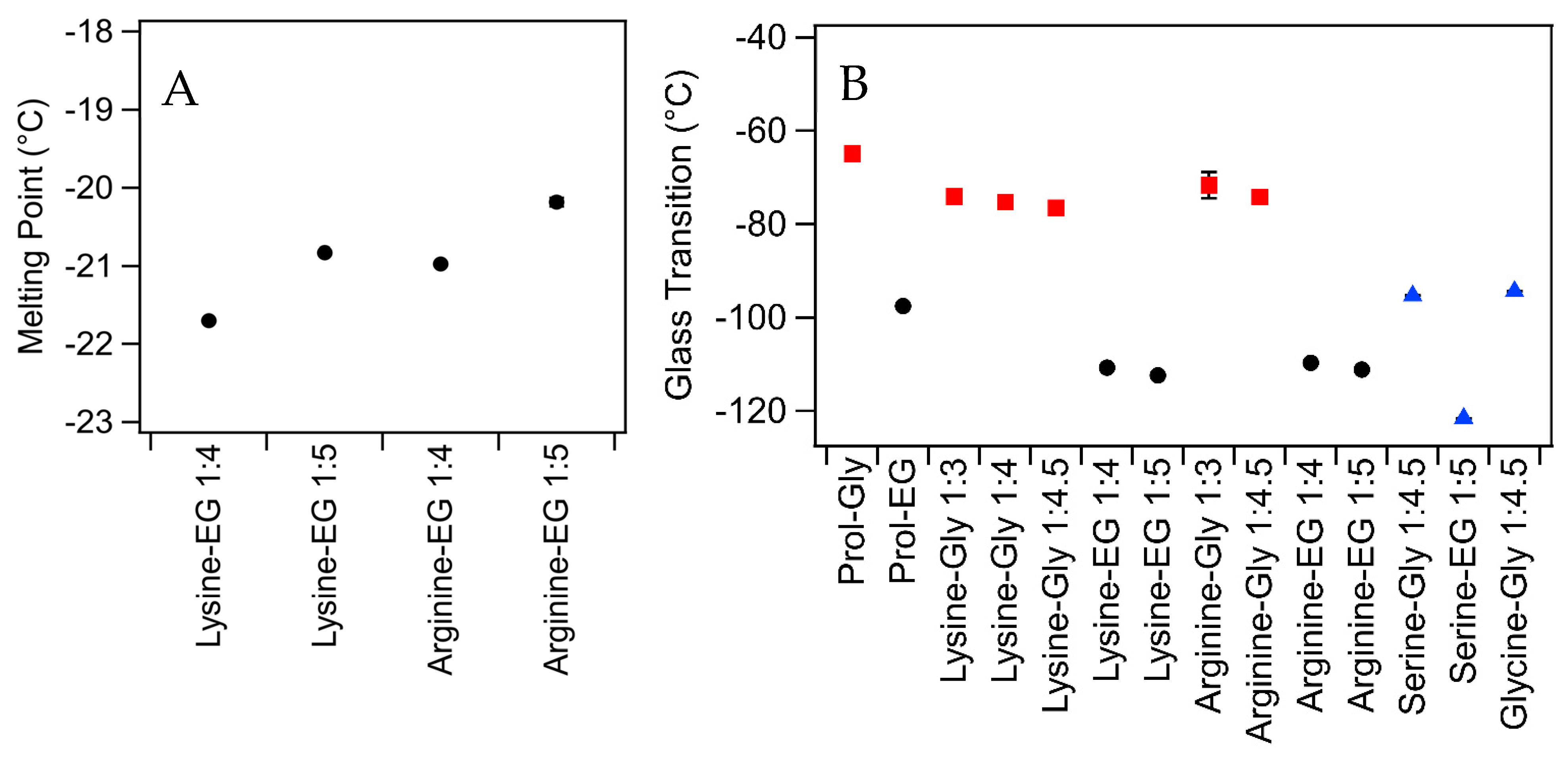
2.2. Thermal Behavior
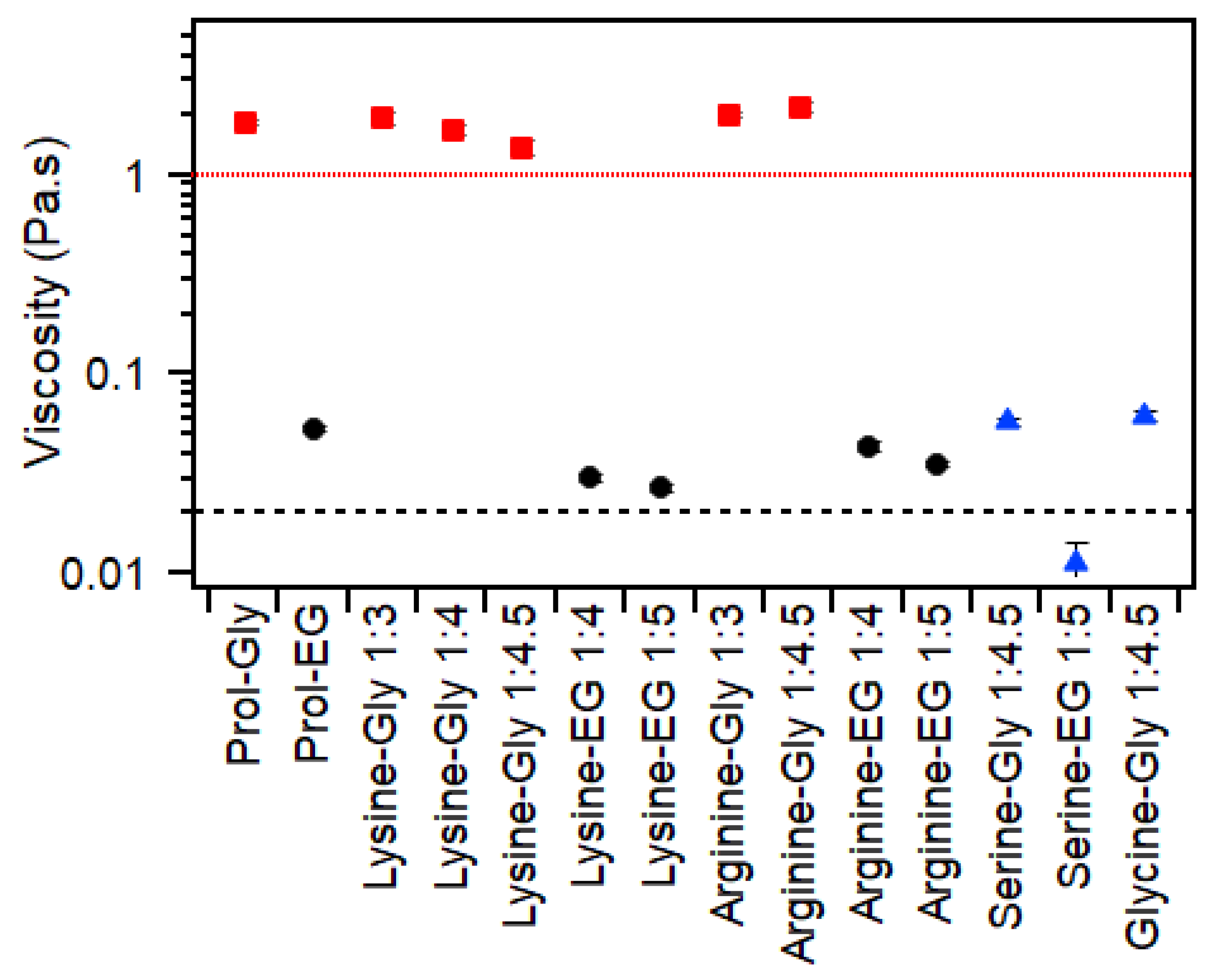
2.3. Viscosity
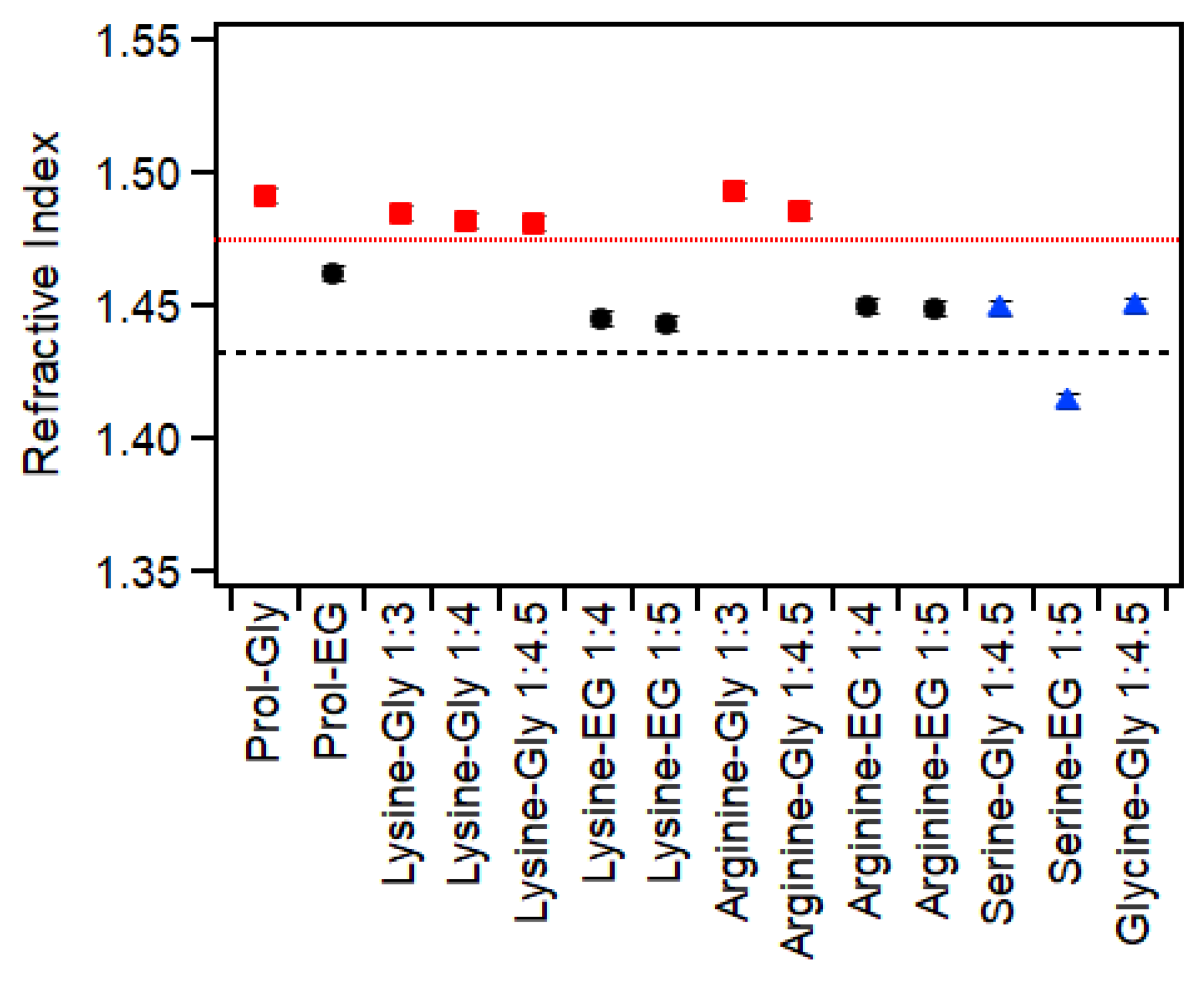
2.4. Refractive Index
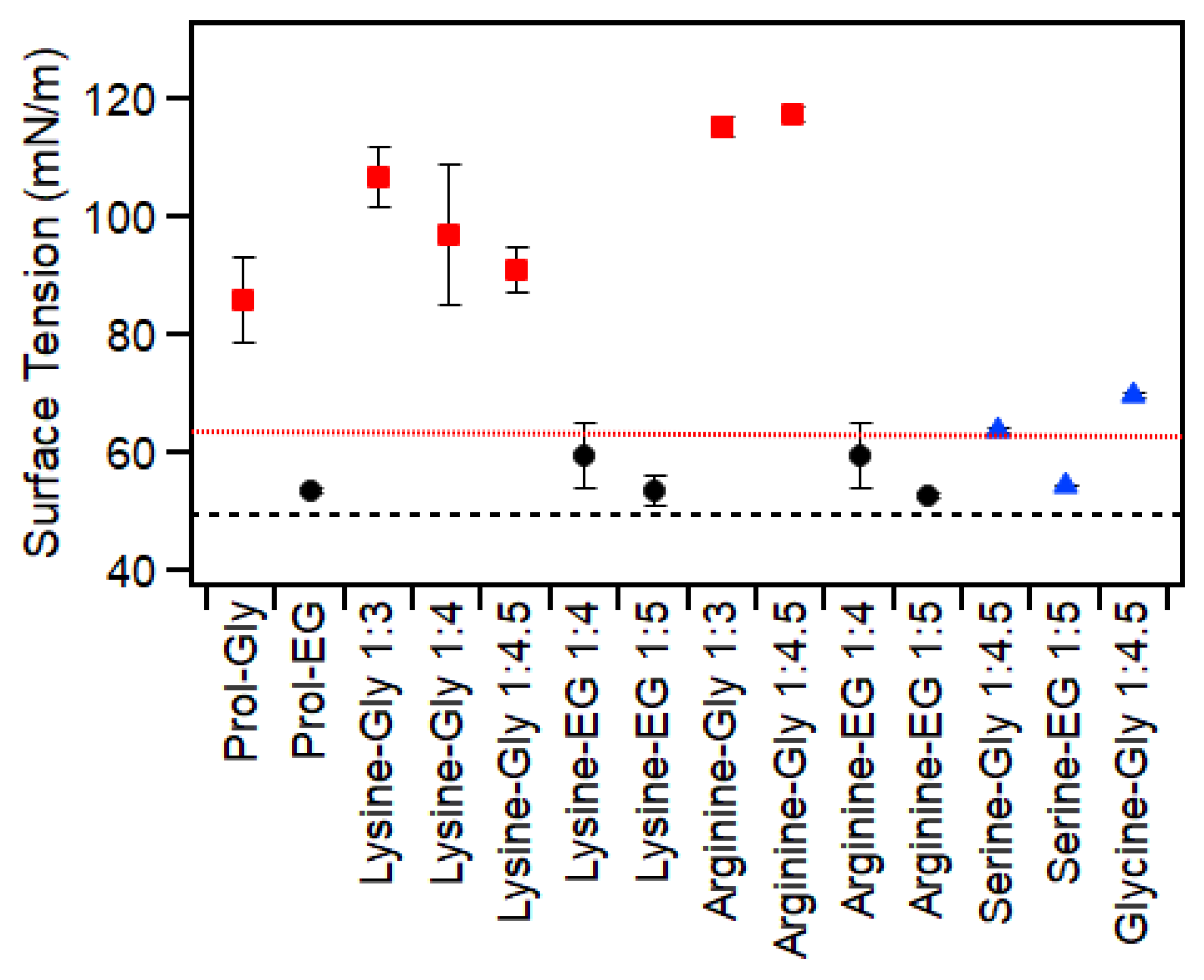
2.5. Surface Tension
3. Materials and Methods
3.1. Synthesis
3.2. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Abranches, D.O.; Coutinho, J.A.P. Everything You Wanted to Know about Deep Eutectic Solvents but Were Afraid to Be Told. Annu. Rev. Chem. Biomol. Eng. 2023, 14, 141–163. [Google Scholar] [CrossRef] [PubMed]
- Meredith, L.; Elbourne, A.; Greaves, T.L.; Bryant, G.; Bryant, S.J. Physico-chemical characterisation of glycerol- and ethylene glycol-based deep eutectic solvents. J. Mol. Liq. 2024, 394, 123777. [Google Scholar] [CrossRef]
- Bryant, S.J.; Christofferson, A.J.; Greaves, T.L.; McConville, C.F.; Bryant, G.; Elbourne, A. Bulk and interfacial nanostructure and properties in deep eutectic solvents: Current perspectives and future directions. J. Colloid Interface Sci. 2022, 608, 2430–2454. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Ballerat-Busserolles, K.; Husson, P.; Andanson, J.-M. Impact of water on the melting temperature of urea + choline chloride deep eutectic solvent. New J. Chem. 2016, 40, 4492–4499. [Google Scholar] [CrossRef]
- Johnson, L.; Bryant, S.J.; Lei, P.; Roan, C.; Marks, D.C.; Bryant, G. A deep eutectic solvent is an effective cryoprotective agent for platelets. Cryobiology 2024, 116, 104913. [Google Scholar] [CrossRef]
- Bryant, S.J.; Awad, M.N.; Elbourne, A.; Christofferson, A.J.; Martin, A.V.; Meftahi, N.; Drummond, C.J.; Greaves, T.L.; Bryant, G. Deep eutectic solvents as cryoprotective agents for mammalian cells. J. Mater. Chem. B 2022, 10, 4546–4560. [Google Scholar] [CrossRef]
- Castro, V.I.B.; Craveiro, R.; Silva, J.M.; Reis, R.L.; Paiva, A.; Duarte, A.R.C. Natural deep eutectic systems as alternative nontoxic cryoprotective agents. Cryobiology 2018, 83, 15–26. [Google Scholar] [CrossRef]
- Shaw, Z.L.; Awad, M.N.; Gharehgozlo, S.; Greaves, T.L.; Haidari, H.; Kopecki, Z.; Bryant, G.; Spicer, P.T.; Walia, S.; Elbourne, A.; et al. Deep Eutectic Solvent Eutectogels for Delivery of Broad-Spectrum Antimicrobials. ACS Appl. Bio. Mater. 2024, 7, 1429–1434. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.M.; Silva, E.; Reis, R.L.; Duarte, A.R.C. A closer look in the antimicrobial properties of deep eutectic solvents based on fatty acids. Sustain. Chem. Pharm. 2019, 14, 100192. [Google Scholar] [CrossRef]
- Bryant, S.J.; Bryant, G.; Greaves, T.L. Getting together without water: Lipid self-assembly in polar non-aqueous solvents. Eur. J. Pharm. Biopharm. 2024, 204, 114472. [Google Scholar] [CrossRef]
- Bryant, S.J.; Elbourne, A.; Greaves, T.L.; Bryant, G. Phytantriol phase behaviour in choline chloride urea and water mixtures. J. Mater. Chem. B 2023, 11, 6868–6880. [Google Scholar] [CrossRef]
- Bryant, S.J.; Atkin, R.; Warr, G.G. Effect of Deep Eutectic Solvent Nanostructure on Phospholipid Bilayer Phases. Langmuir 2017, 33, 6878–6884. [Google Scholar] [CrossRef]
- Hallett, J.E.; Hayler, H.J.; Perkin, S. Nanolubrication in deep eutectic solvents. Phys. Chem. Chem. Phys. 2020, 22, 20253–20264. [Google Scholar] [CrossRef]
- Liu, P.; Hao, J.-W.; Mo, L.-P.; Zhang, Z.-H. Recent advances in the application of deep eutectic solvents as sustainable media as well as catalysts in organic reactions. RSC Adv. 2015, 5, 48675–48704. [Google Scholar] [CrossRef]
- Basu, M.; Hassan, P.A.; Shelar, S.B. Modulation of surfactant self-assembly in deep eutectic solvents and its relevance to drug delivery-A review. J. Mol. Liq. 2023, 375, 121301. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Y.; Jiang, W.; Zhu, C.; Yao, S. Transdermal release behaviors of bioactive deep eutectic solvents as natural skin care and mechanism. J. Mol. Liq. 2022, 367, 120412. [Google Scholar] [CrossRef]
- Maimulyanti, A.; Nurhidayati, I.; Mellisani, B.; Amelia Rachmawati Putri, F.; Puspita, F.; Restu Prihadi, A. Development of natural deep eutectic solvent (NADES) based on choline chloride as a green solvent to extract phenolic compound from coffee husk waste. Arab. J. Chem. 2023, 16, 104634. [Google Scholar] [CrossRef]
- Huang, Y.; Feng, F.; Jiang, J.; Qiao, Y.; Wu, T.; Voglmeir, J.; Chen, Z.-G. Green and efficient extraction of rutin from tartary buckwheat hull by using natural deep eutectic solvents. Food Chem. 2017, 221, 1400–1405. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Ma, C.; Laaksonen, A.; Liu, C.; Lu, X.; Ji, X. The peculiar effect of water on ionic liquids and deep eutectic solvents. Chem. Soc. Rev. 2018, 47, 8685–8720. [Google Scholar] [CrossRef]
- Hammond, O.S.; Bowron, D.T.; Edler, K.J. The Effect of Water upon Deep Eutectic Solvent Nanostructure: An Unusual Transition from Ionic Mixture to Aqueous Solution. Angew. Chem. Int. Ed. 2017, 56, 9782–9785. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ren, H.; Li, B.; Udin, S.M.; Maarof, H.; Zhou, W.; Cheng, F.; Yang, J.; Liu, Y.; Alias, H.; et al. Mechanistic insights into the lignin dissolution behavior in amino acid based deep eutectic solvents. Int. J. Biol. Macromol. 2023, 242, 124829. [Google Scholar] [CrossRef]
- Li, Y.; Luo, J.; Shan, S.; Cao, Y. High toxicity of amino acid-based deep eutectic solvents. J. Mol. Liq. 2023, 370, 121044. [Google Scholar] [CrossRef]
- Dochi, O. Direct transfer of frozen-thawed bovine embryos and its application in cattle reproduction management. J. Reprod Dev. 2019, 65, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Edashige, K. Permeability of the plasma membrane to water and cryoprotectants in mammalian oocytes and embryos: Its relevance to vitrification. Reprod. Med. Biol. 2017, 16, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Lovelock, J.E. Het mechanism of the protective action of glycerol against haemolysis by freezing and thawing. Biochim. Biophys. Acta 1953, 11, 28–36. [Google Scholar] [CrossRef]
- Hayler, H.J.; Perkin, S. The eutectic point in choline chloride and ethylene glycol mixtures. Chem. Commun. 2022, 58, 12728–12731. [Google Scholar] [CrossRef]
- Haynes, W.M. Crc Handbook of Chemistry and Physics; CRC Press LLC: Milton, UK, 2016. [Google Scholar]
- Lane, L.B. Freezing Points of Glycerol and Its Aqueous Solutions. Ind. Eng. Chem. 1925, 17, 924. [Google Scholar] [CrossRef]
- Hayashi, Y.; Puzenko, A.; Balin, I.; Ryabov, Y.E.; Feldman, Y. Relaxation Dynamics in Glycerol−Water Mixtures. 2. Mesoscopic Feature in Water Rich Mixtures. J. Phys. Chem. B 2005, 109, 9174–9177. [Google Scholar] [CrossRef] [PubMed]
- Like, B.D.; Uhlenbrock, C.E.; Panzer, M.J. A quantitative thermodynamic metric for identifying deep eutectic solvents. Phys. Chem. Chem. Phys. 2023, 25, 7946–7950. [Google Scholar] [CrossRef] [PubMed]
- Andruch, V.; Makoś-Chełstowska, P.; Płotka-Wasylka, J. Remarks on use of the term “deep eutectic solvent” in analytical chemistry. Microchem. J. 2022, 179, 107498. [Google Scholar] [CrossRef]
- Joback, K.G.; Reid, R.C. Estimation of pure-component properties from group-contributions. Chem. Eng. Commun. 1987, 57, 233–243. [Google Scholar] [CrossRef]
- Segur, J.B.; Oberstar, H.E. Viscosity of Glycerol and Its Aqueous Solutions. Ind. Eng. Chem. 1951, 43, 2117–2120. [Google Scholar] [CrossRef]
- Sun, T.; Teja, A.S. Density, Viscosity, and Thermal Conductivity of Aqueous Ethylene, Diethylene, and Triethylene Glycol Mixtures between 290 K and 450 K. J. Chem. Eng. Data 2003, 48, 198–202. [Google Scholar] [CrossRef]
- García, G.; Aparicio, S.; Ullah, R.; Atilhan, M. Deep Eutectic Solvents: Physicochemical Properties and Gas Separation Applications. Energy Fuels 2015, 29, 2616–2644. [Google Scholar] [CrossRef]
- Stefanovic, R.; Ludwig, M.; Webber, G.B.; Atkin, R.; Page, A.J. Nanostructure, hydrogen bonding and rheology in choline chloride deep eutectic solvents as a function of the hydrogen bond donor. Phys. Chem. Chem. Phys. 2017, 19, 3297–3306. [Google Scholar] [CrossRef]
- Leron, R.B.; Soriano, A.N.; Li, M.-H. Densities and refractive indices of the deep eutectic solvents (choline chloride+ethylene glycol or glycerol) and their aqueous mixtures at the temperature ranging from 298.15 to 333.15K. J. Taiwan Inst. Chem. Eng. 2012, 43, 551–557. [Google Scholar] [CrossRef]
- Greaves, T.L.; Weerawardena, A.; Fong, C.; Krodkiewska, I.; Drummond, C.J. Protic Ionic Liquids: Solvents with Tunable Phase Behavior and Physicochemical Properties. J. Phys. Chem. B 2006, 110, 22479–22487. [Google Scholar] [CrossRef]
- Takamura, K.; Fischer, H.; Morrow, N.R. Physical properties of aqueous glycerol solutions. J. Pet. Sci. Eng. 2012, 98–99, 50–60. [Google Scholar] [CrossRef]
- Azizian, S.; Hemmati, M. Surface Tension of Binary Mixtures of Ethanol + Ethylene Glycol from 20 to 50 °C. J. Chem. Eng. Data 2003, 48, 662–663. [Google Scholar] [CrossRef]
- Angle, K.J.; Nowak, C.M.; Davasam, A.; Dommer, A.C.; Wauer, N.A.; Amaro, R.E.; Grassian, V.H. Amino Acids Are Driven to the Interface by Salts and Acidic Environments. J. Phys. Chem. Lett. 2022, 13, 2824–2829. [Google Scholar] [CrossRef] [PubMed]
- Raza, M.A.; Hallett, P.D.; Liu, X.; He, M.; Afzal, W. Surface Tension of Aqueous Solutions of Small-Chain Amino and Organic Acids. J. Chem. Eng. Data 2019, 64, 5049–5056. [Google Scholar] [CrossRef]
- Ho, B.K.; Coutsias, E.A.; Seok, C.; Dill, K.A. The flexibility in the proline ring couples to the protein backbone. Protein Sci. 2005, 14, 1011–1018. [Google Scholar] [CrossRef]
- Francis, A.; Carey, R.M.G. Amino acids, peptides and proteins. In Organic Chemistry, 8th ed.; McGraw-Hill: New York, NY, USA, 2011; p. 1247. [Google Scholar]
| Amino Acid | HBD | Ratio | Water (wt%) | Thermal Behavior (°C) | Viscosity (Pa·s) | RI (±0.003) | Surface Tension (mN/m) |
|---|---|---|---|---|---|---|---|
| Lysine | Glycerol | 1:3 | 1.3 | Glass: −74 ± 1 | 1.9 ± 0.2 | 1.485 | 106 ± 5 |
| 1:4 | 1 | Glass: −75 ± 1 | 1.7 ± 0.1 | 1.482 | 97 ± 10 | ||
| 1:4.5 | 0.9 | Glass: −76 ± 1 | 1.4 ± 0.1 | 1.481 | 91 ± 4 | ||
| Ethylene Glycol | 1:4 | 1.1 | Melt: −22 ± 1 Glass: −110 ± 1 | 0.03 ± 0.001 | 1.446 | 60 ± 6 | |
| 1:5 | 0.87 | Melt: −21 ± 1 Glass: −112 ± 1 | 0.027 ± 0.001 | 1.443 | 54 ± 2 | ||
| Arginine | Glycerol | 1:3 | 10 | Glass: −72 ± 3 | 2.0 ± 0.07 | 1.493 | 115 ± 2 |
| 1:4.5 | 0.2 | Glass: −74 ± 1 | 2.2 ± 0.1 | 1.486 | 117 ± 2 | ||
| Ethylene Glycol | 1:4 | 0.3 | Melt: −21 ± 1 Glass: −110 ± 1 | 0.043 ± 0.002 | 1.450 | 60 ± 5 | |
| 1:5 | 0.3 | Melt: −2 ± 1 Glass: −111 ± 1 | 0.035 ± 0.001 | 1.449 | 52.8 ± 0.3 | ||
| Serine | Glycerol | 1:4.5 | 23 | Glass: −95 ± 1 | 0.058 ± 0.002 | 1.449 | 63.8 ± 0.4 |
| Ethylene Glycol | 1:5 | 29 A | Glass: −122 ± 1 | 0.011 ± 0.003 | 1.414 | 54.5 ± 0.1 | |
| Glycine | Glycerol | 1:4.5 | 21 A | Glass: −94 ± 1 | 0.061 ± 0.004 | 1.450 | 69.6 ± 0.2 |
| Mixture | Molar Ratio | Scaled Molar Excess Gibbs Free Energy | Maximum Melting Point to Meet DES Requirement (°C) |
|---|---|---|---|
| Lysine–Glycerol | 1:3 | No melting point in DSC | 13 |
| 1:4 | No melting point in DSC | 10 | |
| 1:4.5 | No melting point in DSC | 10 | |
| Lysine–Ethylene Glycol | 1:4 | −0.4 | −15 |
| 1:5 | −0.4 | −20 | |
| Arginine–Glycerol | 1:4.5 | No melting point in DSC | 17 |
| Arginine–Ethylene Glycol | 1:4 | −2.2 | −4 * |
| 1:5 | −1.8 | −10 * |
| Amino Acid | HBD | Ratio | Result |
|---|---|---|---|
| L-Proline | Glycerol | 1:3 | Homogenous yellow/brown liquid |
| Ethylene Glycol | 1:4 | Homogenous yellow liquid | |
| L-Alanine | Glycerol | 1:3 | Precipitated crystals, even with >50 wt% water. |
| Ethylene Glycol | 1:4 | Precipitated crystals, even with >50 wt% water. | |
| L-Lysine | Glycerol | 1:3 | Homogenous yellow liquid |
| 1:4 | Homogenous yellow liquid | ||
| 1:4.5 | Homogenous yellow liquid | ||
| Ethylene Glycol | 1:4 | Homogenous yellow liquid | |
| 1:5 | Homogenous yellow liquid | ||
| L-Methionine | Glycerol | 1:3 | Precipitated crystals, even with >50 wt% water. |
| Ethylene Glycol | 1:4 | Precipitated crystals, even with >50 wt% water. | |
| L-Threonine | Glycerol | 1:3 | Precipitated crystals, even with >50 wt% water. |
| Ethylene Glycol | 1:4 | Precipitated crystals, even with >50 wt% water. | |
| L-Valine | Glycerol | 1:3 | Precipitated crystals, even with >50 wt% water. |
| Ethylene Glycol | 1:4 | Precipitated crystals, even with >50 wt% water. | |
| L-Tryptophan | Glycerol | 1:3 | Precipitated crystals, even with >50 wt% water. |
| Ethylene Glycol | 1:4 | Precipitated crystals, even with >50 wt% water. | |
| L-Tyrosine | Glycerol | 1:3 | Precipitated crystals, even with >50 wt% water. |
| Ethylene Glycol | 1:4 | Precipitated crystals, even with >50 wt% water. | |
| L-Phenylalanine | Glycerol | 1:3 | Precipitated crystals, even with >50 wt% water. |
| Ethylene Glycol | 1:4 | Precipitated crystals, even with >50 wt% water. | |
| L-Leucine | Glycerol | 1:3 | Precipitated crystals, even with >50 wt% water. |
| Ethylene Glycol | 1:4 | Precipitated crystals, even with >50 wt% water. | |
| L-Glutamic Acid | Glycerol | 1:3 | Precipitated crystals, even with >50 wt% water. |
| Ethylene Glycol | 1:4 | Precipitated crystals, even with >50 wt% water. | |
| L-Glutamine | Glycerol | 1:3 | Precipitated crystals, even with >50 wt% water. |
| Ethylene Glycol | 1:4 | Precipitated crystals, even with >50 wt% water. | |
| Glycine | Glycerol | 1:3 | Precipitated crystals, even with >50 wt% water. |
| 1:4.5 | Homogenous mixture at 21 wt% water * | ||
| Ethylene Glycol | 1:4 | Precipitated crystals, even with >50 wt% water. | |
| L-Histidine | Glycerol | 1:3 | Precipitated crystals, even with >50 wt% water. |
| Ethylene Glycol | 1:4 | Precipitated crystals, even with >50 wt% water. | |
| L-Isoleucine | Glycerol | 1:3 | Precipitated crystals, even with >50 wt% water. |
| Ethylene Glycol | 1:4 | Precipitated crystals, even with >50 wt% water. | |
| L-Cystine | Glycerol | 1:3 | Precipitated crystals, even with >50 wt% water. |
| Ethylene Glycol | 1:4 | Precipitated crystals, even with >50 wt% water. | |
| L-Cysteine | Glycerol | 1:3 | Precipitated crystals, even with >50 wt% water. |
| Ethylene Glycol | 1:4 | Precipitated crystals, even with >50 wt% water. | |
| L-Asparagine | Glycerol | 1:3 | Precipitated crystals, even with >50 wt% water. |
| Ethylene Glycol | 1:4 | Precipitated crystals, even with >50 wt% water. | |
| L-Aspartic Acid | Glycerol | 1:3 | Precipitated crystals, even with >50 wt% water. |
| Ethylene Glycol | 1:4 | Precipitated crystals, even with >50 wt% water. | |
| L-Ascorbic Acid | Glycerol | 1:3 | Precipitated crystals, even with >50 wt% water. |
| Ethylene Glycol | 1:4 | Precipitated crystals, even with >50 wt% water. | |
| L-Arginine | Glycerol | 1:3 | Formed a homogenous mixture at 10 wt% water |
| 1:4.5 | Homogenous, slightly yellow liquid | ||
| Ethylene Glycol | 1:4 | Homogenous, mostly clear liquid | |
| 1:5 | Homogenous, slightly yellow liquid | ||
| L-Serine | Glycerol | 1:3 | Homogenous mixture at 49 wt% water |
| 1:4.5 | Homogenous mixture at 23 wt% water * | ||
| Ethylene Glycol | 1:4 | Precipitated crystals, even with >50 wt% water. | |
| 1:5 | Homogenous mixture at 29 wt% water * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bryant, S.J.; Bryant, G.; Drummond, C.J.; Greaves, T.L. Physico-Chemical Characterization of Amino Acid-Based Deep Eutectic Solvents. Molecules 2025, 30, 818. https://doi.org/10.3390/molecules30040818
Bryant SJ, Bryant G, Drummond CJ, Greaves TL. Physico-Chemical Characterization of Amino Acid-Based Deep Eutectic Solvents. Molecules. 2025; 30(4):818. https://doi.org/10.3390/molecules30040818
Chicago/Turabian StyleBryant, Saffron J., Gary Bryant, Calum J. Drummond, and Tamar L. Greaves. 2025. "Physico-Chemical Characterization of Amino Acid-Based Deep Eutectic Solvents" Molecules 30, no. 4: 818. https://doi.org/10.3390/molecules30040818
APA StyleBryant, S. J., Bryant, G., Drummond, C. J., & Greaves, T. L. (2025). Physico-Chemical Characterization of Amino Acid-Based Deep Eutectic Solvents. Molecules, 30(4), 818. https://doi.org/10.3390/molecules30040818








