Anti-Bacterial and Anti-Fungal Properties of a Set of Transition Metal Complexes Bearing a Pyridine Moiety and [B(C6F5)4]2 as a Counter Anion
Abstract
1. Introduction
2. Results and Discussion
2.1. FT-IR Spectroscopy
2.2. 11B and 1H –NMR Spectral Data
2.3. Elemental Analysis of Complexes 1–6
2.4. Electron Paramagnetic Resonance (EPR) Spectra of 1, 2, and 5
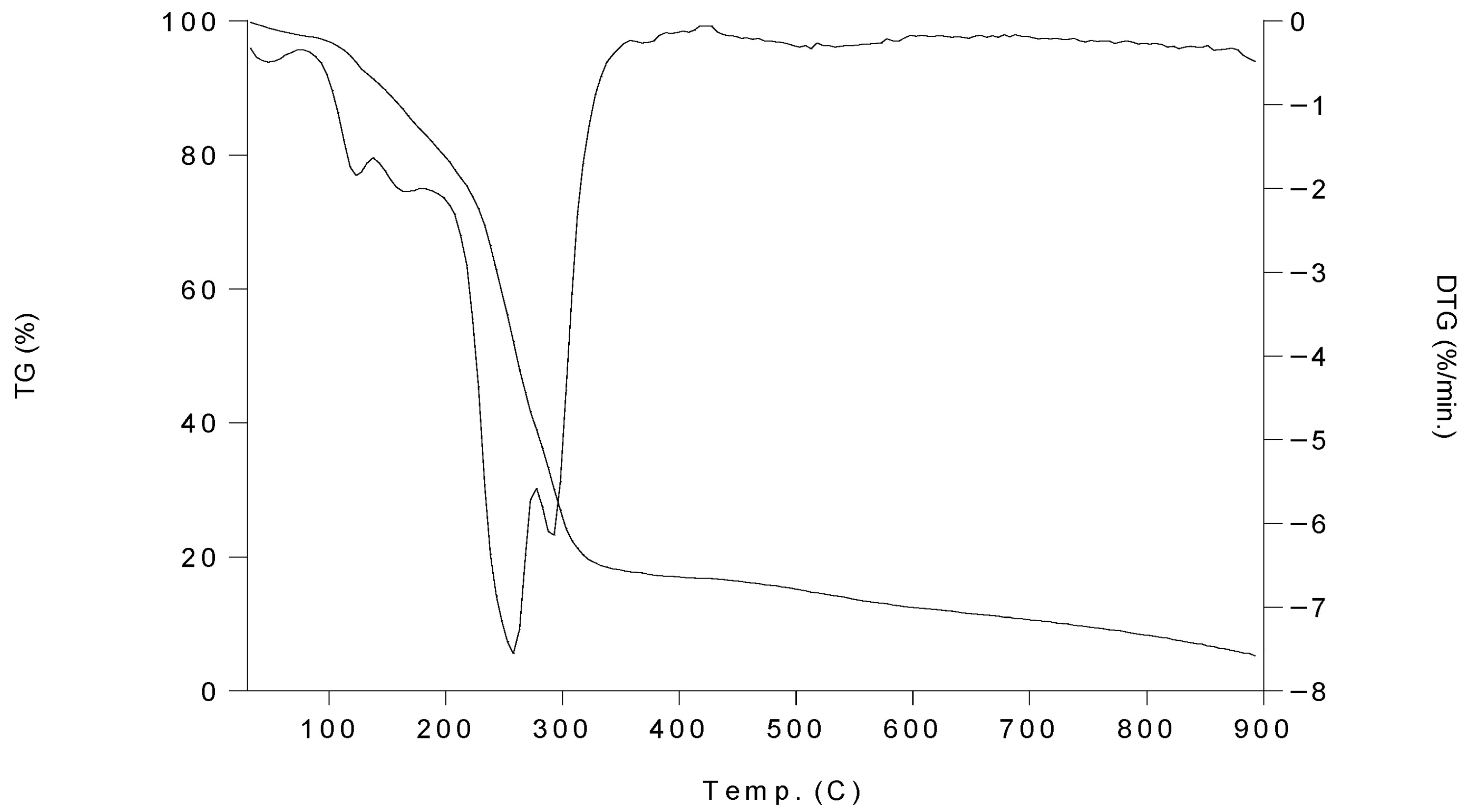
2.5. Thermal Gravimetric Analysis (TGA) of Complexes 1–6
2.6. Ultraviolet–Visible (UV–Vis) Spectra of Complexes 1–6
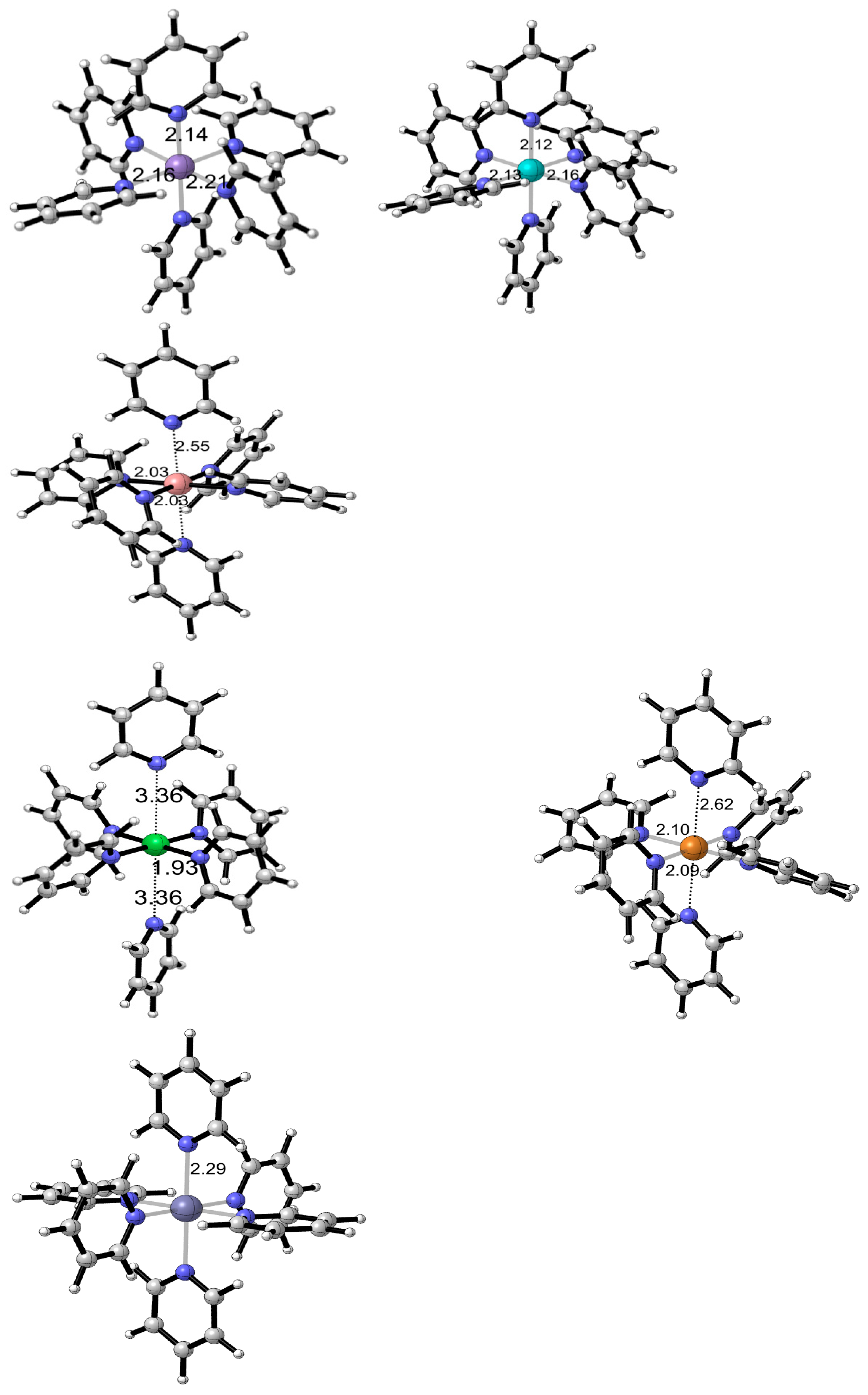
2.7. Structural Analysis of Complexes 1–6
2.8. Antimicrobial Activity of Complexes 1–6
2.8.1. Agar Well Diffusion
2.8.2. Determination of Minimum Inhibitory Concentration
3. Experimental Part
3.1. General
3.2. Synthesis of Complexes 1–6
3.3. Computational Method
3.4. Antimicrobial Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferrari, M.B.; Capacchi, S.; Pelosi, G.; Reffo, G.; Tarasconi, P.; Albertini, R.; Pinelli, S.; Lunghi, P. Synthesis, structural characterization and biological activity of helicin thiosemicarbazone monohydrate and a copper(II) complex of salicylaldehyde thiosemicarbazone. Inorg. Chim. Acta 1999, 286, 134–141. [Google Scholar] [CrossRef]
- Canpolat, E.; Kaya, M. Studies on mononuclear chelates derived from substituted Schiff-base ligands (part 2): Synthesis and characterization of a new 5-bromosalicyliden-p-aminoacetophenoneoxime and its complexes with Co(II), Ni(II), Cu(II) and Zn(II). J. Coord. Chem. 2004, 57, 1217–1223. [Google Scholar] [CrossRef]
- Kumar, M.; Singh, A.K.; Singh, V.K.; Yadav, R.K.; Singh, A.P.; Singh, S. Recent developments in the biological activities of 3d-metal complexes with salicylaldehyde-based N, O-donor Schiff base ligands. Coord. Chem. Rev. 2024, 505, 215663. [Google Scholar] [CrossRef]
- Chohan, Z.H.; Arif, M.; Akhtar, M.A.; Supuran, C.T. Metal-based antibacterial and antifungal agents: Synthesis, characterization, and in-vitro biological evaluation of Co(II), Cu(II), Ni(II), and Zn(II) complexes with amino acid-derived compounds. Bioinorg. Chem. Appl. 2006, 2006, 83131. [Google Scholar] [CrossRef] [PubMed]
- Froux, A.; Anna, L.D.; Rainot, A.; Neybecker, C.; Spinello, R.; Bonsignore, R.; Rouget, R.; Harle, G.; Terenzi, A.; Monari, A.; et al. Metal centers and aromatic moieties in Schiff base complexes: Impact on G-quadruplex stabilization and oncogene downregulation. Inorg. Chem. Front. 2024, 11, 5725–5740. [Google Scholar] [CrossRef]
- Hijazi, A.K.; Taha, Z.A.; Issa, D.K.; Alshare, H.M.; Al-Momani, W.M.; Elrashidi, A.; Barham, A.S. Synthesis, characterization and catalytic/antimicrobial activities of some transition metal complexes derived from 2-floro-N-((2-hydroxyphenyl)methylene)benzohydrazide. Molecules 2024, 29, 5758. [Google Scholar] [CrossRef] [PubMed]
- Ittel, S.D.; Johnson, L.K.; Brookhart, M. Late-metal catalysts for ethylene homo-and copolymerization. Chem. Rev. 2000, 100, 1169–1204. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.Y.-X.; Marks, T.J. Cocatalysts for metal-catalyzed olefin polymerization: Activators, activation processes, and structure— activity relationships. Chem. Rev. 2000, 100, 1391–1434. [Google Scholar] [CrossRef] [PubMed]
- Hagen, K.S. Iron (II) triflate salts as convenient substitutes for perchlorate salts: Crystal structures of [Fe(H2O)6](CF3SO3)2 and Fe(MeCN)4(CF3SO3)2. Inorg. Chem. 2000, 39, 5867–5869. [Google Scholar] [CrossRef] [PubMed]
- Hijazi, A.K.; Radhakrishnan, N.; Jain, K.R.; Herdtweck, E.; Nuyken, O.; Walter, H.M.; Hanefeld, P.; Voit, B.; Kühn, F.E. Molybdenum (III) compounds as catalysts for 2-methylpropene polymerization. Angew. Chem. Int. Engl. Ed. 2007, 46, 7290–7292. [Google Scholar] [CrossRef] [PubMed]
- Storhoff, B.N.; Lewis, H.C., Jr. Organonitrile complexes of transition metals. Coord. Chem. Rev. 1977, 23, 1–29. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Gondo, S.; Kamakura, T.; Konishi, M. Organoamine and organophosphate molybdenum complexes as lubricant additives. Wear 1987, 120, 51–60. [Google Scholar] [CrossRef]
- Konda, S.G.; Khedkar, V.T.; Dawane, B. Synthesis of some new 2-amino-3-cyano-4-aryl-6-(1-naphthylamino)-pyridines as antibacterial agent. J. Chem. Pharm. Res. 2010, 2, 187–191. [Google Scholar]
- Gholap, A.R.; Toti, K.S.; Shirazi, F. Synthesis and evaluation of antifungal properties of a series of the novel 2-amino-5- oxo-4-phenyl-5, 6, 7, 8-tetrahydroquinoline-3-carbonitrile and its analogues. Bioorg. Med. Chem. 2007, 15, 6705–6715. [Google Scholar] [CrossRef] [PubMed]
- Flefel, E.M.; Alsafi, M.A.; Alahmadi, S.M.; Amr, A.E.; Fayed, A.A. Antimicrobial activities of some synthesized macrocyclic pentaazapyridine and dipeptide pyridine derivatives. Biomed. Res. 2018, 29, 1407–1413. [Google Scholar] [CrossRef]
- El-Sayed, H.A.; Moustafa, A.H.; El-Torky, A.E.; Abd El-Salam, E.A. A series of pyridines and pyridine based sulfa-drugs as antimicrobial agents: Design, synthesis and antimicrobial activity. Russ. J. Gen. Chem. 2017, 87, 2401–2408. [Google Scholar] [CrossRef]
- Shi, F.; Tu, S.; Fang, F.; Li, T. One-pot synthesis of 2-amino-3-cyanopyridine derivatives under microwave irradiation without solvent. Arkivoc 2005, 2005, 137–142. [Google Scholar] [CrossRef]
- Mishra, N.; Poonia, K.; Kumar, D. An overview of biological aspects of Schiff base metal complexes. Int. J. Adv. Res. Technol. 2013, 2, 52–66. [Google Scholar]
- Gaballa, A.S.; Asker, M.S.; Barakat, A.S.; Teleb, S.M. Synthesis, characterization and biological activity of some platinum (II) complexes with Schiff bases derived from salicylaldehyde, 2-furaldehyde and phenylenediamine. Spectrochim. Acta A 2007, 67, 114–121. [Google Scholar] [CrossRef] [PubMed]
- El-Gammal, O.A.; Mohamed, F.S.; Rezk, G.N.; El-Bindary, A.A. Synthesis, characterization, catalytic, DNA binding and antibacterial activities of Co (II), Ni (II) and Cu (II) complexes with new Schiff base ligand. J. Mol. Liq. 2021, 326, 115223. [Google Scholar] [CrossRef]
- Kiwaan, H.A.; El-Mowafy, A.S.; El-Bindary, A.A. Synthesis, spectral characterization, DNA binding, catalytic and in vitro cytotoxicity of some metal complexes. J. Mol. Liq. 2021, 326, 115381. [Google Scholar] [CrossRef]
- El-Gammal, O.A.; El-Bindary, A.A.; Mohamed, F.S.; Rezk, G.N.; El-Bindary, M.A. Synthesis, characterization, design, molecular docking, anti COVID-19 activity, DFT calculations of novel Schiff base with some transition metal complexes. J. Mol. Struct. 2022, 346, 117850. [Google Scholar] [CrossRef]
- Beck, W.; Sunkel, K. Metal complexes of weakly coordinating anions. Precursors of strong cationic organometallic Lewis acids. Chem. Rev. 1988, 88, 1405–1421. [Google Scholar]
- Reed, C.A. Carboranes: A new class of weakly coordinating anions for strong electrophiles, oxidants, and superacids. Acc. Chem. Res. 1998, 37, 133–139. [Google Scholar] [CrossRef]
- Strauss, S.H. The search for larger and more weakly coordinating anions. Chem. Rev. 1993, 93, 927–942. [Google Scholar] [CrossRef]
- Sakthivel, A.; Syukri, S.; Hijazi, A.K.; Kühn, F.E. Heterogenization of [Cu(NCCH3)4][BF4]2 on mesoporous AlMCM-41/AlMCM-48 and its application as cyclopropanation catalyst. Catal. Lett. 2006, 111, 43–49. [Google Scholar] [CrossRef]
- Syukri, S.; Hijazi, A.K.; Sakthivel, A.; Al-Hmaideen, A.I.; Kühn, F.E. Heterogenization of solvent-ligated copper (II) complexes on poly(4-vinylpyridine) for the catalytic cyclopropanation of olefins. Inorg. Chim. Acta 2007, 360, 197–202. [Google Scholar] [CrossRef]
- Sakthivel, A.; Hijazi, A.K.; Yeong, H.Y.; Köhler, K.; Nykon, O.; Kühn, F.E. Heterogenization of a manganese(II) acetonitrile complex on AlMCM-41 and AlMCM-48 molecular sieves by ion exchange. J. Mater. Chem. 2005, 15, 4441–4445. [Google Scholar] [CrossRef]
- Vierle, M.; Zhang, Y.; Herdtweck, E.; Bohnenpoll, M.; Nuyken, O.; Kühn, F.E. Highly reactive polyisobutenes prepared with manganese(II) complexes as initiators. Angew. Chem. Int. Engl. Ed. 2003, 42, 1307–1310. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Ando, T.; Kamigaito, M.; Sawamoto, M. Evidence for living radical polymerization of methyl methacrylate with ruthenium complex: Effects of protic and radical compounds and reinitiation from the recovered polymers. Macromolecules 1997, 30, 2244–2248. [Google Scholar] [CrossRef]
- Hijazi, A.K.; Taha, Z.A.; Ajlouni, A.; Radhakrishnan, N.; Voit, B.; Kühn, F.E. Improved synthesis, characterization and catalytic application of [H(OEt2)2][B{C6H3(m-CF3)2}4]. J. Organomet. Chem. 2014, 763–764, 65–68. [Google Scholar] [CrossRef]
- Moineau, G.; Granel, C.; Dubois, P.; Jerome, R.; Teyssie, P. Controlled radical polymerization of methyl methacrylate initiated by an alkyl halide in the presence of the wilkinson catalyst. Macromolecules 1998, 31, 542–544. [Google Scholar] [CrossRef]
- Hijazi, A.K.; Yeong, H.Y.; Zhang, Y.; Herdtweck, E.; Nuyken, O.; Kühn, F.E. Isobutene polymerization using [CuII(NCMe)6]2+ with non coordinating anions as catalysts. Angew. Macro. Rapid Comm. 2007, 28, 670–675. [Google Scholar] [CrossRef]
- Hijazi, A.K.; Al Hmaideen, A.; Syukri, S.; Radhakrishnan, N.; Herdtweck, E.; Voit, B.; Kühn, F.E. Synthesis and characterization of acetonitrile ligated transition metal complexes containing tetrakis{(pentafluorophenyl)} borate as counter anions. Eur. J. Inorg. Chem. 2008, 2008, 2892–2898. [Google Scholar] [CrossRef]
- Mohr, F.; Binfield, S.A.; Fettinger, J.C.; Vedernikov, A.N. A practical, fast, and high-yielding aziridination procedure using simple Cu(II) complexes containing N-donor pyridine-based ligands. J. Org. Chem. 2005, 70, 4833–4839. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, A.; Hijazi, A.K.; Hanzlik, M.; Chiang, A.S.T.; Kühn, F.E. Heterogenization of [Cu(NCCH3)6][B(C6F5)4]2 and its application in catalytic olefin aziridination. Appl. Catal. A 2005, 294, 161–167. [Google Scholar] [CrossRef]
- Gillis, E.P.; Eastman, K.J.; Hill, M.D.; Donnelly, D.J.; Meanwell, N.A. Applications of fluorine in medicinal chemistry. J. Med. Chem. 2015, 58, 8315–8359. [Google Scholar] [CrossRef] [PubMed]
- Champagne, P.A.; Desroches, J.; Hamel, J.-D.; Vandamme, M.; Paquin, J.-F. Monofluorination of organic compounds: 10 years of innovation. Chem. Rev. 2015, 115, 9073–9174. [Google Scholar] [CrossRef] [PubMed]
- Hijazi, A.K.; Taha, Z.A.; Ajlouni, A.M.; Al Momani, W.M.; Ababneh, T.S.; Alshare, H.M.; Kühn, F.E. Synthesis, catalytic and biological Aactivities and computational study of Fe(III) solvent-ligated complexes having B(Ph)4 as counter anio36n. Appl. Organometal. Chem. 2017, 31, e3601. [Google Scholar] [CrossRef]
- Ajlouni, A.M.; Hijazi, A.K.; Taha, Z.A.; Al Momani, W.; Okour, A.; Kühn, F.E. Synthesis, characterization and biological and catalytic activities of propionitrile—Ligated transition metal complexes with [B(C6F5)4] as counter anion. Cat. Lett. 2019, 149, 2317–2324. [Google Scholar] [CrossRef]
- Hijazi, A.K.; Taha, Z.A.; Ababneh, T.S.; Alshare, H.M.; Al-Bataineh, N.; Al Momani, W.M.; Ajlouni, A.M. In vitro biological, catalytic and DFT studies of some iron(III) N-ligated complexes. Chem. Pap. 2020, 74, 1561–1572. [Google Scholar] [CrossRef]
- Hijazi, A.K.; Taha, Z.A.; Al-Dawood, L.A.; Ababneh, T.S.; Al Momani, W.M. Catalytic cyclopropanation, antimicrobial, and DFT properties of some chelated transition metal(II) complexes. J. Mol. Struct. 2021, 1228, 129733. [Google Scholar] [CrossRef]
- Hijazi, A.K.; Taha, Z.A.; Abuhamad, N.J.; Al-Momani, W.M. Synthesis, and characterization of some Cu(I) complexes having propionitrile and pyridine moieties: An investigation on their antibacterial properties. J. Saudi Chem. Soc. 2021, 25, 101387. [Google Scholar] [CrossRef]
- Anacona, J.R.; Martell, T.; Sanchez, I. Metal complexes of a new ligand derived from 2,3-quinoxalinedithiol and 2,6-bis(bromomethyl)pyridine. J. Chil. Chem. Soc. 2005, 50, 375–378. [Google Scholar] [CrossRef]
- Landesman, H.; Williams, R.E. B11 N.m.r. Chemical Shifts. II. Amine-borate ester complexes, alkoxydifluoroborane trimers and tetrahaloborate ions. J. Am. Chem. Soc. 1961, 83, 2663–2666. [Google Scholar] [CrossRef]
- Nöth, H.; Vahrenkamp, H. Kernresonanzuntersuchungen an bor-verbindungen, I. 11B-Kernresonanzspektren von boranen mit substituenten aus der ersten achterperiode des periodensystems. Chem. Ber. 1966, 99, 1049–1067. [Google Scholar] [CrossRef]
- Sinclair, J.A.; Brown, H.C. Synthesis of unsymmetrical conjugated diynes via the reaction of lithium dialkynyldialkylborates with iodine. J. Org. Chem. 1971, 41, 1078–1079. [Google Scholar] [CrossRef]
- Carrasco, A.C.; Rodriguez-Fanjul, V.; Pizarro, A.M. Activation of the Ir–N(pyridine) bond in half-sandwich tethered iridium(III) complexes. Inorg. Chem. 2020, 59, 16454–16466. [Google Scholar] [CrossRef] [PubMed]
- Kurpik, G.; Walczak, A.; Goldyn, M.; Harrowfield, J.; Stefankiewicz, A.R. Pd(II) complexes with pyridine ligands: Substituent effects on the NMR data, crystal structures, and catalytic activity. Inorg. Chem. 2022, 61, 14019–14029. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Goldfarb, D. Manganese incorporation into the mesoporous material MCM-41 under acidic conditions as studied by high field pulsed EPR and ENDOR spectroscopies. J. Am. Chem. Soc. 2000, 122, 7034–7041. [Google Scholar] [CrossRef]
- Chan, S.I.; Fung, B.M.; Lütje, H. Electron paramagnetic resonance of Mn(II) complexes in acetonitrile. J. Chem. Phys. 1967, 47, 2121–2130. [Google Scholar] [CrossRef]
- Misra, S.K.; Diehl, S.; Tipikin, D.; Freed, J.H. A Multifrequency EPR study of Fe2+ and Mn2+ ions in a ZnSiF6.6H2O single crystal at liquid-helium temperatures. J. Magn. Reson. 2010, 205, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Voon, L.T.; Yeong, H.Y.; Hijazi, A.K.; Radhakrishnan, N.; Köhler, K.; Voit, B.; Nuyken, O.; Kühn, F.E. Solvent-ligated copper (II) complexes for the homopolymerization of 2-methylpropene. Chem. Euro. J. 2008, 14, 7997–8003. [Google Scholar] [CrossRef] [PubMed]
- Sawada, T.; Fukumaru, K.; Sakurai, H. Coordination-dependent ESR spectra of copper(II) complexes with a CuN4 type coordination mode: Relationship between ESR parameters and stability constants or redox potentials of the complexes. Chem. Pharm. Bull. 1996, 44, 1009–1016. [Google Scholar] [CrossRef]
- Jahn, H.A.; Teller, E. Stability of polyatomic molecules in degenerate electronic states—I—Orbital degeneracy. Proc. R. Soc. Lond. Ser. A-Math. Phys. Sci. 1937, 161, 220–235. [Google Scholar]
- Shriver, D.F.; Atkins, P.W. Inorganic Chemistry; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sec. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Pal, S. Pyridine: A useful ligand in transition metal complexes. In Pyridine; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Azza, A.; Abou-Hussen, A.; Linert, W. Chromotropism behavior and biological activity of some Schiff base-mixed ligand transition metal complexes. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2009, 39, 570–599. [Google Scholar]
- Chandraleka, S.; Chandramohan, G.; Saha, S.; Dhanasekaran, D.; Panneerselvam, A. Synthesis, characterization and biological activity of Zn(II) Schiff base complex against drug resistant pathogens. Drug Invent. Today 2010, 2, 8–12. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Revision C.01. 2019. Available online: https://gaussian.com/ (accessed on 28 April 2025).
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functional with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- Hijazi, A.K.; Taha, Z.A.; Ajlouni, A.M.; Al Momani, W.M.; Idris, I.M.; Abu Hamra, E. Synthesis and biological activities of Lanthanide(III) nitrate complexes with N-(2-hydroxynaphthalen-1-yl) methylene) nicotinohydrazide Schiff base. Med. Chem. 2017, 13, 77–84. [Google Scholar] [CrossRef] [PubMed]
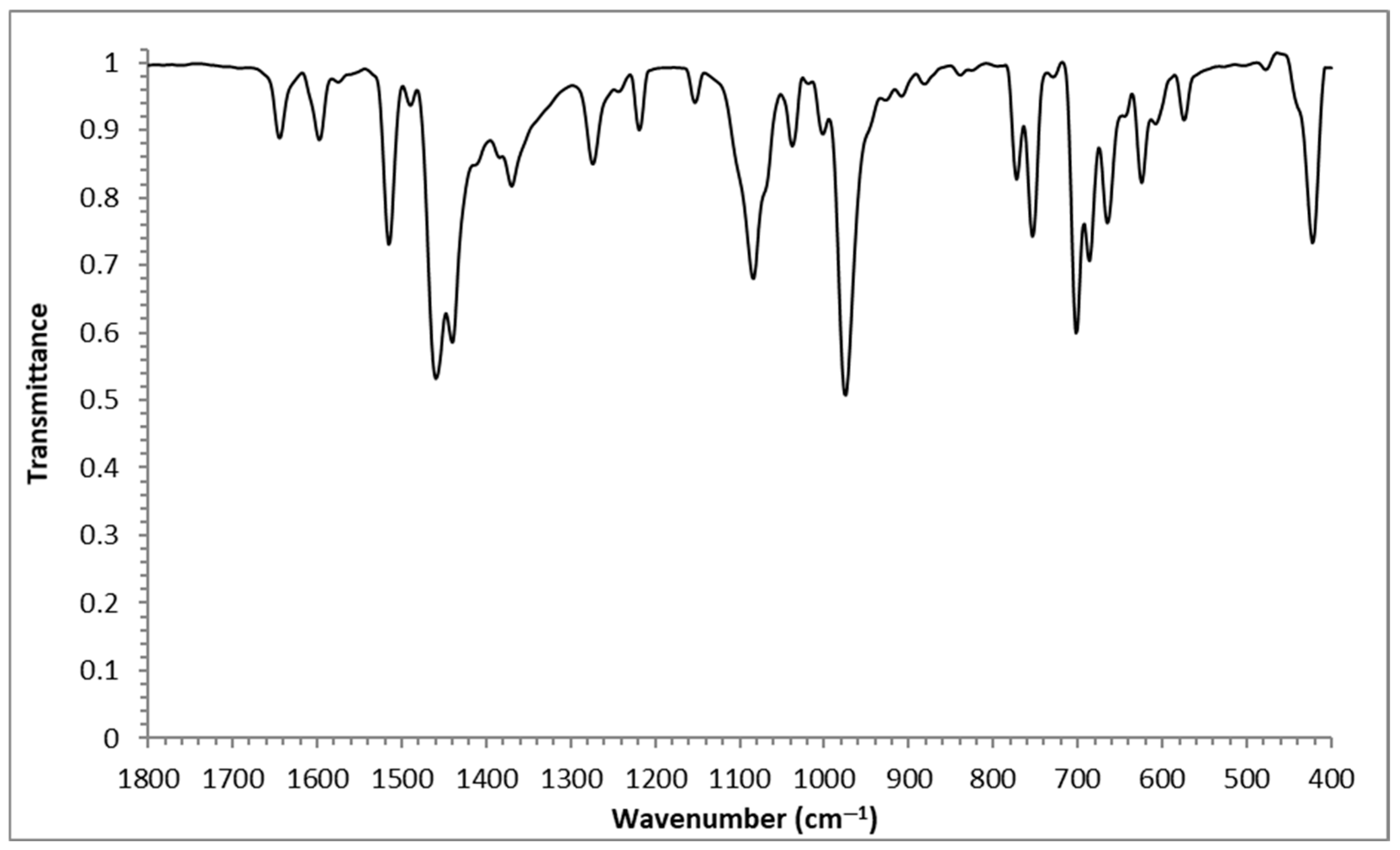

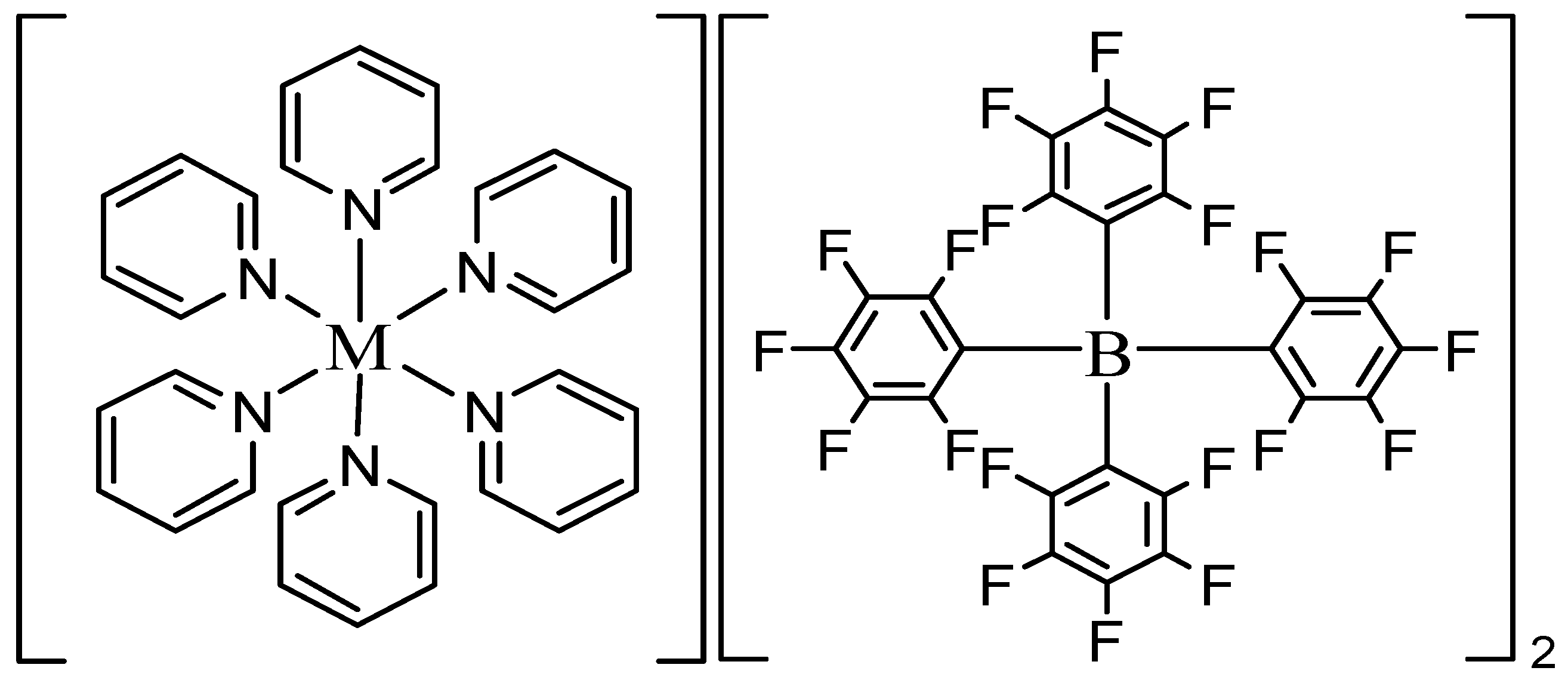
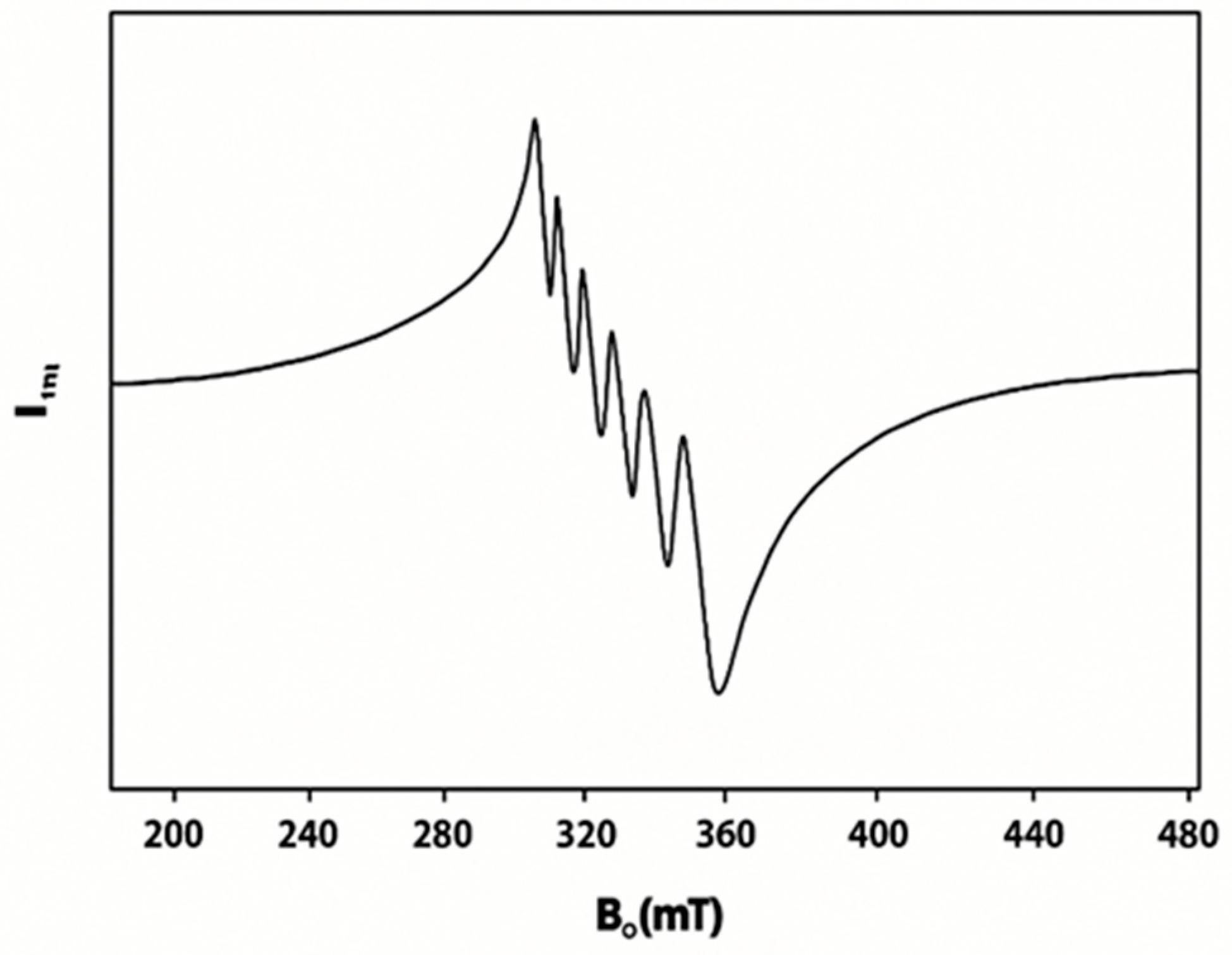

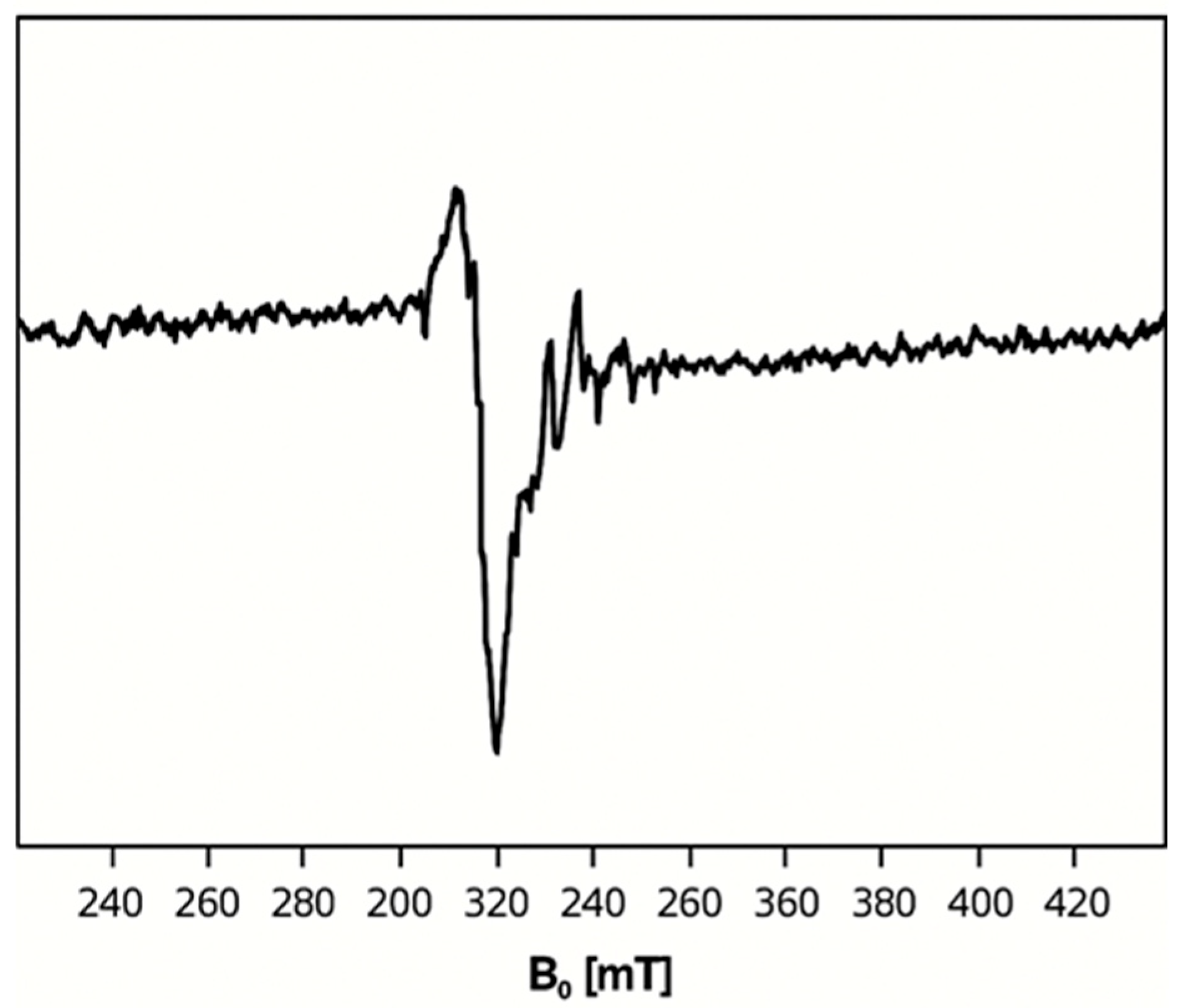
| Complexes | ν(Ar-(C-F)) | ν(C-H) | ν(C=C) | ν(C=N) | ||
|---|---|---|---|---|---|---|
| 1 | 1368 | 1273 | 1083 | 2924 | 1597 | 1643 |
| 2 | 1369 | 1274 | 1084 | 3062 | 1597 | 1643 |
| 3 | 1369 | 1273 | 1085 | 3037 | 1597 | 1644 |
| 4 | 1369 | 1273 | 1084 | 3036 | 1596 | 1642 |
| 5 | 1369 | 1273 | 1084 | 3039 | 1597 | 1644 |
| 6 | 1370 | 1273 | 1085 | 3037 | 1596 | 1644 |
| Complexes | N (%) Found (calc.) | H (%) Found (calc.) | C (%) Found (calc.) | F (%) Found (calc.) | Yields |
|---|---|---|---|---|---|
| 1 C78H30B2F40N6Mn (1887.15 g/mol) | 4.49 | 1.57 | 49.58 | 40.22 | 88.9% 0.802 g |
| (4.45) | (1.60) | (49.63) | (40.26) | ||
| 2 C78H30B2F40N6Fe (1888.14 g/mol) | 4.46 | 1.61 | 49.66 | 40.27 | 86.6% 0.781 g |
| (4.45) | (1.60) | (49.61) | (40.24) | ||
| 3 C78H30B2F40N6Co (1891.14 g/mol) | 4.48 | 1.59 | 49.47 | 40.10 | 98.0% 0.886 g |
| (4.44) | (1.60) | (49.53) | (40.17) | ||
| 4 C78H30B2F40N6Ni (1890.14 g/mol) | 4.41 | 1.67 | 49.46 | 40.13 | 71.4% 0.646 g |
| (4.44) | (1.60) | (49.53) | (40.18) | ||
| 5 C78H30B2F40N6Cu (1895.15 g/mol) | 4.40 | 1.62 | 49.43 | 39.97 | 94.9% 0.860 g |
| (4.43) | (1.59) | (49.41) | (40.08) | ||
| 6 C78H30B2F40N6Zn (1898.06 g/mol) | 4.36 | 1.61 | 49.41 | 40.15 | 60.9% 0.552 g |
| (4.43) | (1.59) | (49.36) | (40.04) |
| Complexes | Stage | Temp. Range (°C) | Mass Loss % | Total Mass Loss % |
|---|---|---|---|---|
| 1 | 1 | 94–196 | 16.88 | 94.96 |
| 2 | 196–281 | 43.10 | ||
| 3 | 281–366 | 19.07 | ||
| 2 | 1 | 34–116 | 16.85 | 92.77 |
| 2 | 116–165 | 8.38 | ||
| 3 | 165–261 | 15.21 | ||
| 4 | 261–287 | 37.59 | ||
| 3 | 1 | 49–81 | 4.66 | 94.82 |
| 2 | 81–114 | 4.67 | ||
| 3 | 114–227 | 16.88 | ||
| 4 | 227–321 | 54.26 | ||
| 4 | 1 | 37–116 | 8.40 | 96.17 |
| 2 | 116–205 | 12.76 | ||
| 3 | 205–278 | 52.85 | ||
| 4 | 278–392 | 12.20 | ||
| 5 | 1 | 36–130 | 8.55 | 90.59 |
| 2 | 130–189 | 12.63 | ||
| 3 | 189–214 | 20.18 | ||
| 4 | 214–247 | 7.87 | ||
| 5 | 247–296 | 35.07 | ||
| 6 | 1 | 41–151 | 8.47 | 99.36 |
| 2 | 151–164 | 16.66 | ||
| 3 | 164–327 | 59.02 |
| Complexes | Wavelength [nm] | Absorptivity | Transitions |
|---|---|---|---|
| 1 | 316.0 | 1.340 | π→π* |
| 364.0 | 0.578 | π→π* | |
| 416.0 | 0.253 | n→π* | |
| 2 | 315.0 | 0.397 | π→π* |
| 388.5 | 0.427 | π→π* | |
| 426.5 | 0.360 | π→π* | |
| 468.0 | 0.396 | n→π* | |
| 3 | 275.5 | 3.084 | π→π* |
| 571.0 | 0.249 | n→π* | |
| 4 | 273.0 | 5.000 | π→π* |
| 321.5 | 0.289 | n→π* | |
| 5 | 272.0 | 2.608 | π→π* |
| 309.5 | 0.215 | π→π* | |
| 322.5 | 0.195 | n→π* | |
| 6 | 273.5 | 2.421 | π→π* |
| 356.5 | 0.079 | π→π* | |
| 397.5 | 0.067 | n→π* |
| Tested Compounds | Gram (−) Bacteria | Gram (+) Bacteria | ||||||
|---|---|---|---|---|---|---|---|---|
| Ec | Pm | Sf | Kp | Pa | Cf | Sa | Sp | |
| 1 | 21.3 | 19.1 | 24 | 15.9 | 14.2 | 21.9 | 17.3 | 20.6 |
| 2 | 13 | 21.8 | 18.8 | 25.1 | 13.5 | 19.4 | 17.6 | 18.5 |
| 3 | 12.7 | 19.7 | 17.8 | 14.9 | 9.7 | 15.2 | 21.9 | 21.3 |
| 4 | 11.2 | 20.6 | 16.4 | 18.3 | 16.2 | 17 | 20.9 | 25.3 |
| 5 | 8.9 | 16.2 | 14.7 | 13.3 | 12.5 | 17.8 | 21.2 | 23.1 |
| 6 | 13.3 | 22.1 | 20.7 | 8.9 | 20.5 | 20 | 20.6 | 27.3 |
| DMSO (−ve control) | Not active | Not active | Not active | Not active | Not active | Not active | Not active | Not active |
| Oxytetracycline (+ve control) | 16 | 15 | 14 | 13 | 15 | 14 | 16 | 14 |
| Tested Compounds | Gram (−) Bacteria | Gram (+) Bacteria | Fungi | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ec | Pm | Sf | Kp | Pa | Cf | Sa | Sp | Ca | |
| 1 | 16 | 16 | 8 | 128 | 128 | 16 | 64 | 32 | 64 |
| 2 | 128 | 16 | 16 | 8 | 128 | 16 | 64 | 64 | 32 |
| 3 | 256 | 16 | 64 | 128 | 512 | 128 | 16 | 16 | 128 |
| 4 | 256 | 16 | 128 | 16 | 128 | 64 | 32 | 8 | 32 |
| 5 | 512 | 128 | 128 | 128 | 128 | 64 | 16 | 16 | 8 |
| 6 | 256 | 16 | 16 | 512 | 32 | 32 | 32 | 4 | 8 |
| DMSO (−ve control) | Not active | Not active | Not active | Not active | Not active | Not active | Not active | Not active | Not active |
| Oxytetracycline (+ve control) | 4 | 2 | 8 | 2 | 8 | 8 | --- | 8 | Not active |
| Fluconazole (+ve control) | Not active | Not active | Not active | Not active | Not active | Not active | Not active | Not active | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hijazi, A.K.; El-Khateeb, M.; Taha, Z.A.; Alomari, M.I.; Khwaileh, N.M.; Alakhras, A.I.; Al-Momani, W.M.; Elrashidi, A.; Barham, A.S. Anti-Bacterial and Anti-Fungal Properties of a Set of Transition Metal Complexes Bearing a Pyridine Moiety and [B(C6F5)4]2 as a Counter Anion. Molecules 2025, 30, 3121. https://doi.org/10.3390/molecules30153121
Hijazi AK, El-Khateeb M, Taha ZA, Alomari MI, Khwaileh NM, Alakhras AI, Al-Momani WM, Elrashidi A, Barham AS. Anti-Bacterial and Anti-Fungal Properties of a Set of Transition Metal Complexes Bearing a Pyridine Moiety and [B(C6F5)4]2 as a Counter Anion. Molecules. 2025; 30(15):3121. https://doi.org/10.3390/molecules30153121
Chicago/Turabian StyleHijazi, Ahmed K., Mohammad El-Khateeb, Ziyad A. Taha, Mohammed I. Alomari, Noor M. Khwaileh, Abbas I. Alakhras, Waleed M. Al-Momani, Ali Elrashidi, and Ahmad S. Barham. 2025. "Anti-Bacterial and Anti-Fungal Properties of a Set of Transition Metal Complexes Bearing a Pyridine Moiety and [B(C6F5)4]2 as a Counter Anion" Molecules 30, no. 15: 3121. https://doi.org/10.3390/molecules30153121
APA StyleHijazi, A. K., El-Khateeb, M., Taha, Z. A., Alomari, M. I., Khwaileh, N. M., Alakhras, A. I., Al-Momani, W. M., Elrashidi, A., & Barham, A. S. (2025). Anti-Bacterial and Anti-Fungal Properties of a Set of Transition Metal Complexes Bearing a Pyridine Moiety and [B(C6F5)4]2 as a Counter Anion. Molecules, 30(15), 3121. https://doi.org/10.3390/molecules30153121





