Polyphosphoramidate Glycohydrogels with Biorecognition Properties and Potential Antibacterial Activity
Abstract
1. Introduction
2. Results and Discussion
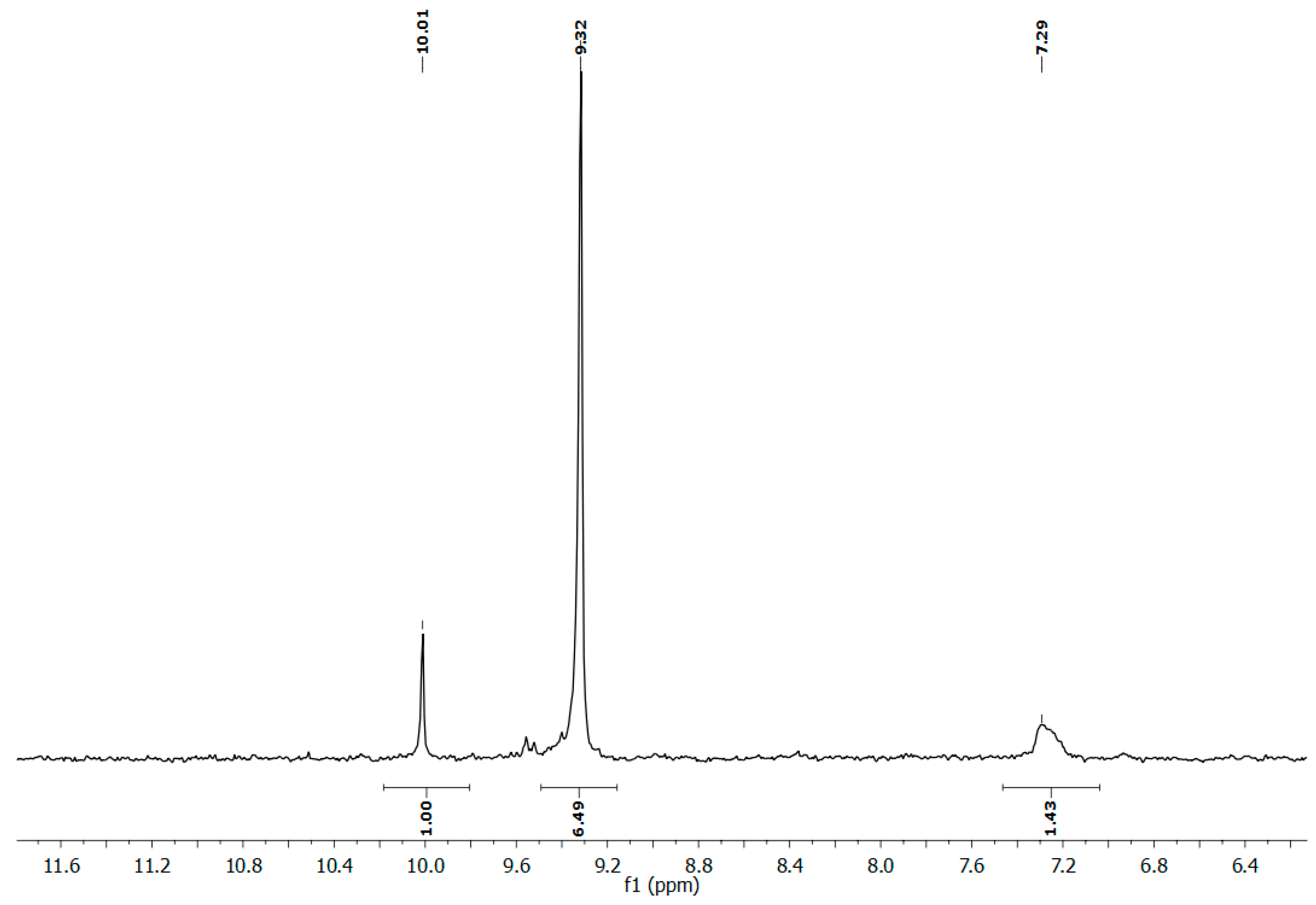
2.1. Synthesis of Poly(oxyethylene H-phosphonate) (POEHP)
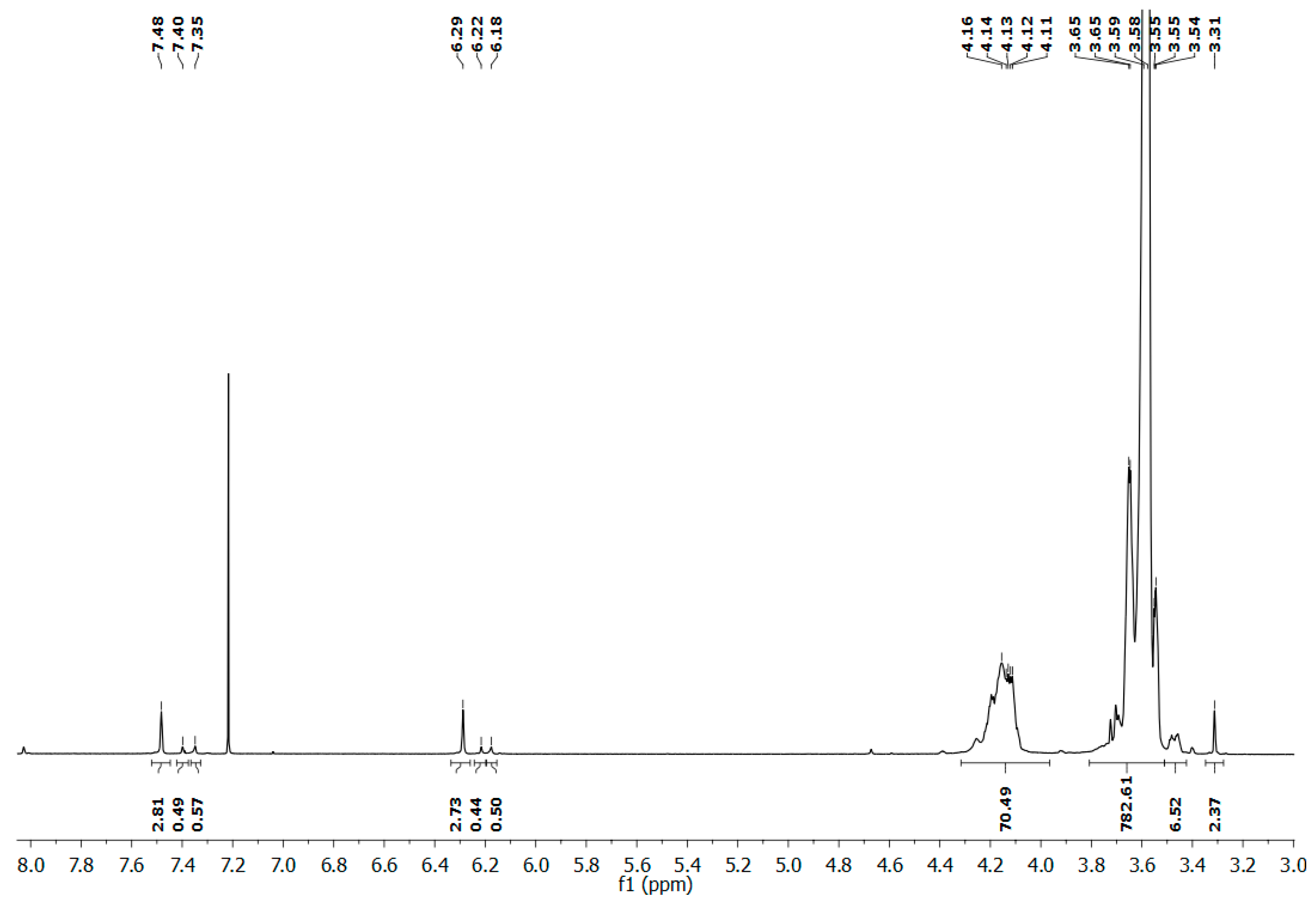
2.2. Synthesis of Poly(phosphoramidate) Glycoconjugate via Staudinger Reaction
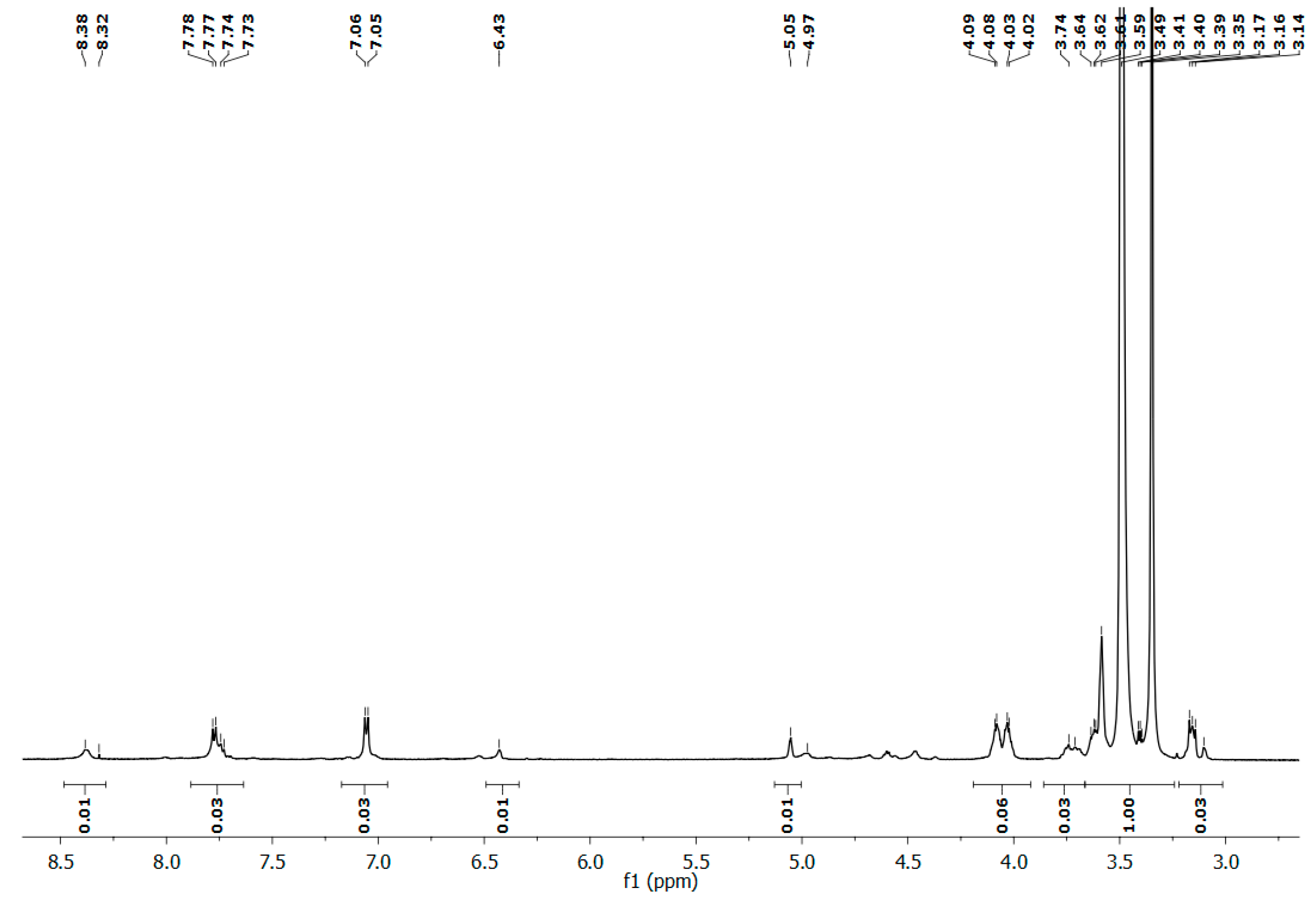
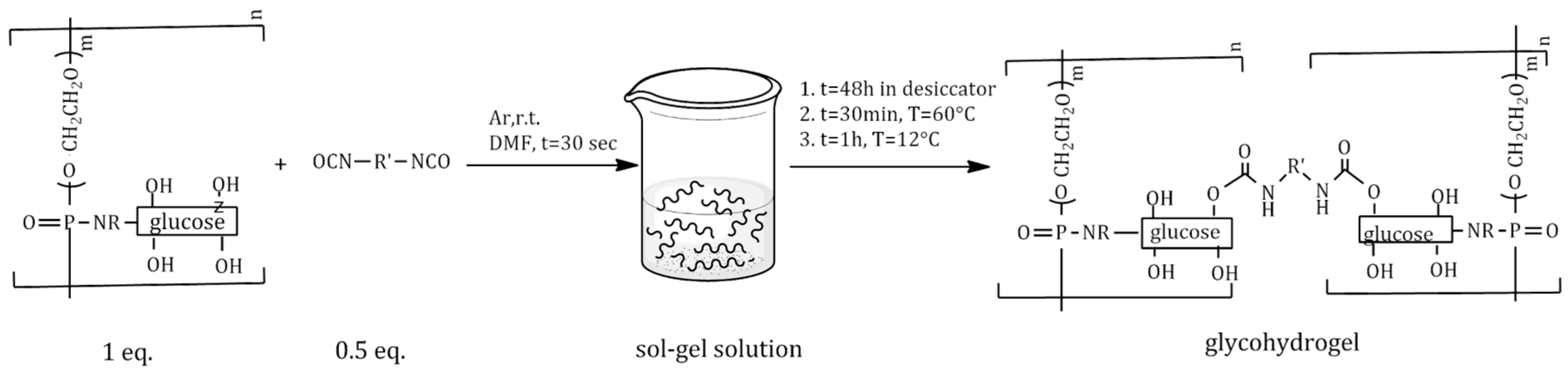
2.3. Synthesis and Characterization of Polyphosphoramidate Glycohydrogel (PPAGHGel)
2.3.1. Swelling and Gel Fraction
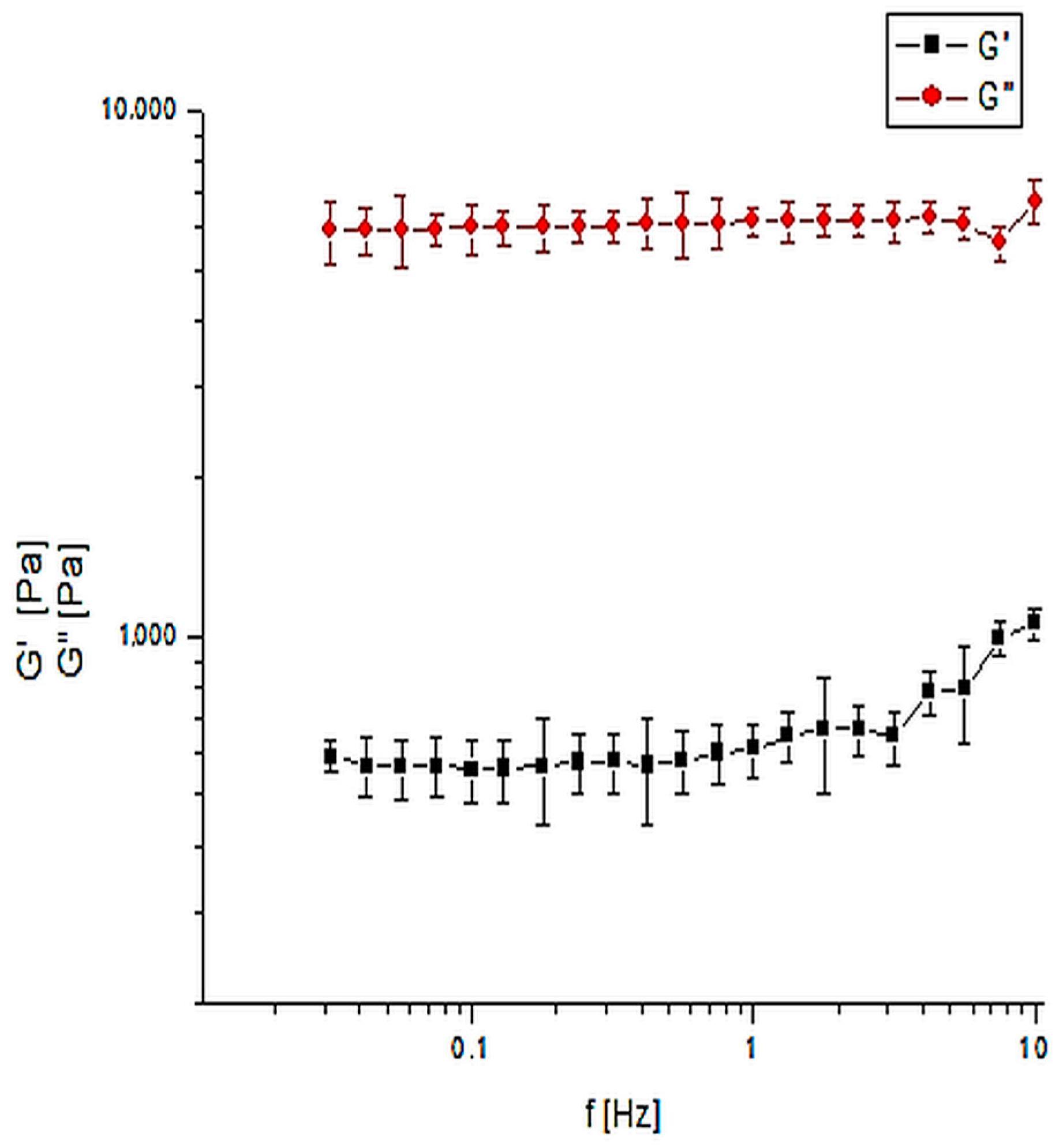
2.3.2. Mechanical Properties
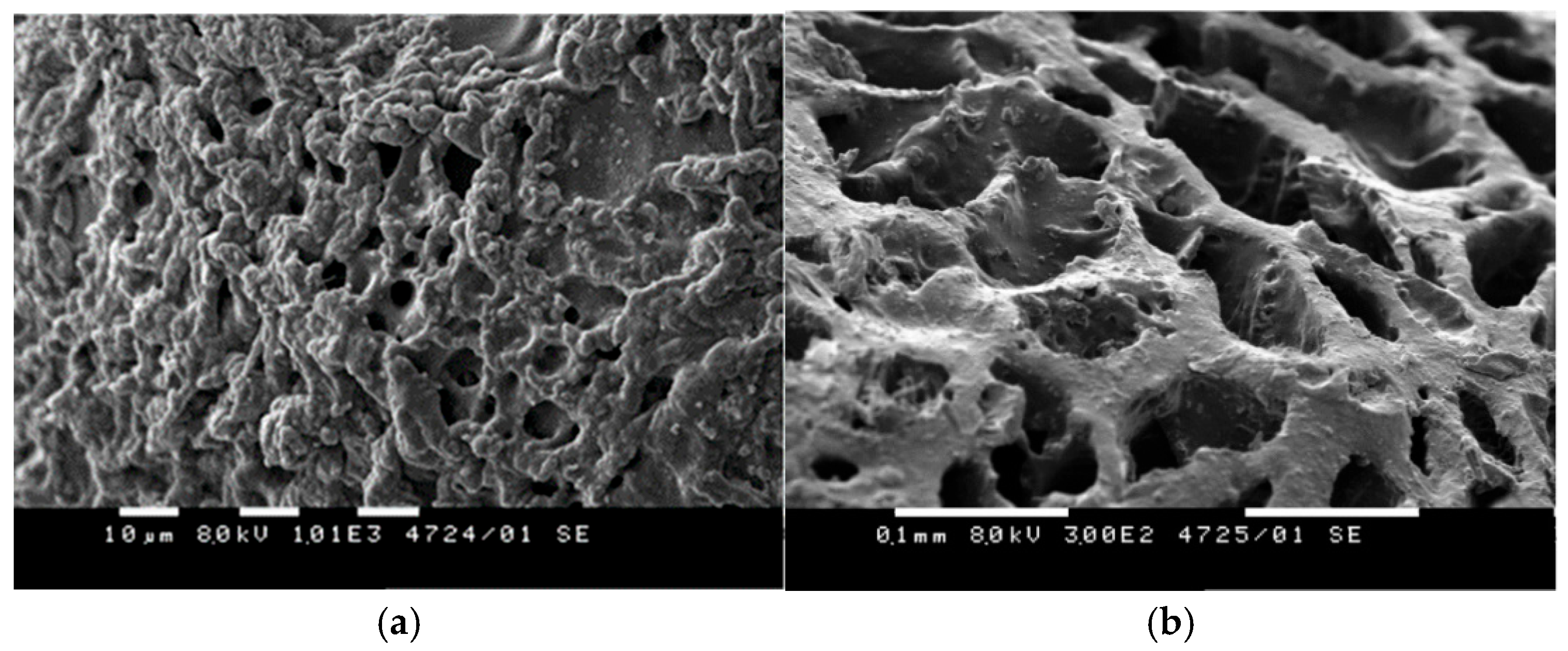
2.3.3. Morphology of PPAGHGel
2.3.4. Thermogravimetric Analysis of PPAGHGel
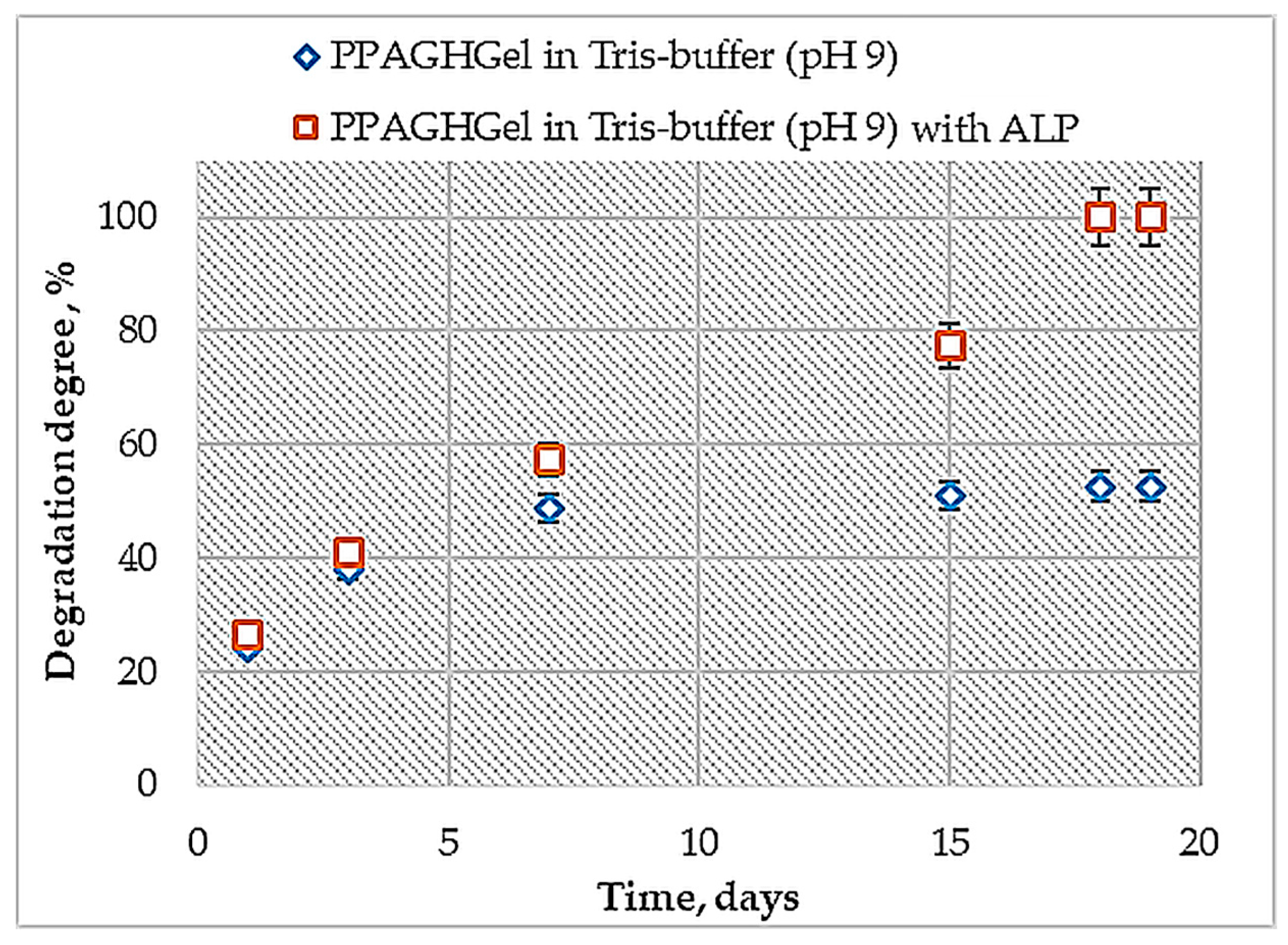
2.4. Enzymatic and Hydrolytic Degradation of PPAGHGel
2.5. Multivalent Studies of Polyphosphoramidate Glycohydrogel
2.6. Preparation of Silver Nanoparticles
2.7. Antibacterial Tests
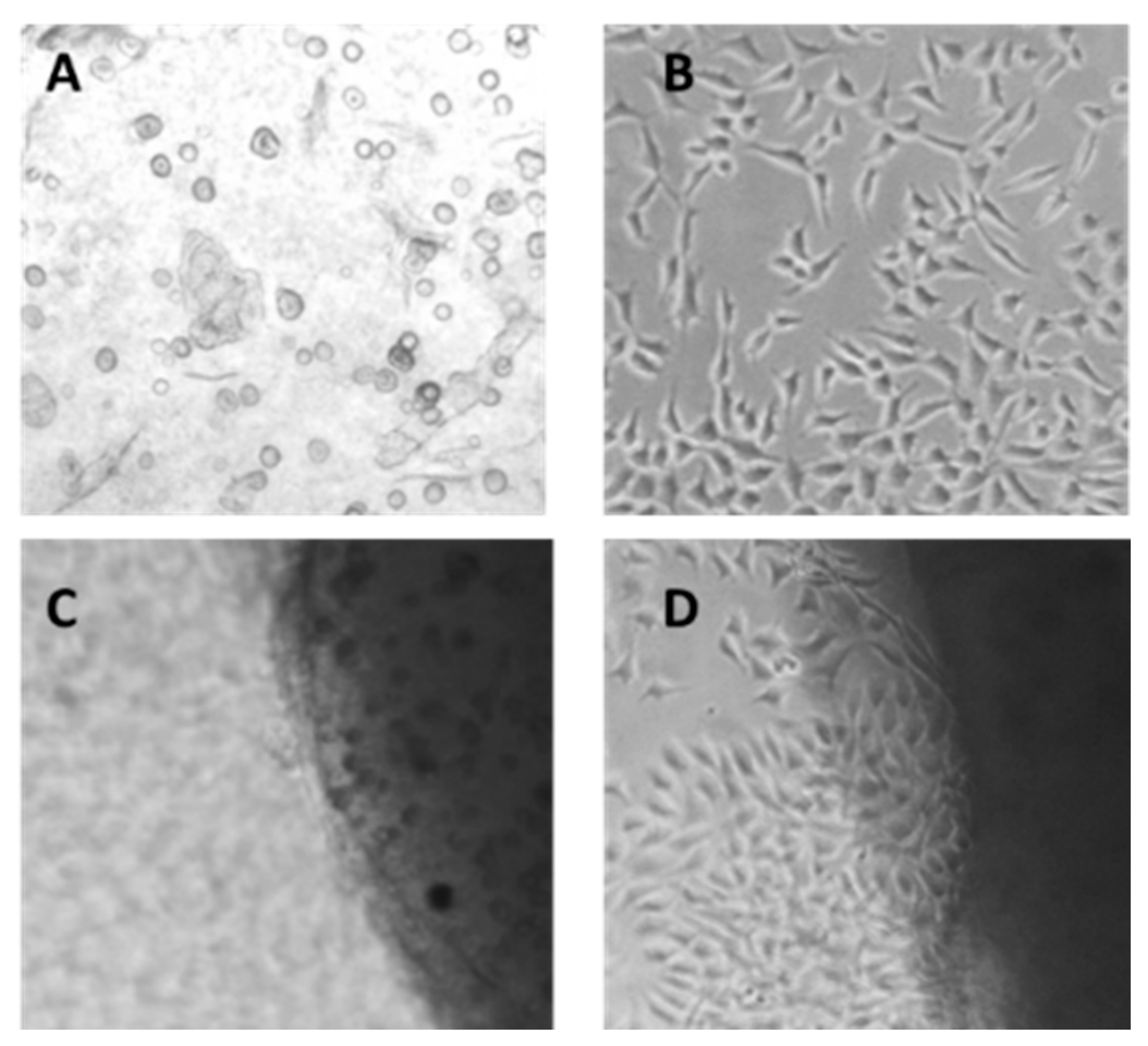
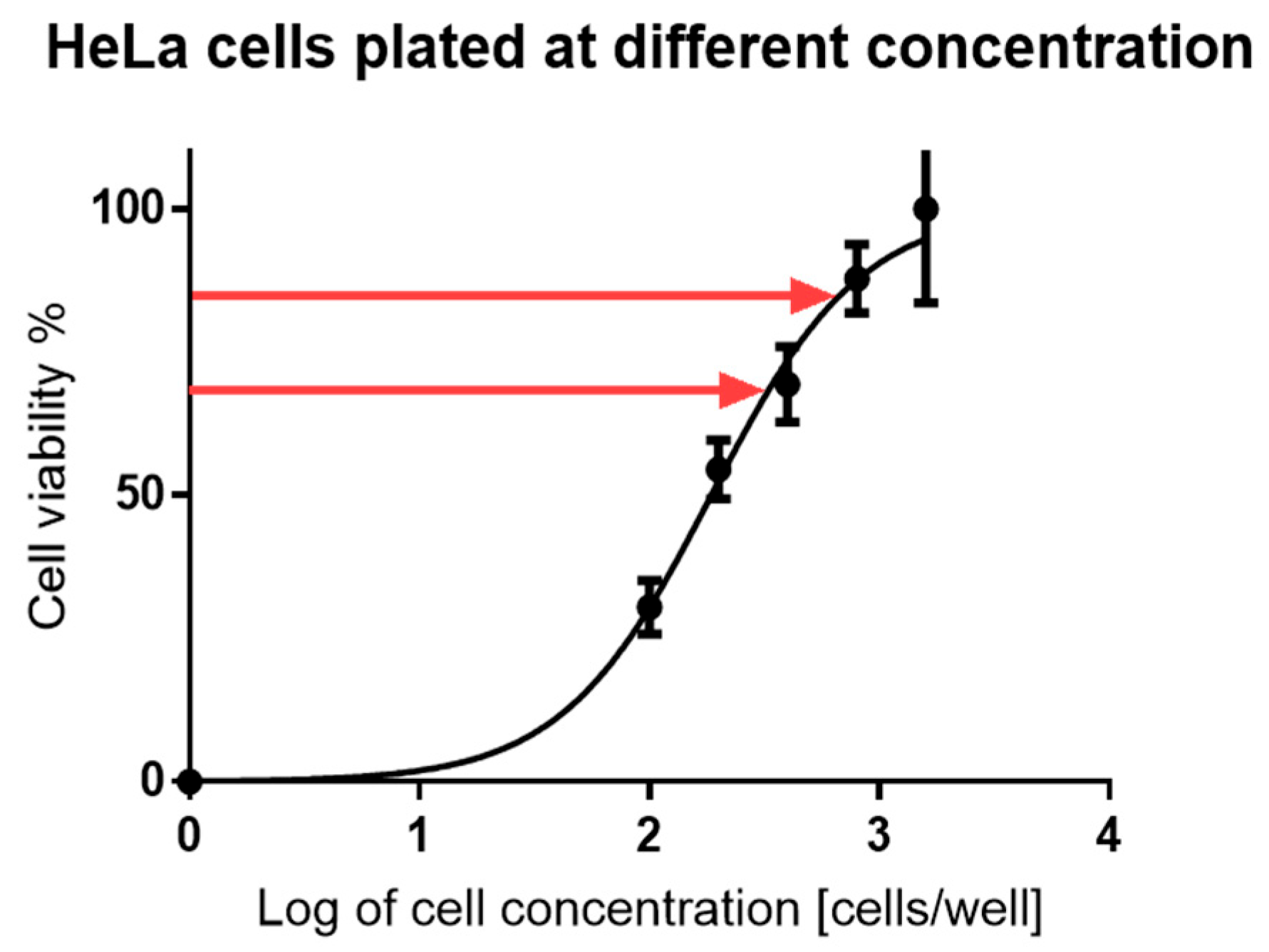
2.8. Cytotoxicity Studies
3. Materials and Methods
3.1. Materials
3.2. Synthetic Procedures
3.2.1. Preparation of Poly(oxyethylene H-phosphonate) (POEHP)
3.2.2. Synthesis of Sugar Azide
3.2.3. Synthesis of Poly(phosphoramidate) Glycoconjugate by Staudinger Reaction
3.2.4. Synthesis of Phosphorus-Containing Polyphosphoramidate Glycohydrogel (PPAGHGel)
3.2.5. Preparation of Silver Nanoparticles Using PPAGHGel
3.3. Methods
3.3.1. NMR and IR
3.3.2. Sol–Gel Fraction
3.3.3. Swelling Degree
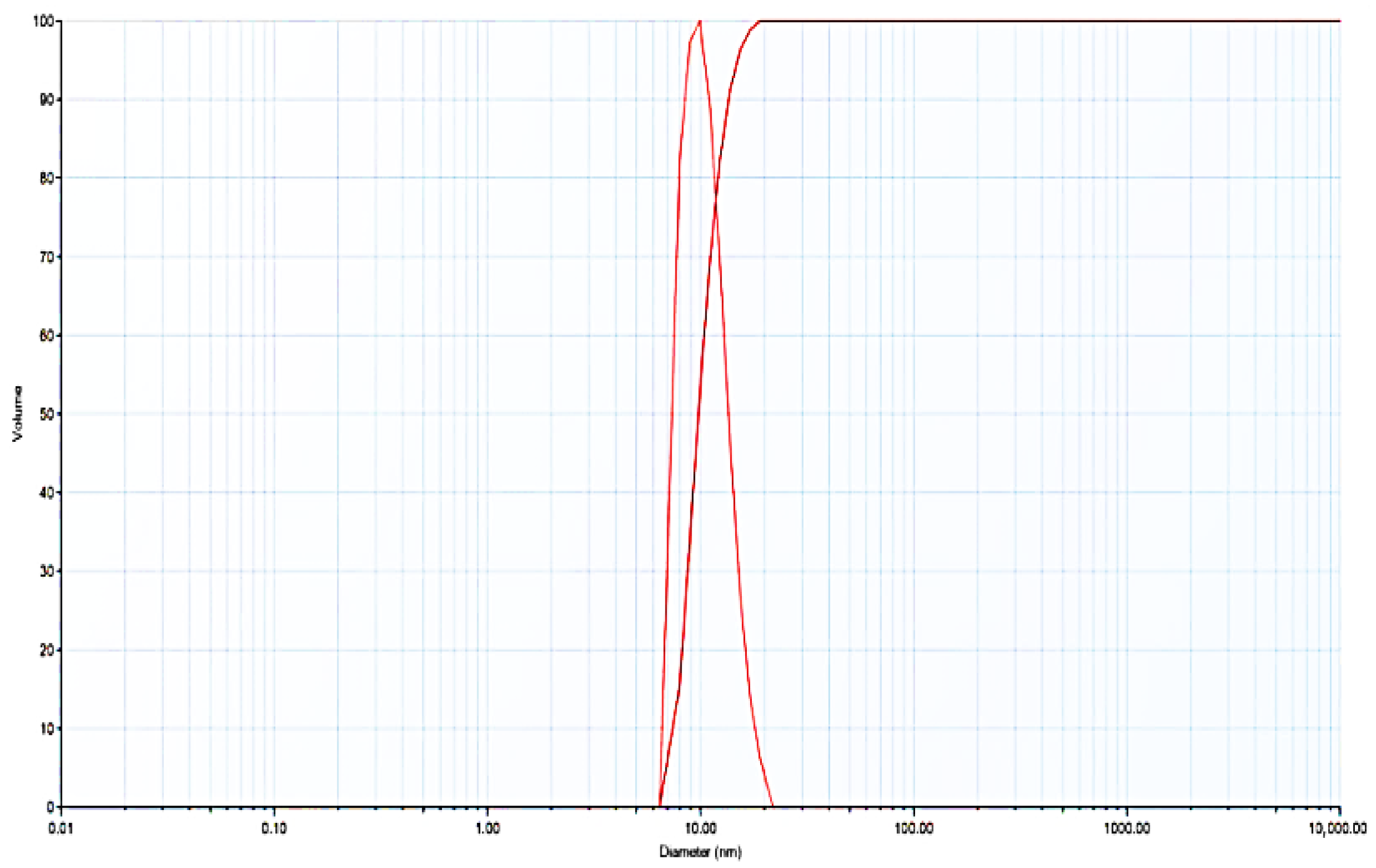
3.3.4. Dynamic Light Scattering
3.3.5. Scanning Electron Microscopy (SEM)
3.3.6. Dynamic–Rheological Measurements
3.3.7. Thermogravimetric Analysis
3.3.8. Turbidimetry
3.3.9. Purification Methods
3.3.10. Enzymatic and Hydrolytic Degradation
3.3.11. Antibacterial Tests
3.3.12. Cytotoxicity Studies
3.3.13. Cell Adhesion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PPAG | Polyphosphoramidate glycoconjugate |
| BSA | N,O-bis-trimethylsilyl acetamide |
| POEHP | Poly(oxyethylene H-phosphonate) |
| PPAGHG | Polyphopsphoramidate glycohydrogel |
| HMDS | Hexamethyldisilazane |
| TBAF | Tetrabutylammonium fluoride |
| PEG | Polyethylene glycol |
| HMDI | Hexamethylene diisocyanate |
| ALP | Alkaline phosphatase |
| PVA | Polyvinyl alcohol |
| AG | Amino-containing glucose |
References
- van Vlierberghe, S.; Dubruel, P.; Schacht, E. Biopolymer-based hydrogels as scaffolds for tissue engineering applications: A review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Rodrigues, J.; Tomás, H. Injectable and biodegradable hydrogels: Gelation, biodegradation and biomedical applications. Chem. Soc. Rev. 2012, 41, 2193–2221. [Google Scholar] [CrossRef] [PubMed]
- Todorova, Z.; Koseva, N.; Ugrinova, I.; Troev, K. Synthesis of poly(oxyethylene phosphoramidate)s and glycopolymers via Staudinger reaction: Multivalent binding studies with Concanavalin A. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1730–1741. [Google Scholar] [CrossRef]
- Nasution, H.; Harahap, H.; Dalimunthe, N.F.; Ginting, M.H.S.; Jaafar, M.; Tan, O.O.H.; Aruan, H.K.; Herfananda, A.L. Hydrogel and Effects of Crosslinking Agent on Cellulose-Based Hydrogels: A Review. Gels 2022, 8, 568. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Zhang, N.; Li, C. Saccharide Modified Pharmaceutical Nanocarriers for Targeted Drug and Gene Delivery. Curr. Pharm. Des. 2009, 15, 3826–3836. [Google Scholar] [CrossRef] [PubMed]
- Pourjavadi, A.; Harzandi, A.M.; Hosseinzadeh, H. Modified carrageenan 3. Synthesis of a novel polysaccharide-based superabsorbent hydrogel via graft copolymerization of acrylic acid onto kappa-carrageenan in air. Eur. Polym. J. 2004, 40, 1363–1370. [Google Scholar] [CrossRef]
- van Dijk, M.; van Nostrum, C.F.; Hennink, W.E.; Rijkers, D.T.S.; Liskamp, R.M.J. Synthesis and characterization of enzymatically biodegradable PEG and peptide-based hydrogels prepared by click chemistry. Biomacromolecules 2010, 11, 1608–1614. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, E.A.; Kenawy, E.-R.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Islam, M.M.; Islam, M.S.; Zaman, A.; Ahmed, T.; Biswas, S.; Sharmeen, S.; Rashid, T.U.; Rahman, M.M. Morphological Characterization of Hydrogels. CB-SAH 2019, 6, 819–863. [Google Scholar] [CrossRef]
- Cometa, S.; Iatta, R.; Ricci, M.A.; Ferretti, C.; de Giglio, E. Analytical characterization and antimicrobial properties of novel copper nanoparticle–loaded electrosynthesized hydrogel coatings. J. Bioact. Compat. Polym. 2013, 28, 508–522. [Google Scholar] [CrossRef]
- Martínez-Castañón, G.A.; Niño-Martínez, N.; Martínez-Gutierrez, F.; Martínez-Mendoza, J.R.; Ruiz, F. Synthesis and antibacterial activity of silver nanoparticles with different sizes. J. Nanopart. Res. 2008, 10, 1343–1348. [Google Scholar] [CrossRef]
- Thiel, J.; Pakstis, L.; Buzby, S.; Raffi, M.; Ni, C.; Pochan, D.J.; Shah, S.I. Antibacterial properties of silver-doped titania. Small 2007, 3, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Guo, H.; Liu, M.; Tang, K.; Li, S.; Fang, Q.; Du, H.; Zhou, X.; Lin, X.; Yang, Y.; et al. Recent design strategies for boosting chemodynamic therapy of bacterial infections. Exploration 2024, 4, 20230087. [Google Scholar] [CrossRef] [PubMed]
- Ofek, I.; Bayer, E.A.; Abraham, S.N. Bacterial Adhesion. In The Prokaryotes; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- Capella, V.; Rivero, R.E.; Liaudat, A.C.; Ibarra, L.E.; Roma, D.A.; Alustiza, F.; Mañas, F.; Barbero, C.A.; Bosch, P.; Rivarola, C.R.; et al. Cytotoxicity and bioadhesive properties of poly-N-isopropylacrylamide hydrogel. Heliyon 2019, 5, e01474. [Google Scholar] [CrossRef] [PubMed]
- Todorova, Z.; Koseva, N.; Troev, K. Sylilation of poly(alkylene H-phosphonate)s—Rapid and efficient method for obtaining poly(alkylene trisilylmethylphosphite)s. Eur. Polym. J. 2015, 62, 87–96. [Google Scholar] [CrossRef]
- Wilkening, I.; del Signore, G.; Hackenberger, C.P.R. Synthesis of phosphonamidate peptides by Staudinger reactions of silylated phosphinic acids and esters. Chem. Commun. 2011, 47, 349–351. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.A.; Dhurandhare, V.M.; Chang, C.-W.; Verma, V.P.; Mishra, G.P.; Ku, C.-C.; Lin, C.-C.; Wang, C.-C. Chemoselective per-O-trimethylsilylation and homogeneous N-functionalisation of amino sugars. Chem. Commun. 2015, 51, 104–106. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Miyano, T.; Endo, T. Synthesis and characteristics of networked polycarbosilanes having urethane-crosslinked glucose groups. Polym. Bull. 2018, 75, 2391–2400. [Google Scholar] [CrossRef]
- Mossman, B.; Light, W.; Wei, E. Asbestos: Mechanisms of toxicity and carcinogenicity in the respiratory tract. Annu. Rev. Pharmacol. Toxicol. 1983, 23, 595–615. [Google Scholar] [CrossRef] [PubMed]
- Schröder, M.; Yusein-Myashkova, S.; Petrova, M.; Dobrikov, G.; Kamenova-Nacheva, M.; Todorova, J.; Pasheva, E.; Ugrinova, I. The Effect of a Ferrocene Containing Camphor Sulfonamide DK-164 on Breast Cancer Cell Lines. Anticancer Agents Med. Chem. 2019, 19, 1874–1886. [Google Scholar] [CrossRef] [PubMed]




| Gel Type | Antibacterial Activity (Sterile Halo in mm) | |
|---|---|---|
| - | P. aeruginosa | S. aureus |
| PPAGHGel-Ag | 22.3 ± 0.4 * | 17.5 ± 0.4 |
| PPAGHGel | 20.3 ± 0.5 | 18.2 ± 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todorova, Z.; Tumurbaatar, O.; Mitova, V.; Koseva, N.; Ugrinova, I.; Petrova, P.; Troev, K. Polyphosphoramidate Glycohydrogels with Biorecognition Properties and Potential Antibacterial Activity. Molecules 2025, 30, 3140. https://doi.org/10.3390/molecules30153140
Todorova Z, Tumurbaatar O, Mitova V, Koseva N, Ugrinova I, Petrova P, Troev K. Polyphosphoramidate Glycohydrogels with Biorecognition Properties and Potential Antibacterial Activity. Molecules. 2025; 30(15):3140. https://doi.org/10.3390/molecules30153140
Chicago/Turabian StyleTodorova, Zornica, Oyundari Tumurbaatar, Violeta Mitova, Neli Koseva, Iva Ugrinova, Penka Petrova, and Kolio Troev. 2025. "Polyphosphoramidate Glycohydrogels with Biorecognition Properties and Potential Antibacterial Activity" Molecules 30, no. 15: 3140. https://doi.org/10.3390/molecules30153140
APA StyleTodorova, Z., Tumurbaatar, O., Mitova, V., Koseva, N., Ugrinova, I., Petrova, P., & Troev, K. (2025). Polyphosphoramidate Glycohydrogels with Biorecognition Properties and Potential Antibacterial Activity. Molecules, 30(15), 3140. https://doi.org/10.3390/molecules30153140








