Olive Pomace Extract Acts as a New Potent Ferroptosis Inhibitor in Human Cells
Abstract
1. Introduction
2. Results
2.1. OPE OH-Tyr and Tyr Quantification
2.1.1. Cell-Free Antioxidant Activity of OPE
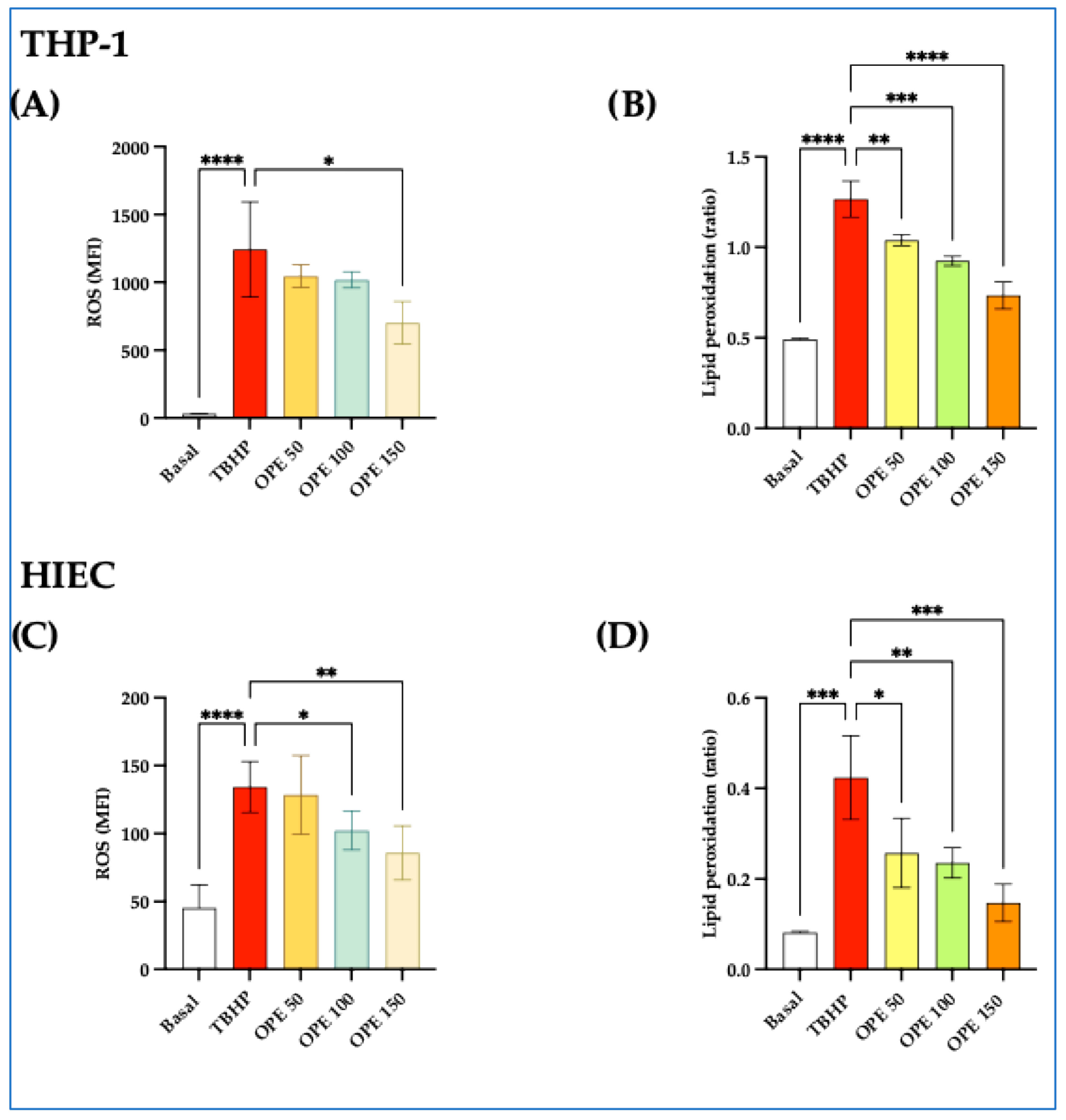
2.1.2. OPE Decreases ROS and Lipid Peroxidation Induced by TBHP in THP-1 and HIEC Cells
2.1.3. Effect of OPE in Reducing the RSL3-Induced Cytotoxicity in THP-1 and HIEC Cells
2.1.4. OPE Reduces ROS and Lipid Peroxidation Triggered by RSL3 in THP-1 and HIEC Cells
2.1.5. OPE Increases GSH and GSH/GSSG Ratio in THP-1 and HIEC Cells Incubated with RSL3
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. OP Phenolic Compound Extraction and Characterization
4.3. Cell-Free Antioxidant Capacity of OPE
4.4. Cell Cultures
4.5. Cell Viability Assay
4.6. Intracellular ROS Measurement
4.7. Determination of Cellular Lipid Peroxidation
4.8. Ferroptosis Induction and the Effect of OPE
4.9. Measurement of GSH and GSSG
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cinardi, G.; D’Urso, P.R.; Arcidiacono, C.; Ingrao, C. Accounting for Circular Economy Principles in Life Cycle Assessments of Extra-Virgin Olive Oil Supply Chains—Findings from a Systematic Literature Review. Sci. Total Environ. 2024, 945, 173977. [Google Scholar] [CrossRef] [PubMed]
- Ntougias, S.; Bourtzis, K.; Tsiamis, G. The Microbiology of Olive Mill Wastes. BioMed Res. Int. 2013, 2013, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Topi, D.; Beqiraj, I.; Seiti, B.; Halimi, E. Environmental Impact From Olive Mills Waste Disposal, Chemical Analysis Of Solid Wastes And Wastewaters. J. Hyg. Eng. Des. 2014, 7, 44–48. [Google Scholar]
- Herrero, M.; Temirzoda, T.N.; Segura-Carretero, A.; Quirantes, R.; Plaza, M.; Ibañez, E. New Possibilities for the Valorization of Olive Oil By-Products. J. Chromatogr. A 2011, 1218, 7511–7520. [Google Scholar] [CrossRef] [PubMed]
- Radić, K.; Jurišić Dukovski, B.; Vitali Čepo, D. Influence of Pomace Matrix and Cyclodextrin Encapsulation on Olive Pomace Polyphenols’ Bioaccessibility and Intestinal Permeability. Nutrients 2020, 12, 669. [Google Scholar] [CrossRef] [PubMed]
- Rigacci, S.; Stefani, M. Nutraceutical Properties of Olive Oil Polyphenols. An Itinerary from Cultured Cells through Animal Models to Humans. Int. J. Mol. Sci. 2016, 17, 843. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Bridges, R.J.; Natale, N.R.; Patel, S.A. System xc− Cystine/Glutamate Antiporter: An Update on Molecular Pharmacology and Roles within the CNS. Br. J. Pharmacol. 2012, 165, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of Ferroptotic Cancer Cell Death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R.; Angeli, J.P.F.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.; Fulda, S.; Gascon, S.; Hatzios, S.K.; Kagan, V.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. How Mitochondria Produce Reactive Oxygen Species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cheff, D.M.; Huang, C.; Scholzen, K.C.; Gencheva, R.; Ronzetti, M.H.; Cheng, Q.; Hall, M.D.; Arnér, E.S.J. The Ferroptosis Inducing Compounds RSL3 and ML162 Are Not Direct Inhibitors of GPX4 but of TXNRD1. Redox Biol. 2023, 62, 102703. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.-J.; Huang, X.; Zhu, B.T. Mechanism of RSL3-Induced Ferroptotic Cell Death in HT22 Cells: Crucial Role of Protein Disulfide Isomerase. Acta Biochim. Biophys. Sin. 2024, 57, 616–632. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yu, C.; Kang, R.; Tang, D. Iron Metabolism in Ferroptosis. Front. Cell Dev. Biol. 2020, 8, 590226. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Ardehali, H.; Min, J.; Wang, F. The Molecular and Metabolic Landscape of Iron and Ferroptosis in Cardiovascular Disease. Nat. Rev. Cardiol. 2023, 20, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Galluzzi, L. Fighting Resilient Cancers with Iron. Trends Cell Biol. 2018, 28, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Gan, B. ACSL4, PUFA, and Ferroptosis: New Arsenal in Anti-Tumor Immunity. Signal Transduct. Target. Ther. 2022, 7, 128. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R. Ferroptosis Turns 10: Emerging Mechanisms, Physiological Functions, and Therapeutic Applications. Cell 2022, 185, 2401–2421. [Google Scholar] [CrossRef] [PubMed]
- Fierri, I.; Chignola, R.; Stranieri, C.; Di Leo, E.G.; Bellumori, M.; Roncoletta, S.; Romeo, A.; Benetti, F.; Fratta Pasini, A.M.; Zoccatelli, G. Formulation, Characterization, and Antioxidant Properties of Chitosan Nanoparticles Containing Phenolic Compounds from Olive Pomace. Antioxidants 2024, 13, 1522. [Google Scholar] [CrossRef] [PubMed]
- Cecchi, L.; Khatib, M.; Bellumori, M.; Civa, V.; Domizio, P.; Innocenti, M.; Balli, D.; Mulinacci, N. Industrial Drying for Agrifood By-Products Re-Use: Cases Studies on Pomegranate Peel (Punica granatum L.) and Stoned Olive Pomace (Pâtè, Olea Europaea, L.). Food Chem. 2023, 403, 134338. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.B.; Oliveira, A.; Coelho, M.; Veiga, M.; Costa, E.M.; Silva, S.; Nunes, J.; Vicente, A.A.; Pintado, M. Are Olive Pomace Powders a Safe Source of Bioactives and Nutrients? J. Sci. Food Agric. 2021, 101, 1963–1978. [Google Scholar] [CrossRef] [PubMed]
- Abbattista, R.; Ventura, G.; Calvano, C.D.; Cataldi, T.R.I.; Losito, I. Bioactive Compounds in Waste By-Products from Olive Oil Production: Applications and Structural Characterization by Mass Spectrometry Techniques. Foods 2021, 10, 1236. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.M.; Barreto-Peixoto, J.; Andrade, N.; Machado, S.; Silva, C.; Lobo, J.C.; Nunes, M.A.; Álvarez-Rivera, G.; Ibáñez, E.; Cifuentes, A.; et al. Comprehensive Analysis of the Phytochemical Composition and Antitumoral Activity of an Olive Pomace Extract Obtained by Mechanical Pressing. Food Bioscience 2024, 61, 104759. [Google Scholar] [CrossRef]
- Türkekul, B.; Günden, C.; Abay, C.; Miran, B. Competitiveness of Mediterranean Countries in the Olive Oil Market. New Medit 2010, 9, 41–46. [Google Scholar]
- Argun, M.E.; Arslan, F.N.; Ateş, H.; Yel, E.; Çakmakcı, Ö.; Dağ, B. A Pioneering Study on the Recovery of Valuable Functional Compounds From Olive Pomace by Using Supercritical Carbon Dioxide Extraction: Comparison of Perlite Addition and Drying. Sep. Purif. Technol. 2023, 306, 122593. [Google Scholar] [CrossRef]
- Antónia Nunes, M.; Pawlowski, S.; Costa, A.S.G.; Alves, R.C.; Oliveira, M.B.P.P.; Velizarov, S. Valorization of Olive Pomace by a Green Integrated Approach Applying Sustainable Extraction and Membrane-Assisted Concentration. Sci. Total Environ. 2019, 652, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Chanioti, S.; Tzia, C. Extraction of Phenolic Compounds from Olive Pomace by Using Natural Deep Eutectic Solvents and Innovative Extraction Techniques. Innov. Food Sci. Emerg. Technol. 2018, 48, 228–239. [Google Scholar] [CrossRef]
- Visioli, F.; Poli, A.; Gall, C. Antioxidant and Other Biological Activities of Phenols from Olives and Olive Oil. Med. Res. Rev. 2002, 22, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Brangoulo, H.L.; Molan, P.C. Assay of the Antioxidant Capacity of Foods Using an Iron(II)-Catalysed Lipid Peroxidation Model for Greater Nutritional Relevance. Food Chem. 2011, 125, 1126–1130. [Google Scholar] [CrossRef]
- Leopoldini, M.; Russo, N.; Toscano, M. The Molecular Basis of Working Mechanism of Natural Polyphenolic Antioxidants. Food Chem. 2011, 125, 288–306. [Google Scholar] [CrossRef]
- Di Benedetto, R.; Varì, R.; Scazzocchio, B.; Filesi, C.; Santangelo, C.; Giovannini, C.; Matarrese, P.; D’Archivio, M.; Masella, R. Tyrosol, the Major Extra Virgin Olive Oil Compound, Restored Intracellular Antioxidant Defences in Spite of Its Weak Antioxidative Effectiveness. Nutr. Metab. Cardiovasc. Dis. NMCD 2007, 17, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Bayram, B.; Ozcelik, B.; Grimm, S.; Roeder, T.; Schrader, C.; Ernst, I.M.A.; Wagner, A.E.; Grune, T.; Frank, J.; Rimbach, G. A Diet Rich in Olive Oil Phenolics Reduces Oxidative Stress in the Heart of SAMP8 Mice by Induction of Nrf2-Dependent Gene Expression. Rejuvenation Res. 2012, 15, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Scoditti, E.; Nestola, A.; Massaro, M.; Calabriso, N.; Storelli, C.; De Caterina, R.; Carluccio, M.A. Hydroxytyrosol Suppresses MMP-9 and COX-2 Activity and Expression in Activated Human Monocytes via PKCα and PKCβ1 Inhibition. Atherosclerosis 2014, 232, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Bigagli, E.; Cinci, L.; Paccosi, S.; Parenti, A.; D’Ambrosio, M.; Luceri, C. Nutritionally Relevant Concentrations of Resveratrol and Hydroxytyrosol Mitigate Oxidative Burst of Human Granulocytes and Monocytes and the Production of Pro-Inflammatory Mediators in LPS-Stimulated RAW 264.7 Macrophages. Int. Immunopharmacol. 2017, 43, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Hur, J.; Lee, Y.; Yoon, B.-R.; Choi, S.Y. Protective Effects of Tyrosol Against Oxidative Damage in L6 Muscle Cells. Food Sci. Technol. Res. 2018, 24, 943–947. [Google Scholar] [CrossRef]
- Sies, H. Glutathione and Its Role in Cellular Functions. Free Radic. Biol. Med. 1999, 27, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Basiricò, L.; Morera, P.; Dipasquale, D.; Bernini, R.; Santi, L.; Romani, A.; Lacetera, N.; Bernabucci, U. (-)-Epigallocatechin-3-Gallate and Hydroxytyrosol Improved Antioxidative and Anti-Inflammatory Responses in Bovine Mammary Epithelial Cells. Anim. Int. J. Anim. Biosci. 2019, 13, 2847–2856. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I. Glutathione Homeostasis and Functions: Potential Targets for Medical Interventions. J. Amino Acids 2012, 2012, 736837. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Stockwell, B.R. Synthetic Lethal Screening Identifies Compounds Activating Iron-Dependent, Nonapoptotic Cell Death in Oncogenic-RAS-Harboring Cancer Cells. Chem. Biol. 2008, 15, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Wong-ekkabut, J.; Xu, Z.; Triampo, W.; Tang, I.-M.; Tieleman, D.P.; Monticelli, L. Effect of Lipid Peroxidation on the Properties of Lipid Bilayers: A Molecular Dynamics Study. Biophys. J. 2007, 93, 4225–4236. [Google Scholar] [CrossRef] [PubMed]
- Esposito, B.P.; Breuer, W.; Sirankapracha, P.; Pootrakul, P.; Hershko, C.; Cabantchik, Z.I. Labile Plasma Iron in Iron Overload: Redox Activity and Susceptibility to Chelation. Blood 2003, 102, 2670–2677. [Google Scholar] [CrossRef] [PubMed]
- Park, E.K.; Jung, H.S.; Yang, H.I.; Yoo, M.C.; Kim, C.; Kim, K.S. Optimized THP-1 Differentiation Is Required for the Detection of Responses to Weak Stimuli. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. Al 2007, 56, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Fratta Pasini, A.; Anselmi, M.; Garbin, U.; Franchi, E.; Stranieri, C.; Nava, M.C.; Boccioletti, V.; Vassanelli, C.; Cominacini, L. Enhanced Levels of Oxidized Low-Density Lipoprotein Prime Monocytes to Cytokine Overproduction via Upregulation of CD14 and Toll-like Receptor 4 in Unstable Angina. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1991–1997. [Google Scholar] [CrossRef] [PubMed]
- Peserico, D.; Stranieri, C.; Garbin, U.; Mozzini, C.C.; Danese, E.; Cominacini, L.; Fratta Pasini, A.M. Ezetimibe Prevents Ischemia/Reperfusion-Induced Oxidative Stress and Up-Regulates Nrf2/ARE and UPR Signaling Pathways. Antioxidants 2020, 9, 349. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.; Nguyen, V.; Coder, D. Assessment of Cell Viability. Curr. Protoc. Cytom. 2013, 64, 9.2.1–9.2.26. [Google Scholar] [CrossRef] [PubMed]
- Adan, A.; Kiraz, Y.; Baran, Y. Cell Proliferation and Cytotoxicity Assays. Curr. Pharm. Biotechnol. 2016, 17, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Khalef, L.; Lydia, R.; Filicia, K.; Moussa, B. Cell Viability and Cytotoxicity Assays: Biochemical Elements and Cellular Compartments. Cell Biochem. Funct. 2024, 42, e4007. [Google Scholar] [CrossRef] [PubMed]
- Vermes, I.; Haanen, C.; Steffens-Nakken, H.; Reutelingsperger, C. A Novel Assay for Apoptosis. Flow Cytometric Detection of Phosphatidylserine Expression on Early Apoptotic Cells Using Fluorescein Labelled Annexin, V. J. Immunol. Methods 1995, 184, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Van Engeland, M.; Ramaekers, F.C.S.; Schutte, B.; Reutelingsperger, C.P.M. A Novel Assay to Measure Loss of Plasma Membrane Asymmetry during Apoptosis of Adherent Cells in Culture. Cytometry 1996, 24, 131–139. [Google Scholar] [CrossRef]
- Celeghini, E.C.C.; Alves, M.B.R.; De Arruda, R.P.; De Rezende, G.M.; Florez-Rodriguez, S.A.; De Sá Filho, M.F. Efficiency of CellROX Deep Red ® and CellROX Orange ® Fluorescent Probes in Identifying Reactive Oxygen Species in Sperm Samples from High and Low Fertility Bulls. Anim. Biotechnol. 2021, 32, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Drummen, G.P.C.; van Liebergen, L.C.M.; Op den Kamp, J.A.F.; Post, J.A. C11-BODIPY(581/591), an Oxidation-Sensitive Fluorescent Lipid Peroxidation Probe: (Micro)Spectroscopic Characterization and Validation of Methodology. Free Radic. Biol. Med. 2002, 33, 473–490. [Google Scholar] [CrossRef] [PubMed]
- Pap, E.H.W.; Drummen, G.P.C.; Winter, V.J.; Kooij, T.W.A.; Rijken, P.; Wirtz, K.W.A.; Op den Kamp, J.A.F.; Hage, W.J.; Post, J.A. Ratio-Fluorescence Microscopy of Lipid Oxidation in Living Cells Using C11-BODIPY581/591. FEBS Lett. 1999, 453, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xie, Y.; Cao, L.; Yang, L.; Yang, M.; Lotze, M.T.; Zeh, H.J.; Kang, R.; Tang, D. The Ferroptosis Inducer Erastin Enhances Sensitivity of Acute Myeloid Leukemia Cells to Chemotherapeutic Agents. Mol. Cell. Oncol. 2015, 2, e1054549. [Google Scholar] [CrossRef] [PubMed]
- Zilka, O.; Shah, R.; Li, B.; Friedmann Angeli, J.P.; Griesser, M.; Conrad, M.; Pratt, D.A. On the Mechanism of Cytoprotection by Ferrostatin-1 and Liproxstatin-1 and the Role of Lipid Peroxidation in Ferroptotic Cell Death. ACS Cent. Sci. 2017, 3, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, A.C.; Schneider, E.; McKinnon, T.; Goldfine, H.; Levy, M.A. Validation of a Simplified Procedure for Convenient and Rapid Quantification of Reduced and Oxidized Glutathione in Human Plasma by Liquid Chromatography Tandem Mass Spectrometry Analysis. Biomed. Chromatogr. BMC 2020, 34, e4854. [Google Scholar] [CrossRef] [PubMed]




| Peak Number | Rt (min) | λ max | MS Peaks | Molecule | Ref. | Mean | SD |
|---|---|---|---|---|---|---|---|
| 1 | 6.98 | 276 | 315–153 | OH-tyr hexose | [21,22,23] | 0.71 | 0.12 |
| 2 | 7.08 | 280 | 153–123 | OH-tyr | STD | 1.14 | 0.11 |
| 3 | 10.21 | 276 | tyr | STD | 0.24 | 0.02 | |
| 4 | 11.36 | 270 | 377–151 | Putative tyr derivate | 0.13 | 0.01 | |
| 5 | 12.34 | 262–292 | 565–467 | Putative tyr derivate | 0.15 | 0.02 | |
| 6 | 13.07 | 300–322 | 179 | Caffeic acid | STD | 0.12 | 0.02 |
| 7 | 17.09 | 288–330 | 639 | OH-acteoside 1 | [22] | 0.13 | 0.02 |
| 8 | 17.32 | 288–330 | 639 | OH-acteoside 2 | [22] | 0.11 | 0.02 |
| 9 | 18.95 | 274–350 | 947–473 | Flavonoid 1 | 0.08 | 0.01 | |
| 10 | 20.04 | 254–354 | 609 | Flavonoid 2 | [22,23] | 0.10 | 0.01 |
| 11 | 21.37 | 288–330 | 623 | verbascoside | STD | 1.42 | 0.15 |
| 12 | 22.78 | 288–330 | 623 | verbascoside iso | [22] | 0.09 | 0.00 |
| 13 | 24.25 | 290–328 | 551 | cafselogoside | [22,23] | 0.24 | 0.04 |
| 14 | 26.83 | 226–310 | 535 | comselogoside | [21,22,23] | 0.28 | 0.03 |
| 15 | 30.45 | 252–350 | 285 | luteolin | STD | 0.306 | 0.030 |
| * total tyr + OH-tyr | 4.96 | 0.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Leo, E.G.; Stranieri, C.; Zoccatelli, G.; Bellumori, M.; Zonfrillo, B.; Cominacini, L.; Fratta Pasini, A.M. Olive Pomace Extract Acts as a New Potent Ferroptosis Inhibitor in Human Cells. Molecules 2025, 30, 3095. https://doi.org/10.3390/molecules30153095
Di Leo EG, Stranieri C, Zoccatelli G, Bellumori M, Zonfrillo B, Cominacini L, Fratta Pasini AM. Olive Pomace Extract Acts as a New Potent Ferroptosis Inhibitor in Human Cells. Molecules. 2025; 30(15):3095. https://doi.org/10.3390/molecules30153095
Chicago/Turabian StyleDi Leo, Edoardo Giuseppe, Chiara Stranieri, Gianni Zoccatelli, Maria Bellumori, Beatrice Zonfrillo, Luciano Cominacini, and Anna Maria Fratta Pasini. 2025. "Olive Pomace Extract Acts as a New Potent Ferroptosis Inhibitor in Human Cells" Molecules 30, no. 15: 3095. https://doi.org/10.3390/molecules30153095
APA StyleDi Leo, E. G., Stranieri, C., Zoccatelli, G., Bellumori, M., Zonfrillo, B., Cominacini, L., & Fratta Pasini, A. M. (2025). Olive Pomace Extract Acts as a New Potent Ferroptosis Inhibitor in Human Cells. Molecules, 30(15), 3095. https://doi.org/10.3390/molecules30153095











