Abstract
Alzheimer’s disease (AD) is one of the main causes of dementia, with an exponential increment in its incidence as years go by. However, since pathophysiological mechanisms are complex and multifactorial, therapeutic strategies remain inconclusive and only provide symptomatic relief to patients. In order to solve this problem, new strategies have been investigated over recent years for AD treatment. This field has been reborn due to epidemiological and preclinical findings that demonstrate the fact that omega-3 polyunsaturated fatty acids (ω-3 PUFAs) can be promising therapeutic agents because of their anti-inflammatory, antioxidant, and neurogenic-promoting activities, thus allowing us to classify these molecules as neuroprotectors. Similarly, ω-3 PUFAs perform important actions in the formation of characteristic AD lesions, amyloid-β plaques (Aβ) and neurofibrillary tangles, reducing the development of these structures. Altogether, the aforementioned actions hinder cognitive decline and possibly reduce AD development. In addition, ω-3 PUFAs modulate the inflammatory response by inhibiting the production of pro-inflammatory molecules and promoting the synthesis of specialised pro-resolving mediators. Consequently, the present review assesses the mechanisms by which ω-3 PUFAs can act as therapeutic molecules and the effectiveness of their use in patients. Clinical evidence so far has shown promising results on ω-3 PUFA effects, both in animal and epidemiological studies, but remains contradictory in clinical trials. More research on these molecules and their neuroprotective effects in AD is needed, as well as the establishment of future guidelines to obtain more reproducible results on this matter.
1. Introduction
Alzheimer’s disease is the most frequent neurodegenerative disorder worldwide and the most prevalent cause of neurocognitive impairment in the elderly population [1], affecting a total of 24 million people, with an estimated duplication of these numbers by 2040 [2]. This clinical entity has a multifactorial origin, conditioned by genetic and environmental factors, in addition to the age of the individual [3]. The disease is characterised by progressive loss of neurocognitive functions, mainly memory, eventually progressing to impairment in other spheres, such as voluntary motricity and vegetative functions [4].
In general, the typical structural changes in AD are a product of the abnormal accumulation of misfolded beta amyloid and tau proteins [2], which leads to the formation of lesions known as amyloid plaques and neurofibrillary tangles, generally located within the temporal lobe. These lesions disrupt neuronal synapses and promote the beginning of inflammatory processes that contribute to disease progression [5]. This sequence of events is known as the amyloid cascade hypothesis (ACH). Despite its wide acceptance, the exact alterations and variability in disease progression remain to be completely elucidated [2]. Thus, effective therapeutic strategies have been difficult to achieve.
Accordingly, the results obtained with current treatments based on the ACH do not seem to disrupt or avoid progressive neurodegeneration. In the hope of sorting out this issue, new approaches focused on inflammatory mechanisms involved in AD have been established, along with AD prevention through interventions on modifiable risk factors such as diet and lifestyle [6]. In this sense, ω-3 PUFAs, key components of a healthy diet, have emerged as possible therapeutic agents for AD treatment. These molecules are essential components of neurons’ lipidic membrane and participate in the modulation of multiple functions, such as cell migration regulation and neurotransmission, as well as inflammatory markers and neuroprotection against oxidative stress. The previously mentioned functions have been confirmed by clinical and epidemiological trials, which have identified an association between ω-3 polyunsaturated fatty acid (ω-3 PUFAs)-rich diets and a lower risk of the development and progression of cognitive impairment [7,8].
Hence, this review aims to explore current knowledge on the neuroprotective mechanisms of ω-3 PUFAs and their implications on AD development, as well as demonstrating the effectiveness of ω-3 PUFA supplementation in patients with Alzheimer’s disease.
2. Materials and Methods
This review provides compiled information regarding the function of ω-3 PUFAs in AD through an extensive and non-systematic literature search in the Scopus, EMBASE, PubMed, ISI Web of Science, ScienceDirect, Medline, Cochrane Library Plus, and Google Scholar databases, from inception to July 2025. Only articles in Spanish and English were used. No restrictions were made based on the type of study. Scientific articles from high-impact journals were selected: Q1, Q2, and Q3. The studies used for the preclinical and clinical evidence section were selected, predominantly, based on the clarity and reproducibility of their methodology and scientific quality. The search strategy included terms such as “Alzheimer’s disease”, “PUFAs”, “neuroprotection”, and “inflammation” combined with Boolean operators (AND/OR).
3. Results
3.1. Key Neurodegenerative Mechanisms in Alzheimer’s Disease
Alzheimer’s disease evolution begins many years before the initiation of clinical symptoms [9]. Within the different theories concerning AD development, the ACH explains that the formation of senile plaques, composed of misfolded Aβ proteins of 40–12 amino acids, as a product of altered amyloid precursor protein (APP) cleavage by β-secretase [10]. These lesions initially develop in the basal, temporal, and orbitofrontal regions of the neocortex. As the disease progresses, the lesions expand to the hippocampus, amygdala, diencephalon, and basal ganglia [11]. The deposition of plaques induces mitochondrial damage, homeostatic dysregulation, and synaptic dysfunction, along with the hyperphosphorylation of tau proteins. As a result, the proteins lose affinity for microtubules and instead form conglomerates of misfolded tau proteins, leading to the formation of neurofibrillary tangles. These structures aggravate axonal transport and mitochondrial dysfunction, with the subsequent loss of synaptic function [12] (Figure 1).
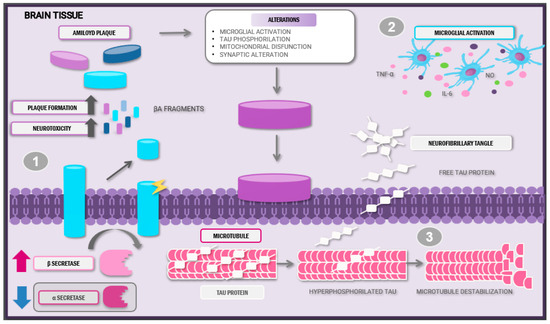
Figure 1.
Anatomopathological alterations and pathophysiology of Alzheimer’s disease. Abbreviations: Aβ: amyloid beta; IL-6: interleukin-6; TNF-α: tumour necrosis factor; NO: nitric oxide.
Amyloid plaque and neurofibrillary tangle formation characterises Alzheimer’s disease. (1) These plaques are a product of the altered cleavage of precursor amyloid protein by the β-secretase enzyme, thus provoking Aβ neurotoxic peptide release and posterior aggregation. (2) Abnormal Aβ accumulation induces microglial activation and pro-inflammatory cytokine release. (3) The hyperphosphorylation of tau proteins leads to microtubule destabilisation and loss of synaptic function. This sequence of events creates a pro-inflammatory and neurotoxic state, aggravating disease evolution.
Due to the high toxicity and capacity to induce neuronal damage, Aβ and hyperphosphorylated tau protein can lead to microglial cell infiltration around amyloid plaques [11]. Once activated, these cells can adopt different phenotypes: M1, induced by interferon-gamma (IFN-γ) and lipopolysaccharide (LPS) stimulation, producing pro-inflammatory cytokines release, and the M2 phenotype, activated by interleukins (ILs) and characterised by the expression of anti-inflammatory molecules such as transforming growth factor β (TGF-β) and extracellular matrix molecules [13].
During the early phases of the disease, the lesions induce microglial polarisation into the M1 phenotype via specific receptors, such as CD14, CD36, and Toll-like receptors (TLRs), which facilitates the release of pro-inflammatory cytokines. These molecules recruit other cells that proceed to organise themselves to phagocytose the pathogen [14]. However, the continual production of these aberrant proteins and their interactions with the microglia do not allow a resolution phase to occur; instead, they promote exacerbated inflammatory responses and defective Aβ plaque removal [15].
Moreover, abnormal Aβ accumulation induces impaired mitochondrial membrane potential by disrupting its homeostasis and enzymatic activity. Aβ enters the mitochondria via the translocase of the outer membrane complex, leading to excessive production of reactive oxygen species and limiting the antioxidant system’s ability to neutralise them, consequently resulting in oxidative stress, subsequent neuronal damage, and further mitochondrial dysfunction [16].
Additionally, epigenetics plays a significant role in Alzheimer’s disease progression. Epigenetic modifications (e.g., DNA methylation and histone alterations) can be influenced by both modifiable (e.g., diet and environmental toxins) and non-modifiable factors (e.g., ageing). Among the non-modifiable genetic factors, mutations in the presenilin genes (PSEN1 and PSEN2), which encode essential subunits of the γ-secretase complex, promote the production of neurotoxic amyloid-β (Aβ42) by altering the proteolytic processing of amyloid precursor protein (APP). Furthermore, the APOE ε4 allele, which impairs Aβ clearance and accelerates its aggregation, is a major genetic risk factor for amyloid plaque deposition [17,18]. Within the non-modifiable factors, APP genetic mutations, presenilin 1 (PSEN1), and presenilin 2 (PSEN2) favour the production of toxic Aβ forms. Moreover, the presence of allele 4 of the APOE protein, which binds to Aβ, and its subsequent polymerisation are also considered relevant factors in plaque deposition [18]. Furthermore, age is considered the main factor in AD development, since this phenomenon is linked to diminished DNA methylation capacity in the brain, leading to mitochondrial decline. In consequence, mitochondrial dysfunction affects the expression and processing of APP, triggering Aβ accumulation [19,20]. On the other hand, acquired factors such as strokes and dyslipidaemias are linked to oligemia, brain–blood barrier dysfunction, and the accumulation of neurotoxic molecules that produce neuronal damage [21].
Lastly, modifiable variables, such as diet and exercise, are associated with beneficial and neuroprotective effects against AD development. Regarding physical activity, its neuroprotective effects could be produced by the release of neurotrophic factors such as insulin-like growth factor (IGF-1), nerve growth factor (NGF), and vascular endothelial growth factor (VEGF), since they exhibit antioxidant properties and promote cerebral perfusion [22]. Likewise, diets that include abundant unsaturated fats and antioxidants contain essential neuroprotective components, such as vitamins and PUFAs. These molecules can stabilise the neuronal membrane since they promote Aβ peptide elimination and phospholipid synthesis to improve and preserve synaptic functions [23,24].
3.2. Omega-3 Fatty Acids as Neuroprotective Agents
The nervous system is rich in fatty acids, and PUFAs, particularly, constitute about 35% of this tissue [25]. Thus, incorporating such molecules is necessary to maintain nervous cells adequately. Among fatty acids, the ω-3 series is mainly represented by eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) [26]. These acids are found in seafood and vegetable sources (ALA) [27]. Both DHA and EPA are part of the lipid membrane and act as substrates for molecule synthesis, improving cognitive processes and performing neuroprotective activities, due to their anti-inflammatory, antioxidant, and neuromodulator activities [28].
ω-3 PUFAs, and particularly DHA, display multiple actions that counteract amyloid plaque formation. Among the mechanisms involved, their capacity to affect amyloidogenic and non-amyloidogenic processing is highly relevant. Regarding the first pathway, PUFAs’ main actions consist of 1. γ-secretase suppression, via PESEN1; 2. β-secretase inhibition; 3. BACE1 altered signalling; and 4. BACE1 downregulation through PPAR activation. Meanwhile, non-amyloidogenic processing is in charge of 5. α-secretase stabilisation, 6. ADAM17 increase, and 7. the interstitial removal of Aβ peptides via aquaporins.
3.2.1. Anti-Inflammatory Role
In pathological conditions such as AD or ageing, pro-inflammatory states as a result of microglial dysfunction are a signature feature [29]. Inflammation is a defence mechanism designed to repair and protect tissues [30] which is usually controlled and self-limited. Nevertheless, under certain circumstances, it can turn into a chronic process and contribute to tissue damage [31].
Different hypotheses have been proposed to explain the anti-inflammatory mechanisms of ω-3 PUFAs [32]. Among these, microglial modulation is highlighted, since microglia are capable of inducing M2 phenotype polarisation and thus decrease the production of pro-inflammatory cytokines such as tumour necrosis factor alpha (TNF-α), interleukin-1beta (IL-1β), and interleukin-6 (I-6) [33]. PUFAs presumably diminish factor nuclear kappa B subunit (IκB) phosphorylation and induce peroxisome proliferator-activated receptor (PPARγ), consequently inhibiting NF-κB translocation to the cellular nucleus [34]. Simultaneously, PUFAs promote the disruption of the P38 of mitogen-activated protein kinase (MAPK)7 signalling pathway [35], involved in the biosynthesis of pro-inflammatory cytokines like TNF-α and IL-1β [36].
Moreover, cyclooxygenase (COX) and lipoxygenase (LOX) enzymes catalyse the oxidation of EPA and DHA molecules, resulting in compounds specialised in inflammation resolution, known as specialised pro-resolving lipid mediators (SPMs) [31]. Within this group, we have molecules such as E series resolvins (RvE1-3), derived from EPA; D series resolvins (RvD1-6); maresins (Mar1-2); and neuroprotectins (NPD1), derived from DHA [37].
Particularly, resolvins (Rvs) are bioactive compounds with chemoselective and focalised activity, whose pro-resolutive and anti-inflammatory effects are exerted through G protein-coupled receptors (GPCRs) [38]. Molecules derived from the E series (RevE1-E3) act as ChemR23 agonists. This receptor is located in microglia and neurons widely distributed within the prefrontal cortex and hippocampus, where it increases the phagocytosis of apoptotic cells, diminishes pro-inflammatory cytokines, and induces beneficial resolving signals [39]. In addition, resolvins act as BLT-1 agonists in order to block polymorphonuclear (PMN) cell chemotaxis and NF-kB activation [40]. RvD exerts similar effects regarding PMN cell infiltration regulation [41], NF-κB activation, and the negative modulation of pro-inflammatory cytokine production via microRNA induction [31].
Concurrently, NPD1 modulates anti-apoptotic protein activity and decreases caspase-3 activation induced by oxidative stress, thus promoting cellular survival. Furthermore, this molecule inhibits COX-2 and NF-κB expression in order to regulate pro-inflammatory molecules [42]. The neuroprotective effects associated with Mars molecules range from the downregulation of inflammatory markers through NF-κB pathway inhibition [43] to the decrease in the levels of neuronal death promoter CD40 and the increase in tissue regeneration [44].
3.2.2. Role of ω-3 PUFAs in Oxidative Stress
The accumulation of the Aβ peptide and hyperphosphorylated tau proteins, along with the persistent activation of microglial cells observed in AD patients, creates an environment of abnormally high oxidative stress levels in the nervous system [28]. Therefore, multiple mechanisms by which ω-3 PUFAs might counteract this environment have been proposed. Their antioxidant activities are a product of cell migration and the induction of nuclear factor erythroid 2-related factor 2 (Nrf-2). PUFAs bind to the antioxidant response element (ARE) and augment the gene transcription of antioxidant enzyme heme oxygenase (HO-1), nicotinamide adenine dinucleotide (NAD), and quinone oxidoreductase 1 (Nqo1) [45]. PUFAs are also able to induce microglial polarisation into the M2 phenotype, favouring phagocytic activity and simultaneously hindering the production of oxidative stress-promoting factors, such as nitric oxide (NO) and reactive oxygen species (ROS) [25].
Similarly, SPMs also act as oxidative stress antagonists through ROS reduction, since these molecules activate antioxidant enzymes that promote homeostatic balance. Rvs suppress NADPH oxidase NOX2 in microglia and antagonise BLT1, thereby regulating ROS production and cell apoptosis [46]. Similarly, NDP1 induces the phosphoinositide 3-kinase/Akt pathway, enabling the activation of cell survival mechanisms [47]. The antioxidant effects linked to Mars result from the modulation of the Nrf-2/HO-1 pathway [48].
3.2.3. Role of PUFAs in Neuronal Generation
After embryogenesis and early postnatal life, neurogenesis is a process exclusively maintained in two areas of the adult brain [49]: the subventricular zone of the lateral ventricles and the subventricular layer of the hippocampus dentate gyrus [50]. However, ageing, pro-inflammatory chronic states, and oxidative stress can potentially diminish this process [51].
In vivo and in vitro studies have demonstrated that DHA supplementation induces an increase in the axonal growth marker Growth-Associated Protein 43 (GAP-43), thereby promoting the growth and ramification of hippocampal dendrites [52]. Furthermore, DHA induces an increase in synapsin levels [53] and binds to the GPCR40 receptor, leading to a cascade of events that culminate in calcium mobilisation, the activation of protein kinase C (PKC), and increased production of substrates required for synaptogenesis.
Moreover, both EPA and DHA exert modulating effects on neurogenesis via transcription factors Hes-1 and Hes-6, which act through a positive feedback cycle to promote neuronal differentiation [50]. Multiple DHA neurogenic effects can be explained by their active mediators, specifically docosahexaenoyl ethanolamandine (DHEA). This endocannabinoid metabolite activates protein kinase A (PKA), allowing the subsequent differentiation of neuronal stem cells [54].
3.3. Preclinical Evidence of Omega-3 Fatty Acids in Alzheimer’s Disease
It is important to note that the neuroprotective effect of ω-3 PUFAs in AD and other neurodegenerative diseases is primarily attributed to their anti-inflammatory, antioxidant, and neuronal growth-promoting mechanisms. In addition to this, DHA (one of the derivatives of PUFAs) has been shown to exhibit molecular mechanisms capable of acting on two key processes of AD development: the formation of Aβ plaques and the aggregation of tau protein. However, further preclinical research is required to demonstrate the role of EPA, another PUFA derivative, in combating AD.
3.3.1. Docosahexaenoic Acid and Aβ
ω-3 PUFAs, particularly DHA and its derivatives, have shown multiple mechanisms capable of inhibiting the formation and neurotoxic effects caused by Aβ. DHA can hinder the formation of neurofibrillary tangles through the alteration in the nucleation and elongation phases, as well as the increased genetic expression of insulin-degrading enzyme (IDE), which mediates Aβ degradation [55,56]. Furthermore, dietary supplementation with DHA can reduce Aβ density in the hippocampus and toxic prefibrillar Aβ oligomers; additionally, it can stabilise soluble fibrillar Aβ oligomers in transgenic models performed in APP/PS1 rats with AD [57]. Additionally, DHA modulates Aβ 16-21 peptide aggregation by redirecting Leu-17, Phe-19, and Phe-20 residues, which eventually form stable and non-structured complexes, according to an extensive molecular dynamics simulation study [58].
In this sense, DHA inhibits the in vitro fibrillation of Aβ 25-35 and neutralises the neurotoxicity related to fibrillar formation [59]. On the other hand, DHA molecules exert anti-amyloidogenic effects on Aβ-42, the most abundant protein linked to amyloid plaque formation in AD [60]. Among the mechanisms involved, the ability of DHA to modulate the amyloidogenic and non-amyloidogenic processing of the APP is highlighted; it could be attributed to the induction of anti-amyloidogenic chaperones such as SorLA and transthyretin based on in vivo models performed in mice [61,62].
One of the main actions of DHA in amyloidogenic processing is the modulation of enzymes implicated in its metabolism. DHA can induce the suppression of PESEN1 and thus γ-secretase [63], as well as the inhibition of β-secretase. On the contrary, DHA stabilises the activity of α-secretase, enhancing a non-amyloidogenic process [64]. Furthermore, DHA modifies BACE1 internalisation in the endosomes, reducing β-secretase activity and subsequently inhibiting amyloidosis, typical of AD [64,65]. Similarly, DHA binds to PPARγ, activating these receptors and leading to reduced transcription and BACE1 expression [66,67] (Figure 2).
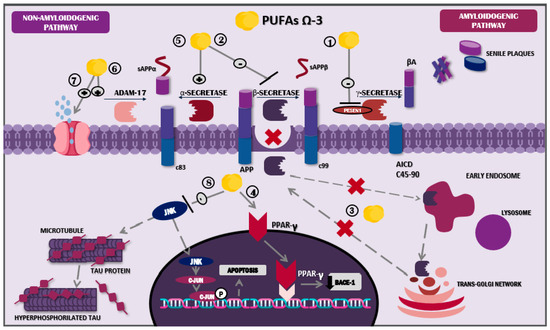
Figure 2.
Mechanism of action of docosahexaenoic acid in the nervous system. Abbreviations: sAPPβ: soluble peptide APPβ, sAPPα: soluble peptide APPα; AICD: amyloid precursor protein intracellular domain; Aβ: amyloid beta; JNK: Jun N-terminal kinase; ω-3 PUFAs: omega-3 polyunsaturated fatty acids.
DHA also diminishes PESEN1 levels in lipid droplets, directly influencing amyloidogenic processes [64]. Additionally, it plays a role in cholesterol microdomains located on the membrane of the droplet, altering its organisation and structure; in other words, it boosts the change from droplet to non-droplet [68]. This process results in a loss of Aβ affinity for lipid droplets, leading to decreased accumulation of this element on neuronal plasma membranes [69].
Furthermore, DHA’s role in non-amyloidogenic processing involves increased levels of ADAM metallopeptidase domain 17 (ADAM17) and, consequently, of soluble amyloid precursor protein-alpha (sAPPα), a molecule associated with neuroprotective effects and β-secretase regulation [64,70]. In a similar manner, the potential activity of the lymphatic system through aquaporin-4 has been described; these molecules showed the ability to promote interstitial Aβ removal, and this mechanism was evidenced in an in vivo study using murine models treated with fish oil, one of the main dietary ω-3 PUFA sources [71] (Figure 2). Likewise, DHA has proven to possess a genetic role, since in vitro studies indicate that its consumption has inhibitory effects on the pro-amyloidogenic activity of the APOE ε4 allele [72].
Among the DHA actions against Aβ, microglial phagocytosis plays an important role, stimulating the activity of the anti-inflammatory phenotype M2 and subsequent Aβ-42 phagocytosis [44]. SPMs derived from DHA exhibit similar mechanisms: MaR1 and RvD1 stimulate Aβ phagocytosis via macrophages and microglial cells that bind to specific receptors for each molecule. Said actions provoke increased intracellular calcium levels and the activation of signalling pathways such as PPAR, extracellular signal-regulated kinase (ERK), protein kinase A (PKA), and phosphoinositide 3-kinase (PI3K) [72,73,74].
Nevertheless, the key SPM against Aβ is NPD1. The actions of NPD1 are very similar to DHA regarding amyloidogenic and non-amyloidogenic processing pathways, including PESEN1 reduction, the modulation of secretases, the augmentation in SorLA/LR11 expression, the down-regulation of BACE1, and the up-regulation of disintegrin and metalloproteinase 10 (ADAM10) activity, in addition to increased PPARγ-mediated activities. Each one of these mechanisms attributed to NPD1 is likely to result in Aβ and sAPPβ decrement, along with increased sAPPα levels [72,73,74], hence stimulating NPD1 synthesis and release, with subsequent positive feedback and long-term neurotrophic benefits [73].
Furthermore, Aβ deposits are linked to neuronal impairment and apoptosis. PPARγ activation by NPD1 can generate anti-apoptotic activities via the up-regulation of anti-apoptotic genes that codify the proteins Bcl-2, Bcl-xl, and Bfl-1 [75]. However, NPD1 is not the only molecule to exhibit anti-apoptotic properties; MaR1 has been implicated in the downregulation of pro-apoptotic proteins such as p38, caspase-3, and mTOR, thereby contributing to the inhibition of neuronal apoptosis [76].
3.3.2. Docosahexaenoic Acid and Tau Protein
Since neurofibrillary tangles are a feature of AD, strategies that inhibit the aggregation of tau proteins or tau kinases to avoid or diminish disease development have been suggested [77]. It has been proposed that ω-3 PUFAs, and DHA specifically, could contribute as potential therapeutic agents with the capacity to inhibit the main kinases involved in tau protein phosphorylation, such as glycogen synthase kinase-3 beta (GSK-3 beta) and Jun N-terminal kinase (JNK) [78].
Experimental in vivo studies carried out in murine models with AD have shown increased JNK activity in the plaques of the neurites that contain phosphorylated tau, as well as a subsequent decrement after DHA supplementation. JNK is a kinase, part of the MAPK family, that can phosphorylate two sites of tau proteins related to AD: ser202/Thr205 and Ser422. The phosphorylation of these elements has been observed in early stages of the disease and is thought to precede neurofibrillary tangle formation [79]. DHA through the Mfsd2a transporter can directly inhibit JNK, leading to a decrease in p-c-Jun levels and thus lower tau hyperphosphorylation [80].
In contrast, DHA acts favourably on the PI3-K/Akt pathway, inhibiting GSK-3 beta and subsequently tau phosphorylation [78]. The GSK-3 enzyme can phosphorylate tau protein in 42 sites, and its activity is regulated by phosphorylation in two specific sites, serine 9 and tyrosine 216, allowing for its inactivation and activation, respectively. In AD, this regulation is altered, promoting the hyperactivity of the enzyme and leading to tau hyperphosphorylation [81].
3.4. Clinical and Epidemiological Evidence of Omega-3 Fatty Acids in Alzheimer’s Disease
Alzheimer’s disease is one of the main causes of dementia, and owing to the impact that it exerts over the patient’s life and its family environment, multiple strategies, such as dietary intervention, pharmacological treatments, gene editing, and monoclonal antibody therapies, have been considered to approach this pathology [82,83,84,85,86]. The nutritional role has gained particular interest. It was observed, in various epidemiological studies regarding Mediterranean diets rich in ω-3 PUFAs, a link between the mentioned dietary lifestyle and a lower prevalence of dementias and specifically AD [87,88,89,90,91,92,93]. This section will focus on discussing clinical and epidemiological findings related to ω-3 PUFAs as potential therapeutic agents in AD.
Under this premise, a cross-sectional study performed in 894 adults above ≥50 years old; it explored the association between diet, lifestyle and cognitive function, respectively, and showed that diets with abundant nuts and vegetables rich in ω-3 PUFAs had beneficial effects on cognition [94]. Moreover, a meta-analysis assessed the dose–response effect of ω-3 PUFAs in adults over 40 years old with normal cognitive functions or mild cognitive impairment. A significant improvement was evidenced 12 months after the intervention with doses over 500 mg. These results were attributed to the effects of ω-3 PUFAs on synaptic plasticity and neurogenesis in cerebral regions exposed to oxidative stress [95].
Likewise, another cross-sectional clinical trial linked greater cortical thickness (reduced in AD pre-symptomatic individuals) and a lower dementia risk to diets such as the Mediterranean diet and ω-3 PUFA-rich diets [96]. Similar results were observed in the Framingham Heart Study [97] and the Atherosclerosis Risk in Communities study [98]. Furthermore, Baierle et al. [99] demonstrated that individuals with diminished levels of ω-3 PUFAs, particularly DHA, exhibited reduced cognition compared with controls, thereby reinforcing the epidemiological evidence previously mentioned. However, this study is limited by a small population sample.
On the other hand, to examine the relationship between diet and AD brain biomarkers, Mosconi et al. [100] showed an association between greater ω-3 PUFA administration and less Aβ peptide development via brain imaging analysis. In addition, the authors have found that low ω-3 PUFA levels in serum were linked to increments in cerebral amyloidosis, while higher levels were correlated with the preservation of brain volume in AD-implicated regions [101]. In this sense, significant correlations were observed between the ω-3 PUFA index and entorhinal cortical volume, a structure tightly associated with the hippocampus and involved in memory and learning, as well as total white matter volume and decreased oxidative stress and inflammation [102].
Consistently, Wei et al. [103] carried out a meta-analysis that pointed out a potential modulating effect of APOE ε4, the most influential genetic factor in AD, through ω-3 PUFA supplementation. Similarly, the efficacy of these supplements showed a threshold concentration of 1.0 g/dL, since cognitive impairment risk declined once the supplementation exceeded this concentration. On the other hand, Sala-Vila et al. evidenced statistically significant associations concerning ω-3 PUFA levels and lower risk of AD and dementia in males and patients ≥60 years of age, in comparison with females between 50 and 59 years of age, suggesting that further investigation is needed due to the lack of clinical trials that effectively compare gender and age [104].
Nevertheless, despite the inverse relationship observed between ω-3 PUFA intake and AD risk in observational studies, the results of clinical trials are controversial. This phenomenon could be explained by factors such as inconsistencies in sample size, methodology, and time or by differences among the interventions [105]. Despite multiple studies showing the beneficial effects of supplementation, other clinical trials do not report any favourable results.
The Multi-domain Alzheimer Preventive Trial (MAPT) is a multicentric, randomised, placebo-controlled study and one of the biggest randomised clinical trials (RCTs) in this area. The MAPT aimed to assess the effects of ω-3 PUFA supplementation (two capsules a day providing a total daily dose of 800 mg of docosahexaenoic acid and 225 mg of eicosapentaenoic acid) on the cognitive function of adults over 70 years old without dementia. Beneficial effects were not demonstrated compared with the placebo group; however, a subgroup population analysis suggested that ω-3 PUFA supplementation, along with multi-domain intervention, could aid in slowing cognitive impairment in patients with a greater dementia risk [106].
It is important to note that other RCTs and secondary analyses of the MAPT have reported similar results, including less cognitive decline, improved orientation, and delayed recall [107,108]. Moreover, a recent clinical trial demonstrated the protective effect of ω-3 PUFA supplementation in individuals with cognitive deficits and dementia. These participants had family-style meals containing an additional 1720 mg of docosahexaenoic acid per day for 12 months [109]. Comparatively, a seven-trial RCT meta-analysis showed that compared with the placebo, ω-3 PUFA supplementation diminishes cognitive deficit significantly in elderly individuals (IC 95%: 0.04–1.67; p = 0.04) [110]. Additionally, various meta-analyses have found similar associations between ω-3 PUFAs and lower cognitive deficits. Overall, an 8 g/d increment in dietary PUFAs was significantly associated with lower risks of dementia [111,112] and AD risk [113] (Table 1).

Table 1.
Efficacy of ω-3 PUFA supplementation in individuals with cognitive impairment or AD.
On the contrary, an RCT secondary to the MAPT did not find significant effects on the cognitive functions of elderly patients subjected to a multi-domain intervention along with ω-3 PUFA supplementation over 36 months [117]. Similar results were found by Phillips et al. [114], where an increase in EPA/DHA concentrations in the serum of subjects did not show benefits on cognition; however, these results can be debated due to the small sample size and short follow-up period. Simultaneously, a meta-analysis of three RCTs pointed out that no beneficial effects on cognition were observed after consuming ω-3 PUFA-rich diets or supplementation in patients with moderate or mild AD [114].
Despite being considered a potential therapeutic strategy against AD due to its molecular properties, clinical evidence of ω-3 PUFA supplementation is mixed and inconclusive. One significant problem is the heterogeneity among these studies. The regimen protocols have not been standardised yet, and they differ in their supplementation composition. Furthermore, some studies assess ω-3 PUFA supplementation based on plasma levels, while others only select participants with a high intake of dietary ω-3 PUFA foods.
Currently, the focus of systematic reviews based on RCT presents several limitations. These studies aim to assess the efficacy of clinical interventions, but lack reproducibility as a result of their strict inclusion criteria, non-randomised nature, and short-term follow-up periods [118]. Likewise, other limitations, such as the use of unequal supplementations, dose, time, population, and diagnostic tools, may explain the incongruence of these results.
4. Conclusions
Alzheimer’s disease is characterised by the formation of amyloid plaques and neurofibrillary tangles that lead to a chronic pro-inflammatory state, neuronal apoptosis, and oxidative stress. To combat this pathology, ω-3 PUFAs might be considered potential therapeutic agents due to the extensive neuroprotective functions they exert. These molecules exhibit antioxidant and anti-inflammatory properties through microglial modulation and SPM production. In addition, ω-3 PUFAs play a key role in synaptogenesis and neurogenesis. Moreover, these fatty acids induce protective effects against AD by counteracting Aβ synthesis and its neurotoxic effects and influence APP processing in order to promote anti-amyloidogenic activities. Lastly, ω-3 PUFAs seem to increase Aβ peptide phagocytosis in microglial cells and hamper tau protein hyperphosphorylation via GSK-3β and JNK inhibition.
So far, current clinical and epidemiological evidence has demonstrated a positive relationship between ω-3 PUFA supplementation and lower risk of cognitive impairment as well as AD development; nevertheless, some results have been inconclusive. Also, the regimen protocols have not been solidly established, but studies demonstrating promising results are based on higher-dosage protocols (e.g., 1000–2000 mg of EPA and DHA combined daily) and long-term supplementation. Thus, it is necessary to perform more randomised clinical trials, with similar supplementation types, doses, and study durations, taking into account that DHA renovation in the brain takes approximately 2 years. Finally, the study subjects should be selected and screening tools adapted according to the specific population.
Even though ω-3 PUFA supplementation shows promise, particularly in the early stages of AD, there are several key limitations to consider in this therapeutic approach. The benefits appear to be stage-dependent, with greater effects observed in individuals in early AD or mild cognitive impairment. Genetic factors, such as the presence of the APOE ε4 allele, may influence individual responses to treatment. Furthermore, there is no standardised dosing protocol, making comparisons across studies difficult. Additionally, therapeutic effects take months to appear, which may be costly and difficult for some patients to maintain over the long term.
It is important to emphasise that although our study focuses on ω-3 PUFAs as a novel therapeutic approach for AD, there are also emerging pharmacological therapies such as anti-amyloid monoclonal antibodies that can slow clinical decline by promoting amyloid clearance. Among these, aducanumab, lecanemab, and donanemab have received US Food and Drug Administration approval. These agents are administered intravenously and exert their effects by activating microglia and inducing them to engulf and consume amyloids [119].
Author Contributions
Conceptualisation, M.C.-C., M.P.G., P.D., E.D.-C., M.P.D., M.N., C.C., G.C., R.C., D.R.-P. and V.B.; methodology, M.C.-C., M.P.G., P.D., M.P.D., M.N., C.C., G.C., R.C., D.R.-P. and V.B.; formal analysis, M.C.-C., M.P.G., P.D., E.D.-C., M.P.D., M.N., C.C., G.C., R.C., D.R.-P. and V.B.; investigation, M.C.-C., M.P.G., P.D., E.D.-C., M.P.D., M.N., C.C., G.C., R.C., D.R.-P. and V.B.; resources, M.C.-C., M.P.G., P.D., M.P.D., M.N., C.C., G.C., R.C., D.R.-P. and V.B.; writing—original draft preparation, M.C.-C., M.P.G., P.D., M.P.D., M.N., C.C., G.C., R.C., D.R.-P. and V.B.; writing—review and editing, M.C.-C., M.P.G., P.D., M.P.D., M.N., C.C., G.C., R.C., D.R.-P. and V.B.; visualisation, M.C.-C., D.R.-P. and V.B.; funding acquisition, V.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research study was funded by Universidad Simón Bolívar, Vicerrectoría de Investigación, Extensión e Innovación, internal funds for research strengthening, Barranquilla, Colombia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analysed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ACH | Amyloid cascade hypothesis |
| APP | Amyloid precursor protein |
| ALA | Alpha-linolenic acid |
| Aβ | Amyloid-β |
| COX | Cyclooxygenase |
| DHA | Docosahexaenoic acid |
| DHEA | Docosahexaenoyl ethanolamide |
| AD | Alzheimer’s disease |
| EPA | Eicosapentaenoic acid |
| ERK | Extracellular signal-regulated kinase |
| GPCR | G protein-coupled receptors |
| GPCR40 | G protein-coupled receptor 40 |
| GSK-3 beta | Glycogen synthase kinase-3 beta |
| HO-1 | Heme oxygenase 1 |
| IDE | Insulin-degrading enzyme |
| IGF-1 | Insulin growth factor |
| IκB | Inhibitory subunit of factor nuclear kappa B |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| JNK | c-Jun N-terminal kinases |
| CSF | Cerebrospinal fluid |
| LOX | Lipoxygenase |
| MAPK | Mitogen-activated protein kinases |
| Mar | Maresin |
| NGF | Nerve growth factor |
| NPD1 | Neuroprotectin 1 |
| NO | Nitric oxide |
| NF-κB | Nuclear factor kappa beta |
| PESEN1 | Presenilin 1 |
| PESEN2 | Presenilin 2 |
| PI3K | Phosphoinositide 3-kinase |
| PUFA | Polyunsaturated fatty acid |
| PKA | Protein kinase A |
| PKC | Protein kinase C |
| PPAR-γ | Peroxisome proliferator-activated receptor γ |
| PMN cells | Polymorphonuclear cells |
| ROS | Reactive oxygen species |
| RvEs | E series resolvins |
| RvDs | D series resolvins |
| sAPPα | Soluble amyloid precursor protein-alpha |
| SPM | Specialised pro-resolving lipid mediator |
| CNS | Central Nervous System |
| TLR | Toll-like receptors |
| TNF-α | Tumour necrosis factor alpha |
| VEGF | Vascular endothelial growth factor |
References
- Cooper, E.L.; Ma, M.J. Alzheimer Disease: Clues from traditional and complementary medicine. J. Tradit. Complement. Med. 2017, 7, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association. 2015 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2015, 11, 332–384. [Google Scholar] [CrossRef] [PubMed]
- Araya-Quintanilla, F.; Gutiérrez-Espinoza, H.; Sánchez-Montoya, U.; Muñoz-Yañez, M.J.; Baeza-Vergara, A.; Petersen-Yanjarí, M.; Fernández-Lecaros, L. Effectiveness of omega-3 fatty acid supplementation in patients with Alzheimer disease: A systematic review and meta-analysis. Neurologia 2020, 35, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Shieh, C.H.; Wu, Y.S.; Kalueff, A.; Gaikwad, S.; Su, K.P. The role of omega-3 polyunsaturated fatty acids eicosapentaenoic and docosahexaenoic acids in the treatment of major depression and Alzheimer’s disease: Acting separately or synergistically? Prog. Lipid Res. 2016, 62, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Devassy, J.G.; Leng, S.; Gabbs, M.; Monirujjaman, M.; Aukema, H.M. Omega-3 polyunsaturated fatty acids and oxylipins in neuroinflammation and management of Alzheimer disease. Adv. Nutr. 2016, 7, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Hou, J.; Ping, J.; Cai, D. Advances in developing novel therapeutic strategies for Alzheimer’s disease 11 Medical and Health Sciences 1109 Neurosciences. Mol. Neurodegener. 2018, 13, 64. [Google Scholar] [CrossRef] [PubMed]
- Lopez, L.B.; Kritz-Silverstein, D.; Barrett-Connor, E. High dietary and plasma levels of the omega-3 fatty acid docosahexaenoic acid are associated with decreased dementia risk: The rancho bernardo study. J. Nutr. Health Aging 2011, 15, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Bo, Y.; Zhang, X.; Wang, Y.; You, J.; Cui, H.; Zhu, Y.; Pang, W.; Liu, W.; Jiang, Y.; Lu, Q. The n-3 polyunsaturated fatty acids supplementation improved the cognitive function in the Chinese elderly with mild cognitive impairment: A double-blind randomised controlled trial. Nutrients 2017, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Esquerda-Canals, G.; Montoliu-Gaya, L.; Güell-Bosch, J.; Villegas, S. Mouse Models of Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 57, 1171–1183. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Q.; Mobley, W.C. Alzheimer disease pathogenesis: Insights from molecular and cellular biology studies of oligomeric Aβ and tau species. Front. Neurosci. 2019, 13, 659. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Atluri, V.; Kaushik, A.; Yndart, A.; Nair, M. Alzheimer’s disease: Pathogenesis, diagnostics, and therapeutics. Int. J. Nanomed. 2019, 14, 5541–5554. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Tan, L.; Yu, J.T.; Tan, L. Tau in Alzheimer’s Disease: Mechanisms and Therapeutic Strategies. Curr. Alzheimer Res. 2018, 15, 283–300. [Google Scholar] [CrossRef] [PubMed]
- Bolós, M.; Perea, J.R.; Avila, J. Alzheimer’s disease as an inflammatory disease. Biomol. Concepts 2017, 8, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Fakhoury, M. Microglia and astrocytes in Alzheimer’s disease: Implications for therapy. Curr. Neuropharmacol. 2018, 16, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Nichols, M.R.; St-Pierre, M.K.; Wendeln, A.C.; Makoni, N.J.; Gouwens, L.K.; Garrad, E.C.; Sohrabi, M.; Neher, J.J.; Tremblay, M.E.; Combs, C.K. Inflammatory mechanisms in neurodegeneration. J. Neurochem. 2019, 149, 562–581. [Google Scholar] [CrossRef] [PubMed]
- Nasb, M.; Tao, W.; Chen, N. Alzheimer’s Disease Puzzle: Delving into Pathogenesis Hypotheses. Aging Dis. 2024, 15, 43–73. [Google Scholar] [PubMed]
- Ma, Y.; Wang, W.; Liu, S.; Qiao, X.; Xing, Y.; Zhou, Q.; Zhang, Z. Epigenetic Regulation of Neuroinflammation in Alzheimer’s Disease. Cells 2023, 13, 79. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Basak, J.M.; Holtzman, D.M. The Role of Apolipoprotein E in Alzheimer’s Disease. Neuron 2009, 63, 287–303. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Huai, J. Role of primary aging hallmarks in Alzheimer’s disease. Theranostics 2023, 13, 197–230. [Google Scholar] [CrossRef] [PubMed]
- Ionescu-Tucker, A.; Cotman, C.W. Emerging roles of oxidative stress in brain aging and Alzheimer’s disease. Neurobiol. Aging 2021, 107, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, M.; Sato, N.; Shimamura, M.; Kurinami, H.; Hamasaki, T.; Chatterjee, A.; Rakugi, H.; Morishita, R. Possible modification of Alzheimer’s disease by statins in midlife: Interactions with genetic and non-genetic risk factors. Front. Aging Neurosci. 2014, 6, 71. [Google Scholar] [CrossRef] [PubMed]
- Di Liegro, C.M.; Schiera, G.; Proia, P.; Di Liegro, I. Physical Activity and Brain Health. Genes 2019, 10, 720. [Google Scholar] [CrossRef] [PubMed]
- Vinicius, M.; De Mello, C.; Vieira, L.; Cruz de Souza, L.; Gomes, K.; Carvalho, M. Alzheimer’s disease: Risk factors and potentially protective measures. J. Biomed. Sci. 2019, 26, 33. [Google Scholar] [CrossRef] [PubMed]
- Reemst, K.; Broos, J.Y.; Abbink, M.R.; Cimetti, C.; Giera, M.; Kooij, G.; Korosi, A. Early-life stress and dietary fatty acids impact the brain lipid/oxylipin profile into adulthood, basally and in response to LPS. Front. Immunol. 2022, 13, 967437. [Google Scholar] [CrossRef] [PubMed]
- Heras-Sandoval, D.; Pedraza-Chaverri, J.; Pérez-Rojas, J.M. Role of docosahexaenoic acid in the modulation of glial cells in Alzheimer’s disease. J. Neuroinflamm. 2016, 13, 61. [Google Scholar] [CrossRef] [PubMed]
- Waitzberg, D.L.; Garla, P. Contribución de los Ácidos grasos omega-3 para la memoria y la función cognitiva. Nutr. Hosp. 2014, 30, 467–477. [Google Scholar] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef] [PubMed]
- Serini, S.; Calviello, G. Reduction of Oxidative/Nitrosative Stress in Brain and its Involvement in the Neuroprotective Effect of n-3 PUFA in Alzheimer’s Disease. Curr. Alzheimer Res. 2016, 13, 123–134. [Google Scholar] [CrossRef] [PubMed]
- McGrattan, A.M.; McGuinness, B.; McKinley, M.C.; Kee, F.; Passmore, P.; Woodside, J.V.; McEvoy, C.T. Diet and Inflammation in Cognitive Ageing and Alzheimer’s Disease. Curr. Nutr. Rep. 2019, 8, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Crupi, R.; Marino, A.; Cuzzocrea, S. n-3 Fatty Acids: Role in Neurogenesis and Neuroplasticity. Curr. Med. Chem. 2013, 20, 2953–2963. [Google Scholar] [CrossRef] [PubMed]
- Fraga, V.G.; Carvalho Mdas, G.; Caramelli, P.; de Sousa, L.P.; Gomes, K.B. Resolution of inflammation, n − 3 fatty acid supplementation and Alzheimer disease: A narrative review. J. Neuroimmunol. 2017, 310, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Szczechowiak, K.; Diniz, B.S.; Leszek, J. Diet and Alzheimer’s dementia—Nutritional approach to modulate inflammation. Pharmacol. Biochem. Behav. 2019, 184, 172743. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Liu, S.; Hu, M.; Sun, X.; Qiu, W.; Zheng, S.; Hu, X.; Lu, Z. Post-stroke DHA Treatment Protects Against Acute Ischemic Brain Injury by Skewing Macrophage Polarity Toward the M2 Phenotype. Transl. Stroke Res. 2018, 9, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Wong, C.Y.; Koh, R.Y.; Lim, C.L.; Kok, Y.Y.; Chye, S.M. Natural Bioactive Compounds from Macroalgae and Microalgae for the Treatment of Alzheimer’s Disease: A Review. Yale J. Biol. Med. 2024, 97, 205–224. [Google Scholar] [CrossRef] [PubMed]
- Antonietta Ajmone-Cat, M.; Lavinia Salvatori, M.; de Simone, R.; Mancini, M.; Biagioni, S.; Bernardo, A.; Cacci, E.; Minghetti, L. Docosahexaenoic acid modulates inflammatory and antineurogenic functions of activated microglial cells. J. Neurosci. Res. 2012, 90, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Kim, N.J. Recent advances in the inhibition of p38 MAPK as a potential strategy for the treatment of Alzheimer’s disease. Molecules 2017, 22, 1287. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wu, X.; Liu, S.; Shen, D.; Zhu, J.; Liu, K. Role of Resolvins in the Inflammatory Resolution of Neurological Diseases. Front. Pharmacol. 2020, 11, 612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, N.; Ding, Y.; Zhang, Y.; Li, Q.; Flores, J.; Haghighiabyaneh, M.; Doycheva, D.; Tang, J.; Zhang, J.H. Chemerin suppresses neuroinflammation and improves neurological recovery via CaMKK2/AMPK/Nrf2 pathway after germinal matrix hemorrhage in neonatal rats. Brain Behav. Immun. 2018, 70, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Spite, M.; Clària, J.; Serhan, C.N. Resolvins, specialised proresolving lipid mediators, and their potential roles in metabolic diseases. Cell Metab. 2014, 19, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Levy, B.D. Resolvins and protectins: Natural pharmacophores for resolution biology. Prostaglandins Leukot. Essent. Fat. Acids 2010, 82, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Bazan, N.G. Docosanoids and elovanoids from omega-3 fatty acids are pro-homeostatic modulators of inflammatory responses, cell damage and neuroprotection. Mol. Asp. Med. 2018, 64, 18–33. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Wang, X.; Hjorth, E.; Colas, R.A.; Schroeder, L.; Granholm, A.C.; Serhan, C.N.; Schultzberg, M. Pro-Resolving Lipid Mediators Improve Neuronal Survival and Increase Aβ42 Phagocytosis. Mol. Neurobiol. 2016, 53, 2733–2749. [Google Scholar] [CrossRef] [PubMed]
- Hjorth, E.; Zhu, M.; Toro, V.C.; Vedin, I.; Palmblad, J.; Cederholm, T.; Freund-Levi, Y.; Faxen-Irving, G.; Wahlund, L.-O.; Basun, H.; et al. Omega-3 fatty acids enhance phagocytosis of alzheimer’s disease-related amyloid-β42 by human microglia and decrease inflammatory markers. J. Alzheimer’s Dis. 2013, 35, 697–713. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.; Lee, M.H.; Lee, S.; Cho, E.J. Neuroprotective Effect of Alpha-Linolenic Acid against Aβ-Mediated Inflammatory Responses in C6 Glial Cell. J. Agric. Food Chem. 2018, 66, 4853–4861. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.N.; Surh, Y.J. Resolvin D1-mediated NOX2 inactivation rescues macrophages undertaking efferocytosis from oxidative stress-induced apoptosis. Biochem. Pharmacol. 2013, 86, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Suzumura, A.; Terao, R.; Kaneko, H. Protective effects and molecular signaling of n-3 fatty acids on oxidative stress and inflammation in retinal diseases. Antioxidants 2020, 9, 920. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Wu, Y.; Zhao, F.; Wang, J. Maresin 1 Ameliorates Lung Ischemia/Reperfusion Injury by Suppressing Oxidative Stress via Activation of the Nrf-2-Mediated HO-1 Signaling Pathway. Oxidative Med. Cell. Longev. 2017, 2017, 9634803. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.; Blackshaw, S. Regulation and function of neurogenesis in the adult mammalian hypothalamus. Prog. Neurobiol. 2018, 170, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Dyall, S.C. Long-chain omega-3 fatty acids and the brain: A review of the independent and shared effects of EPA, DPA and DHA. Front. Aging Neurosci. 2015, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Poulose, S.M.; Miller, M.G.; Scott, T.; Shukitt-Hale, B. Nutritional factors affecting adult neurogenesis and cognitive function. Adv. Nutr. 2017, 8, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, T.; Cansev, M.; Wurtman, R.J. Oral supplementation with docosahexaenoic acid and uridine-5′-monophosphate increases dendritic spine density in adult gerbil hippocampus. Brain Res. 2007, 1182, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Kevala, K.; Kim, J.; Moon, H.S.; Jun, S.B.; Lovinger, D.; Kim, H. Docosahexaenoic acid promotes hippocampal neuronal development and synaptic function. J. Neurochem. 2009, 111, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Díaz, M.; Mesa-Herrera, F.; Marín, R. Dha and its elaborated modulation of antioxidant defenses of the brain: Implications in aging and ad neurodegeneration. Antioxidants 2021, 10, 907. [Google Scholar] [CrossRef] [PubMed]
- Eto, M.; Hashimoto, T.; Shimizu, T.; Iwatsubo, T. Characterization of the unique in Vitro effects of unsaturated fatty acids on the formation of amyloid β fibrils. PLoS ONE 2019, 14, e0219465. [Google Scholar] [CrossRef] [PubMed]
- Grimm, M.O.W.; Mett, J.; Stahlmann, C.P.; Haupenthal, V.J.; Blümel, T.; Stötzel, H.; Grimm, H.S.; Hartmann, T. Eicosapentaenoic acid and docosahexaenoic acid increase the degradation of amyloid-β by affecting insulin-degrading enzyme. Biochem. Cell Biol. 2016, 94, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Teng, E.; Taylor, K.; Bilousova, T.; Weiland, D.; Pham, T.; Zuo, X.; Yang, F.; Chen, P.P.; Glabe, C.G.; Takacs, A.; et al. Dietary DHA supplementation in an APP/PS1 transgenic rat model of AD reduces behavioral and Aβ pathology and modulates Aβ oligomerisation. Neurobiol. Dis. 2015, 82, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Liu, S.; Shao, Q.; Ma, D.; Yang, Z.; Zhou, R. Mechanism by which DHA inhibits the aggregation of KLVFFA peptides: A molecular dynamics study. J. Chem. Phys. 2018, 148, 115102. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Shahdat, H.M.; Katakura, M.; Tanabe, Y.; Gamoh, S.; Miwa, K.; Shimada, T.; Shido, O. Effects of docosahexaenoic acid on in vitro amyloid beta peptide 25–35 fibrillation. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2009, 1791, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Hossain, S.; Al Mamun, A.; Matsuzaki, K.; Arai, H. Docosahexaenoic acid: One molecule diverse functions. Crit. Rev. Biotechnol. 2017, 37, 579–597. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.L.; Teter, B.; Ubeda, O.J.; Morihara, T.; Dhoot, D.; Nyby, M.D.; Tuck, M.L.; Frautschy, S.A.; Cole, G.M. Omega-3 fatty acid docosahexaenoic acid increases SorLA/LR11, a sorting protein with reduced expression in sporadic Alzheimer’s disease (AD): Relevance to AD prevention. J. Neurosci. 2007, 27, 14299–14307. [Google Scholar] [CrossRef] [PubMed]
- Puskás, L.G.; Kitajka, K.; Nyakas, C.; Barcelo-Coblijn, G.; Farkas, T. Short-term administration of omega 3 fatty acids from fish oil results in increased transthyretin transcription in old rat hippocampus. Proc. Natl. Acad. Sci. USA 2003, 100, 1580–1585. [Google Scholar] [CrossRef] [PubMed]
- Green, K.N.; Martinez-Coria, H.; Khashwji, H.; Hall, E.B.; Yurko-Mauro, K.A.; Ellis, L.; LaFerla, F.M. Dietary docosahexaenoic acid and docosapentaenoic acid ameliorate amyloid-β and tau pathology via a mechanism involving presenilin 1 levels. J. Neurosci. 2007, 27, 4385–4395. [Google Scholar] [CrossRef] [PubMed]
- Grimm, M.O.W.; Kuchenbecker, J.; Grosgen, S.; Burg, V.K.; Hundsdorfer, B.; Rothhaar, T.L.; Friess, P.; de Wilde, M.C.; Broersen, L.M.; Penke, B.; et al. Docosahexaenoic acid reduces amyloid β production via multiple pleiotropic mechanisms. J. Biol. Chem. 2011, 286, 14028–14039. [Google Scholar] [CrossRef] [PubMed]
- Che, H.; Zhou, M.; Zhang, T.; Zhang, L.; Ding, L.; Yanagita, T.; Xu, J.; Xue, C.; Wang, Y. Comparative study of the effects of phosphatidylcholine rich in DHA and EPA on Alzheimer’s disease and the possible mechanisms in CHO-APP/PS1 cells and SAMP8 mice. Food Funct. 2018, 9, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Sastre, M.; Dewachter, I.; Rossner, S.; Bogdanovic, N.; Rosen, E.; Borghgraef, P.; Evert, B.O.; Dumitrescu-Ozimek, L.; Thal, D.R.; Landreth, G.; et al. Nonsteroidal anti-inflammatory drugs repress β-secretase gene promoter activity by the activation of PPARγ. Proc. Natl. Acad. Sci. USA 2006, 103, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Sastre, M.; Dewachter, I.; Landreth, G.E.; Willson, T.M.; Klockgether, T.; Van Leuven, F.; Heneka, M.T. Nonsteroidal Anti-Inflammatory Drugs and Peroxisome Proliferator-Activated Receptor-γ Agonists Modulate Immunostimulated Processing of Amyloid Precursor Protein through Regulation of β-Secretase. J. Neurosci. 2003, 23, 9796–9804. [Google Scholar] [CrossRef] [PubMed]
- Stillwell, W.; Shaikh, S.R.; Zerouga, M.; Siddiqui, R.; Wassall, S.R. Docosahexaenoic acid affects cell signaling by altering lipid rafts. Reprod. Nutr. Dev. 2005, 45, 559–579. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.; Price, S.L.; Fiol-Deroque, M.A.; Marcilla-Etxenike, A.; Ahyayauch, H.; Barceló-Coblijn, G.; Terés, S.; Katsouri, L.; Ordinas, M.; López, D.J.; et al. Membrane lipid modifications and therapeutic effects mediated by hydroxydocosahexaenoic acid on Alzheimer’s disease. Biochim. Biophys. Acta (BBA)—Biomembr. 2014, 1838, 1680–1692. [Google Scholar] [CrossRef] [PubMed]
- Obregon, D.; Hou, H.; Deng, J.; Giunta, B.; Tian, J.; Darlington, D.; Shahaduzzaman, M.; Zhu, Y.; Mori, T.; Mattson, M.P.; et al. Soluble amyloid precursor protein-α modulates β-secretase activity and amyloid-β 2 generation. Nat. Commun. 2012, 3, 777. [Google Scholar] [CrossRef] [PubMed]
- Dyall, S.C. Amyloid-beta peptide, oxidative stress and inflammation in Alzheimer’s disease: Potential neuroprotective effects of omega-3 polyunsaturated fatty acids. Int. J. Alzheimer’s Dis. 2010, 2010, 274128. [Google Scholar] [CrossRef][Green Version]
- Zhao, Y.; Calon, F.; Julien, C.; Winkler, J.W.; Petasis, N.A.; Lukiw, W.J.; Bazan, N.G. Docosahexaenoic acid-derived neuroprotectin D1 induces neuronal survival via secretase- and PPARγ-mediated mechanisms in Alzheimer’s disease models. PLoS ONE 2011, 6, e15816. [Google Scholar] [CrossRef] [PubMed]
- Bazan, N.G.; Molina, M.F.; Gordon, W.C. Docosahexaenoic acid signalolipidomics in nutrition: Significance in aging, neuroinflammation, macular degeneration, Alzheimer’s, and other neurodegenerative diseases. Annu. Rev. Nutr. 2011, 31, 321–351. [Google Scholar] [CrossRef] [PubMed]
- Stark, D.T.; Bazan, N.G. Neuroprotectin D1 induces neuronal survival and downregulation of amyloidogenic processing in Alzheimer’s disease cellular models. Mol. Neurobiol. 2011, 43, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Lukiw, W.J.; Cui, J.G.; Marcheselli, V.L.; Bodker, M.; Botkjaer, A.; Gotlinger, K.; Serhan, C.N.; Bazan, N.G. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J. Clin. Investig. 2005, 115, 2774–2783. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.; Wang, X.; Wang, S.; Wei, Y.; Feng, J.; Zhu, M. Maresin 1 Improves Cognitive Decline and Ameliorates Inflammation in a Mouse Model of Alzheimer’s Disease. Front. Cell. Neurosci. 2019, 13, 466. [Google Scholar] [CrossRef] [PubMed]
- Frautschy, S.A.; Cole, G.M. Why pleiotropic interventions are needed for alzheimer’s disease. Mol. Neurobiol. 2010, 41, 392–409. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, P.; Hu, X.; Zhang, F.; Chen, J.; Gao, Y. Omega-3 polyunsaturated fatty acids in the brain: Metabolism and neuroprotection. Front. Biosci. 2011, 16, 2653–2670. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.L.; Yang, F.; Rosario, E.R.; Ubeda, O.J.; Beech, W.; Gant, D.J.; Chen, P.P.; Hudspeth, B.; Chen, C.; Zhao, Y.; et al. β-Amyloid oligomers induce phosphorylation of tau and inactivation of insulin receptor substrate via c-Jun N-terminal kinase signaling: Suppression by omega-3 fatty acids and curcumin. J. Neurosci. 2009, 29, 9078–9089. [Google Scholar] [CrossRef] [PubMed]
- Vela, S.; Sainz, N.; Moreno-Aliaga, M.J.; Solas, M.; Ramirez, M.J. DHA Selectively Protects SAMP-8-Associated Cognitive Deficits Through Inhibition of JNK. Mol. Neurobiol. 2019, 56, 1618–1627. [Google Scholar] [CrossRef] [PubMed]
- Toral-Rios, D.; Pichardo-Rojas, P.S.; Alonso-Vanegas, M.; Campos-Peña, V. GSK3β and Tau Protein in Alzheimer’s Disease and Epilepsy. Front. Cell. Neurosci. 2020, 14, 19. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Li, G.; Chi, X.; Du, L.; Huang, K.; Zhang, H.; Huang, B. Preparation of 99mTc-EDTA-MN and its bioimaging in mouse. Chin. J. Lung Cancer 2015, 18, 422–426. [Google Scholar]
- Boada, M.; Ramos-Fernández, E.; Guivernau, B.; Muñoz, F.J.; Costa, M.; Ortiz, A.M.; Jorquera, J.; Núñez, L.; Torres, M.; Páez, A. Treatment of Alzheimer disease using combination therapy with plasma exchange and haemapheresis with albumin and intravenous immunoglobulin: Rationale and treatment approach of the AMBAR (Alzheimer Management By Albumin Replacement) study. Neurología 2016, 31, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Panza, F.; Solfrizzi, V.; Seripa, D.; Imbimbo, B.P.; Lozupone, M.; Santamato, A.; Zecca, C.; Barulli, M.R.; Bellomo, A.; Pilotto, A.; et al. Tau-Centric Targets and Drugs in Clinical Development for the Treatment of Alzheimer’s Disease. BioMed Res. Int. 2016, 2016, 3245935. [Google Scholar] [CrossRef] [PubMed]
- Upton, N.; Chuang, T.T.; Hunter, A.J.; Virley, D.J. 5-HT6 Receptor Antagonists as Novel Cognitive Enhancing Agents for Alzheimer’s Disease. Neurotherapeutics 2008, 5, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; López-Arrieta, J.; Lipschitz, S.; Triantafyllos, D.; Spiru, L.; Moroz, S.; Venger, O.; Vermersch, P.; Moussy, A.; Mansfield, C.D.; et al. Masitinib for mild-to-moderate Alzheimer’s disease: Results from a randomised, placebo-controlled, phase 3, clinical trial. Alzheimer’s Res. Ther. 2023, 15, 39. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, N.Z.; Hasan, M.K.; Mahmud, Z.; Hossain, M.S.; Rahman, A. Prevention of Alzheimer’s disease through diet: An exploratory review. Metab. Open 2023, 20, 100257. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Cesari, F.; Abbate, R.; Gensini, G.F.; Casini, A. Adherence to Mediterranean diet and health status: Meta-analysis. BMJ 2008, 337, a1344. [Google Scholar] [CrossRef] [PubMed]
- Yusufov, M.; Weyandt, L.L.; Piryatinsky, I. Alzheimer’s disease and diet: A systematic review. Int. J. Neurosci. 2017, 127, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Tangney, C.C. DASH and Mediterranean-Type Dietary Patterns to Maintain Cognitive Health. Curr. Nutr. Rep. 2014, 3, 51–61. [Google Scholar] [CrossRef] [PubMed]
- van de Rest, O.; Berendsen, A.A.M.; Haveman-Nies, A.; de Groot, L.C. Dietary patterns, cognitive decline, and dementia: A systematic review. Adv. Nutr. 2015, 6, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Passeri, E.; Elkhoury, K.; Morsink, M.; Broersen, K.; Linder, M.; Tamayol, A.; Malaplate, C.; Yen, F.T.; Arab-Tehrany, E. Alzheimer’s Disease: Treatment Strategies and Their Limitations. Int. J. Mol. Sci. 2022, 23, 13954. [Google Scholar] [CrossRef] [PubMed]
- Bandiwadekar, A.; Jose, J.; Khayatkashani, M.; Habtemariam, S.; Khayat Kashani, H.R.; Nabavi, S.M. Emerging Novel Approaches for the Enhanced Delivery of Natural Products for the Management of Neurodegenerative Diseases. J. Mol. Neurosci. 2022, 72, 653–676. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Xiao, R.; Cai, C.; Xu, Z.; Wang, S.; Pan, L.; Yuan, L. Diet, lifestyle and cognitive function in old Chinese adults. Arch. Gerontol. Geriatr. 2016, 63, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.W.; Lim, E.; Burm, S.Y.; Lee, H.; Bae, J.B.; Han, J.W.; Kim, K.W. The influence of n-3 polyunsaturated fatty acids on cognitive function in individuals without dementia: A systematic review and dose–response meta-analysis. BMC Med. 2024, 22, 109. [Google Scholar] [CrossRef] [PubMed]
- Staubo, S.C.; Aakre, J.A.; Vemuri, P.; Syrjanen, J.A.; Mielke, M.M.; Geda, Y.E.; Kremers, W.K.; Machulda, M.M.; Knopman, D.S.; Petersen, R.C.; et al. Mediterranean diet, micronutrients and macronutrients, and MRI measures of cortical thickness. Alzheimer’s Dement. 2017, 13, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, E.J.; Bongard, V.; Beiser, A.S.; Lamon-Fava, S.; Robins, S.J.; Au, R.; Tucker, K.L.; Kyle, D.J.; Wilson, P.W.; Wolf, P.A. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and alzheimer disease: The framingham heart study. Arch. Neurol. 2006, 63, 1545–1550. [Google Scholar] [CrossRef] [PubMed]
- Beydoun, M.A.; Kaufman, J.S.; Satia, J.A.; Rosamond, W.; Folsom, A.R. Plasma n-3 fatty acids and the risk of cognitive decline in older adults: The atherosclerosis risk in communities study. Am. J. Clin. Nutr. 2007, 85, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Baierle, M.; Vencato, P.H.; Oldenburg, L.; Bordignon, S.; Zibetti, M.; Trentini, C.M.; Duarte, M.M.M.F.; Veit, J.C.; Somacal, S.; Emanuelli, T.; et al. Fatty acid status and its relationship to cognitive decline and homocysteine levels in the elderly. Nutrients 2014, 6, 3624–3640. [Google Scholar] [CrossRef] [PubMed]
- Mosconi, L.; Murray, J.; Davies, M.; Williams, S.; Pirraglia, E.; Spector, N.; Tsui, W.H.; Li, Y.; Butler, T.; Osorio, R.S.; et al. Nutrient intake and brain biomarkers of Alzheimer’s disease in at-risk cognitively normal individuals: A cross-sectional neuroimaging pilot study. BMJ Open 2014, 4, e004850. [Google Scholar] [CrossRef] [PubMed]
- Yassine, H.N.; Feng, Q.; Azizkhanian, I.; Rawat, V.; Castor, K.; Fonteh, A.N.; Harrington, M.G.; Zheng, L.; Reed, B.R.; DeCarli, C.; et al. Association of serum docosahexaenoic acid with cerebral amyloidosis. JAMA Neurol. 2016, 73, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Loong, S.; Barnes, S.; Gatto, N.M.; Chowdhury, S.; Lee, G.J. Omega-3 Fatty Acids, Cognition, and Brain Volume in Older Adults. Brain Sci. 2023, 13, 1278. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.Z.; Li, L.; Dong, C.W.; Tan, C.C.; Xu, W. The Relationship of Omega-3 Fatty Acids with Dementia and Cognitive Decline: Evidence from Prospective Cohort Studies of Supplementation, Dietary Intake, and Blood Markers. Am. J. Clin. Nutr. 2023, 117, 1096–1109. [Google Scholar] [CrossRef] [PubMed]
- Sala-Vila, A.; Tintle, N.; Westra, J.; Harris, W.S. Plasma Omega-3 Fatty Acids and Risk for Incident Dementia in the UK Biobank Study: A Closer Look. Nutrients 2023, 15, 4896. [Google Scholar] [CrossRef] [PubMed]
- Tomata, Y.; Larsson, S.C.; Hägg, S. Polyunsaturated fatty acids and risk of Alzheimer’s disease: A Mendelian randomization study. Eur. J. Nutr. 2020, 59, 1763–1766. [Google Scholar] [CrossRef] [PubMed]
- Andrieu, S.; Guyonnet, S.; Coley, N.; Cantet, C.; Bonnefoy, M.; Bordes, S.; Bories, L.; Cufi, M.-N.; Dantoine, T.; Dartigues, J.-F.; et al. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multi-domain intervention on cognitive function in elderly adults with memory complaints (MAPT): A randomised, placebo-controlled trial. Lancet Neurol. 2017, 16, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Chhetri, J.K.; De Souto Barreto, P.; Cantet, C.; Pothier, K.; Cesari, M.; Andrieu, S.; Coley, N.; Vellas, B. Effects of a 3-Year Multi-Domain Intervention with or without Omega-3 Supplementation on Cognitive Functions in Older Subjects with Increased CAIDE Dementia Scores. J. Alzheimer’s Dis. 2018, 64, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Hooper, C.; de Souto Barreto, P.; Coley, N.; Cantet, C.; Cesari, M.; Andrieu, S.; Vellas, B.; MAPT/DSA Study Group. Cognitive changes with omega-3 polyunsaturated fatty acids in non-demented older adults with low omega-3 index. J. Nutr. Health Aging 2017, 21, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Kato, S.; Tanabe, Y.; Katakura, M.; Al Mamun, A.; Ohno, M.; Hossain, S.; Onoda, K.; Yamaguchi, S.; Shido, O. Beneficial effects of dietary docosahexaenoic acid intervention on cognitive function and mental health of the oldest elderly in Japanese care facilities and nursing homes. Geriatr. Gerontol. Int. 2017, 17, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Han, H.; Ge, X.; Liu, L.; Wang, T.; Yu, H. Effect of n-3 long-chain polyunsaturated fatty acids on mild cognitive impairment: A meta-analysis of randomized clinical trials. Eur. J. Clin. Nutr. 2020, 74, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, J.; Qiu, J.; Li, Y.; Wang, J.; Jiao, J. Intakes of fish and polyunsaturated fatty acids and mild-to-severe cognitive impairment risks: A dose-response meta-analysis of 21 cohort studies. Am. J. Clin. Nutr. 2016, 103, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.-Z.; Chen, M.-Q.; Zhang, Z.-W.; Wu, T.-Y.; Zhao, W.-H. Dietary fatty acids and risk for Alzheimer’s disease, dementia, and mild cognitive impairment: A prospective cohort meta-analysis. Nutrition 2021, 90, 111355. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Ding, Y.; Wu, F.; Li, R.; Hou, J.; Mao, P. Omega-3 fatty acids intake and risks of dementia and Alzheimer’s disease: A meta-analysis. Neurosci. Biobehav. Rev. 2015, 48, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Burckhardt, M.; Herke, M.; Wustmann, T.; Watzke, S.; Langer, G.; Fink, A. Omega 3 fatty acids for the treatment of dementia. Cochrane Database Syst. Rev. 2016, 2016, CD009002. [Google Scholar] [CrossRef] [PubMed]
- Calderon Martinez, E.; Zachariah Saji, S.; Salazar Ore, J.V.; Borges-Sosa, O.A.; Srinivas, S.; Mareddy, N.S.R.; Manzoor, T.; Di Vanna, M.; Al Shanableh, Y.; Taneja, R.; et al. The effects of omega-3, DHA, EPA, Souvenaid® in Alzheimer’s disease: A systematic review and meta-analysis. Neuropsychopharmacol. Rep. 2024, 44, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Shinto, L.H.; Murchison, C.F.; Silbert, L.C.; Dodge, H.H.; Lahna, D.; Rooney, W.; Kaye, J.; Quinn, J.F.; Bowman, G.L. ω-3 PUFA for Secondary Prevention of White Matter Lesions and Neuronal Integrity Breakdown in Older Adults: A Randomised Clinical Trial. JAMA Netw. Open 2024, 7, e2426872. [Google Scholar] [CrossRef] [PubMed]
- Tabue-Teguo, M.; Barreto de Souza, P.; Cantet, C.; Andrieu, S.; Simo, N.; Fougère, B.; Dartigues, J.F.; Vellas, B.; Fougère, B. Effect of Multi-domain Intervention, Omega-3 Polyunsaturated Fatty Acids Supplementation or their Combinaison on Cognitive Function in Non-Demented Older Adults According to Frail Status: Results from the MAPT Study. J. Nutr. Health Aging 2018, 22, 923–927. [Google Scholar] [CrossRef] [PubMed]
- O’Donovan, F.; Carney, S.; Kennedy, J.; Hayes, H.; Pender, N.; Boland, F.; Stanton, A. Associations and effects of omega-3 polyunsaturated fatty acids on cognitive function and mood in healthy adults: A protocol for a systematic review of observational and interventional studies. BMJ Open 2019, 9, e027167. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.L. Maximizing the benefit and managing the risk of anti-amyloid monoclonal antibody therapy for Alzheimer’s disease: Strategies and research directions. Neurotherapeutics 2025, 22, e00570. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).