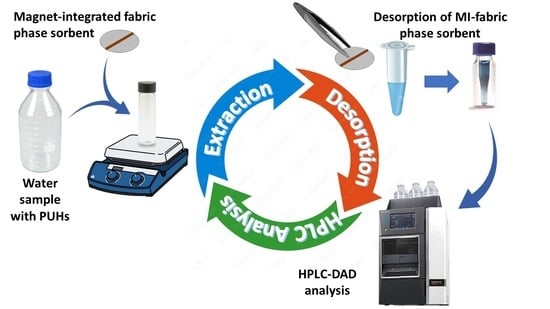
Determination of Phenylurea Herbicides in Water Samples by Magnet-Integrated Fabric Phase Sorptive Extraction Combined with High Performance Liquid Chromatography
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of the MI-FPSE Procedure
2.1.1. Optimization of the Adsorption Step
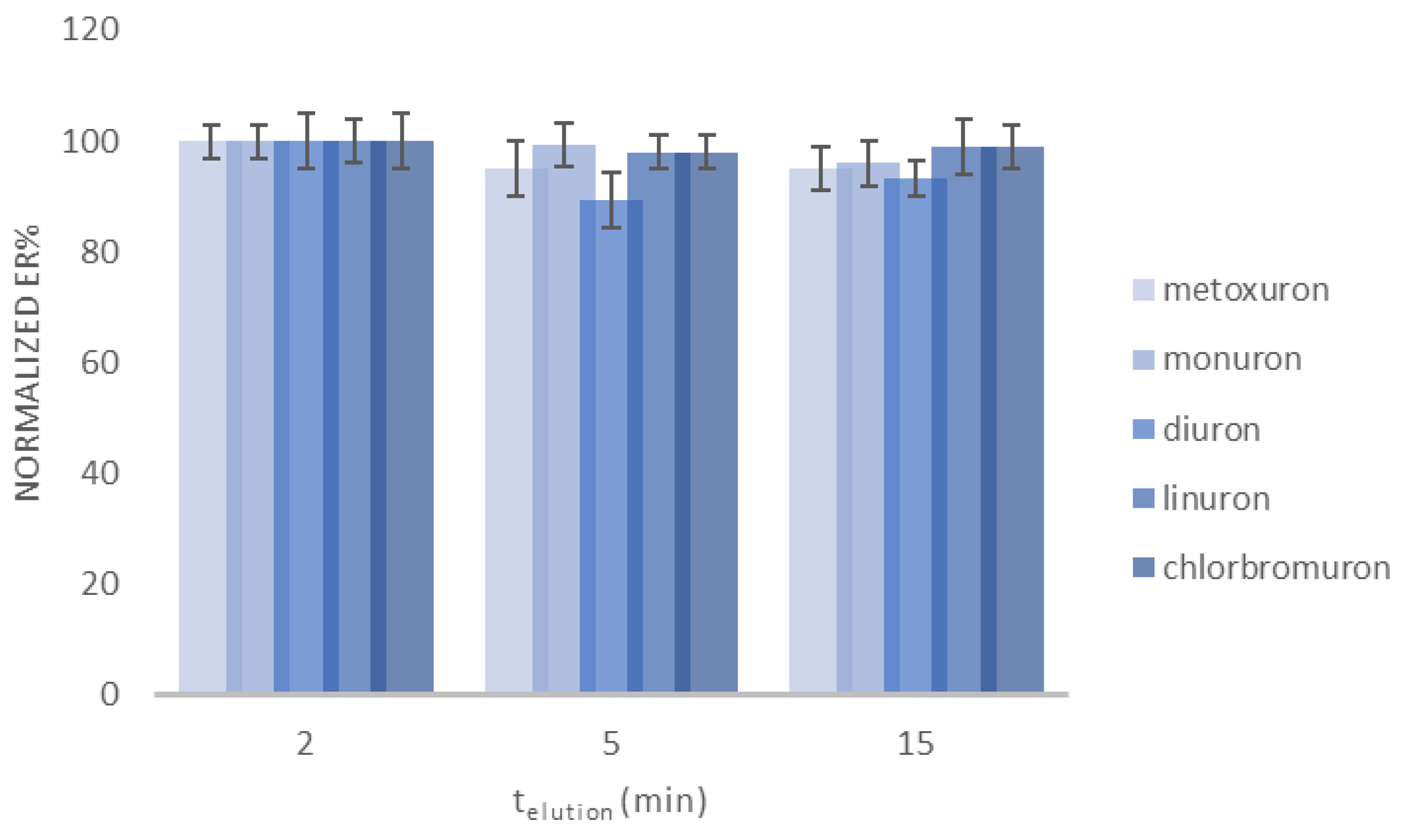
2.1.2. Optimization of the Desorption Step
2.2. Method Validation
2.3. Investigation of Method’s Green Character and Practicality
2.4. Comparison with Other Studies
2.5. Real Sample Analysis
3. Materials and Methods
3.1. Standards, Chemicals, and Samples
3.2. HPLC-DAD Analysis
3.3. MI-FPSE Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACE | Acetone |
| ACN | Acetonitrile |
| BAGI | Blue Applicability Grade Index |
| C18 | Octadecyl |
| ComplexGAPI | Complementary Green Analytical Procedure Index |
| CW 20 M | Carbowax 20 M |
| EF | Enhancement factor |
| EtOH | Ethanol |
| FPSE | Fabric phase sorptive extraction |
| GAC | Green Analytical Chemistry |
| GC | Gas chromatography |
| GSP | Green Sample Preparation |
| HPLC | High performance liquid chromatography |
| HPLC-DAD | High performance liquid chromatography-diode array detection |
| LOD | Limit of Detection |
| LOQ | Limit of Quantification |
| MeOH | Methanol |
| MI-FPSE | Magnet integrated-fabric phase sorptive extraction |
| PF | Preconcentration factor |
| PTHF | Poly(tetrahydrofuran) |
| PUH | Phenylurea herbicide |
| RR | Relative recovery |
| RSD | Relative standard deviation |
References
- Mou, R.X.; Chen, M.X.; Zhi, J.L. Simultaneous determination of 15 phenylurea herbicides in rice and corn using HPLC with fluorescence detection combined with UV decomposition and post-column derivatization. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2008, 875, 437–443. [Google Scholar] [CrossRef]
- Li, J.; Xu, X.; Zhang, F.; Guo, W.; Wang, X.; Xie, Y.; Zhang, F. Urea-based magnetic porous organic frameworks as novel adsorbent for the enrichment of phenylurea herbicides in foods. Food Chem. 2023, 425, 136436. [Google Scholar] [CrossRef]
- Mohanty, S.S.; Singh, P.; Nistala, S.; Mohanty, K. Understanding the environmental fate and removal strategies of phenylurea herbicides: A comprehensive review. J. Hazard. Mater. Adv. 2024, 16, 100496. [Google Scholar] [CrossRef]
- Guo, L.; Hao, L.; Gao, T.; Wang, C.; Wu, Q.; Wang, Z. p-Phenylenediamine-modified graphene oxide as a sorbent for solid-phase extraction of phenylurea herbicides, nitroimidazoles, chlorophenols, phenylurea insecticides and phthalates. Microchim. Acta 2019, 186, 464. [Google Scholar] [CrossRef]
- de Araújo, E.P.; Caldas, E.D.; Oliveira-Filho, E.C. Pesticides in surface freshwater: A critical review. Environ. Monit. Assess. 2022, 194, 452. [Google Scholar] [CrossRef]
- Musarurwa, H.; Chimuka, L.; Tavengwa, N.T. Green pre-concentration techniques during pesticide analysis in food samples. J. Environ. Sci. Health—Part B Pestic. Food Contam. Agric. Wastes 2019, 54, 770–780. [Google Scholar] [CrossRef]
- López-Lorente, Á.I.; Pena-Pereira, F.; Pedersen-Bjergaard, S.; Zuin, V.G.; Ozkan, S.A.; Psillakis, E. The ten principles of green sample preparation. TrAC—Trends Anal. Chem. 2022, 148, 116530. [Google Scholar] [CrossRef]
- Nasiri, M.; Ahmadzadeh, H.; Amiri, A. Sample preparation and extraction methods for pesticides in aquatic environments: A review. TrAC—Trends Anal. Chem. 2020, 123, 115772. [Google Scholar] [CrossRef]
- Ahmad, W.; Al-Sibaai, A.A.; Bashammakh, A.S.; Alwael, H.; El-Shahawi, M.S. Recent advances in dispersive liquid-liquid microextraction for pesticide analysis. TrAC—Trends Anal. Chem. 2015, 72, 181–192. [Google Scholar] [CrossRef]
- Beltran, J.; López, F.J.; Hernández, F. Solid-phase microextraction in pesticide residue analysis. J. Chromatogr. A 2000, 885, 389–404. [Google Scholar] [CrossRef]
- Han, J.H.; Cui, Y.Y.; He, X.Q.; Zhang, Y.; Yang, C.X. Fabrication of carboxyl functionalized microporous organic network coated stir bar for efficient extraction and analysis of phenylurea herbicides in food and water samples. J. Chromatogr. A 2021, 1640, 461947. [Google Scholar] [CrossRef]
- Musarurwa, H.; Chimuka, L.; Tavengwa, N.T. Metal organic framework-based magnetic solid phase extraction of pesticides in complex matrices. Microchem. J. 2021, 171, 106907. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, R.; Rani, S.; Malik, A.K.; Kabir, A.; Furton, K.G.; Samanidou, V.F. Rapid monitoring of organochlorine pesticide residues in various fruit juices and water samples using fabric phase sorptive extraction and gas chromatography-mass spectrometry. Molecules 2019, 24, 1013. [Google Scholar] [CrossRef]
- Kabir, A.; Samanidou, V. Fabric Phase Sorptive Extraction: A Paradigm Shift Approach in Analytical and Bioanalytical Sample Preparation. Molecules 2021, 26, 865. [Google Scholar] [CrossRef]
- Samanidou, V.F.; Kabir, A. Magnet Integrated Fabric Phase Sorptive Extraction (MI-FPSE): A Powerful Green(er) Alternative for Sample Preparation. Analytica 2022, 3, 439–447. [Google Scholar] [CrossRef]
- Manousi, N.; Alampanos, V.; Ferracane, A.; Efstratiadis, G.; Kabir, A.; Furton, K.G.; Tranchida, P.Q.; Zachariadis, G.A.; Mondello, L.; Rosenberg, E.; et al. Magnet integrated fabric phase sorptive extraction as a stand-alone extraction device for the monitoring of benzoyl urea insecticides in water samples by HPLC-DAD. J. Chromatogr. A 2022, 1672, 463026. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Wojnowski, W. Complementary green analytical procedure index (ComplexGAPI) and software. Green Chem. 2021, 23, 8657–8665. [Google Scholar] [CrossRef]
- Manousi, N.; Wojnowski, W.; Płotka-Wasylka, J.; Samanidou, V.F. Blue applicability grade index (BAGI) and software: A new tool for the evaluation of method practicality. Green Chem. 2023, 25, 7598–7604. [Google Scholar] [CrossRef]
- Jain, B.; Jain, R.; Kabir, A.; Sharma, S. Rapid Determination of Non-Steroidal Anti-Inflammatory Drugs in Urine Samples after In-Matrix Derivatization and Fabric Phase Sorptive Extraction-Gas Chromatography-Mass Spectrometry Analysis. Molecules 2022, 27, 7188. [Google Scholar] [CrossRef]
- Ulusoy, H.İ.; Köseoğlu, K.; Kabir, A.; Ulusoy, S.; Locatelli, M. Fabric phase sorptive extraction followed by HPLC-PDA detection for the monitoring of pirimicarb and fenitrothion pesticide residues. Microchim. Acta 2020, 187, 337. [Google Scholar] [CrossRef]
- Wang, S.; Ren, L.; Liu, C.; Ge, J.; Liu, F. Determination of five polar herbicides in water samples by ionic liquid dispersive liquid-phase microextraction. Anal. Bioanal. Chem. 2010, 397, 3089–3095. [Google Scholar] [CrossRef]
- Amir, S.; Shah, J.; Jan, M.R. Supramolecular solvent microextraction of phenylurea herbicides from environmental samples. Desalin. Water Treat. 2019, 148, 202–212. [Google Scholar] [CrossRef]
- Singh, B.; Kaur, M.; Malik, A.K. Determination of Phenylurea Herbicides in Tap Water and Soft Drink Samples by HPLC–UV and Solid-Phase Extraction. LCGC Eur. 2012, 23, 120–129. [Google Scholar]
- Saraji, M.; Mohammadipour, L.; Mehrafza, N. An effective configuration for automated magnetic micro solid-phase extraction of phenylurea herbicides from water samples followed by high-performance liquid chromatography. J. Chromatogr. A 2020, 1617, 460829. [Google Scholar] [CrossRef]
- Hong, K.; Huang, Y.; Zheng, L.; Zheng, X.; Huang, X. One-pot fabrication of poly (ionic liquid)s functionalized magnetic adsorbent for efficient enrichment of phenylurea herbicides in environmental waters. Anal. Chim. Acta 2022, 1198, 339549. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, J.; Zhang, H.; Zhang, Q.; Shen, J.; Wei, Y.; Wang, C. Facile construction of a stable core-shell spherically magnetic polyimide covalent organic framework for efficient extraction of phenylurea herbicides. Talanta 2024, 275, 126184. [Google Scholar] [CrossRef]
- Tartaglia, A.; Locatelli, M.; Kabir, A.; Furton, K.G.; Macerola, D.; Sperandio, E.; Piccolantonio, S.; Ulusoy, H.I.; Maroni, F.; Bruni, P.; et al. Comparison between Exhaustive and Equilibrium Extraction Using Different SPE Sorbents and Sol-Gel Carbowax 20M Coated FPSE Media. Molecules 2019, 24, 382. [Google Scholar] [CrossRef]




| Analyte | Regression Analysis | R2 | Working Range (μg L−1) | LOD (μg L−1) | LOQ (μg L−1) | ER% 1 | EF 2 |
|---|---|---|---|---|---|---|---|
| Metoxuron | y = 437x + 332 | 0.9960 | 1.0–100.0 | 0.3 | 1.0 | 60.3 | 50.1 |
| Monuron | y = 694x − 92 | 0.9991 | 1.0–100.0 | 0.3 | 1.0 | 51.4 | 42.6 |
| Diuron | y = 765x + 712 | 0.9968 | 1.0–100.0 | 0.3 | 1.0 | 61.4 | 50.9 |
| Linuron | y = 728x + 438 | 0.9987 | 1.0–100.0 | 0.3 | 1.0 | 61.6 | 51.1 |
| Chlorbromuron | y = 701x + 1046 | 0.9982 | 1.0–100.0 | 0.3 | 1.0 | 60.5 | 50.2 |
| Analyte | Added (μg L−1) | Intra-Day (n = 5) | Inter-Day (n = 3 × 3) | ||||
|---|---|---|---|---|---|---|---|
| Found (μg L−1) | RSD% | RR% | Found (μg L−1) | RSD% | RR% | ||
| Metoxuron | 5.0 | 4.6 ± 0.3 | 7 | 91.3 | 4.7 ± 0.3 | 7 | 93.1 |
| 50.0 | 51.3 ± 1.2 | 2 | 105.5 | 50.5 ± 0.4 | 8 | 101.1 | |
| Monuron | 5.0 | 4.7 ± 0.3 | 6 | 93.3 | 4.6 ± 0.4 | 8 | 92.6 |
| 50.0 | 55.0 ± 2.0 | 4 | 110.0 | 51.6 ± 0.6 | 11 | 103.2 | |
| Diuron | 5.0 | 4.8 ± 0.3 | 6 | 96.0 | 4.6 ± 0.3 | 7 | 92.8 |
| Linuron | 50.0 | 52.4 ± 1.8 | 3 | 104.8 | 49.1 ± 0.4 | 9 | 98.2 |
| 5.0 | 4.3 ± 0.2 | 4 | 85.2 | 4.5 ± 0.3 | 7 | 89.1 | |
| 50.0 | 52.4 ± 0.9 | 2 | 104.8 | 48.4 ± 0.6 | 12 | 96.8 | |
| Chlorbromuron | 5.0 | 4.3 ± 0.1 | 3 | 85.7 | 4.4 ± 0.3 | 6 | 87.7 |
| 50.0 | 52.1 ± 1.0 | 2 | 104.1 | 47.8 ± 0.6 | 13 | 95.6 | |
| Sample Preparation | PUHs | Analytical Technique | Sample Volume (mL) | RR% | RSD% | EF | LOD (μg L−1) | BAGI Pictogram | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Ionic liquid based on dispersive liquid-phase microextraction | Linuron, diuron | HPLC-DAD | 10 | 93.2–98.6 | 8.68–10.78 | N.A. 1 | 0.66–0.74 |  | [21] |
| Supramolecular solvent microextraction | Monuron, linuron, isoproturon | HPLC-UV | 20 | 80.0–99.0 | <7.2 (intra) <6.5 (inter) | N.A. | 10–30 |  | [22] |
| Automated magnetic micro solid-phase extraction | Monuron, linuron, tebuthiuron, isoproturon, | HPLC-UV | 20 | 80–116 | <3 (intra) <6 (inter) | 40–57 | 0.04 |  | [24] |
| Functionalized coated stir bar sorptive extraction | Chlortoluron, isoproturon, diuron, linuron | HPLC-DAD | 10 | 80.0–114.8 | <4.34 (intra) <8.57 (inter) | 46–49 | 0.025–0.070 |  | [11] |
| Magnetic micro solid-phase extraction | Monuron, chlorotoluron, isoproturon, monolinuron, buturon | HPLC-DAD | 50 | 80.6–118.0 | <9.6 (intra) <9.6 (inter) | 126–227 | 0.0025–0.015 |  | [25] |
| Magnetic micro solid-phase extraction | Monuron, chlortoluron, monolinuron, isoproturon, linuron | HPLC-UV | 10 | 74.5–111.4 | <12.7 (intra) <13.3 (inter) | N.A. | 0.06–0.10 |  | [26] |
| Solid phase extraction | Monuron, diuron, linuron, metoxuron, metazachlor | HPLC-UV | 20 | 75.0–90.1 | N.A. | N.A. | 0.82–1.29 |  | [23] |
| MI-FPSE | Metoxuron, monuron, diuron, linuron, chlorbromuron | HPLC-DAD | 25 | 85.2–110.0 | <7 (intra) <13% (inter) | 51.4–61.6 | 0.3 |  | This study |
| Analyte | Added (μg L−1) | Tap Water | River Water | Lake Water | |||
|---|---|---|---|---|---|---|---|
| Found (μg L−1) | RR% | Found (μg L−1) | RR% | Found (μg L−1) | RR% | ||
| Metoxuron | - | <LOD | - | <LOD | - | <LOD | - |
| 50.0 | 56.0 ± 1.1 | 112.0 | 48.4 ± 2.4 | 96.8 | 48.0 ± 1.4 | 96.1 | |
| Monuron | - | <LOD | - | <LOD | - | <LOD | - |
| 50.0 | 57.1 ± 0.4 | 114.3 | 46.3 ± 1.6 | 92.7 | 47.8 ± 0.3 | 95.6 | |
| Diuron | - | <LOD | - | <LOD | - | <LOD | - |
| Linuron | 50.0 | 49.7 ± 3.4 | 99.5 | 53.6 ± 3.4 | 107.2 | 47.1 ± 3.3 | 94.1 |
| - | <LOD | - | <LOD | - | <LOD | - | |
| 50.0 | 45.3 ± 1.6 | 90.6 | 50.1 ± 2.8 | 100.2 | 47.5 ± 3.4 | 94.9 | |
| Chlorbromuron | - | <LOD | - | <LOD | - | <LOD | - |
| 50.0 | 43.1 ± 2.9 | 86.5 | 46.1 ± 1.4 | 92.2 | 51.2 ± 3.0 | 102.4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manousi, N.; Tsiasioti, A.; Kabir, A.; Rosenberg, E. Determination of Phenylurea Herbicides in Water Samples by Magnet-Integrated Fabric Phase Sorptive Extraction Combined with High Performance Liquid Chromatography. Molecules 2025, 30, 3135. https://doi.org/10.3390/molecules30153135
Manousi N, Tsiasioti A, Kabir A, Rosenberg E. Determination of Phenylurea Herbicides in Water Samples by Magnet-Integrated Fabric Phase Sorptive Extraction Combined with High Performance Liquid Chromatography. Molecules. 2025; 30(15):3135. https://doi.org/10.3390/molecules30153135
Chicago/Turabian StyleManousi, Natalia, Apostolia Tsiasioti, Abuzar Kabir, and Erwin Rosenberg. 2025. "Determination of Phenylurea Herbicides in Water Samples by Magnet-Integrated Fabric Phase Sorptive Extraction Combined with High Performance Liquid Chromatography" Molecules 30, no. 15: 3135. https://doi.org/10.3390/molecules30153135
APA StyleManousi, N., Tsiasioti, A., Kabir, A., & Rosenberg, E. (2025). Determination of Phenylurea Herbicides in Water Samples by Magnet-Integrated Fabric Phase Sorptive Extraction Combined with High Performance Liquid Chromatography. Molecules, 30(15), 3135. https://doi.org/10.3390/molecules30153135