Amino Acid Substitutions in Bacteriocin Lactolisterin BU Reveal Functional Domains Involved in Biological Activity Against Staphylococcus aureus
Abstract
1. Introduction
2. Results
2.1. Lactolisterin LBU Structure Prediction and Variant Design
- 1.
- Helix I stability variants (G3A, G7A, W2E).
- 2.
- Pro-kink and its neighborhood (G13A, P14T, P14A).
- 3.
- Histidine functionality (H23A, H23R).
- 4.
- Double mutant (G3A/H23A).
- 5.
- BHT-B (BHT-B, N23H).
2.2. Antimicrobial Activity Assessment
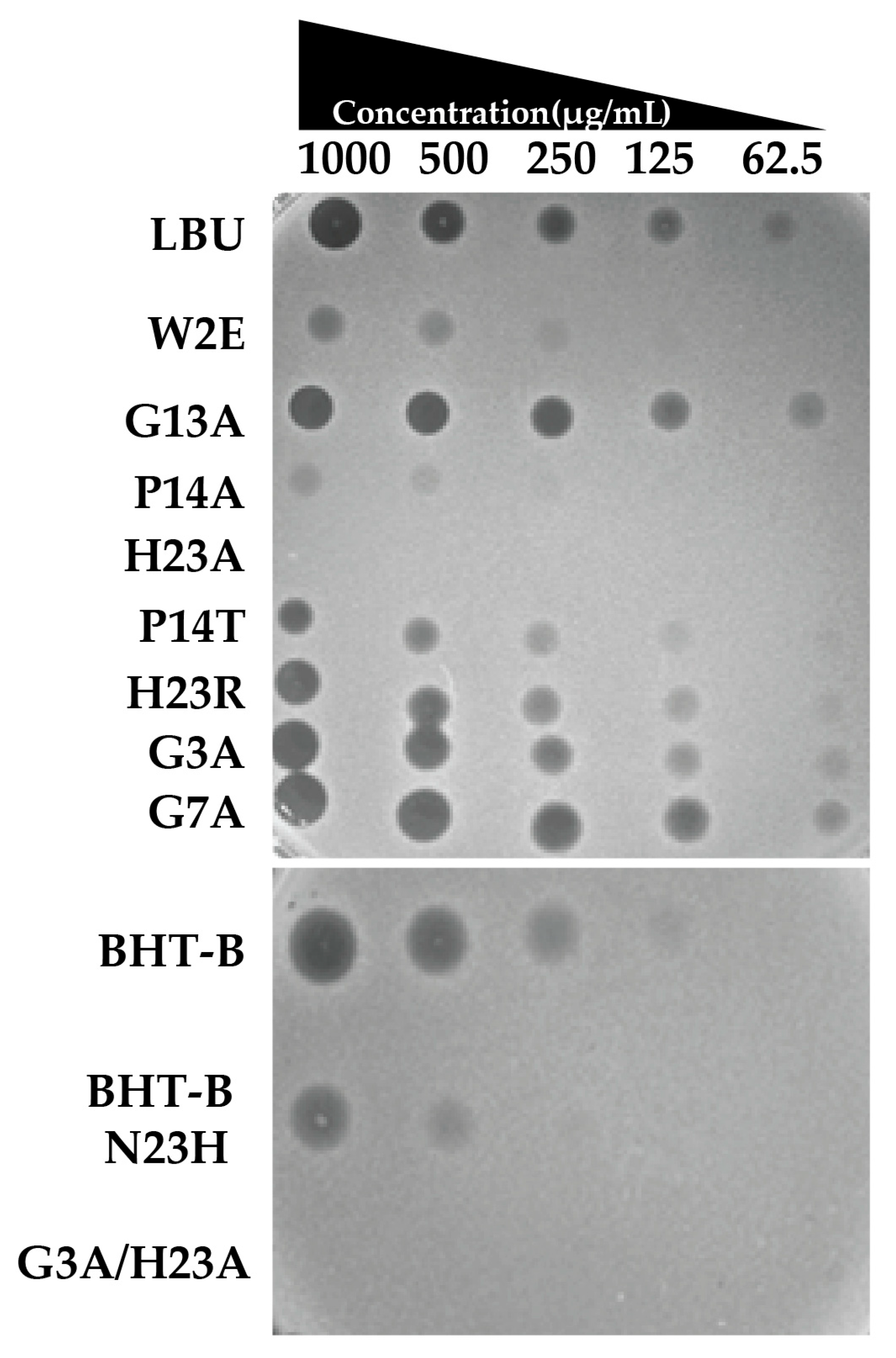
2.2.1. Spot-on-the-Lawn Antimicrobial Testing
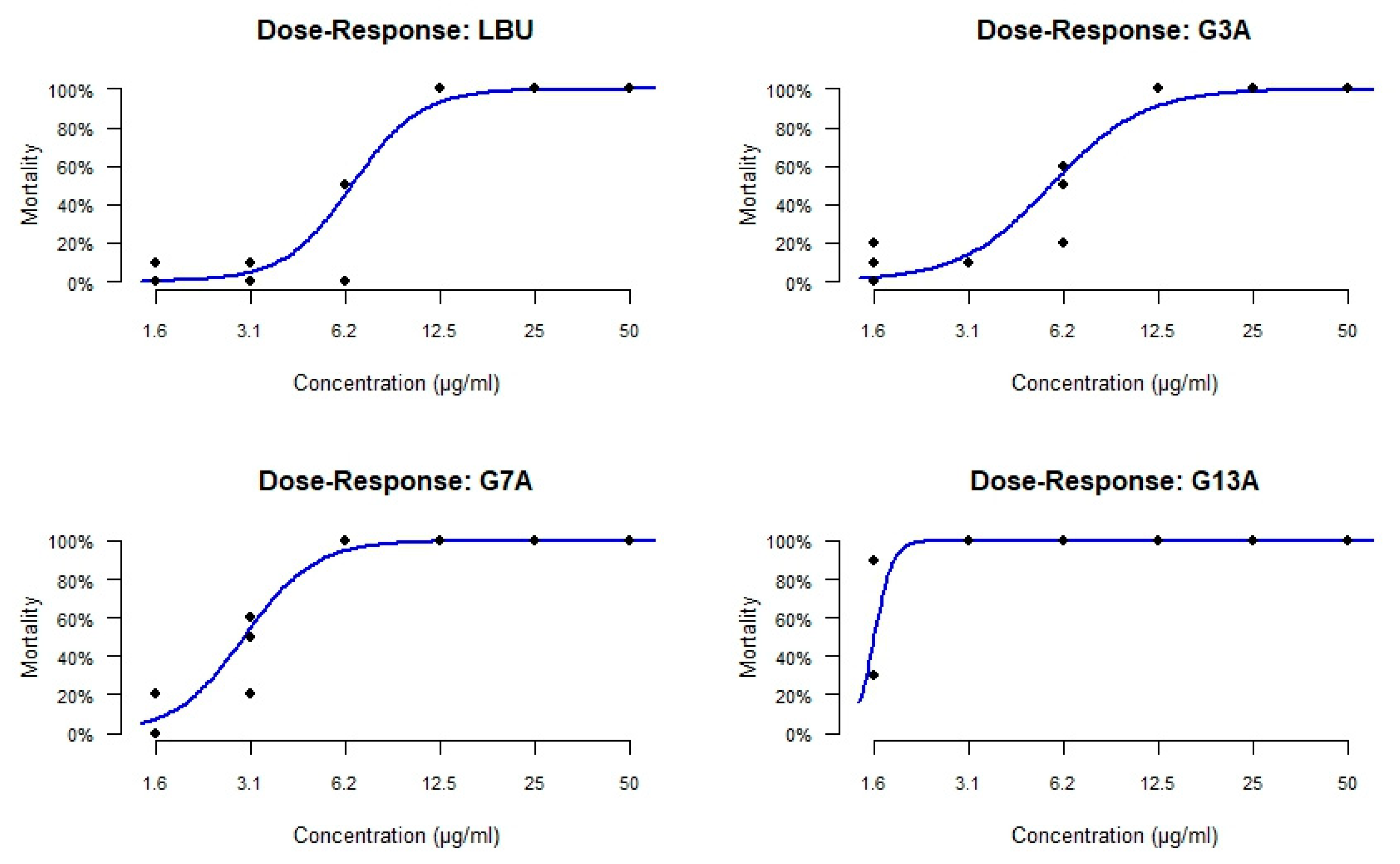
2.2.2. MIC and MBC
2.3. Safety Assessment
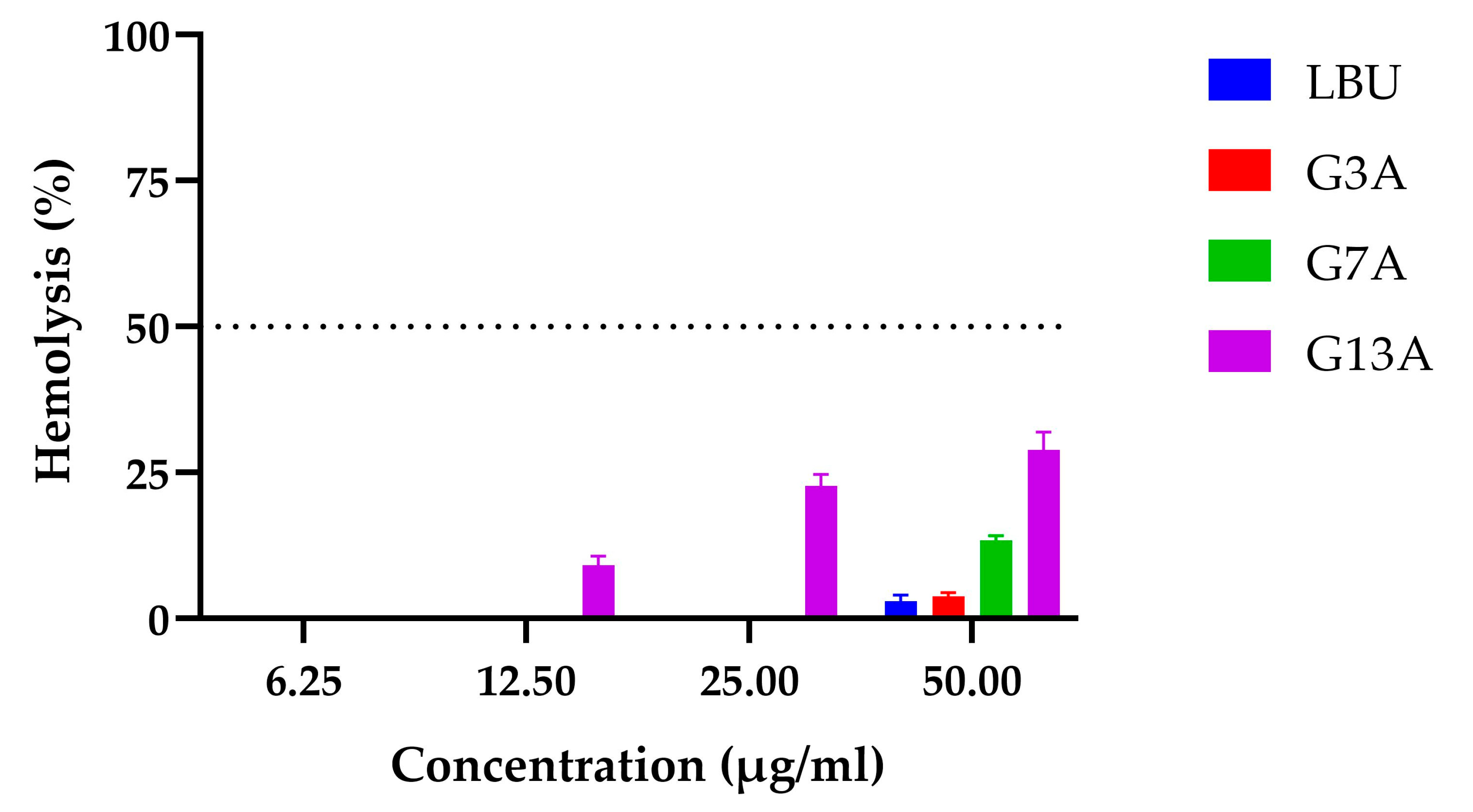
2.3.1. Hemolysis
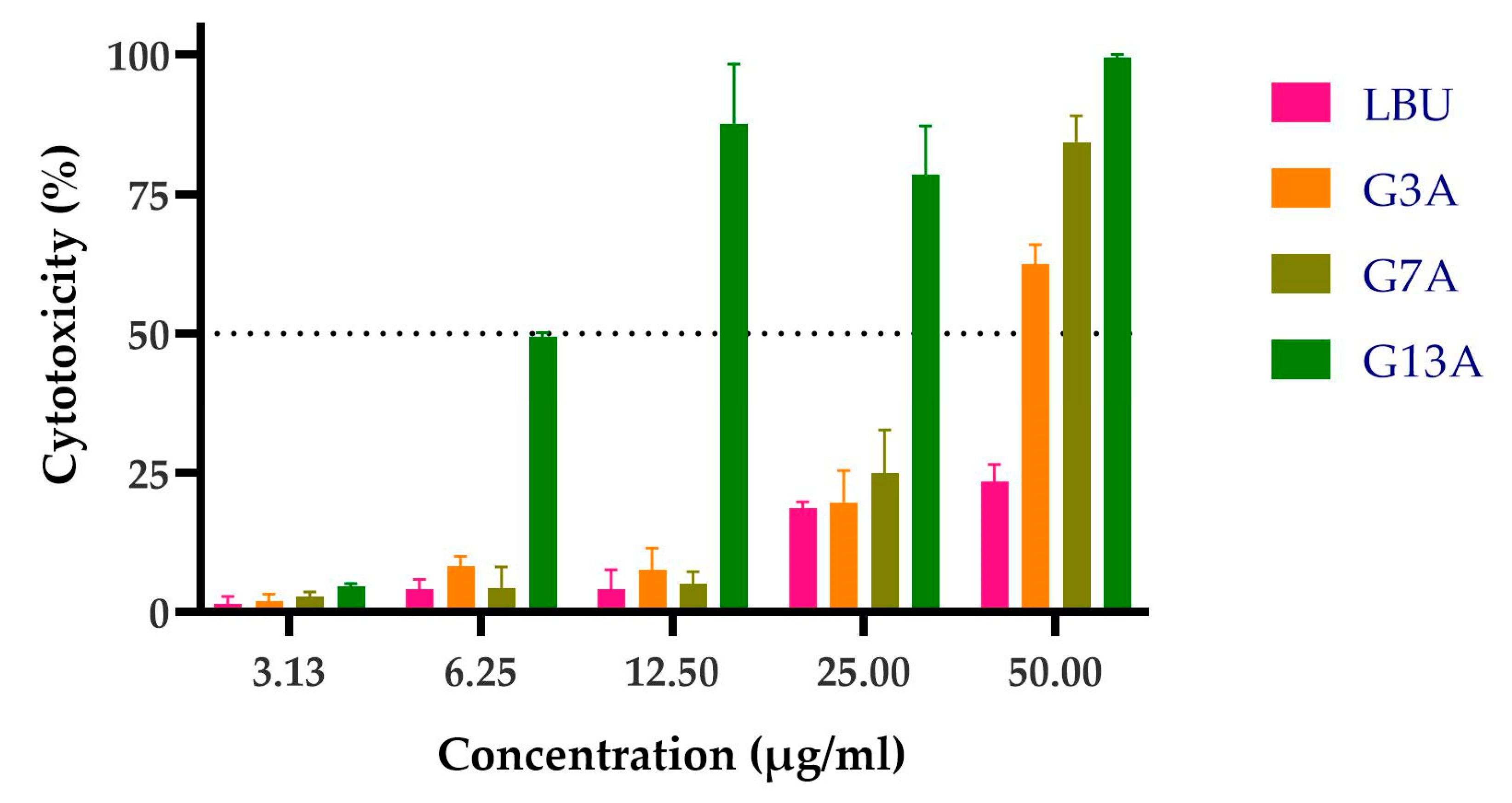
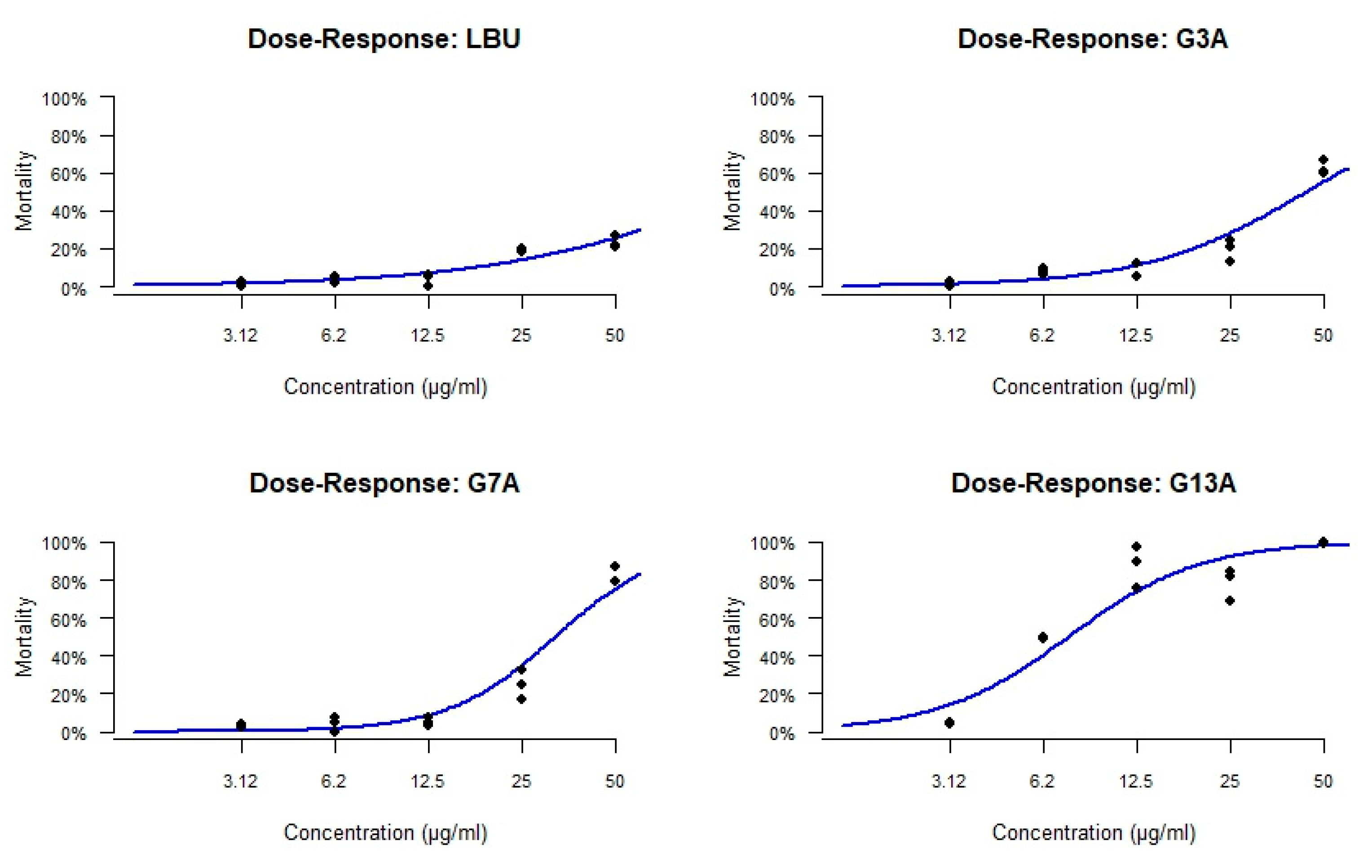
2.3.2. Cytotoxicity Toward Caco-2 Cells
2.3.3. In Vivo Toxicity Assessment
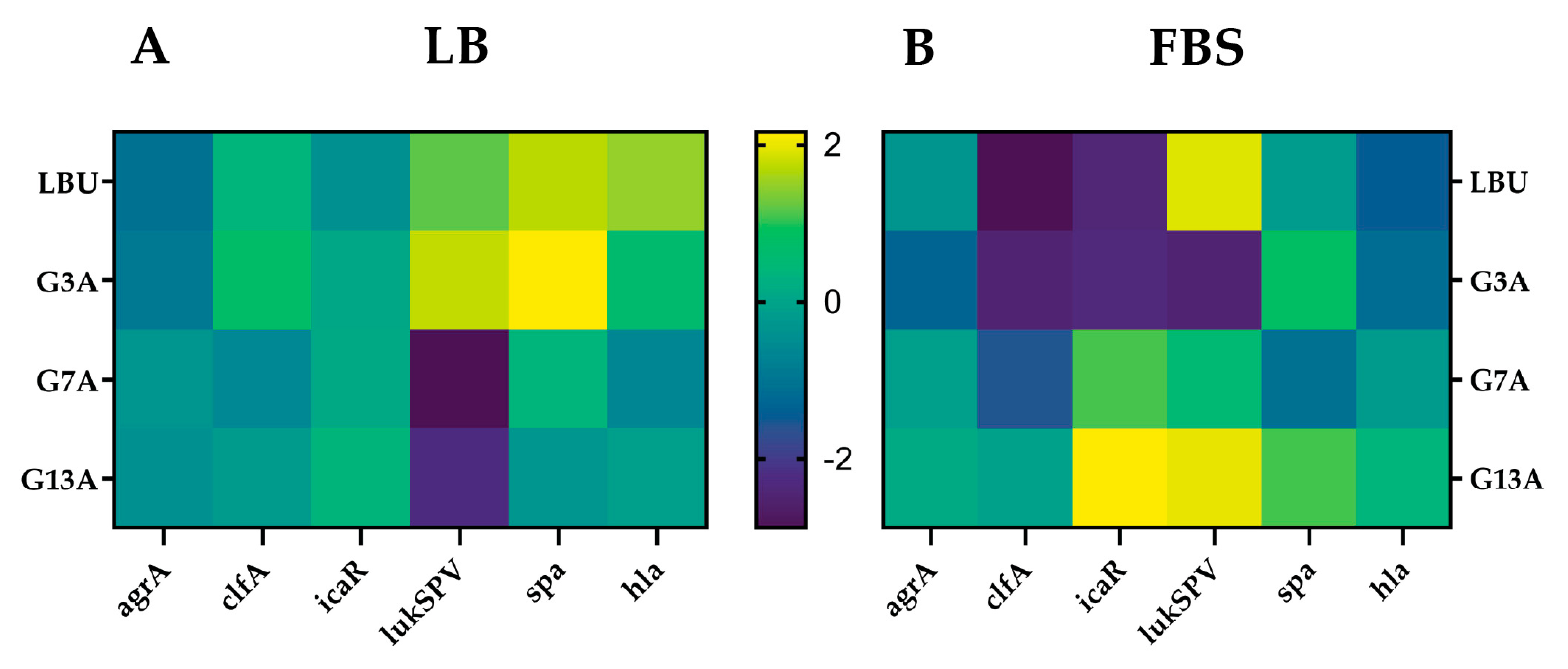
2.4. Exploratory Gene Expression Profiling of LBU and Its Variants
3. Discussion
3.1. Antimicrobial Potency and Mechanistic Implications
3.2. Cytotoxicity, Hemolysis, and In Vivo Safety
3.3. Virulence Modulation
3.4. Future Directions
4. Materials and Methods
4.1. Peptides
4.2. Structure Prediction
4.3. Bacterial Strain and Culture Conditions
4.4. Antimicrobial Activity
4.4.1. Broth Microdilution
4.4.2. Spot-on-Lawn Assay
4.5. Hemolysis Assay
4.6. In Vitro Caco-2 Toxicity
4.7. In Vivo Danio Rerio Embryo Toxicity
4.8. Virulence Gene Expression Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kwiecinski, J.M.; Horswill, A.R. Staphylococcus aureus bloodstream infections: Pathogenesis and regulatory mechanisms. Curr. Opin. Microbiol. 2020, 53, 51–60. [Google Scholar] [CrossRef]
- Naghavi, M.; Vollset, S.E.; Ikuta, K.S.; Swetschinski, L.R.; Gray, A.P.; Wool, E.E.; Robles Aguilar, G.; Mestrovic, T.; Smith, G.; Han, C.; et al. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef]
- Kumar, S.; Mahato, R.P.; Ch, S.; Kumbham, S. Current strategies against multidrug-resistant Staphylococcus aureus and advances toward future therapy. Microbe 2025, 6, 100281. [Google Scholar] [CrossRef]
- Kumar, N.R.; Balraj, T.A.; Kempegowda, S.N.; Prashant, A. Multidrug-Resistant Sepsis: A Critical Healthcare Challenge. Antibiotics 2024, 13, 46. [Google Scholar] [CrossRef]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef]
- Zhang, Q.-Y.; Yan, Z.-B.; Meng, Y.-M.; Hong, X.-Y.; Shao, G.; Ma, J.-J.; Cheng, X.-R.; Liu, J.; Kang, J.; Fu, C.-Y. Antimicrobial peptides: Mechanism of action, activity and clinical potential. Mil. Med. Res. 2021, 8, 48. [Google Scholar] [CrossRef]
- Simons, A.; Alhanout, K.; Duval, R.E. Bacteriocins, Antimicrobial Peptides from Bacterial Origin: Overview of Their Biology and Their Impact against Multidrug-Resistant Bacteria. Microorganisms 2020, 8, 639. [Google Scholar] [CrossRef]
- Meade, E.; Slattery, M.A.; Garvey, M. Bacteriocins, Potent Antimicrobial Peptides and the Fight against Multi Drug Resistant Species: Resistance Is Futile? Antibiotics 2020, 9, 32. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Oulahal, N.; Joly, C.; Degraeve, P. Nisin as a Food Preservative: Part 1: Physicochemical Properties, Antimicrobial Activity, and Main Uses. Crit. Rev. Food Sci. Nutr. 2016, 56, 1262–1274. [Google Scholar] [CrossRef]
- Christmann, J.; Cao, P.; Becker, J.; Desiderato, C.K.; Goldbeck, O.; Riedel, C.U.; Kohlstedt, M.; Wittmann, C. High-efficiency production of the antimicrobial peptide pediocin PA-1 in metabolically engineered Corynebacterium glutamicum using a microaerobic process at acidic pH and elevated levels of bivalent calcium ions. Microb. Cell Factories 2023, 22, 41. [Google Scholar] [CrossRef]
- Naskar, A.; Kim, K.S. Potential Novel Food-Related and Biomedical Applications of Nanomaterials Combined with Bacteriocins. Pharmaceutics 2021, 13, 86. [Google Scholar] [CrossRef]
- Heinzinger, L.R.; Pugh, A.R.; Wagner, J.A.; Otto, M. Evaluating the Translational Potential of Bacteriocins as an Alternative Treatment for Staphylococcus aureus Infections in Animals and Humans. Antibiotics 2023, 12, 1256. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Sun, T.; Xu, J. Bacteriocins in Cancer Treatment: Mechanisms and Clinical Potentials. Biomolecules 2024, 14, 831. [Google Scholar] [CrossRef]
- Hourigan, D.; Miceli de Farias, F.; O’Connor, P.M.; Hill, C.; Ross, R.P. Discovery and synthesis of leaderless bacteriocins from the Actinomycetota. J. Bacteriol. 2024, 206, e0029824. [Google Scholar] [CrossRef]
- Perez, R.H.; Zendo, T.; Sonomoto, K. Circular and Leaderless Bacteriocins: Biosynthesis, Mode of Action, Applications, and Prospects. Front. Microbiol. 2018, 9, 2085. [Google Scholar] [CrossRef]
- Zgheib, H.; Drider, D.; Belguesmia, Y. Broadening and Enhancing Bacteriocins Activities by Association with Bioactive Substances. Int. J. Environ. Res. Public Health 2020, 17, 7835. [Google Scholar] [CrossRef]
- Lozo, J.; Mirkovic, N.; O’Connor, P.M.; Malesevic, M.; Miljkovic, M.; Polovic, N.; Jovcic, B.; Cotter, P.D.; Kojic, M. Lactolisterin BU, a Novel Class II Broad-Spectrum Bacteriocin from Lactococcus lactis subsp. lactis bv. diacetylactis BGBU1-4. Appl. Environ. Microbiol. 2017, 83, e01519-17. [Google Scholar] [CrossRef]
- Malesevic, M.; Gardijan, L.; Miljkovic, M.; O’Connor, P.M.; Mirkovic, N.; Jovcic, B.; Cotter, P.D.; Jovanovic, G.; Kojic, M. Exploring the antibacterial potential of Lactococcus lactis subsp. lactis bv. diacetylactis BGBU1-4 by genome mining, bacteriocin gene overexpression, and chemical protein synthesis of lactolisterin BU variants. Lett. Appl. Microbiol. 2023, 76, ovad004. [Google Scholar] [CrossRef]
- Dong, B.; Zhou, G.; Lin, Y.; Yu, C.; Wang, J.; Sun, C.; Wu, T. Antimicrobial property of recombinant Lactolisterin BU in vitro and its initial application in pork refrigerated storage. Appl. Biol. Chem. 2021, 64, 76. [Google Scholar] [CrossRef]
- Mirkovic, N.; Kulas, J.; Miloradovic, Z.; Miljkovic, M.; Tucovic, D.; Miocinovic, J.; Jovcic, B.; Mirkov, I.; Kojic, M. Lactolisterin BU-producer Lactococcus lactis subsp. lactis BGBU1-4: Bio-control of Listeria monocytogenes and Staphylocococcus aureus in fresh soft cheese and effect on immunological response of rats. Food Control 2020, 111, 107076. [Google Scholar] [CrossRef]
- Bédard, F.; Biron, E. Recent Progress in the Chemical Synthesis of Class II and S-Glycosylated Bacteriocins. Front. Microbiol. 2018, 9, 1048. [Google Scholar] [CrossRef]
- Ferchichi, M.; Fathallah, M.; Mansuelle, P.; Rochat, H.; Sabatier, J.M.; Manai, M.; Mabrouk, K. Chemical synthesis, molecular modeling, and antimicrobial activity of a novel bacteriocin, MMFII. Biochem. Biophys. Res. Commun. 2001, 289, 13–18. [Google Scholar] [CrossRef]
- Bisset, S.W.; Yang, S.-H.; Amso, Z.; Harris, P.W.R.; Patchett, M.L.; Brimble, M.A.; Norris, G.E. Using Chemical Synthesis to Probe Structure–Activity Relationships of the Glycoactive Bacteriocin Glycocin F. ACS Chem. Biol. 2018, 13, 1270–1278. [Google Scholar] [CrossRef]
- Qing, R.; Hao, S.; Smorodina, E.; Jin, D.; Zalevsky, A.; Zhang, S. Protein Design: From the Aspect of Water Solubility and Stability. Chem. Rev. 2022, 122, 14085–14179. [Google Scholar] [CrossRef]
- Edwards, I.A.; Elliott, A.G.; Kavanagh, A.M.; Zuegg, J.; Blaskovich, M.A.T.; Cooper, M.A. Contribution of Amphipathicity and Hydrophobicity to the Antimicrobial Activity and Cytotoxicity of β-Hairpin Peptides. ACS Infect. Dis. 2016, 2, 442–450. [Google Scholar] [CrossRef]
- Strandberg, E.; Bentz, D.; Wadhwani, P.; Bürck, J.; Ulrich, A.S. Terminal charges modulate the pore forming activity of cationic amphipathic helices. Biochim. Biophys. Acta (BBA)—Biomembr. 2020, 1862, 183243. [Google Scholar] [CrossRef]
- Mathath, A.V.; Chakraborty, D. Effect of peptide hydrophilicity on membrane curvature and permeation. J. Chem. Phys. 2024, 161, 164105. [Google Scholar] [CrossRef]
- Varadi, M.; Bertoni, D.; Magana, P.; Paramval, U.; Pidruchna, I.; Radhakrishnan, M.; Tsenkov, M.; Nair, S.; Mirdita, M.; Yeo, J.; et al. AlphaFold Protein Structure Database in 2024: Providing structure coverage for over 214 million protein sequences. Nucleic Acids Res. 2023, 52, D368–D375. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Pirtskhalava, M.; Vishnepolsky, B.; Grigolava, M.; Managadze, G. Physicochemical Features and Peculiarities of Interaction of AMP with the Membrane. Pharmaceuticals 2021, 14, 471. [Google Scholar] [CrossRef]
- Pogozheva, I.D.; Mosberg, H.I.; Lomize, A.L. Life at the border: Adaptation of proteins to anisotropic membrane environment. Protein Sci. 2014, 23, 1165–1196. [Google Scholar] [CrossRef]
- Acedo, J.Z.; van Belkum, M.J.; Lohans, C.T.; Towle, K.M.; Miskolzie, M.; Vederas, J.C. Nuclear Magnetic Resonance Solution Structures of Lacticin Q and Aureocin A53 Reveal a Structural Motif Conserved among Leaderless Bacteriocins with Broad-Spectrum Activity. Biochemistry 2016, 55, 733–742. [Google Scholar] [CrossRef]
- Fujita, K.; Ichimasa, S.; Zendo, T.; Koga, S.; Yoneyama, F.; Nakayama, J.; Sonomoto, K. Structural analysis and characterization of lacticin Q, a novel bacteriocin belonging to a new family of unmodified bacteriocins of gram-positive bacteria. Appl. Environ. Microbiol. 2007, 73, 2871–2877. [Google Scholar] [CrossRef]
- Lefèvre, F.; Rémy, M.-H.; Masson, J.-M. Alanine-stretch scanning mutagenesis: A simple and efficient method to probe protein structure and function. Nucleic Acids Res. 1997, 25, 447–448. [Google Scholar] [CrossRef]
- López-Llano, J.; Campos, L.A.; Sancho, J. Alpha-helix stabilization by alanine relative to glycine: Roles of polar and apolar solvent exposures and of backbone entropy. Proteins 2006, 64, 769–778. [Google Scholar] [CrossRef]
- Högel, P.; Götz, A.; Kuhne, F.; Ebert, M.; Stelzer, W.; Rand, K.D.; Scharnagl, C.; Langosch, D. Glycine Perturbs Local and Global Conformational Flexibility of a Transmembrane Helix. Biochemistry 2018, 57, 1326–1337. [Google Scholar] [CrossRef]
- Meier, S.; Ridgway, Z.M.; Picciano, A.L.; Caputo, G.A. Impacts of Hydrophobic Mismatch on Antimicrobial Peptide Efficacy and Bilayer Permeabilization. Antibiotics 2023, 12, 1624. [Google Scholar] [CrossRef]
- Lander, A.J.; Mercado, L.D.; Li, X.; Taily, I.M.; Findlay, B.L.; Jin, Y.; Luk, L.Y.P. Roles of inter- and intramolecular tryptophan interactions in membrane-active proteins revealed by racemic protein crystallography. Commun. Chem. 2023, 6, 154. [Google Scholar] [CrossRef]
- Rozek, A.; Friedrich, C.L.; Hancock, R.E. Structure of the bovine antimicrobial peptide indolicidin bound to dodecylphosphocholine and sodium dodecyl sulfate micelles. Biochemistry 2000, 39, 15765–15774. [Google Scholar] [CrossRef]
- Tuerkova, A.; Kabelka, I.; Králová, T.; Sukeník, L.; Pokorná, Š.; Hof, M.; Vácha, R. Effect of helical kink in antimicrobial peptides on membrane pore formation. Elife 2020, 9, e47946. [Google Scholar] [CrossRef]
- Rex, S. A Pro→Ala substitution in melittin affects self-association, membrane binding and pore-formation kinetics due to changes in structural and electrostatic properties. Biophys. Chem. 2000, 85, 209–228. [Google Scholar] [CrossRef]
- Ballesteros, J.A.; Deupi, X.; Olivella, M.; Haaksma, E.E.; Pardo, L. Serine and threonine residues bend alpha-helices in the chi(1) = g(-) conformation. Biophys. J. 2000, 79, 2754–2760. [Google Scholar] [CrossRef]
- Hawkins, W.D.; Leary, K.A.; Andhare, D.; Popelka, H.; Klionsky, D.J.; Ragusa, M.J. Dimerization-dependent membrane tethering by Atg23 is essential for yeast autophagy. Cell Rep. 2022, 39, 110702. [Google Scholar] [CrossRef]
- McDonald, M.; Mannion, M.; Pike, D.; Lewis, K.; Flynn, A.; Brannan, A.M.; Browne, M.J.; Jackman, D.; Madera, L.; Power Coombs, M.R.; et al. Structure-function relationships in histidine-rich antimicrobial peptides from Atlantic cod. Biochim. Biophys. Acta (BBA)—Biomembr. 2015, 1848, 1451–1461. [Google Scholar] [CrossRef]
- Kacprzyk, L.; Rydengård, V.; Mörgelin, M.; Davoudi, M.; Pasupuleti, M.; Malmsten, M.; Schmidtchen, A. Antimicrobial activity of histidine-rich peptides is dependent on acidic conditions. Biochim. Biophys. Acta (BBA)—Biomembr. 2007, 1768, 2667–2680. [Google Scholar] [CrossRef]
- Hyink, O.; Balakrishnan, M.; Tagg, J.R. Streptococcus rattus strain BHT produces both a class I two-component lantibiotic and a class II bacteriocin. FEMS Microbiol. Lett. 2005, 252, 235–241. [Google Scholar] [CrossRef]
- Chakrabartty, A.; Schellman, J.A.; Baldwin, R.L. Large differences in the helix propensities of alanine and glycine. Nature 1991, 351, 586–588. [Google Scholar] [CrossRef]
- Ekblad, B.; Kyriakou, P.K.; Oppegård, C.; Nissen-Meyer, J.; Kaznessis, Y.N.; Kristiansen, P.E. Structure-Function Analysis of the Two-Peptide Bacteriocin Plantaricin EF. Biochemistry 2016, 55, 5106–5116. [Google Scholar] [CrossRef]
- Kazazic, M.; Nissen-Meyer, J.; Fimland, G. Mutational analysis of the role of charged residues in target-cell binding, potency and specificity of the pediocin-like bacteriocin sakacin P. Microbiology 2002, 148, 2019–2027. [Google Scholar] [CrossRef][Green Version]
- Gor, M.-C.; Vezina, B.; McMahon, R.M.; King, G.J.; Panjikar, S.; Rehm, B.H.A.; Martin, J.L.; Smith, A.T. Crystal structure and site-directed mutagenesis of circular bacteriocin plantacyclin B21AG reveals cationic and aromatic residues important for antimicrobial activity. Sci. Rep. 2020, 10, 17398. [Google Scholar] [CrossRef]
- Oppegård, C.; Fimland, G.; Thorbaek, L.; Nissen-Meyer, J. Analysis of the two-peptide bacteriocins lactococcin G and enterocin 1071 by site-directed mutagenesis. Appl. Environ. Microbiol. 2007, 73, 2931–2938. [Google Scholar] [CrossRef]
- Zheng, Y.; Du, Y.; Qiu, Z.; Liu, Z.; Qiao, J.; Li, Y.; Caiyin, Q. Nisin Variants Generated by Protein Engineering and Their Properties. Bioengineering 2022, 9, 251. [Google Scholar] [CrossRef]
- Murinda, S.; Rashid, K.A.; Roberts, R. In Vitro Assessment of the Cytotoxicity of Nisin, Pediocin, and Selected Colicins on Simian Virus 40–Transfected Human Colon and Vero Monkey Kidney Cells with Trypan Blue Staining Viability Assays. J. Food Prot. 2003, 66, 847–853. [Google Scholar] [CrossRef]
- Chen, Y.; Mant, C.T.; Farmer, S.W.; Hancock, R.E.; Vasil, M.L.; Hodges, R.S. Rational design of alpha-helical antimicrobial peptides with enhanced activities and specificity/therapeutic index. J. Biol. Chem. 2005, 280, 12316–12329. [Google Scholar] [CrossRef]
- Greco, I.; Molchanova, N.; Holmedal, E.; Jenssen, H.; Hummel, B.D.; Watts, J.L.; Håkansson, J.; Hansen, P.R.; Svenson, J. Correlation between hemolytic activity, cytotoxicity and systemic in vivo toxicity of synthetic antimicrobial peptides. Sci. Rep. 2020, 10, 13206. [Google Scholar] [CrossRef]
- Li, M.; Lai, Y.; Villaruz, A.E.; Cha, D.J.; Sturdevant, D.E.; Otto, M. Gram-positive three-component antimicrobial peptide-sensing system. Proc. Natl. Acad. Sci. USA 2007, 104, 9469–9474. [Google Scholar] [CrossRef]
- Liu, Q.; Zheng, Z.; Kim, W.; Burgwyn Fuchs, B.; Mylonakis, E. Influence of subinhibitory concentrations of NH125 on biofilm formation & virulence factors of Staphylococcus aureus. Future Med. Chem. 2018, 10, 1319–1331. [Google Scholar] [CrossRef]
- Vasilchenko, A.S.; Rogozhin, E.A. Sub-inhibitory Effects of Antimicrobial Peptides. Front. Microbiol. 2019, 10, 1160. [Google Scholar] [CrossRef]
- Gottschalk, S.; Ingmer, H.; Thomsen, L.E. The lysine-peptoid hybrid LP5 maintain activity under physiological conditions and affects virulence gene expression in Staphylococcus aureus. Peptides 2016, 78, 24–29. [Google Scholar] [CrossRef]
- Maali, Y.; Badiou, C.; Martins-Simões, P.; Hodille, E.; Bes, M.; Vandenesch, F.; Lina, G.; Diot, A.; Laurent, F.; Trouillet-Assant, S. Understanding the Virulence of Staphylococcus pseudintermedius: A Major Role of Pore-Forming Toxins. Front. Cell Infect. Microbiol. 2018, 8, 221. [Google Scholar] [CrossRef]
- Abraham, N.M.; Jefferson, K.K. A low molecular weight component of serum inhibits biofilm formation in Staphylococcus aureus. Microb. Pathog. 2010, 49, 388–391. [Google Scholar] [CrossRef]
- Novick, R.P.; Geisinger, E. Quorum sensing in staphylococci. Annu. Rev. Genet. 2008, 42, 541–564. [Google Scholar] [CrossRef]
- Jesus, C.; Soares, R.; Cunha, E.; Grilo, M.; Tavares, L.; Oliveira, M. Influence of Nisin-Biogel at Subinhibitory Concentrations on Virulence Expression in Staphylococcus aureus Isolates from Diabetic Foot Infections. Antibiotics 2021, 10, 1501. [Google Scholar] [CrossRef]
- Tan, L.; Li, S.R.; Jiang, B.; Hu, X.M.; Li, S. Therapeutic Targeting of the Staphylococcus aureus Accessory Gene Regulator (agr) System. Front. Microbiol. 2018, 9, 55. [Google Scholar] [CrossRef]
- Herman-Bausier, P.; Labate, C.; Towell, A.M.; Derclaye, S.; Geoghegan, J.A.; Dufrêne, Y.F. Staphylococcus aureus clumping factor A is a force-sensitive molecular switch that activates bacterial adhesion. Proc. Natl. Acad. Sci. USA 2018, 115, 5564–5569. [Google Scholar] [CrossRef]
- Jenul, C.; Horswill, A.R. Regulation of Staphylococcus aureus Virulence. Microbiol. Spectr. 2019, 7, 2. [Google Scholar] [CrossRef]
- Cerca, N.; Brooks, J.L.; Jefferson, K.K. Regulation of the intercellular adhesin locus regulator (icaR) by SarA, sigmaB, and IcaR in Staphylococcus aureus. J. Bacteriol. 2008, 190, 6530–6533. [Google Scholar] [CrossRef]
- Löffler, B.; Hussain, M.; Grundmeier, M.; Brück, M.; Holzinger, D.; Varga, G.; Roth, J.; Kahl, B.C.; Proctor, R.A.; Peters, G. Staphylococcus aureus panton-valentine leukocidin is a very potent cytotoxic factor for human neutrophils. PLoS Pathog. 2010, 6, e1000715. [Google Scholar] [CrossRef]
- Falugi, F.; Kim, H.K.; Missiakas, D.M.; Schneewind, O. Role of protein A in the evasion of host adaptive immune responses by Staphylococcus aureus. mBio 2013, 4, e00575-13. [Google Scholar] [CrossRef]
- Oogai, Y.; Matsuo, M.; Hashimoto, M.; Kato, F.; Sugai, M.; Komatsuzawa, H. Expression of virulence factors by Staphylococcus aureus grown in serum. Appl. Environ. Microbiol. 2011, 77, 8097–8105. [Google Scholar] [CrossRef]
- Chaffin, D.O.; Taylor, D.; Skerrett, S.J.; Rubens, C.E. Changes in the Staphylococcus aureus Transcriptome during Early Adaptation to the Lung. PLoS ONE 2012, 7, e41329. [Google Scholar] [CrossRef]
- Kumar, N.; Kumar, V.; Waheed, S.M.; Pradhan, D. Efficacy of Reuterin and Bacteriocins Nisin and Pediocin in the Preservation of Raw Milk from Dairy Farms. Food Technol. Biotechnol. 2020, 58, 359–369. [Google Scholar] [CrossRef]
- Gautier, R.; Douguet, D.; Antonny, B.; Drin, G. HELIQUEST: A web server to screen sequences with specific alpha-helical properties. Bioinformatics 2008, 24, 2101–2102. [Google Scholar] [CrossRef]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.e.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein Identification and Analysis Tools on the ExPASy Server. In The Proteomics Protocols Handbook; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2005; pp. 571–607. [Google Scholar]
- Kojic, M.; Strahinic, I.; Fira, D.; Jovcic, B.; Topisirovic, L. Plasmid content and bacteriocin production by five strains of Lactococcus lactis isolated from semi-hard homemade cheese. Can. J. Microbiol. 2006, 52, 1110–1120. [Google Scholar] [CrossRef]
- Popović, N.; Djokić, J.; Brdarić, E.; Dinić, M.; Terzić-Vidojević, A.; Golić, N.; Veljović, K. The Influence of Heat-Killed Enterococcus faecium BGPAS1-3 on the Tight Junction Protein Expression and Immune Function in Differentiated Caco-2 Cells Infected With Listeria monocytogenes ATCC 19111. Front. Microbiol. 2019, 10, 412. [Google Scholar] [CrossRef]
- Aleksic, I.; Ristivojevic, P.; Pavic, A.; Radojević, I.; Čomić, L.R.; Vasiljevic, B.; Opsenica, D.; Milojković-Opsenica, D.; Senerovic, L. Anti-quorum sensing activity, toxicity in zebrafish (Danio rerio) embryos and phytochemical characterization of Trapa natans leaf extracts. J. Ethnopharmacol. 2018, 222, 148–158. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-Response Analysis Using R. PLoS ONE 2016, 10, e0146021. [Google Scholar] [CrossRef]
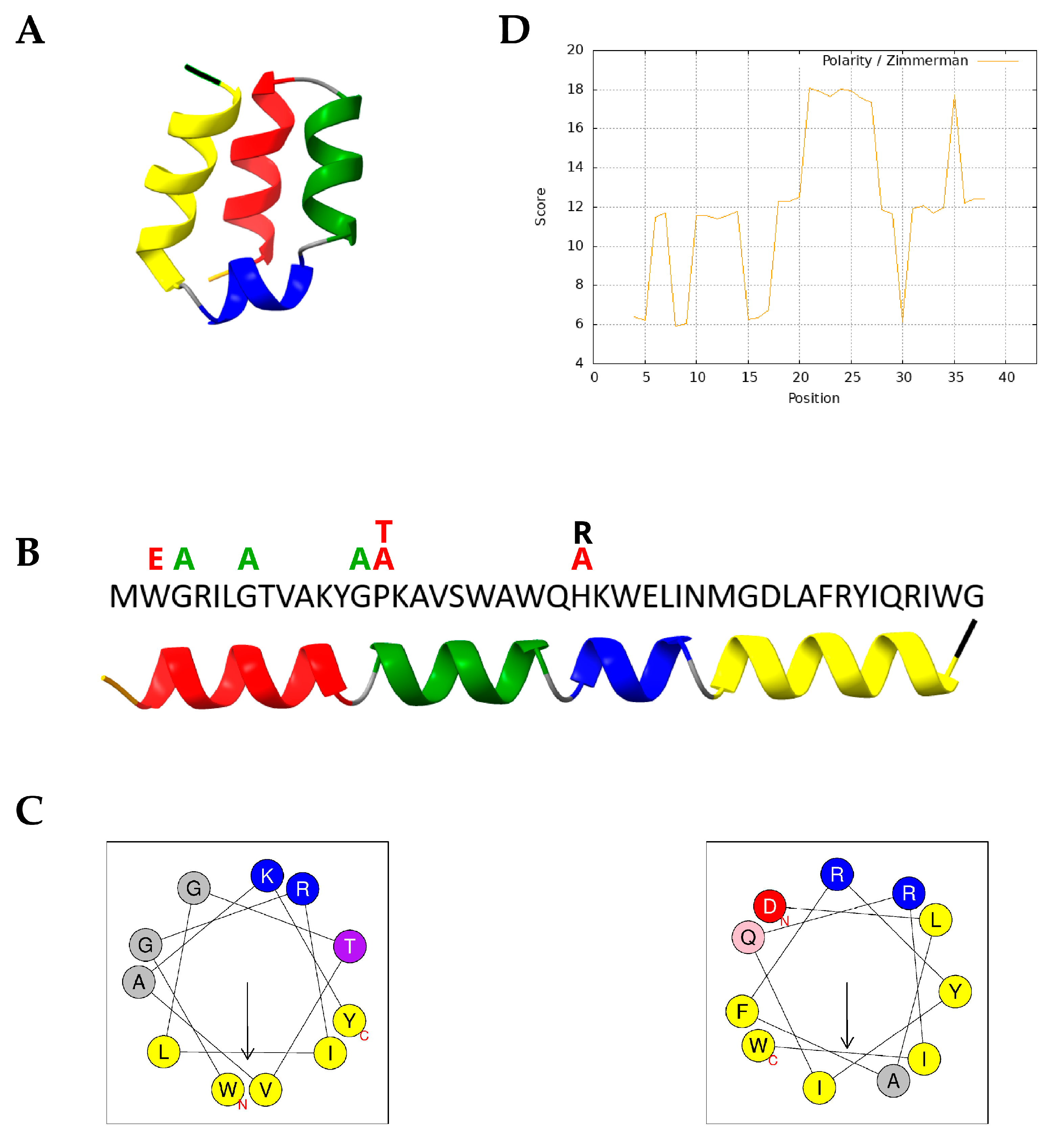

| Group | Variant Name | Variation | Region |
|---|---|---|---|
| I helix stability | G3A | Gly3 → Ala | N-terminus of I helix |
| G7A | Gly7 → Ala | Core of I helix | |
| W2E | Try2 → Glu | N-terminus of I helix | |
| Proline role | G13A | Gly13 → Ala | Adjacent to proline kink |
| P14T | Pro14 → Thr | Proline kink | |
| P14A | Pro14 → Ala | Proline kink | |
| Histidine role | H23A | His23 → Ala | Histidine |
| H23R | His23 → Arg | Histidine | |
| Double mutant | G3A/H23A | Gly3 and H23 → Ala | I helix and histidine |
| BHT-B | BHT-B | Original sequence | - |
| BHT-B_N23H | Asn23 → His | Asparagine |
| Peptide | MIC (µg/mL) | MBC (µg/mL) |
|---|---|---|
| LBU | 12.5 | 25 |
| W2E | >50 | >50 |
| G3A | 6.25 | 25 |
| G7A | 6.25 | 25 |
| G13A | 6.25 | 12.5 |
| P14A | 25 | >50 |
| P14T | 25 | >50 |
| H23R | 12.5 | 50 |
| H23A | >50 | >50 |
| G3A_H23A | >50 | >50 |
| BHT-B | 12.5 | 25 |
| BHT-B_N23H | 50 | >50 |
| Peptide | LC50 | MIC (µg/mL) | TI |
|---|---|---|---|
| LBU | >50 | 12.5 | >4 |
| G3A | 43.91 ± 2.47 | 6.25 | 7.03 |
| G7A | 31.93 ± 1.07 | 6.25 | 5.11 |
| G13A | 7.49 ± 0.26 | 6.25 | 1.20 |
| Peptide | LC50 | MIC (µg/mL) | TI |
|---|---|---|---|
| LBU | 6.54 ± 0.49 | 12.5 | 0.52 |
| G3A | 5.69 ± 0.5 | 6.25 | 0.91 |
| G7A | 2.98 ± 0.23 | 6.25 | 0.48 |
| G13A | 1.56 ± 0.04 | 6.25 | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gardijan, L.; Malešević, M.; Dinić, M.; Pavić, A.; Plačkić, N.; Jovanović, G.; Kojić, M. Amino Acid Substitutions in Bacteriocin Lactolisterin BU Reveal Functional Domains Involved in Biological Activity Against Staphylococcus aureus. Molecules 2025, 30, 3134. https://doi.org/10.3390/molecules30153134
Gardijan L, Malešević M, Dinić M, Pavić A, Plačkić N, Jovanović G, Kojić M. Amino Acid Substitutions in Bacteriocin Lactolisterin BU Reveal Functional Domains Involved in Biological Activity Against Staphylococcus aureus. Molecules. 2025; 30(15):3134. https://doi.org/10.3390/molecules30153134
Chicago/Turabian StyleGardijan, Lazar, Milka Malešević, Miroslav Dinić, Aleksandar Pavić, Nikola Plačkić, Goran Jovanović, and Milan Kojić. 2025. "Amino Acid Substitutions in Bacteriocin Lactolisterin BU Reveal Functional Domains Involved in Biological Activity Against Staphylococcus aureus" Molecules 30, no. 15: 3134. https://doi.org/10.3390/molecules30153134
APA StyleGardijan, L., Malešević, M., Dinić, M., Pavić, A., Plačkić, N., Jovanović, G., & Kojić, M. (2025). Amino Acid Substitutions in Bacteriocin Lactolisterin BU Reveal Functional Domains Involved in Biological Activity Against Staphylococcus aureus. Molecules, 30(15), 3134. https://doi.org/10.3390/molecules30153134







