Immunological Insights into Photodynamic Therapy of Glioblastoma Multiforme
Abstract
1. Introduction
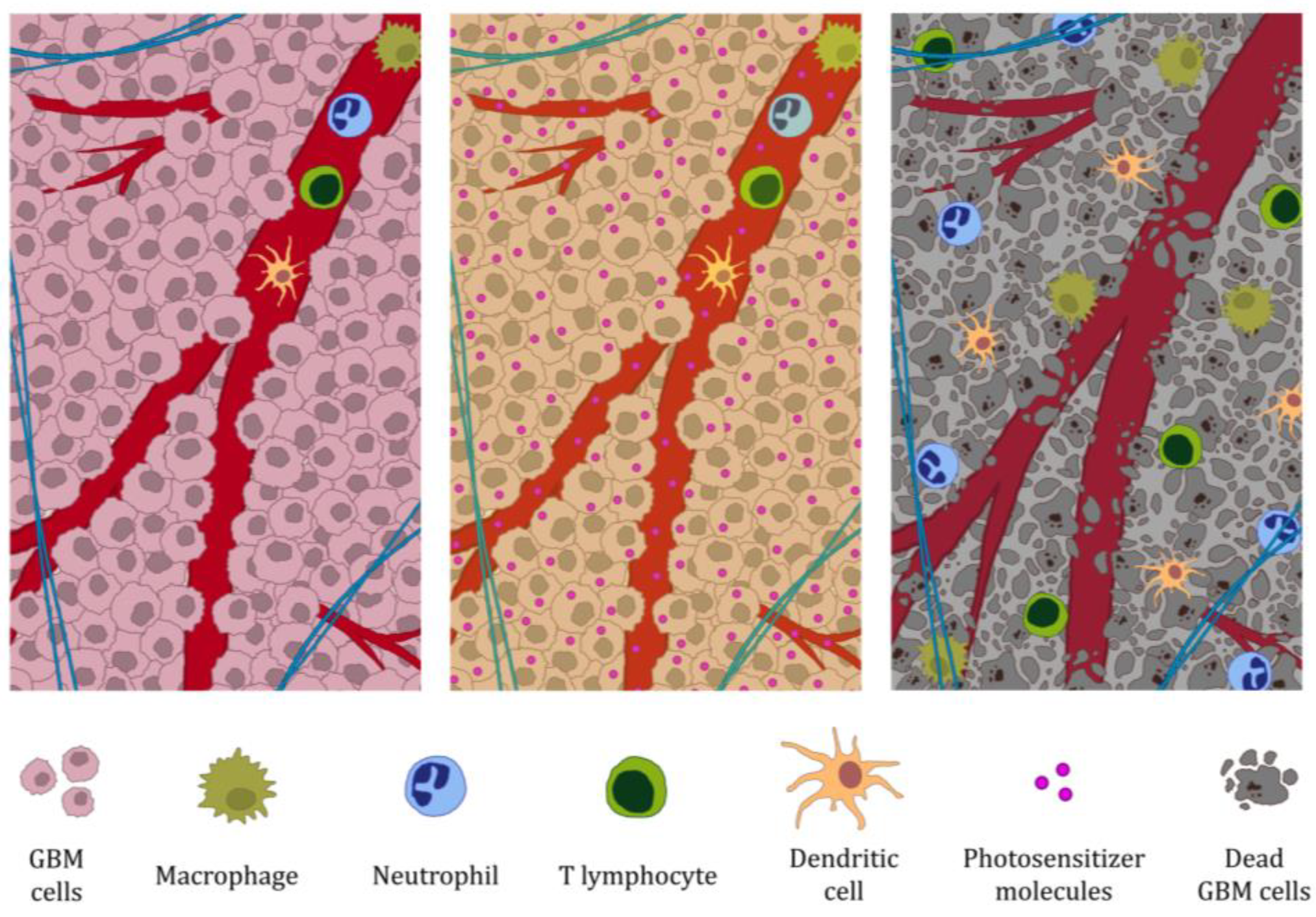
2. Photodynamic Therapy of Glioblastoma Multiforme Induces Immune System Activation
2.1. Photodynamic Therapy Induces Immunogenic GBM Cell Death and the Release of DAMP Signaling Molecules
2.2. Photodynamic Therapy of GBM Induces Non-Specific Immune System Response
2.3. DAMPs Released During Photodynamic Therapy of GBM Induce Activation of Antigen-Presenting Cells
2.4. The Specific Immune Response Against GBM Induced by Photodynamic Therapy Occurs with T Cells
3. Photodynamic Therapy Opens the Blood–Brain Barrier and Induces Clearance of the Brain by Meningeal Lymphatic Vessels
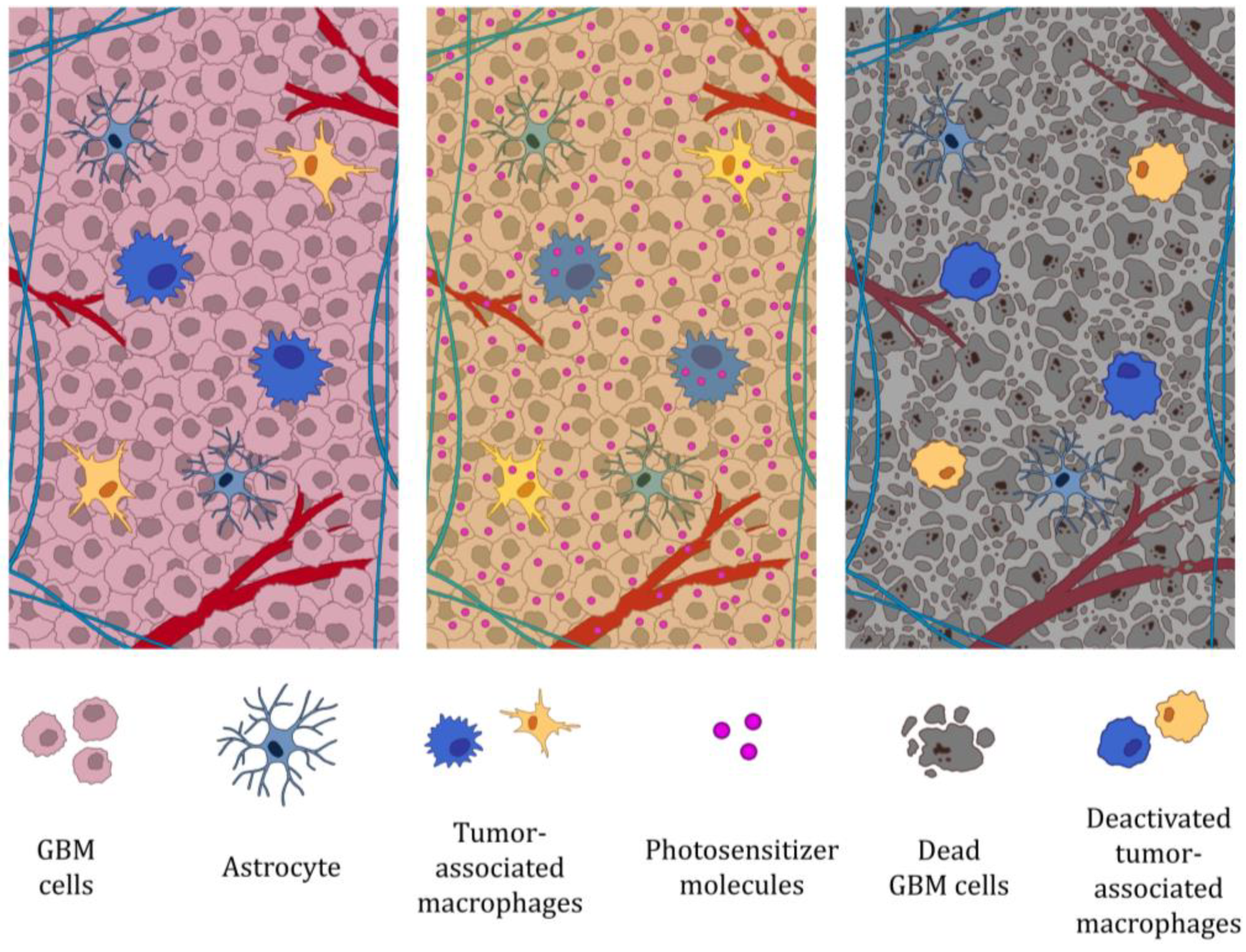
4. Photodynamic Therapy of GBM Impairs Tumor-Associated Macrophage Function
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Ostrom, Q.T.; Gittleman, H.; Truitt, G.; Boscia, A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011–2015. Neuro-Oncol. 2018, 20, 1–86. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A Summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ma, J.; Zou, Z.; Liu, H.; Liu, C.; Gong, S.; Gao, X.; Liang, G. Clinical Characteristics and Prognosis of Patients with Glioblastoma: A Review of Survival Analysis of 1674 Patients Based on SEER Database. Medicine 2022, 101, e32042. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.R.; Slone, S.A.; Dolecek, T.A.; Huang, B.; Neltner, J.H.; Villano, J.L. Primary Central Nervous System Tumor Treatment and Survival in the United States, 2004–2015. J. Neurooncol. 2019, 144, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; van den Bent, M.; Tonn, J.C.; Stupp, R.; Preusser, M.; Cohen-Jonathan-Moyal, E.; Henriksson, R.; Le Rhun, E.; Balana, C.; Chinot, O.; et al. European Association for Neuro-Oncology (EANO) Guideline on the Diagnosis and Treatment of Adult Astrocytic and Oligodendroglial Gliomas. Lancet Oncol. 2017, 18, e315–e329. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wu, Z.; Zhang, H.; Zhang, N.; Wu, W.; Wang, Z.; Dai, Z.; Zhang, X.; Zhang, L.; Peng, Y.; et al. Glioma Targeted Therapy: Insight into Future of Molecular Approaches. Mol. Cancer 2022, 21, 39. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic Therapy–Mechanisms, Photosensitizers and Combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Hsia, T.; Small, J.L.; Yekula, A.; Batool, S.M.; Escobedo, A.K.; Ekanayake, E.; You, D.G.; Lee, H.; Carter, B.S.; Balaj, L. Systematic Review of Photodynamic Therapy in Gliomas. Cancers 2023, 15, 3918. [Google Scholar] [CrossRef] [PubMed]
- Alzeibak, R.; Mishchenko, T.A.; Shilyagina, N.Y.; Balalaeva, I.V.; Vedunova, M.V.; Krysko, D.V. Targeting Immunogenic Cancer Cell Death by Photodynamic Therapy: Past, Present and Future. J. Immunother. Cancer 2021, 9, e001926. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Wei, M.; Yang, B. Recent Advances in Nanomedicines for Photodynamic Therapy (PDT)-Driven Cancer Immunotherapy. Theranostics 2022, 12, 434–458. [Google Scholar] [CrossRef] [PubMed]
- Rui, R.; Zhou, L.; He, S. Cancer Immunotherapies: Advances and Bottlenecks. Front. Immunol. 2023, 14, 1212476. [Google Scholar] [CrossRef] [PubMed]
- Yasinjan, F.; Xing, Y.; Geng, H.; Guo, R.; Yang, L.; Liu, Z.; Wang, H. Immunotherapy: A Promising Approach for Glioma Treatment. Front. Immunol. 2023, 14, 1255611. [Google Scholar] [CrossRef] [PubMed]
- Firczuk, M.; Nowis, D.; Gołąb, J. PDT-Induced Inflammatory and Host Responses. Photochem. Photobiol. Sci. 2011, 10, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.D.; Nowis, D.; Golab, J.; Vandenabeele, P.; Krysko, D.V.; Agostinis, P. Immunogenic Cell Death, DAMPs and Anticancer Therapeutics: An Emerging Amalgamation. Biochim. Et. Biophys. Acta (BBA) Rev. Cancer 2010, 1805, 53–71. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Tait, S.W.G. Targeting Immunogenic Cell Death in Cancer. Mol. Oncol. 2020, 14, 2994–3006. [Google Scholar] [CrossRef] [PubMed]
- Etminan, N.; Peters, C.; Lakbir, D.; Bünemann, E.; Börger, V.; Sabel, M.C.; Hänggi, D.; Steiger, H.-J.; Stummer, W.; Sorg, R.V. Heat-Shock Protein 70-Dependent Dendritic Cell Activation by 5-Aminolevulinic Acid-Mediated Photodynamic Treatment of Human Glioblastoma Spheroids in Vitro. Br. J. Cancer 2011, 105, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Turubanova, V.D.; Balalaeva, I.V.; Mishchenko, T.A.; Catanzaro, E.; Alzeibak, R.; Peskova, N.N.; Efimova, I.; Bachert, C.; Mitroshina, E.V.; Krysko, O.; et al. Immunogenic Cell Death Induced by a New Photodynamic Therapy Based on Photosens and Photodithazine. J. Immunother. Cancer 2019, 7, 350. [Google Scholar] [CrossRef] [PubMed]
- Redkin, T.S.; Sleptsova, E.E.; Turubanova, V.D.; Saviuk, M.O.; Lermontova, S.A.; Klapshina, L.G.; Peskova, N.N.; Balalaeva, I.V.; Krysko, O.; Mishchenko, T.A.; et al. Dendritic Cells Pulsed with Tumor Lysates Induced by Tetracyanotetra(Aryl)Porphyrazines-Based Photodynamic Therapy Effectively Trigger Anti-Tumor Immunity in an Orthotopic Mouse Glioma Model. Pharmaceutics 2023, 15, 2430. [Google Scholar] [CrossRef] [PubMed]
- Stepp, H.; Stummer, W. 5-ALA in the Management of Malignant Glioma. Lasers Surg. Med. 2018, 50, 399–419. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Lai, P.-S.; Lu, Y.-P.; Chen, H.-Y.; Chai, C.-Y.; Tsai, R.-K.; Fang, K.-T.; Tsai, M.-H.; Hsu, C.-Y.; Hung, C.-C.; et al. Real-Time Vascular Imaging and Photodynamic Therapy Efficacy with Micelle-Nanocarrier Delivery of Chlorin E6 to the Microenvironment of Melanoma. J. Dermatol. Sci. 2015, 80, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Kammerer, R.; Buchner, A.; Palluch, P.; Pongratz, T.; Oboukhovskij, K.; Beyer, W.; Johansson, A.; Stepp, H.; Baumgartner, R.; Zimmermann, W. Induction of Immune Mediators in Glioma and Prostate Cancer Cells by Non-Lethal Photodynamic Therapy. PLoS ONE 2011, 6, e21834. [Google Scholar] [CrossRef] [PubMed]
- Al-Khaza’leh, K.A.; Omar, K.; Jaafar, M.S. pH Effect on Cellular Uptake of Sn(IV) Chlorine E6 Dichloride Trisodium Salt by Cancer Cells in Vitro. J. Biol. Phys. 2011, 37, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Obeid, M.; Tesniere, A.; Ghiringhelli, F.; Fimia, G.M.; Apetoh, L.; Perfettini, J.-L.; Castedo, M.; Mignot, G.; Panaretakis, T.; Casares, N.; et al. Calreticulin Exposure Dictates the Immunogenicity of Cancer Cell Death. Nat. Med. 2007, 13, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Aaes, T.L.; Kaczmarek, A.; Delvaeye, T.; De Craene, B.; De Koker, S.; Heyndrickx, L.; Delrue, I.; Taminau, J.; Wiernicki, B.; De Groote, P.; et al. Vaccination with Necroptotic Cancer Cells Induces Efficient Anti-Tumor Immunity. Cell Rep. 2016, 15, 274–287. [Google Scholar] [CrossRef] [PubMed]
- Heras-Murillo, I.; Adán-Barrientos, I.; Galán, M.; Wculek, S.K.; Sancho, D. Dendritic Cells as Orchestrators of Anticancer Immunity and Immunotherapy. Nat. Rev. Clin. Oncol. 2024, 21, 257–277. [Google Scholar] [CrossRef] [PubMed]
- Christie, C.E.; Peng, Q.; Madsen, S.J.; Uzal, F.A.; Hirschberg, H. Increasing the Efficacy of Antitumor Glioma Vaccines by Photodynamic Therapy and Local Injection of Allogeneic Glioma Cells. In Clinical and Translational Neurophotonics; Neural Imaging and Sensing; and Optogenetics and Optical Manipulation, Proceedings of SPIE, San Francisco, CA, USA, 9 March 2016; SPIE: Burlingame, DC, USA, 2016; Volume 9690. [Google Scholar]
- Madsen, S.J.; Christie, C.; Huynh, K.; Peng, Q.; Uzal, F.A.; Krasieva, T.B.; Hirschberg, H. Limiting Glioma Development by Photodynamic Therapy-Generated Macrophage Vaccine and Allo-Stimulation: An in Vivo Histological Study in Rats. J. Biomed. Opt. 2018, 23, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rothe, F.; Patties, I.; Kortmann, R.-D.; Glasow, A. Immunomodulatory Effects by Photodynamic Treatment of Glioblastoma Cells In Vitro. Molecules 2022, 27, 3384. [Google Scholar] [CrossRef] [PubMed]
- Shixiang, Y.; Xi, S.; Junliang, L.; Shanyi, Z.; Xingke, X.; Meiguang, Z.; Kai, W.; Fangcheng, L. Antitumor Efficacy of a Photodynamic Therapy-Generated Dendritic Cell Glioma Vaccine. Med. Oncol. 2011, 28 (Suppl. S1), S453–S461. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, S.; Gross, S.; Brandis, A.; Harmelin, A.; Rosenbach-Belkin, V.; Scherz, A.; Salomon, Y. Local Photodynamic Therapy (PDT) of Rat C6 Glioma Xenografts with Pd-Bacteriopheophorbide Leads to Decreased Metastases and Increase of Animal Cure Compared with Surgery. Int. J. Cancer 2002, 99, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Miki, Y.; Akimoto, J.; Moritake, K.; Hironaka, C.; Fujiwara, Y. Photodynamic Therapy Using Talaporfin Sodium Induces Concentration-Dependent Programmed Necroptosis in Human Glioblastoma T98G Cells. Lasers Med. Sci. 2015, 30, 1739–1745. [Google Scholar] [CrossRef] [PubMed]
- Fettweis, G.; Di Valentin, E.; L’homme, L.; Lassence, C.; Dequiedt, F.; Fillet, M.; Coupienne, I.; Piette, J. RIP3 Antagonizes a TSC2-Mediated pro-Survival Pathway in Glioblastoma Cell Death. Biochim. Et. Biophys. Acta-Mol. Cell Res. 2017, 1864, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Coupienne, I.; Fettweis, G.; Rubio, N.; Agostinis, P.; Piette, J. 5-ALA-PDT Induces RIP3-Dependent Necrosis in Glioblastoma. Photochem. Photobiol. Sci. 2011, 10, 1868–1878. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Jitkaew, S.; Cai, Z.; Choksi, S.; Li, Q.; Luo, J.; Liu, Z.-G. Mixed Lineage Kinase Domain-like Is a Key Receptor Interacting Protein 3 Downstream Component of TNF-Induced Necrosis. Proc. Natl. Acad. Sci. USA 2012, 109, 5322–5327. [Google Scholar] [CrossRef] [PubMed]
- Meier, P.; Legrand, A.J.; Adam, D.; Silke, J. Immunogenic Cell Death in Cancer: Targeting Necroptosis to Induce Antitumour Immunity. Nat. Rev. Cancer 2024, 24, 299–315. [Google Scholar] [CrossRef] [PubMed]
- de Vree, W.J.; Fontijne-Dorsman, A.N.; Koster, J.F.; Sluiter, W. Photodynamic Treatment of Human Endothelial Cells Promotes the Adherence of Neutrophils in Vitro. Br. J. Cancer 1996, 73, 1335–1340. [Google Scholar] [CrossRef] [PubMed]
- Hirschberg, H.; Baek, S.-K.; Kwon, Y.J.; Sun, C.-H.; Madsen, S.J. Bypassing the Blood Brain Barrier: Delivery of Therapeutic Agents by Macrophages. In Proceeding of Photonic Therapeutics and Diagnostics VI, SPIE, San Francisco, CA, USA, 2 March 2010; Volume 7548. [Google Scholar]
- Angell-Petersen, E.; Spetalen, S.; Madsen, S.J.; Sun, C.-H.; Peng, Q.; Carper, S.W.; Sioud, M.; Hirschberg, H. Influence of Light Fluence Rate on the Effects of Photodynamic Therapy in an Orthotopic Rat Glioma Model. J. Neurosurg. 2006, 104, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Hirschberg, H.; Angell-Petersen, E.; Peng, Q.; Sun, C.; Sorensen, D.; Carper, S.; Madsen, S. ALA Mediated Photodynamic Therapy of Experimental Malignant Glioma in the BD-IX Rat Model. Photonic Ther. Diagn. 2005, 5686, 513–521. [Google Scholar]
- Fady, C.; Reisser, D.; Martin, F. Non-Activated Rat Neutrophils Kill Syngeneic Colon Tumor Cells by the Release of a Low Molecular Weight Factor. Immunobiology 1990, 181, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Krosl, G.; Korbelik, M.; Dougherty, G.J. Induction of Immune Cell Infiltration into Murine SCCVII Tumour by Photofrin-Based Photodynamic Therapy. Br. J. Cancer 1995, 71, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, J.; Fukami, S.; Suda, T.; Ichikawa, M.; Haraoka, R.; Kohno, M.; Shishido-Hara, Y.; Nagao, T.; Kuroda, M. First Autopsy Analysis of the Efficacy of Intra-Operative Additional Photodynamic Therapy for Patients with Glioblastoma. Brain Tumor Pathol. 2019, 36, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Muntjewerff, E.M.; Meesters, L.D.; van den Bogaart, G. Antigen Cross-Presentation by Macrophages. Front. Immunol. 2020, 11, 1276. [Google Scholar] [CrossRef] [PubMed]
- Stott, B.; Korbelik, M. Activation of Complement C3, C5, and C9 Genes in Tumors Treated by Photodynamic Therapy. Cancer Immunol. Immunother. 2007, 56, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Cheng, Y.; Lu, J.; Hu, R.; Wan, Q.; Feng, H. Photodynamic Therapy Boosts Anti-Glioma Immunity in Mice: A Dependence on the Activities of T Cells and Complement C3. J. Cell Biochem. 2011, 112, 3035–3043. [Google Scholar] [CrossRef] [PubMed]
- Balan, S.; Saxena, M.; Bhardwaj, N. Chapter One-Dendritic Cell Subsets and Locations. In International Review of Cell and Molecular Biology Immunobiology of Dendritic Cells Part A; Lhuillier, C., Galluzzi, L., Eds.; Academic Press: New York, NY, USA, 2019; Volume 348, pp. 1–68. [Google Scholar]
- Maeding, N.; Verwanger, T.; Krammer, B. Boosting Tumor-Specific Immunity Using PDT. Cancers 2016, 8, 91. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.J.; Tedder, T.F. Human Blood Dendritic Cells Selectively Express CD83, a Member of the Immunoglobulin Superfamily. J. Immunol. 1995, 154, 3821–3835. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Amigorena, S. The Cell Biology of Antigen Presentation in Dendritic Cells. Curr. Opin. Immunol. 2001, 13, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Steinman, R.M. Dendritic Cells and the Control of Immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Junt, T.; Moseman, E.A.; Iannacone, M.; Massberg, S.; Lang, P.A.; Boes, M.; Fink, K.; Henrickson, S.E.; Shayakhmetov, D.M.; Di Paolo, N.C.; et al. Subcapsular Sinus Macrophages in Lymph Nodes Clear Lymph-Borne Viruses and Present Them to Antiviral B Cells. Nature 2007, 450, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Chesnut, R.W.; Grey, H.M. Studies on the Capacity of B Cells to Serve as Antigen-Presenting Cells. J. Immunol. 1981, 126, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- Vedunova, M.; Turubanova, V.; Vershinina, O.; Savyuk, M.; Efimova, I.; Mishchenko, T.; Raedt, R.; Vral, A.; Vanhove, C.; Korsakova, D.; et al. DC Vaccines Loaded with Glioma Cells Killed by Photodynamic Therapy Induce Th17 Anti-Tumor Immunity and Provide a Four-Gene Signature for Glioma Prognosis. Cell Death Dis. 2022, 13, 1062. [Google Scholar] [CrossRef] [PubMed]
- Caux, C.; Massacrier, C.; Vanbervliet, B.; Dubois, B.; Van Kooten, C.; Durand, I.; Banchereau, J. Activation of Human Dendritic Cells through CD40 Cross-Linking. J. Exp. Med. 1994, 180, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Sioud, M.; Fløisand, Y. TLR Agonists Induce the Differentiation of Human Bone Marrow CD34+ Progenitors into CD11c+ CD80/86+ DC Capable of Inducing a Th1-Type Response. Eur. J. Immunol. 2007, 37, 2834–2846. [Google Scholar] [CrossRef] [PubMed]
- Metzger, R.; Mempel, T.; Joppich, I.; Till, H. Organ-Specific Distribution of Major Histocompatibility Antigens in Rats. Pediatr. Surg. Int. 2000, 16, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Asai, J.; Suzuki, R.; Fujimoto, T.; Suzuki, T.; Nakagawa, N.; Nagashima, G.; Miyo, T.; Hokaku, H.; Takei, A. Fluorescence Automatic Cell Sorter and Immunohistochemical Investigation of CD68-Positive Cells in Meningioma. Clin. Neurol. Neurosurg. 1999, 101, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, C.; Zhang, Z.; Han, B.; Shen, Z.; Li, L.; Liu, S.; Zhao, X.; Ye, F.; Zhang, Y. Specific Clinical and Immune Features of CD68 in Glioma via 1,024 Samples. Cancer Manag. Res. 2018, 10, 6409–6419. [Google Scholar] [CrossRef] [PubMed]
- Nishie, A.; Ono, M.; Shono, T.; Fukushi, J.; Otsubo, M.; Onoue, H.; Ito, Y.; Inamura, T.; Ikezaki, K.; Fukui, M.; et al. Macrophage Infiltration and Heme Oxygenase-1 Expression Correlate with Angiogenesis in Human Gliomas. Clin. Cancer Res. 1999, 5, 1107–1113. [Google Scholar] [PubMed]
- Saito, Y.; Fukami, S.; Nagai, K.; Ogawa, E.; Kuroda, M.; Kohno, M.; Akimoto, J. Cytocidal Effects of Interstitial Photodynamic Therapy Using Talaporfin Sodium and a Semiconductor Laser in a Rat Intracerebral Glioma Model. Biomedicines 2024, 12, 2141. [Google Scholar] [CrossRef] [PubMed]
- Platt, A.M.; Randolph, G.J. Dendritic Cell Migration through the Lymphatic Vasculature to Lymph Nodes. Adv. Immunol. 2013, 120, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Austyn, J.M.; Kupiec-Weglinski, J.W.; Hankins, D.F.; Morris, P.J. Migration Patterns of Dendritic Cells in the Mouse. Homing to T Cell-Dependent Areas of Spleen, and Binding within Marginal Zone. J. Exp. Med. 1988, 167, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Engleman, E.G.; Benike, C.J.; Grumet, F.C.; Evans, R.L. Activation of Human T Lymphocyte Subsets: Helper and Suppressor/Cytotoxic T Cells Recognize and Respond to Distinct Histocompatibility Antigens. J. Immunol. 1981, 127, 2124–2129. [Google Scholar] [CrossRef] [PubMed]
- Doyle, C.; Strominger, J.L. Interaction between CD4 and Class II MHC Molecules Mediates Cell Adhesion. Nature 1987, 330, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Balázs, M.; Martin, F.; Zhou, T.; Kearney, J.F. Blood Dendritic Cells Interact with Splenic Marginal Zone B Cells to Initiate T-Independent Immune Responses. Immunity 2002, 17, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Kotsias, F.; Cebrian, I.; Alloatti, A. Antigen Processing and Presentation. Int. Rev. Cell Mol. Biol. 2019, 348, 69–121. [Google Scholar] [CrossRef] [PubMed]
- Kinkhabwala, M.; Sehajpal, P.; Skolnik, E.; Smith, D.; Sharma, V.K.; Vlassara, H.; Cerami, A.; Suthanthiran, M. A Novel Addition to the T Cell Repertory. Cell Surface Expression of Tumor Necrosis Factor/Cachectin by Activated Normal Human T Cells. J. Exp. Med. 1990, 171, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Zhou, Y.; Yu, J.; Mao, L.; Bosco, M.J.; Wang, J.; Lu, Y.; Mao, L.; Wu, X.; Wang, F.; et al. Establishment of the Reference Intervals of Lymphocyte Function in Healthy Adults Based on IFN-γ Secretion Assay upon Phorbol-12-Myristate-13-Acetate/Ionomycin Stimulation. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Kabingu, E.; Vaughan, L.; Owczarczak, B.; Ramsey, K.D.; Gollnick, S.O. CD8+ T Cell-Mediated Control of Distant Tumours Following Local Photodynamic Therapy Is Independent of CD4+ T Cells and Dependent on Natural Killer Cells. Br. J. Cancer 2007, 96, 1839–1848. [Google Scholar] [CrossRef] [PubMed]
- De Boer, R.J.; Homann, D.; Perelson, A.S. Different Dynamics of CD4+ and CD8+ T Cell Responses during and after Acute Lymphocytic Choriomeningitis Virus Infection. J. Immunol. 2003, 171, 3928–3935. [Google Scholar] [CrossRef] [PubMed]
- Preise, D.; Oren, R.; Glinert, I.; Kalchenko, V.; Jung, S.; Scherz, A.; Salomon, Y. Systemic Antitumor Protection by Vascular-Targeted Photodynamic Therapy Involves Cellular and Humoral Immunity. Cancer Immunol. Immunother. 2009, 58, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Rocha, L.B.; Gomes-Da-Silva, L.C.; Dąbrowski, J.M.; Arnaut, L.G. Elimination of Primary Tumours and Control of Metastasis with Rationally Designed Bacteriochlorin Photodynamic Therapy Regimens. Eur. J. Cancer 2015, 51, 1822–1830. [Google Scholar] [CrossRef] [PubMed]
- Mroz, P.; Szokalska, A.; Wu, M.X.; Hamblin, M.R. Photodynamic Therapy of Tumors Can Lead to Development of Systemic Antigen-Specific Immune Response. PLoS ONE 2010, 5, e15194. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Tanaka, M.; Kojima, Y.; Nishie, H.; Shimura, T.; Kubota, E.; Kataoka, H. Anti-Tumor Immunity Enhancement by Photodynamic Therapy with Talaporfin Sodium and Anti-Programmed Death 1 Antibody. Mol. Ther. Oncolytics 2023, 28, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.I.; McKenzie, B.S.; Zhou, L.; Tadokoro, C.E.; Lepelley, A.; Lafaille, J.J.; Cua, D.J.; Littman, D.R. The Orphan Nuclear Receptor RORγt Directs the Differentiation Program of Proinflammatory IL-17+ T Helper Cells. Cell 2006, 126, 1121–1133. [Google Scholar] [CrossRef] [PubMed]
- Kadry, H.; Noorani, B.; Cucullo, L. A Blood–Brain Barrier Overview on Structure, Function, Impairment, and Biomarkers of Integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, H.; Soldati, S.; Mossu, A.; Rosito, M.; Rudolph, H.; Muller, W.A.; Latorre, D.; Sallusto, F.; Sospedra, M.; Martin, R.; et al. Human CD4+ T Cell Subsets Differ in Their Abilities to Cross Endothelial and Epithelial Brain Barriers in Vitro. Fluids Barriers CNS 2020, 17, 3. [Google Scholar] [CrossRef] [PubMed]
- Seitz, R.J.; Wechsler, W. Immunohistochemical Demonstration of Serum Proteins in Human Cerebral Gliomas. Acta Neuropathol. 1987, 73, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Sarkaria, J.N.; Hu, L.S.; Parney, I.F.; Pafundi, D.H.; Brinkmann, D.H.; Laack, N.N.; Giannini, C.; Burns, T.C.; Kizilbash, S.H.; Laramy, J.K.; et al. Is the Blood–Brain Barrier Really Disrupted in All Glioblastomas? A Critical Assessment of Existing Clinical Data. Neuro Oncol. 2018, 20, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Sampson, J.H.; Gunn, M.D.; Fecci, P.E.; Ashley, D.M. Brain Immunology and Immunotherapy in Brain Tumours. Nat. Rev. Cancer 2020, 20, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Belle, V.; Anka, A.; Cross, N.; Thompson, P.; Mott, E.; Sharma, R.; Gray, K.; Zhang, R.; Xu, Y.; Sun, J.; et al. Dynamic Contrast Enhanced-Magnetic Resonance Imaging (DCE-MRI) of Photodynamic Therapy (PDT) Outcome and Associated Changes in the Blood-Brain Barrier Following Pc 4-PDT of Glioma in an Athymic Nude Rat Model. In Proceedings of the Photonic Therapeutics and Diagnostics VIII; SPIE, San Francisco, CA, USA, 9 February 2012; Volume 8207, pp. 674–682. [Google Scholar]
- Luo, Q.; Yang, J.; Yang, M.; Wang, Y.; Liu, Y.; Liu, J.; Kalvakolanu, D.V.; Cong, X.; Zhang, J.; Zhang, L.; et al. Utilization of nanotechnology to surmount the blood-brain barrier in disorders of the central nervous system. Mater. Today Bio. 2025, 31, 101457. [Google Scholar] [CrossRef] [PubMed]
- Hirschberg, H.; Uzal, F.A.; Chighvinadze, D.; Zhang, M.J.; Peng, Q.; Madsen, S.J. Disruption of the Blood-Brain Barrier Following ALA-Mediated Photodynamic Therapy. Lasers Surg. Med. 2008, 40, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Yeung, I.; Lilge, L.D.; Wilson, B.C.; Lee, T.-Y.; Stevens, L.; Cenic, A. Photodynamic-Therapy-Induced Alterations of the Blood-Brain Barrier Transfer Constant of a Tracer Molecule in Normal Brain. In Proceedings of the Optical Methods for Tumor Treatment and Detection: Mechanisms and Techniques in Photodynamic Therapy VI; SPIE, San Francisco, CA, USA, 8 May 1997; Volume 2972, pp. 54–63. [Google Scholar]
- Demyanenko, S.V.; Uzdensky, A.B.; Sharifulina, S.A.; Lapteva, T.O.; Polyakova, L.P. PDT-Induced Epigenetic Changes in the Mouse Cerebral Cortex: A Protein Microarray Study. Biochim. Biophys. Acta 2014, 1840, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Hirschberg, H.; Peng, Q.; Uzal, F.A.; Chighvinadze, D.; Zhang, M.J.; Madsen, S.J. Targeted Opening of the Blood Brain Barrier by ALA-Mediated Photodynamic Therapy. In Proceedings of the Photodynamic Therapy: Back to the Future; SPIE, San Francisco, CA, USA, 13 July 2009; Volume 7380, pp. 212–221. [Google Scholar]
- Mathews, M.S.; Chighvinadze, D.; Gach, H.M.; Uzal, F.A.; Madsen, S.J.; Hirschberg, H. Cerebral Edema Following Photodynamic Therapy Using Endogenous and Exogenous Photosensitizers in Normal Brain. Lasers Surg. Med. 2011, 43, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Semyachkina-Glushkovskaya, O.; Chehonin, V.; Borisova, E.; Fedosov, I.; Namykin, A.; Abdurashitov, A.; Shirokov, A.; Khlebtsov, B.; Lyubun, Y.; Navolokin, N.; et al. Photodynamic Opening of the Blood-Brain Barrier and Pathways of Brain Clearing. J. Biophotonics 2018, 11, e201700287. [Google Scholar] [CrossRef] [PubMed]
- Louveau, A.; Smirnov, I.; Keyes, T.J.; Eccles, J.D.; Rouhani, S.J.; Peske, J.D.; Derecki, N.C.; Castle, D.; Mandell, J.W.; Lee, K.S.; et al. Structural and Functional Features of Central Nervous System Lymphatic Vessels. Nature 2015, 523, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Aspelund, A.; Antila, S.; Proulx, S.T.; Karlsen, T.V.; Karaman, S.; Detmar, M.; Wiig, H.; Alitalo, K. A Dural Lymphatic Vascular System That Drains Brain Interstitial Fluid and Macromolecules. J. Exp. Med. 2015, 212, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Deng, Q.; Ma, L.; Li, Q.; Chen, Y.; Liao, Y.; Zhou, F.; Zhang, C.; Shao, L.; Feng, J.; et al. Meningeal Lymphatic Vessels Regulate Brain Tumor Drainage and Immunity. Cell Res. 2020, 30, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Ma, L.; Xu, H.; Huo, Y.; Luo, J. Meningeal Lymphatics Regulate Radiotherapy Efficacy through Modulating Anti-Tumor Immunity. Cell Res. 2022, 32, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Blokhina, I.; Terskov, A.; Evsiukova, A.; Dubrovsky, A.; Adushkina, V.; Zlatogorskaya, D.; Dmitrenko, A.; Tuzhilkin, M.; Manzhaeva, M.; Krupnova, V.; et al. Photodynamic Opening of the Blood-Brain Barrier Affects Meningeal Lymphatics and the Brain’s Drainage in Healthy Male Mice. Biomed. Opt. Express 2024, 15, 6063–6072. [Google Scholar] [CrossRef] [PubMed]
- Su, P.; Li, O.; Ke, K.; Jiang, Z.; Wu, J.; Wang, Y.; Mou, Y.; Jin, W. Targeting Tumor-associated Macrophages: Critical Players in Tumor Progression and Therapeutic Strategies (Review). Int. J. Oncol. 2024, 64, 60. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Yang, Z.; Chen, P.; Yeh, Y.; Sun, C.; Xie, T.; Huang, W.; Zhang, X. GPR65 Sensing Tumor-Derived Lactate Induces HMGB1 Release from TAM via the cAMP/PKA/CREB Pathway to Promote Glioma Progression. J. Exp. Clin. Cancer Res. 2024, 43, 105. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wu, L.; Wu, W.; Zhu, M.; Li, J.; Wang, Z.; Li, J.; Ding, R.; Liang, Y.; Li, L.; et al. Phagocytosis of Glioma Cells Enhances the Immunosuppressive Phenotype of Bone Marrow-Derived Macrophages. Cancer Res. 2023, 83, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Motevasseli, M.; Darvishi, M.; Khoshnevisan, A.; Zeinalizadeh, M.; Saffar, H.; Bayat, S.; Najafi, A.; Abbaspour, M.J.; Mamivand, A.; Olson, S.B.; et al. Distinct Tumor-TAM Interactions in IDH-Stratified Glioma Microenvironments Unveiled by Single-Cell and Spatial Transcriptomics. Acta Neuropathol. Commun. 2024, 12, 133. [Google Scholar] [CrossRef] [PubMed]
- Pyonteck, S.M.; Akkari, L.; Schuhmacher, A.J.; Bowman, R.L.; Sevenich, L.; Quail, D.F.; Olson, O.C.; Quick, M.L.; Huse, J.T.; Teijeiro, V.; et al. CSF-1R Inhibition Alters Macrophage Polarization and Blocks Glioma Progression. Nat. Med. 2013, 19, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- Korbelik, M.; Hamblin, M.R. The Impact of Macrophage-Cancer Cell Interaction on the Efficacy of Photodynamic Therapy. Photochem. Photobiol. Sci. 2015, 14, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Hoppstädter, J.; Seif, M.; Dembek, A.; Cavelius, C.; Huwer, H.; Kraegeloh, A.; Kiemer, A.K. M2 Polarization Enhances Silica Nanoparticle Uptake by Macrophages. Front. Pharmacol. 2015, 6, 55. [Google Scholar] [CrossRef] [PubMed]
- Maklygina, Y.S.; Romanishkin, I.D.; Skobeltsin, A.S.; Savelyeva, T.A.; Potapov, A.A.; Pavlova, G.V.; Chekhonin, I.V.; Gurina, O.I.; Loschenov, V.B. Time-Resolved Fluorescence Imaging Technique for Rat Brain Tumors Analysis. J. Phys. Conf. Ser. 2021, 2058, 012028. [Google Scholar] [CrossRef]
- Lerouge, L.; Gries, M.; Chateau, A.; Daouk, J.; Lux, F.; Rocchi, P.; Cedervall, J.; Olsson, A.-K.; Tillement, O.; Frochot, C.; et al. Targeting Glioblastoma-Associated Macrophages for Photodynamic Therapy Using AGuIX(®)-Design Nanoparticles. Pharmaceutics 2023, 15, 997. [Google Scholar] [CrossRef] [PubMed]
- Vandenabeele, P.; Vandecasteele, K.; Bachert, C.; Krysko, O.; Krysko, D.V. Immunogenic Apoptotic Cell Death and Anticancer Immunity. Adv. Exp. Med. Biol. 2016, 930, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Cesca, B.A.; Pellicer San Martin, K.; Caverzan, M.D.; Oliveda, P.M.; Ibarra, L.E. State-of-the-Art Photodynamic Therapy for Malignant Gliomas: Innovations in Photosensitizers and Combined Therapeutic Approaches. Explor. Target. Antitumor. Ther. 2025, 6, 1002303. [Google Scholar] [CrossRef] [PubMed]
| DAMP | Photosensitizer | Photosensitizer Concentration [μM] | Incubation Time [h] | Fluence Value [J/cm2] | Cell Line | Reference |
|---|---|---|---|---|---|---|
| HSP-70 | PpIX 1 | 95.3 | 4 | No data available 2 | U251 | [17] |
| U87 | ||||||
| CRT | PHS | 1.4 | 4 | 20 J/cm2 | GL261 | [18] 3 |
| PD | 1.2 | 4 | 20 J/cm2 | GL261 | [18] | |
| HMGB1 | PHS | 1.4 | 4 | 20 J/cm2 | GL261 | [18] |
| PD | 1.2 | 4 | 20 J/cm2 | GL261 | [18] | |
| PZ-I | 2.8 | 4 | 20 J/cm2 | GL261 | [19] 4 | |
| PZ-III | 1.7 | 4 | 20 J/cm2 | GL261 | [19] | |
| ATP | PHS | 1.4 | 4 | 20 J/cm2 | GL261 | [18] |
| PD | 1.2 | 4 | 20 J/cm2 | GL261 | [18] | |
| Pz-I | 2.8 | 4 | 20 J/cm2 | GL261 | [19] | |
| Pz-III | 1.7 | 4 | 20 J/cm2 | GL261 | [19] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woźnicki, P.; Bartusik-Aebisher, D.; Przygórzewska, A.; Aebisher, D. Immunological Insights into Photodynamic Therapy of Glioblastoma Multiforme. Molecules 2025, 30, 3091. https://doi.org/10.3390/molecules30153091
Woźnicki P, Bartusik-Aebisher D, Przygórzewska A, Aebisher D. Immunological Insights into Photodynamic Therapy of Glioblastoma Multiforme. Molecules. 2025; 30(15):3091. https://doi.org/10.3390/molecules30153091
Chicago/Turabian StyleWoźnicki, Paweł, Dorota Bartusik-Aebisher, Agnieszka Przygórzewska, and David Aebisher. 2025. "Immunological Insights into Photodynamic Therapy of Glioblastoma Multiforme" Molecules 30, no. 15: 3091. https://doi.org/10.3390/molecules30153091
APA StyleWoźnicki, P., Bartusik-Aebisher, D., Przygórzewska, A., & Aebisher, D. (2025). Immunological Insights into Photodynamic Therapy of Glioblastoma Multiforme. Molecules, 30(15), 3091. https://doi.org/10.3390/molecules30153091








