Exploring the Therapeutic Value of Some Vegetative Parts of Rubus and Prunus: A Literature Review on Bioactive Profiles and Their Pharmaceutical and Cosmetic Interest
Abstract
1. Introduction
2. Research Methodology
- the expression “Rubus idaeus + shoots + bioactive compounds” produced over 1570 relevant results;
- the search “Prunus avium + twigs + bioactive compounds” provided approximately 2400 studies;
- “Prunus serotina + twigs + bioactive compounds” led to the identification of 540 articles;
- and the formula “Prunus cerasus + twigs + bioactive compounds” returned more than 1000 works.
3. Bioactive Compounds from Plant Waste of Rubus idaeus, Prunus serotina, Prunus avium, and Prunus cerasus
4. Extraction and Isolation Methods
4.1. Extraction
4.2. Isolation
5. Biological Activity
5.1. Pharmacological Importance, Bioavailability, and Toxicity
5.2. Synergistic Activity
5.3. Structure–Activity Relationship Study
5.3.1. Phenolic Compounds
- Simple Phenols
- Hydroquinone, in the phenolic structural form, called 1,4-dihydroxybenzene, due to the presence of the hydroxyl group in the para position, exhibits antioxidant activity [273].
- 2.
- Flavonoids
- Quercetin, named 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one [278], whose basic structure is represented by two phenyl groups connected by three carbon atoms, can be arranged in an open form or in the form of a heterocyclic ring [279]. The antioxidant activity of this compound is determined by the presence of the hydroxyl group, which is why some quercetin derivatives exhibit lower activity. On the other hand, obtaining methylated derivatives can lead to an increase in anti-inflammatory activity, and through glycosylation reactions, compounds with higher bioavailability regarding the antiobesity effect can be obtained [280]. Of the five hydroxyl groups in the structure of quercetin, only those at positions 3, 3′, and 4′ are responsible for the antioxidant activity of this compound, also being involved in its photolability [84]. The presence of the double bond in the heterocyclic ring of quercetin determines the manner in which this compound binds to DNA, by fitting into the helix of deoxyribonucleic acid, compared to naringenin, which does not have that double bond and exhibits a groove-type DNA binding [281]. The inhibitory activity on lipase is influenced by the structure of flavonoids as follows: it decreases through the hydrogenation of the double bond in the C ring, specifically through the glycosylation reaction, and increases with the presence of the carbonyl group or the hydroxylation reaction. Quercetin, due to its chemical structure, exhibits this activity, but it is lower than that of luteolin [282].
- Astragalin is also known as kaempferol 3-O-β-d-glucopyranoside. The substitution of phenolic hydroxyl groups influences the anti-inflammatory activity of astragalin, having a stronger effect than chrysin or luteolin [115].
- Rutin, known as 3′,4′,5,7-tetrahydroxyflavone-3-rutinoside or quercetin-3-rutinoside, is a flavonoid glycoside formed from quercetin and rutin [87]. The antioxidant activity of rutin can be enhanced by complexation with cyclodextrin [283]. On the other hand, glycosylation of this compound leads to an increase in antioxidant, antibacterial, and α-glucosidase inhibitory activities [88].
- Aromadendrin contains four hydroxyl groups in its structure and is also called (2R,3R)-3,5,7-trihydroxy-2-(4-hydroxyphenyl)-2,3-dihydrochromen-4-one. This compound exhibits multiple pharmacological activities, but in the case of antidiabetic and anticancer actions, the 7-O methylated derivative stands out, while methylation at the 4′-O position is noted to be effective for antiulcer activity [157].
- Juglanin (kaempferol 3-O-α-L-arabinofuranoside) contains multiple hydroxyl groups in its structure. This compound exhibits a lower antiradical effect compared to quercetin, the scientific justification being the presence of a single hydroxyl group on ring B [17].
- Kaempferol, also named 3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one, has a diphenylpropane structure and can be obtained through a series of reactions applied to naringenin [134,135]. In the study conducted by Rho et al., it was highlighted that depigmentation activity and cytotoxicity are enhanced by the presence of the hydroxyl group at position 3 [284].
- Prunin, a flavanone glycoside, is obtained following the hydrolysis process of naringenin. In the case of prunin laurate, a strong antibacterial activity against Porphyromonas gingivalis was shown by Wada et al. [285]. Additionally, in another study, when examining naringenin derivatives, it was highlighted that an aliphatic chain of 10–12 carbon atoms attached to ring A has the ability to enhance antimicrobial activity, with alkylprunin being an important representative [286].
- Apigenin or 4′,5,7–trihydroxyflavone, contains a 2-phenylchromen-4-one skeleton [63]. A study aimed at comparing the biological activity of apigenin and one of its derivatives, apigenin-7-O-glucoside, concluded that the presence of the sugar moiety in the derivative resulted in stronger antifungal activity against Candida albicans and Candida glabrata. Additionally, in vitro, the glycosidic derivative exhibits higher cytotoxic activity against cancer cells in the case of colon cancer, compared to apigenin [287].
- Chrysin (5,7-dihydroxyflavone) is a flavone that contains hydroxyl and keto functional groups [176]. The antioxidant activity of this compound is correlated with the lack of hydroxyl in rings B and C, as well as the presence of the carbonyl group on C4 and the double bond between C2 and C3 [288]. Liu et al. highlighted that halogenated derivatives exhibit stronger anticancer activity. Additionally, an enhancement of the effect was observed when the C7-OH of ring A was linked to various hydrophilic amines. Regarding the anti-inflammatory activity, a strong effect was demonstrated in the case of the derivative containing a cyclic pyridine at position 8 [289].
- Naringenin has two hydroxyl groups missing in its chemical structure compared to quercetin, which explains its lower antioxidant activity. Quercetin, on the other hand, has an antioxidant effect comparable to that of vitamin C, and the presence of two hydroxyl groups on ring C, instead of one as in the case of naringenin, leads to the formation of a stabilized quinone structure that contributes to enhancing the effect [281]. The antibacterial activity of naringenin is lower than that of other flavones that contain fewer hydroxyl groups; additionally, the position of these groups also influences the activity, so compounds that have hydroxyl groups in ring A but not in ring B exhibit significant activity. Methylation of hydroxyl groups may contribute to the reduction of the antibacterial effect [290].
- Taxifolin exhibits inhibitory activity against certain protein structures, such as amyloid fibrils, which have been highlighted in the literature as being responsible for the onset of Alzheimer’s disease. This inhibitory activity is due to the presence of the catechol group in ring B [291].
- Catechin is a flavan-3-ol, also named (2R,3S)-2-(3,4-dihydroxyphenyl)-3,4-dihydro-2H-chromene-3,5,7-triol. The position and number of hydroxyl groups influence the antibacterial activity of catechins. Additionally, the polymerization of catechin molecules enhances activity, as is the case with theaflavins [292]. The antioxidant activity is correlated with the presence of the hydroxyl group in position 3 [293].
- Genistein (4′,5,7-trihydroxyisoflavone) is a phytoestrogen that can be synthesized from naringenin in plants. It exhibits characteristics similar to those of the estrogen estradiol-17β, due to structural similarities consisting of the presence of the phenolic ring and the distance between the hydroxyl groups [294].
- Phlorizin, phloretin 2′-β-D-glucoside, according to Li et al., exhibits lower antioxidant activity than the parent compound because the glycosylation reaction reduces the number of phenolic hydroxyl groups [295].
- -
- at a concentration of 500 µM: hesperetin, genistein, epicatechin, naringenin, apigenin, kaempferol, quercetin, and rutin;
- -
- at a concentration of 100 µM: quercetin, rutin, kaempferol, and luteolin.
- 3.
- Tannins
- Sanguiin H6, an ellagitannin derived from ellagic acid, has multiple biological activities that are influenced by the presence of hydroxyl groups and the galloyl configuration [299].
- 4.
- Phenolic Acids
- 3,4-Dihydroxycinnamic acid exhibits hepatoprotective activity that can be enhanced through methoxylation at positions 3 or 4 [300]. The esterification gives rise to derivatives that exhibit remarkable antileishmanial activity. Otero et al. highlighted in a study that the bioactivity of cinnamic acid derivatives depends on the degree of oxygenation at positions 3 and 4, the presence of a double bond in the side chain, and hydroxyl groups, as well as the length of the alkyl chain [301].
- Caffeic acid is a hydroxycinnamic acid that contains an aromatic ring and three hydroxyl groups, along with the double bond in the carbon chain, with anticancer activity [212]. Anilides and aliphatic amides of caffeic acid enhance its antioxidant activity [302]. The attachment of a naphthyl ring increases the capacity of caffeic acid to inhibit monoamine oxidase, an enzyme responsible for multiple neurological disorders [301,303].
- Ferulic acid, also called 4-hydroxy-3-methoxycinnamic acid, is responsible for some biological activities [306]. The anticancer activity of some ferulic acid derivatives was investigated; thus, although some derivatives exhibit lower activity compared to caffeic acid derivatives, the phenylsulfonylfuroxan nitrates of ferulic acid stand out as having strong anticancer activity [307]. Ferulic acid, found in raspberry plant parts, has the ability to stabilize anthocyanins, but it is also recognized for its involvement in flavonoid catabolism, particularly in the spontaneous carboxylation of caffeic acid [308].
- Chlorogenic acid is derived from caffeic acid and quinic acid, and the hydroxyl groups present in its structure are responsible for the strong antioxidant effect it exhibits [58].
- Ellagic acid or 2,3,7,8-tetrahydroxy [1]-benzopyrano [5,4,3-cde] benzopyran-5,10-dione, structurally contains a hydrophilic part, represented by phenolic groups and lactone-type groups, as well as a lipophilic part represented by the four phenolic rings [50…60]. The anticancer activity of this compound is closely related to its chemical structure, specifically the presence of hydroxyl groups at positions 3 and 4, as well as the presence of lactone groups [309,310].
- Salicylic acid or 2-hydroxybenzoic acid is a plant hormone, being the main precursor of aspirin. From a structural perspective, it is notable for the ortho arrangement of the hydroxyl and carboxyl groups [311]. The inhibition of luciferase by salicylic acid is enhanced by the amidation of the carboxyl group or the substitution of chlorine at position 5 [312].
- Anacardic acid, a derivative of salicylic acid, has a side chain with different degrees of unsaturation, which is responsible for its varied biological activity. Regarding antioxidant activity, trienic anacardic acid (15:3) stands out, while for antifungal activity, monoenic anacardic acid (15:1) is highlighted [313]. The biological activity of anacardic acid is closely related to the structure of the side chain; thus, the presence of the trienic alkyl side chain determines a strong bactericidal activity against Streptococcus mutans and Staphylococcus aureus, while the saturated alkyl chain acts against Propionibacterium acnes. The antioxidant activity is synergistically influenced by the length of the alkyl chain, the presence of the salicylic acid moiety, as well as the stereochemistry of the side chain [56]. Some researchers have noted that the anticancer activity of anacardic acid largely depends on the molecular volume of the hydrophobic side chain, in addition to its metal-chelating ability and its action as a surfactant [93].
5.3.2. Coumarins
- Scopoletin, 6-methoxy-7-hydroxycoumarin, is characterized by the presence of a single hydroxyl group, a methoxy group, and a keto group [210]. Liu et al. demonstrated that derivatives containing a Δ3,4 olefinic bond, as well as naphthyl or phenyl groups with a sulfate ester at the C7 position, enhance insecticidal activity against Tetranychus cinnabarinus and Artemia salina, respectively [314].
5.3.3. Cyanogenic Glycosides
- Prunasin, the glucoside of (R)-mandelonitrile, can be glycosylated with the formation of amygdalin, and it can be converted into mandelonitrile by α-glucosidase or a hydrolase, and subsequently hydrolyzed into benzaldehyde and hydrocyanic acid [315].
5.3.4. Aldehyde
- Vanillin is an important flavor molecule, being named 4-hydroxy-3-methoxybenzaldehyde, and constitutes the major component of vanilla [316]. The aldehyde group in the structure of vanillin, as well as the position of the side group on the benzene ring, supports the antifungal activity exhibited by this compound [317]. Furthermore, this compound also exhibits antioxidant activity, stronger than that of ascorbic acid, justified by its self-dimerization in contact with free radicals [318].
5.3.5. Terpenoid
- Squalene is a precursor of cholesterol, and not only a triterpene that contains 30 carbon atoms in its structure. In the synthesis of cholesterol, the process was initially proposed to be described as a cyclization of squalene to lanosterol; later, it was demonstrated that it oxidizes to form monooxidosqualene before cyclization [319]. It exhibits a high detoxification capacity due to its ability to attach to uncharged substances, owing to its nonpolarity [320].
5.3.6. Vitamins
- Ascorbic acid, better known as vitamin C, is a compound with multiple bioactive activities, including antioxidant activity. This activity is justified on one hand by the acid’s ability to donate single hydrogen atoms, and on the other hand by the interaction between radicals and the monodehydroascorbate anion [321]. It is also worth mentioning the importance of vitamin C in collagen synthesis, a compound extremely important for human health, as well as in the fixation of vitamin E or iron [322]. The structure of lactone, with two ionizable hydroxyl groups, makes this compound an excellent reducing agent. It oxidizes successively, forming ascorbate radical and then dehydroascorbic acid, a mechanism that underlies many biological activities [323].
- Tocopherol, belonging to the vitamin E family, has a chemical structure that contains a polar chromanol ring and a lipophilic phytyl chain, and its antioxidant activity is justified by its ability to form tocopherol quinone [324]. Just like in the case of vitamin C, the presence of hydroxyl groups in the chemical structure of tocopherols, which act as hydrogen donors for peroxyl radicals, reveals other biological activities, such as cellular signaling properties [325].
6. Conclusions
- -
- presentation of the bioactive compounds representative of these species and highlighting their extraction and isolation methodology;
- -
- correlation between biological activity and their chemical structure, with emphasis on the possible synergistic action of some compounds common to the four species.
7. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brás, I.; Silva, E.; Raimondo, R.; Saetta, R.; Mignano, V.; Fabbricino, M.; Ferreira, J. Valorisation of forest and agriculture residual biomass—The application of life cycle assessment to analyse composting, mulching, and energetic valorisation strategies. Sustainability 2024, 16, 630. [Google Scholar] [CrossRef]
- Rousou, I.S. Importance of Reusing Wood from Pruning and Promotion of Circular Economy Principles in Agricultural Sector in Tripolis, Greece. Master Thesis, Agricultural University of Athens, Athens, Greece, 2025. Available online: http://hdl.handle.net/10329/8412 (accessed on 23 July 2025).
- Sanoja-López, K.A.; Guamán-Marquines, C.W.; Luque, R. Advanced processes in biomass/waste valorization: A Review. Sustain. Chem. Pharm. 2024, 41, 101704. [Google Scholar] [CrossRef]
- Syrodoy, S.V.; Yu, M.D.; Nigay, N.A.; Purin, M.V. Influence of the type of woody biomass on energy and environmental characteristics of the thermal preparation processes and ignition of bio-water-coal fuel particles. Process Saf. Environ. Protect. 2024, 184, 736–746. [Google Scholar] [CrossRef]
- Tomlin, A.S. Air quality and climate impacts of biomass use as an energy source: A review. Energy Fuels 2021, 35, 14213–14240. [Google Scholar] [CrossRef]
- Tran, H.; Jino, E.; Arunachalam, S. Emissions of wood pelletization and bioenergy use in the United States. Renew. Energy 2023, 219, 119536. [Google Scholar] [CrossRef]
- Aliaño-González, M.J.; Gabaston, J.; Ortiz-Somovilla, V.; Cantos-Villar, E. Wood waste from fruit trees: Biomolecules and their applications in agri-food industry. Biomolecules 2022, 12, 238. [Google Scholar] [CrossRef]
- Del Toro-Gipson, R.S.; Rizzo, P.V.; Hanson, D.J.; Drake, M. Sensory characterization of specific wood smoke aromas and their contributions to smoked Cheddar cheese flavor. J. Sens. Stud. 2020, 35, e12564. [Google Scholar] [CrossRef]
- Swaney-Stueve, M.; Talavera, M.; Jepsen, T.; Severns, B.; Wise, R.; Deubler, G. Sensory and consumer evaluation of smoked pulled pork prepared using different smokers and different types of wood. J. Food Sci. 2019, 84, 640–649. [Google Scholar] [CrossRef]
- Racovita, R.C.; Secuianu, C.; Ciuca, M.D.; Israel-Roming, F. Effects of smoking temperature, smoking time, and type of wood sawdust on polycyclic aromatic hydrocarbon accumulation levels in directly smoked pork sausages. J. Agric. Food Chem. 2020, 68, 9530–9536. [Google Scholar] [CrossRef]
- Öncül, M.; Atagür, M.; Atan, E.; Sever, K. A preliminary evaluation of bing cherry tree (Prunus avium L.) pruning waste as an alternative lignocellulosic filler for lightweight composite material applications. Polym. Compos. 2025, 46, 3655–3667. [Google Scholar] [CrossRef]
- Memete, A.R.; Sărac, I.; Teusdea, A.C.; Budău, R.; Bei, M.; Vicas, S.I. Bioactive compounds and antioxidant capacity of several blackberry (Rubus spp.) fruits cultivars grown in Romania. Horticulturae 2023, 9, 556. [Google Scholar] [CrossRef]
- Buczyński, K.; Kapłan, M.; Jarosz, Z. Review of the report on the nutritional and health-promoting values of species of the Rubus L. genus. Agriculture 2024, 14, 1324. [Google Scholar] [CrossRef]
- Azzini, E.; Barnaba, L.; Mattera, M.; Calina, D.; Sharifi-Rad, J.; Cho, W.C. Updated evidence on raspberries as functional foods: Anticancer bioactivity and therapeutic implications. Food Front. 2024, 5, 2351–2382. [Google Scholar] [CrossRef]
- Ispiryan, A.; Viškelis, J.; Viškelis, P.; Urbonavičienė, D.; Raudonė, L. Biochemical and antioxidant profiling of raspberry plant parts for sustainable processing. Plants 2023, 12, 2424. [Google Scholar] [CrossRef]
- Telichowska, A.; Kobus-Cisowska, J.; Szulc, P. Phytopharmacological possibilities of bird cherry Prunus padus L. and Prunus serotina L. species and their bioactive phytochemicals. Nutrients 2020, 12, 1966. [Google Scholar] [CrossRef]
- Rutkowska, M.; Witek, M.; Olszewska, M.A. A comprehensive review of molecular mechanisms, pharmacokinetics, toxicology and plant sources of juglanin: Current landscape and future perspectives. Int. J. Mol. Sci. 2024, 25, 10323. [Google Scholar] [CrossRef]
- Ademović, Z.; Hodžić, S.; Halilić-Zahirović, Z.; Husejnagić, D.; Džananović, J.; Šarić-Kundalić, B.; Suljagić, J. Phenolic compounds, antioxidant and antimicrobial properties of the wild cherry (Prunus avium L.) stem. Acta Period. Technol. 2017, 48, 1–13. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Chakraborty, S. A review on medicinal plants and its importance from glycosides. Int. J. Res. Appl. Sci. Eng. Technol. 2024, 12, 715–729. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Zymonė, K.; Liaudanskas, M.; Lanauskas, J.; Nagelytė, M.; Janulis, V. Variability in the qualitative and quantitative composition of phenolic compounds and the in vitro antioxidant activity of sour cherry (Prunus cerasus L.) leaves. Antioxidants 2024, 13, 553. [Google Scholar] [CrossRef]
- Tomar, O.; Akarca, G.; Gök, V.; İstek, Ö. Chemical composition and antifungal potential of apricot, sour cherry, and cherry tree bio-products (resins) against food-borne molds. Food Biosci. 2022, 47, 101627. [Google Scholar] [CrossRef]
- Krauze-Baranowska, M.; Głód, D.; Kula, M.; Majdan, M.; Hałasa, R.; Matkowski, A.; Kozłowska, W.; Kawiak, A. Chemical composition and biological activity of Rubus idaeus shoots—A traditional herbal remedy of Eastern Europe. BMC Complement. Altern. Med. 2014, 14, 480. [Google Scholar] [CrossRef]
- Cyboran-Mikołajczyk, S.; Męczarska, K.; Solarska-Ściuk, K.; Ratajczak-Wielgomas, K.; Oszmiański, J.; Jencova, V.; Bonarska-Kujawa, D. Protection of erythrocytes and microvascular endothelial cells against oxidative damage by Fragaria vesca L. and Rubus idaeus L. leaves extracts—The mechanism of action. Molecules 2022, 27, 5865. [Google Scholar] [CrossRef]
- Raal, A.; Vahtra, A.; Koshovyi, O.; Ilina, T.; Kovalyova, A.; Püssa, T. Polyphenolic compounds in the stems of raspberry (Rubus idaeus) growing wild and cultivated. Molecules 2024, 29, 5016. [Google Scholar] [CrossRef]
- Nastić, N.; Lozano-Sánchez, J.; Borrás-Linares, I.; Švarc-Gajić, J.; Segura-Carretero, A. New technological approaches for recovering bioactive food constituents from sweet cherry (Prunus avium L.) stems. Phytochem. Anal. 2020, 31, 119–130. [Google Scholar] [CrossRef]
- Brozdowski, J.; Waliszewska, B.; Gacnik, S.; Hudina, M.; Veberic, R.; Mikulic-Petkovsek, M. Phenolic composition of leaf and flower extracts of black cherry (Prunus serotina Ehrh.). Ann. For. Sci. 2021, 78, 66. [Google Scholar] [CrossRef]
- Jesus, F.; Gonçalves, A.C.; Alves, G.; Silva, L.R. Health benefits of Prunus avium plant parts: An unexplored source rich in phenolic compounds. Food Rev. Int. 2022, 38, 118–146. [Google Scholar] [CrossRef]
- Nunes, A.R.; Gonçalves, A.C.; Alves, G.; Falcão, A.; Garcia-Viguera, C.; Moreno, D.A.; Silva, L.R. Valorisation of Prunus avium L. by-products: Phenolic composition and effect on Caco-2 cells viability. Foods 2021, 10, 1185. [Google Scholar] [CrossRef]
- Costea, T.; Vlase, L.; Gostin, I.N.; Olah, N.K.; Predan, G.M.I. Botanical characterization, phytochemical analysis and antioxidant activity of indigenous red raspberry (Rubus idaeus L.) leaves. Stud. Univ. Vasile Goldis Ser. Stiintele Vietii 2016, 26, 463–472. [Google Scholar]
- Bastos, C.; Barros, L.; Dueñas, M.; Calhelha, R.C.; Queiroz, M.J.R.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Chemical characterisation and bioactive properties of Prunus avium L.: The widely studied fruits and the unexplored stems. Food Chem. 2015, 173, 1045–1053. [Google Scholar] [CrossRef]
- Dudzinska, D.; Luzak, B.; Boncler, M.; Rywaniak, J.; Sosnowska, D.; Podsedek, A.; Watala, C. CD39/NTPDase-1 expression and activity in human umbilical vein endothelial cells are differentially regulated by leaf extracts from Rubus caesius and Rubus idaeus. Cell. Mol. Biol. Lett. 2014, 19, 361–380. [Google Scholar] [CrossRef]
- Jakopič, J.; Štampar, F.; Veberič, R. Influence of hail net and reflective foil on cyanidin glycosides and quercetin glycosides in ‘Fuji’apple skin. HortScience 2010, 45, 1447–1452. [Google Scholar] [CrossRef]
- Alkhudaydi, H.M.S.; Muriuki, E.N.; Spencer, J.P. Determination of the polyphenol composition of raspberry leaf using LC-MS/MS. Molecules 2025, 30, 970. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Turkiewicz, I.P.; Tkacz, K. Profiling of polyphenols by LC-QTOF/ESI-MS, characteristics of nutritional compounds and in vitro effect on pancreatic lipase, α-glucosidase, α-amylase, cholinesterase and cyclooxygenase activities of sweet (Prunus avium) and sour (P. cerasus) cherries leaves and fruits. Ind. Crops Prod. 2021, 174, 114214. [Google Scholar] [CrossRef]
- Tian, Y.; Liimatainen, J.; Alanne, A.L.; Lindstedt, A.; Liu, P.; Sinkkonen, J.; Kallio, H.; Yang, B. Phenolic compounds extracted by acidic aqueous ethanol from berries and leaves of different berry plants. Food Chem. 2017, 220, 266–281. [Google Scholar] [CrossRef]
- Kanoun, K.; Belyagoubi-Benhammou, N.; Ghembaza, N.; Atik Bekkara, F. Comparative studies on antioxidant activities of extracts from the leaf, stem and berry of Myrtus communis L. Int. Food Res. J. 2014, 21, 1957–1962. [Google Scholar]
- Cvetanović, A.; Zengin, G.; Zeković, Z.; Švarc-Gajić, J.; Ražić, S.; Damjanović, A.; Mašković, P.; Mitić, M. Comparative in vitro studies of the biological potential and chemical composition of stems, leaves and berries Aronia melanocarpa’s extracts obtained by subcritical water extraction. Food Chem. Toxicol. 2018, 121, 458–466. [Google Scholar] [CrossRef]
- Willig, G.; Brunissen, F.; Brunois, F.; Godon, B.; Magro, C.; Monteux, C.; Peyrot, C.; Ioannou, I. Phenolic compounds extracted from cherry tree (Prunus avium) branches: Impact of the process on cosmetic properties. Antioxidants 2022, 11, 813. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Q.; Qiao, G.; Qiu, Z.; Wen, Z.; Wen, X. Optimizing the supercritical carbon dioxide extraction of sweet cherry (Prunus avium L.) leaves and UPLC-MS/MS analysis. Anal. Methods 2020, 12, 3004–3013. [Google Scholar] [CrossRef]
- Kniepkamp, K.; Errico, M.; Yu, M.; Roda-Serrat, M.C.; Eilers, J.G.; Wark, M.; van Haren, R. Lipid extraction of high-moisture sour cherry (Prunus cerasus L.) stones by supercritical carbon dioxide. J. Chem. Technol. Biotechnol. 2024, 99, 810–819. [Google Scholar] [CrossRef]
- Pavlić, B.; Aćimović, M.; Sknepnek, A.; Miletić, D.; Mrkonjić, Ž.; Kljakić, A.C.; Jerković, J.; Mišan, A.; Pojić, M.; Stupar, A.; et al. Sustainable raw materials for efficient valorization and recovery of bioactive compounds. Ind. Crops Prod. 2023, 193, 116167. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, H.; Lu, C.; Lin, H.; Li, Q. Optimization of ultrasound and microwave-assisted extraction of sweet cherry tree branches and chemical component analysis by UPLC–MS/MS. Trees—Struct. Funct. 2021, 35, 1247–1256. [Google Scholar] [CrossRef]
- Wang, L.; Lin, X.; Zhang, J.; Zhang, W.; Hu, X.; Li, W.; Li, C.; Liu, S. Extraction methods for the releasing of bound phenolics from Rubus idaeus L. leaves and seeds. Ind. Crops Prod. 2019, 135, 1–9. [Google Scholar] [CrossRef]
- Hałasa, R.; Turecka, K.; Mizerska, U.; Krauze-Baranowska, M. Anti-Helicobacter pylori biofilm extracts from Rubus idaeus and Rubus occidentalis. Pharmaceutics 2024, 16, 501. [Google Scholar] [CrossRef]
- Kotuła, M.; Kapusta-Duch, J.; Smoleń, S.; Doskočil, I. Phytochemical composition of the fruits and leaves of raspberries (Rubus idaeus L.)—Conventional vs. organic and those wild grown. Appl. Sci. 2022, 12, 11783. [Google Scholar] [CrossRef]
- Plasencia, P.; Finimundy, T.C.; Carocho, M.; Calhelha, R.C.; Añibarro-Ortega, M.; Pires, T.C.S.P.; Barreiro, F.; Garcia, P.A.; Barros, L.; Heleno, S.A. Extraction of bioactive compounds from Rubus idaeus bioresidues: A full screening on phenolic composition and bioactive potential. Waste Biomass Valor. 2025, 16, 737–747. [Google Scholar] [CrossRef]
- Maslov, O.; Komisarenko, M.; Kolisnyk, S.; Kostina, T.; Golik, M.; Moroz, V.; Tarasenko, D.; Akhmedov, E. Investigation of the extraction dynamic of the biologically active substances of the raspberry (Rubus idaeus L.) shoots. Curr. Issues Pharm. Med. Sci. 2023, 36, 194–198. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Z.; Li, X.; Abubaker, M.A.; Liu, X.; Li, Z.; Wang, X.; Zhu, X.; Zhang, J.; Chen, X. Comparative study of three raspberry cultivar (Rubus idaeus L.) leaves metabolites: Metabolome profiling and antioxidant activities. Appl. Sci. 2022, 12, 990. [Google Scholar] [CrossRef]
- Ruiz-Aquino, F.; Feria-Reyes, R.; Rutiaga-Quiñones, J.G.; Robledo-Taboada, L.H.; Gabriel-Parra, R. Characterization of tannin extracts derived from the bark of four tree species by HPLC and FTIR. For. Sci. Technol. 2023, 19, 38–46. [Google Scholar] [CrossRef]
- Agarwal, C.; Hofmann, T.; Vršanská, M.; Schlosserová, N.; Visi-Rajczi, E.; Voběrková, S.; Pásztory, Z. In vitro antioxidant and antibacterial activities with polyphenolic profiling of wild cherry, the european larch and sweet chestnut tree bark. Eur. Food Res. Technol. 2021, 247, 2355–2370. [Google Scholar] [CrossRef]
- Švarc-Gajić, J.; Clavijo, S.; Suárez, R.; Cvetanović, A.; Cerdà, V. Simultaneous dispersive liquid-liquid microextraction derivatisation and gas chromatography mass spectrometry analysis of subcritical water extracts of sweet and sour cherry stems. Anal. Bioanal. Chem. 2018, 410, 1943–1953. [Google Scholar] [CrossRef]
- Voss, M.; Gaudino, E.C.; Tabasso, S.; Forte, C.; Cravotto, G. Current emerging green technologies for the valorization of grape and cherry wastes. Curr. Food Sci. Technol. Rep. 2023, 1, 47–61. [Google Scholar] [CrossRef]
- Evtyugin, D.D.; Magina, S.; Evtuguin, D.V. Recent advances in the production and applications of ellagic acid and its derivatives. A review. Molecules 2020, 25, 2745. [Google Scholar] [CrossRef]
- Švarc-Gajić, J.; Cerdà, V.; Clavijo, S.; Suárez, R.; Mašković, P.; Cvetanović, A.; Delerue-Matos, C.; Carvalho, A.P.; Novakov, V. Bioactive compounds of sweet and sour cherry stems obtained by subcritical water extraction. J. Chem. Technol. Biotechnol. 2018, 93, 1627–1635. [Google Scholar] [CrossRef]
- Hamad, F.B.; Mubofu, E.B. Potential biological applications of bio-based anacardic acids and their derivatives. Int. J. Mol. Sci. 2015, 16, 8569–8590. [Google Scholar] [CrossRef]
- Li, Z.; Niu, L.; Chen, Y.; Qiu, X.; Du, T.; Zhu, M.; Wang, M.; Mo, H.; Xiao, S. Recent advance in the biological activity of chlorogenic acid and its application in food industry. Int. J. Food Sci. Technol. 2023, 58, 4931–4947. [Google Scholar] [CrossRef]
- Wang, L.; Pan, X.; Jiang, L.; Chu, Y.; Gao, S.; Jiang, X.; Zhang, Y.; Chen, Y.; Luo, S.; Peng, C. The biological activity mechanism of chlorogenic acid and its applications in food industry: A review. Front. Nutr. 2022, 9, 943911. [Google Scholar] [CrossRef]
- Peng, R.; Wu, Q.; Chen, J.; Ghosh, R.; Chen, X. Isolation of ellagic acid from pomegranate peel extract by hydrophobic interaction chromatography using graphene oxide grafted cotton fiber adsorbent. J. Sep. Sci. 2018, 41, 747–755. [Google Scholar] [CrossRef]
- García-Niño, W.R.; Zazueta, C. Ellagic acid: Pharmacological activities and molecular mechanisms involved in liver protection. Pharmacol. Res. 2015, 97, 84–103. [Google Scholar] [CrossRef]
- Tomou, E.M.; Papakyriakopoulou, P.; Skaltsa, H.; Valsami, G.; Kadoglou, N.P.E. Bio-actives from natural products with potential cardioprotective properties: Isolation, identification, and pharmacological actions of apigenin, quercetin, and silibinin. Molecules 2023, 28, 2387. [Google Scholar] [CrossRef]
- Kumar, K.S.; Sabu, V.; Sindhu, G.; Rauf, A.A.; Helen, A. Isolation, identification and characterization of apigenin from Justicia gendarussa and its anti-inflammatory activity. Int. Immunopharmacol. 2018, 59, 157–167. [Google Scholar] [CrossRef]
- Cvetanović, A.; Švarc-Gajić, J.; Gašić, U.; Tešić, Ž.; Zengin, G.; Zeković, Z.; Đurović, S. Isolation of apigenin from subcritical water extracts: Optimization of the process. J. Supercrit. Fluids 2017, 120, 32–42. [Google Scholar] [CrossRef]
- Mehdi, A.; Al-ani, W.M.K.; Raoof, A. Isolation of astragalin from IRAQI Chenopodium album. Asian J. Pharm. Clin. Res. 2018, 11, 530–535. [Google Scholar] [CrossRef]
- Ruan, J.; Shi, Z.; Cao, X.; Dang, Z.; Zhang, Q.; Zhang, W.; Wu, L.; Zhang, Y.; Wang, T. Research progress on anti-inflammatory effects and related mechanisms of astragalin. Int. J. Mol. Sci. 2024, 25, 4476. [Google Scholar] [CrossRef]
- Jeyanthi, V.; Anbu, P.; Vairamani, M.; Velusamy, P. Isolation of hydroquinone (benzene-1,4-diol) metabolite from halotolerant Bacillus methylotrophicus MHC10 and its inhibitory activity towards bacterial pathogens. Bioprocess Biosyst. Eng. 2016, 39, 429–439. [Google Scholar] [CrossRef]
- Enguita, F.J.; Leitão, A.L. Hydroquinone: Environmental pollution, toxicity, and microbial answers. BioMed Res. Int. 2013, 2013, 542168. [Google Scholar] [CrossRef]
- Raza, M.J.; Jalil, O.; Kumar, D.; Pandey, C.M. Highly sensitive electrochemical detection of hydroquinone in wastewater using ionic liquid grafted rGO-ZrO2 nanohybrid-based conducting paper. J. Electrochem. Soc. 2025, 172, 067508. [Google Scholar] [CrossRef]
- Cao, X.; Wei, Y.; Ito, Y. Preparative isolation of isorhamnetin from Stigma Maydis using high speed countercurrent chromatography. J. Liq. Chromatogr. Relat. Technol. 2009, 32, 273–280. [Google Scholar] [CrossRef]
- Mei, C.; Liu, Y.; Lyu, X.; Jiang, Z.; Liu, Z.; Zhi, Y.; Xu, X.; Wang, H. Advances in isorhamnetin treatment of malignant tumors: Mechanisms and applications. Nutrients 2025, 17, 1853. [Google Scholar] [CrossRef]
- Duan, M.; Wang, X.; Feng, J.; Xiao, X.; Zhang, L.; He, S.; Ma, L.; Wang, X.; Yang, S.; Rao, M.J. From agricultural waste to functional tea: Optimized processing enhances bioactive flavonoid recovery and antioxidant capacity with multifaceted health benefits in loquat (Eriobotrya japonica Lindl.) flowers. Horticulturae 2025, 11, 766. [Google Scholar] [CrossRef]
- Nguelefack, T.B.; Mbakam, F.H.K.; Tapondjou, L.A.; Watcho, P.; Nguelefack-Mbuyo, E.P.; Ponou, B.K.; Kamanyi, A.; Park, H.J. A dimeric triterpenoid glycoside and flavonoid glycosides with free radical-scavenging activity isolated from Rubus rigidus var. camerunensis. Arch. Pharmacal Res. 2011, 34, 543–550. [Google Scholar] [CrossRef]
- Gajjar, N.D.; Dhameliya, T.M.; Shah, G.B. In search of RdRp and Mpro inhibitors against SARS CoV-2: Molecular docking, molecular dynamic simulations and ADMET analysis. J. Mol. Struct. 2021, 1239, 130488. [Google Scholar] [CrossRef]
- Wahab, A.; Begum, S.; Mahmood, I.; Mahmood, T.; Ahmad, A.; Fayyaz, N. Luteolin and kaempferol from Cassia Alata. Antimicrobial and antioxidant activity of its methanolic extracts. FUUAST J. Biol. 2014, 4, 1–5. [Google Scholar]
- Bangar, S.P.; Chaudhary, V.; Sharma, N.; Bansal, V.; Ozogul, F.; Lorenzo, J.M. Kaempferol: A flavonoid with wider biological activities and its applications. Crit. Rev. Food Sci. Nutr. 2023, 63, 9580–9604. [Google Scholar] [CrossRef]
- Montenegro, I.; Pérez, C.; González, B.; Domínguez, Á.; Gómez, E. Thermal characterization and heat capacities of seven polyphenols. Molecules 2025, 30, 199. [Google Scholar] [CrossRef]
- Sudto, K.; Pornpakakul, S.; Wanichwecharungruang, S. An efficient method for the large scale isolation of naringin from pomelo (Citrus grandis) peel. Int. J. Food Sci. Technol. 2009, 44, 1737–1742. [Google Scholar] [CrossRef]
- Koirala, M.; Lee, Y.K.; Kim, M.S.; Chung, Y.C.; Park, J.S.; Kim, S.Y. Biotransformation of naringenin by Bacillus amyloliquefaciens into three naringenin derivatives. Nat. Prod. Commun. 2019, 14, 465–472. [Google Scholar] [CrossRef]
- Zhang, L.; Song, L.; Zhang, P.; Liu, T.; Zhou, L.; Yang, G.; Lin, R.; Zhang, J. Solubilities of naringin and naringenin in different solvents and dissociation constants of naringenin. J. Chem. Eng. Data 2015, 60, 932–940. [Google Scholar] [CrossRef]
- Kylli, P.; Nohynek, L.; Puupponen-Pimiä, R.; Westerlund-Wikström, B.; Leppänen, T.; Welling, J.; Moilanen, E.; Heinonen, M. Lingonberry (Vaccinium vitis-idaea) and European cranberry (Vaccinium microcarpon) proanthocyanidins: Isolation, identification, and bioactivities. J. Agric. Food Chem. 2011, 59, 3373–3384. [Google Scholar] [CrossRef]
- Chen, S.; Song, J.; Du, L.; Ma, Y.; Ren, S.; Ren, J.; Li, S. Quantitative analysis of solubility parameters and surface properties of larch bark proanthocyanidins. Polymers 2020, 12, 2800. [Google Scholar] [CrossRef]
- Choi, J.S.; Yokozawa, T.; Oura, H. Improvement of hyperglycemia and hyperlipemia in streptozotocin-diabetic rats by a methanolic extract of Prunus davidiana stems and its main component, prunin. Planta Med. 1991, 57, 208–211. [Google Scholar] [CrossRef]
- Céliz, G.; Daz, M. Biocatalytic preparation of alkyl esters of citrus flavanone glucoside prunin in organic media. Process Biochem. 2011, 46, 94–100. [Google Scholar] [CrossRef]
- Dall’Acqua, S.; Miolo, G.; Innocenti, G.; Caffieri, S. The photodegradation of quercetin: Relation to oxidation. Molecules 2012, 17, 8898–8907. [Google Scholar] [CrossRef]
- Ramešová, Š.; Sokolová, R.; Degano, I.; Bulíčková, J.; Žabka, J.; Gál, M. On the stability of the bioactive flavonoids quercetin and luteolin under oxygen-free conditions. Anal. Bioanal. Chem. 2012, 402, 975–982. [Google Scholar] [CrossRef]
- Yingyuen, P.; Sukrong, S.; Phisalaphong, M. Isolation, separation and purification of rutin from banana leaves (Musa balbisiana). Ind. Crops Prod. 2020, 149, 112307. [Google Scholar] [CrossRef]
- Baldisserotto, A.; Vertuani, S.; Bino, A.; De Lucia, D.; Lampronti, I.; Milani, R.; Gambari, R.; Manfredini, S. Design, synthesis and biological activity of a novel rutin analogue with improved lipid soluble properties. Bioorg. Med. Chem. 2015, 23, 264–271. [Google Scholar] [CrossRef]
- Choi, S.S.; Park, H.R.; Lee, K.A.A. Comparative study of rutin and rutin glycoside: Antioxidant activity, anti-inflammatory effect, effect on platelet aggregation and blood coagulation. Antioxidants 2021, 10, 1696. [Google Scholar] [CrossRef]
- Choe, U.; Li, Y.; Yu, L.; Gao, B.; Wang, T.T.Y.; Sun, J.; Chen, P.; Yu, L. Chemical composition of cold-pressed blackberry seed flour extract and its potential health-beneficial properties. Food Sci. Nutr. 2020, 8, 1215–1225. [Google Scholar] [CrossRef]
- Firmansyah, A.; Winingsih, W.; Manobi, J.D.Y. Review of scopoletin: Isolation, analysis process, and pharmacological activity. Biointerface Res. Appl. Chem. 2021, 11, 12006–12019. [Google Scholar] [CrossRef]
- Galán-Pérez, J.A.; Gámiz, B.; Celis, R. Determining the effect of soil properties on the stability of scopoletin and its toxicity to target plants. Biol. Fertil. Soils 2021, 57, 643–655. [Google Scholar] [CrossRef]
- Chaaban, H.; Ioannou, I.; Chebil, L.; Slimane, M.; Gérardin, C.; Paris, C.; Charbonnel, C.; Chekir, L.; Ghoul, M. Effect of heat processing on thermal stability and antioxidant activity of six flavonoids. J. Food Process. Preserv. 2017, 41, e13203. [Google Scholar] [CrossRef]
- Hemshekhar, M.; Sebastin Santhosh, M.; Kemparaju, K.; Girish, K.S. Emerging roles of anacardic acid and its derivatives: A pharmacological overview. Basic Clin. Pharmacol. Toxicol. 2012, 110, 122–132. [Google Scholar] [CrossRef]
- Rosa, M.E.P.; Rebouças, L.M.; Marques, S.P.D.; Silva, L.M.R.; Cunha, F.E.T.; Costa, P.M.S.; de Assis, D.A.; Silveira, K.B.; Muniz, C.R.; Trevisan, M.T.S.; et al. Sodium hyaluronate microcapsules to promote antitumor selectivity of anacardic acid. Int. J. Biol. Macromol. 2025, 296, 139616. [Google Scholar] [CrossRef]
- Schultz, D.J.; Krishna, A.; Vittitow, S.L.; Alizadeh-Rad, N.; Muluhngwi, P.; Rouchka, E.C.; Klinge, C.M. Transcriptomic response of breast cancer cells to anacardic acid. Sci. Rep. 2018, 8, 8063. [Google Scholar] [CrossRef]
- Anjum, M.M.; Patel, K.K.; Pandey, N.; Tilak, R.; Agrawal, A.K.; Singh, S. Development of anacardic acid/hydroxypropyl-β-cyclodextrin inclusion complex with enhanced solubility and antimicrobial activity. J. Mol. Liq. 2019, 296, 112085. [Google Scholar] [CrossRef]
- Jit, T.; Deb Roy, S.; Shil, D.; Chakraborty, J.; Paul, A.; Roy, S.; De, D. The potential of tannins from medicinal plants as anti-cancer agents. J. Med. Plants Stud. 2024, 12, 414423. [Google Scholar]
- Radha, R.; Prakash, S.; Kumari, N.; Sharma, N.; Puri, S.; Singh, J.; Thakur, M.; Kumar, M. Bioactives and bioactivities from food byproducts. Curr. Food Sci. Technol. Rep. 2024, 2, 297–308. [Google Scholar] [CrossRef]
- Ko, H.; Jeon, H.; Lee, D.; Choi, H.K.; Kang, K.S.; Choi, K.C. Sanguiin H6 suppresses TGF-β induction of the epithelial–mesenchymal transition and inhibits migration and invasion in A549 lung cancer. Bioorg. Med. Chem. Lett. 2015, 25, 5508–5513. [Google Scholar] [CrossRef]
- Gesek, J.; Jakimiuk, K.; Atanasov, A.G.; Tomczyk, M. Sanguiins—Promising molecules with broad biological potential. Int. J. Mol. Sci. 2021, 22, 12972. [Google Scholar] [CrossRef]
- Park, E.J.; Lee, D.; Baek, S.E.; Kim, K.H.; Kang, K.S.; Jang, T.S.; Lee, H.L.; Song, J.H.; Yoo, J.E. Cytotoxic effect of sanguiin H-6 on MCF-7 and MDA-MB-231 human breast carcinoma cells. Bioorg. Med. Chem. Lett. 2017, 27, 4389–4392. [Google Scholar] [CrossRef]
- Huang, Z.Q.; Chen, P.; Su, W.W.; Wang, Y.G.; Wu, H.; Peng, W.; Li, P.B. Antioxidant activity and hepatoprotective potential of quercetin 7-rhamnoside in vitro and in vivo. Molecules 2018, 23, 1188. [Google Scholar] [CrossRef]
- Deng, D.; Zhao, B.; Yang, H.; Wang, S.; Geng, Z.; Zhou, J.; Yang, G.; Han, L. Investigating the effect and potential mechanism of rhamnetin 3-o-α-rhamnoside on acute liver injury in vivo and in vitro. Pharmaceuticals 2025, 18, 116. [Google Scholar] [CrossRef]
- Li, L.; Lei, X.; Chen, L.; Ma, Y.; Luo, J.; Liu, X.; Xu, X.; Zhou, G.; Feng, X. Protective Mechanism of quercetin compounds against acrylamide-induced hepatotoxicity. Food Sci. Hum. Wellness 2024, 13, 225–240. [Google Scholar] [CrossRef]
- Choi, H.J.; Song, J.H.; Park, K.S.; Kwon, D.H. Inhibitory effects of quercetin 3-rhamnoside on influenza a virus replication. Eur. J. Pharm. Sci. 2009, 37, 329–333. [Google Scholar] [CrossRef]
- Meng, X.; Xia, C.; Wu, H.; Gu, Q.; Li, P. Metabolism of quercitrin in the colon and its beneficial regulatory effects on gut microbiota. J. Sci. Food Agric. 2024, 104, 9255–9264. [Google Scholar] [CrossRef]
- Reinboth, M.; Wolffram, S.; Abraham, G.; Ungemach, F.R.; Cermak, R. Oral bioavailability of quercetin from different quercetin glycosides in dogs. Br. J. Nutr. 2010, 104, 198–203. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, inflammation and immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Cao, M.M.; Guo, Z.; Wang, J.; Ma, H.Y.; Qin, X.Y.; Hu, Y.; Lan, R. Astragalin alleviates lipopolysaccharide-induced depressive-like behavior in mice by preserving blood-brain barrier integrity and suppressing neuroinflammation. Free Radic. Biol. Med. 2025, 232, 340–352. [Google Scholar] [CrossRef]
- Li, Q.; Yang, Z.; Lu, H.; Liu, F.; Zhou, D.; Zou, Y. Astragalin Exerted hypoglycemic effect by both inhibiting α-glucosidase and modulating AMPK signaling pathway. Nutrients 2025, 17, 406. [Google Scholar] [CrossRef]
- Riaz, A.; Rasul, A.; Hussain, G.; Zahoor, M.K.; Jabeen, F.; Subhani, Z.; Younis, T.; Ali, M.; Sarfraz, I.; Selamoglu, Z. Astragalin: A bioactive phytochemical with potential therapeutic activities. Adv. Pharmacol. Pharm. Sci. 2018, 2018, 9794625. [Google Scholar] [CrossRef]
- Zeng, W.; Chen, L. Astragalin inhibits the proliferation of high-risk HPV-positive cervical epithelial cells and attenuates malignant cervical lesions. Cytotechnology 2025, 77, 80. [Google Scholar] [CrossRef]
- Li, C.; Hu, M.; Jiang, S.; Liang, Z.; Wang, J.; Liu, Z.; Wang, H.M.D.; Kang, W. Evaluation procoagulant activity and mechanism of astragalin. Molecules 2020, 25, 177. [Google Scholar] [CrossRef]
- Yang, C.Z.; Wang, S.H.; Zhang, R.H.; Lin, J.H.; Tian, Y.H.; Yang, Y.Q.; Liu, J.; Ma, Y.X. Neuroprotective effect of astragalin via activating PI3K/Akt-MTOR-mediated autophagy on APP/PS1 mice. Cell Death Discov. 2023, 9, 15. [Google Scholar] [CrossRef]
- Chen, J.; Zhong, K.; Qin, S.; Jing, Y.; Liu, S.; Li, D.; Peng, C. Astragalin: A food-origin flavonoid with therapeutic effect for multiple diseases. Front. Pharmacol. 2023, 14, 1265960. [Google Scholar] [CrossRef]
- Duszka, K.; Clark, B.F.C.; Massino, F.; Barciszewski, J. Biological activities of kinetin. In Herbal Drugs: Ethnomedicine to Modern Medicine; Ramawat, K.G., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 369–380. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, L.; Chen, G.; Feng, X.; Liu, Y.; Gao, Q.; Mai, M.; Chen, C.; Yu, C.; Ye, S.; et al. Discovery of kinetin in inhibiting colorectal cancer progression via enhancing PSMB1-mediated RAB34 degradation. Cancer Lett. 2024, 584, 216600. [Google Scholar] [CrossRef]
- Souza, T.M.L.; Pinho, V.D.; Setim, C.F.; Sacramento, C.Q.; Marcon, R.; Fintelman-Rodrigues, N.; Chaves, O.A.; Heller, M.; Temerozo, J.R.; Ferreira, A.C.; et al. Preclinical development of kinetin as a safe error-prone SARS-CoV-2 antiviral able to attenuate virus-induced inflammation. Nat. Commun. 2023, 14, 199. [Google Scholar] [CrossRef]
- Chandorkar, Y.; Valeske, M.; Kolrosova, B.; Elbs-Glatz, Y.; Zuber, F.; Schoeller, J.; Kummer, N.; Ren, Q.; Rottmar, M.; Maniura-Weber, K. Bioactive salicylic acid containing coating for dental implants to combat infection and inflammation. Adv. Mater. Interfaces 2024, 11, 2300750. [Google Scholar] [CrossRef]
- Miclaus, M.O.; Borodi, G.; Turza, A. Four polymorphs of the bioactive diuretic drug 4-chloro-5-chlorosulfonyl salicylic acid. Crystals 2025, 15, 136. [Google Scholar] [CrossRef]
- Nagelschmitz, J.; Blunck, M.; Kraetzschmar, J.; Ludwig, M.; Wensing, G.; Hohlfeld, T. Pharmacokinetics and pharmacodynamics of acetylsalicylic acid after intravenous and oral administration to healthy volunteers. Clin. Pharmacol.: Adv. Appl. 2014, 6, 51–59. [Google Scholar] [CrossRef]
- Madan, R.K.; Levitt, J. A review of toxicity from topical salicylic acid preparations. J. Am. Acad. Dermatol. 2014, 70, 788–792. [Google Scholar] [CrossRef]
- Varvara, M.; Bozzo, G.; Celano, G.; Disanto, C.; Pagliarone, C.N.; Celano, G.V. The use of ascorbic acid as a food additive: Technical-legal issues. Ital. J. Food Saf. 2016, 5, 4313. [Google Scholar] [CrossRef]
- Davis, J.L.; Paris, H.L.; Beals, J.W.; Binns, S.E.; Giordano, G.R.; Scalzo, R.L.; Schweder, M.M.; Blair, E.; Bell, C. Liposomal-encapsulated ascorbic acid: Influence on vitamin C bioavailability and capacity to protect against ischemia-reperfusion injury. Nutr. Metab. Insights 2016, 9, 25–30. [Google Scholar] [CrossRef]
- Stephenson, C.M.; Levin, R.D.; Spector, T.; Lis, C.G. Phase I clinical trial to evaluate the safety, tolerability, and pharmacokinetics of high-dose intravenous ascorbic acid in patients with advanced cancer. Cancer Chemother. Pharmacol. 2013, 72, 139–146. [Google Scholar] [CrossRef]
- Bhatt, S.C.; Naik, B.; Kumar, V.; Kumar Gupta, A.; Kumar, S.; Singh Preet, M.; Sharma, N.; Rustagi, S. Untapped potential of non-conventional Rubus species: Bioactivity, nutrition, and livelihood opportunities. Plant Methods 2023, 19, 114. [Google Scholar] [CrossRef]
- Borel, P.; Preveraud, D.; Desmarchelier, C. Bioavailability of vitamin E in humans: An update. Nutr. Rev. 2013, 71, 319–331. [Google Scholar] [CrossRef]
- Gad, S.C. Hydroquinone. In Encyclopedia of Toxicology, 4th ed.; Wexler, P., Ed.; Academic Press: Cambridge, MA, USA, 2024; Volume 5, pp. 425–430. [Google Scholar] [CrossRef]
- Fabian, I.M.; Sinnathamby, E.S.; Flanagan, C.J.; Lindberg, A.; Tynes, B.; Kelkar, R.A.; Varrassi, G.; Ahmadzadeh, S.; Shekoohi, S.; Kaye, A.D. Topical hydroquinone for hyperpigmentation: A narrative review. Cureus 2023, 15, e48840. [Google Scholar] [CrossRef]
- Banodkar, P.D.; Banodkar, K.P. History of hydroquinone. Indian J. Dermatol. Venereol. Leprol. 2022, 88, 696–699. [Google Scholar] [CrossRef]
- Shivaram, K.; Edwards, K.; Mohammad, T.F. An update on the safety of hydroquinone. Arch. Dermatol. Res. 2024, 316, 378. [Google Scholar] [CrossRef]
- Serrano, D.R.; Gordo, M.J.; Matji, A.; González, S.; Lalatsa, A.; Torrado, J.J. Tuning the transdermal delivery of hydroquinone upon formulation with novel permeation enhancers. Pharmaceutics 2019, 11, 167. [Google Scholar] [CrossRef]
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Gondal, T.A.; Saeed, F.; Imran, A.; Shahbaz, M.; Fokou, P.V.T.; Arshad, M.U.; Khan, H.; et al. Kaempferol: A key emphasis to its anticancer potential. Molecules 2019, 24, 2277. [Google Scholar] [CrossRef]
- Periferakis, A.; Periferakis, K.; Badarau, I.A.; Petran, E.M.; Popa, D.C.; Caruntu, A.; Costache, R.S.; Scheau, C.; Caruntu, C.; Costache, D.O. Kaempferol: Antimicrobial properties, sources, clinical, and traditional applications. Int. J. Mol. Sci. 2022, 23, 15054. [Google Scholar] [CrossRef]
- Calderón-Montaño, J.M.; Burgos-Morón, E.; Pérez-Guerrero, C.; López-Lázaro, M. A review on the dietary flavonoid kaempferol. Mini-Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef]
- Li, Q.; Ge, C.; Tan, J.; Sun, Y.; Kuang, Q.; Dai, X.; Zhong, S.; Yi, C.; Hu, L.F.; Lou, D.S.; et al. Juglanin protects against high fat diet-induced renal injury by suppressing inflammation and dyslipidemia via regulating NF-ΚB/HDAC3 signaling. Int. Immunopharmacol. 2021, 95, 107340. [Google Scholar] [CrossRef]
- Ren, Y.; Hu, S.; Pu, H.; Zhou, Y.; Jiang, M.; Li, Y.; Deng, C.; Gao, J.; Xu, M.; Ge, C. Juglanin Ameliorates depression-like behavior in chronic unpredictable mild stress-induced mice by improving AMPK signaling. J. Funct. Foods 2022, 98, 105263. [Google Scholar] [CrossRef]
- Wang, P.; Hu, M.; Wang, L.; Qu, J.; Liu, Y.; Li, C.; Liu, Z.; Ma, C.; Kang, W. Chemical constituents and coagulation effects of the flowers of Rosa chinensis Jacq. J. Future Foods 2023, 3, 155–162. [Google Scholar] [CrossRef]
- Gong, G.; Guan, Y.Y.; Zhang, Z.L.; Rahman, K.; Wang, S.J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A review of pharmacological effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef]
- González-Arceo, M.; Gomez-Lopez, I.; Carr-Ugarte, H.; Eseberri, I.; González, M.; Cano, M.P.; Portillo, M.P.; Gómez-Zorita, S. Anti-obesity effects of isorhamnetin and isorhamnetin conjugates. Int. J. Mol. Sci. 2022, 24, 299. [Google Scholar] [CrossRef]
- Dayem, A.A.; Choi, H.Y.; Kim, Y.B.; Cho, S.G. Antiviral effect of methylated flavonol isorhamnetin against influenza. PLoS ONE 2015, 10, e0121610. [Google Scholar] [CrossRef]
- Ku, S.K.; Kim, T.H.; Bae, J.S. Anticoagulant activities of persicarin and isorhamnetin. Vascul. Pharmacol. 2013, 58, 272–279. [Google Scholar] [CrossRef]
- Scarlata, G.G.M.; Lopez, I.; Gambardella, M.L.; Milanović, M.; Milić, N.; Abenavoli, L. Preventive and therapeutic effects of baicalein, galangin, and isorhamnetin in chronic liver diseases: A narrative review. Molecules 2025, 30, 1253. [Google Scholar] [CrossRef]
- Huang, Z.R.; Lin, Y.K.; Fang, J.Y. Biological and pharmacological activities of squalene and related compounds: Potential uses in cosmetic dermatology. Molecules 2009, 14, 540–554. [Google Scholar] [CrossRef]
- Shalu, S.; Raveendranathan, P.K.; Vaidyanathan, V.K.; Blank, L.M.; Germer, A.; Balakumaran, P.A. Microbial squalene: A sustainable alternative for the cosmetics and pharmaceutical industry – A review. Eng. Life Sci. 2024, 24, e202400003. [Google Scholar] [CrossRef]
- Maxim, C.; Turcov, D.; Bulgariu, A.G.; Șuteu, D. Squalene—Background and perspectives in cosmeceuticals formulas. Bul. Inst. Polit. Iasi 2024, 70, 47–57. [Google Scholar] [CrossRef]
- Hussain, S.; Javed, M.; Abid, M.A.; Khan, M.A.; Syed, S.K.; Faizan, M.; Feroz, F. Prunus avium L.; Phytochemistry, nutritional and pharmacological review. Adv. Life Sci. 2021, 8, 307–314. [Google Scholar] [CrossRef]
- Kumari, N.; Radha; Kumar, M.; Puri, S.; Zhang, B.; Rais, N.; Pundir, A.; Chandran, D.; Raman, P.; Dhumal, S.; et al. Peach (Prunus persica (L.) Batsch) seeds and kernels as potential plant-based functional food ingredients: A review of bioactive compounds and health-promoting activities. Food Biosci. 2023, 54, 102914. [Google Scholar] [CrossRef]
- Kajla, V.; Singh, B.; Muskaan, S.K.D.; Sharma, P.; Vishali, M.; Sayam, A. Harnessing amygdalin in integrative medicine: Novel insights for endocrine disorders. Rev. Argent. Clin. Psicol. 2024, 33, 54–73. [Google Scholar] [CrossRef]
- Mungamuri, S.K.; Chatterjee, N.; Ara, D. Phytotherapy for liver fibrosis: Insights from the biology of hepatic stellate cells—A narrative review. Liver Int. Commun. 2025, 6, e70015. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, S.; Wen, Q.; Xia, Q.; Wang, S.; Chen, G.; Sun, J.; Shen, C.; Song, S. Interactions between Ephedra sinica and Prunus armeniaca: From stereoselectivity to deamination as a metabolic detoxification mechanism of amygdalin. Front. Pharmacol. 2021, 12, 744624. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Acute health risks related to the presence of cyanogenic glycosides in raw apricot kernels and products derived from raw apricot kernels. EFSA J. 2016, 14, e04424. [Google Scholar] [CrossRef]
- Das, A.; Baidya, R.; Chakraborty, T.; Samanta, A.K.; Roy, S. Pharmacological basis and new insights of taxifolin: A comprehensive review. Biomed. Pharmacother. 2021, 142, 112004. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, X.; Tian, Y.; Zhai, S.; Liu, Y.; Xiong, Z.; Chu, S. An insight into novel therapeutic potentials of taxifolin. Front. Pharmacol. 2023, 14, 1173855. [Google Scholar] [CrossRef]
- Zu, Y.; Wu, W.; Zhao, X.; Li, Y.; Wang, W.; Zhong, C.; Zhang, Y.; Zhao, X. Enhancement of solubility, antioxidant ability and bioavailability of taxifolin nanoparticles by liquid antisolvent precipitation technique. Int. J. Pharm. 2014, 471, 366–376. [Google Scholar] [CrossRef]
- Rajnochová Svobodová, A.; Ryšavá, A.; Psotová, M.; Kosina, P.; Zálešák, B.; Ulrichová, J.; Vostálová, J. The phototoxic potential of the flavonoids, taxifolin and quercetin. Photochem. Photobiol. 2017, 93, 1240–1247. [Google Scholar] [CrossRef]
- Patel, K.; Patel, D.K. Biological potential of aromadendrin against human disorders: Recent development in pharmacological activities and analytical aspects. Pharmacol. Res. Mod. Chin. Med. 2024, 11, 100424. [Google Scholar] [CrossRef]
- El-Shiekh, R.A.; Radi, M.H.; Abdel-Sattar, E. Unveiling the therapeutic potential of aromadendrin (AMD): A promising anti-inflammatory agent in the prevention of chronic diseases. Inflammopharmacology 2025, 33, 1209–1220. [Google Scholar] [CrossRef]
- Fernandes, F.H.; Guterres, Z.D.R.; Corsino, J.; Garcez, W.S.; Garcez, F.R. Assessment of the mutagenicity of propolis compounds from the Brazilian Cerrado biome in somatic cells of Drosophila melanogaster. Orbital: Electron. J. Chem. 2019, 11, 307–313. [Google Scholar] [CrossRef]
- Salehi, B.; Fokou, P.V.T.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The therapeutic potential of naringenin: A review of clinical trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef]
- Orhan, I.E.; Nabavi, S.F.; Daglia, M.; Tenore, G.C.; Mansouri, K.; Nabavi, S.M. Naringenin and atherosclerosis: A review of literature. Curr. Pharm. Biotechnol. 2015, 16, 245–251. [Google Scholar] [CrossRef]
- Hernández-Aquino, E.; Muriel, P. Beneficial effects of naringenin in liver diseases: Molecular mechanisms. World J. Gastroenterol. 2018, 24, 1679. [Google Scholar] [CrossRef]
- Abdelkawy, Y.S.; Elharoun, M.; Sheta, E.; Abdel-Raheem, I.T.; Nematalla, H.A. Liraglutide and naringenin relieve depressive symptoms in mice by enhancing neurogenesis and reducing inflammation. Eur. J. Pharmacol. 2024, 971, 176525. [Google Scholar] [CrossRef]
- Rebello, C.J.; Beyl, R.A.; Lertora, J.J.L.; Greenway, F.L.; Ravussin, E.; Ribnicky, D.M.; Poulev, A.; Kennedy, B.J.; Castro, H.F.; Campagna, S.R.; et al. Safety and pharmacokinetics of naringenin: A randomized, controlled, single-ascending-dose clinical trial. Diabetes Obes. Metab. 2020, 22, 91–98. [Google Scholar] [CrossRef]
- Pérez-Coll, C.S.; Herkovits, J. Lethal and teratogenic effects of naringenin evaluated by means of an amphibian embryo toxicity test (AMPHITOX). Food Chem. Toxicol. 2004, 42, 299–306. [Google Scholar] [CrossRef]
- Monadi, T.; Mohajer, Z.; Soltani, A.; Khazeei Tabari, M.A.; Manayi, A.; Azadbakht, M. The influence of apigenin on cellular responses to radiation: From protection to sensitization. Biofactors 2024, 51, e2113. [Google Scholar] [CrossRef]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The therapeutic potential of apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef]
- Allemailem, K.S.; Almatroudi, A.; Alharbi, H.O.A.; AlSuhaymi, N.; Alsugoor, M.H.; Aldakheel, F.M.; Khan, A.A.; Rahmani, A.H. Apigenin: A bioflavonoid with a promising role in disease prevention and treatment. Biomedicines. 2024, 12, 1353. [Google Scholar] [CrossRef]
- DeRango-Adem, E.F.; Blay, J. Does oral apigenin have real potential for a therapeutic effect in the context of human gastrointestinal and other cancers? Front. Pharmacol. 2021, 12, 681477. [Google Scholar] [CrossRef]
- Lee, J.A.; Ha, S.K.; Kim, Y.C.; Choi, I. Effects of friedelin on the intestinal permeability and bioavailability of apigenin. Pharmacol. Rep. 2017, 69, 1044–1048. [Google Scholar] [CrossRef]
- Rana, J.N.; Mumtaz, S. Prunin: An emerging anticancer flavonoid. Int. J. Mol. Sci. 2025, 26, 2678. [Google Scholar] [CrossRef]
- Gunaseelan, S.; Wong, K.Z.; Min, N.; Sun, J.; Ismail, N.K.B.M.; Tan, Y.J.; Lee, R.C.H.; Chu, J.J.H. Prunin suppresses viral IRES activity and is a potential candidate for treating enterovirus A71 infection. Sci. Transl. Med. 2019, 11, eaar5759. [Google Scholar] [CrossRef]
- Patel, D.K.; Patel, K. Biological importance of prunin in the medicine for the treatment of diabetes related complication: Therapeutic benefit through data analysis. Metabolism 2022, 128, 155057. [Google Scholar] [CrossRef]
- Guo, F.; Yan, D.; Qin, Z.; Bais, S. Prunin modulates the expression of cerebral serotonin induced by anxiety-like behavior in mice. Nat. Prod. Commun. 2021, 16, 1–9. [Google Scholar] [CrossRef]
- Pan, L.; Ye, H.; Pi, X.; Liu, W.; Wang, Z.; Zhang, Y.; Zheng, J. Effects of several flavonoids on human gut microbiota and its metabolism by in vitro simulated fermentation. Front. Microbiol. 2023, 14, 1092729. [Google Scholar] [CrossRef]
- Mani, R.; Natesan, V. Chrysin: Sources, beneficial pharmacological activities, and molecular mechanism of action. Phytochemistry 2018, 145, 187–196. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Braidy, N.; Habtemariam, S.; Orhan, I.E.; Daglia, M.; Manayi, A.; Gortzi, O.; Nabavi, S.M. Neuroprotective effects of chrysin: From chemistry to medicine. Neurochem. Int. 2015, 90, 224–231. [Google Scholar] [CrossRef]
- Siddiqui, A.; Badruddeen; Akhtar, J.; Shahab Uddin, M.S.; Khan, M.I.; Khalid, M.; Ahmad, M. A Naturally occurring flavone (chrysin): Chemistry, occurrence, pharmacokinetic, toxicity, molecular targets and medicinal properties. J. Biol. Act. Prod. Nat. 2018, 8, 208–227. [Google Scholar] [CrossRef]
- Islam, A.; Islam, M.S.; Uddin, M.N.; Hasan, M.M.I.; Akanda, M.R. The potential health benefits of the isoflavone glycoside genistin. Arch. Pharmacal Res. 2020, 43, 395–408. [Google Scholar] [CrossRef]
- Tang, X.; Liao, R.; Zhou, L.; Yi, T.; Ran, M.; Luo, J.; Huang, F.; Wu, A.; Mei, Q.; Wang, L.; et al. Genistin: A novel estrogen analogue targeting ERβ to alleviate thrombocytopenia. Int. J. Biol. Sci. 2024, 20, 2236–2260. [Google Scholar] [CrossRef]
- Jaiswal, N.; Akhtar, J.; Singh, S.P.; Ahsan, F. An overview on genistein and its various formulations. Drug Res. 2019, 69, 305–313. [Google Scholar] [CrossRef]
- Arya, S.S.; Rookes, J.E.; Cahill, D.M.; Lenka, S.K. Vanillin: A review on the therapeutic prospects of a popular flavouring molecule. Adv. Tradit. Med. 2021, 21, 1–17. [Google Scholar] [CrossRef]
- Huang, W.; Yang, Y.; Wen, W.; Luo, Y.; Wu, J.; Xiang, L.; Hu, Y.; Xu, S.; Chen, S.; Wang, P. Vanillin enhances the passive transport rate and absorption of drugs with moderate oral bioavailability in vitro and in vivo by affecting the membrane structure. Food Funct. 2020, 11, 700–710. [Google Scholar] [CrossRef]
- Ruwizhi, N.; Aderibigbe, B.A. Cinnamic acid derivatives and their biological efficacy. Int. J. Mol. Sci. 2020, 21, 5712. [Google Scholar] [CrossRef]
- Safaeian, L.; Asghari-Varzaneh, M.; Alavi, S.S.; Halvaei-Varnousfaderani, M.; Laher, I. Cardiovascular protective effects of cinnamic acid as a natural phenolic acid: A review. Arch. Physiol. Biochem. 2025, 131, 52–62. [Google Scholar] [CrossRef]
- Adisakwattana, S. Cinnamic acid and its derivatives: Mechanisms for prevention and management of diabetes and its complications. Nutrients 2017, 9, 163. [Google Scholar] [CrossRef]
- Bickers, D.; Calow, P.; Greim, H.; Hanifin, J.M.; Rogers, A.E.; Saurat, J.H.; Sipes, I.G.; Smith, R.L.; Tagami, H. A Toxicologic and dermatologic assessment of cinnamyl alcohol, cinnamaldehyde and cinnamic acid when used as fragrance ingredients. Food Chem. Toxicol. 2005, 43, 799–836. [Google Scholar] [CrossRef]
- Albuquerque, C.F.B.; de Souza, D.A.A.; Figueiredo, P.L.B.; Rocha, C.Q.; Maia, J.G.S.; Kato, M.J.; Chisté, R.C.; da Silva, J.K.R. Optimization of extraction conditions for improving gallic acid and quercetin content in Pouteria macrophylla fruits: A promising cosmetic ingredient. ACS Omega 2025, 10, 7371–7380. [Google Scholar] [CrossRef]
- Jiang, Y.; Pei, J.; Zheng, Y.; Miao, Y.J.; Duan, B.Z.; Huang, L.F. Gallic acid: A potential anti-cancer agent. Chin. J. Integr. Med. 2022, 28, 661–671. [Google Scholar] [CrossRef]
- Bhuia, M.S.; Rahaman, M.M.; Islam, T.; Bappi, M.H.; Sikder, M.I.; Hossain, K.N.; Akter, F.; Al Shamsh Prottay, A.; Rokonuzzman, M.; Gürer, E.S.; et al. Neurobiological effects of gallic acid: Current perspectives. Chin. Med. 2023, 18, 27. [Google Scholar] [CrossRef]
- Wianowska, D.; Olszowy-Tomczyk, M. A concise profile of gallic acid—From its natural sources through biological properties and chemical methods of determination. Molecules 2023, 28, 1186. [Google Scholar] [CrossRef]
- Verma, S.; Singh, A.; Mishra, A. Gallic acid: Molecular rival of cancer. Environ. Toxicol. Pharmacol. 2013, 35, 473–485. [Google Scholar] [CrossRef]
- Kahkeshani, N.; Farzaei, F.; Fotouhi, M.; Alavi, S.S.; Bahramsoltani, R.; Naseri, R.; Momtaz, S.; Abbasabadi, Z.; Rahimi, R.; Farzaei, M.H.; et al. pharmacological effects of gallic acid in health and diseases: A mechanistic review. Iran J. Basic Med. Sci. 2019, 22, 225. [Google Scholar] [CrossRef]
- Hadidi, M.; Liñán-Atero, R.; Tarahi, M.; Christodoulou, M.C.; Aghababaei, F. The potential health benefits of gallic acid: Therapeutic and food applications. Antioxidants 2024, 13, 1001. [Google Scholar] [CrossRef]
- Zhao, X.L.; Cao, Z.J.; Li, K.-D.; Tang, F.; Xu, L.-Y.; Zhang, J.-N.; Liu, D.; Peng, C.; Ao, H. Gallic acid: A dietary metabolite’s therapeutic potential in the management of atherosclerotic cardiovascular disease. Front. Pharmacol. 2024, 15, 1515172. [Google Scholar] [CrossRef]
- Kaliora, A.C.; Kanellos, P.T.; Kalogeropoulos, N. Gallic acid bioavailability in humans. In Handbook on Gallic Acid; Thompson, M.A., Collins, P.B., Eds.; Nova Science: New York, NY, USA, 2013; pp. 301–312. [Google Scholar]
- Sarimahmut, M.; Vekshari, S.; Karaali, D.; Çelikler, S. In vitro evaluation of antigenotoxic effects of phloridzin. Cumhur. Sci. J. 2022, 43, 358–364. [Google Scholar] [CrossRef]
- Lv, F.; Chen, Y.; Xie, H.; Gao, M.; He, R.; Deng, W.Y.; Chen, W. Therapeutic potential of phloridzin carbomer gel for skin inflammatory healing in atopic dermatitis. Arch. Dermatol. Res. 2025, 317, 352. [Google Scholar] [CrossRef]
- Wang, L.; Wu, X.; Wan, Q.; Yang, Y.; Gao, C. Phloridzin reduces synovial hyperplasia and inflammation in rheumatoid arthritis rat by modulating MTOR pathway. Int. Immunopharmacol. 2024, 133, 111727. [Google Scholar] [CrossRef]
- Tian, L.; Cao, J.; Zhao, T.; Liu, Y.; Khan, A.; Cheng, G. The bioavailability, extraction, biosynthesis and distribution of natural dihydrochalcone: Phloridzin. Int. J. Mol. Sci. 2021, 22, 962. [Google Scholar] [CrossRef]
- Londzin, P.; Siudak, S.; Cegieła, U.; Pytlik, M.; Janas, A.; Waligóra, A.; Folwarczna, J. Phloridzin, an apple polyphenol, exerted unfavorable effects on bone and muscle in an experimental model of type 2 diabetes in rats. Nutrients 2018, 10, 1701. [Google Scholar] [CrossRef]
- Rampogu, S.; Gajula, R.G.; Lee, K.W. A comprehensive review on chemotherapeutic potential of galangin. Biomed. Pharmacother. 2021, 141, 111808. [Google Scholar] [CrossRef]
- Thapa, R.; Afzal, O.; Alfawaz Altamimi, A.S.; Goyal, A.; Almalki, W.H.; Alzarea, S.I.; Kazmi, I.; Jakhmola, V.; Singh, S.K.; Dua, K.; et al. Galangin as an inflammatory response modulator: An updated overview and therapeutic potential. Chem. Biol. Interact. 2023, 378, 110482. [Google Scholar] [CrossRef]
- Khawaja, G.; El-Orfali, Y.; Shoujaa, A.; Abou Najem, S. Galangin: A promising flavonoid for the treatment of rheumatoid arthritis—Mechanisms, evidence, and therapeutic potential. Pharmaceuticals 2024, 17, 963. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, B.; Deng, H.; Zhang, C.; Wang, Y.; Chen, L.; Teng, H. Galangin alleviates alcohol-provoked liver injury associated with gut microbiota disorder and intestinal barrier dysfunction in mice. J. Agric. Food Chem. 2024, 72, 22336–22348. [Google Scholar] [CrossRef]
- Chen, F.; Tan, Y.F.; Li, H.L.; Qin, Z.M.; Cai, H.D.; Lai, W.Y.; Zhang, X.P.; Li, Y.H.; Guan, W.W.; Li, Y.B.; et al. Differential systemic exposure to galangin after oral and intravenous administration to rats. Chem. Cent. J. 2015, 9, 14. [Google Scholar] [CrossRef]
- Çakır, D.K.; Zannou, O.; Koca, I. Scopoletin contents and antioxidant properties of some edible plants of black sea regions. Discov. Food 2022, 2, 1–10. [Google Scholar] [CrossRef]
- Antika, L.D.; Tasfiyati, A.N.; Hikmat, H.; Septama, A.W. Scopoletin: A review of its source, biosynthesis, methods of extraction, and pharmacological activities. Z. Naturforsch. C J. Biosci. 2022, 77, 303–316. [Google Scholar] [CrossRef]
- Gao, X.Y.; Li, X.Y.; Zhang, C.Y.; Bai, C.Y. Scopoletin: A review of its pharmacology, pharmacokinetics, and toxicity. Front. Pharmacol. 2024, 15, 1268464. [Google Scholar] [CrossRef]
- Zeng, Y.C.; Li, S.; Liu, C.; Gong, T.; Sun, X.; Fu, Y.; Zhang, Z.R. Soluplus micelles for improving the oral bioavailability of scopoletin and their hypouricemic effect in vivo. Acta Pharmacol. Sin. 2017, 38, 424–433. [Google Scholar] [CrossRef]
- Parama, D.; Girisa, S.; Khatoon, E.; Kumar, A.; Alqahtani, M.S.; Abbas, M.; Sethi, G.; Kunnumakkara, A.B. An overview of the pharmacological activities of scopoletin against different chronic diseases. Pharmacol. Res. 2022, 179, 106202. [Google Scholar] [CrossRef]
- Monteiro Espíndola, K.M.; Ferreira, R.G.; Mosquera Narvaez, L.E.; Rocha Silva Rosario, A.C.; Machado Da Silva, A.H.; Bispo Silva, A.G.; Oliveira Vieira, A.P.; Chagas Monteiro, M. Chemical and pharmacological aspects of caffeic acid and its activity in hepatocarcinoma. Front. Oncol. 2019, 9, 467241. [Google Scholar] [CrossRef]
- Pavlíková, N. Caffeic acid and diseases—Mechanisms of action. Int. J. Mol. Sci. 2022, 24, 588. [Google Scholar] [CrossRef]
- Bhuia, M.S.; Ferdous, J.; Chowdhury, R.; Ansari, S.A.; Ansari, I.A.; Al Hasan, M.S.; Sheikh, S.; Islam, M.T. Exploring the antiemetic potential of caffeic acid: A combined in vivo and computational approach. Neurogastroenterol. Motil. 2025, 37, e70003. [Google Scholar] [CrossRef]
- Muhammad Abdul Kadar, N.N.; Ahmad, F.; Teoh, S.L.; Yahaya, M.F. Caffeic acid on metabolic syndrome: A review. Molecules 2021, 26, 5490. [Google Scholar] [CrossRef]
- Boo, Y.C. p-Coumaric acid as an active ingredient in cosmetics: A review focusing on its antimelanogenic effects. Antioxidants 2019, 8, 275. [Google Scholar] [CrossRef]
- Pei, K.; Ou, J.; Huang, J.; Ou, S. p-Coumaric acid and its conjugates: Dietary sources, pharmacokinetic properties and biological activities. J. Sci. Food Agric. 2016, 96, 2952–2962. [Google Scholar] [CrossRef]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant properties of ferulic acid and its possible application. Ski. Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef]
- Markowska, A.; Markowska, J.; Stanisławiak-Rudowicz, J.; Kozak, K.; Roubinek, O.K.; Jasińska, M. The role of ferulic acid in selected malignant neoplasms. Molecules 2025, 30, 1018. [Google Scholar] [CrossRef]
- Zheng, M.; Liu, Y.; Zhang, G.; Yang, Z.; Xu, W.; Chen, Q. The antioxidant properties, metabolism, application and mechanism of ferulic acid in medicine, food, cosmetics, livestock and poultry. Antioxidants 2024, 13, 853. [Google Scholar] [CrossRef]
- Gortzi, O.; Patsios, S.I.; Pyrzynska, K. Ferulic acid—A brief review of its extraction, bioavailability and biological activity. Separations 2024, 11, 204. [Google Scholar] [CrossRef]
- Raj, N.D.; Singh, D. A critical appraisal on ferulic acid: Biological profile, biopharmaceutical challenges and nano formulations. Health Sci. Rev. 2022, 5, 100063. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Nguyen, V.; Taine, E.G.; Meng, D.; Cui, T.; Tan, W. Chlorogenic acid: A systematic review on the biological functions, mechanistic actions, and therapeutic potentials. Nutrients 2024, 16, 924. [Google Scholar] [CrossRef]
- Golmei, P.; Kasna, S.; Roy, K.P.; Kumar, S. A review on pharmacological advancement of ellagic acid. J. Pharmacol. Pharmacother. 2024, 15, 93–104. [Google Scholar] [CrossRef]
- Lu, G.; Wang, X.; Cheng, M.; Wang, S.; Ma, K. The multifaceted mechanisms of ellagic acid in the treatment of tumors: State-of-the-art. Biomed. Pharmacother. 2023, 165, 115132. [Google Scholar] [CrossRef]
- Naraki, K.; Ghasemzadeh Rahbardar, M.; Ajiboye, B.O.; Hosseinzadeh, H. The effect of ellagic acid on the metabolic syndrome: A review article. Heliyon 2023, 9, e21844. [Google Scholar] [CrossRef]
- Ríos, J.L.; Giner, R.M.; Marín, M.; Recio, M.C. A Pharmacological update of ellagic acid. Planta Med. 2018, 84, 1068–1093. [Google Scholar] [CrossRef]
- Lafay, S.; Gil-Izquierdo, A. Bioavailability of phenolic acids. Phytochem. Rev. 2008, 7, 301–311. [Google Scholar] [CrossRef]
- Przybylska-Balcerek, A.; Stuper-Szablewska, K. The effect of phenolic acids on living organisms. Indian J. Med. Res. Pharm. Sci. 2019, 6, 1–14. [Google Scholar] [CrossRef]
- Jian, X.; Shi, C.; Luo, W.; Zhou, L.; Jiang, L.; Liu, K. Therapeutic effects and molecular mechanisms of quercetin in gynecological disorders. Biomed. Pharmacother. 2024, 173, 116418. [Google Scholar] [CrossRef]
- Pei, J.; Kumarasamy, R.V.; Jayaraman, S.; Kanniappan, G.V.; Long, Q.; Palanisamy, C.P. Quercetin-functionalized nanomaterials: Innovative therapeutic avenues for Alzheimer’s disease management. Ageing Res. Rev. 2025, 104, 102665. [Google Scholar] [CrossRef]
- Kasahara, K.; Kerby, R.L.; Aquino-Martinez, R.; Evered, A.H.; Cross, T.-W.L.; Everhart, J.; Ulland, T.K.; Kay, C.D.; Bolling, B.W.; Bäckhed, F.; et al. Gut microbes modulate the effects of the flavonoid quercetin on atherosclerosis. NPJ Biofilms Microbiomes. 2025, 11, 12. [Google Scholar] [CrossRef]
- Bian, X.; Ge, Z.; Chen, X.; Zhong, S.; Li, L.; Xu, W.; Li, B.; Chen, S.; Lv, G. Protective effects and mechanisms of quercetin in animal models of hyperuricemia: A systematic review and meta-analysis. Pharmacol. Res. 2025, 213, 107665. [Google Scholar] [CrossRef]
- Carrillo-Martinez, E.J.; Flores-Hernández, F.Y.; Salazar-Montes, A.M.; Nario-Chaidez, H.F.; Hernández-Ortega, L.D. Quercetin, a flavonoid with great pharmacological capacity. Molecules 2024, 29, 1000. [Google Scholar] [CrossRef]
- Aghababaei, F.; Hadidi, M. Recent advances in potential health benefits of quercetin. Pharmaceuticals 2023, 16, 1020. [Google Scholar] [CrossRef]
- Zou, H.; Ye, H.; Kamaraj, R.; Zhang, T.; Zhang, J.; Pavek, P. A review on pharmacological activities and synergistic effect of quercetin with small molecule agents. Phytomedicine 2021, 92, 153736. [Google Scholar] [CrossRef]
- Harwood, M.; Danielewska-Nikiel, B.; Borzelleca, J.F.; Flamm, G.W.; Williams, G.M.; Lines, T.C. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem. Toxicol. 2007, 45, 2179–2205. [Google Scholar] [CrossRef]
- Kandemir, K.; Tomas, M.; McClements, D.J.; Capanoglu, E. Recent advances on the improvement of quercetin bioavailability. Trends Food Sci. Technol. 2022, 119, 192–200. [Google Scholar] [CrossRef]
- Isemura, M. Catechin in human health and disease. Molecules 2019, 24, 528. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A. The pharmacological potential of catechin. Indian J. Biochem. Biophys. 2020, 57, 505–511. [Google Scholar] [CrossRef]
- Bernal-Mercado, A.T.; Vazquez-Armenta, F.J.; Tapia-Rodriguez, M.R.; Islas-Osuna, M.A.; Mata-Haro, V.; Gonzalez-Aguilar, G.A.; Lopez-Zavala, A.A.; Ayala-Zavala, J.F. Comparison of single and combined use of catechin, protocatechuic, and vanillic acids as antioxidant and antibacterial agents against uropathogenic Escherichia coli at planktonic and biofilm levels. Molecules 2018, 23, 2813. [Google Scholar] [CrossRef]
- Peters, C.M.; Green, R.J.; Janle, E.M.; Ferruzzi, M.G. Formulation with ascorbic acid and sucrose modulates catechin bioavailability from green tea. Food Res. Int. 2010, 43, 95–102. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The pharmacological potential of rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef]
- Wang, X.; Xia, X.; Song, X.; Zhou, Y.; Ma, M.; Ren, Y.; Chen, X.; Xia, Z.; Guo, Y.; Song, C. Therapeutic potential of rutin in premenstrual depression: Evidence from in vivo and in vitro studies. Front. Pharmacol. 2024, 15, 1525753. [Google Scholar] [CrossRef]
- Hamid, Z.M.; Sahib, H.B. The acute toxicity of rutin in mice. Iraqi J. Pharm. Sci. 2021, 30, 231–240. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham-Ul-Haq; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Qi, Q.; Chu, M.; Yu, X.; Xie, Y.; Li, Y.; Du, Y.; Liu, X.; Zhang, Z.; Shi, J.; Yan, N. Anthocyanins and proanthocyanidins: Chemical structures, food sources, bioactivities, and product development. Food Rev. Int. 2023, 39, 4581–4609. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Li, D.; Ho, C.T.; Li, J.; Wan, X. The absorption, distribution, metabolism and excretion of procyanidins. Food Funct. 2016, 7, 1273–1281. [Google Scholar] [CrossRef]
- Yamakoshi, J.; Saito, M.; Kataoka, S.; Kikuchi, M. Safety evaluation of proanthocyanidin-rich extract from grape seeds. Food Chem. Toxicol. 2002, 40, 599–607. [Google Scholar] [CrossRef]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; da Silva Pinto, M. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef]
- Colunga Biancatelli, R.M.L.; Berrill, M.; Catravas, J.D.; Marik, P.E. Quercetin and vitamin C: An experimental, synergistic therapy for the prevention and treatment of SARS-CoV-2 related disease (COVID-19). Front. Immunol. 2020, 11, 1451. [Google Scholar] [CrossRef]
- Ge, C.; Wei, X.; Xu, Y.; Jiang, Y.; Yang, X.; Lin, J.; Li, M.; Tian, Y.; Fan, S.; Ye, T.; et al. Natural ellagic acid-polyphenol ″sandwich biscuit″ self-assembled solubilizing system for formation mechanism and antibacterial synergia. ACS Appl. Mater. Interfaces 2025, 17, 27772–27787. [Google Scholar] [CrossRef]
- Liu, Y.; Guan, L.; Yang, D.; Luo, H.; Zhang, H. Investigating the synergistic antibacterial effects of chlorogenic and p-coumaric acids on Shigella dysenteriae. Food Chem. 2025, 462, 141011. [Google Scholar] [CrossRef]
- Guan, H.; Zhang, W.; Liu, H.; Jiang, Y.; Li, F.; Wang, D.; Liu, Y.; He, F.; Wu, M.; Waterhouse, G.I.N.; et al. Simultaneous binding of quercetin and catechin to FOXO3 enhances IKKα transcription inhibition and suppression of oxidative stress-induced acute alcoholic liver injury in rats. J. Adv. Res. 2025, 67, 71–92. [Google Scholar] [CrossRef]
- Mukai, K.; Mitani, S.; Ohara, K.; Nagaoka, S.I. Structure–activity relationship of the tocopherol-regeneration reaction by catechins. Free Radic. Biol. Med. 2005, 38, 1243–1256. [Google Scholar] [CrossRef]
- Jaramillo Carmona, S.M.; López Martín, S.; Abia, R.; Rodriguez-Arcos, R.; Jiménez Araujo, A.; Guillén Bejarano, R.; Muriana, F.J. Combination of quercetin and kaempferol enhances in vitro cytotoxicity on human colon cancer (HCT-116) cells. Rec. Nat. Prod. 2014, 8, 262–271. [Google Scholar]
- Mok, J.Y.; Jeong, S.I.; Kim, J.H.; Jang, S.I. Synergic effect of quercetin and astragalin from mulberry leaves on anti-inflammation. Korean J. Orient. Physiol. Pathol. 2011, 25, 830–836. [Google Scholar]
- Hajimehdipoor, H.; Shahrestani, R.; Shekarchi, M. Investigating the synergistic antioxidant effects of some flavonoid and phenolic compounds. Res. J. Pharmacogn. 2014, 1, 35–40. [Google Scholar]
- Liu, X.; Zhao, T.; Shi, Z.; Hu, C.; Li, Q.; Sun, C. Synergism antiproliferative effects of apigenin and naringenin in NSCLC cells. Molecules 2023, 28, 4947. [Google Scholar] [CrossRef]
- Biswas, P.; Kaium, M.A.; Islam Tareq, M.M.; Tauhida, S.J.; Hossain, M.R.; Siam, L.S.; Parvez, A.; Bibi, S.; Hasan, M.H.; Rahman, M.M.; et al. The experimental significance of isorhamnetin as an effective therapeutic option for cancer: A comprehensive analysis. Biomed. Pharmacother. 2024, 176, 116860. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, W.; Chen, F.; Lu, Z. Combined effect of chrysin and apigenin on inhibiting the development and progression of colorectal cancer by suppressing the activity of P38-MAPK/AKT pathway. IUBMB Life 2021, 73, 774–783. [Google Scholar] [CrossRef]
- Harasstani, O.A.; Tham, C.L.; Israf, D.A. Kaempferol and chrysin synergies to improve septic mice survival. Molecules 2017, 22, 92. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, M.; Tao, Q.; Li, Y.; Wang, H.; Zhang, M.; Liu, X.; Zhang, J. Betaine–salicylic acid cocrystal for enhanced skincare and acne treatment. RSC Med. Chem. 2025, 16, 1705–1714. [Google Scholar] [CrossRef]
- Bezerra, C.F.; Camilo, C.J.; do Nascimento Silva, M.K.; de Freitas, T.S.; Ribeiro-Filho, J.; Coutinho, H.D.M. Vanillin selectively modulates the action of antibiotics against resistant bacteria. Microb. Pathog. 2017, 113, 265–268. [Google Scholar] [CrossRef]
- Catanzaro, E.; Greco, G.; Potenza, L.; Calcabrini, C.; Fimognari, C. Natural products to fight cancer: A focus on Juglans regia. Toxins 2018, 10, 469. [Google Scholar] [CrossRef]
- Jafari, S.; Dabiri, S.; Mehdizadeh Aghdam, E.; Fathi, E.; Saeedi, N.; Montazersaheb, S.; Farahzadi, R. Synergistic effect of chrysin and radiotherapy against triple-negative breast cancer (TNBC) cell lines. Clin. Transl. Oncol. 2023, 25, 2559–2568. [Google Scholar] [CrossRef]
- Sarg, N.H.; Hersi, F.H.; Zaher, D.M.; Hamouda, A.O.; Ibrahim, S.I.; El-Seedi, H.R.; Omar, H.A. Unveiling the therapeutic potential of taxifolin in cancer: From molecular mechanisms to immune modulation and synergistic combinations. Phytomedicine 2024, 133, 155934. [Google Scholar] [CrossRef]
- Materska, M. Quercetin and its derivatives: Chemical structure and bioactivity - a review. Pol. J. Food Nutr. Sci. 2008, 58, 407–413. [Google Scholar]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Sanchez-Maldonado, A.F. Mode of Action, Interaction and Recovery of Plant Secondary Metabolites for Potential Applications as Food Preservatives. Ph.D. Thesis, University of Alberta, Edmonton, Canada, 2014. [Google Scholar] [CrossRef]
- Bhuyan, D.J.; Basu, A. Phenolic compounds potential health benefits and toxicity. In Utilisation of Bioactive Compounds from Agricultural and Food Waste, 1st ed.; Vuong, Q.V., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 27–59. [Google Scholar] [CrossRef]
- Pizzimenti, S.; Muzio, G.; Barrera, G.; Giner, R.M.; Ríos, J.L.; Máñez, S. Antioxidant activity of natural hydroquinones. Antioxidants 2022, 11, 343. [Google Scholar] [CrossRef]
- Xiang, H.H.; Seong, S.H.; Ji, S.H.; Myung, K.L.; Bang, Y.H.; Ro, J.S. Monoamine oxidase inhibitory components from Cayratia japonica. Arch. Pharm. Res. 2007, 30, 13–17. [Google Scholar] [CrossRef]
- Abou Baker, D.H. An ethnopharmacological review on the therapeutical properties of flavonoids and their mechanisms of actions: A comprehensive review based on up to date knowledge. Toxicol. Rep. 2022, 9, 445–469. [Google Scholar] [CrossRef]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef]
- Latos-Brozio, M.; Masek, A. Structure-activity relationships analysis of monomeric and polymeric polyphenols (quercetin, rutin and catechin) obtained by various polymerization methods. Chem. Biodivers. 2019, 16, e1900426. [Google Scholar] [CrossRef]
- Zahoor, M.; Shafiq, S.; Ullah, H.; Sadiq, A.; Ullah, F. Isolation of quercetin and mandelic acid from Aesculus indica fruit and their biological activities. BMC Biochem. 2018, 19, 5. [Google Scholar] [CrossRef]
- Bentz, A.B. A review of quercetin: Chemistry, antioxidant properties, and bioavailability. J. Young Investig. 2009. Available online: https://www.jyi.org/2009-april/2017/10/15/a-review-of-quercetin-chemistry-antioxidant-properties-and-bioavailability (accessed on 1 October 2024).
- Magar, R.T.; Sohng, J.K. A review on structure, modifications and structure-activity relation of quercetin and its derivatives. J. Microbiol. Biotechnol. 2020, 30, 11–20. [Google Scholar] [CrossRef]
- Tu, B.; Liu, Z.-J.; Chen, Z.-F.; Ouyang, Y.; Hu, Y.-J. Understanding the structure–activity relationship between quercetin and naringenin: In vitro. RSC Adv. 2015, 5, 106171–106181. [Google Scholar] [CrossRef]
- Li, M.M.; Chen, Y.T.; Ruan, J.C.; Wang, W.J.; Chen, J.G.; Zhang, Q.F. Structure-activity relationship of dietary flavonoids on pancreatic lipase. Curr. Res. Food Sci. 2023, 6, 100424. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Liu, B.; Zhao, J.; Thomas, D.S.; Hook, J.M. An investigation into the supramolecular structure, solubility, stability and antioxidant activity of rutin/cyclodextrin inclusion complex. Food Chem. 2013, 136, 186–192. [Google Scholar] [CrossRef]
- Rho, H.S.; Ghimeray, A.K.; Yoo, D.S.; Ahn, S.M.; Kwon, S.S.; Lee, K.H.; Cho, D.H.; Cho, J.Y. Kaempferol and kaempferol rhamnosides with depigmenting and anti-inflammatory properties. Molecules 2011, 16, 3338–3344. [Google Scholar] [CrossRef]
- Wada, E.; Ito, C.; Shinohara, M.; Handa, S.; Maetani, M.; Yasugi, M.; Miyake, M.; Sakamoto, T.; Yazawa, A.; Kamitani, S. Prunin laurate derived from natural substances shows antibacterial activity against the periodontal pathogen Porphyromonas gingivalis. Foods 2024, 13, 1917. [Google Scholar] [CrossRef]
- Céliz, G.; Daz, M.; Audisio, M.C. Antibacterial activity of naringin derivatives against pathogenic strains. J. Appl. Microbiol. 2011, 111, 731–738. [Google Scholar] [CrossRef]
- Smiljkovic, M.; Stanisavljevic, D.; Stojkovic, D.; Petrovic, I.; Vicentic, J.M.; Popovic, J.; Golic Grdadolnik, S.G.; Markovic, D.; Sanković-Babić, S.; Glamoclija, J.; et al. Apigenin-7-O-glucoside versus apigenin: Insight into the modes of anticandidal and cytotoxic actions. EXCLI J. 2017, 16, 795–807. [Google Scholar] [CrossRef]
- Naz, S.; Imran, M.; Rauf, A.; Orhan, I.E.; Shariati, M.A.; Iahtisham-Ul-Haq; IqraYasmin; Shahbaz, M.; Qaisrani, T.B.; Shah, Z.A.; et al. Chrysin: Pharmacological and therapeutic properties. Life Sci. 2019, 235, 116797. [Google Scholar] [CrossRef]
- Liu, Y.; Song, X.; He, J.; Zheng, X.; Wu, H. Synthetic derivatives of chrysin and their biological activities. Med. Chem. Res. 2014, 23, 555–563. [Google Scholar] [CrossRef]
- Shamsudin, N.F.; Ahmed, Q.U.; Mahmood, S.; Shah, S.A.A.; Khatib, A.; Mukhtar, S.; Alsharif, M.A.; Parveen, H.; Zakaria, Z.A. Antibacterial effects of flavonoids and their structure-activity relationship study: A comparative interpretation. Molecules 2022, 27, 1149. [Google Scholar] [CrossRef]
- Sato, M.; Murakami, K.; Uno, M.; Ikubo, H.; Nakagawa, Y.; Katayama, S.; Akagi, K.; Irie, K. Structure-activity relationship for (+)-taxifolin isolated from silymarin as an inhibitor of amyloid β aggregation. Biosci. Biotechnol. Biochem. 2013, 77, 1100–1103. [Google Scholar] [CrossRef]
- Renzetti, A.; Betts, J.W.; Fukumoto, K.; Rutherford, R.N. Antibacterial green tea catechins from a molecular perspective: Mechanisms of action and structure–activity relationships. Food Funct. 2020, 11, 9370–9396. [Google Scholar] [CrossRef]
- Ahmadi, S.M.; Farhoosh, R.; Sharif, A.; Rezaie, M. Structure-antioxidant activity relationships of luteolin and catechin. J. Food Sci. 2020, 85, 298–305. [Google Scholar] [CrossRef]
- Dixon, R.A.; Ferreira, D. Genistein. Phytochemistry 2002, 60, 205–211. [Google Scholar] [CrossRef]
- Li, X.; Chen, B.; Xie, H.; He, Y.; Zhong, D.; Chen, D. Antioxidant structure–activity relationship analysis of five dihydrochalcones. Molecules 2018, 23, 1162. [Google Scholar] [CrossRef]
- Guerrero, L.; Castillo, J.; Quiñones, M.; Garcia-Vallvé, S.; Arola, L.; Pujadas, G.; Muguerza, B. Inhibition of angiotensin-converting enzyme activity by flavonoids: Structure-activity relationship studies. PLoS ONE 2012, 7, e49493. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Coburn, R.A.; Morris, M.E. Structure activity relationships and quantitative structure activity relationships for the flavonoid-mediated inhibition of breast cancer resistance protein. Biochem. Pharmacol. 2005, 70, 627–639. [Google Scholar] [CrossRef]
- Li, Z.; Liu, J.; You, J.; Li, X.; Liang, Z.; Du, J. Proanthocyanidin structure-activity relationship analysis by path analysis model. Int. J. Mol. Sci. 2023, 24, 6379. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, L.; Wang, Y.; Chen, Z.; Zhang, M.; Panichayupakaranant, P.; Chen, H. Study on the active polyphenol constituents in differently colored Rubus chingii Hu and the structure-activity relationship of the main ellagitannins and ellagic acid. LWT 2020, 121, 108967. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020, 10, 2611. [Google Scholar] [CrossRef]
- Otero, E.; Robledo, S.M.; Díaz, S.; Carda, M.; Muñoz, D.; Paños, J.; Vélez, I.D.; Cardona, W. Synthesis and leishmanicidal activity of cinnamic acid esters: Structure-activity relationship. Med. Chem. Res. 2014, 23, 1378–1386. [Google Scholar] [CrossRef]
- Fernandez-Martinez, E.; Bobadilla, R.A.; Morales-Rios, M.S.; Muriel, P.; Perez-Alvarez, V.M. Trans-3-phenyl-2-propenoic acid (cinnamic acid) derivatives: Structure-activity relationship as hepatoprotective agents. Med. Chem. 2007, 3, 475–479. [Google Scholar] [CrossRef]
- Razzaghi-Asl, N.; Garrido, J.; Khazraei, H.; Borges, F.; Firuzi, O. Antioxidant properties of hydroxycinnamic acids: A review of structure- activity relationships. Curr. Med. Chem. 2013, 20, 4436–4450. [Google Scholar] [CrossRef]
- De Armas-Ricard, M.; Ruiz-Reyes, E.; Ramírez-Rodríguez, O. Caffeates and caffeamides: Synthetic methodologies and their antioxidant properties. Int. J. Med. Chem. 2019, 2019, 2592609. [Google Scholar] [CrossRef]
- Dhiman, P.; Malik, N.; Khatkar, A. Hybrid caffeic acid derivatives as monoamine oxidases inhibitors: Synthesis, radical scavenging activity, molecular docking studies and in silico ADMET analysis. Chem. Cent. J. 2018, 12, 112. [Google Scholar] [CrossRef]
- Kiliç, I.; Yeşiloǧlu, Y. Spectroscopic studies on the antioxidant activity of p-coumaric acid. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 115, 719–724. [Google Scholar] [CrossRef]
- Khatkar, A.; Nanda, A.; Kumar, P.; Narasimhan, B. Synthesis, antimicrobial evaluation and QSAR studies of p-coumaric acid derivatives. Arab. J. Chem. 2017, 10, S3804–S3815. [Google Scholar] [CrossRef]
- de Oliveira Silva, E.; Batista, R. Ferulic acid and naturally occurring compounds bearing a feruloyl moiety: A review on their structures, occurrence, and potential health benefits. Compr. Rev. Food Sci. Food Saf. 2017, 16, 580–616. [Google Scholar] [CrossRef]
- Li, W.; Li, N.; Tang, Y.; Li, B.; Liu, L.; Zhang, X.; Fu, H.; Duan, J.A. Biological activity evaluation and structure–activity relationships analysis of ferulic acid and caffeic acid derivatives for anticancer. Bioorg. Med. Chem. Lett. 2012, 22, 6085–6088. [Google Scholar] [CrossRef]
- Varzaru, I.; Oancea, A.G.; Vlaicu, P.A.; Saracila, M.; Untea, A.E. Exploring the antioxidant potential of blackberry and raspberry leaves: Phytochemical analysis, scavenging activity, and in vitro polyphenol bioaccessibility. Antioxidants 2023, 12, 2125. [Google Scholar] [CrossRef]
- Macario, A.; López, J.C.; Blanco, S. Molecular structure of salicylic acid and its hydrates: A rotational spectroscopy study. Int. J. Mol. Sci. 2024, 25, 4074. [Google Scholar] [CrossRef]
- Kim, J.; Kang, S.; Hong, S.; Yum, S.; Kim, Y.M.; Jung, Y. Structure–Activity relationship of salicylic acid derivatives on inhibition of TNF-α dependent NFκB activity: Implication on anti-inflammatory effect of n-(5-chlorosalicyloyl)phenethylamine against experimental colitis. Eur. J. Med. Chem. 2012, 48, 36–44. [Google Scholar] [CrossRef]
- Morais, S.M.; Silva, K.A.; Araujo, H.; Vieira, I.G.P.; Alves, D.R.; Fontenelle, R.O.S.; Silva, A.M.S. Anacardic acid constituents from cashew nut shell liquid: NMR characterization and the effect of unsaturation on its biological activities. Pharmaceuticals 2017, 10, 31. [Google Scholar] [CrossRef]
- Liu, C.; Zheng, P.; Wang, H.; Wei, Y.; Wang, C.; Hao, S. Design and synthesis of scopoletin sulfonate derivatives as potential insecticidal agents. Molecules 2023, 28, 530. [Google Scholar] [CrossRef]
- Shalayel, M.H.F.; Al-Mazaideh, G.M.; Alanezi, A.A.; Almuqati, A.F.; Alotaibi, M. The potential anti-cancerous activity of Prunus amygdalus var. amara extract. Processes 2023, 11, 1277. [Google Scholar] [CrossRef]
- Walton, N.J.; Mayer, M.J.; Narbad, A. Vanillin. Phytochemistry 2003, 63, 505–515. [Google Scholar] [CrossRef]
- Fitzgerald, D.J.; Stratford, M.; Gasson, M.J.; Narbad, A. Structure-function analysis of the vanillin molecule and its antifungal properties. J. Agric. Food Chem. 2005, 53, 1769–1775. [Google Scholar] [CrossRef]
- Tai, A.; Sawano, T.; Yazama, F.; Ito, H. Evaluation of antioxidant activity of vanillin by using multiple antioxidant assays. Biochim. Biophys. Acta 2011, 1810, 170–177. [Google Scholar] [CrossRef]
- Chua, N.K.; Coates, H.W.; Brown, A.J. Squalene monooxygenase: A journey to the heart of cholesterol synthesis. Prog. Lipid Res. 2020, 79, 101033. [Google Scholar] [CrossRef]
- Güneş, F.E. Medical use of squalene as a natural antioxidant. J. Marmara Univ. Inst. Health Sci. 2013, 3, 220–228. [Google Scholar] [CrossRef]
- Njus, D.; Kelley, P.M.; Tu, Y.J.; Schlegel, H.B. Ascorbic acid: The chemistry underlying its antioxidant properties. Free Radic. Biol. Med. 2020, 159, 37–43. [Google Scholar] [CrossRef]
- Hon, S.L. Vitamin C (ascorbic acid). In Encyclopedia of Toxicology, 4th ed.; Wexler, P., Ed.; Academic Press: Cambridge, MA, USA, 2024; Volume 9, pp. 805–807. [Google Scholar] [CrossRef]
- Du, J.; Cullen, J.J.; Buettner, G.R. Ascorbic acid: Chemistry, biology and the treatment of cancer. Biochim. Biophys. Acta 2012, 1826, 443–457. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Semchuk, N.M. Tocopherol biosynthesis: Chemistry, regulation and effects of environmental factors. Acta Physiol. Plant. 2012, 34, 1607–1628. [Google Scholar] [CrossRef]
- Rimbach, G.; Moehring, J.; Huebbe, P.; Lodge, J.K. Gene-regulatory activity of α-tocopherol. Molecules 2010, 15, 1746–1761. [Google Scholar] [CrossRef]
- Mamoun, C.; GhanemI, F.Z.; Yelles, N.; Chenni, F.Z.; Essid, R.Y.M.; Patoli, D.; El Haci, I.A.; Benariba, K.; Rahmoun, A.; Kharoubi, O.; et al. Exploring the therapeutic potential of sour cherry leaf extract (Prunus cerasus L.): A comprehensive in vitro and in vivo evaluation. Farmacia 2025, 73, 2. [Google Scholar] [CrossRef]
- Nunes, A.R.; Gonçalves, A.C.; Falcão, A.; Alves, G.; Silva, L.R. Prunus avium L. (sweet cherry) by-products: A source of phenolic compounds with antioxidant and anti-hyperglycemic properties—A review. Appl. Sci. 2021, 11, 8516. [Google Scholar] [CrossRef]
- Roșcan, A.G.; Ifrim, I.-L.; Patriciu, O.-I.; Fînaru, A.-L. Bioactive Compounds from Orchard Waste: A Pharmacological Perspective on Rubus and Prunus Species. In Proceedings of the Conference Proceedings Abstracts of the 20th International Conference OPROTEH 2025, Bacău, Romania, 21–23 May 2025. [Google Scholar]

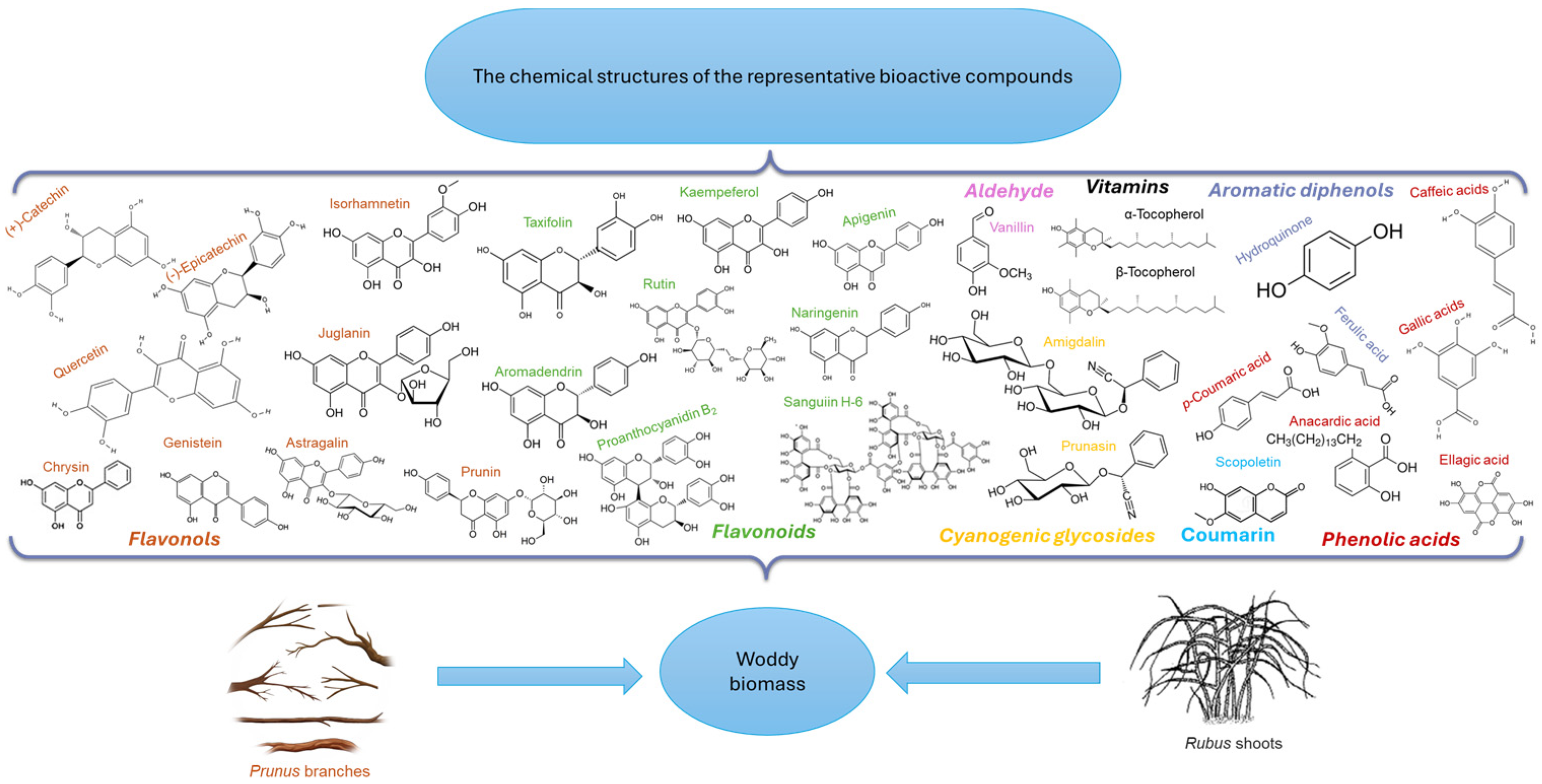
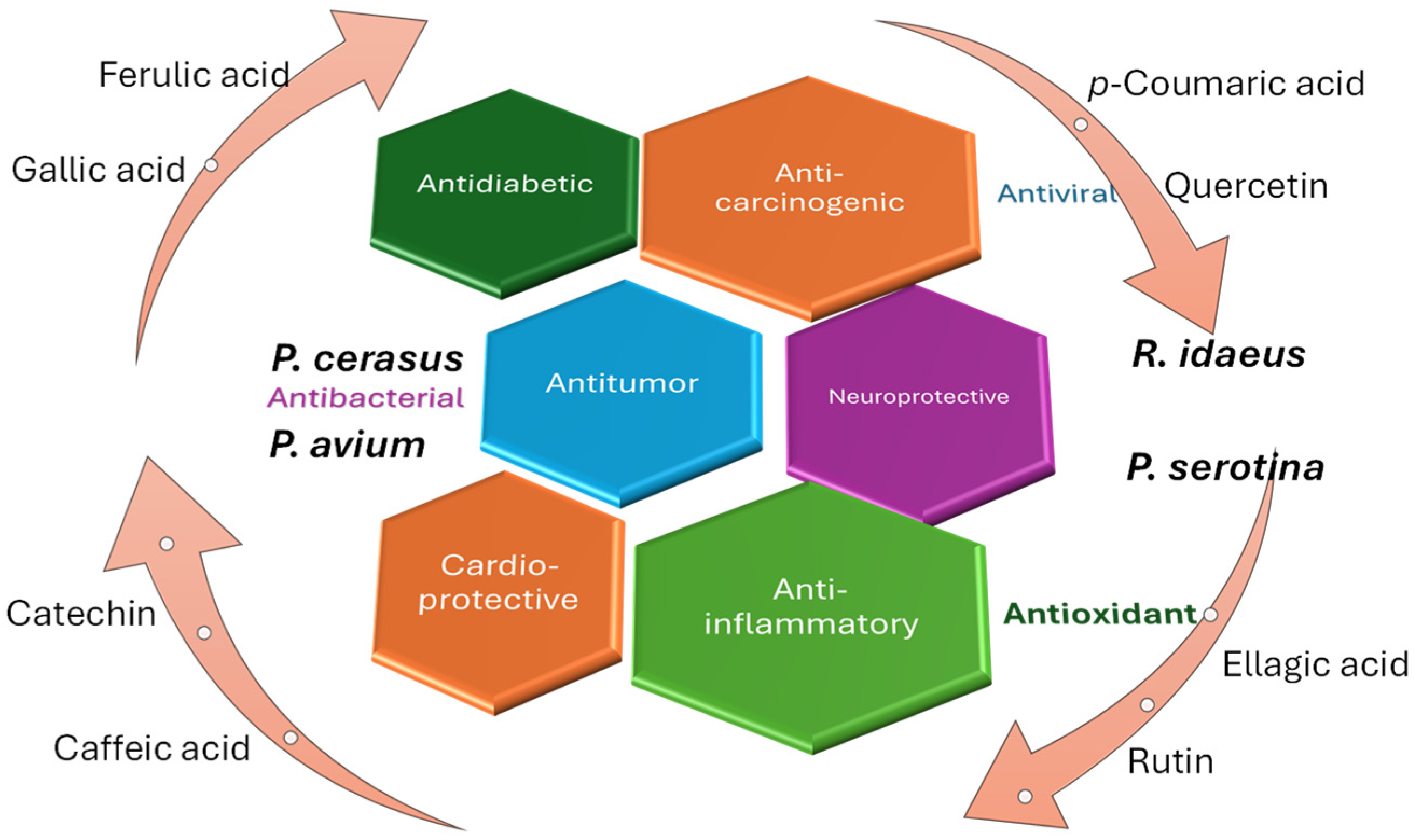
| Bioactive Compound | Source | Content | References |
|---|---|---|---|
| Flavonols | |||
| Catechin | P. serotina (dry leaves) | 605–2342 mg/kg | [27] |
| R. idaeus (Willlamette: non-lignified dry shoots) | 129.3 mg/100 g | [23] | |
| P. avium (wood) | 0.32–30.15 mg/g | [7] | |
| P. avium (dry leaves and branches) | 6.879 mg/g and 0.42–3.74 mg/g | [28] | |
| P. cerasus (resin) | 1.91 mg/L | [22] | |
| P. avium (resin) | 0.33 mg/L | [22] | |
| Epicatechin | R. idaeus (non-lignified dry shoots) | 10.9–85.3 mg/100 g | [23] |
| P. avium (wood) | 0.36 mg/g | [7] | |
| P. avium (dry branches) | 0.0873–0.1102 mg/g | [28] | |
| Quercetin | P. avium (resin) | 1.77 mg/L | [22] |
| P. cerasus (resin) | 0.63 mg/L | [22] | |
| Quercetin derivates | R. idaeus (non-lignified dry shoots) | 10.3–67.4 mg/100 g | [23] |
| Quercetin 3-O-rutinoside | P. avium (dry branches) | 404.39–767 µg/g | [29] |
| Quercetin 3-O-hexosides | P. avium (dry branches) | 665.76–1025.78 µg/g | [29] |
| Chrysin | P. avium (resin) | 1.57 mg/L | [22] |
| P. cerasus (resin) | 0.16 mg/L | [22] | |
| Genistein (genistein-7-O-glucoside) | P. avium (dry branches) | 0.42–3.74 mg/g | [28] |
| Flavonoids | |||
| Kaempferol-3-O-rutinoside | R. idaeus (dry leaves) | 37.92 mg/g | [24] |
| P. avium (dry leaves and branches) | 6.6 mg/g and 0.88 mg/g | [28] | |
| Kaempeferol | R. idaeus (dry leaves) | 1.88–82.28 g/100 g | [30] |
| P. cerasus (resin) | 1.04 mg/L | [22] | |
| P. avium (resin) | 0.22 mg/L | [22] | |
| Rutin | P. cerasus (resin) | 0.22 mg/L | [22] |
| P. avium (resin) | 0.19 mg/L | [22] | |
| Apigenin hidroxihexoside | P. serotina (dry leaves) | 57.4–63.4 mg/kg | [27] |
| Apigenin | P. avium (dry branches) | 0.033 mg/g | [28] |
| Aromadendrin | P. avium (wood) | 0.08–4.54 g/kg | [7] |
| Aromadendrin-7-O-hexoside | P. avium (dry branches) | 0.86–2.66 mg/g | [28] |
| Naringenin | P. avium (resin) | 5.01 mg/L | [22] |
| P. cerasus (resin) | 4.73 mg/L | [22] | |
| P. avium (dry leaves) | 0.74 mg/g | [28] | |
| P. avium (wood) | 0.17–0.41 mg/g | [7] | |
| P. serotina (lyophilized leaves) | 0.13 mg/100 g | [16] | |
| Naringenin-7-O-hexoside | P. avium (dry branches) | 1482.67–1940.77 µg/g | [29] |
| Taxifolin | P. avium (wood) | 0.09–8.46 mg/g | [7] |
| P. avium (dry branches) | 0.19–0.79 mg/g | [28] | |
| Tannins | |||
| Proanthocyanidin B1 | R. idaeus (Willlamette: non-lignified dry shoots) | 229 mg/100 g | [23] |
| P. avium (wood) | 0.15 mg/g | [7] | |
| Proanthocyanidin B2 | R. idaeus (Willlamette: non-lignified dry shoots) | 646 mg/100 g | [23] |
| P. avium (wood) | 0.72 mg/g | [7] | |
| Proanthocyanidin dimer B type 2 | P. avium (dry branches) | 7149.5–8810.67 µg/g | [29] |
| Proanthocyanidin dimer type B | P. avium (wood) | 3.65 mg/g | [7] |
| Proanthocyanidin trimer type B | P. avium (wood) | 1.25 mg/g | [7] |
| Sanguiin H-6 | R. idaeus (non-lignified dry shoots) | 139.2–633.1 mg/100 g | [23] |
| Cyanogenic glycosides | |||
| Prunasin | P. serotina (leaves) | 59.49 mg/g | [16] |
| Amigdalin | P. serotina (leaves) | 20.95 mg/g | [16] |
| Vitamins | |||
| α-Tocopherol | P. avium (lyophilized branches) | 512.58 µg/100 g | [31] |
| β-Tocopherol | P. avium (lyophilized branches) | 31.94 µg/100 g | [31] |
| γ-Tocopherol | P. avium (lyophilized branches) | 23.58 µg/100 g | [31] |
| Aldehyde | |||
| Vanillin | P. avium (wood) | 4.68 mg/g | [7] |
| P. avium (dry branches) | 0.079 mg/g | [28] | |
| Phenolic acids | |||
| Ellagic acid | R. idaeus (non-lignified dry shoots) | 26.1–106.8 mg/100 g | [23] |
| P. avium (wood) | 0.27 mg/g | [7] | |
| Chlorogenic acid | R. idaeus (Willlamette: non-lignified dry shoots) | 177.4 mg/100 g | [23] |
| P. serotina (lyophilized leaves) | 29.5 mg/100 g | [16] | |
| P. avium (dry leaves) | 17.06 mg/g | [28] | |
| P. avium (resin) | 0.62 mg/L | [22] | |
| P. cerasus (resin) | 0.27 mg/L | [22] | |
| Gallic acid | R. idaeus (Willlamette: non-lignified dry shoots) | 72.2 mg/100 g | [23] |
| P. serotina (lyophilized leaves) | 19.56 mg/100 g | [16] | |
| P. avium (dry branches) | 0.041–0.05 mg/g | [28] | |
| Caffeic acid | R. idaeus (dry leaves) | 0.64–7.21 mg/g | [32] |
| P. serotina (dry leaves) | 75–158.8 mg/kg | [27] | |
| Ferulic acid | P. serotina (lyophilized leaves) | 185.3 mg/100 g | [16] |
| P. avium (dry branches) | 0.22–0.23 mg/g | [28] | |
| R. idaeus (dry leaves) | 0.1–0.49 mg/g | [32] | |
| p-Coumaric acid | P. serotina (lyophilized leaves) | 103.6 mg/100 g | [16] |
| P. avium (wood) | 26.3 mg/g | [7] | |
| R. idaeus (dry leaves) | 0.07–0.95 mg/g | [32] | |
| P. avium (dry branches) | 0.038–0.161 mg/g | [28] | |
| Bioactive Compound/Class of Compounds | Sources | Extraction Methods | References |
|---|---|---|---|
| R. idaeus | |||
| Non-extractable/bound phenolic compounds | leaves | Acid and enzymatic hydrolysis (especially for ellagic acid) | [44] |
| Phenolic compounds | dry, non-lignified shoots | Soxhlet, using chloroform and methanol | [23,45] |
| dry leaves | Reflux extraction | [30] | |
| dry leaves | Aqueous extraction in a mass ratio of 3:1 | [24] | |
| dry leaves | Extraction with acetone and trichloromethane | [32] | |
| lyophilized leaves | Alcoholic extraction (2 g sample in 80 mL 70% methanol) with stirring | [46] | |
| dried leaves and woody material | Ultrasound-assisted extraction, using 1 g of dried and ground sample, 30 mL of 80:20 ethanol–water solution | [47] | |
| Polyphenols, catechins, hydroxycinnamic acids, flavonoids | shoots | Reflux extraction (solid–liquid) | [48] |
| Quercetin 3-glucosid and K2 vitamin | frozen leaves | Ultrasound-assisted extraction (solvent: methanol–acetonitrile–water solution in a volume ratio of 2:2:1) | [49] |
| Carotenoids | lyophilized leaves | Extraction with acetone and hexane (4:6 v/v) | [46] |
| P. serotina | |||
| Phenolic compounds | dry leaves | Ultrasound-assisted methanol extraction | [27] |
| lyophilized leaves | Aqueous extraction | [16] | |
| Tannins | dry bark | Reflux extraction (25 g sample + 200 mL 90% ethyl alcohol + 200 mL glacial acetic acid) | [50] |
| P. avium | |||
| Phenolic compounds | dry branches | Pressurized liquid extraction (ethanol–water) Supercritical fluid extraction (CO2) | [26] |
| lyophilized branches | Hydromethanolic extraction (80/20 v/v)–decoction Infusion (distilled water) | [31] | |
| lyophilized leaves | Extraction with water/methanol/ascorbic acid/hydrochloric acid 37% (6.8:3:0.1:0.1; v/v/g/v), assisted by ultrasound | [35] | |
| dry bark | Ultrasound-assisted extraction (solvent: 80% aqueous ethanol solution) | [51] | |
| Fatty acids, organic and phenolic acids, aromatic aldehydes, isoprenoids | dry branches | Subcritical water extraction | [52] |
| Hydroxycinnamic acids | salks and leaves | Maceration Supercritical fluid extraction–CO2 | [53] |
| Caffeic acid | salks | Solvent extraction, maceration | [53] |
| Proanthocyanidin | salks | Accelerated solvent extraction | [53] |
| Catechin | salks | Solvent extraction, supercritical fluid extraction, ultrasound-assisted extraction | [53] |
| P. cerasus | |||
| Phenolic compounds | lyophilized leaves | Extraction with water/methanol/ascorbic acid/hydrochloric acid 37% (6.8:3:0.1:0.1; v/v/g/v), assisted by ultrasound | [35] |
| Fatty acids, organic and phenolic acids, aromatic aldehydes, isoprenoids | dry branches | Subcritical water extraction | [52] |
| Bioactive Compound | Isolation Methods | Observations | References |
|---|---|---|---|
| Anacardic acid | Obtaining the extract using supercritical carbon dioxide, followed by precipitation in the form of calcium anacardate, which, after treatment with hydrochloric acid, is converted back into anacardic acid | It is a thermolabile compound, and distillation under low pressure favors the acid thermal decomposition into cardanol | [56] |
| Chlorogenic acid | Surface imprint polymerization based on hyper-branched amino magnetic nanoparticles | Soluble in water It is a thermosensitive compound and easily oxidized | [57,58] |
| Ellagic acid | The use of cotton fibers grafted with graphene oxide promotes insulation through hydrophobic interaction, serving as a stationary absorbent | It is thermally stable Slightly soluble in water, alcohol, and ether Soluble in potassium hydroxide High solubility in pyridine | [54,59,60] |
| Apigenin | The hydroalcoholic, methanolic, or ethyl acetate fractions of the aqueous extract are subjected to column chromatography and preparative HPLC The methanolic extract is subjected to partitioning with ethyl acetate, followed by column chromatography on silica gel for separation, thin-layer chromatography for purification, and NMR spectroscopy for compound confirmation | Low solubility in lipophilic and highly hydrophilic solvents High solubility in phosphate buffers with pH 7.5 Low solubility in water | [61,62,63] |
| Astragalin | The ethyl acetate fraction is concentrated and isolated by column chromatography (TLC and HPLC) on silica gel, using a mixture of ethyl acetate, methanol, and water as the eluent | Solubility is reduced in water | [64,65] |
| Hydroquinone | The crude extract is loaded onto a silica gel column (ethyl acetate and hexane), followed by purification through semi-preparative HPLC (methanol-water) | Soluble in methanol, ether, and water It oxidizes in contact with air and light Significant thermal sensitivity | [66,67,68] |
| Isorhamnetin | High-speed countercurrent preparative chromatography | Low solubility in water Thermal stability | [69,70,71] |
| Juglanin | The alcoholic extract is subjected to Sephadex column chromatography on silica gel, using a mixture of chloroform, methanol, and water as the eluent | Great solubility in water | [72,73] |
| Kaempeferol | The alcohol is evaporated under vacuum from the methanolic extract, yielding an ethyl acetate fraction that is separated with n-hexane and subjected to vacuum liquid chromatography, followed by other chromatographic techniques (Sephadex column and TLC) until the target compound is isolated | Low solubility in water Thermal stability | [74,75,76] |
| Naringenin | Methanolic extraction followed by crystallization in water containing 14–15% dichloromethane | Low solubility in water Solubility in different solvents: ethyl acetate > isopropanol > methanol > n-butanol > petroleum ether > hexane | [77,78,79] |
| Proanthocyanidins | Sephadex column chromatography | Solubility varies directly proportionally with temperature; in alcohol, it decreases with increasing molecular weight; it exhibits shorter interaction times with tetrahydrofuran and ethyl acetate | [80,81] |
| Prunin | The methanolic extract is divided into several fractions, the one soluble in ethyl acetate is subjected to silica gel chromatography, a mixture of chloroform and methanol is used as the eluent, followed by a separation using Sephadex, with methanol as the solvent | Low solubility in lipophilic media | [82,83] |
| Quercetin | The chloroform fraction of the ethanolic extract is subjected to column chromatography on silica gel, using a mixture of methanol, chloroform, and ethyl acetate as solvents. The use of a mixture of formic acid, water, and methanol in a gradient system, through the HPLC-DAD-MS/MS method. The application of column chromatography on polyamide of the ethyl acetate fraction | Insoluble in water. Stability to light in concentrations greater than 10% Unstable when exposed to atmospheric oxygen Thermal stability | [61,76,84,85] |
| Rutin | Dichloromethane fractions or aqueous fractions with a higher rutin content are obtained, which are chromatographically separated on a Sephadex column with methanol as the mobile phase | Low liposolubility. Increases water solubility through glycosylation | [86,87,88] |
| Sanguiin H6 | Quantification from the hydrolytic solution, by HPLC | Soluble in water Hydrolyzes in acidic or basic environments, giving rise to ellagic acid | [89] |
| Scopoletin | Chromatographic separation (TLC or HPLC) with elution in a mixture of methanol and other compounds (chloroform, acetonitrile, acetic acid) | It is soluble in water and stable in solution at a pH between 3 and 10, a stability that can be extended in time and pH range by the addition of methanol | [90,91] |
| Bioactive Compound | Pharmacological Activity | Ref | Bioavailability and Toxicity | Ref | |
|---|---|---|---|---|---|
| R. idaeus | |||||
| Phenols | Anacardic acid | Bactericide, anticancerogenic, fungicide, insecticide, anti-termite, and molluscicidal Tyrosinase and urease inhibition | [93,94] | Physicochemical stability and low water solubility result in limited bioavailability One of the main culprits of cashew allergy is due to the presence of the carboxyl group and the unsaturated side chain May induce allergic contact dermatitis | [93,96] |
| Prevention and treatment of breast cancer | [95] | ||||
| Sanguiin H6 | Antioxidant Anticancerigenic (breast) | [97] | Low upon oral administration, being stable in the acidic environment of the stomach, hydrolysis is possible in the intestinal environment It has no adverse effects | [100,101] | |
| Anti-inflammatory | [98] | ||||
| Antiangiogenic | [99] | ||||
| Flavonoids | Quercetin ramnoside | Antioxidant and liver protection | [102,103,104] | Higher than quercetin Does not present any potential toxicity to animals | [107,108] |
| Antivirals | [105] | ||||
| Restoring the intestinal microbiota | [106] | ||||
| Astragalin (kaempferol 3-glucoside) | Antidepressant | [109] | Low, structural modification by enzymatic synthesis is suggested Studies conducted to date have not revealed any toxic activity of this compound | [65,115] | |
| Hypoglycemic | [110] | ||||
| Anti-inflammatory, antioxidant, neuroprotective, cardioprotective, antiobesity, antiosteoporotic, anticancer, antiulcer, and antidiabetic | [111,112] | ||||
| Analgesic, procoagulant, antibacterial, antiallergic, and antihepatotoxic | [113] | ||||
| Neuroprotective | [114] | ||||
| Vegetal hormones | Kinetin | Antioxidant | [116] | Good oral absorption. Is not mutagenic nor cardiotoxic | [118] |
| Inhibition of colorectal cancer | [117] | ||||
| Salicylic acid | Antimicrobial and anti-inflammatory | [119] | Higher bioavailability in intravenous form compared to oral administration Topical toxicity is rare | [121,122] | |
| Obtaining the 4-chloro-5-chlorosulfonyl salicylic acid derivative–diuretic agent | [120] | ||||
| Vitamins | Ascorbic Acid (Vitamin C) | Antioxidant, anticancer Wound healing | [123] | Intravenous bioavailability is higher compared to oral Does not show toxicity even in higher doses | [124,125] |
| Tocopherol (Vitamin E) | Antioxidant, platelet anticoagulant | [126] | The absorption efficiency can be close to 80%, but it depends on many factors (pH, presence of proteins, etc.) | [127] | |
| P. serotina | |||||
| Phenols | Hydroquinone (1,4 dihydroxybenzene) | Reduces hyperpigmentation (possible side effects) May cause ochronosis | [128,129,130] | Improved permeability may be associated with an increase in toxicity due to poor physicochemical stability The instability of this compound can lead to the formation of potentially carcinogenic products, but skin and eye side effects can also be recorded It has high toxicity for the aquatic environment and soil In human and animal organisms, it promotes the occurrence of cancer, damages DNA, and favors allergic immune responses | [67,131,132] |
| Antiphotoaging | [131] | ||||
| Flavonoids | Kaempferol | Antimicrobial, anti-inflammatory, antioxidant, antitumor, cardioprotective, neuroprotective, and antidiabetic, anticarcinogenic | [133] | Low May react with iron and decrease bioavailability May decrease the action of anticancer drugs Possible genotoxic action | [75,135] |
| Antifungal and antiprotozoal, hepatoprotective, renoprotective, gastroprotective, and antimutagenic | [134] | ||||
| Effective in treating cervical cancer | [112] | ||||
| Flavonols | Juglanin (kaempferol 3-O-α-L-arabinofuranoside) | Anti-inflammatory, antioxidant, antifibrotic, antithrombotic, antiangiogenic, hepatoprotective, hypolipidemic, hypoglycemic | [17] | Good, especially in glycoside form. Low probability of presenting toxicity when ingested, but significant when applied topically. | [17] |
| Renal protection | [136] | ||||
| Antidepressant | [137] | ||||
| Procoagulant effect | [138] | ||||
| Isorhamnetin | Neuroprotective, cardioprotective, antioxidant, anti-inflammatory, and antiapoptosis | [139] | Higher than quercetin as a liver protector It has no adverse effects and reduces those associated with classic cancer treatment | [70,143] | |
| Antiobesity | [140] | ||||
| Antiviral | [141] | ||||
| Anticoagulants | [142] | ||||
| Terpen | Squalen | Antitumor, antioxidant, and emollient activity on the skin | [144] | High cutaneous availability | [146] |
| Vaccine adjuvant | [145] | ||||
| Cyanogenic glycoside | Prunasin | Treating respiratory conditions (risk of toxicity–cyanide release) | [146] | Larger in the form of decoction. Cyanide can be eliminated through hydrolysis, the lethal dose of which is 0.5 – 3.5 mg/kg body weight | [151,152] |
| Anticarcinogenic properties | [147] | ||||
| Anti-inflammatory and antioxidant | [148] | ||||
| Hepatoprotective and antifibrogenic | [149,150] | ||||
| P. avium | |||||
| Flavonoids | Taxifolin | Anticarcinogenic (minimal adverse effects) Anti-inflammatory, hepatoprotective, antioxidant, cardioprotective, antimicrobial, antiviral, antifungal, antiangiogenic, antihyperglycemic, antipsoriatic, anti-Alzheimer | [153,154] | Low bioavailability Compared to quercetin, this compound is not phototoxic. | [155,156] |
| Aromadendrin | Anti-inflammatory, antioxidant, antidiabetic, antiproliferative, antimicrobial, hepatoprotective, and gastroprotective | [157] | Studies suggest that it is not a mutagenic compound, but may exhibit promutagenic activity | [159] | |
| Antityrosinase, neuroprotective, cardioprotective, antiviral, immunomodulatory, antiacetylcholinesterase, antiapoptotic, antityrosinase, neuroprotective, cardioprotective, antiviral, immunomodulatory, antiacetylcholinesterase, antiapoptotic | [158] | ||||
| Naringenin | Antioxidant, antitumor, antiviral, antibacterial, anti-inflammatory, antiadipogenic, anticancer, antiproliferative, and cardioprotective anti-HCV (hepatitis C virus) | [160] | Small, but still larger than that of the plum There are no adverse reactions recorded when administered to humans. In amphibian embryos, it produced mutations or death in fairly low doses. | [78,164,165] | |
| Antidiabetic | [161] | ||||
| Antifibrogenic | [162] | ||||
| Neuroprotective, antidiabetic, antidepressant | [163] | ||||
| Apigenin | Radioprotective and radiosensitive | [166] | Oral bioavailability approaches 30% and increases with coadministration with friedelin. | [169,170] | |
| Neuroprotective, antidiabetic, antidepressant, anti-insomnia | [167] | ||||
| Hepatoprotective, renoprotective, cardioprotective, antimicrobial, dermatoprotective (anti-UV, antiaging, combats dermatitis and supports wound healing), antiarthritic Supports oral and ocular health | [168] | ||||
| Prunin | Antioxidant, anti-inflammatory, anticancer, immune regulation, antiosteoporosis, antihypoxia, and protective effects for the lungs, liver and kidneys | [171] | Exhibits selective toxicity for cancer cells, but glycosylated derivatives develop lower toxicity on human cells. Glycosylation at position 7 is responsible for increasing bioavailability | [171] | |
| Antiviral effect (Human Enterovirus A17) | [172] | ||||
| Antidiabetic | [173] | ||||
| Antianxiety | [174] | ||||
| Broad-spectrum antibacterial activity | [175] | ||||
| Flavone | Chrysin (5,7-dihydroxyflavone) | Anti-inflammatory, anticancer, antidiabetic, antirachitic, antiasthmatic, antidepressant, neuroprotective | [176] | Low in oral administration caused by poor absorption, metabolism and rapid elimination At a dose of 400–500 mg, it does not cause any notable adverse effects, but it is likely to induce liver toxicity at the cellular level and inhibit de novo DNA synthesis | [176] |
| Antihypercholesterolemic, cardioprotective, antiepileptic, antiamyloidogenic, antiatherogenic | [177] | ||||
| Antidiabetic, antioxidant, antihyperlipidemic | [178] | ||||
| Genistein | Effects of reducing the risk of osteoporosis and post-menopausal symptoms, as well as anticancer, antioxidant, cardioprotective, antiapoptotic, neuroprotective, hepatoprotective, and antimicrobial activities. | [179] | It grows in glycosylated form. Minimal toxicity at doses up to 16 mg/kg body weight | [181] | |
| Treating thrombocytopenia | [180] | ||||
| Aldehydes | Vanillin | Neuroprotective, anti-inflammatory, antifungal, antibacterial, antiviral, and anticancer Modulates the activities of antibiotics | [182] | May pose health risks by increasing the absorption of drugs with moderate oral bioavailability. | [183] |
| Carboxylic acids | Cinamic acid | Antioxidant, antimicrobial, anticancer, neuroprotective, anti-inflammatory | [184] | Reduced for antidiabetic activity. Compared to some derivatives, it has reduced toxicity or no dermatological toxicity. | [186,187] |
| Lipid-lowering, antiobesity, antihyperglycemic, cardioprotective, and vasorelaxant | [185] | ||||
| P. cerasus | |||||
| Phenols | Gallic acid | Antioxidant | [188] | Compared to other polyphenols, it has a high absorption | [196] |
| Anti-inflammatory, antiobesity | [189] | ||||
| It can be used to manage several neurological diseases and disorders, such as Alzheimer’s disease, Parkinson’s disease, stroke, sedation, depression, psychosis, neuropathic pain, anxiety, and memory loss, as well as neuroinflammation. | [190] | ||||
| Anti-HIV, antiulcer, UV protection | [191] | ||||
| Anticarcinogenic | [192] | ||||
| Antioxidant and antineoplastic | [193] | ||||
| Antiviral, antimicrobial, antiallergic, anti-melanogenic, neuroprotective, anti-Alzheimer’s, antidiabetic, and antiobesity | [194] | ||||
| It can be used to treat atherosclerotic cardiovascular disease, coronary artery disease, and cerebral ischemia | [195] | ||||
| Flavonoids | Phloridzin | Antigenotoxic, antioxidant, anti-inflammatory, and anticarcinogenic | [197] | Possible adverse effects on the musculoskeletal system in conditions of hyperglycemia. | [201] |
| Antiaging | [198] | ||||
| Antiarthritic effect | [199] | ||||
| Antidiabetic, antihyperglycemic, antibacterial, cardioprotective, neuroprotective, hepatoprotective, immunomodulatory, and antiobesity | [200] | ||||
| Galangin | Anticancer (breast, renal, lung, esophageal, laryngeal, ovarian, cervical, colon) | [202] | Very low oral bioavailability | [206] | |
| Strong ability to control apoptosis and inflammation | [203] | ||||
| Antioxidant, anti-inflammatory, antiarthritic | [204] | ||||
| Hepatoprotectors | [205] | ||||
| Coumarin | Scopoletin | Antioxidant | [207] | Low, but which can be positively influenced by encapsulation in Solupus micelles It does not present toxicity | [210,211] |
| Antimicrobial, immunomodulatory, anti-inflammatory, anticarcinogenic, neuroprotective | [208] | ||||
| Antibacterial, antifungal, antiparasitic, hepatoprotective, antihyperlipidemic, antidiabetic, antiangiogenesis, antihypertensive, analgesic, anti-immunomodropozic, antiallergic, antiaging, and antigout | [209] | ||||
| Common bioactive compounds (Rubus and Prunus) | |||||
| Phenolic acids | Caffeic acid | Anticancer (hepatocarcinoma) | [212] | Raised in free form. Some phenolic acids may present moderate toxicity, promoting irritation of the gastric mucosa, skin, or eyes | [229,230] |
| Antidiabetic, antiobesity, antiarteriosclerotic, antidepressant, antibacterial, antiviral | [213] | ||||
| Mild antiemetic properties | [214] | ||||
| Antiproliferative, immunomodulatory and neuroprotective | [215] | ||||
| p-Coumaric acid | Antioxidant, efficacy in hypopigmentation and depigmentation | [216] | Raised in free form. Some phenolic acids may present moderate toxicity, promoting irritation of the gastric mucosa, skin, or eyes | [229,230] | |
| Antioxidant, anticancer, antimicrobial, antiviral, anti-inflammatory, antiplatelet, anxiolytic, antipyretic, analgesic and antiarthritic | [217] | ||||
| Ferulic acid | Dermato-protectors (UV, antipigmentation, regeneration) Used as a stabilizer in cosmetic products for vitamins C and E | [218] | Raised in free form. Some phenolic acids may present moderate toxicity, promoting irritation of the gastric mucosa, skin, or eyes | [229,230] | |
| Antioxidant, anti-inflammatory, antiangiogenic, antiallergic, antimicrobial, antiviral, neuroprotective, and anticancer | [219] | ||||
| Pro-angiogenesis, antithrombosis, antiaging, analgesic, antithrombotic | [220] | ||||
| Antidiabetic, cardioprotective, neuroprotective, and antiapoptotic | [221] | ||||
| Hepatoprotectors | [222] | ||||
| Chlorogenic acid | Antioxidant, antibacterial, hepatoprotective, cardioprotective, anti-inflammatory, antipyretic, neuroprotective, anti-obesity, antiviral, antimicrobial, antihypertensive, free radical scavenger, and central nervous system stimulant | [223] | Raised in free form. Some phenolic acids may present moderate toxicity, promoting irritation of the gastric mucosa, skin, or eyes | [229,230] | |
| Antidiabetic, antifibrotic, antimelanogenesis, antiallergic, antifungal, antiatherosclerosis, dermal protection | [224] | ||||
| Ellagic acid | Effective in treating insomnia, fatigue, ischemia, colorectal cancer, and multiple sclerosis; improves physical endurance and lowers blood glucose levels | [225] | Raised in free form. Some phenolic acids may present moderate toxicity, promoting irritation of the gastric mucosa, skin, or eyes | [229,230] | |
| Antitumor, antioxidant, anti-inflammatory, antimutation, antiallergic | [226] | ||||
| Role in treating/managing metabolic syndrome | [227] | ||||
| Neuroprotectors, hepatoprotectors, cardioprotectors, antiphotoaging, depigmenting agent | [228] | ||||
| Flavonols | Quercetin | Anti-inflammatory, antiviral, antioxidant, and psychostimulant, immune-supporting properties | [102,103,104] | Low due to partial solubility in water, but also chemical stability It is not toxic to the human body at a consumption of 3–1000 mg/day. | [238,239] |
| Anticancer effect against malignant gynecological tumors May improve hyperandrogenemia and insulin resistance | [231] | ||||
| Antioxidant and neuroprotector for Alzheimer’s therapy | [232] | ||||
| Antiatherosclerosis (increased absorption in association with lipids) | [233] | ||||
| Liver and kidney protection | [234] | ||||
| Antihypertensive, ability to protect low-density lipoprotein (LDL) oxidation, and ability to inhibit angiogenesis | [235] | ||||
| Antifungal, antiasthmatic, antiallergic, antiobesity | [236] | ||||
| Antiaging, antiviral (COVID-19) | [237] | ||||
| Catechin | Antioxidant, anticancer, antiobesity, (in excess, it can promote hepatitis) | [240] | Intestinal absorption and bioavailability are increased by formulations with sucrose and ascorbic acid | [243] | |
| Antihypertensive, anticoagulant, antiulcer, antithyroid, antihyperlipidemic, antidiabetic, antiosteoporotic, antiosteopenic, hepatoprotective, nephroprotective, neuroprotective, antiallergic, anxiolytic, antimicrobial | [241] | ||||
| It can chelate metals essential for bacterial growth, which supports bactericidal action. | [242] | ||||
| Flavonoids | Rutin | Sedative, diuretic, analgesic, anticonvulsant, anticancer, antidepressant, antiarthritic, antidiabetic, antiulcer, antiasthmatic, anticataract, antiosteoporotic, antiosteopenic, antimicrobial, antifungal, antiviral, larvicidal, antimalarial, effective in atopic dermatitis | [244] | Limited. The lethal dose in mice is between 1.49 and 1.51 g/kg | [87,246] |
| Effective in combating premenstrual dysphoric disorder | [245] | ||||
| Tannins | Proantho-cyanidins (oligo- or polymers of monomeric flavan-3-ols) | Antioxidant, anticancer, antidiabetic, neuroprotective, and antimicrobial | [247] | Low in oral administration Only monomers and oligomeric procyanidins with a degree of polymerization of less than 4 are absorbed. There is no evidence of toxicity when administered orally or of potential mutagenic action at a consumption of 2% of the diet. | [247,249,250] |
| Hypolipidemic, anti-inflammatory, metabolic, and intestinal flora regulation, DNA repair | [248] | ||||
| Chemical Compound | Synergistic Activity | References |
|---|---|---|
| Quercetin + vitamin C | Antiviral | [252] |
| Ellagic acid + gallic acid + catechin | Antibacterial | [253] |
| p-Coumaric acid + chlorogenic acid | Antibacterial | [254] |
| Proanthocyanidins + vitamin C + vitamin E | Synergistic effect for skin whitening | [248] |
| Catechin + quercetin | Against alcoholic liver damage | [255] |
| Catechin + vitamin E | Antioxidant | [256] |
| Ferulic acid + δ-tocotrienol (a derivative of vitamin E) | Against prostate cancer | [220] |
| Kaempferol + apigenin | Cytotoxic efficacy for colon cancer | [257] |
| Quercetin + astragalin | Anti-inflammatory | [258] |
| Quercetin + gallic acid + caffeic acid Quercetin + gallic acid + rutin | Antioxidant | [259] |
| Apigenin + naringenin | Anticarcinogenic | [260] |
| Flavonoids + tocopherol | Inhibitors in the case of lipid peroxidation | [256] |
| Isorhamnetin + quercetin | Potentiation of anticancer activity and broadening of the spectrum | [261] |
| Isorhamnetin + caffeic acid | Increased antioxidant activity | [261] |
| Chrysin + apigenin | Antitumor | [262] |
| Kaempeferol + chrysin | Anti-inflammatory and antioxidant | [263] |
| Betaine-salicylic acid cocrystal | Acne treatment | [264] |
| Vanillin + norfloxacin (antibiotic) | Antibacterial | [265] |
| Juglanin + doxorubicin (antitumor drug) | High cytotoxicity | [266] |
| Chrysin + radiotherapy | Anticarcinogenic | [267] |
| Toxifolin + chemotherapy | Anticarcinogenic | [268] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roșcan, A.G.; Ifrim, I.-L.; Patriciu, O.-I.; Fînaru, A.-L. Exploring the Therapeutic Value of Some Vegetative Parts of Rubus and Prunus: A Literature Review on Bioactive Profiles and Their Pharmaceutical and Cosmetic Interest. Molecules 2025, 30, 3144. https://doi.org/10.3390/molecules30153144
Roșcan AG, Ifrim I-L, Patriciu O-I, Fînaru A-L. Exploring the Therapeutic Value of Some Vegetative Parts of Rubus and Prunus: A Literature Review on Bioactive Profiles and Their Pharmaceutical and Cosmetic Interest. Molecules. 2025; 30(15):3144. https://doi.org/10.3390/molecules30153144
Chicago/Turabian StyleRoșcan, Andreea Georgiana, Irina-Loredana Ifrim, Oana-Irina Patriciu, and Adriana-Luminița Fînaru. 2025. "Exploring the Therapeutic Value of Some Vegetative Parts of Rubus and Prunus: A Literature Review on Bioactive Profiles and Their Pharmaceutical and Cosmetic Interest" Molecules 30, no. 15: 3144. https://doi.org/10.3390/molecules30153144
APA StyleRoșcan, A. G., Ifrim, I.-L., Patriciu, O.-I., & Fînaru, A.-L. (2025). Exploring the Therapeutic Value of Some Vegetative Parts of Rubus and Prunus: A Literature Review on Bioactive Profiles and Their Pharmaceutical and Cosmetic Interest. Molecules, 30(15), 3144. https://doi.org/10.3390/molecules30153144







