South Tyrol (Italy) Pastinaca sativa L. subsp. sativa Essential Oil: GC-MS Composition, Antimicrobial, Anti-Biofilm, and Antioxidant Properties
Abstract
1. Introduction
2. Results and Discussion
2.1. Gas Chromatography and Mass Spectrometry (GC-MS) Analysis of the EO
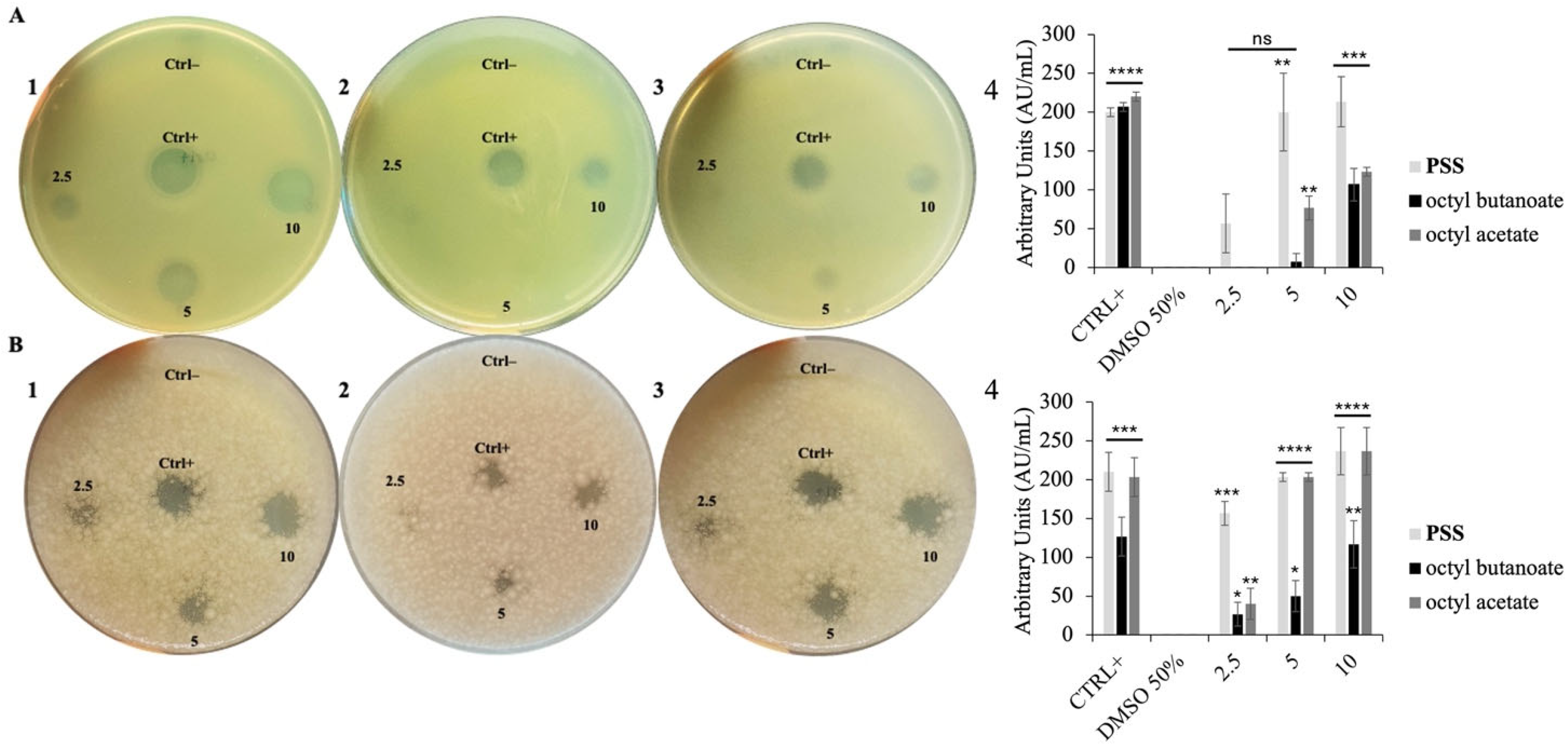
2.2. Antimicrobial Properties of PSS
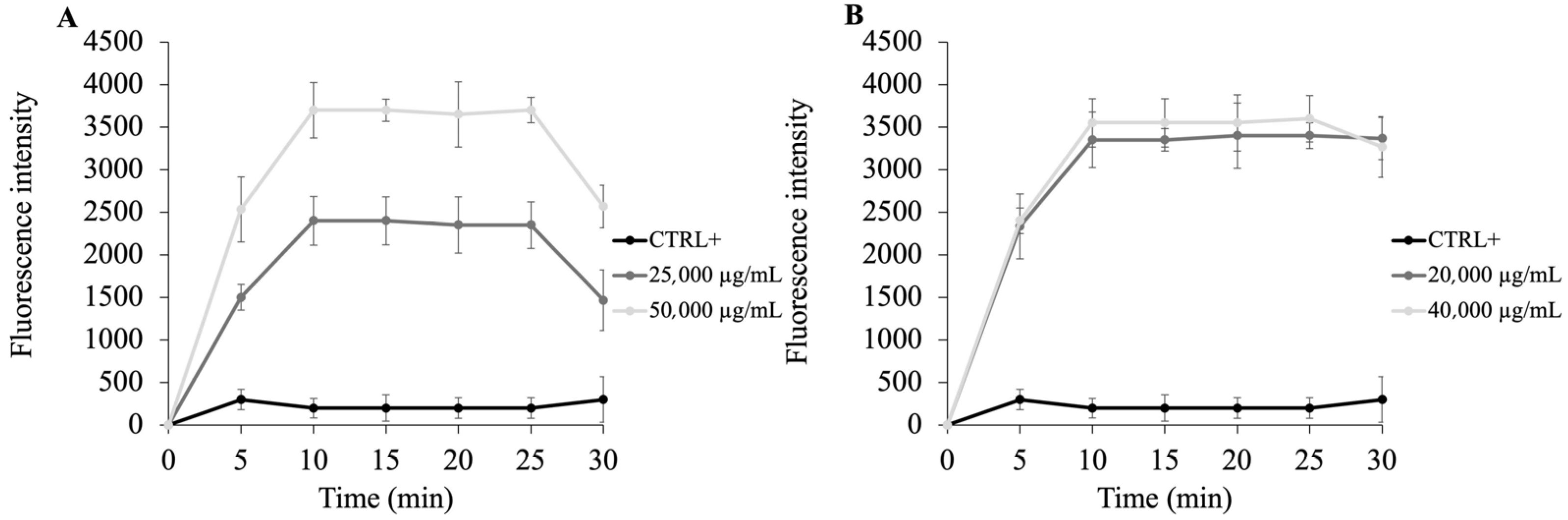
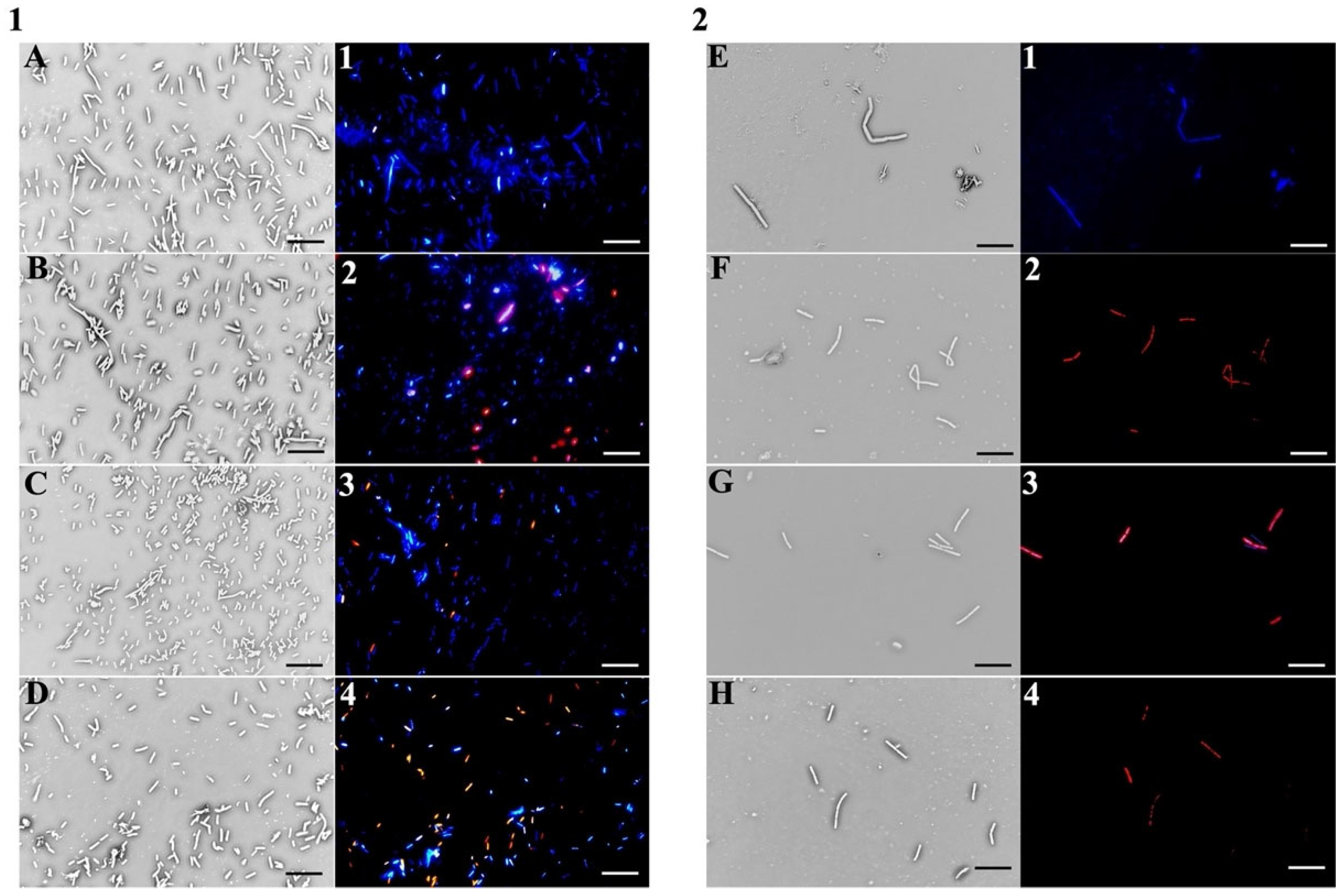
2.3. PSS Target Determination in Bacteria
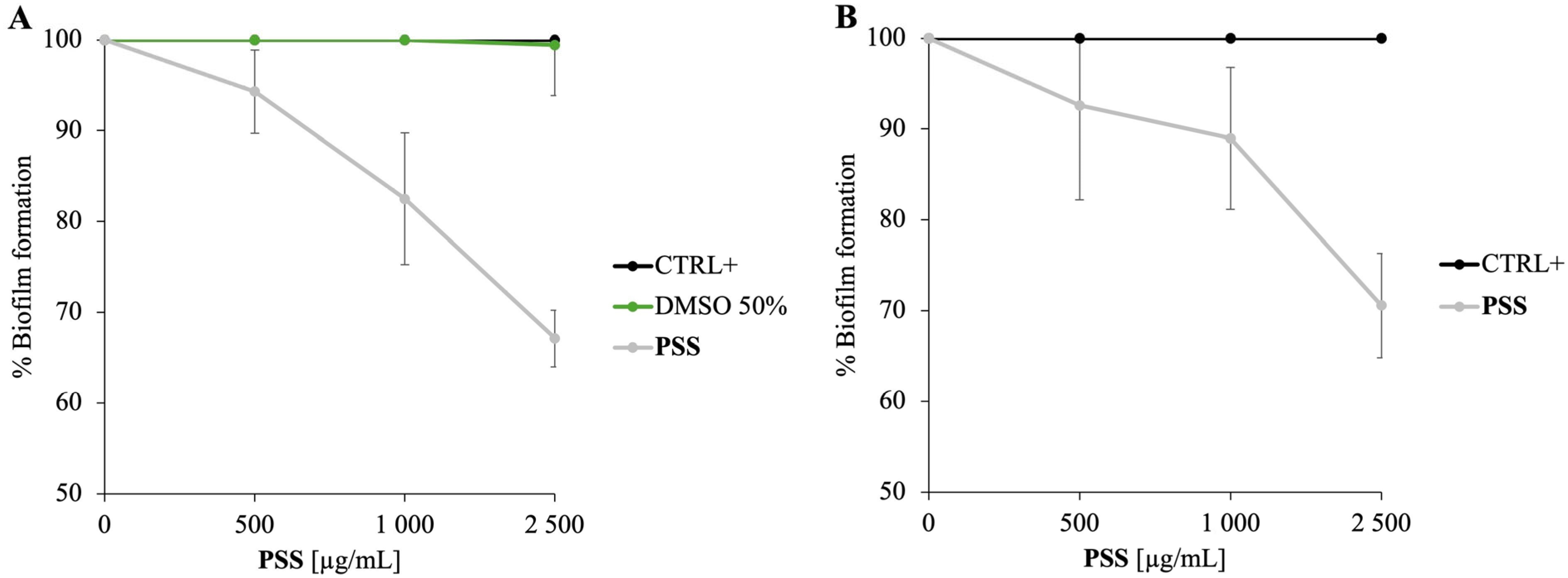
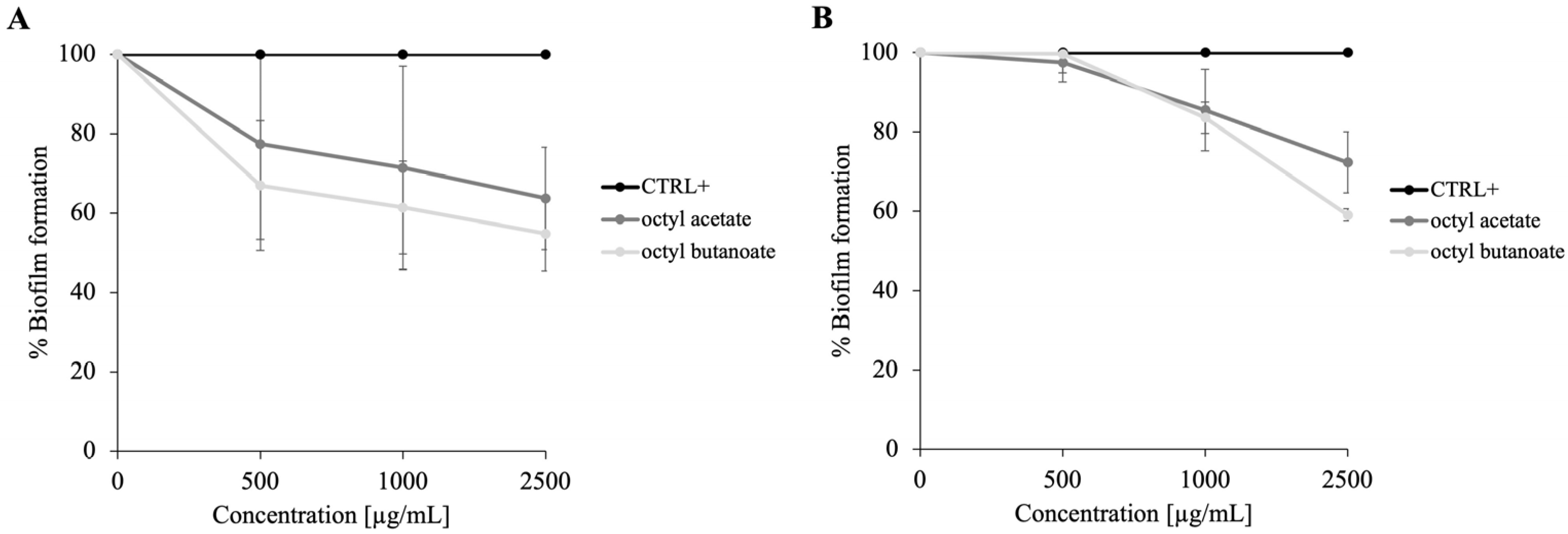
2.4. Antibiofilm Activity of PSS
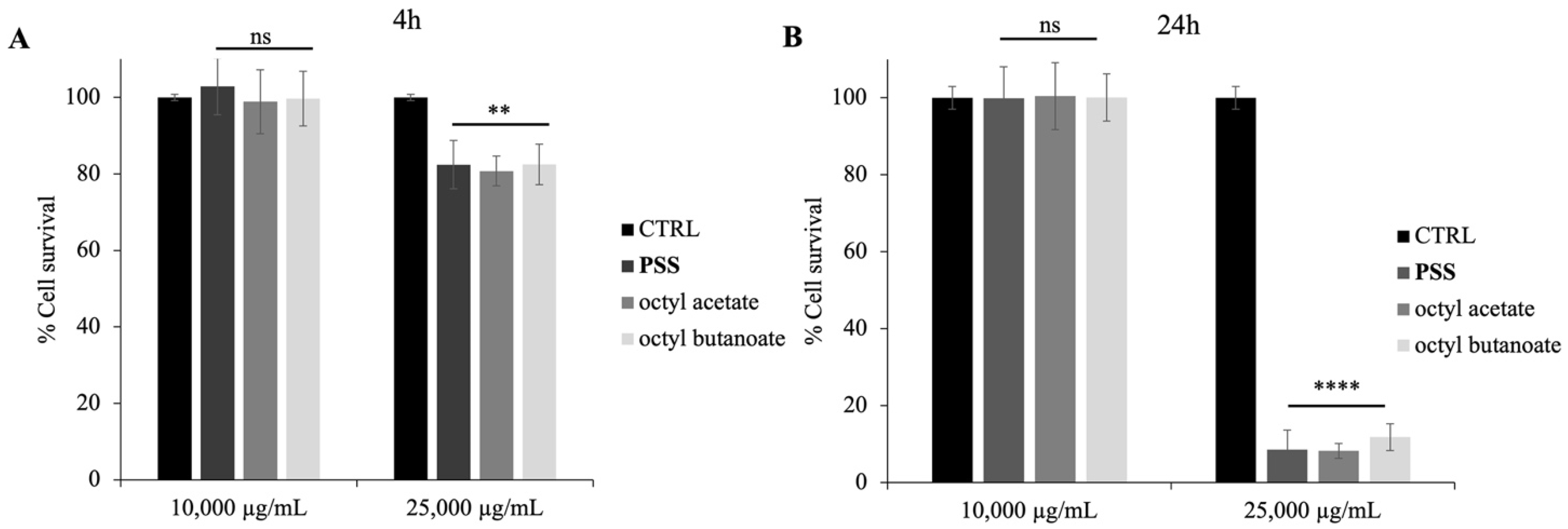
2.5. Cytotoxic Activity of PSS
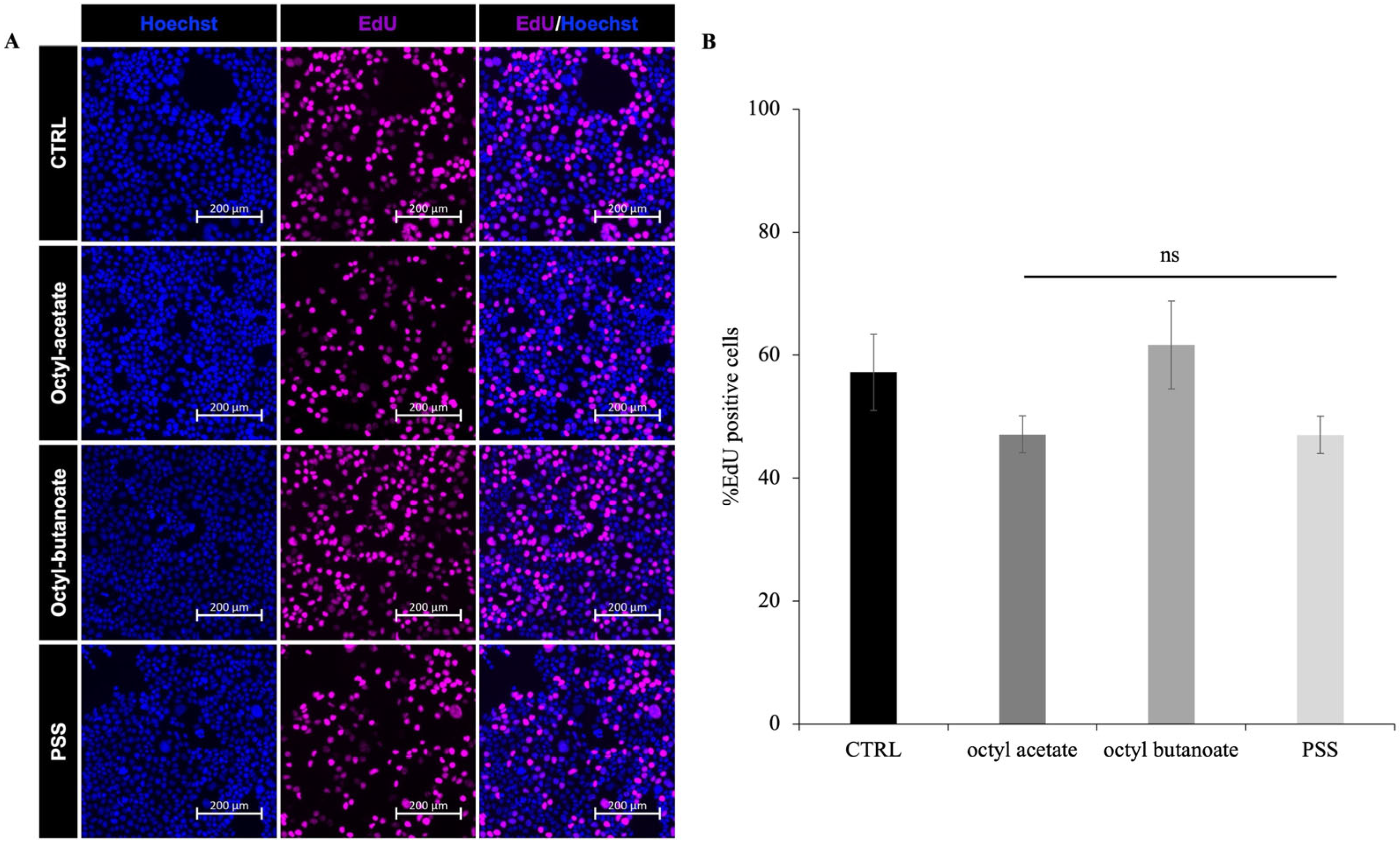
2.6. Effect of PSS on Cell Proliferation
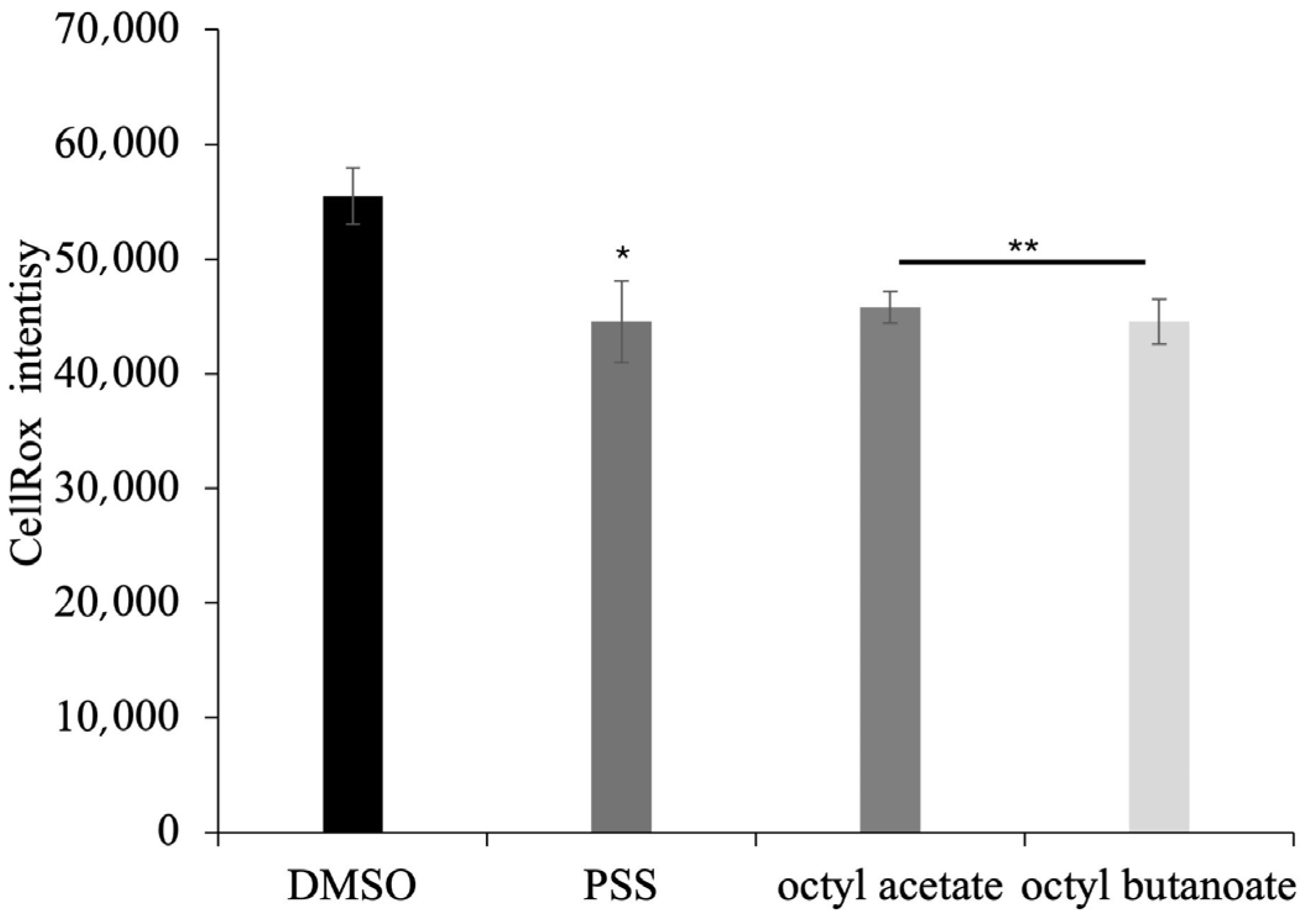
2.7. Antioxidant Activity of PSS on Eukaryotic Cells
3. Materials and Methods
3.1. Plant Material
3.2. Isolation of EO
3.3. GC-MS Analysis
3.4. Pure Compounds
3.5. Microorganisms
3.6. Sample Preparation
3.7. Kirby–Bauer Disk Diffusion Assay
3.8. Determination of Minimum Inhibitory Concentrations (MIC)
3.9. N-Phenyl Naphthylamine (NPN) Assay
3.10. Fluorescence-Based Viability Assay Using DAPI and PI
3.11. Inhibition of Biofilm Development Assays
3.12. Eukaryotic Cell Culture
3.13. MTT Assay
3.14. Oxidative Stress Measurement
3.15. EdU Incorporation Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding

Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- POWO. Plants of the World Online. Available online: https://powo.science.kew.org/ (accessed on 22 March 2025).
- Averill, K.M.; Di Tommaso, A. Wild parsnip (Pastinaca sativa): A troublesome species of increasing concern. Weed Technol. 2007, 21, 279–287. [Google Scholar] [CrossRef]
- Baykan, S.; Ozturk, B.; Sahin, B.; Senol, S.G. Ethnobotanical study of medicinal plants in Nemrut Mountain, Adiyaman-Türkiye. J. Res. Pharm. 2023, 27, 2250–2269. [Google Scholar] [CrossRef]
- Polat, R.; Cakilcioglu, U.; Satil, F. Traditional uses of medicinal plants in Solhan (Bingöl–Turkey). J. Ethnopharmacol. 2013, 148, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Grieve, M. A Modern Herbal; Penguin: Dover, NY, USA, 1971; Volume 2. [Google Scholar]
- Foster, S.; Duke, J.A. A Field Guide to Medicinal Plants in Eastern and Central N. America; Houghton Mifflin: Boston, MA, USA, 1998; p. 366. [Google Scholar]
- Avicenna, H. Canon of Medicine; Ehyaol Toras al-Arabi Press: Beirut, Lebanon, 2010; pp. 263–264. [Google Scholar]
- Akramo Sadat, A.; Fatemeh, N.; Fatemeh, E.; Laila, S.; Hoorie Mohammadi, K.; Mohammad Hossein, A. Persian medicine nonpharmacological therapies for headache: Phlebotomy and wet cupping. J. Tradit. Chin. Med. 2018, 38, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Mahdizadeh, S.; Khaleghi Ghadiri, M.; Gorji, A. Avicenna’s Canon of Medicine: A review of analgesics and anti-inflammatory substances. Avicenna J. Phytomed. 2015, 5, 182–202. [Google Scholar] [PubMed]
- Allardice, P. A—Z of Companion Planting; Cassell Publishers Ltd.: London, UK, 1993; ISBN 0-304-34324-2. [Google Scholar]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea; Cambridge University Press: Cambridge, UK, 1981; Volume 2. [Google Scholar]
- Stolarczyk, J.; Janick, J. Carrot: History and iconography. Chron. Horticult. 2011, 51, 13–18. [Google Scholar]
- Bahrami, R.; Ghobadi, A.; Behnoud, N.; Akhtari, E. Medicinal properties of Daucus carota in traditional Persian medicine and modern phytotherapy. J. Biochem. Technol. 2018, 9, 107–114. [Google Scholar]
- Launert, E. Edible and Medicinal Plants; Hamlyn: London, UK, 1981; ISBN 0-600-37216-2. [Google Scholar]
- Facciola, S. Cornucopia-A Source Book of Edible Plants; Kampong Publications: Vasta, CA, USA, 1990; ISBN 0-9628087-0-9. [Google Scholar]
- Di Napoli, M.; Castagliuolo, G.; Badalamenti, N.; Maresca, V.; Basile, A.; Bruno, M.; Varcamonti, M.; Zanfardino, A. Antimicrobial, antibiofilm, and antioxidant properties of essential oil of Foeniculum vulgare Mill. leaves. Plants 2022, 11, 3573. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, S.E.; Jovanovic, O.P.; Petrovic, G.M.; Stojanovic, G.S. Endemic Balkan parsnip Pastinaca hirsuta: The chemical profile of essential oils, headspace volatiles and extracts. Nat. Prod. Commun. 2015, 10, 661–664. [Google Scholar] [CrossRef] [PubMed]
- Kapetanos, C.; Karioti, A.; Bojović, S.; Marin, P.; Veljić, M.; Skaltsa, H. Chemical and principal-component analyses of the essential oils of Apioideae taxa (Apiaceae) from Central Balkan. Chem. Biodivers. 2008, 5, 101–119. [Google Scholar] [CrossRef] [PubMed]
- Matejić, J.S.; Džamić, A.M.; Mihajilov-Krstev, T.; Ranđelović, V.N.; Krivošej, Z.Đ.; Marin, P.D. Antimicrobial potential of essential oil from Pastinaca sativa L. Biol. Nyssana 2014, 5, 31–35. [Google Scholar]
- Petrović, S.; Pavlović, M.; Milenković, M.; Vučićević, D.; Couladis, M.; Tzakou, O.; Niketić, M. Pastinaca hirsuta essential oils: Composition and antimicrobial activity. Planta Med. 2008, 74, PI7. [Google Scholar] [CrossRef]
- Semerdjieva, I.; Piperkova, N.; Maneva, V.; Dincheva, I.; Zheljaz, V.D. Biopesticidal potential and phytochemical composition of Pastinaca hirsuta Pančić essential oil from Bulgaria. Ind. Crops Prod. 2024, 217, 118843. [Google Scholar] [CrossRef]
- Ušjak, L.; Drobac, M.; Ivanov, M.; Soković, M.; Milenković, M.T.; Niketić, M.; Petrović, S. Composition and antimicrobial activity of Pastinaca sativa subsp. sativa, P. sativa subsp. urens and P. hirsuta essential oils. J. Essent. Oil Res. 2023, 35, 154–167. [Google Scholar] [CrossRef]
- Kubeczka, K.H.; Stahl, E. Volatile oils from Apiaceae (Umbelliferae). I. Oil of Pastinaca sativa roots. Planta Med. 1975, 27, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Kubeczka, K.H.; Stahl, E. On the essential oils from the Apiaceae (Umbelliferae) II. The essential oils from the above ground parts of Pastinaca sativa. Planta Med. 1977, 31, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Zykova, I.D.; Efremov, A.A. Antimicrobial activity and component composition of essential oil from seeds of Pastinaca silvestris Mill. of Siberian region. Sib. Med. J. 2014, 5, 96–98. [Google Scholar]
- Zykova, I.D.; Efremov, A.A. Chemical composition and antimicrobial activity of essential oils from different bodies of Pastinaca silvestris. Am. Sci. J. 2017, 11, 16–18. [Google Scholar]
- Stahl-Biskup, E.; Wichtmann, E.M. Composition of the essential oils from roots of some Apiaceae in relation to the development of their oil duct systems. Flav. Fragr. J. 1991, 6, 249–255. [Google Scholar] [CrossRef]
- Jianu, C.; Golet, I.; Stoin, D.; Cocan, I.; Lukinich-Gruia, A.T. Antioxidant activity of Pastinaca sativa L. ssp. sylvestris [Mill.] Rouy and Camus essential oil. Molecules 2000, 25, 869. [Google Scholar] [CrossRef]
- Kurkcuoglu, M.; Baser, K.H.C.; Vural, M. Composition of the essential oil of Pastinaca sativa L. subsp. urens (Req. ex Godron) Celak. Chem. Nat. Comp. 2006, 42, 114–115. [Google Scholar] [CrossRef]
- Tosum, B.; Karadoğan, T.; Şanli, A. Determination of essential oil content and composition, total phenolic content and antioxidant activities of Pastinaca sativa L. subsp. urens (Req. Ex Gordon). Curr. Pers. MAPs 2019, 2, 125–132. [Google Scholar]
- Skalicka-Woźniak, K.; Grzegorczyk, A.; Świątek, Ł.; Walasek, M.; Widelski, J.; Rajtar, B.; Polz-Dacewicz, M.; Malm, A.; Elansary, H.O. Biological activity and safety profile of the essential oil from fruits of Heracleum mantegazzianum Sommier & Levier (Apiaceae). Food Chem. Toxicol. 2017, 109 Pt 2, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Fang, C.; Zhang, J.; Wang, M.; Luo, X.; Hou, Z. Contemporaneous measurement of outer and inner membrane permeability in gram-negative bacteria. Bio-Protocol 2020, 10, e3548. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.G.; Zhu, Y.J.; Shao, S.Y.; Zhang, R.R.; Wu, Y.; Zhu, C.M.; Liang, X.R.; Cai, W.Q. Alkyl Ferulate Esters as Multifunctional Food Additives: Antibacterial Activity and Mode of Action against Escherichia coli in Vitro. J. Agric. Food Chem. 2018, 66, 12088–12101. [Google Scholar] [CrossRef] [PubMed]
- Campana, R.; Merli, A.; Verboni, M.; Biondo, F.; Favi, G.; Duranti, A.; Lucarini, S. Synthesis and Evaluation of Saccharide-Based Aliphatic and Aromatic Esters as Antimicrobial and Antibiofilm Agents. Pharmaceuticals 2019, 12, 186. [Google Scholar] [CrossRef] [PubMed]
- Pillai, A.D.; Rathod, P.D.; Franklin, P.X.; Padh, H.; Vasu, K.K.; Sudarsanam, V. Design, synthesis, and SAR studies of some 5-aliphatic oximino esters of thiophene as potential anti-inflammatory leads: Comparative biological activity profile of aliphatic oximes vs aromatic oximes. Biochem. Biophys. Res. Commun. 2004, 317, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- El-Tarabily, K.A.; El-Saadony, M.T.; Alagawany, M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Elwan, H.A.M.; Elnesr, S.S.; Abd El-Hack, M.E. Using essential oils to overcome bacterial biofilm formation and their antimicrobial resistance. Saudi J. Biol. Sci. 2021, 28, 5145–5156. [Google Scholar] [CrossRef] [PubMed]
- Khan, P.; Waheed, A.; Azeem, M.; Parveen, A.; Yameen, M.A.; Iqbal, J.; Ali, M.; Wang, S.; Qayyum, S.; Noor, A.; et al. Essential oil from Tagetes minuta has antiquorum sensing and antibiofilm potential against Pseudomonas aeruginosa Strain PAO1. ACS Omega 2023, 8, 35866–35873. [Google Scholar] [CrossRef] [PubMed]
- Kerekes, E.-B.; Deák, É.; Takó, M.; Tserennadmid, R.; Petkovits, T.; Vágvölgyi, C.; Krisch, J. Anti-Biofilm Forming and Anti-Quorum Sensing Activity of Selected Essential Oils and Their Main Components on Food-Related Micro-Organisms. J. Appl. Microbiol. 2013, 115, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Düşgün, C.; Kankılıç, T.; İşlek, C.; Balı, D.F.; Kankılıç, Özgür. Antioxidant and cytotoxic potential of local endemic plant Pastinaca zozimoides Fenzl. Turk. J. Agric.—Food Sci. Technol. 2021, 9, 646–649. [Google Scholar] [CrossRef]
- Kozics, K.; Bučková, M.; Puškárová, A.; Kalászová, V.; Cabicarová, T.; Pangallo, D. The effect of ten essential oils on several cutaneous drug-resistant microorganisms and their cyto/genotoxic and antioxidant properties. Molecules 2019, 24, 4570. [Google Scholar] [CrossRef] [PubMed]
- European Pharmacopoeia 10.3. 2020. Determination of Essential Oils in Herbal Drugs. 2.8.12., 2020, 307. Available online: https://www.edqm.eu/ (accessed on 11 May 2025).
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turk, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Di Napoli, M.; Castagliuolo, G.; Pio, S.; Di Nardo, I.; Russo, T.; Antonini, D.; Notomista, E.; Varcamonti, M.; Zanfardino, A. study of the antimicrobial activity of the human peptide SQQ30 against pathogenic bacteria. Antibiotics 2024, 13, 145. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Wang, J.; Zhang, L.; Zhou, J.; He, Y.; Lu, Y.; Liu, K.; Yan, W.; Wang, K. Multiple action mechanism and in vivo antimicrobial efficacy of antimicrobial peptide Jelleine-I. J. Pept. Sci. 2021, 27, e3294. [Google Scholar] [CrossRef] [PubMed]
- Di Napoli, M.; Castagliuolo, G.; Badalamenti, N.; Maresca, V.; Basile, A.; Bruno, M.; Varcamonti, M.; Zanfardino, A. Citrus aurantium ‘Crispifolia’ Essential Oil: A Promise for Nutraceutical Applications. Nutraceuticals 2023, 3, 153–164. [Google Scholar] [CrossRef]
- Di Napoli, M.; Badalamenti, N.; Castagliuolo, G.; Merra, R.; Varcamonti, M.; Zanfardino, A.; Bruno, M.; Sottile, F. Chemical composition, antimicrobial, and antioxidant activities of Opuntia stricta (Haw.) Haw.mucilage collected in Sicily, Italy. Nat. Prod. Res. 2023, 38, 4077–4085. [Google Scholar] [CrossRef] [PubMed]
| No. | Compound a | LRI b | LRI c | Relative Area (%) d |
|---|---|---|---|---|
| 1 | 1-Hexanol | 865 | 865 | 0.1 |
| 2 | α-Pinene | 929 | 931 | 0.1 |
| 3 | β-Pinene | 970 | 969 | 0.2 |
| 4 | Butyl butanoate | 996 | 997 | 0.6 |
| 5 | Octanal | 1002 | 1004 | 1.0 |
| 6 | Limonene | 1024 | 1025 | 0.3 |
| 7 | trans-Ocimene | 1035 | 1037 | 1.3 |
| 8 | cis-Ocimene | 1047 | 1051 | 0.9 |
| 9 | γ-Terpinene | 1084 | 1089 | 0.1 |
| 10 | cis-5-Octen-1-ol | 1061 | 1057 | 0.2 |
| 11 | 1-Octanol | 1072 | 1068 | 5.5 |
| 12 | Terpinolene | 1084 | 1089 | 0.4 |
| 13 | Hexyl butanoate | 1194 | 1197 | 7.9 |
| 14 | Decanal | 1204 | 1204 | 0.3 |
| 15 | Octyl acetate | 1217 | 1221 | 38.7 |
| 16 | α-Terpinyl acetate | 1344 | 1337 | 2.3 |
| 17 | Octyl butanoate | 1393 | 1396 | 26.7 |
| 18 | Decyl acetate | 1410 | 1413 | 0.7 |
| 19 | Caryophyllene | 1427 | 1429 | 0.3 |
| 20 | β-Phenylethyl butyrate | 1437 | 1441 | 1.0 |
| 21 | β-Farnesene | 1456 | 1458 | 1.1 |
| 22 | α-Amorphene | 1475 | 1479 | 0.2 |
| 23 | Methylisoeugenol | 1495 | 1492 | 0.9 |
| 24 | α-Farnese | 1506 | 1509 | 0.1 |
| 25 | Myristicin | 1516 | 1520 | 2.9 |
| 26 | trans-Nerolidol | 1556 | 1560 | 0.2 |
| 27 | Octyl hexanoate | 1581 | 1578 | 0.7 |
| 28 | Phenethyl hexanoate | 1639 | 1628 | 0.1 |
| 29 | γ-Palmitolactone | 2178 | 2185 | 0.6 |
| Monoterpene Hydrocarbons | 3.3 | |||
| Oxygenated Monoterpenes | 2.3 | |||
| Sesquiterpene Hydrocarbons | 1.9 | |||
| Alcohols | 5.8 | |||
| Esters | 77.3 | |||
| Other Compounds | 4.8 | |||
| Total Composition | 95.4 |
| Taxa | Origin and Sample Parts | Compounds | References |
|---|---|---|---|
| P. hirsuta Pančić. | Serbia, roots at the flowering stage | apiole (33.4%), (Z)-falcarinol (22.7%), myristicin (17.0%), β-bisabolene (4.9%), γ-palmitolactone (3.6%) | [17] |
| Serbia, roots at the fruiting stage | apiole (45.3%), myristicin (30.1%), (Z)-falcarinol (8.1%), terpinolene (3.1%) | [17] | |
| Serbia, stems at the flowering stage, | γ-palmitolactone (51.9%), propyl linoleate (23.3%), (E)-β-ocimene (8.6%), methyl linoleate (3.0%) | [17] | |
| Serbia, stems at the fruiting stage | γ-palmitolactone (45.7%), propyl linoleate (16.7%), (E)-β-ocimene (8.1%), methyl linoleate (4.8%), (E)-nerolidol (3.2%) | [17] | |
| Serbia, flowers | hexyl butanoate (31.1%), hexyl hexanoate (15.9%), γ-palmitolactone (13.6%) (E)-β-ocimene (8.7%), propyl linoleate (5.3%), terpinolene (3.4%) | [17] | |
| Serbia, fruits | hexyl butanoate (80.4%), hexyl hexanoate (12.1%) | [17] | |
| Serbia, aerial parts | (Z)-β-ocimene (10.8%), hexyl butanoate (10.4%), (E)-β-farnesene (6.1%), lavandulyl acetate (5.2%), (+)-γ-terpinene (3.7%), germacrene D (3.7%), ar-curcumene (3.3%), α-zingiberene (3.3%) | [18,19] | |
| Serbia, roots | apiole (56.0%), myristicin (21.0%), β-bisabolene (7.2%) | [20] | |
| Serbia, aerial parts | hexyl hexanoate (59.8%), hexyl butanoate (21.4%) | [20] | |
| Bulgaria, Loc 1, aerial parts | n-octyl butanoate (46.5%), n-hexyl butanoate (16.0%), n-tricosane (10.7%), guaiol (7.2%), n-octanal (3.2%) | [21] | |
| Bulgaria, Loc 2, aerial parts | neryl acetate (28.4%), (Z)-hexenyl benzoate (5.4%), germacrene D-4-ol (4.3%), nerol (4.3%), α-pinene (4.1%), β-himachalene (3.8%), geranyl butanoate (3.1%), neryl propanoate (3.1%), italicene (3.1%) | [21] | |
| Bulgaria, Loc 2, flowers | neryl acetate (24.9%), tetrahydro-lavandulol acetate (13.4%), neryl propanoate (5.7%), α-terpinyl acetate (5.6%), (E,E)-α-farnesene (5.6%), italicene (4.1%), (2E)-tridecenol (3.7%), guaiol (3.7%), n-hexyl butanoate (3.6%), lavandulyl isobutanoate (3.1%) | [21] | |
| Bulgaria, Loc 2, seeds | neryl acetate (15.9%), n-tricosane (15.2%), tetrahydro-lavandulol acetate (10.6%), neryl propanoate (8.8%), geranyl butanoate (4.5%), α-pinene (3.6%), n-octadecane (3.3%) | [21] | |
| Serbia, flowers | hexyl butanoate (61.9%), hexyl hexanoate (17.0%), γ-palmitolactone (6.0%) | [22] | |
| Serbia, fruits | hexyl butanoate (22.9–58.4%), hexyl hexanoate (29.1–59.8%), octyl acetate (1.5–3.3%) | [22] | |
| Serbia, leaves | γ-palmitolactone (47.5%), octadecadienoic acid (24.3%), β-pinene (3.9%), (E)-β-ocimene (3.9%) | [22] | |
| Serbia, stems | γ-palmitolactone (53.3–60.4%), octadecadienoic acid (25.5–34.0%), hexadecanoic acid (2.8–4.1%) | [22] | |
| Serbia, roots | apiole (25.8–30.9%), (Z)-falcarinol (12.2–25.9%), myristicin (11.6–20.3%), γ-palmitolactone (7.9–12.4%), octadecadienoic acid (5.0–6.7%), β-bisabolene (2.4–4.0%) | [22] | |
| P. sativa L. ssp. sativa | Serbia, aerial parts | hexyl butanoate (55.4%) | [18] |
| Germany, roots | terpinolene (40.3–69.0%), myristicin (17.2–40.1%), β-pinene (2.4–8.6%), limonene (1.7–3.2%), (Z)-β-ocimene (0.7–3.7%) | [23] | |
| Germany, fruits | octyl butanoate (46.2%), octyl acetate (32.8%), n-hexyl butanoate (6.4%) | [24] | |
| Germany, leaves | (Z)-β-ocimene (18.3%), (E)-β-farnesene (17.1%), γ-palmitolactone (16.2%), (E)-β-ocimene (12.6%) | [24] | |
| Germany, petiole | (Z)-β-ocimene (40.6%), γ-palmitolactone (18.3%), (E)-β-ocimene (17.1%), (E)-β-farnesene (7.2%), terpinolene (5.3%) | [24] | |
| Germany, stems | (Z)-β-ocimene (30.5%), terpinolene (22.6%), γ-palmitolactone (15.1%), (E)-β-ocimene (14.4%), (E)-β-farnesene (5.0%) | [24] | |
| Serbia, cult. flowers | octyl butanoate (31.4%), myristicin (21.5%), (E)-β-farnesene (10.3%), γ-palmitolactone (7.9%), (Z)-β-ocimene (3.7%) | [22] | |
| Serbia, cult. fruits | octyl butanoate (70.9–79.0%), octyl hexanoate (6.5–8.1%), n-octanol (1.4–9.1%), octyl acetate (0.6–5.1%), myristicin (0.5–3.8%) | [22] | |
| Serbia, cult. leaves | myristicin (41.4–42.8%), (E)-β-farnesene (22.3–22.4%), (Z)-β-ocimene (2.8–9.0%), α-(E)-bergamotene (6.6–7.7%), (E)-β-ocimene (1.5–3.8%), germacrene D (2.0–3.3%), γ-palmitolactone (1.2–3.2%) | [22] | |
| Serbia, cult. stems | myristicin (63.3–64.9%), γ-palmitolactone (11.8–18.4%), (E)-β-farnesene (7.0–14.4%) | [22] | |
| Serbia, cult. roots | myristicin (59.3–82.5%), terpinolene (1.2–28.7%), β-pinene (0.1–4.6%), γ-palmitolactone (0–4.5%), (Z)-falcarinol (0–3.6%) | [22] | |
| P. silvestris Mill. (Syn: P. sativa ssp. sativa) | Siberia, seeds | n-octyl butanoate (32.0%), octyl acetate (27.0%), Z-asarone (14.1), n-hexyl butanoate (5.2%), n-octyl hexanoate (4.2%) | [25] |
| Siberia, seeds | phytol (48.7%), (E)-β-farnesene (12.1%), trans-muurola-3,5-dien (10.8%) | [26] | |
| Siberia, leaves | (E)-β-farnesene (17.8%), (E)-caryophyllene (16.0%), cis-β-ocimene (10.7%) | [26] | |
| Siberia, flowers | octyl acetate (17.8%), cis-β-ocimene (16.0%), (E)-β-farnesene (11.3%) | [26] | |
| P. sativa ssp. sativa var. hortensis (Syn: P. sativa ssp. sativa) | Cultivated, roots | terpinolene (36.0%), myristicin (24.7%), apiole (22.9%), dill-apiole (5.3%) | [27] |
| P. sativa L. ssp. sylvestris [Mill.] Rouy and Camus (Syn: P. sativa ssp. sativa) | Romania, seeds | octyl acetate (78.5%), octyl hexanoate (6.7%) | [28] |
| P. sativa L. subsp. urens (Req. ex Godron) Celak. | Turkey, aerial parts | octyl butanoate (79.5%), octyl hexanoate (5.3%), hexyl butanoate (3.3%) | [29] |
| Turkey, fruits | octyl butanoate (90.4%) | [30] | |
| Turkey, aerial parts | cis-β-ocimene (38.2%), octadecanoic acid (14.1%), octyl butanoate (13.2%), butanoic acid (11.1%), trans-β-ocimene (5.7%) | [30] | |
| Serbia, flowers | octyl butanoate (26.1–29.7%), γ-palmitolactone (13.9–24.0%), hexyl butanoate (1.8–12.7%), octyl acetate (4.2–8.3%), cis-β-ocimene (3.2–6.6%), (E)-β-farnesene (3.4–6.0%), myristicin (3.8–4.0%), caryophyllene oxide (0.3–3.2%) | [22] | |
| Serbia, fruits | octyl butanoate (53.6–65.1%), octyl acetate (1.1–28.9%), n-octyl hexanoate (4.6–15.4%) hexyl butanoate (3.1–10.6%), n-octanol (1.0–3.5%) | [22] | |
| Serbia, leaves | γ-palmitolactone (22.6–29.5%), (E)-β-farnesene (4.4–13.8%), caryophyllene oxide (8.0–10.6%), (E)-caryophyllene (8.2–9.9%), (Z)-β-ocimene (3.2–7.4%), octyl butanoate (2.0–7.1%), (E)-β-ocimene (4.1–6.3%), germacrene D (3.4–4.6%), β-bourbonene (1.7–3.1%), | [22] | |
| Serbia, stems | γ-palmitolactone (50.6–53.4%), (E)-β-farnesene (4.9–6.5%), caryophyllene oxide (2.1–6.1%), (Z)-β-ocimene (3.2–5.5%), (E)-nerolidol (0–4.5%), myristicin (1.1–3.8%), hexadecanoic acid (2.3–3.3%) | [22] | |
| Serbia, roots | myristicin (39.7–62.1%), terpinolene (1.7–23.4%), (Z)-falcarinol (10.5–15.9%), γ-palmitolactone (1.5–15.6%) | [22] |
| Strains | MIC [µg/mL] ± SD | p-Value |
|---|---|---|
| E. coli | 24,333.3 ± 1527.5 | ** |
| P. aeruginosa | 25,000 ± 1732.1 | **** |
| S. typhimurium | >25,000 ± 0 | **** |
| B. subtilis | 22,666.7 ± 2516.6 | *** |
| B. cereus | 18,333.3 ± 2886.8 | **** |
| M. smegmatis | 15,000 ± 5000 | **** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Girolamo, D.; Badalamenti, N.; Castagliuolo, G.; Ilardi, V.; Varcamonti, M.; Bruno, M.; Zanfardino, A. South Tyrol (Italy) Pastinaca sativa L. subsp. sativa Essential Oil: GC-MS Composition, Antimicrobial, Anti-Biofilm, and Antioxidant Properties. Molecules 2025, 30, 3033. https://doi.org/10.3390/molecules30143033
Di Girolamo D, Badalamenti N, Castagliuolo G, Ilardi V, Varcamonti M, Bruno M, Zanfardino A. South Tyrol (Italy) Pastinaca sativa L. subsp. sativa Essential Oil: GC-MS Composition, Antimicrobial, Anti-Biofilm, and Antioxidant Properties. Molecules. 2025; 30(14):3033. https://doi.org/10.3390/molecules30143033
Chicago/Turabian StyleDi Girolamo, Daniela, Natale Badalamenti, Giusy Castagliuolo, Vincenzo Ilardi, Mario Varcamonti, Maurizio Bruno, and Anna Zanfardino. 2025. "South Tyrol (Italy) Pastinaca sativa L. subsp. sativa Essential Oil: GC-MS Composition, Antimicrobial, Anti-Biofilm, and Antioxidant Properties" Molecules 30, no. 14: 3033. https://doi.org/10.3390/molecules30143033
APA StyleDi Girolamo, D., Badalamenti, N., Castagliuolo, G., Ilardi, V., Varcamonti, M., Bruno, M., & Zanfardino, A. (2025). South Tyrol (Italy) Pastinaca sativa L. subsp. sativa Essential Oil: GC-MS Composition, Antimicrobial, Anti-Biofilm, and Antioxidant Properties. Molecules, 30(14), 3033. https://doi.org/10.3390/molecules30143033








