Catechins and Human Health: Breakthroughs from Clinical Trials
Abstract
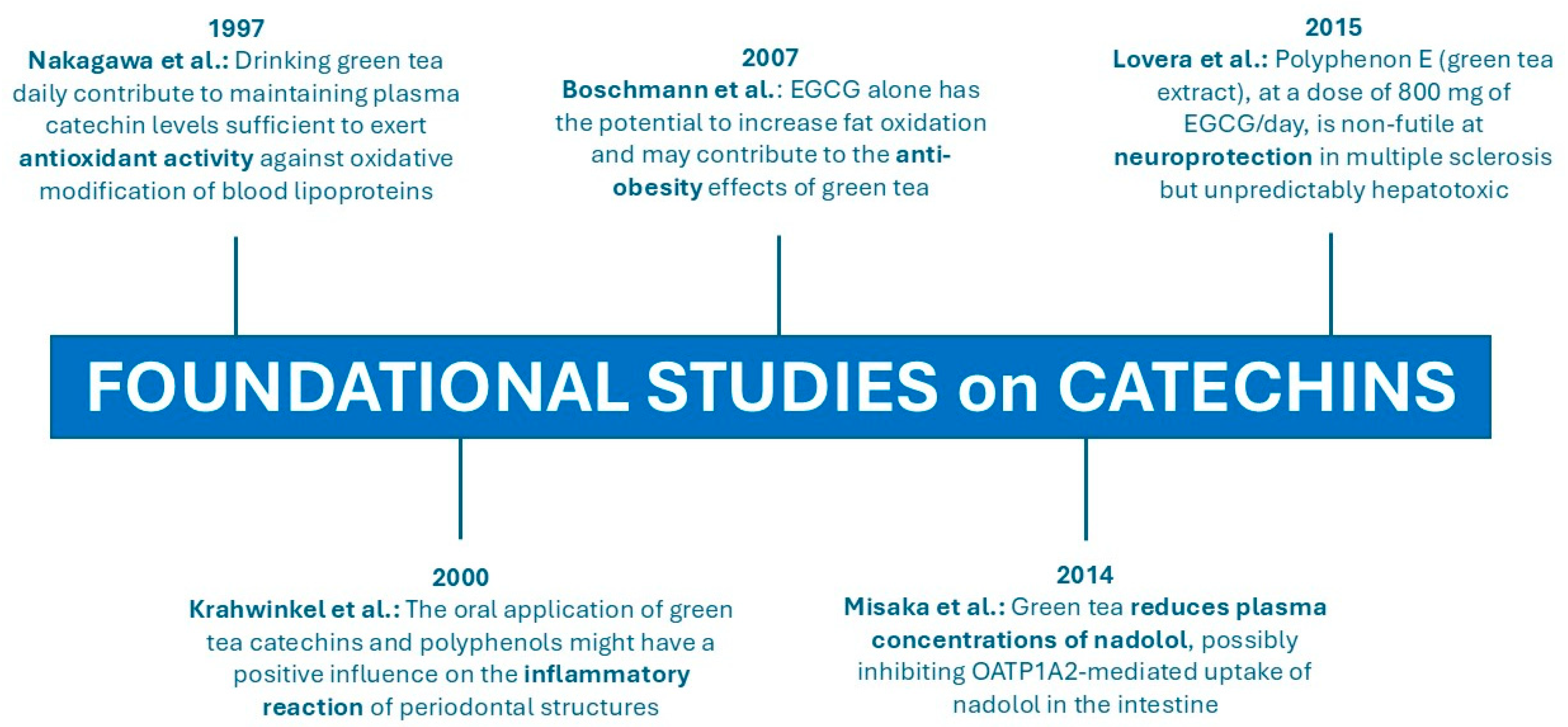
1. Introduction
1.1. Epigallocatechin Gallate: Molecular Targets, Bioavailability, and Safety Considerations
1.1.1. Molecular Targets
1.1.2. Bioavailability
1.1.3. Evidence and Variables of Hepatotoxicity
| Population | Catechin Dose | Treatment Period | Hepatotoxicity (Cases/Grade) * | Reference |
|---|---|---|---|---|
| Breast cancer patients (n = 30 women) | 2–4 capsules Polyphenon E (1600 mg EGCG/day) | 6 months | 2/grade 1 1/grade 3 | Crew et al. (2012) [33] |
| Chronic lymphocytic leukemia patients (n = 42) | 5 or 10 capsules Polyphenon E (2000 mg EGCG and 4000 mg EGCG/day) with meals | 6 months | 13/grade 1 6/grade 2 1/grade 3 | Shanafelt et al. (2013) [34] |
| Healthy women (n = 41) | Capsules of Polyphenon E (800 mg EGCG/day) with meals | 4 months | 1/grade 3 (9 with elevated enzymes) | Garcia et al. (2014) [35] |
| Postmenopausal women (n = 799) | 843 + 44 mg/day GTE capsules (n = 400) vs. placebo (n = 399) with meals | 12 months | 43/grade 1 (4 placebo group) 7/grade 2 6/grade 3 1/grade 4 | Dostal et al. (2015) [36] |
| Multiple sclerosis patients (n = 13) | 2 capsules Polyphenon E (800 mg/day EGCG) (n = 8) vs. placebo (n = 5) with meals | 6 months | 1/grade 3 | Lovera et al. (2015) [37] |
| Multiple sclerosis patients (n = 7) | 2 capsules Polyphenon E (800 mg/day EGCG) with meals | 1 year | 4/grade 1 1/grade 4 | Lovera et al. (2015) [37] |
1.1.4. Long-Term Safety
2. Main Topics Informing Clinical Study Categorization
3. Major Findings from the Clinical Trials Reviewed
3.1. Toxicity and Detoxification Effect of Catechins
3.2. Effect of Catechins on Drug Pharmacokinetics
3.3. Effect of Catechins on Cognitive Functions
3.4. Anti-Inflammatory and Antioxidant Effects of EGCG
3.5. Effect of EGCG on Obesity and Metabolism
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABCB1 | ATP-binding cassette (ABC) transporter B1 |
| ALT | Alanine transaminase |
| AKT1 | Serine/threonine kinase 1 |
| AST | Aspartate transaminase |
| BMI | Body mass index |
| C | Catechin |
| COMT | Catechol-O-methyltransferase (COMT) |
| DBP | Diastolic blood pressure |
| DILIN | Drug-Induced Liver Injury Network |
| DS | Down syndrome |
| EC | Epicatechin |
| ECG | Epicatechin gallate |
| EFSA | European Food Safety Authority |
| EGC | Epigallocatechin |
| EGCG | Epigallocatechin-3-gallate |
| EGFR | Epidermal growth factor receptor |
| ESR1 | Estrogen Receptor 1 |
| GSH | Reduced glutathione |
| GSSG | Oxidized glutathione |
| GTE | Green tea extract |
| HDL-C | High-density lipoprotein cholesterol |
| HF | High frequency |
| IGF1 | Insulin-like growth factor |
| ILD | Interstitial lung disease |
| IL-1β | Interleukin 1beta |
| IL-6 | Interleukin 6 |
| JAK | Janus kinase |
| LDL-C | Low-density lipoprotein cholesterol |
| LF | Low frequency |
| MAP | Mean arterial pressure |
| MAPK1 | Mitogen-activated protein kinase 1 |
| NCI CTCAE | National Cancer Institute Common Terminology Criteria for Adverse Events |
| NF-kB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NLC | Nanostructured lipid carrier |
| OATP | Organic anion-transporting polypeptide |
| PI3K | Phosphoinositide 3-kinase |
| PLPro | Papain-like protease |
| RES | Resveratrol |
| RID | Radiation-induced dermatitis |
| ROS | Reactive oxygen species |
| SBP | Systolic blood pressure |
| SkMOx | Mitochondrial respiration in permeabilized skeletal muscle fibers |
| SLN | Solid lipid nanoparticle |
| STAT3 | Signal transducer and activator of transcription 3 |
| TGF-ß1 | Transforming growth factor beta 1 |
| TP | Tea Polyphenols |
| UGT1A4 | Uridine 5′-diphospho-glucuronosyltransferase 1A4 |
| 67LR | 67-kDa laminin receptor |
References
- Franks, M.; Lawrence, P.; Abbaspourrad, A.; Dando, R. The Influence of Water Composition on Flavor and Nutrient Extraction in Green and Black Tea. Nutrients 2019, 11, 80. [Google Scholar] [CrossRef]
- Farhan, M. Green Tea Catechins: Nature’s Way of Preventing and Treating Cancer. Int. J. Mol. Sci. 2022, 23, 10713. [Google Scholar] [CrossRef]
- Cardoso, R.R.; Neto, R.O.; dos Santos D’Almeida, C.T.; do Nascimento, T.P.; Pressete, C.G.; Azevedo, L.; Martino, H.S.D.; Cameron, L.C.; Ferreira, M.S.L.; Barros, F.A.R.d. Kombuchas from Green and Black Teas Have Different Phenolic Profile, Which Impacts Their Antioxidant Capacities, Antibacterial and Antiproliferative Activities. Food Res. Int. 2020, 128, 108782. [Google Scholar] [CrossRef]
- Pervin, M.; Unno, K.; Takagaki, A.; Isemura, M.; Nakamura, Y. Function of Green Tea Catechins in the Brain: Epigallocatechin Gallate and Its Metabolites. Int. J. Mol. Sci. 2019, 20, 3630. [Google Scholar] [CrossRef]
- Sivanesan, I.; Muthu, M.; Kannan, A.; Pushparaj, S.S.C.; Oh, J.W.; Gopal, J. Identification of Epigallocatechin-3-Gallate (EGCG) from Green Tea Using Mass Spectrometry. Separations 2022, 9, 209. [Google Scholar] [CrossRef]
- Mehmood, S.; Maqsood, M.; Mahtab, N.; Khan, M.I.; Sahar, A.; Zaib, S.; Gul, S. Epigallocatechin Gallate: Phytochemistry, Bioavailability, Utilization Challenges, and Strategies. J. Food Biochem. 2022, 46, e14189. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, L.; Li, M. Effects of Green Tea Extract Epigallocatechin-3-Gallate on Oral Diseases: A Narrative Review. Pathogens 2024, 13, 634. [Google Scholar] [CrossRef]
- Capasso, L.; De Masi, L.; Sirignano, C.; Maresca, V.; Basile, A.; Nebbioso, A.; Rigano, D.; Bontempo, P. Epigallocatechin Gallate (EGCG): Pharmacological Properties, Biological Activities and Therapeutic Potential. Molecules 2025, 30, 654. [Google Scholar] [CrossRef] [PubMed]
- Mokra, D.; Joskova, M.; Mokry, J. Therapeutic Effects of Green Tea Polyphenol (−)-Epigallocatechin-3-Gallate (EGCG) in Relation to Molecular Pathways Controlling Inflammation, Oxidative Stress, and Apoptosis. Int. J. Mol. Sci. 2023, 24, 340. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.Y.; Meng, X.; Gan, R.Y.; Zhao, C.N.; Liu, Q.; Feng, Y.B.; Li, S.; Wei, X.L.; Atanasov, A.G.; Corke, H.; et al. Health Functions and Related Molecular Mechanisms of Tea Components: An Update Review. Int. J. Mol. Sci. 2019, 20, 6196. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Ali, S.; Ashraf, G.M.; Bilgrami, A.L.; Yadav, D.K.; Hassan, M.I. Epigallocatechin 3-Gallate: From Green Tea to Cancer Therapeutics. Food Chem. 2022, 379, 132135. [Google Scholar] [CrossRef]
- Almatrood, S.A.; Almatroudi, A.; Khan, A.A.; Alhumaydh, F.A.; Alsahl, M.A.; Rahmani, A.H. Potential Therapeutic Targets of Epigallocatechin Gallate (EGCG), the Most Abundant Catechin in Green Tea, and Its Role in the Therapy of Various Types of Cancer. Molecules 2020, 25, 3146. [Google Scholar] [CrossRef]
- Naponelli, V.; Ramazzina, I.; Lenzi, C.; Bettuzzi, S.; Rizzi, F. Green Tea Catechins for Prostate Cancer Prevention: Present Achievements and Future Challenges. Antioxidants 2017, 6, 26. [Google Scholar] [CrossRef]
- Randisi, F.; Perletti, G.; Marras, E.; Gariboldi, M.B. Green Tea Components: In Vitro and In Vivo Evidence for Their Anticancer Potential in Colon Cancer. Cancers 2025, 17, 623. [Google Scholar] [CrossRef]
- Ferrari, E.; Bettuzzi, S.; Naponelli, V. The Potential of Epigallocatechin Gallate (EGCG) in Targeting Autophagy for Cancer Treatment: A Narrative Review. Int. J. Mol. Sci. 2022, 23, 6075. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Pezzani, R.; Redaelli, M.; Zorzan, M.; Imran, M.; Khalil, A.A.; Salehi, B.; Sharopov, F.; Cho, W.C.; Sharifi-Rad, J. Preclinical Pharmacological Activities of Epigallocatechin-3-Gallate in Signaling Pathways: An Update on Cancer. Molecules 2020, 25, 467. [Google Scholar] [CrossRef]
- Alam, M.; Gulzar, M.; Akhtar, M.S.; Rashid, S.; Zulfareen; Tanuja; Shamsi, A.; Hassan, M.I. Epigallocatechin-3-Gallate Therapeutic Potential in Human Diseases: Molecular Mechanisms and Clinical Studies. Mol. Biomed. 2024, 5, 73. [Google Scholar] [CrossRef]
- Negri, A.; Naponelli, V.; Rizzi, F.; Bettuzzi, S. Molecular Targets of Epigallocatechin—Gallate (EGCG): A Special Focus on Signal Transduction and Cancer. Nutrients 2018, 10, 1936. [Google Scholar] [CrossRef] [PubMed]
- Panji, M.; Behmard, V.; Zare, Z.; Malekpour, M.; Nejadbiglari, H.; Yavari, S.; Nayerpour dizaj, T.; Safaeian, A.; Maleki, N.; Abbasi, M.; et al. Suppressing Effects of Green Tea Extract and Epigallocatechin-3-Gallate (EGCG) on TGF-β- Induced Epithelial-to-Mesenchymal Transition via ROS/Smad Signaling in Human Cervical Cancer Cells. Gene 2021, 794, 145774. [Google Scholar] [CrossRef] [PubMed]
- Chourasia, M.; Koppula, P.R.; Battu, A.; Ouseph, M.M.; Singh, A.K. EGCG, a Green Tea Catechin, as a Potential Therapeutic Agent for Symptomatic and Asymptomatic SARS-CoV-2 Infection. Molecules 2021, 26, 1200. [Google Scholar] [CrossRef] [PubMed]
- Bimonte, S.; Forte, C.A.; Cuomo, M.; Esposito, G.; Cascella, M.; Cuomo, A. An Overview on the Potential Roles of Egcg in the Treatment of COVID-19 Infection. Drug Des. Devel Ther. 2021, 15, 4447–4454. [Google Scholar] [CrossRef]
- Bakun, P.; Mlynarczyk, D.T.; Koczorowski, T.; Cerbin-Koczorowska, M.; Piwowarczyk, L.; Kolasiński, E.; Stawny, M.; Kuźmińska, J.; Jelińska, A.; Goslinski, T. Tea-Break with Epigallocatechin Gallate Derivatives—Powerful Polyphenols of Great Potential for Medicine. Eur. J. Med. Chem. 2023, 261, 115820. [Google Scholar] [CrossRef]
- Fernández, V.A.; Toledano, L.A.; Lozano, N.P.; Tapia, E.N.; Roig, M.D.G.; Fornell, R.D.L.T.; Algar, Ó.G. Bioavailability of Epigallocatechin Gallate Administered with Different Nutritional Strategies in Healthy Volunteers. Antioxidants 2020, 9, 440. [Google Scholar] [CrossRef] [PubMed]
- Andrade, E.D.S.; Santos, R.A.; Guillermo, L.V.C.; Miyoshi, N.; Ferraz da Costa, D.C. Immunomodulatory Effects of Green Tea Catechins and Their Ring Fission Metabolites in a Tumor Microenvironment Perspective. Molecules 2024, 29, 4575. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Mao, B.; Cui, S.; Zhang, Q.; Zhao, J.; Tang, X.; Chen, W. Absorption, Metabolism, Bioactivity, and Biotransformation of Epigallocatechin Gallate. Crit. Rev. Food Sci. Nutr. 2024, 64, 6546–6566. [Google Scholar] [CrossRef]
- Bhalani, D.V.; Nutan, B.; Kumar, A.; Singh Chandel, A.K. Bioavailability Enhancement Techniques for Poorly Aqueous Soluble Drugs and Therapeutics. Biomedicines 2022, 10, 2055. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Yang, Y.; Wang, C.; Liu, M.; Wang, J.; Qiao, S.; Jiang, P.; Sun, C.; Jiang, S. Epigallocatechin-3-Gallate at the Nanoscale: A New Strategy for Cancer Treatment. Pharm. Biol. 2024, 62, 676–690. [Google Scholar] [CrossRef]
- Nag, S.; Bhunia, A.; Mohanto, S.; Ahmed, M.G.; Subramaniyan, V. Rising Potentials of Epigallocatechin Gallate (EGCG) Loaded Lipid-Based Delivery Platforms for Breast Cancer. Discov. Appl. Sci. 2024, 6, 426. [Google Scholar] [CrossRef]
- Farabegoli, F.; Pinheiro, M. Epigallocatechin-3-Gallate Delivery in Lipid-Based Nanoparticles: Potentiality and Perspectives for Future Applications in Cancer Chemoprevention and Therapy. Front. Pharmacol. 2022, 13, 809706. [Google Scholar] [CrossRef]
- Wong, C.N.; Lim, Y.M.; Liew, K.B.; Chew, Y.L.; Chua, A.L.; Lee, S.K. EGCG as a Therapeutic Agent: A Systematic Review of Recent Advances and Challenges in Nanocarrier Strategies. J. Zhejiang Univ. Sci. B 2025, 1–24. [Google Scholar] [CrossRef]
- Hu, J.; Webster, D.; Cao, J.; Shao, A. The Safety of Green Tea and Green Tea Extract Consumption in Adults—Results of a Systematic Review. Regul. Toxicol. Pharmacol. 2018, 95, 412–433. [Google Scholar] [CrossRef]
- Yu, Z.; Samavat, H.; Dostal, A.M.; Wang, R.; Torkelson, C.J.; Yang, C.S.; Butler, L.M.; Kensler, T.W.; Wu, A.H.; Kurzer, M.S.; et al. Effect of Green Tea Supplements on Liver Enzyme Elevation: Results from a Randomized Intervention Study in the United States. Cancer Prev. Res. 2017, 10, 571–579. [Google Scholar] [CrossRef]
- Crew, K.D.; Brown, P.; Greenlee, H.; Bevers, T.B.; Arun, B.; Hudis, C.; McArthur, H.L.; Chang, J.; Rimawi, M.; Vornik, L.; et al. Phase IB Randomized, Double-Blinded, Placebo-Controlled, Dose Escalation Study of Polyphenon E in Women with Hormone Receptor-Negative Breast Cancer. Cancer Prev. Res. 2012, 5, 1144–1154. [Google Scholar] [CrossRef] [PubMed]
- Shanafelt, T.D.; Call, T.G.; Zent, C.S.; Leis, J.F.; Laplant, B.; Bowen, D.A.; Roos, M.; Laumann, K.; Ghosh, A.K.; Lesnick, C.; et al. Phase 2 Trial of Daily, Oral Polyphenon e in Patients with Asymptomatic, Rai Stage 0 to II Chronic Lymphocytic Leukemia. Cancer 2013, 119, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Garcia, F.A.R.; Cornelison, T.; Nuño, T.; Greenspan, D.L.; Byron, J.W.; Hsu, C.H.; Alberts, D.S.; Chow, H.H.S. Results of a Phase II Randomized, Double-Blind, Placebo-Controlled Trial of Polyphenon e in Women with Persistent High-Risk HPV Infection and Low-Grade Cervical Intraepithelial Neoplasia. Gynecol. Oncol. 2014, 132, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Dostal, A.M.; Samavat, H.; Bedell, S.; Torkelson, C.; Wang, R.; Swenson, K.; Le, C.; Wu, A.H.; Ursin, G.; Yuan, J.M.; et al. The Safety of Green Tea Extract Supplementation in Postmenopausal Women at Risk for Breast Cancer: Results of the Minnesota Green TeaTrial. Food Chem. Toxicol. 2015, 83, 26–35. [Google Scholar] [CrossRef]
- Lovera, J.; Ramos, A.; Devier, D.; Garrison, V.; Kovner, B.; Reza, T.; Koop, D.; Rooney, W.; Foundas, A.; Bourdette, D. Polyphenon E, Non-Futile at Neuroprotection in Multiple Sclerosis but Unpredictably Hepatotoxic: Phase I Single Group and Phase II Randomized Placebo-Controlled Studies. J. Neurol. Sci. 2015, 358, 46–52. [Google Scholar] [CrossRef]
- Toolsee, N.A.; Aruoma, O.I.; Gunness, T.K.; Kowlessur, S.; Dambala, V.; Murad, F.; Googoolye, K.; Daus, D.; Indelicato, J.; Rondeau, P.; et al. Effectiveness of Green Tea in a Randomized Human Cohort: Relevance to Diabetes and Its Complications. Biomed. Res. Int. 2013, 2013, 412379. [Google Scholar] [CrossRef]
- Ullmann, U.; Haller, J.; Decourt, J.D.; Girault, J.; Spitzer, V.; Weber, P. Plasma-Kinetic Characteristics of Purified and Isolated Green Tea Catechin Epigallocatechin Gallate (EGCG) after 10 Days Repeated Dosing in Healthy Volunteers. Int. J. Vitam. Nutr. Res. 2004, 74, 269–278. [Google Scholar] [CrossRef]
- Chantre, P.; Lairon, D. Recent Findings of Green Tea Extract AR25 (Exolise) and Its Activity for the Treatment of Obesity. Phytomedicine 2002, 9, 3–8. [Google Scholar] [CrossRef]
- Joe, A.K.; Schnoll-Sussman, F.; Bresalier, R.S.; Abrams, J.A.; Hibshoosh, H.; Cheung, K.; Friedman, R.A.; Yang, C.S.; Milne, G.L.; Liu, D.D.; et al. Phase Ib Randomized, Double-Blinded, Placebo- Controlled, Dose Escalation Study of Polyphenon e in Patients with Barrett’s Esophagus. Cancer Prev. Res. 2015, 8, 1131–1137. [Google Scholar] [CrossRef]
- James, A.; Wang, K.; Wang, Y. Therapeutic Activity of Green Tea Epigallocatechin-3-Gallate on Metabolic Diseases and Non-Alcoholic Fatty Liver Diseases: The Current Updates. Nutrients 2023, 15, 3022. [Google Scholar] [CrossRef] [PubMed]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Scientific Opinion on the Safety of Green Tea Catechins. EFSA J. 2018, 16, e05239. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zhu, J.X.; Guo, Y.M.; Niu, M.; Zhang, L.; Tu, C.; Huang, Y.; Li, P.Y.; Zhao, X.; Zhang, Z.T.; et al. Epigallocatechin Gallate During Dietary Restriction—Potential Mechanisms of Enhanced Liver Injury. Front. Pharmacol. 2021, 11, 609378. [Google Scholar] [CrossRef] [PubMed]
- Kamboj, N.; Sharma, S.; Kumar, R. Neuroprotective Insights into Epigallocatechin Gallate (EGCG) for Neurodegenerative Disorders. Explor. Neurosci. 2025, 4, 100673. [Google Scholar] [CrossRef]
- García-Cortés, M.; Robles-Díaz, M.; Ortega-Alonso, A.; Medina-Caliz, I.; Andrade, R.J. Hepatotoxicity by Dietary Supplements: A Tabular Listing and Clinical Characteristics. Int. J. Mol. Sci. 2016, 17, 537. [Google Scholar] [CrossRef]
- Sarma, D.N.; Barrett, M.L.; Chavez, M.L.; Gardiner, P.; Ko, R.; Mahady, G.B.; Marles, R.J.; Pellicore, L.S.; Giancaspro, G.I.; Low Dog, T. Safety of Green Tea Extracts: A Systematic Review by the US Pharmacopeia. Drug Saf. 2008, 31, 469–484. [Google Scholar] [CrossRef]
- Miller, R.J.; Jackson, K.G.; Dadd, T.; Mayes, A.E.; Louise Brown, A.; Minihane, A.M. The Impact of the Catechol-O-Methyltransferase Genotype on the Acute Responsiveness of Vascular Reactivity to a Green Tea Extract. Br. J. Nutr. 2011, 105, 1138–1144. [Google Scholar] [CrossRef]
- Chow, H.H.S.; Hakim, I.A.; Vining, D.R.; Crowell, J.A.; Cordova, C.A.; Chew, W.M.; Xu, M.J.; Hsu, C.H.; Ranger-Moore, J.; Alberts, D.S. Effects of Repeated Green Tea Catechin Administration on Human Cytochrome P450 Activity. Cancer Epidemiol. Biomark. Prev. 2006, 15, 2473–2476. [Google Scholar] [CrossRef]
- Albassam, A.A.; Markowitz, J.S. An Appraisal of Drug-Drug Interactions with Green Tea (Camellia sinensis). Planta Med. 2017, 83, 496–508. [Google Scholar] [CrossRef]
- Knop, J.; Misaka, S.; Singer, K.; Hoier, E.; Muller, F.; Glaeser, H.; Konig, J.; Fromm, M.F. Inhibitory Effects of Green Tea and (-)-Epigallocatechin Gallate on Transport by OATP1B1, OATP1B3, OCT1, OCT2, MATE1, MATE2-K and P-Glycoprotein. PLoS ONE 2015, 10, e0139370. [Google Scholar] [CrossRef]
- Misaka, S.; Yatabe, J.; Müller, F.; Takano, K.; Kawabe, K.; Glaeser, H.; Yatabe, M.S.; Onoue, S.; Werba, J.P.; Watanabe, H.; et al. Green Tea Ingestion Greatly Reduces Plasma Concentrations of Nadolol in Healthy Subjects. Clin. Pharmacol. Ther. 2014, 95, 432–438. [Google Scholar] [CrossRef]
- Misaka, S.; Ono, Y.; Uchida, A.; Ono, T.; Abe, O.; Ogata, H.; Sato, H.; Suzuki, M.; Onoue, S.; Shikama, Y.; et al. Impact of Green Tea Catechin Ingestion on the Pharmacokinetics of Lisinopril in Healthy Volunteers. Clin. Transl. Sci. 2021, 14, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Golden, E.B.; Lam, P.Y.; Kardosh, A.; Gaffney, K.J.; Cadenas, E.; Louie, S.G.; Petasis, N.A.; Chen, T.C.; Schönthal, A.H. Green Tea Polyphenols Block the Anticancer Effects of Bortezomib and Other Boronic Acid-Based Proteasome Inhibitors. Blood 2009, 113, 5927–5937. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Kopustinskiene, D.M. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules 2018, 23, 965. [Google Scholar] [CrossRef]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial Properties of Green Tea Catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef]
- Zwolak, I. Epigallocatechin Gallate for Management of Heavy Metal-Induced Oxidative Stress: Mechanisms of Action, Efficacy, and Concerns. Int. J. Mol. Sci. 2021, 22, 4027. [Google Scholar] [CrossRef]
- Afzal, O.; Dalhat, M.H.; Altamimi, A.S.A.; Rasool, R.; Alzarea, S.I.; Almalki, W.H.; Murtaza, B.N.; Iftikhar, S.; Nadeem, S.; Nadeem, M.S.; et al. Green Tea Catechins Attenuate Neurodegenerative Diseases and Cognitive Deficits. Molecules 2022, 27, 7604. [Google Scholar] [CrossRef] [PubMed]
- Kesse-Guyot, E.; Fezeu, L.; Andreeva, V.A.; Touvier, M.; Scalbert, A.; Hercberg, S.; Galan, P. Total and Specific Polyphenol Intakes in Midlife Are Associated with Cognitive Function Measured 13 Years Later. J. Nutr. 2012, 142, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Biasibetti, R.; Tramontina, A.C.; Costa, A.P.; Dutra, M.F.; Quincozes-Santos, A.; Nardin, P.; Bernardi, C.L.; Wartchow, K.M.; Lunardi, P.S.; Gonçalves, C.A. Green Tea (-)Epigallocatechin-3-Gallate Reverses Oxidative Stress and Reduces Acetylcholinesterase Activity in a Streptozotocin-Induced Model of Dementia. Behav. Brain Res. 2013, 236, 186–193. [Google Scholar] [CrossRef]
- Baba, Y.; Inagaki, S.; Nakagawa, S.; Kaneko, T.; Kobayashi, M.; Takihara, T. Effect of Daily Intake of Green Tea Catechins on Cognitive Function in Middle-Aged and Older Subjects: A Randomized, Placebo-Controlled Study. Molecules 2020, 25, 4265. [Google Scholar] [CrossRef]
- Ramanan, M.; Sinha, S.; Sudarshan, K.; Aidhen, I.S.; Doble, M. Inhibition of the Enzymes in the Leukotriene and Prostaglandin Pathways in Inflammation by 3-Aryl Isocoumarins. Eur. J. Med. Chem. 2016, 124, 428–434. [Google Scholar] [CrossRef]
- Sudarshan, K.; Boda, A.K.; Dogra, S.; Bose, I.; Yadav, P.N.; Aidhen, I.S. Discovery of an Isocoumarin Analogue That Modulates Neuronal Functions via Neurotrophin Receptor TrkB. Bioorganic Med. Chem. Lett. 2019, 29, 585–590. [Google Scholar] [CrossRef]
- Xu, X.Y.; Zhao, C.N.; Li, B.Y.; Tang, G.Y.; Shang, A.; Gan, R.Y.; Feng, Y.B.; Li, H. Bin Effects and Mechanisms of Tea on Obesity. Crit. Rev. Food Sci. Nutr. 2023, 63, 3716–3733. [Google Scholar] [CrossRef]
- Guo, J.; Li, K.; Lin, Y.; Liu, Y. Protective Effects and Molecular Mechanisms of Tea Polyphenols on Cardiovascular Diseases. Front. Nutr. 2023, 10, 1202378. [Google Scholar] [CrossRef] [PubMed]
- Chatree, S.; Sitticharoon, C.; Maikaew, P.; Pongwattanapakin, K.; Keadkraichaiwat, I.; Churintaraphan, M.; Sripong, C.; Sririwichitchai, R.; Tapechum, S. Epigallocatechin Gallate Decreases Plasma Triglyceride, Blood Pressure, and Serum Kisspeptin in Obese Human Subjects. Exp. Biol. Med. 2021, 246, 163–176. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakagawa, K.; Okuda, S.; Miyazawat, T. Dose-Dependent Incorporation of Tea Catechins, (–)-Epigallocatechin-3-Gallate and (–)-Epigallocatechin, into Human Plasma. Biosci. Biotechnol. Biochem. 1997, 61, 1981–1985. [Google Scholar] [CrossRef]
- Krahwinkel, T.; Willershausen, B. The Effect of Sugar-Free Green Tea Chew Candies on the Degree of Inflammation of the Gingiva. Eur. J. Med. Res. 2000, 5, 463–467. [Google Scholar]
- Boschmann, M.; Thielecke, F. The Effects of Epigallocatechin-3-Gallate on Thermogenesis and Fat Oxidation in Obese Men: A Pilot Study. J. Am. Coll. Nutr. 2007, 26, 389S–395S. [Google Scholar] [CrossRef]
- Siblini, H.; Al-Hendy, A.; Segars, J.; González, F.; Taylor, H.S.; Singh, B.; Flaminia, A.; Flores, V.A.; Christman, G.M.; Huang, H.; et al. Assessing the Hepatic Safety of Epigallocatechin Gallate (EGCG) in Reproductive-Aged Women. Nutrients 2023, 15, 320. [Google Scholar] [CrossRef] [PubMed]
- Acosta, L.; Byham-Gray, L.; Kurzer, M.; Samavat, H. Hepatotoxicity with High-Dose Green Tea Extract: Effect of Catechol-O-Methyltransferase and Uridine 5′-Diphospho-Glucuronosyltransferase 1A4 Genotypes. J. Diet. Suppl. 2023, 20, 850–869. [Google Scholar] [CrossRef] [PubMed]
- Bathgate, J.R.; Radler, D.R.; Kurzer, M.; Samavat, H. Green Tea Extract Supplementation Does Not Modify Plasma Concentration of F2-Isoprostanes in Women Who Are Postmenopause: Findings from a Randomized Controlled Trial. Nutr. Res. 2023, 113, 29–38. [Google Scholar] [CrossRef]
- Wan, X.; Jia, W.; Wang, Q.; Chen, X.; Wang, A.; Zhu, L.; Liu, X.; Zhang, L.; Zhuang, P.; Jiao, J.; et al. Metabolomics Strategy Comprehensively Unveils the Effect of Catechins Intervention on the Biomarkers of Exposure to Acrylamide and Biomarkers of Cardiometabolic Risk. Environ. Int. 2022, 169, 107517. [Google Scholar] [CrossRef]
- Goempel, K.; Tedsen, L.; Ruenz, M.; Bakuradze, T.; Schipp, D.; Galan, J.; Eisenbrand, G.; Richling, E. Biomarker Monitoring of Controlled Dietary Acrylamide Exposure Indicates Consistent Human Endogenous Background. Arch. Toxicol. 2017, 91, 3551–3560. [Google Scholar] [CrossRef]
- Rietjens, I.M.C.M.; Dussort, P.; Günther, H.; Hanlon, P.; Honda, H.; Mally, A.; O’Hagan, S.; Scholz, G.; Seidel, A.; Swenberg, J.; et al. Exposure Assessment of Process-Related Contaminants in Food by Biomarker Monitoring. Arch. Toxicol. 2018, 92, 15–40. [Google Scholar] [CrossRef]
- Misaka, S.; Ono, Y.; Taudte, R.V.; Hoier, E.; Ogata, H.; Ono, T.; König, J.; Watanabe, H.; Fromm, M.F.; Shimomura, K. Exposure of Fexofenadine, but Not Pseudoephedrine, Is Markedly Decreased by Green Tea Extract in Healthy Volunteers. Clin. Pharmacol. Ther. 2022, 112, 627–634. [Google Scholar] [CrossRef]
- Veerman, G.D.M.; van der Werff, S.C.; Koolen, S.L.W.; Miedema, J.R.; Oomen-de Hoop, E.; van der Mark, S.C.; Chandoesing, P.P.; de Bruijn, P.; Wijsenbeek, M.S.; Mathijssen, R.H.J. The Influence of Green Tea Extract on Nintedanib’s Bioavailability in Patients with Pulmonary Fibrosis. Biomed. Pharmacother. 2022, 151, 113101. [Google Scholar] [CrossRef]
- Uchida, K.; Meno, K.; Korenaga, T.; Liu, S.; Suzuki, H.; Baba, Y.; Tagata, C.; Araki, Y.; Tsunemi, S.; Aso, K.; et al. Effect of Matcha Green Tea on Cognitive Functions and Sleep Quality in Older Adults with Cognitive Decline: A Randomized Controlled Study over 12 Months. PLoS ONE 2024, 19, e0309287. [Google Scholar] [CrossRef] [PubMed]
- Cieuta-Walti, C.; Cuenca-Royo, A.; Langohr, K.; Rakic, C.; López-Vílchez, M.Á.; Lirio, J.; González-Lamuño Leguina, D.; González, T.B.; García, J.G.; Roure, M.R.; et al. Safety and Preliminary Efficacy on Cognitive Performance and Adaptive Functionality of Epigallocatechin Gallate (EGCG) in Children with Down Syndrome. A Randomized Phase Ib Clinical Trial (PERSEUS Study). Genet. Med. 2022, 24, 2004–2013. [Google Scholar] [CrossRef]
- Zeng, J.; Wang, Y.; Yuan, Q.; Luan, Q. The Effect of (-)-Epigallocatechin Gallate as an Adjunct to Non-Surgical Periodontal Treatment: A Randomized Clinical Trial. Trials 2022, 23, 368. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhu, W.; Zhao, X.; Li, X.; Zhou, Z.; Zheng, M.; Meng, X.; Kong, L.; Zhang, S.; He, D.; et al. Efficacy of Epigallocatechin-3-Gallate in Preventing Dermatitis in Patients with Breast Cancer Receiving Postoperative Radiotherapy: A Double-Blind, Placebo-Controlled, Phase 2 Randomized Clinical Trial. JAMA Dermatol. 2022, 158, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Jia, L.; Xie, P.; Yin, X.; Zhu, W.; Zhao, H.; Wang, X.; Meng, X.; Xing, L.; Zhao, H.; et al. Efficacy and Safety of Epigallocatechin-3-Gallate in Treatment Acute Severe Dermatitis in Patients with Cancer Receiving Radiotherapy: A Phase I Clinical Trial. Sci. Rep. 2023, 13, 13865. [Google Scholar] [CrossRef]
- Yin, X.; Zhu, W.; Tang, X.; Yang, G.; Zhao, X.; Zhao, K.; Jiang, L.; Li, X.; Zhao, H.; Wang, X.; et al. Phase I/II Clinical Trial of Efficacy and Safety of EGCG Oxygen Nebulization Inhalation in the Treatment of COVID-19 Pneumonia Patients with Cancer. BMC Cancer 2024, 24, 486. [Google Scholar] [CrossRef]
- Cohen, M.L.; Brumwell, A.N.; Ho, T.C.; Garakani, K.; Montas, G.; Leong, D.; Ding, V.W.; Golden, J.A.; Trinh, B.N.; Jablons, D.M.; et al. A Fibroblast-Dependent TGFβ1/SFRP2 Noncanonical Wnt Signaling Axis Promotes Epithelial Metaplasia in Idiopathic Pulmonary Fibrosis. J. Clin. Investig. 2024, 134, e174598. [Google Scholar] [CrossRef]
- Wilasrusmee, K.T.; Sitticharoon, C.; Keadkraichaiwat, I.; Maikaew, P.; Pongwattanapakin, K.; Chatree, S.; Sririwichitchai, R.; Churintaraphan, M. Epigallocatechin Gallate Enhances Sympathetic Heart Rate Variability and Decreases Blood Pressure in Obese Subjects: A Randomized Control Trial. Sci. Rep. 2024, 14, 21628. [Google Scholar] [CrossRef]
- Gu, Q.; Xia, L.; Du, Q.; Shao, Y.; He, J.; Wu, P.; Liang, L.; Shen, X. The Therapeutic Role and Potential Mechanism of EGCG in Obesity-Related Precocious Puberty as Determined by Integrated Metabolomics and Network Pharmacology. Front. Endocrinol. 2023, 14, 1159657. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jardon, K.M.; Goossens, G.H.; Most, J.; Galazzo, G.; Venema, K.; Penders, J.; Blaak, E.E. Examination of Sex-Specific Interactions between Gut Microbiota and Host Metabolism after 12-Week Combined Polyphenol Supplementation in Individuals with Overweight or Obesity. Gut Microbes 2024, 16, 2392875. [Google Scholar] [CrossRef]
- Churm, R.; Williams, L.M.; Dunseath, G.; Prior, S.L.; Bracken, R.M. The Polyphenol Epigallocatechin Gallate Lowers Circulating Catecholamine Concentrations and Alters Lipid Metabolism during Graded Exercise in Man: A Randomized Cross-over Study. Eur. J. Nutr. 2023, 62, 1517–1526. [Google Scholar] [CrossRef]
- Bonkovsky Md, H.L. Hepatotoxicity Associated with Supplements Containing Chinese Green Tea (Camellia sinensis). Ann. Intern. Med. 2006, 144, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Galati, G.; Lin, A.; Sultan, A.M.; O’Brien, P.J. Cellular and in Vivo Hepatotoxicity Caused by Green Tea Phenolic Acids and Catechins. Free Radic. Biol. Med. 2006, 40, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Saenz, M.; Martinez-Sanchez, C. Green Tea Extracts and Acute Liver Failure: The Need for Caution in Their Use and Diagnostic Assessment. Liver Transplant. 2007, 13, 1067. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, X.; Xiao, D.; Wu, C.; Shi, W.; Yang, H.; Bai, Z.; Xiao, X. Hepatotoxicity of Green Tea Extract: Idiosyncratic or Indirect Drug-Induced Liver Injury? Future Integr. Med. 2022, 1, 43–45. [Google Scholar] [CrossRef]
- Matoso, V.; Bargi-Souza, P.; Ivanski, F.; Romano, M.A.; Romano, R.M. Acrylamide: A Review about Its Toxic Effects in the Light of Developmental Origin of Health and Disease (DOHaD) Concept. Food Chem. 2019, 283, 422–430. [Google Scholar] [CrossRef]
- Tepe, Y.; Çebi, A. Acrylamide in Environmental Water: A Review on Sources, Exposure, and Public Health Risks. Expo. Health 2019, 11, 3–12. [Google Scholar] [CrossRef]
- Kyriacou, N.M.; Gross, A.S.; McLachlan, A.J. Green Tea Catechins as Perpetrators of Drug Pharmacokinetic Interactions. Clin. Pharmacol. Ther. 2025, 118, 45–61. [Google Scholar] [CrossRef]
- Abdelkawy, K.S.; Abdelaziz, R.M.; Abdelmageed, A.M.; Donia, A.M.; El-Khodary, N.M. Effects of Green Tea Extract on Atorvastatin Pharmacokinetics in Healthy Volunteers Key Points. Eur. J. Drug Metab. Pharmacokinet. 2020, 45, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Braal, C.L.; Hussaarts, K.G.A.M.; Seuren, L.; Oomen-de Hoop, E.; de Bruijn, P.; Buck, S.A.J.; Bos, M.E.M.M.; Thijs-Visser, M.F.; Zuetenhorst, H.J.M.; Mathijssen-van Stein, D.; et al. Influence of Green Tea Consumption on Endoxifen Steady-State Concentration in Breast Cancer Patients Treated with Tamoxifen. Breast Cancer Res. Treat. 2020, 184, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Frasheri, L.; Schielein, M.C.; Tizek, L.; Mikschl, P.; Biedermann, T.; Zink, A. Great Green Tea Ingredient? A Narrative Literature Review on Epigallocatechin Gallate and Its Biophysical Properties for Topical Use in Dermatology. Phytother. Res. 2020, 34, 2170–2179. [Google Scholar] [CrossRef]
- Alsenani, F.; Alotaiq, N.; Dermawan, D.; Elwali, N.E.; Nasrullah, M.Z.; Almalki, R.H.; Alfarsi, N.T.; Almatrafi, A.S.; Alsulami, M.S. Understanding the Role of Green Tea and Matcha Consumption in Cardiovascular Health, Obesity, and Diabetes: Insights from a Saudi Arabian Population. Hum. Nutr. Metab. 2025, 40, 200302. [Google Scholar] [CrossRef]
- Lin, Y.; Shi, D.; Su, B.; Wei, J.; Găman, M.A.; Sedanur Macit, M.; Borges do Nascimento, I.J.; Guimaraes, N.S. The Effect of Green Tea Supplementation on Obesity: A Systematic Review and Dose–Response Meta-Analysis of Randomized Controlled Trials. Phytother. Res. 2020, 34, 2459–2470. [Google Scholar] [CrossRef]
- Liu, Z.; De Bruijn, W.J.C.; Bruins, M.E.; Vincken, J.P. Reciprocal Interactions between Epigallocatechin-3-Gallate (EGCG) and Human Gut Microbiota in Vitro. J. Agric. Food Chem. 2020, 68, 9804–9815. [Google Scholar] [CrossRef]
- Che, S.; Qin, B.; Wu, K.; Zhu, M.; Hu, H.; Peng, C.; Wang, Z.; Yin, Y.; Xia, Y.; Wu, M. EGCG Drives Gut Microbial Remodeling-Induced Epithelial GPR43 Activation to Lessen Th1 Polarization in Colitis. Redox Biol. 2024, 75, 103291. [Google Scholar] [CrossRef]
- Singh, N.A.; Mandal, A.K.A.; Khan, Z.A. Potential Neuroprotective Properties of Epigallocatechin-3-Gallate (EGCG). Nutr. J. 2016, 15, 60. [Google Scholar] [CrossRef]
- De la Torre, R.; De Sola, S.; Pons, M.; Duchon, A.; de Lagran, M.M.; Farré, M.; Fitó, M.; Benejam, B.; Langohr, K.; Rodriguez, J.; et al. Epigallocatechin-3-Gallate, a DYRK1A Inhibitor, Rescues Cognitive Deficits in Down Syndrome Mouse Models and in Humans. Mol. Nutr. Food Res. 2014, 58, 278–288. [Google Scholar] [CrossRef]
- Stalmach, A.; Troufflard, S.; Serafini, M.; Crozier, A. Absorption, Metabolism and Excretion of Choladi Green Tea Flavan-3-Ols by Humans. Mol. Nutr. Food Res. 2009, 53, S44–S53. [Google Scholar] [CrossRef]
- Del Rio, D.; Calani, L.; Cordero, C.; Salvatore, S.; Pellegrini, N.; Brighenti, F. Bioavailability and Catabolism of Green Tea Flavan-3-Ols in Humans. Nutrition 2010, 26, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Unno, K.; Pervin, M.; Nakagawa, A.; Iguchi, K.; Hara, A.; Takagaki, A.; Nanjo, F.; Minami, A.; Nakamura, Y. Blood–Brain Barrier Permeability of Green Tea Catechin Metabolites and Their Neuritogenic Activity in Human Neuroblastoma SH-SY5Y Cells. Mol. Nutr. Food Res. 2017, 61, 1700294. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Gan, R.Y.; Chen, D.; Zheng, L.; Ng, S.B.; Rietjens, I.M.C.M. Gut Microbiota-Mediated Metabolism of Green Tea Catechins and the Biological Consequences: An Updated Review. Crit. Rev. Food Sci. Nutr. 2024, 64, 7067–7084. [Google Scholar] [CrossRef] [PubMed]
- Mereles, D.; Hunstein, W. Epigallocatechin-3-Gallate (EGCG) for Clinical Trials: More Pitfalls than Promises? Int. J. Mol. Sci. 2011, 12, 5592–5603. [Google Scholar] [CrossRef]
- Cai, Z.Y.; Li, X.M.; Liang, J.P.; Xiang, L.P.; Wang, K.R.; Shi, Y.L.; Yang, R.; Shi, M.; Ye, J.H.; Lu, J.L.; et al. Bioavailability of Tea Catechins and Its Improvement. Molecules 2018, 23, 2346. [Google Scholar] [CrossRef]

| Author (Year) | Country | Purpose | Trial Registry Number | Sample | Intervention | Major Findings |
|---|---|---|---|---|---|---|
| Siblini et al. (2023) [70] | U.S.A. | To assess the hepatic safety profile of EGCG in reproductive-aged women | NCT 04177693 | 39 women w/wo uterine fibroids | Capsules of GTE for a daily dose of 720 mg EGCG, w/wo an ovarian stimulation medication | No subject had evidence of drug-induced liver injury or serum folate levels outside the normal range |
| Acosta et al. (2023) [71] | U.S.A. | To investigate the influence of COMT and UGT1A4 genotypes on changes in liver injury biomarkers | NCT 00917735 | 1075 healthy postmenopausal women | Capsules of GTE (EGCG 843 ± 44 mg/day) or placebo for 12 months | COMT and UGT1A4 genotypes affected serum ALT levels and AST:ALT ratios at different time intervals |
| Bathgate et al. (2023) [72] | U.S.A. | To test whether subjects taking EGCG supplements had reduced plasma F2-isoprostane concentrations | NCT 00917735 (secondary analysis) | A subset (N = 252) of 1075 healthy postmenopausal women | Capsules of GTE (EGCG 843 ± 44 mg/day) or placebo for 12 months | GTE supplement did not result in a significant decrease in plasma F2-isoprostanes levels. COMT genotype did not modify the effect of GTE on F2-isoprostanes |
| Wan et al. (2022) [73] | China | To investigate the protective effect of TP against acrylamide exposure by measuring urine and blood mercapturic acid and hemoglobin adducts of acrylamide | NCT 03118167 | 78 young volunteers exposed to acrylamide through potato chip consumption | Capsules containing 200 mg, 100 mg, 50 mg, or 0 mg of TP | TP supplementation attenuated the toxicity of acrylamide exposure by promoting the mercapturic acid detoxification pathway |
| Author (Year) | Country | Purpose | Trial Registry Number | Sample | Intervention | Major Findings |
|---|---|---|---|---|---|---|
| Misaka et al. (2022) [76] | Japan | To evaluate whether the pharmacokinetics of fexofenadine and pseudoephedrine are affected when administered orally in a GTE aqueous solution | UMIN 000032828 | 10 healthy volunteers | Fexofenadine and pseudoephedrine dissolved in water or in a solution containing 325 mg GTE (92.5% of EGCG) | Plasma concentrations and urinary excretions of fexofenadine were markedly decreased when co-administered with GTE |
| Veerman et al. (2022) [77] | The Netherlands | To study the interaction nintedanib/GTE in patients with fibrotic ILD | NL 8913 | 26 patients treated with nintedanib both in period A and B | 500 mg GTE (60.7% EGCG) administered with 250 mL of water only in period B | Exposure to nintedanib decreased by 21% when administered 60 min after GTE for 7 days |
| Author (Year) | Country | Purpose | Trial Reg. Identifier | Sample | Intervention | Major Findings |
|---|---|---|---|---|---|---|
| Uchida et al. (2024) [78] | Japan | To test the effect of matcha green tea on cognitive function and sleep quality | UMIN 000035658 | 99 older adults with cognitive decline or mild cognitive impairment | Daily supplementation with 2 g matcha (containing 105.3 mg of EGCG/170.8 mg catechins) or placebo for 12 months | Regular consumption of matcha could improve emotional perception and sleep quality in older adults with mild cognitive decline |
| Cieuta-Walti et al. (2022) [79] | Spain and France | To evaluate safety and tolerability of a dietary supplement of EGCG and if EGCG improves cognitive and functional performance in DS | NCT 03624556 | 66 children with DS (aged 6–12 years) | FontUp (at a daily dose of 10 mg/kg EGCG) or placebo for 6 months | EGCG was safe and well tolerated in children with DS, but the efficacy results did not support its use in this population |
| Author (Year) | Country | Purpose | Trial Reg. Identifier | Sample | Intervention | Major Findings |
|---|---|---|---|---|---|---|
| Zeng et al. (2022) [80] | China | To evaluate the adjunctive effect of EGCG solution as a coolant during scaling and root planing in the management of chronic periodontitis | ChiCTR 2000029831 | 15 patients with moderate to severe chronic periodontitis; bilateral maxillary teeth were randomly divided into test side and control side | On the test side, the distilled water in the ultrasonic scaler was replaced with 5 mg/mL (≈10 mM) EGCG solution | EGCG solution revealed an additional benefit on the bleeding index at the 12-week review |
| Zhao et al. (2022) [81] | China | To determine whether EGCG solution reduces the incidence of RID in patients undergoing radiotherapy | NCT 02580279 | 180 patients after breast cancer surgery and receiving radiotherapy | EGCG solution (660 μmol/L) or placebo (0.9% NaCl saline) was sprayed into the radiation field | Prophylactic use of EGCG solution significantly reduced the incidence and severity of RID |
| Xie et al. (2023) [82] | China | To evaluate the safety and efficacy of an EGCG solution for the treatment of acute severe dermatitis in patients receiving radiotherapy | NCT 02580279 | 19 patients with thoracic cancer receiving radiotherapy | EGCG solution (max. concentration: 2574 µmol/L) was sprayed into the radiation field when RID level III appeared for the first time | A decreasing trend in RID was observed, and the associated symptoms were significantly reduced |
| Yin et al. (2024) [83] | China | To evaluate the safety and efficacy of EGCG aerosol for the control of COVID-19 pneumonia in cancer patients | NCT 05758571 | 54 patients diagnosed with malignant tumor, SARS-CoV-2 infection, and moderate pneumonia | Patients were treated with EGCG nebulization (10 mL) three times daily for at least seven days; adverse events were registered | EGCG may be effective in COVID-19-induced pneumonia, promoting an improvement in moderate pneumonia or preventing the development of severe pneumonia |
| Cohen et al. (2024) [84] | U.S.A. | To investigate molecular and cellular basis of ILD using lung biopsy and single-cell RNA-Seq | NCT 03928847 | 8 ILD patients (4 in test and 4 in control group) | 600 mg of EGCG by mouth once daily for 2 weeks prior to biopsy in the treatment group | EGCG downregulated TGF-β1 signaling and several pro-inflammatory and stress pathways in biopsy samples |
| Author (Year) | Country | Purpose | Trial Reg. Identifier | Sample | Intervention | Major Findings |
|---|---|---|---|---|---|---|
| Wilasrusmee et al. (2024) [85] | Thailand | To investigate the effects of EGCG on BP and autonomic nervous system, as indicated by 5 min heart rate variability measurement in obese subjects | TCTR 20200422001 | 30 obese subjects | Capsules of 150 mg EGCG (n = 15) or placebo (n = 15) twice a day without dietary restrictions | 8-week EGCG treatment decreased BP and increased the LF/HF power ratio, reflecting increased sympathetic activity |
| Gu et al. (2023) [86] | China | To investigate the mechanism of EGCG in the prevention of obesity-related precocious puberty by means of serum metabolomics and network pharmacology | NCT 03628937 | 34 obese girls (6 to 10 years old) | Capsules of EGCG (200 mg, 50% EGCG, n = 18) or placebo (n = 16) for 12 weeks | EGCG may contribute to the prevention through AKT1, EGFR, ESR1, STAT3, IGF1, and MAPK1 targets and multiple signaling pathways, including the estrogen pathway |
| Jardon et al. (2024) [87] | The Netherlands | To investigate sex-specific differences in microbiota composition and interactions with cardiometabolic parameters after polyphenol supplementation in overweight/obese individuals | NCT 02381145 | 18 healthy Caucasian men and 19 women with overweight or obesity (BMI > 25 kg/m2) | Capsules of EGCG and resveratrol (EGCG + RES, 282 + 80 mg/d) or placebo for 12 weeks | EGCG + RES supplementation did not induce changes in the gut microbiota of men and women. Microbiota composition seemed to be predictive for polyphenol-induced changes in SkMOx in men but not in women |
| Churm et al. (2023) [88] | UK | To explore the impact of EGCG ingestion on catecholamine metabolism during graded cycle exercise (to exhaustion) in men | NCT 03199430 | 8 healthy males performing exercise 3–5 times per week (30–90 min per session) | 2 capsules (1450 mg) with at least 94% EGCG and <0.1% caffeine or a placebo | Acute EGCG supplementation reduced circulating catecholamines but not metanephrine, glucose, or lactate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrari, E.; Naponelli, V. Catechins and Human Health: Breakthroughs from Clinical Trials. Molecules 2025, 30, 3128. https://doi.org/10.3390/molecules30153128
Ferrari E, Naponelli V. Catechins and Human Health: Breakthroughs from Clinical Trials. Molecules. 2025; 30(15):3128. https://doi.org/10.3390/molecules30153128
Chicago/Turabian StyleFerrari, Elena, and Valeria Naponelli. 2025. "Catechins and Human Health: Breakthroughs from Clinical Trials" Molecules 30, no. 15: 3128. https://doi.org/10.3390/molecules30153128
APA StyleFerrari, E., & Naponelli, V. (2025). Catechins and Human Health: Breakthroughs from Clinical Trials. Molecules, 30(15), 3128. https://doi.org/10.3390/molecules30153128








