Ap4A in Cancer: A Multifaceted Regulator and Emerging Therapeutic Target
Abstract
1. Introduction
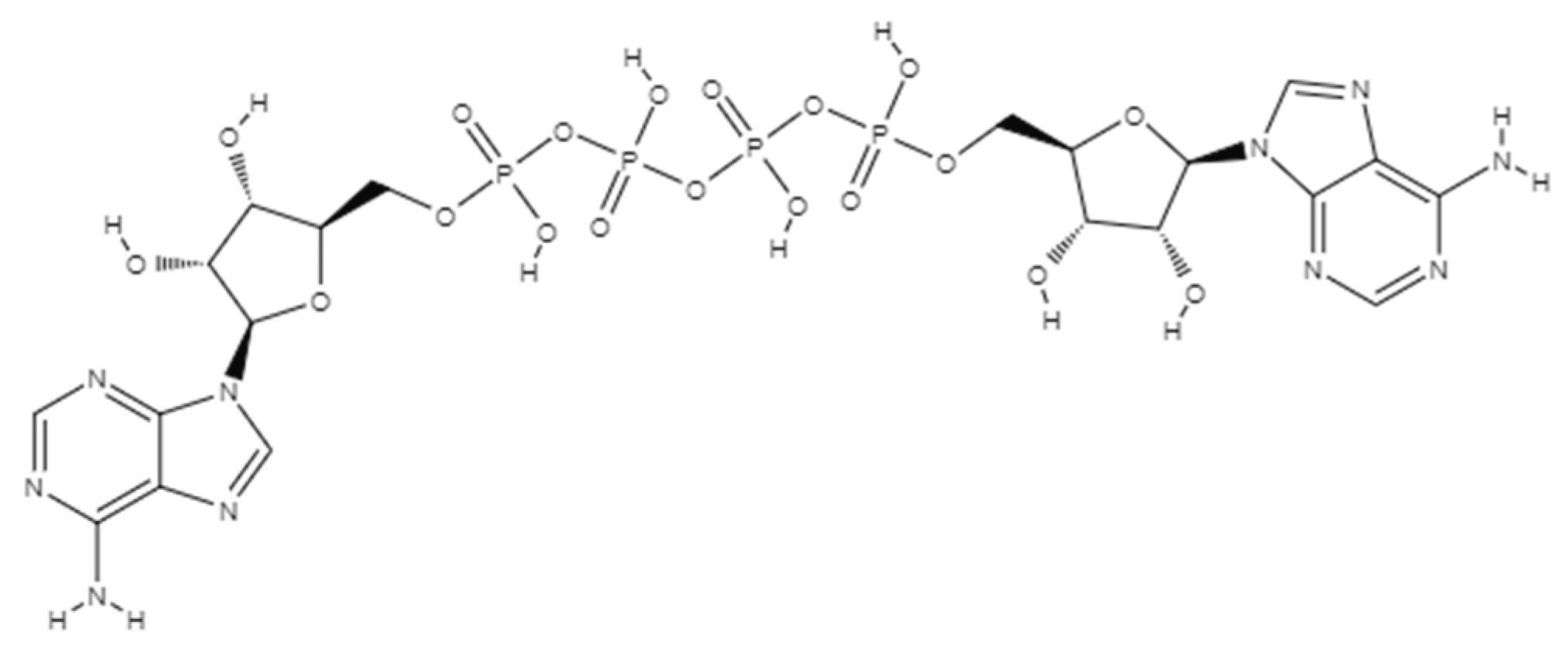
2. AP4A Structural Conformation
3. Regulation of Intracellular Ap4A Levels
3.1. Biosynthesis of Ap4A
3.2. Degradation of Ap4A
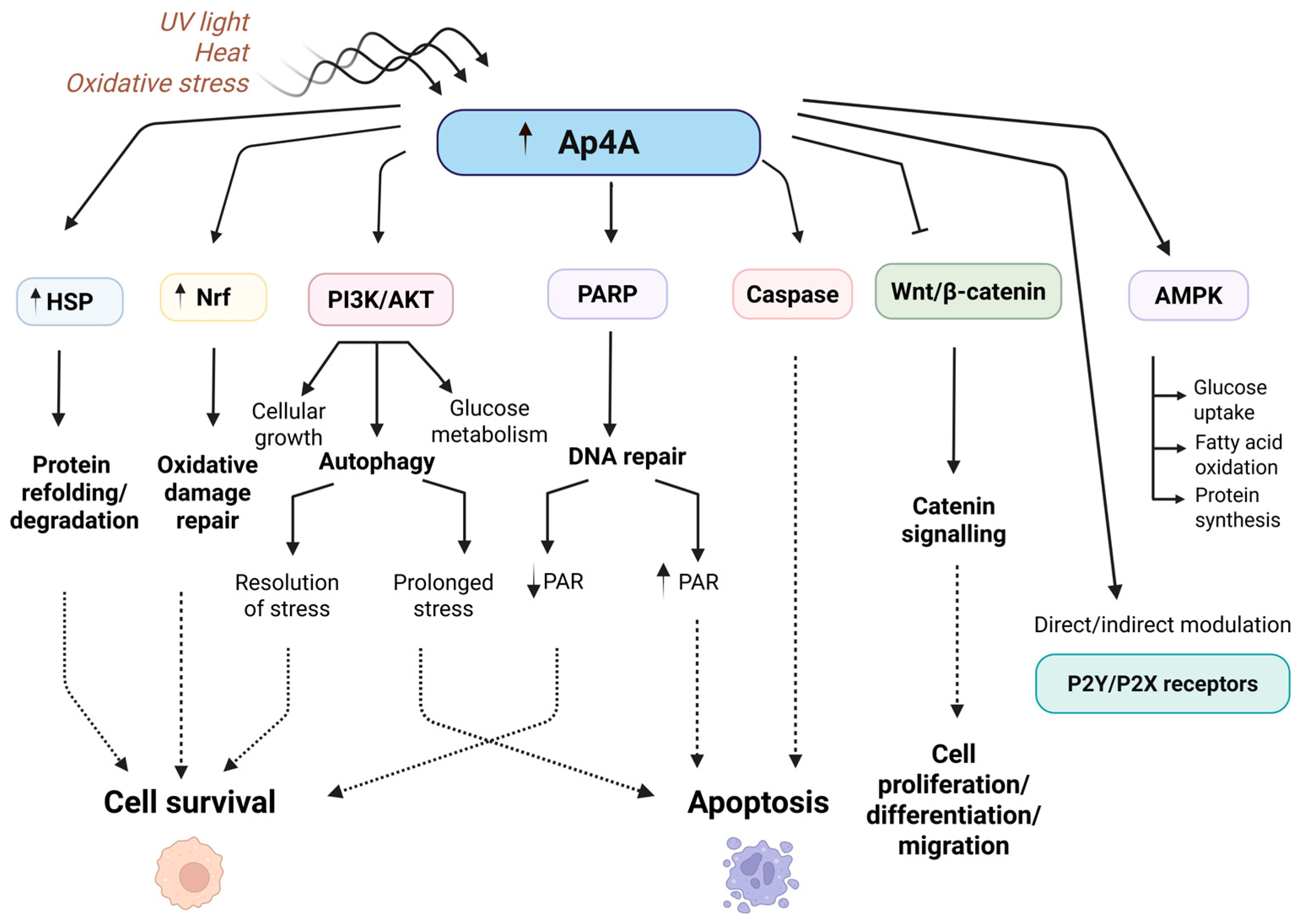
4. Role of Ap4A: Functions in Normal and Cancer Cells
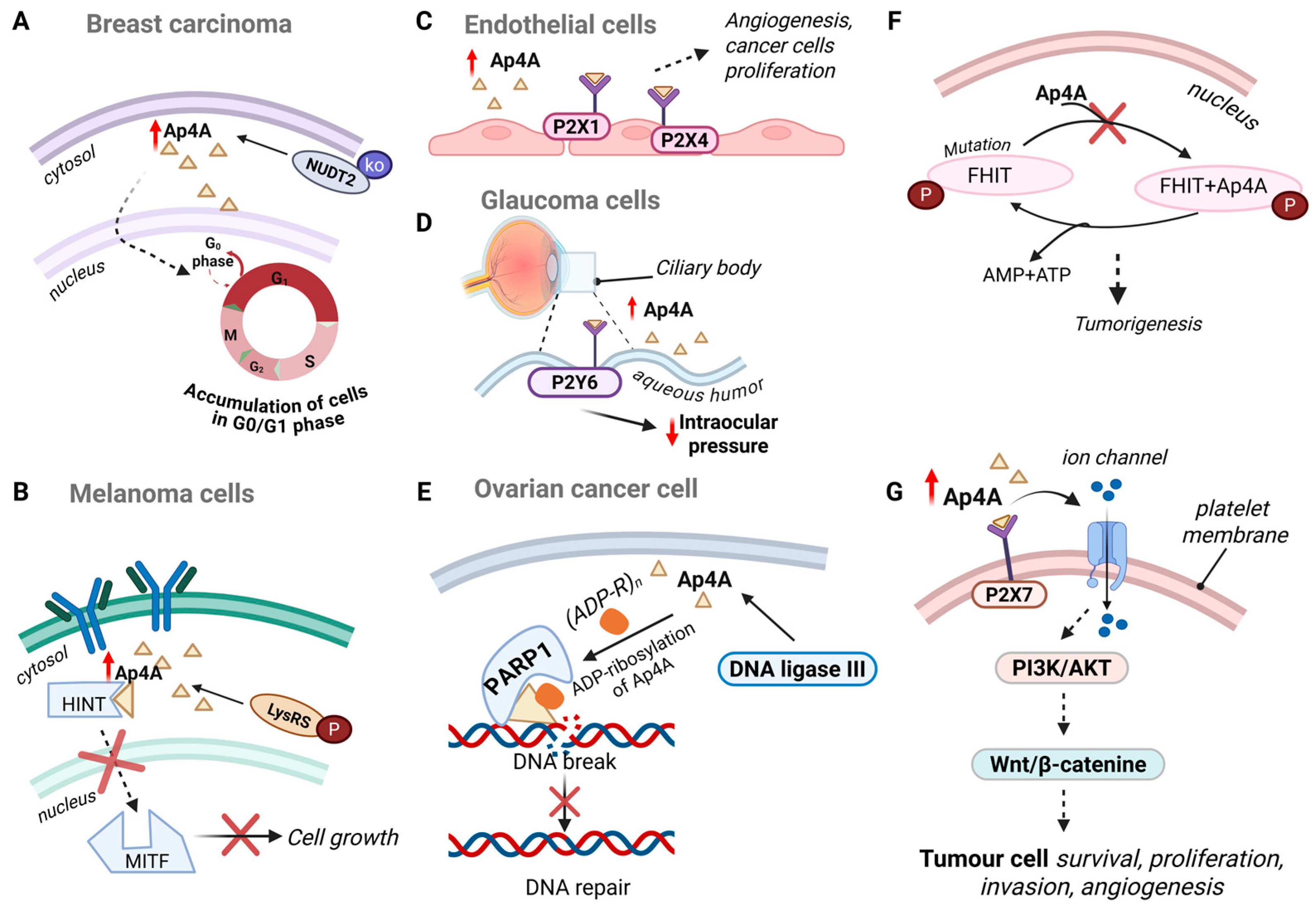
4.1. Apoptosis Evasion
4.2. Metabolic Adaption
4.3. Stress Response Modulation
4.4. Cell Growth and Proliferation
4.5. Interaction with Purinergic Receptors
4.5.1. P2X Receptors
4.5.2. P2X1 Receptors
4.5.3. P2X4 Receptors
4.5.4. P2X7 Receptors
4.5.5. P2X3 and P2X5 Receptors
4.5.6. P2Y Receptors
4.6. Interaction with Other Proteins
5. AP4A-Role in Gene Expression Regulation
5.1. Transcriptional Control
5.2. AP4A-Mediated Post-Transcriptional Gene Expression Regulation
6. The Therapeutic Potential of Ap4A
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AARS | Aminoacyl-tRNA synthetase |
| AMPK | AMP-activated protein kinase |
| Ap4A | Diadenosine tetraphosphate |
| ApaH | Ap4A hydrolase |
| CDKs | Cyclin-dependent kinases |
| CML | Chronic myelogenous leukemia |
| DC | Dendritic cells |
| FHIT | Fragile Histidine Triad |
| GLUT-4 | Glucose Transporter Type 4 |
| HCC | Hepatocellular carcinoma |
| HINT1 | Histidine Triad Nucleotide Binding Protein 1 |
| HSPs | Heat Shock Proteins |
| IOP | Intraocular pressure |
| LysRS | Lysyl-tRNA synthetase |
| MITF | Microphthalmia-associated Transcription Factor |
| NCC | Non-canonical cap |
| NPnN | Nucleoside Polyphosphates |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| Nudix hydrolase 2 | Nucleoside Diphosphate Linked Moiety X)-Type Motif 2 |
| PARP1 | Poly (ADP-ribose) polymerase |
| TME | Tumor microenvironment |
| UBA1 | Ubiquitin-like Modifier-Activating Enzyme 1 |
References
- Srivatsan, A.; Wang, J.D. Control of Bacterial Transcription, Translation and Replication by (p) PpGpp. Curr. Opin. Microbiol. 2008, 11, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Farr, S.B.; Arnosti, D.N.; Chamberlin, M.J.; Amest, B.N. An ApaH Mutation Causes AppppA to Accumulate and Affects Motility and Catabolite Repression in Escherichia coli. Proc. Natl. Acad. Sci. USA 1989, 86, 5010–5014. [Google Scholar] [CrossRef] [PubMed]
- Marriott, A.S.; Copeland, N.A.; Cunningham, R.; Wilkinson, M.C.; McLennan, A.G.; Jones, N.J. Diadenosine 5’, 5’’’-P1, P4-Tetraphosphate (Ap4A) Is Synthesized in Response to DNA Damage and Inhibits the Initiation of DNA Replication. DNA Repair 2015, 33, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Rapaport, E.; Zamecnikt, P.C.; Barilt, E.F. HeLa Cell DNA Polymerase α Is Tightly Associated with Tryptophanyl-TRNA Synthetase and Diadenosine 5’, 5’’’-PlP4-Tetraphosphate Binding Activities (DNA Replication/Protein Synthesis/Growth Regulation/L-Tryptophan/DNA Nucleotidyltransferase). Proc. Natl. Acad. Sci. USA 1981, 78, 838–842. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Liu, Z.; Liang, Y.; Luo, F.; Zhang, J.; Tian, C.; Motzik, A.; Zheng, M.; Kang, J.; Zhong, G.; et al. Second Messenger Ap4A Polymerizes Target Protein HINT1 to Transduce Signals in FcεRI-Activated Mast Cells. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hoyle, C.H.V.; Hilderman, R.H.; Pintor, J.J.; Schlüter, H.; King, B.F. Diadenosine Polyphosphates as Extracellular Signal Molecules. Drug Dev. Res. 2001, 52, 260–273. [Google Scholar] [CrossRef]
- Pojoga, L.H.; Haghiac, M.; Hilderman, R.H. Inhibition by Adenine Dinucleotides of ATP-Induced Prostacyclin Release by Bovine Aortic Endothelial Cells. Biochem. Pharmacol. 2002, 64, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Reigada, D.; Navarro-Ruiz, R.M.; Caballero-López, M.J.; Del Águila, Á.; Muñoz-Galdeano, T.; Maza, R.M.; Nieto-Díaz, M. Diadenosine Tetraphosphate (Ap4A) Inhibits ATP-Induced Excitotoxicity: A Neuroprotective Strategy for Traumatic Spinal Cord Injury Treatment. Purinergic Signal 2017, 13, 75–87. [Google Scholar] [CrossRef] [PubMed]
- McLennan, A.G. The Nudix Hydrolase Superfamily. Cell. Mol. Life Sci. 2006, 63, 123–143. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, F.; McLennan, A.G.; Urbaniak, M.D.; Jones, N.J.; Copeland, N.A. Re-Evaluation of Diadenosine Tetraphosphate (Ap4A) From a Stress Metabolite to Bona Fide Secondary Messenger. Front. Mol. Biosci. 2020, 7, 606807. [Google Scholar] [CrossRef] [PubMed]
- Zamecnik, P.C.; Stephenson, M.L.; Janeway, C.M.; Randerath, K. Enzymatic synthesis of diadenosine tetraphosphate and diadenosine triphosphate with a purified lysyl-sRNA synthetase. Biochem. Biophys. Res. Commun. 1966, 24, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Finamore, F.J.; Warner, A.H. The Occurrence of P’, P4-Diguanosine 5’-Tetraphosphate in Brine Shrimp Eggs. J. Biol. Chem. 1963, 238, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Alle, C.; Tinoco, I. Calculation of the Optical Rotatory Dispersion of Dinucleoside Phosphates. J. Mol. Biol. 1967, 23, 601–614. [Google Scholar] [CrossRef] [PubMed]
- Warshaw, M.M.; Tinoco, I. Absorption and Optical Rotatory Dispersion of Six Dinucleoside Phosphates. J. Mol. Biol. 1965, 13, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Stern, N.; Major, D.T.; Gottlieb, H.E.; Weizman, D.; Fischer, B. What Is the Conformation of Physiologically-Active Dinucleoside Polyphosphates in Solution? Conformational Analysis of Free Dinucleoside Polyphosphates by NMR and Molecular Dynamics Simulations. Org. Biomol. Chem. 2010, 8, 4637–4652. [Google Scholar] [CrossRef] [PubMed]
- Carracedo, G.; Crooke, A.; Guzman-Aranguez, A.; Pérez de Lara, M.J.; Martin-Gil, A.; Pintor, J. The Role of Dinucleoside Polyphosphates on the Ocular Surface and Other Eye Structures. Prog. Retin. Eye Res. 2016, 55, 182–205. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-N.; Nechushtan, H.; Figov, N.; Razin, E. The Function of Lysyl-TRNA Synthetase and Ap4A as Signaling Regulators of MITF Activity in FcRI-Activated Mast Cells. Immunity 2004, 20, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Sillero, M.A.G.; Guranowski, A.; Sillero, A. Synthesis of Dinucleoside Polyphosphates Catalyzed by Firefly Luciferase. Eur. J. Biochem. 1991, 202, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Zegarra, V.; Mais, C.N.; Freitag, J.; Bange, G. The Mysterious Diadenosine Tetraphosphate (AP4A). Micro. Life 2023, 4, uqad016. [Google Scholar] [CrossRef] [PubMed]
- Brevet, A.; Plateau, P.; Best-Belpommey, M.; Blanquets, S. Variation of Ap4A and Other Dinucleoside Drosophila Cells* Polyphosphates in Stressed. Biol. Chemistry 1985, 260, 15566–15570. [Google Scholar] [CrossRef]
- Avila, D.M.; Robinson, A.K.; Kaushal, V.A.R.S.H.A.; Barnes, L.D. A Paradoxical Increase of a Metabolite upon Increased Expression of Its Catabolic Enzyme: The Case of Diadenosine Tetraphosphate (Ap4A) and Ap4A Phosphorylase I in Saccharomyces Cerevisiae. J. Bacteriol. 1991, 173, 7875–7880. [Google Scholar] [CrossRef] [PubMed]
- Cervoni, M.; Sposato, D.; Ferri, G.; Bähre, H.; Leoni, L.; Rampioni, G.; Visca, P.; Recchiuti, A.; Imperi, F. The Diadenosine Tetraphosphate Hydrolase ApaH Contributes to Pseudomonas Aeruginosa Pathogenicity. PLoS Pathog. 2024, 20, e1012486. [Google Scholar] [CrossRef] [PubMed]
- Minazzato, G.; Gasparrini, M.; Amici, A.; Cianci, M.; Mazzola, F.; Orsomando, G.; Sorci, L.; Raffaelli, N. Functional Characterization of COG1713 (YqeK) as a Novel Diadenosine Tetraphosphate Hydrolase Family. J. Bacteriol. 2020, 202, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Gasmi, L.; Mclennan, A.G.; Edwards, S.W. Neutrophil Apoptosis Is Delayed by the Diadenosine Polyphosphates, Ap 5 A and Ap 6 A: Synergism with Granulocyte-Macrophage Colony-Stimulating Factor. Br. J. Haematol. 1996, 95, 637–639. [Google Scholar] [CrossRef] [PubMed]
- Harvey, B.K.; Chou, J.; Shen, H.; Hoffer, B.J.; Wang, Y. Diadenosine Tetraphosphate Reduces Toxicity Caused by High-Dose Methamphetamine Administration. Neurotoxicology 2009, 30, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shao, C.; Lu, W.; Yan, C.; Yao, Q.; Zhu, M.; Chen, P.; Gu, P.; Fu, Y.; Fan, X. Adenosine Triphosphate-Induced Rabbit Corneal Endothelial Cell Proliferation in Vitro via the P2Y2-PI3K/Akt Signaling Axis. Cells Tissues Organs 2014, 199, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Linder, B.; Kögel, D. Autophagy in Cancer Cell Death. Biology 2019, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. AMP-Activated Protein Kinase—An Energy Sensor That Regulates All Aspects of Cell Function. Genes Dev. 2011, 25, 1895–1908. [Google Scholar] [CrossRef] [PubMed]
- Manning, B.D.; Cantley, L.C. AKT/PKB Signaling: Navigating Downstream. Cell 2007, 129, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.N.; Mele, J.; Hayes, J.D.; Buffenstein, R. Nrf2, a Guardian of Healthspan and Gatekeeper of Species Longevity. Integr. Comp. Biology. 2010, 50, 829–843. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Purine and Pyrimidine Receptors. Cell. Mol. Life Sci. 2007, 64, 1471–1483. [Google Scholar] [CrossRef] [PubMed]
- Malumbres, M.; Barbacid, M. Cell Cycle, CDKs and Cancer: A Changing Paradigm. Nat. Rev. Cancer 2009, 9, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Sherr, C.J.; Roberts, J.M. CDK Inhibitors: Positive and Negative Regulators of G 1-Phase Progression. Genes Dev. 1999, 13, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Bartek, J.; Lukas, J. DNA Damage Checkpoints: From Initiation to Recovery or Adaptation. Curr. Opin Cell Biol. 2007, 19, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Neelsen, K.J.; Zanini, I.M.Y.; Herrador, R.; Lopes, M. Oncogenes Induce Genotoxic Stress by Mitotic Processing of Unusual Replication Intermediates. J. Cell Biol. 2013, 200, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Abu-Rahmah, R.; Nechushtan, H.; Hidmi, S.; Meirovitz, A.; Razin, E.; Peretz, T. The Functional Role of Nudt2 in Human Triple Negative Breast Cancer. Front. Oncol. 2024, 14, 1364663. [Google Scholar] [CrossRef] [PubMed]
- Mittag, S.; Wetzel, F.; Müller, S.Y.; Huber, O. The Rosetta Stone Hypothesis-Based Interaction of the Tumor Suppressor Proteins Nit1 and Fhit. Cells 2023, 12, 353. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Zou, J.; Peng, H.; Stolle, A.S.; Xie, R.; Zhang, H.; Peng, B.; Mekalanos, J.J.; Zheng, J. Alarmone Ap4A Is Elevated by Aminoglycoside Antibiotics and Enhances Their Bactericidal Activity. Proc. Natl. Acad. Sci. USA 2019, 116, 9578–9585. [Google Scholar] [CrossRef] [PubMed]
- Mclennan, A.G. Dinucleoside Polyphosphates-Friend or Foe? Pharmacol. Ther. 2000, 87, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Wildman, S.S.; Brown, S.G.; King, B.F.; Burnstock, G. Selectivity of Diadenosine Polyphosphates for Rat P2X Receptor Subunits. Eur. J. Pharmacol. 1999, 367, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Ledderose, S.; Rodler, S.; Eismann, L.; Ledderose, G.; Rudelius, M.; Junger, W.G.; Ledderose, C. P2X1 and P2X7 Receptor Overexpression Is a Negative Predictor of Survival in Muscle-Invasive Bladder Cancer. Cancers 2023, 15, 2321. [Google Scholar] [CrossRef] [PubMed]
- Mai, Y.; Guo, Z.; Yin, W.; Zhong, N.; Dicpinigaitis, P.V.; Chen, R. P2X Receptors: Potential Therapeutic Targets for Symptoms Associated with Lung Cancer—A Mini Review. Front. Oncol. 2021, 11, 691956. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Purinergic Signalling: Therapeutic Developments. Front. Pharmacol. 2017, 8, 661. [Google Scholar] [CrossRef] [PubMed]
- Di Virgilio, F.; Dal Ben, D.; Sarti, A.C.; Giuliani, A.L.; Falzoni, S. The P2X7 Receptor in Infection and Inflammation. Immunity 2017, 47, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J.; Pi, X.W.; Le, Y.G.; Li, T.Z. Role of P2X7 Receptor in the Progression and Clinicopathological Characteristics of Gastric Cancer. Sci. Rep. 2024, 14, 31673. [Google Scholar] [CrossRef] [PubMed]
- Aria, H.; Rezaei, M.; Nazem, S.; Daraei, A.; Nikfar, G.; Mansoori, B.; Bahmanyar, M.; Tavassoli, A.; Vakil, M.K.; Mansoori, Y. Purinergic Receptors Are a Key Bottleneck in Tumor Metabolic Reprogramming: The Prime Suspect in Cancer Therapeutic Resistance. Front. Immunol. 2022, 13, 947885. [Google Scholar] [CrossRef] [PubMed]
- Adinolfi, E.; Capece, M.; Amoroso, F.; De Marchi, E.; Franceschini, A. Emerging Roles of P2X Receptors in Cancer. Curr. Med. Chem. 2015, 22, 877–890. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Gartland, A. Targeting P2 Receptors—Current Progress in Treating Musculoskeletal Diseases. Curr. Opin. Pharmacol. 2014, 16, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Barańska, J.; Czajkowski, R.; Pomorski, P. P2Y1 Receptors—Properties and Functional Activities. In Advances in Experimental Medicine and Biology; Springer New York LLC: New York, NY, USA, 2017; Volume 1051, pp. 71–89. [Google Scholar]
- Vázquez-Cuevas, F.G.; Reyna-Jeldes, M.; Velázquez-Miranda, E.; Coddou, C. Transactivation of Receptor Tyrosine Kinases by Purinergic P2Y and Adenosine Receptors. Purinergic Signal 2023, 19, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhang, S.; Zhang, Y.; Zhou, Q.; Peng, S.; Zhang, T.; Yang, C.; Zhu, Z.; Zhang, F. The Inhibitory Effects of Extracellular ATP on the Growth of Nasopharyngeal Carcinoma Cells via P2Y2 Receptor and Osteopontin. J. Exp. Clin. Cancer Res. 2014, 33, 53. [Google Scholar] [CrossRef] [PubMed]
- Castany, M.; Jordi, I.; Catala, J.; Gual, A.; Morales, M.; Gasull, X.; Pintor, J. Glaucoma Patients Present Increased Levels of Diadenosine Tetraphosphate, Ap4A, in the Aqueous Humour. Exp. Eye Res. 2011, 92, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, B.; Martínez-Águila, A.; de Lara, M.J.P.; Pintor, J. Diadenosine Tetraphosphate as a Potential Therapeutic Nucleotide to Treat Glaucoma. Purinergic Signal 2017, 13, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Ming, L.G.; Hu, D.X.; Zuo, C.; Zhang, W.J. G Protein-Coupled P2Y12 Receptor Is Involved in the Progression of Neuropathic Pain. Biomed. Pharmacother. 2023, 162, 114713. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; Bhatt, D.L. Simultaneous Platelet P2Y12 and P2Y1 ADP Receptor Blockade: Are Two Better than One? Arter. Thromb Vasc. Biol. 2016, 36, 427–428. [Google Scholar] [CrossRef] [PubMed]
- Boison, D.; Yegutkin, G.G. Adenosine Metabolism: Emerging Concepts for Cancer Therapy. Cancer Cell 2019, 36, 582–596. [Google Scholar] [CrossRef] [PubMed]
- Paquola, A.; Mañé, N.; Giron, M.C.; Jimenez, M. Diadenosine Tetraphosphate Activates P2Y1 Receptors That Cause Smooth Muscle Relaxation in the Mouse Colon. Eur. J. Pharmacol 2019, 855, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Purinergic Signaling in the Cardiovascular System. Circ. Res. 2017, 120, 207–228. [Google Scholar] [CrossRef] [PubMed]
- Ruparel, S.B.; Akopian, A.N. Editorial: Mechanisms of Orofacial Pain. Front. Pain. Res. 2024, 5, 1496188. [Google Scholar] [CrossRef] [PubMed]
- Maynard, J.P.; Lee, J.-S.; Sohn, B.H.; Yu, X.; Lopez-Terrada, D.; Finegold, M.J.; Goss, J.A.; Thevananther, S. P2X3 Purinergic Receptor Overexpression Is Associated with Poor Recurrence-Free Survival in Hepatocellular Carcinoma Patients. Oncotarget 2015, 6, 41162–41179. [Google Scholar] [CrossRef] [PubMed]
- Maynard, J.P.; Lu, J.; Vidal, I.; Hicks, J.; Mummert, L.; Ali, T.; Kempski, R.; Carter, A.M.; Sosa, R.Y.; Peiffer, L.B.; et al. P2X4 Purinergic Receptors Offer a Therapeutic Target for Aggressive Prostate Cancer. J. Pathol. 2022, 256, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Overes, I.M.; De Rijke, B.; Van Horssen-Zoetbrood, A.; Fredrix, H.; De Graaf, A.O.; Jansen, J.H.; Van Krieken, J.H.J.M.; Raymakers, R.A.P.; Van Der Voort, R.; De Witte, T.M.; et al. Expression of P2X5 in Lymphoid Malignancies Results in LRH-1-Specific Cytotoxic T-Cell-Mediated Lysis. Br. J. Haematol. 2008, 141, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.-T.; Li, J.-Y.; Chai, J.-Y.; Hu, Y.-S.; Zhang, W.-J.; Zhang, Q. The Impact of the P2X7 Receptor on the Tumor Immune Microenvironment and Its Effects on Tumor Progression. Biochem. Biophys. Res. Commun. 2024, 707, 149513. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, L.; Guan, R.; Zhu, D.; Song, N.; Shen, L. Emodin Inhibits ATP-Induced Proliferation and Migration by Suppressing P2Y Receptors in Human Lung Adenocarcinoma Cells. Cell. Physiol. Biochem. 2017, 44, 1337–1351. [Google Scholar] [CrossRef] [PubMed]
- Le, H.T.T.; Rimpilainen, T.; Konda Mani, S.; Murugesan, A.; Yli-Harja, O.; Candeias, N.R.; Kandhavelu, M. Synthesis and Preclinical Validation of Novel P2Y1 Receptor Ligands as a Potent Anti-Prostate Cancer Agent. Sci. Rep. 2019, 9, 18938. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Xu, J.; Wen, G.; Jin, H.; Liu, X.; Yang, Y.; Ji, B.; Jiang, Y.; Song, P.; Dong, H.; et al. The P2Y2 Nucleotide Receptor Mediates the Proliferation and Migration of Human Hepatocellular Carcinoma Cells Induced by ATP. J. Biol. Chem. 2014, 289, 19137–19149. [Google Scholar] [CrossRef] [PubMed]
- Forti, K.M.; Woods, L.T.; Jasmer, K.J.; Camden, J.M.; Weisman, G.A. Tumoral P2Y2 Receptor Modulates Tumor Growth and Host Anti-Tumor Immune Responses in a Syngeneic Murine Model of Oral Cancer. Purinergic Signal 2024, 20, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Campos-Contreras, A.D.R.; Díaz-Muñoz, M.; Vázquez-Cuevas, F.G. Purinergic Signaling in the Hallmarks of Cancer. Cells 2020, 9, 1612. [Google Scholar] [CrossRef] [PubMed]
- Placet, M.; Arguin, G.; Molle, C.M.; Babeu, J.P.; Jones, C.; Carrier, J.C.; Robaye, B.; Geha, S.; Boudreau, F.; Gendron, F.P. The G Protein-Coupled P2Y6 Receptor Promotes Colorectal Cancer Tumorigenesis by Inhibiting Apoptosis. Biochim. Biophys. Acta. Mol. Basis Dis. 2018, 1864, 1539–1551. [Google Scholar] [CrossRef] [PubMed]
- Faria, P.C.L.; Resende, R.S.; Cardoso, A.M. Metastasis and Angiogenesis in Cervical Cancer: Key Aspects of Purinergic Signaling in Platelets and Possible Therapeutic Targets. Purinergic Signal 2024, 20, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Acedo, A.L.; Mezouar, S.; Mège, D.; Crescence, L.; Dubois, C.; Panicot-Dubois, L. P2RY12-Inhibitors Reduce Cancer-Associated Thrombosis and Tumor Growth in Pancreatic Cancers. Front. Oncol. 2021, 11, 704945. [Google Scholar] [CrossRef] [PubMed]
- La Shu, S.; Paruchuru, L.B.; Tay, N.Q.; Chua, Y.L.; Yun Foo, A.S.; Yang, C.M.; Liong, K.H.; Liang Koh, E.G.; Lee, A.; Nechushtan, H.; et al. Ap4A Regulates Directional Mobility and Antigen Presentation in Dendritic Cells. iScience 2019, 16, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Krüger, L.; Albrecht, C.J.; Schammann, H.K.; Stumpf, F.M.; Niedermeier, M.L.; Yuan, Y.; Stuber, K.; Wimmer, J.; Stengel, F.; Scheffner, M.; et al. Chemical Proteomic Profiling Reveals Protein Interactors of the Alarmones Diadenosine Triphosphate and Tetraphosphate. Nat. Commun. 2021, 12, 5808. [Google Scholar] [CrossRef] [PubMed]
- Loma, P.; Guzman-Aranguez, A.; Perez de Lara, M.J.; Pintor, J. Lactoferrin Levels in Tears Are Increased by the Topical Application of Diadenosine Tetraphosphate. Curr. Eye Res. 2016, 41, 1150–1152. [Google Scholar] [CrossRef] [PubMed]
- Frese, M.; Saumer, P.; Yuan, Y.; Herzog, D.; Höpfner, D.; Itzen, A.; Marx, A. The Alarmone Diadenosine Tetraphosphate as a Cosubstrate for Protein AMPylation. Angew. Chem. Int. Ed. 2023, 62, e202213279. [Google Scholar] [CrossRef] [PubMed]
- Verspohl, E.J.; Hohmeier, N.; Lempka, M. Diadenosine Tetraphosphate (Ap 4 A) Induces a Diabetogenic Situation: Its Impact on Blood Glucose, Plasma Insulin, Gluconeogenesis, Glucose Uptake and GLUT-4 Transporters. Die Pharm.-Int. J. Pharm. Sci. 2003, 58, 910–915. [Google Scholar]
- Motzik, A.; Amir, E.; Erlich, T.; Wang, J.; Kim, B.G.; Han, J.M.; Kim, J.H.; Nechushtan, H.; Guo, M.; Razin, E.; et al. Post-Translational Modification of HINT1 Mediates Activation of MITF Transcriptional Activity in Human Melanoma Cells. Oncogene 2017, 36, 4732–4738. [Google Scholar] [CrossRef] [PubMed]
- Ofir-Birin, Y.; Fang, P.; Bennett, S.P.; Zhang, H.M.; Wang, J.; Rachmin, I.; Shapiro, R.; Song, J.; Dagan, A.; Pozo, J.; et al. Structural Switch of Lysyl-TRNA Synthetase between Translation and Transcription. Mol. Cell 2013, 49, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Draganescu, A.; Hodawadekar, S.C.; Gee, K.R.; Brenner, C. Fhit-Nucleotide Specificity Probed with Novel Fluorescent and Fluorogenic Substrates. J. Biol. Chem. 2000, 275, 4555–4560. [Google Scholar] [CrossRef] [PubMed]
- Simón-Carrasco, L.; Pietrini, E.; López-Contreras, A.J. Integrated Analysis of FHIT Gene Alterations in Cancer. Cell Cycle 2024, 23, 92–113. [Google Scholar] [CrossRef] [PubMed]
- Cowling, V.H. Regulation of MRNA Cap Methylation. Biochem. J. 2010, 425, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Izaurralde, E.; Lewis, J.; Mcguigan, C.; Jankowska, M.; Darzynkiewicz, E.; Mattaj, W. A Nuclear Cap Binding Protein Complex Involved in Pre-MRNA Splicing. Cell 1994, 78, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Doamekpor, S.K.; Sharma, S.; Kiledjian, M.; Tong, L. Recent Insights into Noncanonical 5′ Capping and Decapping of RNA. J. Biol. Chem. 2022, 298, 102171. [Google Scholar] [CrossRef] [PubMed]
- František Potužník, J.; Nešuta, O.; Škríba, A.; Voleníková, B.; Mititelu, M.B.; Mancini, F.; Serianni, V.; Fernandez, H.; Spustová, K.; Trylčová, J.; et al. Diadenosine Tetraphosphate (Ap4A) Serves as a 5′ RNA Cap in Mammalian Cells. Angew. Chem. Int. Ed. 2024, 63, e202314951. [Google Scholar] [CrossRef] [PubMed]
- Batool, A.; Aashaq, S.; Andrabi, K.I. Eukaryotic Initiation Factor 4E (EIF4E): A Recap of the Cap-Binding Protein. J. Cell. Biochem. 2019, 120, 14201–14212. [Google Scholar] [CrossRef] [PubMed]
- Lewis’, J.D.; Izaurfl4lde2, E. The Role of the Cap Structure in RNA Processing and Nuclear Export. Eur. J. Biochem. 1997, 247, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Cahová, H.; Winz, M.L.; Höfer, K.; Nübel, G.; Jäschke, A. NAD CaptureSeq Indicates NAD as a Bacterial Cap for a Subset of Regulatory RNAs. Nature 2015, 519, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Glisovic, T.; Bachorik, J.L.; Yong, J.; Dreyfuss, G. RNA-Binding Proteins and Post-Transcriptional Gene Regulation. FEBS Lett. 2008, 582, 1977–1986. [Google Scholar] [CrossRef] [PubMed]
- Luciano, D.J.; Levenson-Palmer, R.; Belasco, J.G. Stresses That Raise Np4A Levels Induce Protective Nucleoside Tetraphosphate Capping of Bacterial RNA. Mol. Cell 2019, 75, 957–966.e8. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Adinolfi, E.; Raffaghello, L.; Giuliani, A.L.; Cavazzini, L.; Capece, M.; Chiozzi, P.; Bianchi, G.; Kroemer, G.; Pistoia, V.; Di Virgilio, F. Expression of P2X7 Receptor Increases in Vivo Tumor Growth. Cancer Res. 2012, 72, 2957–2969. [Google Scholar] [CrossRef] [PubMed]
- Jon Swartz, K.; Kaczmarek-Hajek, K.; Zhang, J.; Kopp, R.; Grosche, A.; Rissiek, B.; Saul, A.; Bruzzone, S.; Engel, T.; Jooss, T.; et al. Re-Evaluation of Neuronal P2X7 Expression Using Novel Mouse Models and a P2X7-Specific Nanobody. eLife 2018, 7, e36217. [Google Scholar] [CrossRef]
- Koganov, E.S.; Michelson, A.D.; Yanachkov, I.B.; Yanachkova, M.I.; Wright, G.E.; Przyklenk, K.; Frelinger, A.L. GLS-409, an Antagonist of Both P2Y1 and P2Y12, Potently Inhibits Canine Coronary Artery Thrombosis and Reversibly Inhibits Human Platelet Activation. Sci. Rep. 2018, 8, 14529. [Google Scholar]
- SGrudzien-Nogalska, E.; Kiledjian, M. New Insights into Decapping Enzymes and Selective MRNA Decay. Wiley Interdiscip. Rev. RNA 2017, 8, e1379. [Google Scholar] [CrossRef] [PubMed]
- Despotović, D.; Brandis, A.; Savidor, A.; Levin, Y.; Fumagalli, L.; Tawfik, D.S. Diadenosine Tetraphosphate (Ap4A)—An E. Coli Alarmone or a Damage Metabolite? FEBS J. 2017, 284, 2194–2215. [Google Scholar] [CrossRef] [PubMed]
| Receptor | Type | Ap4A Role | Cancer Implications | Therapeutic Avenues | Ref. |
|---|---|---|---|---|---|
| P2X1 | Ionotropic | Acts as an agonist; promotes endothelial cell proliferation and migration | Facilitates angiogenesis, supporting tumor growth by supplying oxygen and nutrients. | Proliferation of highly malignant T24 bladder cancer cells depended on autocrine signaling through P2X receptors | [41,58] |
| P2X3 | Ionotropic | Possible interaction with cancer pain pathways | May influence tumor-induced pain and tissue remodelling. | P2X3 purinergic receptor overexpression is associated with poor recurrence-free survival in hepatocellular carcinoma patients | [59,60] |
| P2X4 | Ionotropic | Activates endothelial cells; enhances pro-angiogenic cytokine release | Promotes vascularization within tumors, aiding their survival in hypoxic environments. | P2X4R activation enhances the invasiveness of prostate cancer | [58,61] |
| P2X5 | Ionotropic | Supports tissue proliferation | Potentially contributes to uncontrolled tumor cell growth | P2X5 is highly expressed in a broad range of lymphoid malignancies | [47,62] |
| P2X7 | Ionotropic | Indirect modulation; triggers pro-inflammatory cytokine release | Contributes to the tumor’s inflammatory microenvironment and immune evasion. | P2X7R was highly expressed in gastric cancer tissues and gastric cancer cells | [45,63] |
| P2Y1 | Metabotropic | Agonist; regulates cell proliferation and migration | Modulates intracellular signaling pathways critical for tumor progression. | Novel P2Y1 receptor ligands as a potent anti-prostate cancer agent | [64,65] |
| P2Y2 | Metabotropic | Regulates cell survival and apoptosis | Promotes tumor resistance to cell death and enhances survival under stress conditions. | P2Y2 antagonism promoted apoptosis in HCC cells Human and murine oral cancer cell lines express numerous P2 receptors | [66,67] |
| P2Y6 | Metabotropic | Activates during stress; influences metabolism and immune response | Modulates tumor adaptation and immune system interactions. | P2Y6R may represent a prime target for reducing colorectal carcinogenesis | [68,69] |
| P2Y12 | Metabotropic | Affects platelet aggregation and tumor cell interactions | Facilitates metastasis by enabling dissemination via the circulatory system. | P2RY12 inhibitors could reduce the tumor spread in melanoma, ovarian, breast, lung, and pancreatic cancers | [70,71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tkachenko, K.; Bachetti, T.; Rosano, C. Ap4A in Cancer: A Multifaceted Regulator and Emerging Therapeutic Target. Molecules 2025, 30, 3056. https://doi.org/10.3390/molecules30153056
Tkachenko K, Bachetti T, Rosano C. Ap4A in Cancer: A Multifaceted Regulator and Emerging Therapeutic Target. Molecules. 2025; 30(15):3056. https://doi.org/10.3390/molecules30153056
Chicago/Turabian StyleTkachenko, Kateryna, Tiziana Bachetti, and Camillo Rosano. 2025. "Ap4A in Cancer: A Multifaceted Regulator and Emerging Therapeutic Target" Molecules 30, no. 15: 3056. https://doi.org/10.3390/molecules30153056
APA StyleTkachenko, K., Bachetti, T., & Rosano, C. (2025). Ap4A in Cancer: A Multifaceted Regulator and Emerging Therapeutic Target. Molecules, 30(15), 3056. https://doi.org/10.3390/molecules30153056








