Focused Solar-Induced Construction of Activated Solar Carbon@Carbon Fiber Coaxial Electrode from Waste Carbon Fiber-Reinforced Polymer and Its Supercapacitor Performance
Abstract
1. Introduction
2. Experimental Section
2.1. Reagents and Materials
2.2. Preparation of A-SC@CF Composites
2.3. Characterization of A-SC@CFs
2.4. Electrochemical Measurements of A-SC@CFs Electrode
3. Results and Discussion
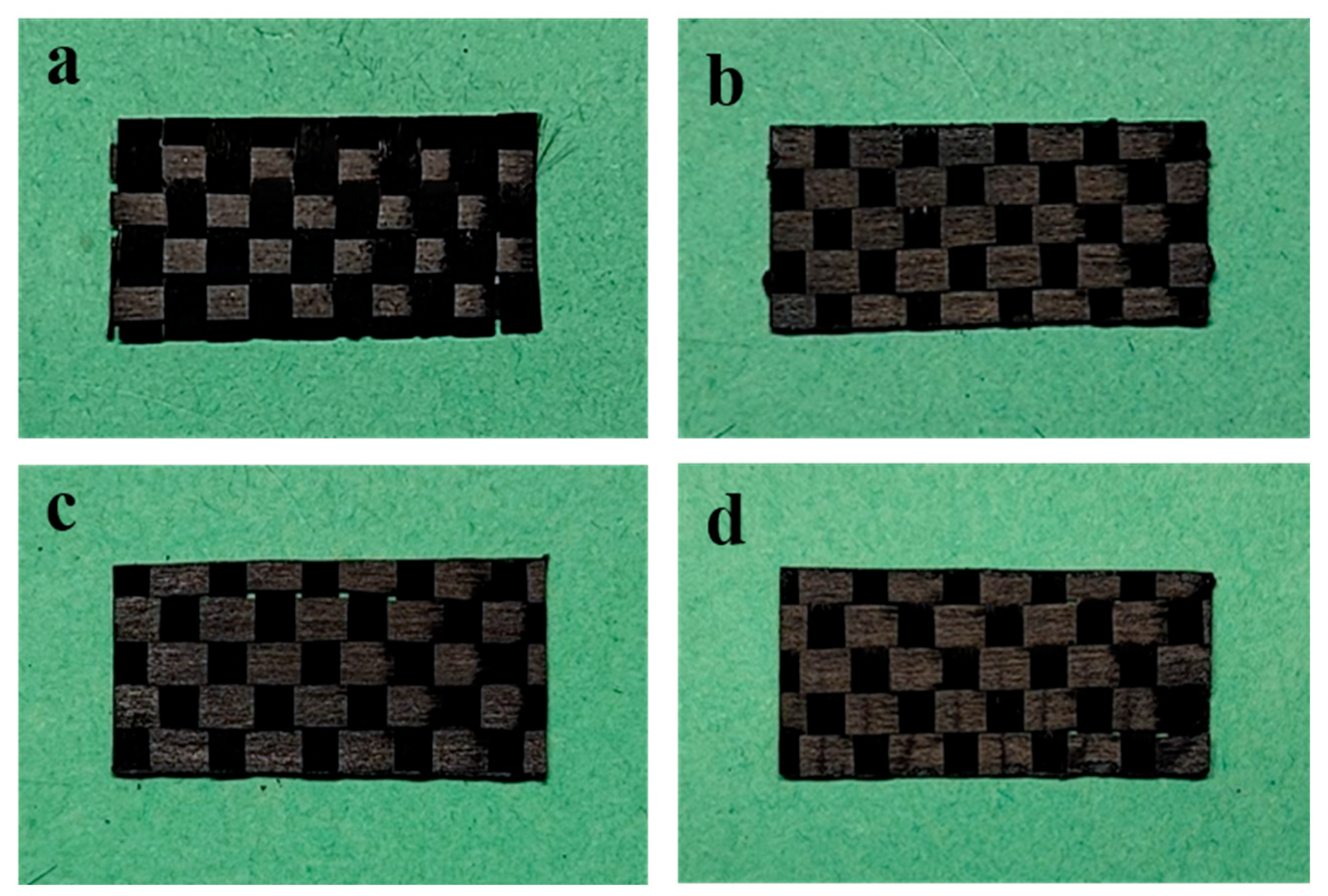
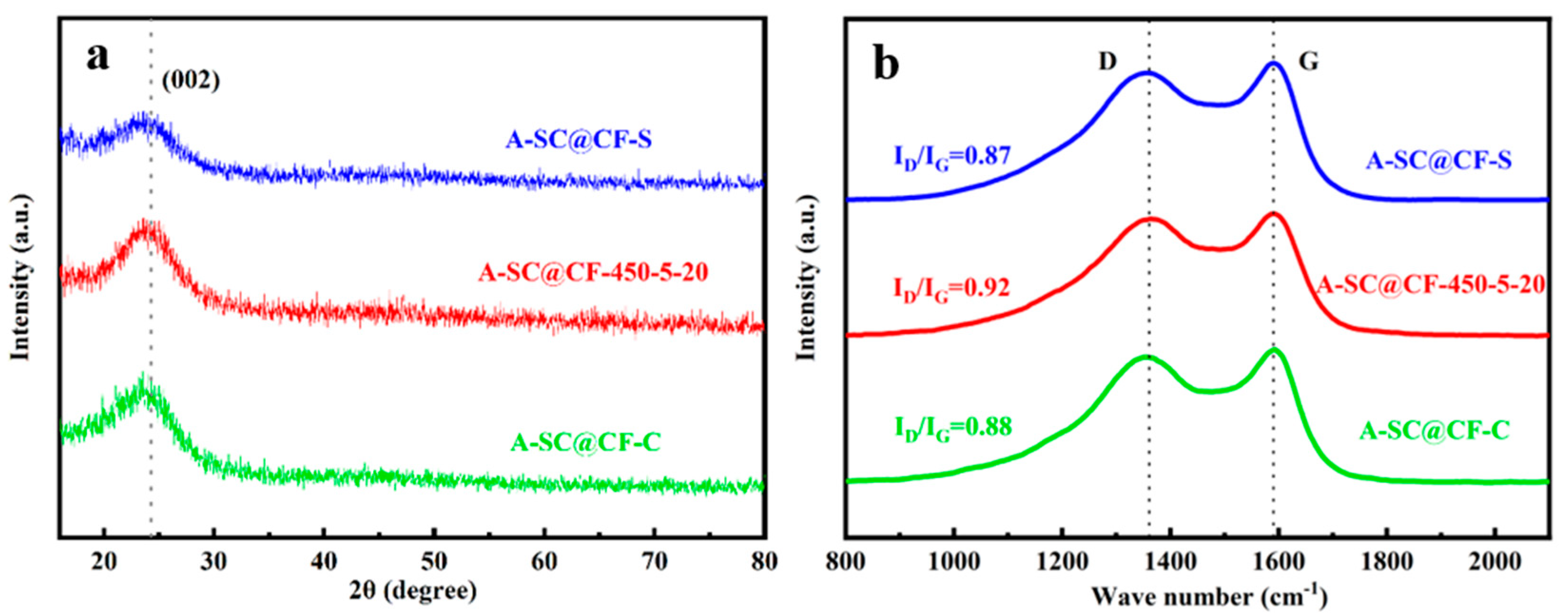
3.1. Surface Structure of A-SC@CFs
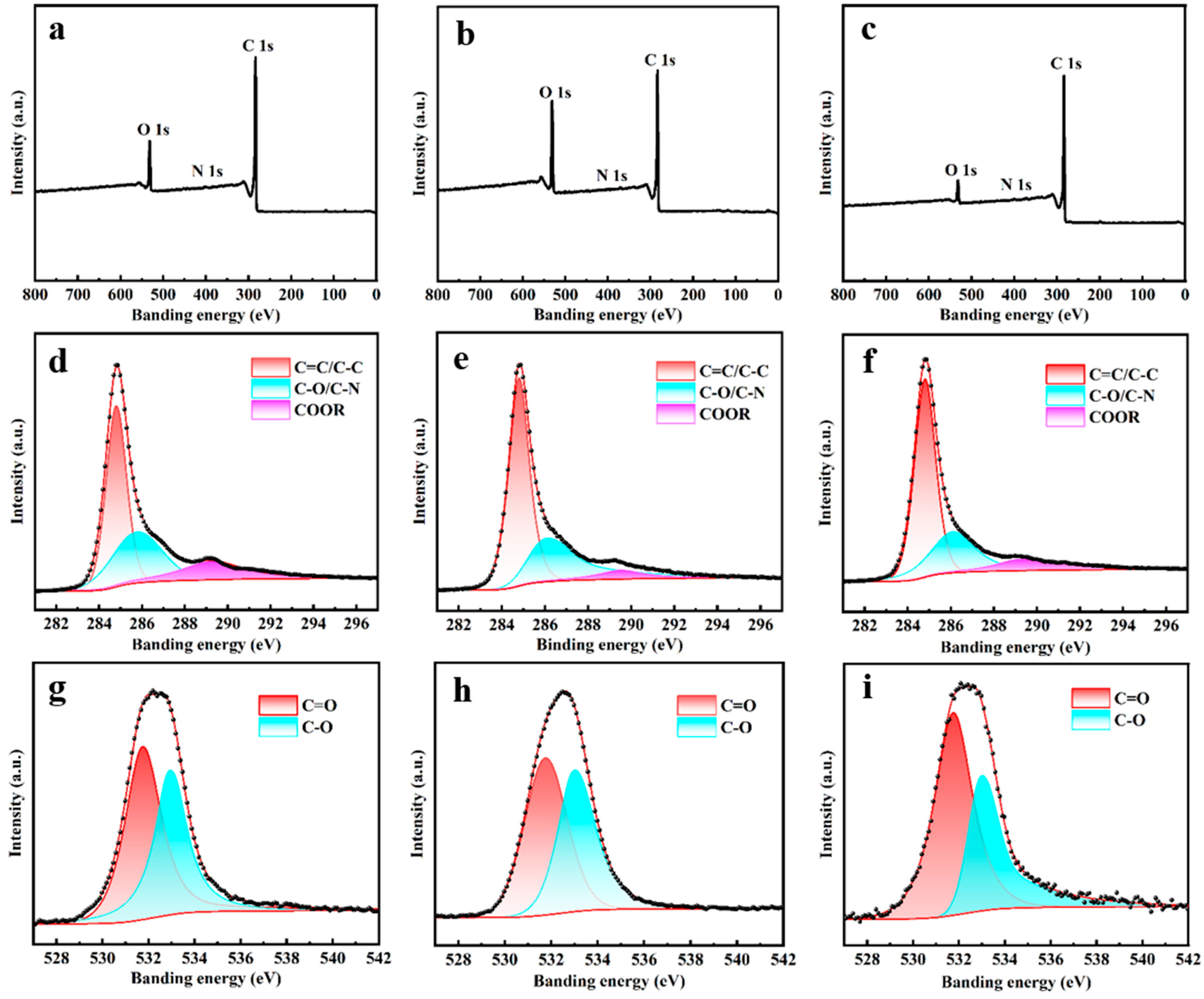
3.2. Composition of ASC@CFs
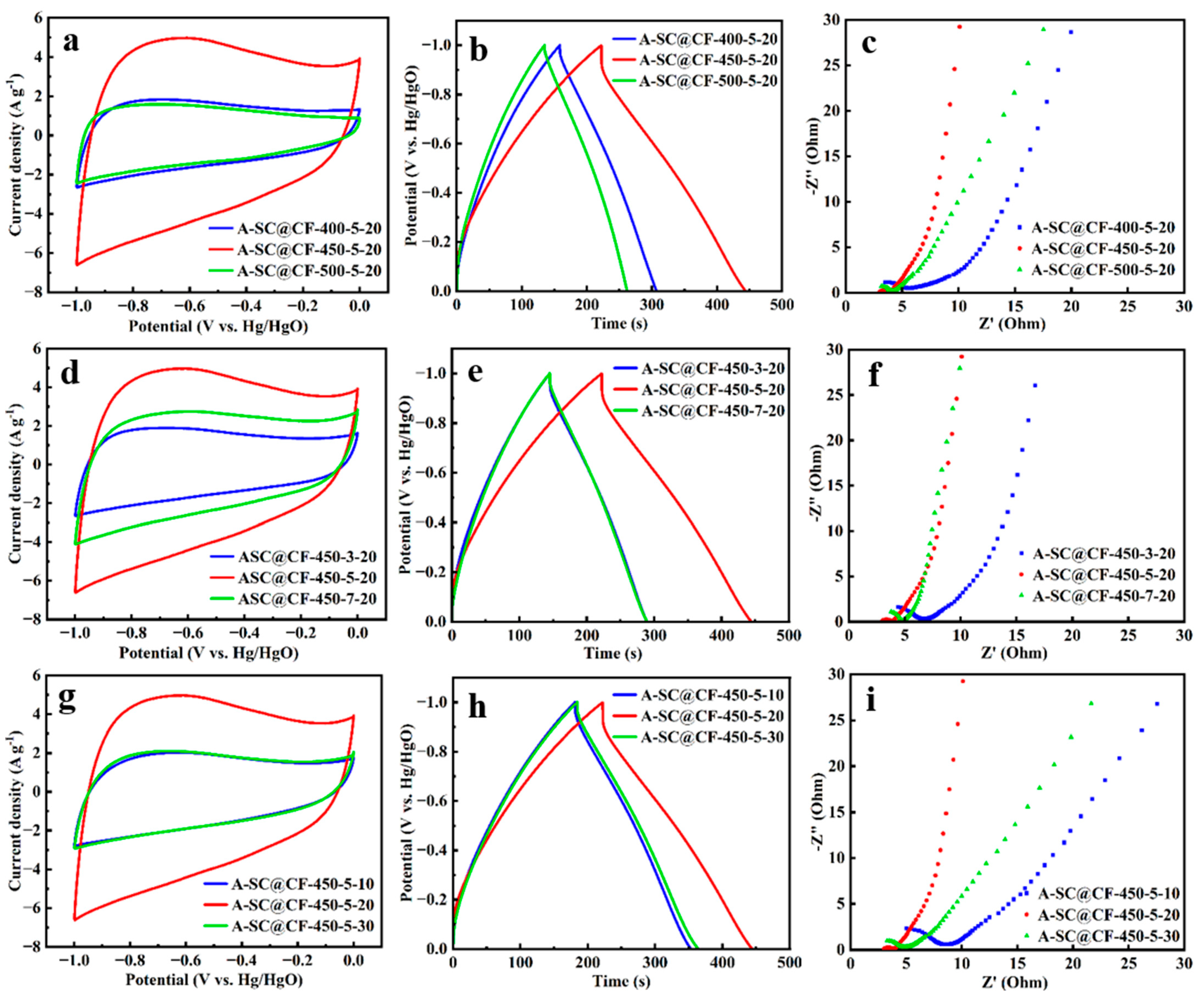
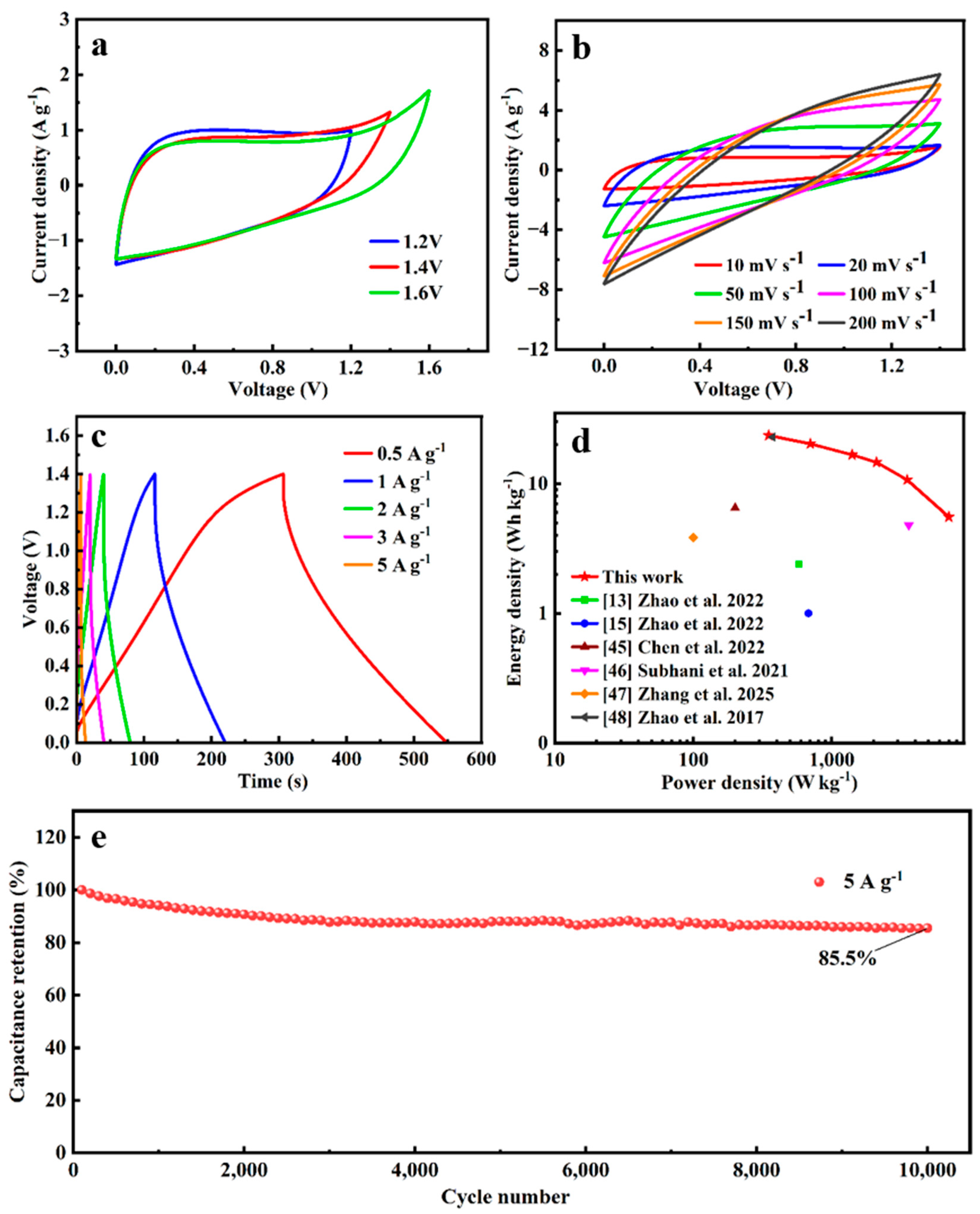
3.3. Electrochemical Performances of A-SC@CF Coaxial Electrode
3.4. Electrochemical Performances of SSC of A-SC@CF//A-SC@CF
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, Z.; Luo, S.J.; Li, Q.; Wu, Z.S.; Liu, S.Z. Recent Advances in Flexible wearable supercapacitors: Properties, fabrication, and applications. Adv. Sci. 2024, 11, 2302172. [Google Scholar] [CrossRef] [PubMed]
- Oyedotun, K.O.; Ighalo, J.O.; Amaku, J.F.; Olisah, C.; Adeola, A.O.; Iwuozor, K.O.; Akpomie, K.G.; Conradie, J.; Adegoke, K.A. Advances in supercapacitor development: Materials, processes, and applications. J. Electron. Mater. 2023, 52, 96–129. [Google Scholar] [CrossRef]
- Olabi, A.G.; Abbas, Q.; Abdelkareem, M.A.; Alami, A.H.; Mirzaeian, M.; Sayed, E.T. Carbon-based materials for supercapacitors: Recent progress. challenges and barriers. Batteries 2023, 9, 19. [Google Scholar] [CrossRef]
- Kumar, Y.A.; Koyyada, G.; Ramachandran, T.; Kim, J.H.; Sajid, S.; Moniruzzaman, M.; Alzahmi, S.; Obaidat, I.M. Carbon materials as a conductive skeleton for supercapacitor electrode applications: A review. Nanomaterials 2023, 13, 1049. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.Q.; Zhang, H.; Zhai, S.L.; Sun, L.; Huang, Z.; Guo, M.Y.; Liu, Y.K.; Zhang, D.Y.; Terrones, M.; Wang, Y.Q. Coaxial fabrication of Ni-Co layered double hydroxide into 3D carbon nanotube networks for high-performance flexible fiber supercapacitors. J. Alloys Compd. 2022, 909, 164664. [Google Scholar] [CrossRef]
- Youssry, S.M.; Elkodous, M.A.; Kumar, R.; Kawamura, G.; Tan, W.K.; Matsuda, A. Thermal-assisted synthesis of reduced graphene oxide-embedded Ni nanoparticles as high-performance electrode material for supercapacitor. Electrochim. Acta 2023, 463, 142814. [Google Scholar] [CrossRef]
- Zhao, C.H.; Chen, S.; Sun, N.; Jiang, W.B.; Cai, W.L.; Zhao, C.J. Bisphenol A epoxy resin-derived activated carbon with high performance for supercapacitors. J. Phys. Chem. C 2023, 127, 18821–18831. [Google Scholar] [CrossRef]
- Yao, B.; Peng, H.; Zhang, H.; Kang, J.; Zhu, C.; Delgado, G.; Byrne, D.; Faulkner, S.; Freyman, M.; Lu, X.; et al. Printing porous carbon aerogels for low temperature supercapacitors. Nano Lett. 2021, 21, 3731–3737. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.H.; Zhou, Y.N.; Ge, Z.X.; Zhao, C.J.; Qian, X.Z. Facile construction of MoS2/RCF electrode for high-performance supercapacitor. Carbon 2018, 127, 699–706. [Google Scholar] [CrossRef]
- Yang, H.; Xiong, T.Z.; Zhu, Z.X.; Xiao, R.; Yao, X.C.; Huang, Y.C.; Balogun, M.S. Deciphering the lithium storage chemistry in flexible carbon fiber-based self-supportive electrodes. Carbon Energy 2022, 4, 820–832. [Google Scholar] [CrossRef]
- Li, H.J.; Wang, X.M.; Zhao, Z.X.; Pathak, R.; Hao, S.Y.; Qiu, X.M.; Qiao, Q.Q. Microstructure controlled synthesis of Ni, N-codoped CoP/carbon fiber hybrids with improving reaction kinetics for superior sodium storage. J. Mater. Sci. Technol. 2022, 99, 184–192. [Google Scholar] [CrossRef]
- Han, J.Y.; Deng, N.P.; Chi, H.; Wang, G.; Wang, Y.L.; Zeng, Q.; Peng, Z.Z.; Cheng, B.W.; Zhou, B.M.; Kang, W.M. Recent advances of carbon fiber-based self-supported electrocatalysts in oxygen electrocatalysis. J. Energy Chem. 2024, 98, 334–363. [Google Scholar] [CrossRef]
- Zhao, C.J.; Guo, J.J.; Fan, J.T.; Zhang, X.; Zhao, C.H.; Chen, S. Surface engineering of reclaimed carbon fiber (RCF) electrode for superimposed supercapacitor performance. J. Energy Storage 2022, 46, 103786. [Google Scholar] [CrossRef]
- Zhou, Y.N.; Zhu, Z.Q.; Zhao, C.H.; Zhang, K.F.; Wang, B.L.; Zhao, C.J.; Chen, G.R. Reclaimed carbon fiber-based 2.4V aqueous symmetric supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 5095–5102. [Google Scholar] [CrossRef]
- Zhao, C.J.; Zheng, J.X.; Wang, Y.X.; Rui, P.F.; Yang, G.; Zhao, C.H.; Xu, S.J. Vaporized hydrothermal functionalization of carbon fiber and its superior supercapacitor performance. Energy Fuels 2022, 36, 4052–4064. [Google Scholar] [CrossRef]
- Xu, M.X.; Meng, X.X.; Ji, H.W.; Yang, J.; Di, J.Y.; Wu, Y.C.; Lu, Q. Evolution of pyrolysis char during the recovery of carbon fiber reinforced polymer composite and its effects on the recovered carbon fiber. J. Environ. Chem. Eng. 2024, 12, 112214. [Google Scholar] [CrossRef]
- Zhao, C.H.; Gao, W.J.; Zhao, Y.P.; Li, M.K.; Tong, X.Z.; Guo, H.M.; Zhu, Y.P.; Zhao, C.J. Nanoarchitectonics with expired carbon fiber reinforced polymer-derived carbon layer@carbon fiber coaxial electrodes for superior supercapacitor performance. J. Phys. Chem. C 2024, 128, 21280–21291. [Google Scholar] [CrossRef]
- Lu, X.H.; Zhai, T.; Zhang, X.H.; Shen, Y.Q.; Yuan, L.Y.; Hu, B.; Gong, L.; Chen, J.; Gao, Y.H.; Zhou, J.; et al. WO3–x@Au@MnO2 core–shell nanowires on carbon fabric for high-performance flexible supercapacitors. Adv. Mater. 2012, 24, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Kim, B.J. Recovery of carbon fibers from carbon fiber-reinforced epoxy-isophorone diamine composites via step thermolysis. Compos. Part B: Eng. 2023, 260, 110757. [Google Scholar] [CrossRef]
- Irisawa, T.; Aratake, R.; Hanai, M.; Sugimoto, Y.; Tanabe, Y. Elucidation of damage factors to recycled carbon fibers recovered from CFRPs by pyrolysis for finding optimal recovery conditions. Compos. Part B: Eng. 2021, 218, 108939. [Google Scholar] [CrossRef]
- Kim, Y.N.; Kim, Y.O.; Kim, S.Y.; Park, M.; Yang, B.; Kim, J.; Jung, Y.C. Application of supercritical water for green recycling of epoxy-based carbon fiber reinforced plastic. Compos. Sci. Technol. 2019, 173, 66–72. [Google Scholar] [CrossRef]
- Khalil, Y.F. Sustainability assessment of solvolysis using supercritical fluids for carbon fiber reinforced polymers waste management. Sustain. Prod. Consum. 2019, 17, 74–84. [Google Scholar] [CrossRef]
- Zhao, Q.; Jiang, J.J.; Li, C.B.; Li, Y.J. Efficient recycling of carbon fibers from amine-cured CFRP composites under facile condition. Polym. Degrad. Stab. 2020, 179, 109268. [Google Scholar] [CrossRef]
- Zhao, Q.; An, L.; Li, C.B.; Zhang, L.J.; Jiang, J.J.; Li, Y.J. Environment-friendly recycling of CFRP composites via gentle solvent system at atmospheric pressure. Compos. Sci. Technol. 2022, 224, 109461. [Google Scholar] [CrossRef]
- Zabihi, O.; Ahmadi, M.; Liu, C.; Mahmoodi, R.; Li, Q.X.; Naebe, M. Development of a low cost and green microwave assisted approach towards the circular carbon fibre composites. Compos. Part B: Eng. 2020, 184, 107750. [Google Scholar] [CrossRef]
- Deng, J.Y.; Xu, L.; Zhang, L.B.; Peng, J.H.; Guo, S.H.; Liu, J.H.; Koppala, S. Recycling of carbon fibers from CFRP waste by microwave thermolysis. Processes 2019, 7, 207. [Google Scholar] [CrossRef]
- Sahu, S.K.; Kopalakrishnaswami, A.S.; Natarajan, S.K. Historical overview of power generation in solar parabolic dish collector system. Environ. Sci. Pollut. Res. 2022, 29, 64404–64446. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.T.; Niu, X.Q.; Yang, L.D.; Mi, N.; Zhao, L. Controllable nitrogen-doped hollow carbon nano-cage structures as supercapacitor electrode materials. Molecules 2025, 30, 2130. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.H.; Wang, R.; Pang, G.L.; Dong, L.L.; Lei, T.Z.; Ren, S.X. Exploring the connection between the structure and activity of lignin-derived porous carbon across various electrolytic environments. Molecules 2025, 30, 494. [Google Scholar] [CrossRef] [PubMed]
- Yakaboylu, G.A.; Jiang, C.L.; Yumak, T.; Zondlo, J.W.; Wang, J.X.; Sabolsky, E.M. Engineered hierarchical porous carbons for supercapacitor applications through chemical pretreatment and activation of biomass precursors. Renew. Energy 2021, 163, 276–287. [Google Scholar] [CrossRef]
- Keppetipola, N.M.; Dissanayake, M.; Dissanayake, P.; Karunarathne, B.; Dourges, M.A.; Talaga, D.; Servant, L.; Olivier, C.; Toupance, T.; Uchida, S.; et al. Graphite-type activated carbon from coconut shell: A natural source for eco-friendly non-volatile storage devices. RSC Adv. 2021, 11, 2854–2865. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.G.; Wang, D.N.; Li, H.F.; Ma, M.; Zhang, Y.; Yan, Z.F.; Agnoli, S.; Zhang, G.X.; Sun, X.M. Single-atom Zn for boosting supercapacitor performance. Nano Res. 2022, 15, 1715–1724. [Google Scholar] [CrossRef]
- Shamoradi, F.; Panjepour, M.; Emadi, R.; Ghiaci, M. Study of fabrication and CNT growth mechanisms of hybrid CFF/CNT composites. J. Nanoparticle Res. 2022, 24, 192. [Google Scholar] [CrossRef]
- Wang, Y.G.; Song, Y.F.; Xia, Y.Y. Electrochemical capacitors: Mechanism, materials, systems, characterization and applications. Chem. Soc. Rev. 2016, 45, 5925–5950. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.L.; Chen, C.; Sun, Y.J.; Gan, S.C.; Dong, L.B.; Zhao, J.H.; Rong, J.H. Flexible, all-hydrogel supercapacitor with self-healing ability. Chem. Eng. J. 2021, 418, 128616. [Google Scholar] [CrossRef]
- Zhao, Z.; Miao, Y.D.; Lu, Q.S. Electrospun nickel cobalt phosphide/carbon nanofibers as high-performance electrodes for supercapacitors. J. Power Sources 2024, 606, 234587. [Google Scholar] [CrossRef]
- He, Y.T.; Zhang, Y.H.; Li, X.F.; Lv, Z.; Wang, X.J.; Liu, Z.G.; Huang, X.Q. Capacitive mechanism of oxygen functional groups on carbon surface in supercapacitors. Electrochim. Acta 2018, 282, 618–625. [Google Scholar] [CrossRef]
- Liu, H.; Liu, R.M.; Xu, C.; Ren, Y.M.; Tang, D.X.; Zhang, C.G.; Li, F.; Wei, X.L.; Zhang, R.L. Oxygen–nitrogen–sulfur self-doping hierarchical porous carbon derived from lotus leaves for high-performance supercapacitor electrodes. J. Power Sources 2020, 479, 228799. [Google Scholar] [CrossRef]
- Tang, B.; Zheng, L.P.; Dai, X.C.; Chen, H.J. Nitrogen/oxygen co-doped porous carbons derived from a facilely-synthesized Schiff-base polymer for high-performance supercapacitor. J. Energy Storage 2019, 26, 100961. [Google Scholar] [CrossRef]
- Liu, Y.J.; Wang, X.; Jiang, X.H.; Li, X.; Yu, L.M. Shape-controlled synthesis of porous carbons for flexible asymmetric supercapacitors. Nanoscale 2018, 10, 22848–22860. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Li, X.; Li, N.; Xie, M.J.; Du, C.; Zhang, Y.; Chen, J. Multi-heteroatom-doped hierarchical porous carbon derived from chestnut shell with superior performance in supercapacitors. J. Alloys Compd 2019, 790, 760–771. [Google Scholar] [CrossRef]
- Sandhiya, M.; Nadira, M.P.; Sathish, M. Fabrication of flexible supercapacitor using n-doped porous activated carbon derived from poultry waste. Energy Fuels 2021, 35, 15094–15100. [Google Scholar] [CrossRef]
- Zeng, Y.K.; Xue, Y.; Gong, X.; Gao, X.; E, J.Q.; Chen, J.W.; Leng, E.W. Pyrolytic performance and kinetics studys of epoxy resin in carbon fiber reinforced composites: Synergistic effects of epoxy resin and carbon fiber. J. Anal. Appl. Pyrolysis 2023, 176, 106255. [Google Scholar] [CrossRef]
- Xia, H.F.; Zhang, B.; Wang, C.H.; Cao, L.; Luo, B.; Fan, X.M.; Zhang, J.F.; Ou, X. Surface engineered carbon-cloth with broadening voltage window for boosted energy density aqueous supercapacitors. Carbon 2020, 162, 136–146. [Google Scholar] [CrossRef]
- Chen, J.J.; Xie, J.X.; Jia, C.Q.; Song, C.Y.; Hu, J.Y.; Li, H.L. Economical preparation of high-performance activated carbon fiber papers as self-supporting supercapacitor electrodes. Chem. Eng. J. 2022, 450, 137938. [Google Scholar] [CrossRef]
- Subhani, K.; Jin, X.; Mahon, P.J.; Lau, A.K.T.; Salim, N.V. Graphene aerogel modified carbon fiber reinforced composite structural supercapacitors. Compos. Commun. 2021, 24, 100663. [Google Scholar] [CrossRef]
- Zhang, S.J.; Lu, Z.C.; Li, Y.X.; Qiu, Z.H.; Bai, Y.J.; Liu, G.G.; Xu, L.Q.; Liao, Y.Y.; Chang, S.S.; Hu, J.B. N-doped porous carbon network anchoring on hollow wooden carbon fibers for high-performance electrode materials in supercapacitors. Biomass Convers. Biorefinery 2025, 15, 271–283. [Google Scholar] [CrossRef]
- Zhao, C.J.; Ge, Z.X.; Zhou, Y.N.; Huang, Y.F.; Wang, G.F.; Qian, X.Z. Solar-assisting pyrolytically reclaimed carbon fiber and their hybrids of MnO2/RCF for supercapacitor electrodes. Carbon 2017, 114, 230–241. [Google Scholar] [CrossRef]
- Pang, S.; Lin, L.; Shen, Y.; Chen, S.; Chen, W.; Tan, N.; Ahmad, A.; Al-Kahtani, A.A.; Tighezza, A.M. Surface Activated Commercial Carbon Cloth as Superior Electrodes for Symmetric Supercapacitors. Mater. Lett. 2022, 315, 131985. [Google Scholar] [CrossRef]






| Sample | SBET (m2 g−1) | Pore Volume (cm3 g−1) |
|---|---|---|
| A-SC@CF-S | 450.81 | 0.20 |
| A-SC@CF-450-5-20 | 619.92 | 0.22 |
| A-SC@CF-C | 313.21 | 0.17 |
| Sample | C 1s | O 1s | N 1s |
|---|---|---|---|
| A-SC@CF-S | 85.55 | 13.96 | 0.49 |
| A-SC@CF-450-5-20 | 75.83 | 23.76 | 0.42 |
| A-SC@CF-C | 88.84 | 10.24 | 0.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Huang, T.; Rong, Y.; Guo, Y.; Geng, P.; Zhao, C. Focused Solar-Induced Construction of Activated Solar Carbon@Carbon Fiber Coaxial Electrode from Waste Carbon Fiber-Reinforced Polymer and Its Supercapacitor Performance. Molecules 2025, 30, 3093. https://doi.org/10.3390/molecules30153093
Zhao C, Huang T, Rong Y, Guo Y, Geng P, Zhao C. Focused Solar-Induced Construction of Activated Solar Carbon@Carbon Fiber Coaxial Electrode from Waste Carbon Fiber-Reinforced Polymer and Its Supercapacitor Performance. Molecules. 2025; 30(15):3093. https://doi.org/10.3390/molecules30153093
Chicago/Turabian StyleZhao, Chongjun, Tenghui Huang, Yingying Rong, Yanyu Guo, Puqi Geng, and Chunhua Zhao. 2025. "Focused Solar-Induced Construction of Activated Solar Carbon@Carbon Fiber Coaxial Electrode from Waste Carbon Fiber-Reinforced Polymer and Its Supercapacitor Performance" Molecules 30, no. 15: 3093. https://doi.org/10.3390/molecules30153093
APA StyleZhao, C., Huang, T., Rong, Y., Guo, Y., Geng, P., & Zhao, C. (2025). Focused Solar-Induced Construction of Activated Solar Carbon@Carbon Fiber Coaxial Electrode from Waste Carbon Fiber-Reinforced Polymer and Its Supercapacitor Performance. Molecules, 30(15), 3093. https://doi.org/10.3390/molecules30153093






