An Ultrasonication-Assisted Green Process for Simultaneous Production of a Bioactive Compound-Rich Extract and a Multifunctional Fibrous Ingredient from Spent Coffee Grounds
Abstract
1. Introduction
2. Results and Discussion
2.1. Bioactive Compounds and Antioxidant Activity of Spent Coffee Grounds (SCGs), Liquid Aqueous Extract (LAE), and Modified Spent Coffee Grounds (MSCGs)
2.2. Chemical Characterization of the Solid Fractions: SCGs and MSCGs
2.3. Techno-Functional Properties of the Solid Fractions: SCGs and MSCGs
2.4. Cholesterol (CAC) and Glucose (GAC) Adsorption Capacities of the Solid Fractions: SCGs and MSCGs
3. Materials and Methods
3.1. Materials
3.2. Ultrasonication-Assisted Process in an Aqueous Medium
3.3. Bioactive Compounds in the SCGs, LAE, and MSCGs
3.3.1. Preparation of Extracts for Bioactive Compound Quantification
3.3.2. Caffeine (CA)
3.3.3. Total Polyphenol Content (TPC)
3.3.4. Melanoidins (MEs)
3.3.5. Antioxidant Properties: Free Radical Scavenging Activity
3.4. Chemical Characterization of the Solid Fractions: SCGs and MSCGs
3.4.1. Chemical Composition
3.4.2. Cellulose, Hemicellulose, and Lignin
3.5. Techno-Functional Properties of the Solid Fractions: SCGs and MSCGs
3.5.1. Water Holding (WHC) and Oil Holding Capacity (OHC)
3.5.2. Swelling Capacity (SC)
3.5.3. Hygroscopicity (HI)
3.5.4. Emulsifying Activity (EA)
3.6. Cholesterol (CAC) and Glucose (GAC) Adsorption Capacities of the Solid Fractions: SCGs and MSCGs
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Solomakou, N.; Loukri, A.; Tsafrakidou, P.; Michaelidou, A.M.; Mourtzinos, I.; Goula, A.M. Recovery of phenolic compounds from spent coffee grounds through optimized extraction process. Sustain. Chem. Pharm. 2022, 25, 100592. [Google Scholar] [CrossRef]
- International Coffee Organization. ICO 2025. Available online: https://icocoffee.org/ (accessed on 20 January 2025).
- Dossi, N.; Tubaro, F.; Paglierani, L.; Vignocchi, M.; Furlanetto, E.; Bontempelli, G. Evaluation of the possible effect of the grinders installed in fully automatic espresso coffee machines on the element content in brewed coffees. ACS Food Sci. Technol. 2024, 4, 426–435. [Google Scholar] [CrossRef]
- Freitas, V.V.; Borges, L.L.R.; Vidigal, M.C.T.R.; Santos, M.H.; Stringheta, M.C. Coffee: A comprehensive overview of origin, market, and the quality process. Trends Food Sci. Technol. 2024, 146, 104411. [Google Scholar] [CrossRef]
- Allen, L. Coffee Statistics: Consumption, Preferences, & Spending. 2025. Available online: https://www.driveresearch.com/market-research-company-blog/coffee-survey/ (accessed on 20 January 2025).
- Gebreeyessus, G.G. Towards the sustainable and circular bioeconomy: Insights on spent coffee grounds valorization. Sci. Total Environ. 2022, 833, 155113. [Google Scholar] [CrossRef] [PubMed]
- Santanatoglia, A.; Alessandroni, L.; Mannozzi, C.; Marconi, R.; Piatti, D.; Sagratini, G.; Vittori, S.; Capioli, G. Valorization of spent coffee ground and coffee silverskin as a source of nutrients and bioactive compounds. Future Postharvest Food 2024, 1, 61–81. [Google Scholar] [CrossRef]
- Lomolino, G.; Dal Zotto, V.; Zannoni, S.; De Iseppi, A. Foam characteristics and sensory analysis of arabica coffee, extracted by espresso capsule and moka methods. Beverages 2022, 8, 28. [Google Scholar] [CrossRef]
- Bouhzam, I.; Cantero, R.; Margallo, M.; Aldaco, R.; Bala, A.; Fullana i Palmer, P.; Puig, R. Extraction of bioactive compounds from spent coffee grounds using ethanol and acetone aqueous solutions. Foods 2023, 12, 4400. [Google Scholar] [CrossRef]
- Johnson, K.; Liu, Y.; Lu, M.A. A Review of recent advances in spent coffee grounds upcycle technologies and practices. Front. Chem. Eng. 2022, 4, 838605. [Google Scholar] [CrossRef]
- Mofijur, M.; Kosumo, F.; Fattah, I.M.R.; Mahmudul, H.M.; Rasul, M.G.; Shamsuddin, A.H.; Mahlia, T.M.I. Resource recovery from waste coffee grounds using ultrasonic-assisted technology for bioenergy production. Energies 2020, 13, 1770. [Google Scholar] [CrossRef]
- Yeoh, L.; Ng, K.S. Future prospects of spent coffee ground valorisation using a biorefinery approach. Resour. Conserv. Recycl. 2022, 79, 106123. [Google Scholar] [CrossRef]
- Bevilacqua, E.; Cruzat, V.; Singh, I.; Rose’Meyer, R.B.; Panchal, S.K.; Brown, L. The potential of spent coffee grounds in functional food development. Nutrients 2023, 15, 994. [Google Scholar] [CrossRef]
- Girotto, F.; Piazza, L.; Ratti, S.; Giovanelli, G. Application of ultrasonic intensification technology in the extraction of bio-actives from spent coffee grounds and spent tea leaves. Chem. Eng. Trans. 2023, 102, 265–270. [Google Scholar] [CrossRef]
- Bijla, L.; Aissa, R.; Laknifli, A.; Bouyahya, A.; Harhar, H.; Gharby, S. Spent coffee grounds: A sustainable approach toward novel perspectives of valorization. J. Food Biochem. 2022, 46, 14190. [Google Scholar] [CrossRef] [PubMed]
- Bijla, L.; Hmitti, A.; Fadda, A.; Oubannin, S.; Gagour, J.; Aissa, R.; Laknifli, R.A.; Sakar, E.H.; Gharby, S. Valorization of spent coffee ground as a natural antioxidant and its use for sunflower oil shelf-life extension. Eur. J. Lipid Sci. Technol. 2024, 126, 2300115. [Google Scholar] [CrossRef]
- Torres-Valenzuela, L.S.; Serna-Jiménez, J.Á.; Martínez, K. Coffee by-Products: Nowadays and Perspectives. In Coffee–Production and Research 2020; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Zabaniotou, A.; Kamaterou, P. Food waste valorization advocating circular bioeconomy—A critical review of potentialities and perspectives of spent coffee grounds biorefinery. J. Clean. Prod. 2019, 211, 1553–1566. [Google Scholar] [CrossRef]
- He, C.; Sampers, I.; Raes, K. Dietary fiber concentrates recovered from agro-industrial by-products: Functional properties and application as physical carriers for probiotics. Food Hydrocoll. 2021, 111, 106175. [Google Scholar] [CrossRef]
- Shaikh-Ibrahim, A.; Curci, N.; De Lise, F.; Di Fenza, M.; Castaldi, S.; Isticato, R.; Oliveira, A.; Aniceto, J.P.S.; Silva, C.M.; Serafim, L.S.; et al. Carbohydrate conversion in spent coffee grounds: Pretreatment strategies and novel enzymatic cocktail to produce value-added saccharides and prebiotic mannooligosaccharides. Biotechnol. Biofuels Bioprod. 2025, 18, 2. [Google Scholar] [CrossRef]
- Ahmed, H.; Abolore, R.S.; Jaiswal, S.; Jaiswal, A.K. Toward circular economy: Potentials of spent coffee grounds in bioproducts and chemical production. Biomass 2024, 4, 286–312. [Google Scholar] [CrossRef]
- Arias, A.; Ioannidou, S.M.; Giannakis, N.; Feijoo, G.; Moreira, M.T.; Koutinas, A. Review of potential and prospective strategies for the valorization of coffee grounds within the framework of a sustainable and circular bioeconomy. Ind. Crops Prod. 2023, 205, 117504. [Google Scholar] [CrossRef]
- Mediani, A.; Kamal, N.; Lee, S.Y.; Abas, F.; Farag, M. Green extraction methods for isolation of bioactive substances from coffee seed and spent. Sep. Purif. Rev. 2023, 52, 24–42. [Google Scholar] [CrossRef]
- Dias, E.C.P.P.; Macedo, G.A.; Camargo, G.A.; Macedo, J.A.; Chiocchetti, G.M. Effects of extraction processes on recovery, the phenolic profile, and antiglycation activity from green coffee residues (Coffea arabica and Coffea canephora Pierre). ACS Sustain. Chem. Eng. 2024, 12, 13464–13474. [Google Scholar] [CrossRef]
- Chindapan, N.; Puangngoen, C.; Devahastin, S. Caffeine removal and compositions losses from whole Robusta coffee beans during conventional and ultrasound-assisted aqueous decaffeination. J. Food Eng. 2025, 387, 112349. [Google Scholar] [CrossRef]
- Myo, H.; Khat-Udomkiri, N. Optimization of ultrasound-assisted extraction of bioactive compounds from coffee pulp using propylene glycol as a solvent and their antioxidant activities. Ultrason. Sonochem. 2022, 89, 106127. [Google Scholar] [CrossRef] [PubMed]
- Al-Dhabi, N.A.; Ponmurugan, K.; Maran Jeganathan, P. Development and validation of ultrasound-assisted solid-liquid extraction of phenolic compounds from waste spent coffee grounds. Ultrason. Sonochem. 2017, 34, 206–213. [Google Scholar] [CrossRef]
- Yusoff, I.M.; Taher, Z.M.; Rahmat, Z.; Chua, L.S.A. A review of ultrasound-assisted extraction for plant bioactive compounds: Phenolics, flavonoids, thymols, saponins and proteins. Food Res. Inter. 2022, 157, 111268. [Google Scholar] [CrossRef]
- Okur, I.; Soyler, B.; Sezer, P.; Oztop, M.H.; Alpas, H. Improving the recovery of phenolic compounds from spent coffee grounds (SCG) by environmentally friendly extraction techniques. Molecules 2021, 26, 613. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, J.; Wang, S.; Rupasinghe, H.V. Experimental exploration of processes for deriving multiple products from spent coffee grounds. Food Bioprod. Process 2021, 128, 21–29. [Google Scholar] [CrossRef]
- Samsalee, N.; Sothornvit, R. Physicochemical, functional properties and antioxidant activity of protein extract from spent coffee grounds using ultrasonic-assisted extraction. AIMS Agric. Food 2021, 6, 864–878. [Google Scholar] [CrossRef]
- Vignoli, J.A.; Bassoli, D.G.; Benassi, M.T. Antioxidant activity of roasted and instant coffees: Standardization and validation of methodologies. Coffee Sci. 2012, 7, 68–75. Available online: https://coffeescience.ufla.br/index.php/Coffeescience/article/view/224 (accessed on 20 January 2025).
- Chatzimitakos, T.; Athanasiadis, V.; Kotsou, K.; Palaiogiannis, D.; Bozinou, E.; Lalas, S.I. Optimized isolation procedure for the extraction of bioactive compounds from spent coffee grounds. Appl. Sci. 2023, 13, 2819. [Google Scholar] [CrossRef]
- Seo, H.S.; Park, B.H. Phenolic compound extraction from spent coffee grounds for antioxidant recovery. Korean J. Chem. Eng. 2019, 36, 186–190. [Google Scholar] [CrossRef]
- Pujol, D.; Liu, C.; Gominho, J.; Olivella, M.A.; Fiol, N.; Villaescusa, I.; Pereira, H. The chemical composition of exhausted coffee waste. Ind. Crops Prod. 2013, 50, 423–429. [Google Scholar] [CrossRef]
- Martínez-Inda, B.; Jiménez-Moreno, N.; Esparza, I.; Ancín-Azpilicueta, C. Coffee and cocoa by-products as valuable sources of bioactive compounds: The influence of ethanol on extraction. Antioxidants 2025, 14, 42. [Google Scholar] [CrossRef]
- Papageorgiou, C.; Dermesonlouoglou, E.; Tsimogiannis, D.; Taoukis, P. Enrichment of bakery products with antioxidant and dietary fiber ingredients obtained from spent coffee ground. Appl. Sci. 2024, 14, 6863. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Casas, A.R.; del Castillo, M.D. Interest of coffee melanoidins as sustainable healthier food ingredients. Front. Nutr. 2021, 8, 730343. [Google Scholar] [CrossRef]
- Xu, H.; Wang, W.; Liu, X.; Yuan, F.; Gao, Y. Antioxidative phenolics obtained from spent coffee grounds (Coffea arabica L.) by subcritical water extraction. Ind. Crops Prod. 2015, 76, 946–954. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, functional, and structural properties of spent coffee grounds and coffee silverskin. Food Bioprocess. Technol. 2014, 7, 3493–3503. [Google Scholar] [CrossRef]
- McNutt, J.; He, Q.S. Spent coffee grounds: A review on current utilization. J. Ind. Eng. Chem. 2019, 71, 78–88. [Google Scholar] [CrossRef]
- Vandeponseele, A.; Draye, M.; Piot, C.; Chatel, G. Study of influential parameters of the caffeine extraction from spent coffee grounds: From brewing coffee method to the waste treatment conditions. Clean. Technol. 2021, 3, 335–350. [Google Scholar] [CrossRef]
- Viencz, T.; Acre, L.B.; Rocha, R.B.; Alves, E.A.; Ramalho, A.R.; Benassi, M.T. Caffeine, trigonelline, chlorogenic acids, melanoidins, and diterpenes contents of Coffea canephora coffees produced in the Amazon. J. Food Compos. Anal. 2023, 117, 105140. [Google Scholar] [CrossRef]
- Panusa, A.; Zuorro, A.; Lavecchia, R.; Marrosu, G.; Petrucci, R. Recovery of natural antioxidants from spent coffee grounds. J. Agric. Food Chem. 2013, 61, 4162–4168. [Google Scholar] [CrossRef]
- Sant’Anna, V.; Biondo, E.; Kolchinski, E.M.; Silva, L.F.S. Total polyphenols, antioxidant, antimicrobial and allelopathic activities of spent coffee ground aqueous extract. Waste Biomass Valor. 2017, 8, 439–442. [Google Scholar] [CrossRef]
- Severini, C.; Derossi, A.; Fiore, A.G. Ultrasound-assisted extraction to improve the recovery of phenols and antioxidants from spent espresso coffee ground: A study by response surface methodology and desirability approach. Eur. Food Res. Technol. 2017, 243, 835–847. [Google Scholar] [CrossRef]
- Ripper, B.; Kaiser, C.R.; Perrone, D. Use of NMR techniques to investigate the changes on the chemical composition of coffee melanoidins. J. Food Compos. Anal. 2020, 87, 103399. [Google Scholar] [CrossRef]
- Jiménez-Zamora, A.; Pastoriza, S.; Rufián-Henares, J.A. Revalorization of coffee by-products. Prebiotic, antimicrobial and antioxidant properties. LWT-Food Sci. Technol. 2015, 61, 12–18. [Google Scholar] [CrossRef]
- Brzezińska, R.; Górska, A.; Wirkowska-Wojdyła, M.; Piasecka, I. Response surface methodology as a tool for optimization of extraction process of bioactive compounds from spent coffee grounds. Appl. Sci. 2023, 13, 7634. [Google Scholar] [CrossRef]
- Page, J.C.; Arruda, N.P.; Freitas, S.P. Crude ethanolic extract from spent coffee grounds: Volatile and functional properties. Waste Manag. 2017, 69, 463–469. [Google Scholar] [CrossRef]
- Batista, M.J.P.A.; Torres, S.S.; Franca, A.S.; Oliveira, L.S. Effect of zinc chloride solution assisted by ultrasound on polysaccharides of spent coffee grounds. Carbohydr. Polym. Technol. Appl. 2023, 5, 100298. [Google Scholar] [CrossRef]
- Benítez, V.; Rebollo-Hernanz, M.; Hernanz, S.; Chantres, S.; Aguilera, Y.; Martin-Cabrejas, M.A. Coffee parchment as a new dietary fiber ingredient: Functional and physiological characterization. Food Res. Int. 2019, 122, 105–113. [Google Scholar] [CrossRef]
- Go, A.W.; Conag, A.T.; Cuizon, D.E.S. Recovery of sugars and lipids from spent coffee grounds: A new approach. Waste Biomass Valor. 2016, 7, 1047–1053. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Machado, E.M.; Martins, S.; Teixeira, J.A. Production, composition, and application of coffee and its industrial residues. Food Bioprocess. Technol. 2011, 4, 661–672. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Biorefinery approach for spent coffee grounds valorization. Bioresour. Technol. 2018, 247, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Kafková, V.; Kubinec, R.; Mikulec, J.; Variny, M.; Ondrejíčková, P.; Ház, A.; Brisudová, A. Integrated approach to spent coffee grounds valorization in biodiesel biorefinery. Sustainability 2023, 15, 5612. [Google Scholar] [CrossRef]
- Silva, J.B.M.D.; Paiva, M.T.P.; Pavanello, A.C.L.; Mantovan, J.; Mali, S. Fiber-rich ingredients obtained from agroindustrial residues through combined hydrothermal-chemical processes. Food Chem. Adv. 2022, 1, 100149. [Google Scholar] [CrossRef]
- Chen, Q.; Dong, W.; Wei, C.; Hu, R.; Long, Y. Combining integrated ultrasonic-microwave technique with ethanol to maximise extraction of green coffee oil from Arabica coffee beans. Ind. Crops Prod. 2020, 151, 112405. [Google Scholar] [CrossRef]
- Valdés, A.; Castro-Puyana, M.; Marina, M.L. Isolation of proteins from spent coffee grounds. Polyphenol removal and peptide identification in the protein hydrolysates by RP-HPLC-ESI-Q-TOF. Food Res. Int. 2020, 137, 109368. [Google Scholar] [CrossRef]
- Benincá, D.B.; Carmo, L.B.; Grancieri, M.; Aguiar, L.L.; Lima Filho, T.; Costa, A.G.; Oliveira, D.S.; Saraiva, S.H.; Silva, P.I. Incorporation of spent coffee grounds in muffins: A promising industrial application. Food Chem. Adv. 2023, 3, 100329. [Google Scholar] [CrossRef]
- Trà, T.T.T.; Phúc, L.N.; Yên, V.T.N.; Sang, L.T.; Thu’, T.A.; Nguyêt, T.N.M.; Mãn, L.V. Use of wheat flour and spent coffee grounds in the production of cookies with high fiber and antioxidant content: Effects of spent coffee grounds ratio on the product quality. IOP Conf. Ser. Earth Environ. Sci. 2021, 947, 012044. [Google Scholar] [CrossRef]
- Oliveira, L.S.; Franca, A.S. Potential of spent coffee grounds as sources of dietary fiber with antioxidant activity. In Food Waste: Practices, Management and Challenges; Riley, G.L., Ed.; Nova Publishers: New York, NY, USA, 2016; pp. 51–70. [Google Scholar]
- Panzella, L.; Pérez-Burillo, S.; Pastoriza, S.; Martín, M.A.; Cerutti, P.; Goya, L.; Ramos, S.; Rufián-Henares, J.A.; Napolitano, A.; D’Ischia, M. High antioxidant action and prebiotic activity of hydrolyzed spent coffee grounds (HSCG) in a simulated digestion–fermentation model: Toward the development of a novel food supplement. J. Agric. Food Chem. 2017, 65, 6452–6459. [Google Scholar] [CrossRef]
- López-Barrera, D.M.; Vázquez-Sánchez, K.; Loarca-Piña, M.G.F.; Campos-Vega, R. Spent coffee grounds, an innovative source of colonic fermentable compounds, inhibit inflammatory mediators in vitro. Food Chem. 2016, 212, 282–290. [Google Scholar] [CrossRef]
- Meng, X.; Liu, F.; Xiao, Y.; Cao, J.; Wang, M.; Duan, X. Alterations in physicochemical and functional properties of buckwheat straw insoluble dietary fiber by alkaline hydrogen peroxide treatment. Food Chem. X 2019, 3, 100029. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, H.; Yuan, F.; Pan, Q.; Fan, R.; Gao, Y. Physicochemical characterization of five types of citrus dietary fibers. Biocatal. Agric. Biotechnol. 2015, 4, 250–258. [Google Scholar] [CrossRef]
- Vargas-Sánchez, R.D.; Torres-Martínez, B.M.; Torrescano-Urrutia, G.R.; Sánchez-Escalante, A. Physicochemical, techno-functional and antioxidant characterization of coffee silverskin. Biotecnia 2023, 25, 43–50. [Google Scholar] [CrossRef]
- Gea Niro Research Laboratory. Gea Niro Research Laboratory 2005 Hygroscopicity–Method N°14A; Gea Niro Research Laboratory: Düsseldorf, Germany, 2005. [Google Scholar]
- Olorunsola, E.O.; Akpabio, E.I.; Adedokun, M.O.; Ajibola, D.O. Emulsifying Properties of Hemicelluloses; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Portela, C.S.; Almeida, I.F.; Reis, T.A.D.; Hickmann, B.R.B.; Benassi, M.T. Effects of brewing conditions and coffee species on the physicochemical characteristics, preference and dynamics of sensory attributes perception in cold brews. Food Res. Int. 2022, 151, 110860. [Google Scholar] [CrossRef] [PubMed]
- Folin, O.; Ciocalteu, V. On tyrosine and tryptophane determinations in proteins. J. Biol. Chem. 1927, 73, 627–650. [Google Scholar] [CrossRef]
- Mori, A.L.B.; Viegas, M.C.; Ferrão, M.A.G.; Fonseca, A.F.A.; Ferrão, R.G.; Benassi, M.T. Coffee brews composition from Coffea canephora cultivars with different fruit ripening seasons. Br. Food J. 2020, 122, 827–840. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Dinis, T.C.; Maderia, V.M.; Almeida, L.M. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Bio. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of Association of Official Analytical Chemists, 19th ed; AOAC: Arlington, VA, USA, 2012. [Google Scholar]
- Van Soest, P.J. Symposium on factors influencing the voluntary intake of herbage by ruminants: Voluntary intake in relation to chemical composition and digestibility. J. Anim. Sci. 1965, 24, 834–843. [Google Scholar] [CrossRef]
- TAPPI. TAPPI. TAPPI test method T222 om-88: Acid-insoluble lignin in wood and pulp. In TAPPI Test Methods; TAPPI: Atlanta, GA, USA, 1999. [Google Scholar]
- Benítez, V.; Cantera, S.; Aguilera, Y.; Mollá, E.; Esteban, R.M.; Díaz, M.F.; Martín-Cabrejas, M.A. Impact of germination on starch, dietary fiber and physicochemical properties in non-conventional legumes. Food Res. Int. 2013, 50, 64–69. [Google Scholar] [CrossRef]
- Mateos-Aparicio, I.; Mateos-Peinado, C.; Rupérez, P. High hydrostatic pressure improves the functionality of dietary fiber in okara by-product from soybean. Innov. Food Sci. Emerg. Technol. 2010, 11, 445–450. [Google Scholar] [CrossRef]
- Castro-Muñhoz, R.; Barragán-Huerta, B.E.; Yáñez-Fernández, J. Use of gelatin-maltodrextrin composite as an encapsulation support for clarified juice from purple cactus pear (Opuntia stricta). LWT-Food Sci. Technol. 2015, 62, 242–248. [Google Scholar] [CrossRef]
- Seibel, N.F.; Beléia, A.D.P. The chemical characteristics and technological functionality of soybean-based ingredients [Glycine max (L.) Merrill]: Carbohydrates and proteins. Braz. J. Food Technol. 2009, 12, 113–122. [Google Scholar] [CrossRef]
- Daou, C.; Zhang, H. Functional and physiological properties of total, soluble, and insoluble dietary fibres derived from defatted rice bran. J. Food Sci. Technol. 2013, 51, 3878–3885. [Google Scholar] [CrossRef]
- Miller, G. Use of dinitrosalicilic acid reagent for determination of reducing sugars. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
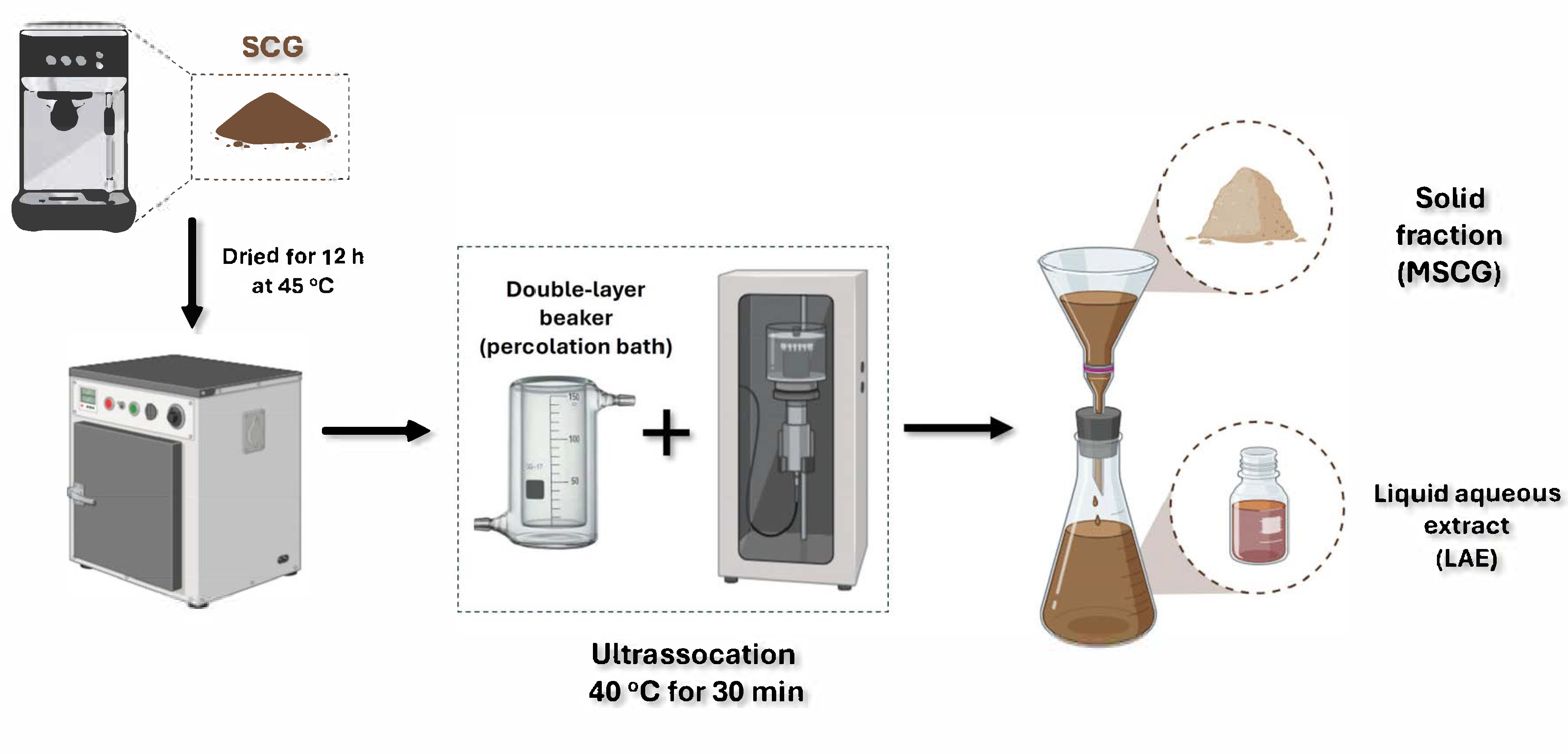
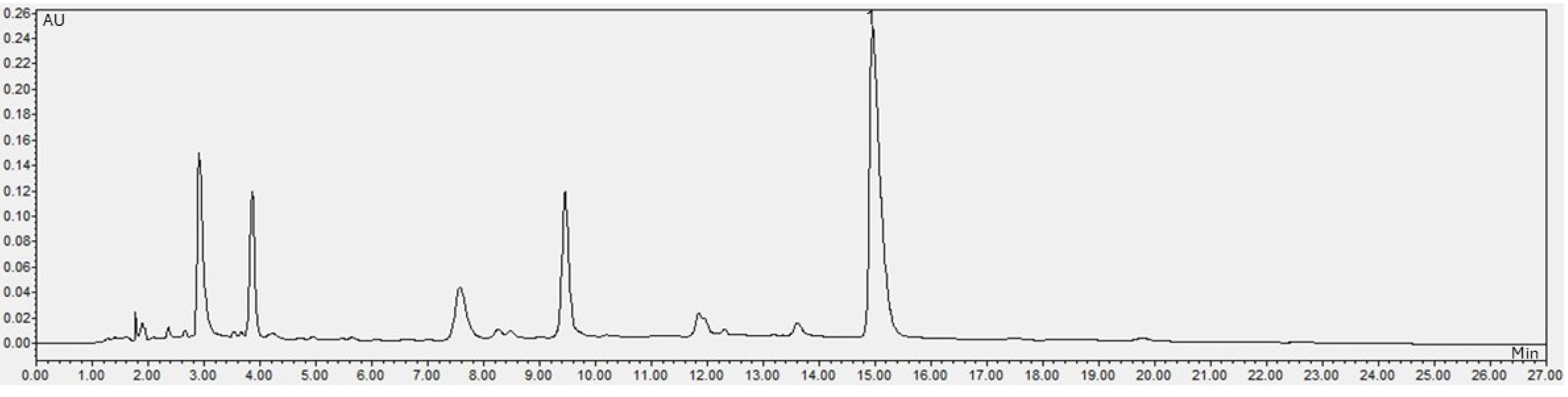
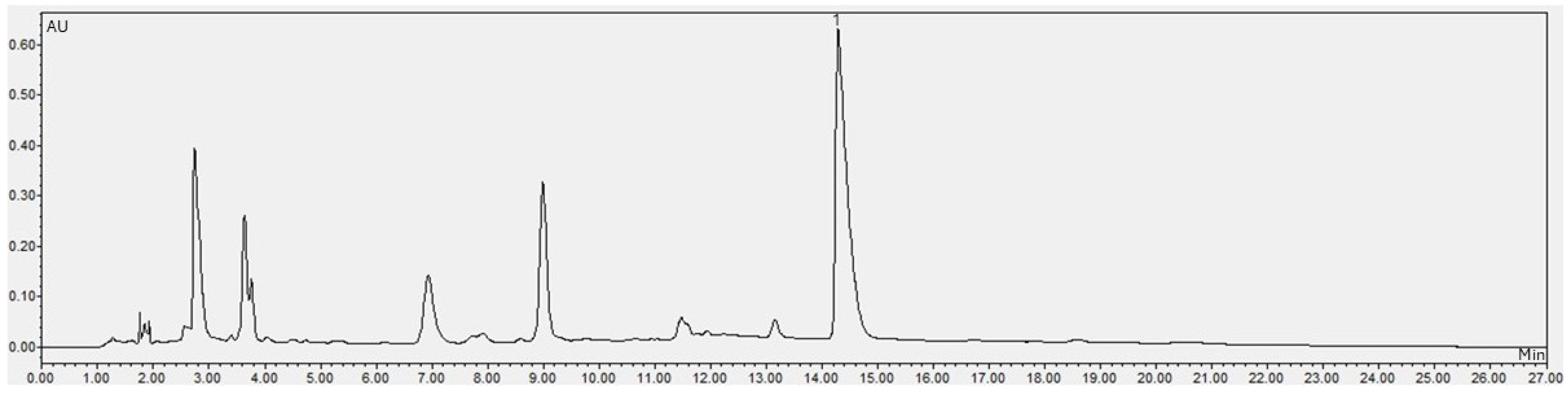
| Compound | SCGs | LAE | MSCGs |
| CA (mg/100 g) | 519.0 ± 0.3 a | 400.1 ± 0.3 b | 403.0 ± 1.6 b |
| TPC (mg GAE/100 g) | 1380.0 ± 0.1 a | 800.4 ± 0.1 b | 821.0 ± 0.1 b |
| MEs (mg/100 g) | 6640.8 ± 0.1 a | 2100.2 ± 0.1 c | 3439.6 ± 0.1 b |
| Antioxidant activity | SCGs | LAE | MSCGs |
| DPPH (mg TE/100 g) | 2015.2 ± 33 a | 154.5 ± 0.5 b | 93.2 ± 3.0 b |
| ABTS (mg TE/100 g) | 267.4 ± 2.2 b | 490.5 ± 0.1 a | 29.7 ± 0.7 c |
| Compound (g/100 g) | SCGs | MSCGs |
|---|---|---|
| Moisture | 4.4 ± 0.1 a | 2.9 ± 0.1 b |
| Ash | 1.9 ± 0.1 a | 0.9 ± 0.1 b |
| Lipids | 10.8 ± 0.1 a | 7.3 ± 0.2 b |
| Proteins | 1.2 ± 0.1 b | 2.9 ± 0.3 a |
| Total fibers | 65.5 ± 0.7 b | 73.0 ± 0.5 a |
| Soluble fibers | 1.4 ± 0.2 a | 1.6 ± 0.3 a |
| Insoluble fibers | 64.1 ± 0.9 b | 71.4 ± 0.4 a |
| Cellulose | 14.1 ± 0.6 b | 17.0 ±0.7 a |
| Hemicellulose | 23.0 ± 1.0 b | 27.4 ± 0.5 a |
| Lignin | 36.0 ± 1.2 b | 39.1 ± 1.4 a |
| Properties | SCGs | MSCGs |
|---|---|---|
| WHC (g/g) | 3.5 ± 0.1 b | 3.7 ± 0.1 a |
| OHC (g/g) | 2.8 ± 0.1 a | 2.8 ± 0.1 a |
| SC (mL/g) | 3.9 ± 0.2 b | 4.2 ± 0.2 a |
| HI (%) | 11.0 ± 0.2 a | 10.6 ± 0.5 a |
| EA (%) | 2.0 ± 0.1 b | 3.3 ± 1.2 a |
| Properties | SCGs | MSCGs |
|---|---|---|
| CAC pH 2 (mg/g) | 2.7 ± 0.9 a | 1.6 ± 0.3 b |
| CAC pH 7 (mg/g) | 1.6 ± 0.8 b | 8.0 ± 0.5 a |
| GAC 50 mmol/L (mmol/L) | 4.7 ± 0.8 b | 9.3 ± 1.1 a |
| GAC100 mmol/L (mmol/L) | 6.7 ± 3.2 b | 17.7 ± 2.6 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, J.B.M.D.; Paiva, M.T.P.; Fuzinato, H.F.; Silvestre, N.; Benassi, M.T.; Mali, S. An Ultrasonication-Assisted Green Process for Simultaneous Production of a Bioactive Compound-Rich Extract and a Multifunctional Fibrous Ingredient from Spent Coffee Grounds. Molecules 2025, 30, 3117. https://doi.org/10.3390/molecules30153117
Silva JBMD, Paiva MTP, Fuzinato HF, Silvestre N, Benassi MT, Mali S. An Ultrasonication-Assisted Green Process for Simultaneous Production of a Bioactive Compound-Rich Extract and a Multifunctional Fibrous Ingredient from Spent Coffee Grounds. Molecules. 2025; 30(15):3117. https://doi.org/10.3390/molecules30153117
Chicago/Turabian StyleSilva, Jaquellyne B. M. D., Mayara T. P. Paiva, Henrique F. Fuzinato, Nathalia Silvestre, Marta T. Benassi, and Suzana Mali. 2025. "An Ultrasonication-Assisted Green Process for Simultaneous Production of a Bioactive Compound-Rich Extract and a Multifunctional Fibrous Ingredient from Spent Coffee Grounds" Molecules 30, no. 15: 3117. https://doi.org/10.3390/molecules30153117
APA StyleSilva, J. B. M. D., Paiva, M. T. P., Fuzinato, H. F., Silvestre, N., Benassi, M. T., & Mali, S. (2025). An Ultrasonication-Assisted Green Process for Simultaneous Production of a Bioactive Compound-Rich Extract and a Multifunctional Fibrous Ingredient from Spent Coffee Grounds. Molecules, 30(15), 3117. https://doi.org/10.3390/molecules30153117








