Cyclodextrins as Multifunctional Platforms in Drug Delivery and Beyond: Structural Features, Functional Applications, and Future Trends
Abstract
1. Introduction
2. Structural Features and Types of Cyclodextrins
2.1. Chemical Structure and Conformation
2.2. Inner Hydrophobic Cavity and Outer Hydrophilic Surface
2.3. Solubility and Inclusion Complexes
2.4. Natural vs. Chemically Modified Cyclodextrins
3. Synthesis and Derivatization
3.1. Enzymatic Production via Cyclodextrin Glucanotransferase (CGTase)
3.2. Strategies for Chemical Modification
3.3. CDs Stimuli-Responsive Derivatives
4. Analytical Techniques for Cyclodextrin Characterization
4.1. Characterization Techniques
4.2. Host/Guest Interaction Studies
4.3. Stoichiometry and Binding Constants
5. Applications in Drug Delivery
5.1. Solubility Enhancement
5.2. Stabilization of Labile Drugs
5.3. Controlled Release Systems
5.4. Targeted Delivery Strategies
5.5. Market for Mulations and Clinical Applications
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Villiers, A. Sur la transformation de la fécule en dextrine par le ferment butyrique. Bull. Société Chim. Paris 1891, 45, 468–470. [Google Scholar]
- Crini, G. The contribution of Franz Schardinger to cyclodextrins: A tribute on the occasion of the centenary of his death. J. Incl. Phenom. Macrocycl. Chem. 2020, 97, 19–28. [Google Scholar] [CrossRef]
- Cunha, J.S.; Pacheco, F.C.; Martins, C.C.N.; Pacheco, A.F.C.; Tribst, A.A.L.; Leite Junior, B.R.C. Use of ultrasound to improve the activity of cyclodextrin glycosyltransferase in the producing of beta-cyclodextrins: Impact on enzyme activity, stability and insights into changes on enzyme macrostructure. Food Res. Int. 2024, 191, 114662. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Bai, Y.; Liu, Y.; Wang, Y.; Zhan, X.; Long, J.; Chen, L.; Qiu, C.; Jin, Z. Deciphering external chain length and cyclodextrin production with starch catalyzed by cyclodextrin glycosyltransferase. Carbohydr. Polym. 2022, 284, 119156. [Google Scholar] [CrossRef] [PubMed]
- Ogunbadejo, B.A.; Al-Zuhair, S. Bioconversion of starch to Cyclodextrin using Cyclodextrin glycosyltransferase immobilized on metal organic framework. Biocatal. Agric. Biotechnol. 2023, 53, 102878. [Google Scholar] [CrossRef]
- Tao, X.; Su, L.; Wang, L.; Chen, X.; Wu, J. Improved production of cyclodextrin glycosyltransferase from Bacillus stearothermophilus NO2 in Escherichia coli via directed evolution. Appl. Microbiol. Biotechnol. 2020, 104, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, F.M.; Lis, M.J.; Firmino, H.B.; Dias da Silva, J.G.; Curto Valle, R.C.S.; Borges Valle, J.A.; Scacchetti, F.A.P.; Tessaro, A.L. The Role of beta-Cyclodextrin in the Textile Industry-Review. Molecules 2020, 25, 3624. [Google Scholar] [CrossRef] [PubMed]
- Wupper, S.; Luersen, K.; Rimbach, G. Cyclodextrins, Natural Compounds, and Plant Bioactives-A Nutritional Perspective. Biomolecules 2021, 11, 401. [Google Scholar] [CrossRef] [PubMed]
- Sevim, S.; Sanlier, N. Cyclodextrin as a singular oligosaccharide: Recent advances of health benefit and in food applications. J. Food Sci. 2024, 89, 8215–8230. [Google Scholar] [CrossRef] [PubMed]
- Puebla-Duarte, A.L.; Santos-Sauceda, I.; Rodriguez-Felix, F.; Iturralde-Garcia, R.D.; Fernandez-Quiroz, D.; Perez-Cabral, I.D.; Del-Toro-Sanchez, C.L. Active and Intelligent Packaging: A Review of the Possible Application of Cyclodextrins in Food Storage and Safety Indicators. Polymers 2023, 15, 4317. [Google Scholar] [CrossRef] [PubMed]
- Mohamadhoseini, M.; Mohamadnia, Z. Supramolecular self-healing materials via host-guest strategy between cyclodextrin and specific types of guest molecules. Coord. Chem. Rev. 2021, 432, 213711. [Google Scholar] [CrossRef]
- Yi, S.; Zheng, J.; Lv, P.; Zhang, D.; Zheng, X.; Zhang, Y.; Liao, R. Controlled Drug Release from Cyclodextrin-Gated Mesoporous Silica Nanoparticles Based on Switchable Host-Guest Interactions. Bioconjug. Chem. 2018, 29, 2884–2891. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.; Yang, X.; Chen, S.; Wang, Q.; Zhang, A.; Tang, B. Cyclodextrin-based host–guest supramolecular hydrogels for local drug delivery. Coord. Chem. Rev. 2022, 454, 214352. [Google Scholar] [CrossRef]
- Cid-Samamed, A.; Rakmai, J.; Mejuto, J.C.; Simal-Gandara, J.; Astray, G. Cyclodextrins inclusion complex: Preparation methods, analytical techniques and food industry applications. Food Chem. 2022, 384, 132467. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sameen, D.E.; Ahmed, S.; Wang, Y.; Lu, R.; Dai, J.; Li, S.; Qin, W. Recent advances in cyclodextrin-based films for food packaging. Food Chem. 2022, 370, 131026. [Google Scholar] [CrossRef] [PubMed]
- Bragança Carvalho, L.; Abreu Venceslau, A.d.F.; Luz Ambrosio Breisch, D.; Fernandes Fraceto, L.; Jaime, C.; Matos Alves Pinto, L. Heterocyclic agrochemical hosted by cyclodextrin and hybrid cyclodextrin-silica materials: Characterization, release behavior, and mobility in soil. Colloids Surf. A Physicochem. Eng. Asp. 2023, 656, 130470. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, W.; Kovaleva, E.G.; Cheng, J.; Li, H. Recent progress in encapsulation and controlled release of pesticides based on cyclodextrin derivative carriers. Adv. Agrochem. 2022, 1, 89–99. [Google Scholar] [CrossRef]
- Yadav, M.; Thakore, S.; Jadeja, R. A review on remediation technologies using functionalized Cyclodextrin. Environ. Sci. Pollut. Res. Int. 2022, 29, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Crini, G.; Wilson, L.D.; Balasubramanian, P.; Li, F. Removal of contaminants present in water and wastewater by cyclodextrin-based adsorbents: A bibliometric review from 1993 to 2022. Environ. Pollut. 2024, 348, 123815. [Google Scholar] [CrossRef] [PubMed]
- Majd, M.; Yazdanpanah, M.; Bayatloo, M.R.; Nojavan, S. Recent advances and applications of cyclodextrins in magnetic solid phase extraction. Talanta 2021, 229, 122296. [Google Scholar] [CrossRef] [PubMed]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of Cyclodextrins and Drug/Cyclodextrin Complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef] [PubMed]
- Jambhekar, S.S.; Breen, P. Cyclodextrins in pharmaceutical formulations I: Structure and physicochemical properties, formation of complexes, and types of complex. Drug Discov. Today 2016, 21, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Crini, G. Review: A history of cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef] [PubMed]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Aiassa, V.; Garnero, C.; Longhi, M.R.; Zoppi, A. Cyclodextrin Multicomponent Complexes: Pharmaceutical Applications. Pharmaceutics 2021, 13, 1099. [Google Scholar] [CrossRef] [PubMed]
- Crini, G.; Fourmentin, S.; Fenyvesi, É.; Torri, G.; Fourmentin, M.; Morin-Crini, N. Cyclodextrins, from molecules to applications. Environ. Chem. Lett. 2018, 16, 1361–1375. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, B.; Xu, J.; Pan, K.; Hou, H.; Hu, J.; Yang, J. Cross-linked chitosan/beta-cyclodextrin composite for selective removal of methyl orange: Adsorption performance and mechanism. Carbohydr. Polym. 2018, 182, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.; Mascarenhas-Melo, F.; Rabaca, S.; Mathur, A.; Sharma, A.; Giram, P.S.; Pawar, K.D.; Rahdar, A.; Raza, F.; Veiga, F.; et al. Cyclodextrin-based dermatological formulations: Dermopharmaceutical and cosmetic applications. Colloids Surf. B Biointerfaces 2023, 221, 113012. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Liu, J.; Yu, J.; Wang, J.; Li, H.; Zhong, T.; Hao, Y.; Li, Z.; Wang, J.; Huang, X.; et al. Methyl-beta-cyclodextrin Enhances Tumor Cellular Uptake and Accumulation of alpha-Linolenic Acid-Paclitaxel Conjugate Nanoparticles. Mol. Pharm. 2024, 21, 6109–6122. [Google Scholar] [CrossRef] [PubMed]
- De Gaetano, F.; Pastorello, M.; Pistara, V.; Rescifina, A.; Margani, F.; Barbera, V.; Ventura, C.A.; Marino, A. Rutin/Sulfobutylether-beta-Cyclodextrin as a Promising Therapeutic Formulation for Ocular Infection. Pharmaceutics 2024, 16, 233. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Zhang, X.; Cao, S.; Liu, C.; Fu, X.; Zhang, R.; Li, X.; Xue, J.; Li, Y.; Wang, X.; et al. Pharmacokinetic studies of hyperoside-2-hydroxypropyl-beta-cyclodextrin inclusion complex and ameliorated DSS-induced colitis in mice. Biosci. Rep. 2023, 43, BSR20230003. [Google Scholar] [CrossRef] [PubMed]
- Sah, S.K.; Ajay, S.A.; Donadkar, A.D.; Kamath, A.J.; Devan, A.R.; Soman, R.; Kumar, A.R.; Unni, A.R.; Sithara, M.S.; Sudheesh, M.S.; et al. Ternary complex of Kaempferol-Hydroxypropyl-beta-Cyclodextrin-Liposomes against hepatocellular carcinoma: Preparation, validation, pharmacokinetics and efficacy studies. Int. J. Pharm. 2025, 671, 125261. [Google Scholar] [CrossRef] [PubMed]
- Carmona, T.; Fernández-Clavero, C.; Marcelo, G.; Mendicuti, F. Encapsulation of doxorubicin in carboxymethyl-β-cyclodextrin in aqueous medium mediated by pH-modulated electrostatics interactions. J. Mol. Liq. 2023, 376, 121512. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Qiang, J.; Cao, Y.; Qu, G.; Zhang, S.; Yu, X. Inclusion of alpha-Linolenic acid ethyl ester in flaxseed oil with beta-Cyclodextrin by hydrogen bonding. Food Chem. 2025, 472, 142860. [Google Scholar] [CrossRef] [PubMed]
- Saha, P.; Rafe, M.R. Cyclodextrin: A prospective nanocarrier for the delivery of antibacterial agents against bacteria that are resistant to antibiotics. Heliyon 2023, 9, e19287. [Google Scholar] [CrossRef] [PubMed]
- Ke, Q.; Zhu, J.; Su, D.; Pan, F.; Meng, Q.; Kou, X. Preparation, physicochemical characterization, and computational studies of aldehyde aroma compounds/cyclodextrin inclusion complexes. Ind. Crops Prod. 2024, 211, 118254. [Google Scholar] [CrossRef]
- Commey, K.L.; Enaka, A.; Nakamura, R.; Yamamoto, A.; Tsukigawa, K.; Nishi, K.; Iohara, D.; Hirayama, F.; Otagiri, M.; Yamasaki, K. Development of alpha-Cyclodextrin-Based Orally Disintegrating Tablets for 4-Phenylbutyrate. Pharmaceutics 2024, 16, 82. [Google Scholar] [CrossRef] [PubMed]
- Muankaew, C.; Loftsson, T. Cyclodextrin-Based Formulations: A Non-Invasive Platform for Targeted Drug Delivery. Basic Clin. Pharmacol. Toxicol. 2018, 122, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Xia, L.; Ji, H.; Jin, Z.; Bai, Y. A Cyclodextrin-Based Controlled Release System in the Simulation of In Vitro Small Intestine. Molecules 2020, 25, 1212. [Google Scholar] [CrossRef] [PubMed]
- Aiassa, V.; Garnero, C.; Zoppi, A.; Longhi, M.R. Cyclodextrins and Their Derivatives as Drug Stability Modifiers. Pharmaceutics 2023, 16, 1074. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, Z.; Xiong, J.; Wu, D.; Li, S.; Tao, Y.; Qin, Y.; Kong, Y. Facile synthesis of chitosan-grafted beta-cyclodextrin for stimuli-responsive drug delivery. Int. J. Biol. Macromol. 2019, 125, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhang, P.; Li, C.; Chen, Y.; Sun, J.; Wang, Y.; Xiao, Y. A “Hand-In-Hand” Thermal-Responsive Cyclodextrin Material Designed for Liquid Smart Windows. Adv. Funct. Mater. 2024, 34, 2406937. [Google Scholar] [CrossRef]
- Rodriguez-Varillas, S.; Fontanil, T.; Espina Casado, J.; Fernandez-Gonzalez, A.; Badia Laino, R. Surface modification of carbon dots with cyclodextrins as potential biocompatible photoluminescent delivery/bioimaging nanoplatform. Anal. Chim. Acta 2024, 1318, 342948. [Google Scholar] [CrossRef] [PubMed]
- Mou, N.; Duan, X.; Qu, K.; Chen, Q.; He, Z.; Cao, Y.; Zhang, K.; Qin, X.; Zhu, L.; Han, Z.; et al. Macrophage Membrane Spontaneously Encapsulated Cyclodextrin-Based Nanomedicines for Improving Lipid Metabolism and Inflammation in Atherosclerosis. ACS Appl. Mater. Interfaces 2024, 16, 49660–49672. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.X.; Feng, S.S.; Zheng, C.H. A comparison between conventional liposome and drug-cyclodextrin complex in liposome system. Int. J. Pharm. 2016, 513, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Topuz, F.; Uyar, T. Advances in the development of cyclodextrin-based nanogels/microgels for biomedical applications: Drug delivery and beyond. Carbohydr. Polym. 2022, 297, 120033. [Google Scholar] [CrossRef] [PubMed]
- Ameli, H.; Alizadeh, N. Targeted delivery of capecitabine to colon cancer cells using nano polymeric micelles based on beta cyclodextrin. RSC Adv. 2022, 12, 4681–4691. [Google Scholar] [CrossRef] [PubMed]
- De Los Reyes-Berbel, E.; Ortiz-Gomez, I.; Ortega-Munoz, M.; Salinas-Castillo, A.; Capitan-Vallvey, L.F.; Hernandez-Mateo, F.; Lopez-Jaramillo, F.J.; Santoyo-Gonzalez, F. Carbon dots-inspired fluorescent cyclodextrins: Competitive supramolecular “off-on” (bio)sensors. Nanoscale 2020, 12, 9178–9185. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, H.; Liu, Y. Cyclodextrin supramolecular assembly confined luminescent materials. Chem. Sci. 2024, 15, 18259–18271. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Huang, X.; Zhang, Y.; Wang, M.; Xue, H.; Wang, X.; Jia, L. Cyclodextrin-based dispersive liquid-liquid microextraction for the determination of fungicides in water, juice, and vinegar samples via HPLC. Food Chem. 2022, 367, 130664. [Google Scholar] [CrossRef] [PubMed]
- Markina, N.E.; Markin, A.V.; Cialla-May, D. Cyclodextrin-assisted SERS determination of fluoroquinolone antibiotics in urine and blood plasma. Talanta 2023, 254, 124083. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Song, Y.; Lu, Y.L.; Zhang, Y.M.; Yu, Z.; Xu, X.; Liu, Y. Cyclodextrin-Activated Porphyrin Photosensitization for Boosting Self-Cleavable Drug Release. J. Med. Chem. 2022, 65, 6764–6774. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Liu, Y.H.; Liu, Y. Cyclodextrin-Based Multistimuli-Responsive Supramolecular Assemblies and Their Biological Functions. Adv. Mater. 2020, 32, e1806158. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Qian, A.; Yan, J.; Li, W.; Liu, K.; Masuda, T.; Zhang, A. OEGylated Cyclodextrins Responsive to Temperature, Redox, and Metal Ions. ACS Appl. Mater. Interfaces 2018, 10, 13258–13263. [Google Scholar] [CrossRef] [PubMed]
- Samuelsen, L.; Larsen, D.; Schonbeck, C.; Beeren, S.R. pH-Responsive templates modulate the dynamic enzymatic synthesis of cyclodextrins. Chem. Commun. 2022, 58, 5152–5155. [Google Scholar] [CrossRef] [PubMed]
- Fenyvesi, E.; Sohajda, T. Cyclodextrin-enabled green environmental biotechnologies. Environ. Sci. Pollut. Res. Int. 2022, 29, 20085–20097. [Google Scholar] [CrossRef] [PubMed]
- Wankar, J.; Kotla, N.G.; Gera, S.; Rasala, S.; Pandit, A.; Rochev, Y.A. Recent Advances in Host–Guest Self-Assembled Cyclodextrin Carriers: Implications for Responsive Drug Delivery and Biomedical Engineering. Adv. Funct. Mater. 2020, 30, 1909049. [Google Scholar] [CrossRef]
- Brettner, F.E.B.; Schreiner, J.; Vogel-Kindgen, S.; Windbergs, M. Engineered Self-Assembly of Amphiphilic Cyclodextrin Conjugates for Drug Encapsulation. ACS Biomater. Sci. Eng. 2024, 10, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Przybyla, M.A.; Yilmaz, G.; Becer, C.R. Natural cyclodextrins and their derivatives for polymer synthesis. Polym. Chem. 2020, 11, 7582–7602. [Google Scholar] [CrossRef]
- Lagiewka, J.; Girek, T.; Ciesielski, W. Cyclodextrins-Peptides/Proteins Conjugates: Synthesis, Properties and Applications. Polymers 2021, 13, 1759. [Google Scholar] [CrossRef] [PubMed]
- Devi, L.S.; Casadidio, C.; Gigliobianco, M.R.; Di Martino, P.; Censi, R. Multifunctionality of cyclodextrin-based polymeric nanoparticulate delivery systems for chemotherapeutics, combination therapy, and theranostics. Int. J. Pharm. 2024, 654, 123976. [Google Scholar] [CrossRef] [PubMed]
- Ok, H.W.; Jin, S.; Park, G.; Jana, B.; Ryu, J.H. Folic Acid-Functionalized beta-Cyclodextrin for Delivery of Organelle-Targeted Peptide Chemotherapeutics in Cancer. Mol. Pharm. 2024, 21, 4498–4509. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Barbarroja, G.; Benito, J.M.; Ortiz Mellet, C.; Garcia Fernandez, J.M. Cyclodextrin-Based Functional Glyconanomaterials. Nanomaterials 2020, 10, 2517. [Google Scholar] [CrossRef] [PubMed]
- Nicolaescu, O.E.; Belu, I.; Mocanu, A.G.; Manda, V.C.; Rau, G.; Pirvu, A.S.; Ionescu, C.; Ciulu-Costinescu, F.; Popescu, M.; Ciocilteu, M.V. Cyclodextrins: Enhancing Drug Delivery, Solubility and Bioavailability for Modern Therapeutics. Pharmaceutics 2025, 17, 288. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Pereira, A.; Carpena, M.; Garcia Oliveira, P.; Mejuto, J.C.; Prieto, M.A.; Simal Gandara, J. Main Applications of Cyclodextrins in the Food Industry as the Compounds of Choice to Form Host-Guest Complexes. Int. J. Mol. Sci. 2021, 22, 1339. [Google Scholar] [CrossRef] [PubMed]
- Kali, G.; Haddadzadegan, S.; Bernkop-Schnurch, A. Cyclodextrins and derivatives in drug delivery: New developments, relevant clinical trials, and advanced products. Carbohydr. Polym. 2024, 324, 121500. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Mahar, R. Cyclodextrin in drug delivery: Exploring scaffolds, properties, and cutting-edge applications. Int. J. Pharm. 2024, 662, 124485. [Google Scholar] [CrossRef] [PubMed]
- Astray, G.; Mejuto, J.C.; Simal-Gandara, J. Latest developments in the application of cyclodextrin host-guest complexes in beverage technology processes. Food Hydrocoll. 2020, 106, 105882. [Google Scholar] [CrossRef]
- Anghel, N.; Melinte, V.; Spiridon, I.; Pertea, M. Antioxidant, Antimicrobial, and Kinetic Studies of Beta-Cyclodextrin Crosslinked with Lignin for Drug Delivery. Pharmaceutics 2022, 14, 2260. [Google Scholar] [CrossRef] [PubMed]
- Christaki, S.; Spanidi, E.; Panagiotidou, E.; Athanasopoulou, S.; Kyriakoudi, A.; Mourtzinos, I.; Gardikis, K. Cyclodextrins for the Delivery of Bioactive Compounds from Natural Sources: Medicinal, Food and Cosmetics Applications. Pharmaceutics 2023, 16, 1274. [Google Scholar] [CrossRef] [PubMed]
- Morin-Crini, N.; Fourmentin, S.; Fenyvesi, É.; Lichtfouse, E.; Torri, G.; Fourmentin, M.; Crini, G. 130 years of cyclodextrin discovery for health, food, agriculture, and the industry: A review. Environ. Chem. Lett. 2021, 19, 2581–2617. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Gao, X.; Fu, J.; Hu, L. Application of cyclodextrin in food industry. Crit. Rev. Food Sci. Nutr. 2022, 62, 2627–2640. [Google Scholar] [CrossRef] [PubMed]
- Musuc, A.M. Cyclodextrins: Advances in Chemistry, Toxicology, and Multifaceted Applications. Molecules 2024, 29, 5319. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, G.; Shen, J.; Matai, I.; Sachdev, A.; Boy, R.; Tonelli, A.E. Cyclodextrin-based nanostructures. Prog. Mater. Sci. 2022, 124, 100869. [Google Scholar] [CrossRef]
- Tang, W.; Zou, C.; Da, C.; Cao, Y.; Peng, H. A review on the recent development of cyclodextrin-based materials used in oilfield applications. Carbohydr. Polym. 2020, 240, 116321. [Google Scholar] [CrossRef] [PubMed]
- Waris, K.H.; Lee, V.S.; Mohamad, S. Pesticide remediation with cyclodextrins: A review. Environ. Sci. Pollut. Res. 2021, 28, 47785–47799. [Google Scholar] [CrossRef] [PubMed]
- Sandilya, A.A.; Natarajan, U.; Priya, M.H. Molecular View into the Cyclodextrin Cavity: Structure and Hydration. ACS Omega 2020, 5, 25655–25667. [Google Scholar] [CrossRef] [PubMed]
- Suárez, D.; Díaz, N. Amphiphilic cyclodextrins: Dimerization and diazepam binding explored by molecular dynamics simulations. J. Mol. Liq. 2022, 349, 118457. [Google Scholar] [CrossRef]
- Dos Santos Silva Araujo, L.; Lazzara, G.; Chiappisi, L. Cyclodextrin/surfactant inclusion complexes: An integrated view of their thermodynamic and structural properties. Adv. Colloid. Interface Sci. 2021, 289, 102375. [Google Scholar] [CrossRef] [PubMed]
- López-Méndez, L.J.; Rojas-Aguirre, Y.; Vázquez-Lima, H.; Cassani, J.; Enríquez, R.G.; Rojo-Domínguez, A.; Guadarrama, P. On the conformational search of a βCD dendritic derivative: NMR and theoretical calculations working together reveal a donut-like amphiphilic structure. J. Mol. Struct. 2020, 1204, 127535. [Google Scholar] [CrossRef]
- Lebedinskiy, K.; Barvik, I.; Tosner, Z.; Cisarova, I.; Jindrich, J.; Hrdina, R. Spatial arrangements of cyclodextrin host-guest complexes in solution studied by 13C NMR and molecular modelling. Beilstein, J. Org. Chem. 2024, 20, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Nelumdeniya, N.R.M.; Ranatunga, R.J.K.U. Complex forming behaviour of α, βand γ-cyclodextrins with varying size probe particles in silico. Ceylon J. Sci. 2021, 50, 329–339. [Google Scholar] [CrossRef]
- Bethanis, K.; Christoforides, E.; Andreou, A.; Eliopoulos, E. Molecular Symmetry of Permethylated β-Cyclodextrins upon Complexation. Symmetry 2022, 14, 2214. [Google Scholar] [CrossRef]
- Sala, A.; Hoossen, Z.; Bacchi, A.; Caira, M.R. Two Crystal Forms of a Hydrated 2:1 beta-Cyclodextrin Fluconazole Complex: Single Crystal X-ray Structures, Dehydration Profiles, and Conditions for Their Individual Isolation. Molecules 2021, 26, 4427. [Google Scholar] [CrossRef] [PubMed]
- Sarabia-Vallejo, A.; Caja, M.D.M.; Olives, A.I.; Martin, M.A.; Menendez, J.C. Cyclodextrin Inclusion Complexes for Improved Drug Bioavailability and Activity: Synthetic and Analytical Aspects. Pharmaceutics 2023, 15, 2345. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Puspasari, T.; Nunes, S.P.; Peinemann, K.V. Ultrathin 2D-Layered Cyclodextrin Membranes for High- Performance Organic Solvent Nanofiltration. Adv. Funct. Mater. 2019, 30, 1906797. [Google Scholar] [CrossRef]
- Abdi, Z.G.; Chen, J.-C.; Chung, T.-S. Novel amino-beta cyclodextrin-based organic solvent nanofiltration membranes with fast and stable solvent transport. J. Membr. Sci. 2024, 689, 122197. [Google Scholar] [CrossRef]
- Liu, J.; Hua, D.; Zhang, Y.; Japip, S.; Chung, T.S. Precise Molecular Sieving Architectures with Janus Pathways for Both Polar and Nonpolar Molecules. Adv. Mater. 2018, 30, 1705933. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; He, Y.; Dai, Y.; Shi, Y.; Li, L.; Zhang, Z.; Xia, F.; Zhang, X. Cyclodextrin membranes prepared by interfacial polymerization for separation. Chem. Eng. J. 2024, 492, 152165. [Google Scholar] [CrossRef]
- Kabouche, Z.; Belhocine, Y.; Benlecheb, T.; Assaba, I.M.; Litim, A.; Lalalou, R.; Mechhoud, A. A DFT-D4 investigation of the complexation phenomenon between pentachlorophenol and β-cyclodextrin. Chimica Techno Acta 2023, 10, 202310209. [Google Scholar] [CrossRef]
- Swarna, S.B.; Gupta, P. Effect of substituted cyclodextrins on ibrutinib solubility and characterization of IBR-cyclodextrin inclusion complexes by isothermal titration calorimetry in solution state. J. Therm. Anal. Calorim. 2024, 150, 237–244. [Google Scholar] [CrossRef]
- Mani, A.; Ramasamy, P.; Prabhu, A.A.M.; Senthilraja, P.; Rajendiran, N. Synthesis and characterization of Ag/Co/chloroquine/cyclodextrin inclusion complex nanomaterials. J. Sol-Gel Sci. Technol. 2024. [Google Scholar] [CrossRef]
- Kumar, P.; Bhardwaj, V.K.; Purohit, R. Highly robust quantum mechanics and umbrella sampling studies on inclusion complexes of curcumin and beta-cyclodextrin. Carbohydr. Polym. 2024, 323, 121432. [Google Scholar] [CrossRef] [PubMed]
- Demircan Ozelcaglayan, E.; Honek, J.F.; Parker, W.J. Molecular level investigation of interactions between pharmaceuticals and beta-cyclodextrin (beta-CD) functionalized adsorption sites for removal of pharmaceutical contaminants from water. Chemosphere 2024, 347, 140639. [Google Scholar] [CrossRef] [PubMed]
- Geue, N.; Alcazar, J.J.; Campodonico, P.R. Influence of beta-Cyclodextrin Methylation on Host-Guest Complex Stability: A Theoretical Study of Intra- and Intermolecular Interactions as Well as Host Dimer Formation. Molecules 2023, 28, 2625. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Han, Z.; Liu, L.; Wang, Y.; Xin, S.; Zhang, H.; Yu, Z. Solubility Enhancement of Myricetin by Inclusion Complexation with Heptakis-O-(2-Hydroxypropyl)-beta-Cyclodextrin: A Joint Experimental and Theoretical Study. Int. J. Mol. Sci. 2020, 21, 766. [Google Scholar] [CrossRef]
- Hanayama, H.; Yamada, J.; Tomotsuka, I.; Harano, K.; Nakamura, E. Rim Binding of Cyclodextrins in Size-Sensitive Guest Recognition. J. Am. Chem. Soc. 2021, 143, 5786–5792. [Google Scholar] [CrossRef] [PubMed]
- Ganjali Koli, M.; Fogolari, F. Exploring the role of cyclodextrins as a cholesterol scavenger: A molecular dynamics investigation of conformational changes and thermodynamics. Sci. Rep. 2023, 13, 21765. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Bhardwaj, V.K.; Shende, P.; Purohit, R. Computational and experimental analysis of Luteolin-beta-cyclodextrin supramolecular complexes: Insights into conformational dynamics and phase solubility. Eur. J. Pharm. Biopharm. 2024, 205, 114569. [Google Scholar] [CrossRef] [PubMed]
- Sandilya, A.A.; Priya, M.H. The counteracting influence of 2-hydroxypropyl substitution and the presence of a guest molecule on the shape and size of the beta-cyclodextrin cavity. Phys. Chem. Chem. Phys. 2024, 26, 11531–11544. [Google Scholar] [CrossRef] [PubMed]
- Cesari, A.; Recchimurzo, A.; Fabiano, A.; Balzano, F.; Rossi, N.; Migone, C.; Uccello-Barretta, G.; Zambito, Y.; Piras, A.M. Improvement of Peptide Affinity and Stability by Complexing to Cyclodextrin-Grafted Ammonium Chitosan. Polymers 2020, 12, 474. [Google Scholar] [CrossRef] [PubMed]
- Szente, L.; Fenyvesi, E. Cyclodextrin-Enabled Polymer Composites for Packaging (dagger). Molecules 2018, 23, 1556. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Han, Z.; Liu, L.; Xin, S.; Yu, Z. Improved Kaempferol Solubility via Heptakis-O-(2-hydroxypropyl)-beta-cyclodextrin Complexation: A Combined Spectroscopic and Theoretical Study. Int. J. Mol. Sci. 2024, 25, 12492. [Google Scholar] [CrossRef] [PubMed]
- Petitprez, J.; Legrand, F.-X.; Tams, C.; Pipkin, J.D.; Antle, V.; Kfoury, M.; Fourmentin, S. Huge solubility increase of poorly water-soluble pharmaceuticals by sulfobutylether-β-cyclodextrin complexation in a low-melting mixture. Environ. Chem. Lett. 2022, 20, 1561–1568. [Google Scholar] [CrossRef]
- Szabo, Z.I.; Orban, G.; Borbas, E.; Csicsak, D.; Kadar, S.; Fiser, B.; Dobo, M.; Horvath, P.; Kiss, E.; Budai, L.; et al. Inclusion complexation of the anticancer drug pomalidomide with cyclodextrins: Fast dissolution and improved solubility. Heliyon 2021, 7, e07581. [Google Scholar] [CrossRef] [PubMed]
- Samuelsen, L.; Holm, R.; Lathuile, A.; Schonbeck, C. Correlation between the stability constant and pH for beta-cyclodextrin complexes. Int. J. Pharm. 2019, 568, 118523. [Google Scholar] [CrossRef] [PubMed]
- Kinart, Z. Stability of the Inclusion Complexes of Dodecanoic Acid with alpha-Cyclodextrin, beta-Cyclodextrin and 2-HP-beta-Cyclodextrin. Molecules 2023, 28, 3113. [Google Scholar] [CrossRef] [PubMed]
- Kfoury, M.; Landy, D.; Fourmentin, S. Characterization of Cyclodextrin/Volatile Inclusion Complexes: A Review. Molecules 2018, 23, 1204. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Nath, S.; Singh, T.S.; Chattopadhyay, N. Cavity size dependent stoichiometry of probe–cyclodextrin complexation: Experimental and molecular docking demonstration. J. Photochem. Photobiol. A Chem. 2020, 388, 112158. [Google Scholar] [CrossRef]
- Ondo, D. Thermodynamic study on complexation of long-chain fatty acid anions with α-cyclodextrin in water. J. Mol. Liq. 2020, 311, 113172. [Google Scholar] [CrossRef]
- Raffaini, G.; Elli, S.; Catauro, M.; D’Angelo, A. Different Drug Mobilities in Hydrophobic Cavities of Host-Guest Complexes between beta-Cyclodextrin and 5-Fluorouracil at Different Stoichiometries: A Molecular Dynamics Study in Water. Int. J. Mol. Sci. 2024, 25, 5888. [Google Scholar] [CrossRef] [PubMed]
- Triamchaisri, N.; Toochinda, P.; Lawtrakul, L. Structural Investigation of Beta-Cyclodextrin Complexes with Cannabidiol and Delta-9-Tetrahydrocannabinol in 1:1 and 2:1 Host-Guest Stoichiometry: Molecular Docking and Density Functional Calculations. Int. J. Mol. Sci. 2023, 24, 1525. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T. Cyclodextrins in Parenteral Formulations. J. Pharm. Sci. 2021, 110, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Nair, A.B. Cyclodextrin complexes: Perspective from drug delivery and formulation. Drug Dev. Res. 2018, 79, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Taupitz, T.; Dressman, J.B.; Buchanan, C.M.; Klein, S. Cyclodextrin-water soluble polymer ternary complexes enhance the solubility and dissolution behaviour of poorly soluble drugs. Case example: Itraconazole. Eur. J. Pharm. Biopharm. 2013, 83, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Ajaz, N.; Abbas, A.; Afshan, R.; Irfan, M.; Khalid, S.H.; Asghar, S.; Munir, M.U.; Rizg, W.Y.; Majrashi, K.A.; Alshehri, S.; et al. In Vitro and In Vivo Evaluation of Hydroxypropyl-beta-cyclodextrin-grafted-poly(acrylic acid)/poly(vinyl pyrrolidone) Semi-Interpenetrating Matrices of Dexamethasone Sodium Phosphate. Pharmaceutics 2022, 15, 1399. [Google Scholar] [CrossRef]
- Varga, E.; Benkovics, G.; Darcsi, A.; Varnai, B.; Sohajda, T.; Malanga, M.; Beni, S. Comparative analysis of the full set of methylated beta-cyclodextrins as chiral selectors in capillary electrophoresis. Electrophoresis 2019, 40, 2789–2798. [Google Scholar] [CrossRef] [PubMed]
- Shankar, V.K.; Police, A.; Pandey, P.; Cuny, Z.G.; Repka, M.A.; Doerksen, R.J.; Murthy, S.N. Optimization of sulfobutyl-ether-beta-cyclodextrin levels in oral formulations to enhance progesterone bioavailability. Int. J. Pharm. 2021, 596, 120212. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Yang, X.; Guo, W.; Wang, J.; Chen, G. Inclusion Complex of Docetaxel with Sulfobutyl Ether beta-Cyclodextrin: Preparation, In Vitro Cytotoxicity and In Vivo Safety. Polymers 2020, 12, 2336. [Google Scholar] [CrossRef] [PubMed]
- Sadaquat, H.; Akhtar, M. Comparative effects of β-cyclodextrin, HP-β-cyclodextrin and SBE7-β-cyclodextrin on the solubility and dissolution of docetaxel via inclusion complexation. J. Incl. Phenom. Macrocycl. Chem. 2020, 96, 333–351. [Google Scholar] [CrossRef]
- Pardeshi, C.V.; Kothawade, R.V.; Markad, A.R.; Pardeshi, S.R.; Kulkarni, A.D.; Chaudhari, P.J.; Longhi, M.R.; Dhas, N.; Naik, J.B.; Surana, S.J.; et al. Sulfobutylether-beta-cyclodextrin: A functional biopolymer for drug delivery applications. Carbohydr. Polym. 2023, 301, 120347. [Google Scholar] [CrossRef] [PubMed]
- Kalambate, P.K.; Upadhyay, S.S.; Shen, Y.; Laiwattanapaisal, W.; Huang, Y. Chiral nanocomposite of sulfobutyl ether-β-cyclodextrin embedded in carbon nanofibers for enantioselective electrochemical discrimination of amlodipine, metoprolol and clenbuterol enantiomers. J. Mater. 2021, 7, 226–235. [Google Scholar] [CrossRef]
- Mi, Y.; Zhang, J.; Tan, W.; Miao, Q.; Li, Q.; Guo, Z. Preparation of Doxorubicin-Loaded Carboxymethyl-beta-Cyclodextrin/Chitosan Nanoparticles with Antioxidant, Antitumor Activities and pH-Sensitive Release. Mar. Drugs 2022, 20, 278. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Tu, J.; Zhao, J.; Zhang, Y.; Li, T.; Zhang, Y.; Zhang, P.; Ling, G.; Ji, J. Dual-targeted and esterase-responsive cyclodextrin-based host-guest nanocomposites for enhanced antitumor therapy. Colloids Surf. B Biointerfaces 2025, 246, 114371. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Solanki, A.; Joshi, A.; Devkar, R.; Seshadri, S.; Thakore, S. beta-cyclodextrin based dual-responsive multifunctional nanotheranostics for cancer cell targeting and dual drug delivery. Carbohydr. Polym. 2019, 206, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Huang, J.L.; Wang, Y.T.; Zheng, A.Q.; Shu, Y.; Wang, J.H. β-Cyclodextrin-Decorated Carbon Dots Serve as Nanocarriers for Targeted Drug Delivery and Controlled Release. ChemNanoMat 2019, 5, 479–487. [Google Scholar] [CrossRef]
- Ehsanimehr, S.; Moghadam, P.N.; Dehaen, W.; Shafiei-Irannejad, V. Redox and pH-Responsive NCC/L-Cysteine/CM-β-CD/FA Contains Disulfide Bond-Bridged as Nanocarriers for Biosafety and Anti-Tumor Efficacy System. Starch–Stärke 2021, 73, 2100061. [Google Scholar] [CrossRef]
- Hu, Y.; Qiu, C.; McClements, D.J.; Qin, Y.; Fan, L.; Xu, X.; Wang, J.; Jin, Z. Simple Strategy Preparing Cyclodextrin Carboxylate as a Highly Effective Carrier for Bioactive Compounds. J. Agric. Food Chem. 2021, 69, 11006–11014. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, P.F.P.; Cumba, L.R.; Andrade, R.D.A.; do Carmo, D.R. Chemical Modifications of Cyclodextrin and Chitosan for Biological and Environmental Applications: Metals and Organic Pollutants Adsorption and Removal. J. Polym. Environ. 2019, 27, 1352–1366. [Google Scholar] [CrossRef]
- Roy, A.; Manna, K.; Dey, S.; Pal, S. Chemical modification of beta-cyclodextrin towards hydrogel formation. Carbohydr. Polym. 2023, 306, 120576. [Google Scholar] [CrossRef] [PubMed]
- Muniz, I.d.C.B.; Santos, J.B.; de Oliveira, R.M.; Santos, F.G.; Souza Junior, E.C.d.; Oyama, L.; Fontan, R.d.C.I.; Bonomo, R.C.F. Current advances in obtaining novel cyclodextrin glycosyltransferases for optimizing the synthesis of cyclodextrins. Process Biochem. 2024, 145, 195–209. [Google Scholar] [CrossRef]
- Rabadiya, K.; Pardhi, D.; Thaker, K.; Patoliya, J.; Rajput, K.; Joshi, R. A review on recent upgradation and strategies to enhance cyclodextrin glucanotransferase properties for its applications. Int. J. Biol. Macromol. 2024, 259, 129315. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Yu, P.; Sun, P.; Kang, Y.; Niu, Y.; She, Y.; Zhao, D. Inclusion complexes of β-cyclodextrin with isomeric ester aroma compounds: Preparation, characterization, mechanism study, and controlled release. Carbohydr. Polym. 2024, 333, 121977. [Google Scholar] [CrossRef] [PubMed]
- Saffarionpour, S.; Diosady, L.L. Preparation and characterization of an iron-beta-cyclodextrin inclusion complex: Factors influencing the host-guest interaction. Food Funct. 2023, 14, 5062–5077. [Google Scholar] [CrossRef] [PubMed]
- Rojas, M.J.; Amaral-Fonseca, M.; Zanin, G.M.; Fernandez-Lafuente, R.; Giordano, R.d.L.C.; Tardioli, P.W. Preparation of Crosslinked Enzyme Aggregates of a Thermostable Cyclodextrin Glucosyltransferase from Thermoanaerobacter sp. Critical Effect of the Crosslinking Agent. Catalysts 2019, 9, 120. [Google Scholar] [CrossRef]
- Li, C.; You, Y.; Zhang, Y.; Xie, X.; Xu, Q.; Gu, Z.; Ban, X.; Tang, X.; Hong, Y.; Cheng, L.; et al. Maltose binding site 2 mutations affect product inhibition of Bacillus circulans STB01 cyclodextrin glycosyltransferase. Int. J. Biol. Macromol. 2021, 175, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Sonnendecker, C.; Melzer, S.; Zimmermann, W. Engineered cyclodextrin glucanotransferases from Bacillus sp. G-825-6 produce large-ring cyclodextrins with high specificity. Microbiologyopen 2019, 8, e00757. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Wu, Y.; Ji, H.; Jin, Z. Synthesis, separation, and purification of glucosyl-beta-cyclodextrin by one-pot method. J. Food Biochem. 2019, 43, e12890. [Google Scholar] [CrossRef] [PubMed]
- Baqing, L.; He, X.; Ni, Q.; Zhang, H.; Li, T.; Lin, X.; Guo, T.; Garba, B.M.; Chen, X.; Zhang, J.; et al. Purification of gamma-cyclodextrin via selective coordination with potassium ions to form metal-organic frameworks. Carbohydr. Polym. 2024, 338, 122193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, M.; Mao, H. Cross-linked enzyme aggregates of recombinant cyclodextrin glycosyltransferase for high-purity β-cyclodextrin production. J. Chem. Technol. Biotechnol. 2019, 94, 1528–1533. [Google Scholar] [CrossRef]
- Ozcan, B.D.; Zimmermann, M.L.; Ren, M.; Bols, M. New methods of modification of alpha-cyclodextrin. Org. Biomol. Chem. 2024, 22, 7092–7102. [Google Scholar] [CrossRef] [PubMed]
- Řezanka, M. Synthesis of substituted cyclodextrins. Environ. Chem. Lett. 2018, 17, 49–63. [Google Scholar] [CrossRef]
- Wang, F.; Bao, X.; Fang, A.; Li, H.; Zhou, Y.; Liu, Y.; Jiang, C.; Wu, J.; Song, X. Nanoliposome-Encapsulated Brinzolamide-hydropropyl-beta-cyclodextrin Inclusion Complex: A Potential Therapeutic Ocular Drug-Delivery System. Front. Pharmacol. 2018, 9, 91. [Google Scholar] [CrossRef]
- Batool, N.; Sarfraz, R.M.; Mahmood, A.; Zaman, M.; Zafar, N.; Salawi, A.; Almoshari, Y.; Alshamrani, M. Orally Administered, Biodegradable and Biocompatible Hydroxypropyl-beta-Cyclodextrin Grafted Poly(methacrylic acid) Hydrogel for pH Sensitive Sustained Anticancer Drug Delivery. Gels 2022, 8, 190. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Liu, Y.; Jiang, J.; Li, X.; Ye, F.; Fu, Y.; Zhao, L. Thiram/hydroxypropyl-beta-cyclodextrin inclusion complex electrospun nanofibers for a fast dissolving water-based drug delivery system. Colloids Surf. B Biointerfaces 2021, 201, 111625. [Google Scholar] [CrossRef] [PubMed]
- Grassiri, B.; Knoll, P.; Fabiano, A.; Piras, A.M.; Zambito, Y.; Bernkop-Schnurch, A. Thiolated Hydroxypropyl-beta-cyclodextrin: A Potential Multifunctional Excipient for Ocular Drug Delivery. Int. J. Mol. Sci. 2022, 23, 2612. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Choi, Y.J.; Woo, M.R.; Cheon, S.; Ji, S.H.; Im, D.; Ud Din, F.; Kim, J.O.; Youn, Y.S.; Oh, K.T.; et al. New potential application of hydroxypropyl-beta-cyclodextrin in solid self-nanoemulsifying drug delivery system and solid dispersion. Carbohydr. Polym. 2021, 271, 118433. [Google Scholar] [CrossRef] [PubMed]
- Grassiri, B.; Cesari, A.; Balzano, F.; Migone, C.; Kali, G.; Bernkop-Schnurch, A.; Uccello-Barretta, G.; Zambito, Y.; Piras, A.M. Thiolated 2-Methyl-beta-Cyclodextrin as a Mucoadhesive Excipient for Poorly Soluble Drugs: Synthesis and Characterization. Polymers 2022, 14, 3170. [Google Scholar] [CrossRef] [PubMed]
- De Gaetano, F.; Mannino, D.; Celesti, C.; Bulzomi, M.; Iraci, N.; Vincenzo Giofre, S.; Esposito, E.; Paterniti, I.; Anna Ventura, C. Randomly methylated beta-cyclodextrin improves water–solubility, cellular protection and mucosa permeability of idebenone. Int. J. Pharm. 2024, 665, 124718. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Higashi, K.; Ueda, K.; Moribe, K. Supersaturation maintenance of carvedilol and chlorthalidone by cyclodextrin derivatives: Pronounced crystallization inhibition ability of methylated cyclodextrin. Int. J. Pharm. 2023, 637, 122876. [Google Scholar] [CrossRef] [PubMed]
- Gholami, R.; Azizi, K.; Ganjali Koli, M. Unveiling the dynamic and thermodynamic interactions of hydrocortisone with beta-cyclodextrin and its methylated derivatives through insights from molecular dynamics simulations. Sci. Rep. 2024, 14, 12495. [Google Scholar] [CrossRef] [PubMed]
- Salgin, S.; Eke, H.H.; Soyer, N.; Salgin, U. Effect of Reaction Parameters on the Synthesis of Cyclodextrin-Based Nanostructured Polymers for Drug Delivery. Polymers 2025, 17, 709. [Google Scholar] [CrossRef] [PubMed]
- Jicsinszky, L.; Bucciol, F.; Manzoli, M.; Cravotto, G. Comparative Studies of Mechanochemically Synthesized Insoluble Beta-Cyclodextrin Polymers. Curr. Org. Chem. 2021, 25, 1923–1936. [Google Scholar] [CrossRef]
- Xu, Y.; Dong, W.; Jia, M.; Shen, J.; Wang, C.; Wei, Y. Supramolecular phenylboronic acid@CD-MOFs: Enrichment integrated derivatization strategy for diols-containing short-chain aliphatic compounds. Sep. Purif. Technol. 2024, 332, 125772. [Google Scholar] [CrossRef]
- Kawashima, K.; Lu, X.; Kuninobu, Y.; Mori, T. Mechanistic insights into the role of cyclodextrin in the regioselective radical C—H trifluoromethylation of aromatic compounds. J. Comput. Chem. 2024, 45, 2112–2118. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Zhang, J.; Huang, J.; Wang, X.; Yang, H.; Jin, Q. Decreased Penetration Mechanism of Ranitidine Due to Application of Sodium Sulfobutyl Ether-beta-Cyclodextrin. Pharmaceutics 2023, 15, 2593. [Google Scholar] [CrossRef] [PubMed]
- Soe, H.; Kerdpol, K.; Rungrotmongkol, T.; Pruksakorn, P.; Autthateinchai, R.; Wet-Osot, S.; Loftsson, T.; Jansook, P. Voriconazole Eye Drops: Enhanced Solubility and Stability through Ternary Voriconazole/Sulfobutyl Ether beta-Cyclodextrin/Polyvinyl Alcohol Complexes. Int. J. Mol. Sci. 2023, 24, 2343. [Google Scholar] [CrossRef] [PubMed]
- Stella, V.J.; Rajewski, R.A. Sulfobutylether-beta-cyclodextrin. Int. J. Pharm. 2020, 583, 119396. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Sha, X.; Zhou, X.; Guo, D.; Han, B.; Huang, S.; Zhu, Y. Cyclodextrin-dendrimers nanocomposites functionalized high performance liquid chromatography stationary phase for efficient separation of aromatic compounds. J. Chromatogr. A 2022, 1662, 462730. [Google Scholar] [CrossRef] [PubMed]
- Yakavets, I.; Guereschi, C.; Lamy, L.; Kravchenko, I.; Lassalle, H.P.; Zorin, V.; Bezdetnaya, L. Cyclodextrin nanosponge as a temoporfin nanocarrier: Balancing between accumulation and penetration in 3D tumor spheroids. Eur. J. Pharm. Biopharm. 2020, 154, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Pivato, R.V.; Rossi, F.; Ferro, M.; Castiglione, F.; Trotta, F.; Mele, A. β-Cyclodextrin Nanosponge Hydrogels as Drug Delivery Nanoarchitectonics for Multistep Drug Release Kinetics. ACS Appl. Polym. Mater. 2021, 3, 6562–6571. [Google Scholar] [CrossRef]
- Utzeri, G.; Matias, P.M.C.; Murtinho, D.; Valente, A.J.M. Cyclodextrin-Based Nanosponges: Overview and Opportunities. Front. Chem. 2022, 10, 859406. [Google Scholar] [CrossRef] [PubMed]
- Lucio, D.; Martinez-Oharriz, M.C.; Gu, Z.; He, Y.; Aranaz, P.; Vizmanos, J.L.; Irache, J.M. Cyclodextrin-grafted poly(anhydride) nanoparticles for oral glibenclamide administration. In vivo evaluation using C. elegans. Int. J. Pharm. 2018, 547, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Serres-Gomez, M.; Gonzalez-Gaitano, G.; Kaldybekov, D.B.; Mansfield, E.D.H.; Khutoryanskiy, V.V.; Isasi, J.R.; Dreiss, C.A. Supramolecular Hybrid Structures and Gels from Host-Guest Interactions between alpha-Cyclodextrin and PEGylated Organosilica Nanoparticles. Langmuir 2018, 34, 10591–10602. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Zhang, X.; Tian, B.R. Selective modifications at the different positions of cyclodextrins: A review of strategies. Turk. J. Chem. 2020, 44, 261–278. [Google Scholar] [CrossRef] [PubMed]
- Blaj, D.A.; Balan-Porcarasu, M.; Harabagiu, V.; Peptu, C. Synthesis of beta-cyclodextrin derivatives substituted at larger or smaller rims via amine-catalyzed ring-opening oligomerization of epsilon-caprolactone. Carbohydr. Polym. 2024, 334, 122032. [Google Scholar] [CrossRef] [PubMed]
- Puglisi, A.; Bayir, E.; Timur, S.; Yagci, Y. pH-Responsive Polymersome Microparticles as Smart Cyclodextrin-Releasing Agents. Biomacromolecules 2019, 20, 4001–4007. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Wang, J.; He, X.; Yu, D.; Qiu, Y.; Liao, Y.; Xie, X. A quadruple-stimuli responsive supramolecular hydrogel constructed from a poly(acrylic acid) derivative and beta-cyclodextrin dimer. Soft Matter. 2024, 20, 5343–5350. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Guha Ray, P.; Bose, A.; Dhara, S.; Pal, S. pH-Responsive Copolymeric Network Gel Using Methacrylated beta-Cyclodextrin for Controlled Codelivery of Hydrophilic and Hydrophobic Drugs. ACS Appl. Bio Mater. 2022, 5, 3530–3543. [Google Scholar] [CrossRef] [PubMed]
- Li, B.-L.; Zhang, J.; Jin, W.; Chen, X.-Y.; Yang, J.-M.; Chi, S.-M.; Ruan, Q.; Zhao, Y. Oral administration of pH-responsive polyamine modified cyclodextrin nanoparticles for controlled release of anti-tumor drugs. React. Funct. Polym. 2022, 172, 105175. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, G.; Wang, N.; Guo, F.; Guo, L.; Liu, X. Synthesis of temperature/pH dual-sensitive supramolecular micelles from beta-cyclodextrin-poly(N-isopropylacrylamide) star polymer for drug delivery. Colloids Surf. B Biointerfaces 2018, 172, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, Y.; Hirose, T.; Kawauchi, S.; Yamaguchi, T. High Temperature-Responsive Autonomous Reswelling Polymer Based on Dynamic Host–Guest (De)complexation of Cyclodextrin. Macromolecules 2023, 56, 4823–4834. [Google Scholar] [CrossRef]
- Gayathry, T.C.; Gaur, M.; Mishra, L.; Mishra, M.; Barooah, N.; Bhasikuttan, A.C.; Mohanty, J. Supramolecular assembly of coumarin 7 with sulfobutylether-beta-cyclodextrin for biomolecular applications. Front. Chem. 2023, 11, 1245518. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Tian, M.; Zhang, H.; Liu, Y. Reversible dynamic optical sensing based on coumarin modified beta-cyclodextrin for glutathione in living cells. Chem. Commun. 2023, 59, 896–899. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ye, H.J.; Xiang, H.M.; Zhou, X.; Wang, P.Y.; Liu, S.S.; Yang, B.X.; Yang, H.B.; Liu, L.W.; Yang, S. Photo-Stimuli Smart Supramolecular Self-Assembly of Azobenzene/β-Cyclodextrin Inclusion Complex for Controlling Plant Bacterial Diseases. Adv. Funct. Mater. 2023, 33, 3206. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, J.; Chen, C.; Wang, D.; Tian, G.; Zhang, G.; Cai, D.; Wu, Z. Infrared-Light-Responsive Controlled-Release Pesticide Using Hollow Carbon Microspheres@Polyethylene Glycol/alpha-Cyclodextrin Gel. J. Agric. Food Chem. 2021, 69, 6981–6988. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.-B.; Zhou, X.-L.; Zheng, H.; Fan, Z.-W.; Pan, D.-W.; Liu, Z.; Wang, W.; Xie, R.; Ju, X.-J.; Chu, L.-Y. Regulatory effects of cyclodextrins on light-responsive phase transition behaviors of poly(N-isopropylacrylamide-co-N-(4-phenylazophenyl)methylacrylamide). Polymers 2024, 313, 127676. [Google Scholar] [CrossRef]
- Palminteri, M.; Dhakar, N.K.; Ferraresi, A.; Caldera, F.; Vidoni, C.; Trotta, F.; Isidoro, C. Cyclodextrin nanosponge for the GSH-mediated delivery of Resveratrol in human cancer cells. Nanotheranostics 2021, 5, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q. Bioresponsive and multifunctional cyclodextrin-based non-viral nanocomplexes in cancer therapy: Building foundations for gene and drug delivery, immunotherapy and bioimaging. Environ. Res. 2023, 234, 116507. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chang, B.; Li, Q.; Xu, L.; Liu, X.; Wang, G.; Wang, Z.; Wang, L. Redox-Responsive Dual Drug Delivery Nanosystem Suppresses Cancer Repopulation by Abrogating Doxorubicin-Promoted Cancer Stemness, Metastasis, and Drug Resistance. Adv. Sci. 2019, 6, 1801987. [Google Scholar] [CrossRef] [PubMed]
- Degirmenci, A.; Ipek, H.; Sanyal, R.; Sanyal, A. Cyclodextrin-containing redox-responsive nanogels: Fabrication of a modular targeted drug delivery system. Eur. Polym. J. 2022, 181, 111645. [Google Scholar] [CrossRef]
- Xu, M.; Zha, H.; Han, R.; Cheng, Y.; Chen, J.; Yue, L.; Wang, R.; Zheng, Y. Cyclodextrin-Derived ROS-Generating Nanomedicine with pH-Modulated Degradability to Enhance Tumor Ferroptosis Therapy and Chemotherapy. Small 2022, 18, e2200330. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Ma, X.; Lu, Y.; Li, X.; Hou, S.; Gao, Y.; Xue, P.; Kang, Y.; Xu, Z. ROS-responsive cyclodextrin nanoplatform for combined photodynamic therapy and chemotherapy of cancer. Chin. Chem. Lett. 2021, 32, 162–167. [Google Scholar] [CrossRef]
- Zhang, S.; Tamura, A.; Yui, N. Enhanced Tumor Targeting and Antitumor Activity of Methylated beta-Cyclodextrin-Threaded Polyrotaxanes by Conjugating Cyclic RGD Peptides. Biomolecules 2024, 14, 223. [Google Scholar] [CrossRef]
- Zhang, D.; Lv, P.; Zhou, C.; Zhao, Y.; Liao, X.; Yang, B. Cyclodextrin-based delivery systems for cancer treatment. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 96, 872–886. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Liu, Y.; Liu, J. Smart stimuli-responsive drug delivery systems based on cyclodextrin: A review. Carbohydr. Polym. 2021, 251, 116871. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Nie, T.; Fang, Y.; You, X.; Huang, H.; Wu, J. Stimuli-responsive cyclodextrin-based supramolecular assemblies as drug carriers. J. Mater. Chem. B 2022, 10, 2077–2096. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Yuan, Q.; Hollett, G.; Zhao, W.; Kang, Y.; Wu, J. Cyclodextrin-based host–guest supramolecular hydrogel and its application in biomedical fields. Polym. Chem. 2018, 9, 3436–3449. [Google Scholar] [CrossRef]
- Tummala, S.; Sandhya, S.; Devika, V.; Rajeev, N.; Sreelekshmi, P.J.; Chandran, A.; Goutami, G.B.; Aiswarya Lakshmi, S.; Kosaraju, S.; Bobba, P.; et al. Multiple stimuli responsive cyclodextrin based smart materials for drug delivery: A review. E3S Web Conf. 2021, 309, 01014. [Google Scholar] [CrossRef]
- Bhattacharya, T.; Teja, C.; Kumar, N.; Bhagat, K.K.; Lahiri, G.K.; Gupta, P.; Tyagi, S.; Maiti, D. Harnessing the “Methyl Effect” in the Development of Novel meta-Directing Template for C–H Cyanation. ACS Catal. 2024, 14, 2216–2228. [Google Scholar] [CrossRef]
- Jiao, K.J.; Xing, Y.K.; Yang, Q.L.; Qiu, H.; Mei, T.S. Site-Selective C-H Functionalization via Synergistic Use of Electrochemistry and Transition Metal Catalysis. Acc. Chem. Res. 2020, 53, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, R.; Dong, Z.; Liu, P.; Dong, G. Complementary site-selectivity in arene functionalization enabled by overcoming the ortho constraint in palladium/norbornene catalysis. Nat. Chem. 2018, 10, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Tadele, D.T.; Mekonnen, T.H. Co-encapsulation of Quercetin and α-Tocopherol Bioactives in Zein Nanoparticles: Synergistic Interactions, Stability, and Controlled Release. ACS Appl. Polym. Mater. 2024, 6, 3767–3777. [Google Scholar] [CrossRef]
- Thambiraj, S.; Vijayalakshmi, R.; Ravi Shankaran, D. An effective strategy for development of docetaxel encapsulated gold nanoformulations for treatment of prostate cancer. Sci. Rep. 2021, 11, 2808. [Google Scholar] [CrossRef] [PubMed]
- Roshan, Z.; Haddadi-Asl, V.; Ahmadi, H.; Moussaei, M. Curcumin-Encapsulated Poly(lactic-co-glycolic acid) Nanoparticles: A Comparison of Drug Release Kinetics from Particles Prepared via Electrospray and Nanoprecipitation. Macromol. Mater. Eng. 2024, 309, 2400040. [Google Scholar] [CrossRef]
- Akar, S.; Fardindoost, S.; Hoorfar, M. High throughput microfluidics-based synthesis of PEGylated liposomes for precise size control and efficient drug encapsulation. Colloids Surf. B Biointerfaces 2024, 238, 113926. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Hua, S.; Tian, Y.; Liu, J. Cyclodextrin-based adsorbents for the removal of pollutants from wastewater: A review. Environ. Sci. Pollut. Res. Int. 2021, 28, 1317–1340. [Google Scholar] [CrossRef] [PubMed]
- Berdimurodov, E.; Eliboyev, I.; Berdimuradov, K.; Kholikov, A.; Akbarov, K.; Dagdag, O.; Rbaa, M.; El Ibrahimi, B.; Verma, D.K.; Haldhar, R.; et al. Green beta-cyclodextrin-based corrosion inhibitors: Recent developments, innovations and future opportunities. Carbohydr. Polym. 2022, 292, 119719. [Google Scholar] [CrossRef] [PubMed]
- Goyal, N.; Amar, A.; Gulati, S.; Varma, R.S. Cyclodextrin-Based Nanosponges as an Environmentally Sustainable Solution for Water Treatment: A Review. ACS Appl. Nano Mater. 2023, 6, 13766–13791. [Google Scholar] [CrossRef]
- Fouda-Mbanga, B.G.; Tywabi-Ngeva, Z.; Badawy, W.M.; Ebite, C.; Onotu, O.P.; Abogidi, C.; Uzordinma, A.P.; Kaba, S. Green cyclodextrins-derivatives for sustainable remediation of pesticides and heavy metals: A review. J. Mol. Struct. 2025, 1328, 141326. [Google Scholar] [CrossRef]
- Szczupaj, G.; Wójcik, J.; Ejchart, A.; Nowakowski, M. NMR studies of complex formation between natural cyclodextrins and benzene. J. Incl. Phenom. Macrocycl. Chem. 2024, 104, 129–136. [Google Scholar] [CrossRef]
- Jopa, S.; Wojcik, J.; Ejchart, A.; Nowakowski, M. NMR studies of inclusion complexes: Naphthalene and natural cyclodextrins. Phys. Chem. Chem. Phys. 2022, 24, 13690–13697. [Google Scholar] [CrossRef] [PubMed]
- Floare, C.G.; Bogdan, M.; Tomoaia-Cotişel, M.; Mocanu, A. 1H NMR spectroscopic characterization of inclusion complex of desferrioxamine B chelator and β-cyclodextrin. J. Mol. Struct. 2022, 1248, 131477. [Google Scholar] [CrossRef]
- Betlejewska-Kielak, K.; Bednarek, E.; Budzianowski, A.; Michalska, K.; Maurin, J.K. Comprehensive Characterisation of the Ketoprofen-beta-Cyclodextrin Inclusion Complex Using X-ray Techniques and NMR Spectroscopy. Molecules 2021, 26, 4089. [Google Scholar] [CrossRef] [PubMed]
- Paladini, G.; Caridi, F.; Crupi, V.; De Gaetano, F.; Majolino, D.; Tommasini, S.; Ventura, C.A.; Venuti, V.; Stancanelli, R. Temperature-Dependent Dynamical Evolution in Coum/SBE-beta-CD Inclusion Complexes Revealed by Two-Dimensional FTIR Correlation Spectroscopy (2D-COS). Molecules 2021, 26, 3749. [Google Scholar] [CrossRef] [PubMed]
- Hadaruga, N.G.; Popescu, G.; Gligor Pane, D.; Mitroi, C.L.; Stanciu, S.M.; Hadaruga, D.I. Discrimination of beta-cyclodextrin/hazelnut (Corylus avellana L.) oil/flavonoid glycoside and flavonolignan ternary complexes by Fourier-transform infrared spectroscopy coupled with principal component analysis. Beilstein, J. Org. Chem. 2023, 19, 380–398. [Google Scholar] [CrossRef] [PubMed]
- Peptu, C.; Blaj, D.A.; Balan-Porcarasu, M.; Rydz, J. Cyclodextrin-Oligocaprolactone Derivatives-Synthesis and Advanced Structural Characterization by MALDI Mass Spectrometry. Polymers 2022, 14, 1436. [Google Scholar] [CrossRef] [PubMed]
- Blaj, D.-A.; Balan-Porcarasu, M.; Petre, B.A.; Harabagiu, V.; Peptu, C. MALDI mass spectrometry monitoring of cyclodextrin-oligolactide derivatives synthesis. Polymers 2021, 233, 124186. [Google Scholar] [CrossRef]
- Blaj, D.A.; Kowalczuk, M.; Peptu, C. Mass Spectrometry of Esterified Cyclodextrins. Molecules 2023, 28, 2001. [Google Scholar] [CrossRef] [PubMed]
- Rigaud, S.; Dosso, A.; Lesur, D.; Cailleu, D.; Mathiron, D.; Pilard, S.; Cezard, C.; Djedaini-Pilard, F. Mass Spectrometry, Ion Mobility Separation and Molecular Modelling: A Powerful Combination for the Structural Characterisation of Substituted Cyclodextrins Mixtures. Int. J. Mol. Sci. 2022, 23, 13352. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Yang, J.; Wang, Q.; Ren, L.; Zhou, J. Physicochemical properties of catechin/β-cyclodextrin inclusion complex obtained via co-precipitation. CyTA–J. Food 2019, 17, 544–551. [Google Scholar] [CrossRef]
- Chang, C.; Song, M.; Ma, M.; Song, J.; Cao, F.; Qin, Q. Preparation, Characterization and Molecular Dynamics Simulation of Rutin-Cyclodextrin Inclusion Complexes. Molecules 2023, 28, 955. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, S.; Zhu, H.; Wang, S.; Xing, J. Inclusion Complexes of Lycopene and beta-Cyclodextrin: Preparation, Characterization, Stability and Antioxidant Activity. Antioxidants 2019, 8, 314. [Google Scholar] [CrossRef] [PubMed]
- Vicatos, A.I.; Hoossen, Z.; Caira, M.R. Inclusion complexes of the steroid hormones 17beta-estradiol and progesterone with beta- and gamma-cyclodextrin hosts: Syntheses, X-ray structures, thermal analyses and API solubility enhancements. Beilstein J. Org. Chem. 2022, 18, 1749–1762. [Google Scholar] [CrossRef] [PubMed]
- Czapnik, P.; Stępniak, A.; Buczkowski, A.; Zawisza, A.; Małecka, M. Inclusion complexes of α-cyclodextrin with p-aminobenzoic acid and nicotinic acid: Crystal structure, DSC and IR spectroscopy analysis in solid state and 1H NMR and ITC calorimetric studies of complexation in solutions. J. Mol. Struct. 2025, 1320, 139671. [Google Scholar] [CrossRef]
- Betlejewska-Kielak, K.; Bednarek, E.; Budzianowski, A.; Michalska, K.; Maurin, J.K. Comprehensive characterisation of the flurbiprofen/β-cyclodextrin inclusion complex using X-ray techniques and NMR spectroscopy. J. Mol. Struct. 2023, 1285, 135450. [Google Scholar] [CrossRef]
- Gasbarri, C.; Angelini, G. Combined calorimetric, spectroscopic and microscopic investigation on the inclusion complex from cyclocurcumin and sulfobutylether-β-cyclodextrin in aqueous solution and Kinetics of thermal cis-trans isomerization. Colloids Surf. A Physicochem. Eng. Asp. 2023, 664, 131149. [Google Scholar] [CrossRef]
- Yao, S.; Falaise, C.; Khlifi, S.; Leclerc, N.; Haouas, M.; Landy, D.; Cadot, E. Redox-Responsive Host-Guest Association between gamma-Cyclodextrin and Mixed-Metal Keggin-Type Polyoxometalates. Inorg. Chem. 2021, 60, 7433–7441. [Google Scholar] [CrossRef] [PubMed]
- D’Aria, F.; Pagano, B.; Giancola, C. Thermodynamic properties of hydroxypropyl-β-cyclodextrin/guest interaction: A survey of recent studies. J. Therm. Anal. Calorim. 2021, 147, 4889–4897. [Google Scholar] [CrossRef]
- Goren, E.; Iron, M.A.; Diskin-Posner, Y.; Falkovich, A.; Avram, L.; Bar-Shir, A. NMR exchange dynamics studies of metal-capped cyclodextrins reveal multiple populations of host-guest complexes in solution. Chem. Sci. 2023, 14, 11351–11358. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, S.; Daftardar, S.; Fratev, F.; Rivera, M.; Sirimulla, S.; Alexander, K.; Boddu, S.H.S. Elucidation of the orientation of selected drugs with 2-hydroxylpropyl-beta-cyclodextrin using 2D-NMR spectroscopy and molecular modeling. Int. J. Pharm. 2018, 545, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Rajamohan, R.; Viswalingam, M.; Lee, Y.R.; Prabu, S.; Sivakumar, K. Molecular encapsulation of nortriptyline in the β-cyclodextrin cavity: In-vitro cytotoxic potential against MCF-7 cell line. Korean J. Chem. Eng. 2023, 40, 1715–1724. [Google Scholar] [CrossRef]
- Banjare, M.K.; Behera, K.; Banjare, R.K.; Pandey, S.; Ghosh, K.K. Inclusion complexation of imidazolium-based ionic liquid and β-cyclodextrin: A detailed spectroscopic investigation. J. Mol. Liq. 2020, 302, 112530. [Google Scholar] [CrossRef]
- Periasamy, R.; Kothainayaki, S.; Sivakumar, K. Host-guest inclusion complex of β-cyclodextrin and 4,4′-(1,4-phenylenediisopropylidene)bisaniline: Spectral, structural and molecular modeling studies. J. Mol. Struct. 2021, 1224, 129050. [Google Scholar] [CrossRef]
- Alramadhan, H.; Elbashir, A.A.; Alnajjar, A.O. Supramolecular Interaction of Atenolol and Propranolol with beta-Cyclodextrin Spectroscopic Characterization and Analytical Application. Molecules 2024, 29, 2875. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Sun, L.; Qiao, C.; Liu, Y.; Wang, Y.; Feng, R.; Zhang, H.; Zhang, Y. Cyclodextrin-based delivery systems for chemical and genetic drugs: Current status and future. Carbohydr. Polym. 2025, 352, 123174. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, M.C.; Sun, W.Q.; Lin, R.L.; Lin, R.G.; Liu, J.X. Solid-State Supramolecular Inclusion Complexes of β-Cyclodextrin with Carboxyphenyl Viologens Showing Photochromic Properties. J. Phys. Chem. 2022, 126, 844–850. [Google Scholar] [CrossRef]
- Nakhle, L.; Kfoury, M.; Ruellan, S.; Greige-Gerges, H.; Landy, D. Insights on cyclodextrin inclusion complexes in deep eutectic solvents:Water mixtures. J. Mol. Struct. 2023, 1293, 136260. [Google Scholar] [CrossRef]
- Zlibut, E.; May, J.C.; Wei, Y.; Gessmann, D.; Wood, C.S.; Bernat, B.A.; Pugh, T.E.; Palmer-Jones, L.; Cosquer, R.P.; Dybeck, E.; et al. Noncovalent Host–Guest Complexes of Artemisinin with α-, β-, and γ- Cyclodextrin Examined by Structural Mass Spectrometry Strategies. Anal. Chem. 2023, 95, 8180–8188. [Google Scholar] [CrossRef] [PubMed]
- Bouhedja, M.; Dinar, K.; Sahra, K.; Habila, T.; Stiti, M.Z. Theoretical study of inclusion complex between R/S-1-[1-(5-chloro-2-methoxyphenyl)ethyl]-3-methyl-3-phenylsulfonylurea and β-cyclodextrin. Braz. J. Health Rev. 2024, 7, e76228. [Google Scholar] [CrossRef]
- Jeyadurai, J.A.; Muthuselvan, P.; Prabhu, A.A.M.; Rajendiran, N.; Dasan, A. Formation of Inclusion Complexes of 2-Hydroxypropyl β-Cyclodextrin with 2-Hydroxychalcone: Experimental and Theoretical Study. Polycycl. Aromat. Compd. 2024, 44, 5038–5059. [Google Scholar] [CrossRef]
- Esmaeilpour, D.; Hussein, A.A.; Almalki, F.A.; Shityakov, S.; Bordbar, A.K. Probing inclusion complexes of 2-hydroxypropyl-β-cyclodextrin with mono-amino mono-carboxylic acids: Physicochemical specification, characterization and molecular modeling. Heliyon 2020, 6, e03360. [Google Scholar] [CrossRef] [PubMed]
- Shumilin, I.; Tanbuz, A.; Harries, D. Self-association of cyclodextrin inclusion complexes in a deep eutectic solvent enhances guest solubility. Carbohydr. Polym. 2025, 351, 123067. [Google Scholar] [CrossRef] [PubMed]
- Raffaini, G.; Elli, S.; Ganazzoli, F.; Catauro, M. Inclusion Complexes Between β-cyclodextrin and the Anticancer Drug 5-Fluorouracil for its Solubilization: A Molecular Dynamics Study at Different Stoichiometries. Macromol. Symp. 2022, 404, 2100305. [Google Scholar] [CrossRef]
- Potti, L.; Avula, P.R. Quality by Design approach for Optimization and Development of Cyclodextrin-Surfactant Complex Based Formulations for Bioavailability Enhancement of Valsartan. Biointerface Res. Appl. Chem. 2022, 13, 388. [Google Scholar] [CrossRef]
- Cerutti, J.P.; Quevedo, M.A.; Buhlman, N.; Longhi, M.R.; Zoppi, A. Synthesis and characterization of supramolecular systems containing nifedipine, beta-cyclodextrin and aspartic acid. Carbohydr. Polym. 2019, 205, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Srabovic, M.; Huremovic, M.; Salihovic, M.; Catovic, B. Inclusion complexation of carbamazepine with different β-cyclodextrins buffer solutions. J. Chem. Biol. Phys. Sci. 2022, 12, 112–126. [Google Scholar] [CrossRef]
- Suta, L.M.; Ridichie, A.; Ledeti, A.; Temereanca, C.; Ledeti, I.; Muntean, D.; Radulescu, M.; Varut, R.M.; Watz, C.; Craineanu, F.; et al. SsssHost-Guest Complexation of Itraconazole with Cyclodextrins for Bioavailability Enhancement. Pharmaceutics 2024, 16, 560. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhu, W.; Ge, X.; Li, R.; Li, S.; Chen, X.; Song, J.; Xie, J.; Chen, X.; Yang, H. A New Class of NIR-II Gold Nanocluster-Based Protein Biolabels for In Vivo Tumor-Targeted Imaging. Angew. Chem. Int. Ed. Engl. 2021, 60, 1306–1312. [Google Scholar] [CrossRef] [PubMed]
- Tai, W.; Khanal, D.; Arnold, J.C.; Chan, H.K.; Kwok, P.C.L. Solubilising and Aerosolising Cannabidiol Using Methyl beta-Cyclodextrin and Human Serum Albumin. AAPS PharmSciTech 2025, 26, 120. [Google Scholar] [CrossRef] [PubMed]
- Schoeman, C.; van Niekerk, S.; Liebenberg, W.; Hamman, J. Cyclodextrin inclusion complex and amorphous solid dispersions as formulation approaches for enhancement of curcumin’s solubility and nasal epithelial membrane permeation. Future J. Pharm. Sci. 2024, 10, 85. [Google Scholar] [CrossRef]
- Devangan, P.; Mourya, A.; Arya, S.; Patel, D.; Jaiswal, A.; Dewangan, A. Molecular Encapsulation of Abiraterone Acetate in 2,6 Di-O-Methyl-β-Cyclodextrin for Solubility Enhancement: Synthesis, Characterization, In Vitro Drug Release, and Molecular Docking. BioNanoScience 2024, 14, 2801–2816. [Google Scholar] [CrossRef]
- Hadadian, M.; Allahyari, R.; Mahdavi, B.; Mohammadhosseini, M. Comparative study of β-cyclodextrin and carboxymethyl-β-cyclodextrin as effective drug delivery systems for oxymetholone: Design, preparation, characterization, phase solubility and in vitro drug release studies. J. Sci. Adv. Mater. Devices 2024, 9, 100751. [Google Scholar] [CrossRef]
- Javanbakht, S.; Poursadegh, H.; Darvishi, S.; Mohammadzadeh, A.; Saboury, A.; Joulaei, M.; Mohammadi, R. Application or function of cyclodextrin in insulin and cell delivery for efficient diabetic treatment. Hybrid Adv. 2025, 10, 100462. [Google Scholar] [CrossRef]
- Sampathi, S.; Kulkarni, N.; Bhikshapathi, D.V.R.N.; Tawade, J.V.; Tarakaramu, N.; Rashid, R.F.; Kubaev, A. Optimizing ibrutinib bioavailability: Formulation and assessment of hydroxypropyl-β-cyclodextrin-based nanosponge delivery systems. Curr. Res. Pharmacol. Drug Discov. 2025, 8, 100213. [Google Scholar] [CrossRef] [PubMed]
- Di Donato, C.; Iacovino, R.; Isernia, C.; Malgieri, G.; Varela-Garcia, A.; Concheiro, A.; Alvarez-Lorenzo, C. Polypseudorotaxanes of Pluronic(R) F127 with Combinations of alpha- and beta-Cyclodextrins for Topical Formulation of Acyclovir. Nanomaterials 2020, 10, 613. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Minhas, M.U.; Ahmad, M.; Sohail, M. Self-assembled supramolecular thermoreversible beta-cyclodextrin/ethylene glycol injectable hydrogels with difunctional Pluronic((R))127 as controlled delivery depot of curcumin. Development, characterization and in vitro evaluation. J. Biomater. Sci. Polym. Ed. 2018, 29, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zhang, Z.; Ding, C.; Xu, S.; Xu, Z. The Use of Cyclodextrin Inclusion Complexes to Increase the Solubility and Pharmacokinetic Profile of Albendazole. Molecules 2023, 28, 7295. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.; Alruwaili, N.K.; Imam, S.S.; Alsaidan, O.A.; Alharbi, K.S.; Mostafa, E.M.; Musa, A.; Gilani, S.J.; Ghoneim, M.M.; Alshehri, S.; et al. Formulation of ternary genistein β-cyclodextrin inclusion complex: In vitro characterization and cytotoxicity assessment using breast cancer cell line. J. Drug Deliv. Sci. Technol. 2022, 67, 102932. [Google Scholar] [CrossRef]
- Zafar, A.; Alruwaili, N.K.; Imam, S.S.; Alsaidan, O.A.; Alkholifi, F.K.; Alharbi, K.S.; Mostafa, E.M.; Alanazi, A.S.; Gilani, S.J.; Musa, A.; et al. Formulation of Genistein-HP beta Cyclodextrin-Poloxamer 188 Ternary Inclusion Complex: Solubility to Cytotoxicity Assessment. Pharmaceutics 2021, 13, 1997. [Google Scholar] [CrossRef] [PubMed]
- Wdowiak, K.; Rosiak, N.; Tykarska, E.; Zarowski, M.; Plazinska, A.; Plazinski, W.; Cielecka-Piontek, J. Amorphous Inclusion Complexes: Molecular Interactions of Hesperidin and Hesperetin with HP-Beta-CD and Their Biological Effects. Int. J. Mol. Sci. 2022, 23, 4000. [Google Scholar] [CrossRef] [PubMed]
- Appleton, S.L.; Tannous, M.; Argenziano, M.; Muntoni, E.; Rosa, A.C.; Rossi, D.; Caldera, F.; Scomparin, A.; Trotta, F.; Cavalli, R. Nanosponges as protein delivery systems: Insulin, a case study. Int. J. Pharm. 2020, 590, 119888. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Guo, T.; Wang, Y.; Yang, X.; Liao, Y.; Tang, X.; Zhang, X. A micrometer sized porous beta-cyclodextrin polymer for improving bioavailability of poorly soluble drug. Carbohydr. Polym. 2025, 350, 123042. [Google Scholar] [CrossRef] [PubMed]
- Azeez, M.; Sumer, S.; Suryakanta, S.; Debashish, G. Quality by Design Enabled-Cyclodextrin Complexes of Lisinopril by Kneading Method: Improved Solubility and Bioavailability. Curr. Mater. Sci. 2024, 17, 135–147. [Google Scholar] [CrossRef]
- Hoseini Aghdam, S.; Allahyari, S. Enhancing the Dissolution of Flutamide Through Supersaturation Using Beta-Cyclodextrin: A Promising Approach for Improved Solubility of Poorly Water-Soluble Drugs. J. Pharm. Innov. 2023, 18, 2294–2304. [Google Scholar] [CrossRef]
- Chen, W.; Zheng, X.; Lao, W.; Wang, H.; Chen, S.; Liu, C.; Chen, Z.; Bai, Y.; Zhang, H.; Zhan, X.; et al. Enhancement of the solubility and oral bioavailability of altrenogest through complexation with hydroxypropyl-beta-cyclodextrin. Eur. J. Pharm. Sci. 2024, 194, 106691. [Google Scholar] [CrossRef] [PubMed]
- Mashaqbeh, H.; Obaidat, R.; Al-Shar’i, N. Evaluation and Characterization of Curcumin-beta-Cyclodextrin and Cyclodextrin-Based Nanosponge Inclusion Complexation. Polymers 2021, 13, 4073. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lee, Y.Y.; Tan, C.P.; Wang, Y.; Qiu, C. Improved solubility and bioavailability of cyclolinopeptides by diacylglycerol in the beta-cyclodextrin Pickering emulsions. Food Chem. 2025, 464, 141553. [Google Scholar] [CrossRef] [PubMed]
- Thiberville, L.; Faivre, V.; Sizun, C.; Dehouck, M.P.; Landry, C.; Baati, R.; Tsapis, N. Cyclodextrin-based formulations for delivering broad-spectrum nerve agent antidote to the central nervous system: Stability, physicochemical characterization and application in a human blood-brain barrier model. Int. J. Pharm. 2025, 674, 125505. [Google Scholar] [CrossRef] [PubMed]
- Saita, M.G.; Aleo, D.; Melilli, B.; Patti, A. Effect of cyclodextrin additives on azithromycin in aqueous solution and insight into the stabilization mechanism by sulfobutyl ether-beta-cyclodextrin. Int. J. Pharm. 2019, 566, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Rajamohan, R.; Murugavel, K.; Almoajel, A.; Elsadek, M.F.; Subramanian, S.A.; Kim, S.J.; Dhandapani, S.; Lee, Y.R. Fabrication of biocompatible water-soluble supramolecular nanofibers of oseltamivir with methyl-β-cyclodextrin for enhanced drug release. J. Drug Deliv. Sci. Technol. 2024, 97, 105769. [Google Scholar] [CrossRef]
- Hadadian, M.; Mahdavi, B.; Rezaei-Seresht, E. Exploring modified cyclodextrins for enhanced encapsulation and release of ethinyl estradiol: Physicochemical characterization and kinetic modeling. J. Sci. Adv. Mater. Devices 2025, 10, 100837. [Google Scholar] [CrossRef]
- Yang, J.; Jia, L.; He, Z.; Wang, Y. Recent advances in SN-38 drug delivery system. Int. J. Pharm. 2023, 637, 122886. [Google Scholar] [CrossRef] [PubMed]
- Soe, H.; Junthip, J.; Chamni, S.; Chansriniyom, C.; Limpikirati, P.; Thanusuwannasak, T.; Asasutjarit, R.; Pruksakorn, P.; Autthateinchai, R.; Wet-Osot, S.; et al. A promising synthetic citric crosslinked beta-cyclodextrin derivative for antifungal drugs: Solubilization, cytotoxicity, and antifungal activity. Int. J. Pharm. 2023, 645, 123394. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Aazam, E.S.; Jain, S.K. Synthesis and Characterization of β-Cyclodextrin/poly(1-naphthylamine) Inclusion Complex and In-Vitro Release Studies of Metformin Hydrochloride. J. Polym. Environ. 2020, 28, 1106–1116. [Google Scholar] [CrossRef]
- Tian, B.; Liu, Y.; Liu, J. Cyclodextrin as a magic switch in covalent and non-covalent anticancer drug release systems. Carbohydr. Polym. 2020, 242, 116401. [Google Scholar] [CrossRef] [PubMed]
- Lou, C.; Tian, X.; Deng, H.; Wang, Y.; Jiang, X. Dialdehyde-beta-cyclodextrin-crosslinked carboxymethyl chitosan hydrogel for drug release. Carbohydr. Polym. 2020, 231, 115678. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Y.; Chen, S.; Cheong, K.L.; Teng, B. Carboxymethyl beta-cyclodextrin grafted carboxymethyl chitosan hydrogel-based microparticles for oral insulin delivery. Carbohydr. Polym. 2020, 246, 116617. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Zhang, B.; Zhou, W.; Liu, Y. High-Efficiency Synergistic Effect of Supramolecular Nanoparticles Based on Cyclodextrin Prodrug on Cancer Therapy. Biomacromolecules 2020, 21, 4998–5007. [Google Scholar] [CrossRef] [PubMed]
- Pistone, M.; Racaniello, G.F.; Rizzi, R.; Iacobazzi, R.M.; Arduino, I.; Lopalco, A.; Lopedota, A.A.; Denora, N. Direct cyclodextrin based powder extrusion 3D printing of budesonide loaded mini-tablets for the treatment of eosinophilic colitis in paediatric patients. Int. J. Pharm. 2023, 632, 122592. [Google Scholar] [CrossRef] [PubMed]
- Celesti, C.; Mele, A.; Espro, C.; Raffaini, G.; Lagana, A.; Visalli, G.; Giofre, S.V.; Gaetano, F.; Neri, G.; Caronna, T.; et al. A smart beta-Cyclodextrin-Aza[5]Helicene system for enhanced gemcitabine delivery and tracking in cancer cells. Int. J. Pharm. 2025, 676, 125611. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, Z.; Abdouss, M. Electrospun nanofibers using beta-cyclodextrin grafted chitosan macromolecules loaded with indomethacin as an innovative drug delivery system. Int. J. Biol. Macromol. 2023, 233, 123518. [Google Scholar] [CrossRef] [PubMed]
- Rajamohan, R.; Muthuraja, P.; Murugavel, K.; Mani, M.K.; Prabakaran, D.S.; Seo, J.H.; Malik, T.; Lee, Y.R. Significantly improving the solubility and anti-inflammatory activity of fenofibric acid with native and methyl-substituted beta-cyclodextrins via complexation. Sci. Rep. 2025, 15, 853. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cronin, M.F.; Mendonca, M.C.P.; Guo, J.; O’Driscoll, C.M. Sialic acid-targeted cyclodextrin-based nanoparticles deliver CSF-1R siRNA and reprogram tumour-associated macrophages for immunotherapy of prostate cancer. Eur. J. Pharm. Sci. 2023, 185, 106427. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.T.; Cagno, V.; Janecek, M.; Ortiz, D.; Gasilova, N.; Piret, J.; Gasbarri, M.; Constant, D.A.; Han, Y.; Vukovic, L.; et al. Modified cyclodextrins as broad-spectrum antivirals. Sci. Adv. 2020, 6, eaax9318. [Google Scholar] [CrossRef] [PubMed]
- Braga, S.S.; Barbosa, J.S.; Santos, N.E.; El-Saleh, F.; Paz, F.A.A. Cyclodextrins in Antiviral Therapeutics and Vaccines. Pharmaceutics 2021, 13, 409. [Google Scholar] [CrossRef] [PubMed]
- Almeida, B.; Domingues, C.; Mascarenhas-Melo, F.; Silva, I.; Jarak, I.; Veiga, F.; Figueiras, A. The Role of Cyclodextrins in COVID-19 Therapy-A Literature Review. Int. J. Mol. Sci. 2023, 24, 2974. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Hong, S.; Goh, L.Y.H.; Zhang, H.; Peng, T.; Chow, K.T.; Gokhale, R.; Tuliani, V. Investigation on the Combined Effect of Hydroxypropyl Beta-Cyclodextrin (HPbetaCD) and Polysorbate in Monoclonal Antibody Formulation. Pharmaceuticals 2024, 17, 528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Chen, G.; Wang, Z.; Ma, J.; Jia, Q. Design and synthesis of Fe3O4@Au@cyclodextrin-molecularly imprinted polymers labeled with SERS nanotags for ultrasensitive detection of transferrin. Sens. Actuators B Chem. 2022, 361, 131669. [Google Scholar] [CrossRef]
- Yang, H.; Wang, N.; Yang, R.; Zhang, L.; Jiang, X. Folic Acid-Decorated beta-Cyclodextrin-Based Poly(epsilon-caprolactone)-dextran Star Polymer with Disulfide Bond-Linker as Theranostic Nanoparticle for Tumor-Targeted MRI and Chemotherapy. Pharmaceutics 2021, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- Ou, C.; Li, C.; Feng, C.; Tong, X.; Vasta, G.R.; Wang, L.X. Synthesis, binding affinity, and inhibitory capacity of cyclodextrin-based multivalent glycan ligands for human galectin-3. Bioorg. Med. Chem. 2022, 72, 116974. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Yang, H.; Zhang, M.; Li, Z.; Sun, N.; Han, Z.; Shu, C.; Li, Z.; Wang, D. Inhalable cyclodextrin metal-organic framework dry powder enhanced direct pulmonary delivery of paclitaxel for the treatment of lung cancer. J. Drug Deliv. Sci. Technol. 2024, 101, 106310. [Google Scholar] [CrossRef]
- Fu, J.; Zhao, W.; Liang, N.; Sun, S. Functionalized hydroxypropyl-beta-cyclodextrin inclusion complex for combined tumor therapy through intelligent delivery of paclitaxel and polarization of M2-like tumor associated macrophages. Colloids Surf. B Biointerfaces 2025, 252, 114654. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, N.; Wu, T.; Qiu, C.; Wang, X.; Jiang, S.; Zhang, Z.; Liu, T.; Wei, C.; Wang, T. Preparation of curcumin-hydroxypropyl-beta-cyclodextrin inclusion complex by cosolvency-lyophilization procedure to enhance oral bioavailability of the drug. Drug Dev. Ind. Pharm. 2018, 44, 1966–1974. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.R.P.; Sakharwade, S.A. Enhancement of Solubility of Albendazole by Inclusion Complexation with Nanosponges and β-Cyclodextrin. Indian J. Pharm. Educ. Res. 2024, 58, s306–s315. [Google Scholar] [CrossRef]
- Bechairia, W. Role of abiraterone acetate in the management of metastatic prostate cancer. Department experience. Batna J. Med. Sci. (BJMS) 2023, 10, 37–40. [Google Scholar] [CrossRef]
- Gala, U.H.; Miller, D.A.; Su, Y.; Spangenberg, A.; Williams, R.O.B., 3rd. The effect of drug loading on the properties of abiraterone-hydroxypropyl beta cyclodextrin solid dispersions processed by solvent free KinetiSol(R) technology. Eur. J. Pharm. Biopharm. 2021, 165, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Tamizhmathy, M.; Gupta, U.; Shettiwar, A.; Kumar, G.S.; Daravath, S.; Aalhate, M.; Mahajan, S.; Maji, I.; Sriram, A.; Modak, C.; et al. Formulation of inclusion complex of Abiraterone acetate with 2-Hydroxypropyl-Beta-Cyclodextrin: Physiochemical characterization, molecular docking and bioavailability evaluation. J. Drug Deliv. Sci. Technol. 2023, 82, 104321. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, J.; Yu, Y.; Ren, W.; Yuan, R.; Liu, X.; Shang, X.; Du, Z.; Xu, M.-L.; Zhang, T. Alleviates effects of γ-cyclodextrin/genistein inclusion complex on oxidative stress injury induced by organic hydrogen peroxide in PC12 cells via Nrf2/GPx4 signaling pathway. Food Biosci. 2025, 63, 105631. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, L.; Zhu, P.; Liu, Q.; Liao, X.; Si, T.; Yang, B. Preparation, Characterization and Anticancer Activity of Inclusion Complexes between Genistein and Amino-Appended β-Cyclodextrins. ChemistrySelect 2022, 7, e202201125. [Google Scholar] [CrossRef]
- Donalisio, M.; Argenziano, M.; Ritta, M.; Bastiancich, C.; Civra, A.; Lembo, D.; Cavalli, R. Acyclovir-loaded sulfobutyl ether-beta-cyclodextrin decorated chitosan nanodroplets for the local treatment of HSV-2 infections. Int. J. Pharm. 2020, 587, 119676. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Kaur, B.; Thakur, K.; Mahajan, A.; Amarji, B.; Singh, M.P.; Katare, O.P. Pluronic F127-tailored lecithin organogel of acyclovir: Preclinical evidence of antiviral activity using BALB/c murine model of cutaneous HSV-1 infection. Drug Deliv. Transl. Res. 2022, 12, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liang, N.; Yan, P.; Kawashima, Y.; Sun, S. Inclusion complex based on N-acetyl-L-cysteine and arginine modified hydroxypropyl-beta-cyclodextrin for oral insulin delivery. Carbohydr. Polym. 2021, 252, 117202. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, M.; Sun, Y.; Zhang, L. A cell-penetrating peptide conjugated carboxymethyl-beta-cyclodextrin to improve intestinal absorption of insulin. Int. J. Biol. Macromol. 2018, 111, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Song, W.; Xue, S.; Sheng, Y.; Gao, B.; Dang, Y.; Zhang, Y.; Zhang, G. β-Cyclodextrin/dialdehyde glucan-coated keratin nanoparticles for oral delivery of insulin. Int. J. Biol. Macromol. 2024, 276, 133805. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Liang, X.; Zhou, C.; Qin, J.; Hou, C.; Zhu, Z.; Zhang, W.; Wang, S.; Zhong, D. Glutamine-beta-cyclodextrin for targeted doxorubicin delivery to triple-negative breast cancer tumors via the transporter ASCT2. J. Mater. Chem. B 2019, 7, 5363–5375. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Li, M.; Liu, T.; Liu, J.; Xie, Y.; Zhang, J.; Xu, S.; Liu, H. A dual-functional HER2 aptamer-conjugated, pH-activated mesoporous silica nanocarrier-based drug delivery system provides in vitro synergistic cytotoxicity in HER2-positive breast cancer cells. Int. J. Nanomed. 2019, 14, 4029–4044. [Google Scholar] [CrossRef] [PubMed]
- Karimi, S.; Namazi, H. Targeted co-delivery of doxorubicin and methotrexate to breast cancer cells by a pH-sensitive biocompatible polymeric system based on β-cyclodextrin crosslinked glycodendrimer with magnetic ZnO core. Eur. Polym. J. 2022, 176, 111435. [Google Scholar] [CrossRef]
- Khin, S.Y.; Soe, H.; Chansriniyom, C.; Pornputtapong, N.; Asasutjarit, R.; Loftsson, T.; Jansook, P. Development of Fenofibrate/Randomly Methylated beta-Cyclodextrin-Loaded Eudragit((R)) RL 100 Nanoparticles for Ocular Delivery. Molecules 2022, 27, 4755. [Google Scholar] [CrossRef] [PubMed]
- Ai, F.; Shen, W.; Shen, Q.; Yang, H.; Wang, J.; Yang, M.; Zhang, Z.; Zhang, N.; Xue, Y. Deciphering the multi-state binding and dissociation of fenofibrate with β-cyclodextrin and its derivatives: Insights from phase solubility analysis and molecular modeling. J. Mol. Liq. 2024, 415, 126374. [Google Scholar] [CrossRef]
- Imperio, D.; Grolla, A.A.; Moro, M.; Bortolotto, V.; Del Grosso, E.; Genazzani, A.A.; Panza, L. Functionalization of beta-cyclodextrin with a urea-based PSMA ligand and preliminary studies on targeting prostate cancer cells. Bioorg. Med. Chem. Lett. 2022, 73, 128890. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Liu, Y.; Zhao, L.; Zhao, L.; Wang, C.; Liu, C.; Xu, B. Construction of a pH-sensitive self-assembly in aqueous solutions based on a dansyl-modified beta-cyclodextrin. Soft Matter. 2021, 17, 7516–7523. [Google Scholar] [CrossRef] [PubMed]
- Mendonca, M.C.P.; Sun, Y.; Cronin, M.F.; Lindsay, A.J.; Cryan, J.F.; O’Driscoll, C.M. Cyclodextrin-Based Nanoparticles for Delivery of Antisense Oligonucleotides Targeting Huntingtin. Pharmaceutics 2023, 15, 520. [Google Scholar] [CrossRef] [PubMed]
- Jarazo, J.; Barmpa, K.; Modamio, J.; Saraiva, C.; Sabate-Soler, S.; Rosety, I.; Griesbeck, A.; Skwirblies, F.; Zaffaroni, G.; Smits, L.M.; et al. Parkinson’s Disease Phenotypes in Patient Neuronal Cultures and Brain Organoids Improved by 2-Hydroxypropyl-beta-Cyclodextrin Treatment. Mov. Disord. 2022, 37, 80–94. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, V.; Pandey, S.P.; Singh, P.K. Prospects of charged cyclodextrins in biomedical applications. Carbohydr. Polym. 2024, 323, 121348. [Google Scholar] [CrossRef] [PubMed]
- Abbas, Z.S.; Sulaiman, G.M.; Jabir, M.S.; Mohammed, S.A.A.; Khan, R.A.; Mohammed, H.A.; Al-Subaiyel, A. Galangin/beta-Cyclodextrin Inclusion Complex as a Drug-Delivery System for Improved Solubility and Biocompatibility in Breast Cancer Treatment. Molecules 2022, 27, 4521. [Google Scholar] [CrossRef] [PubMed]
- Scantlebery, A.M.L.; Ochodnicky, P.; Kors, L.; Rampanelli, E.; Butter, L.M.; El Boumashouli, C.; Claessen, N.; Teske, G.J.; van den Bergh Weerman, M.A.; Leemans, J.C.; et al. beta-Cyclodextrin counteracts obesity in Western diet-fed mice but elicits a nephrotoxic effect. Sci. Rep. 2019, 9, 17633. [Google Scholar] [CrossRef] [PubMed]
- Ali, W.A.; Moselhy, W.A.; Ibrahim, M.A.; Amin, M.M.; Kamel, S.; Eldomany, E.B. Protective effect of rutin and β-cyclodextrin against hepatotoxicity and nephrotoxicity induced by lambda-cyhalothrin in Wistar rats: Biochemical, pathological indices and molecular analysis. Biomarkers 2022, 27, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.H.; Garidel, P.; Michaela, B. HP-beta-CD for the formulation of IgG and Ig-based biotherapeutics. Int. J. Pharm. 2021, 601, 120531. [Google Scholar] [CrossRef] [PubMed]
- Ishitsuka, Y.; Irie, T.; Matsuo, M. Cyclodextrins applied to the treatment of lysosomal storage disorders. Adv. Drug Deliv. Rev. 2022, 191, 114617. [Google Scholar] [CrossRef] [PubMed]
- Al-Heibshy, F.N.S.; Basaran, E.; Ozturk, N.; Demirel, M. Preparation and in vitro characterization of rosuvastatin calcium incorporated methyl beta cyclodextrin and Captisol((R)) inclusion complexes. Drug Dev. Ind. Pharm. 2020, 46, 1495–1506. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Slathia, G.; Saneja, A. Unveiling the complexation mechanism of phloretin with Sulfobutylether–β–cyclodextrin (Captisol®) and its impact on anticancer activity. J. Mol. Liq. 2023, 391, 123348. [Google Scholar] [CrossRef]
- Geyer, F.A.; Domjan, J.; Szalai, T.V.; Rapi, Z.; Varga, Z.; Marosi, G.; Nagy, Z.K.; Hirsch, E. Stabilizing effect of HP-beta-CD on infliximab in liquid formulations and a solid formulation produced by electrospinning. Eur. J. Pharm. Sci. 2025, 206, 107014. [Google Scholar] [CrossRef] [PubMed]
- Sharapova, A.; Ol’khovich, M.; Blokhina, S. Complexation of telmisartan with cyclodextrins as a tool of solubility enhancement: A comparative analysis of influence of the cyclodextrin type and method of solid dispersion preparation. Colloids Surf. A Physicochem. Eng. Asp. 2025, 705, 135662. [Google Scholar] [CrossRef]
- Saxena, S.; Lis, M.J. Native Cyclodextrin-Based Metal-Organic Frameworks (MOFs): Synthesis, Characterization, and Potential Applications in Food Industry. Molecules 2025, 30, 293. [Google Scholar] [CrossRef] [PubMed]
- Garibyan, A.; Terekhova, I. Complex formation with modified cyclodextrins for improving biopharmaceutical properties of baricitinib, a novel immunomodulatory drug. J. Mol. Liq. 2024, 406, 125016. [Google Scholar] [CrossRef]
- Lachowicz, M.; Stanczak, A.; Kolodziejczyk, M. Characteristic of Cyclodextrins: Their Role and Use in the Pharmaceutical Technology. Curr. Drug Targets 2020, 21, 1495–1510. [Google Scholar] [CrossRef] [PubMed]
- Trotta, F.; Loftsson, T.; Gaud, R.S.; Trivedi, R.; Shende, P. Integration of cyclodextrins and associated toxicities: A roadmap for high quality biomedical applications. Carbohydr. Polym. 2022, 295, 119880. [Google Scholar] [CrossRef] [PubMed]
- Vass, P.; Demuth, B.; Farkas, A.; Hirsch, E.; Szabo, E.; Nagy, B.; Andersen, S.K.; Vigh, T.; Verreck, G.; Csontos, I.; et al. Continuous alternative to freeze drying: Manufacturing of cyclodextrin-based reconstitution powder from aqueous solution using scaled-up electrospinning. J. Control. Release 2019, 298, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Mousazadeh, H.; Pilehvar-Soltanahmadi, Y.; Dadashpour, M.; Zarghami, N. Cyclodextrin based natural nanostructured carbohydrate polymers as effective non-viral siRNA delivery systems for cancer gene therapy. J. Control. Release 2021, 330, 1046–1070. [Google Scholar] [CrossRef] [PubMed]

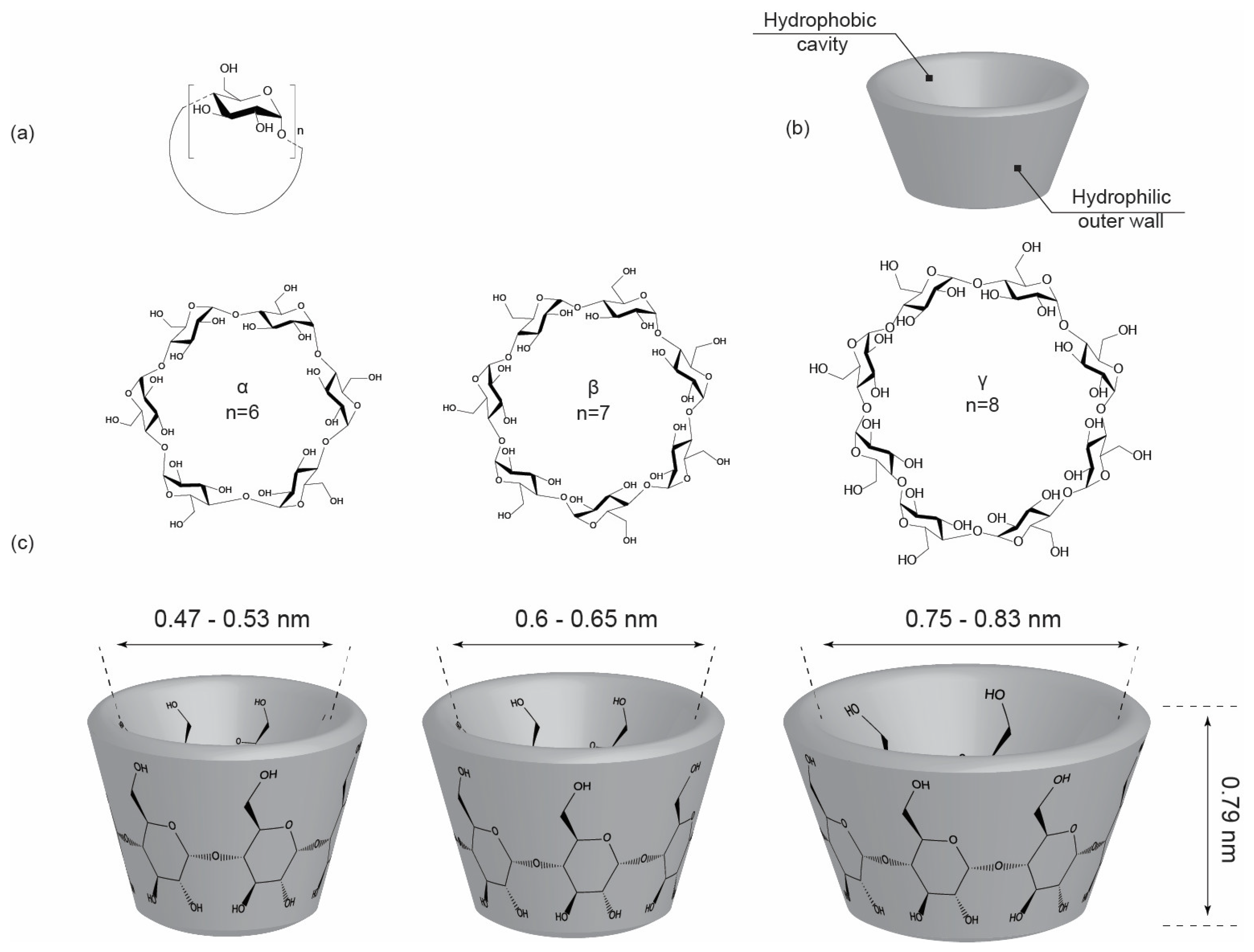
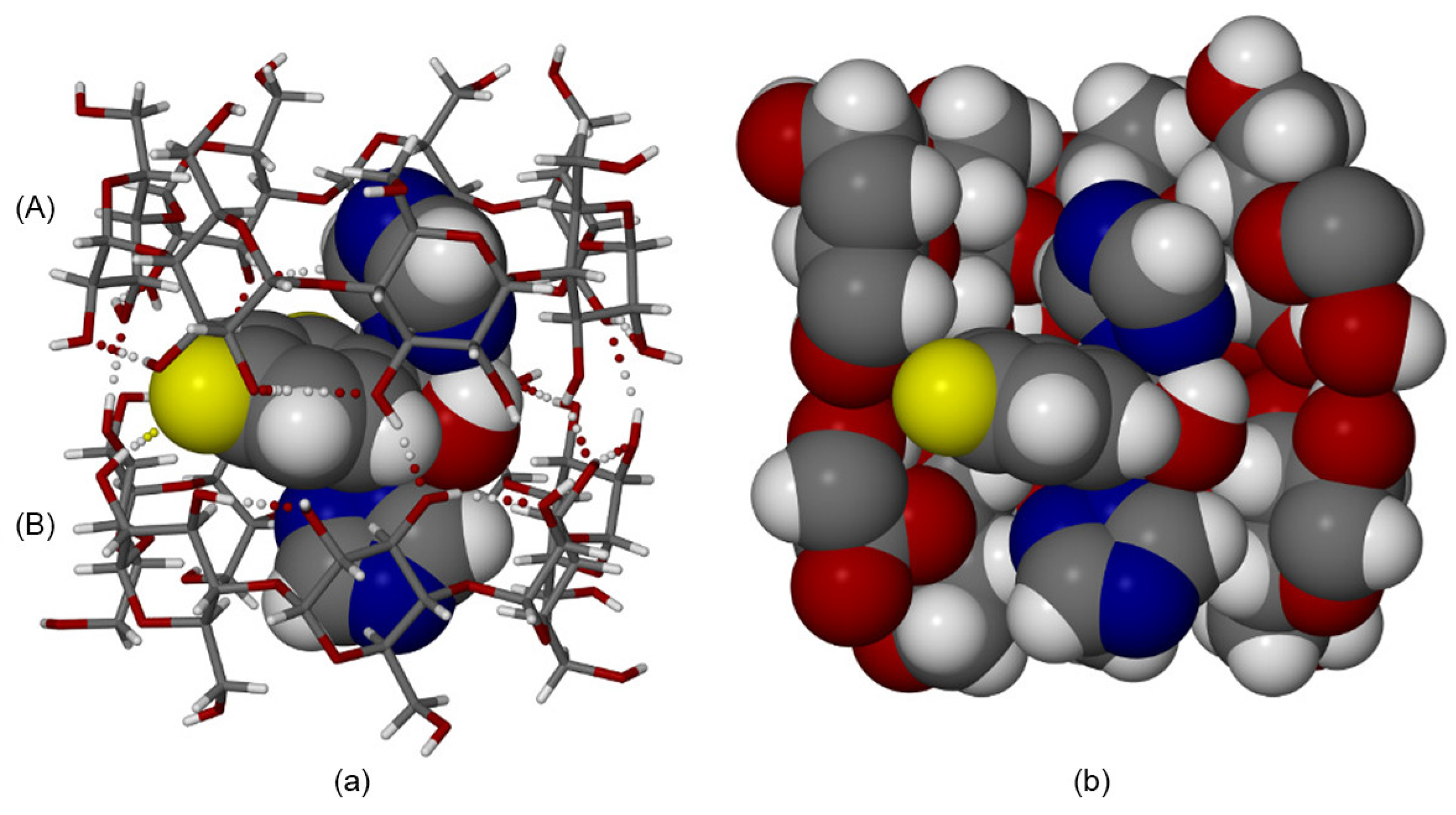
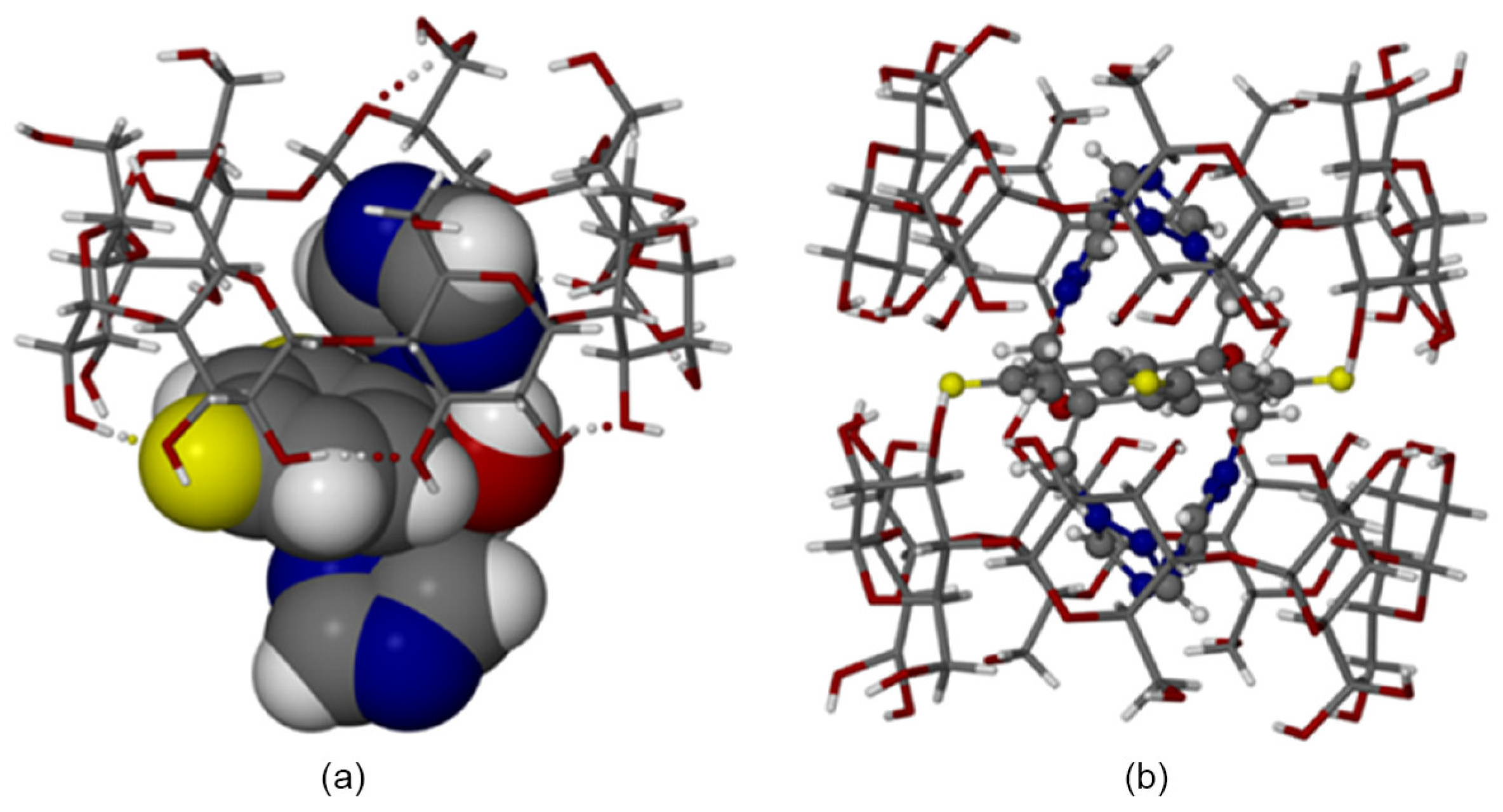
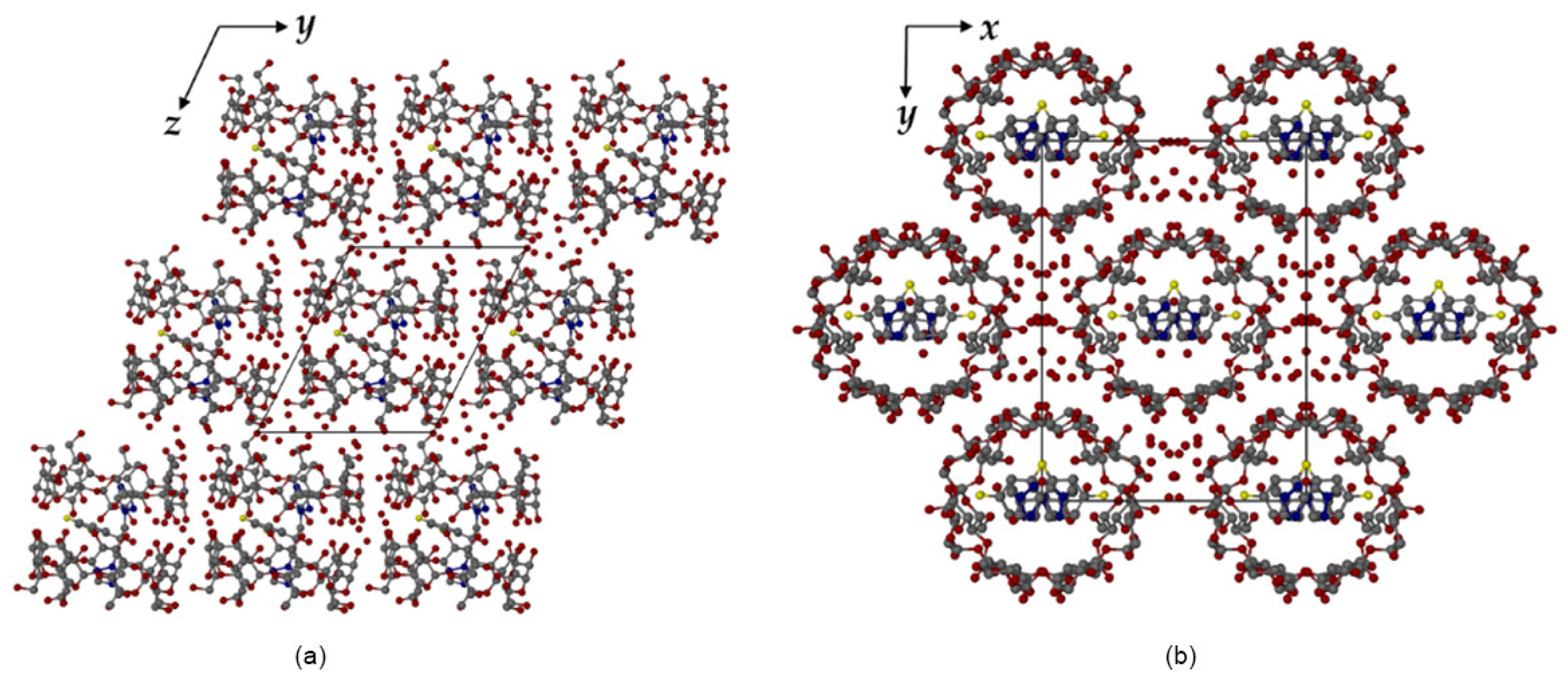
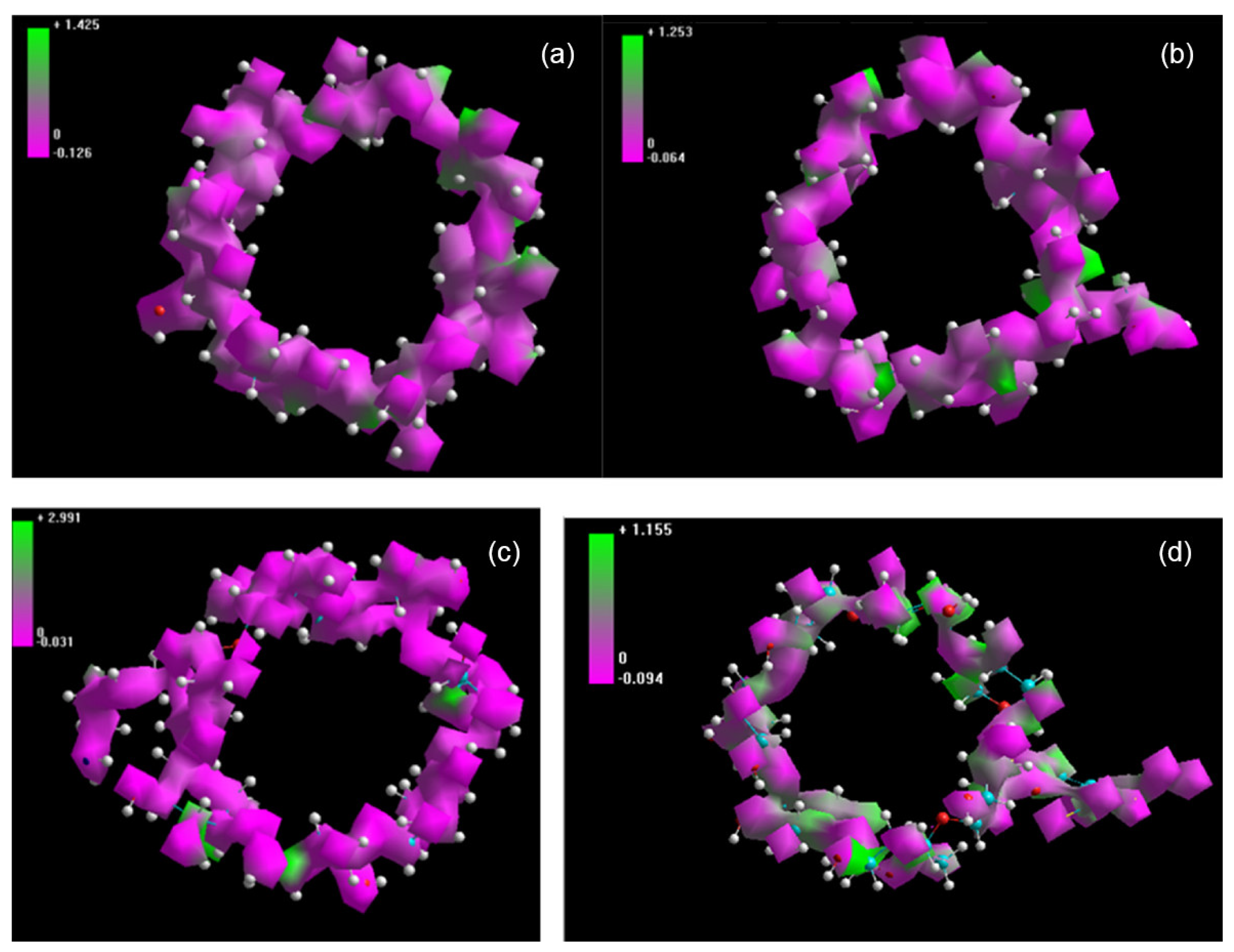
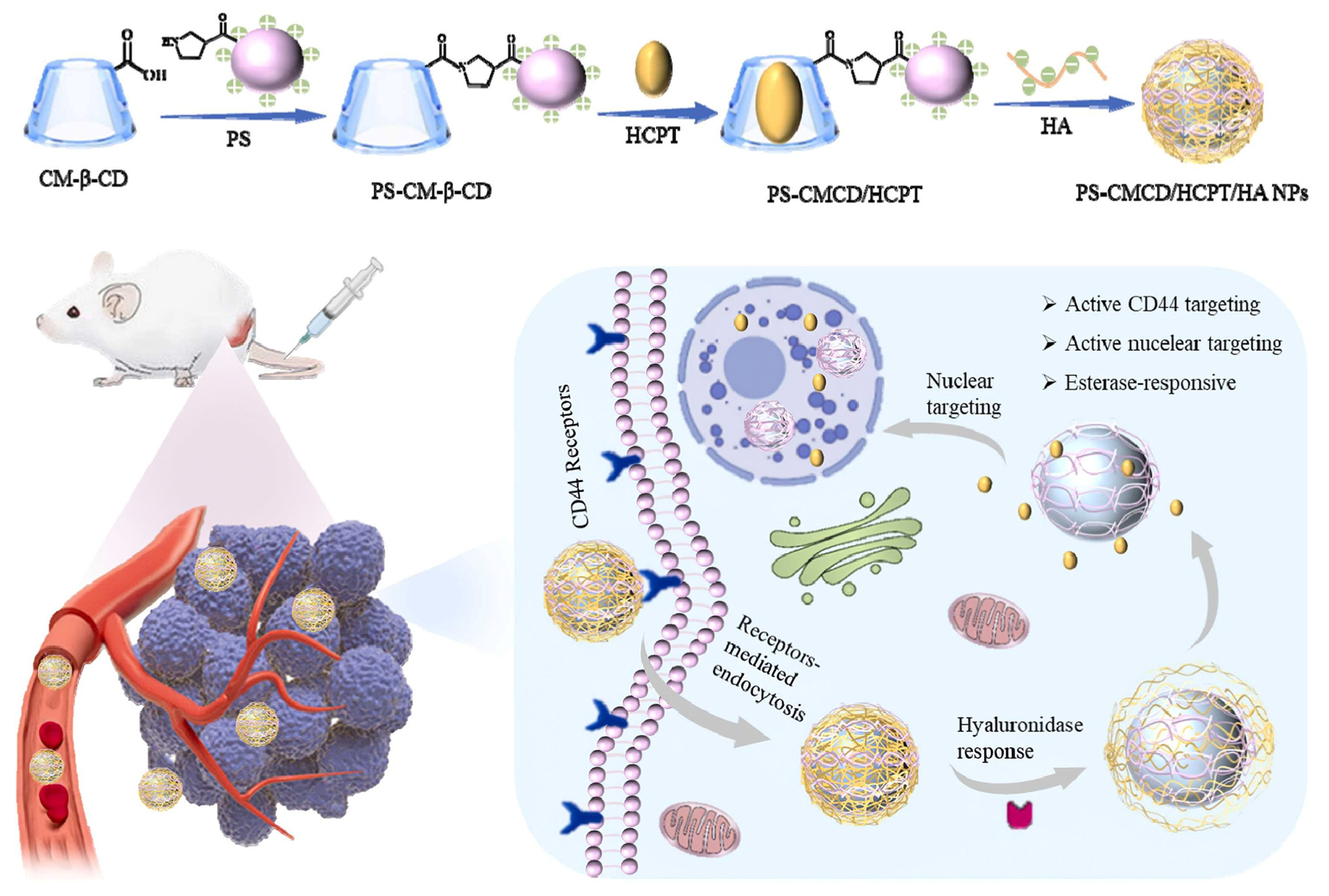
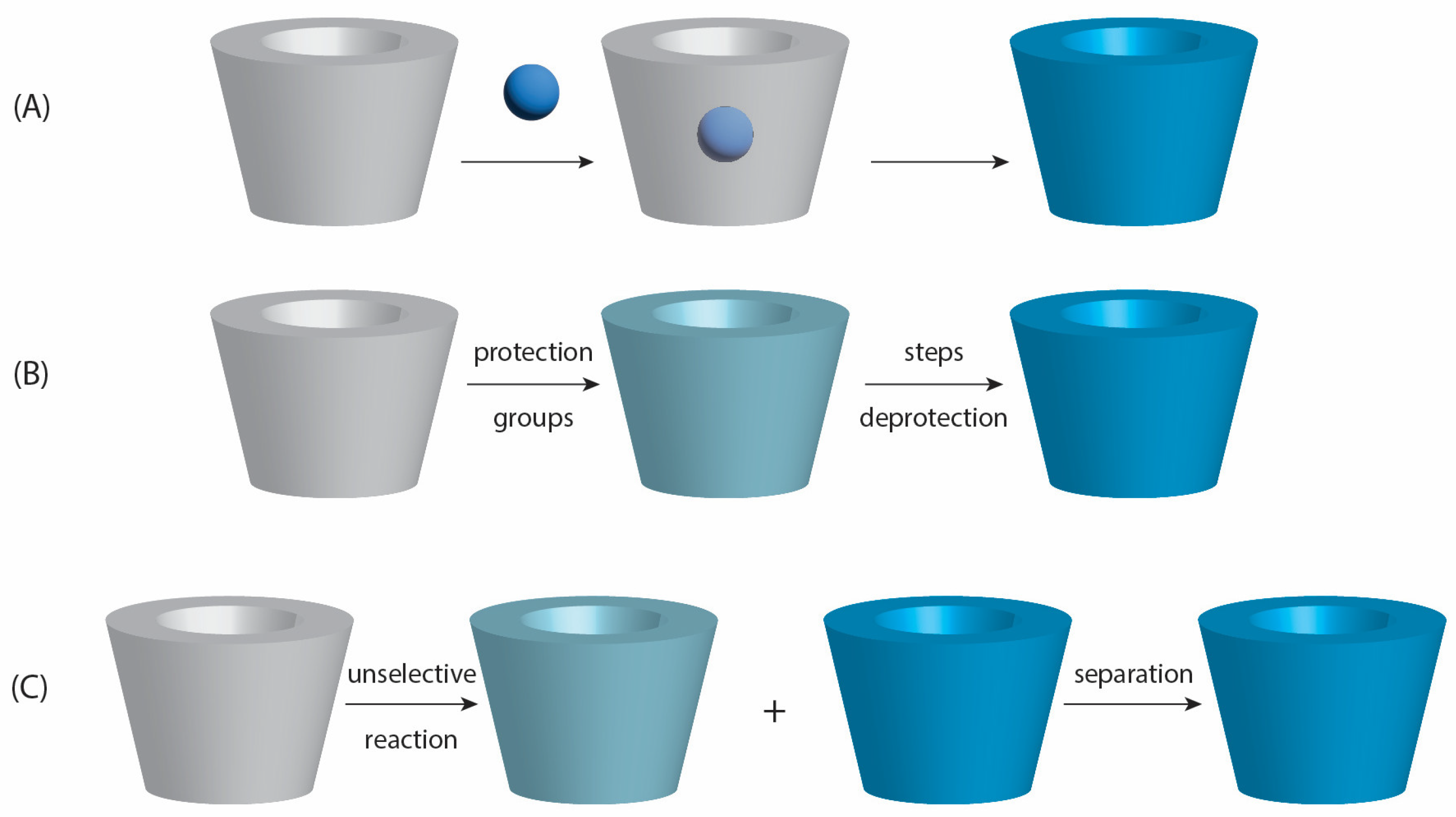
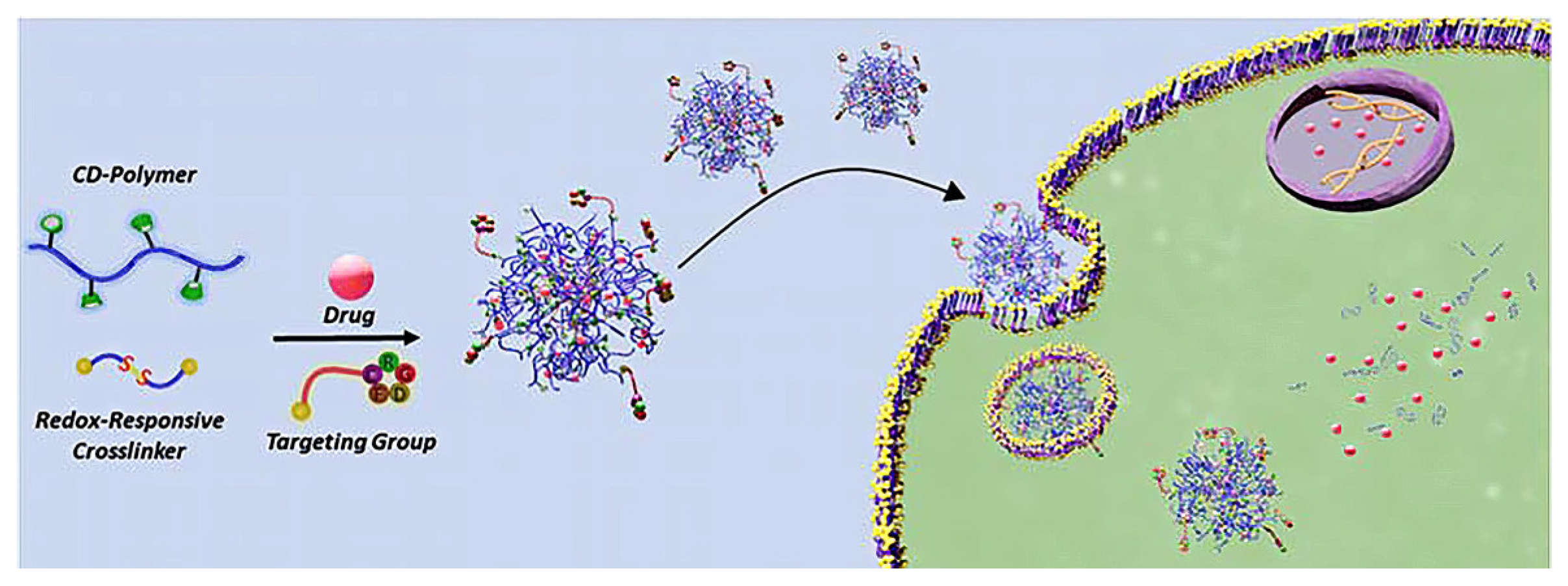
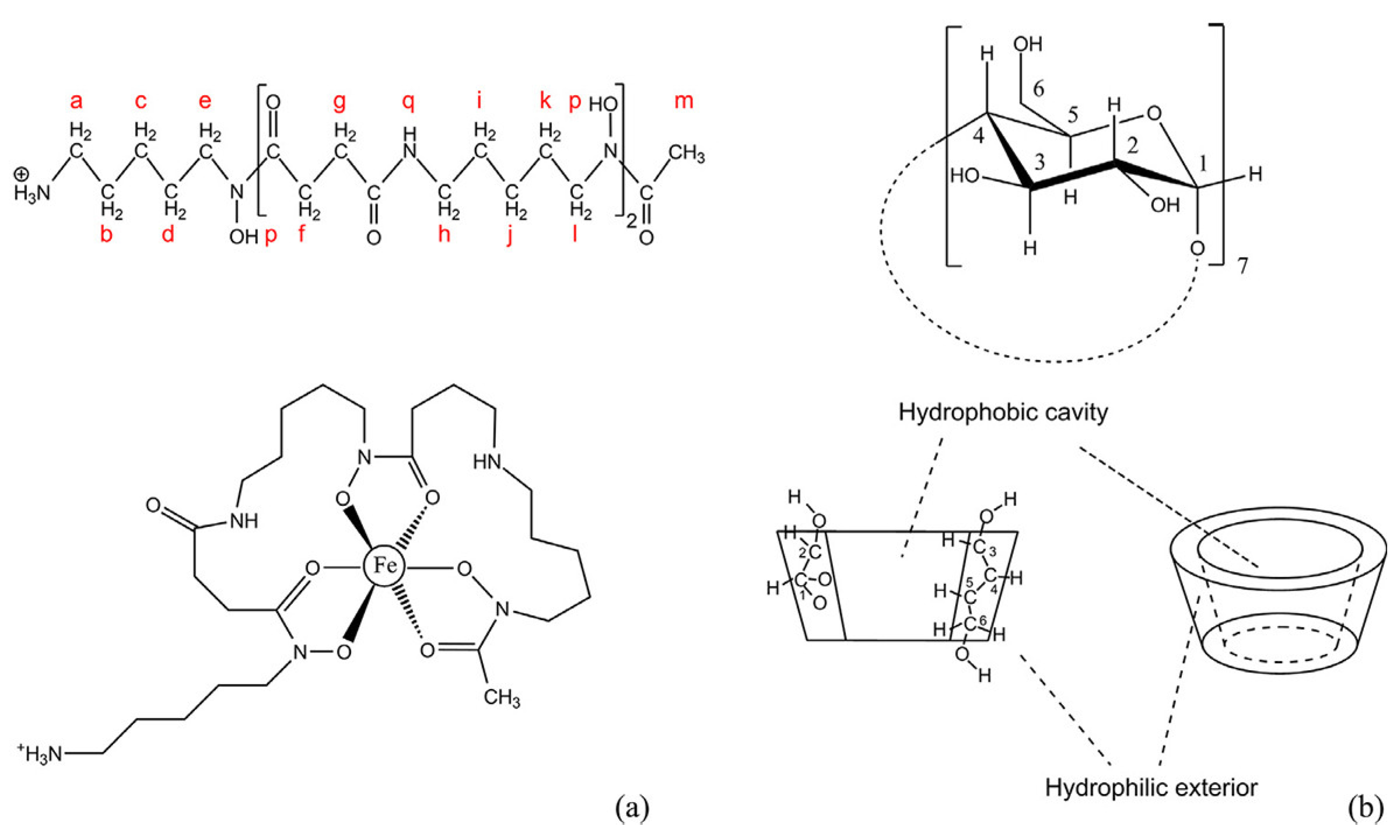
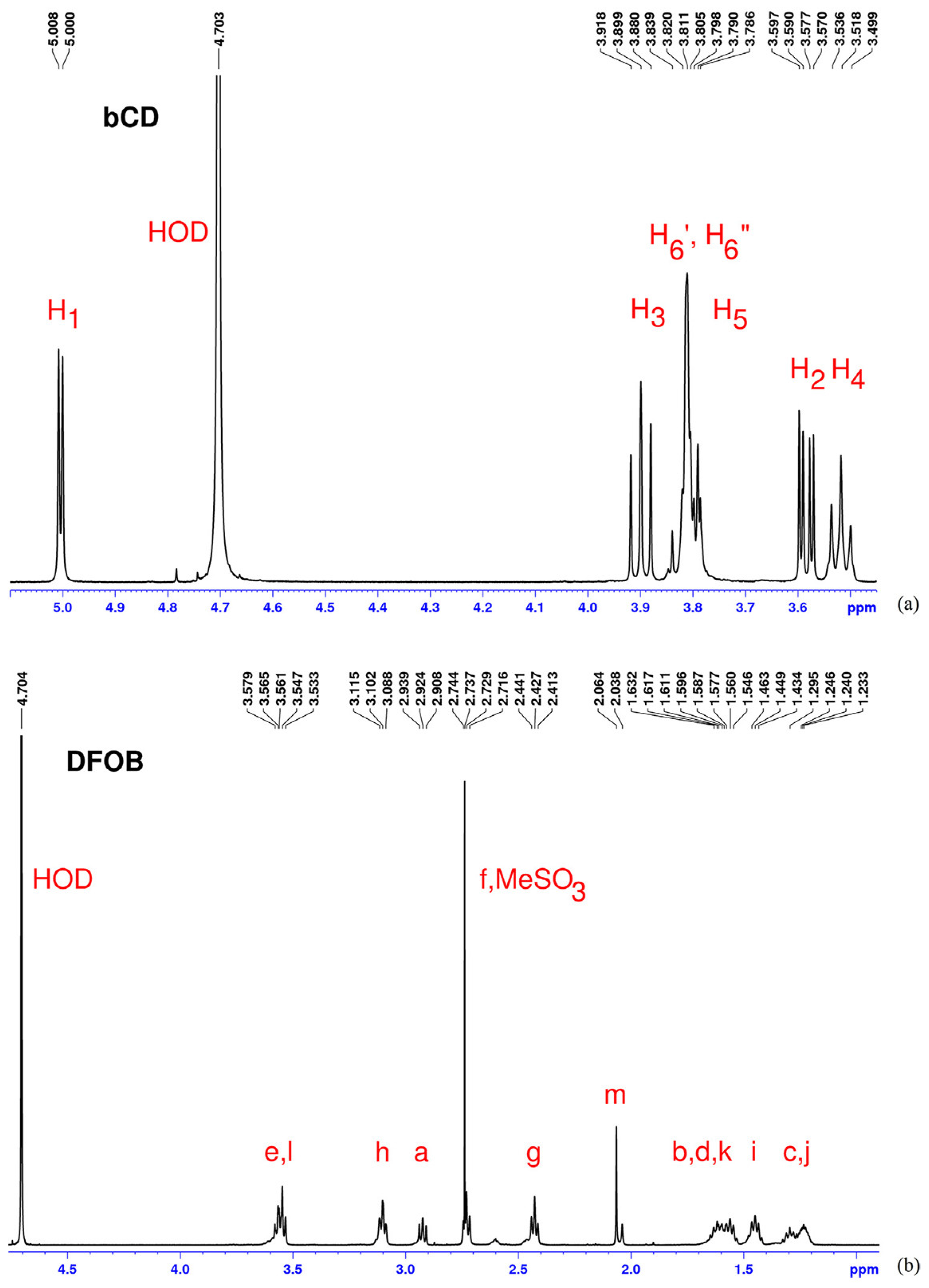
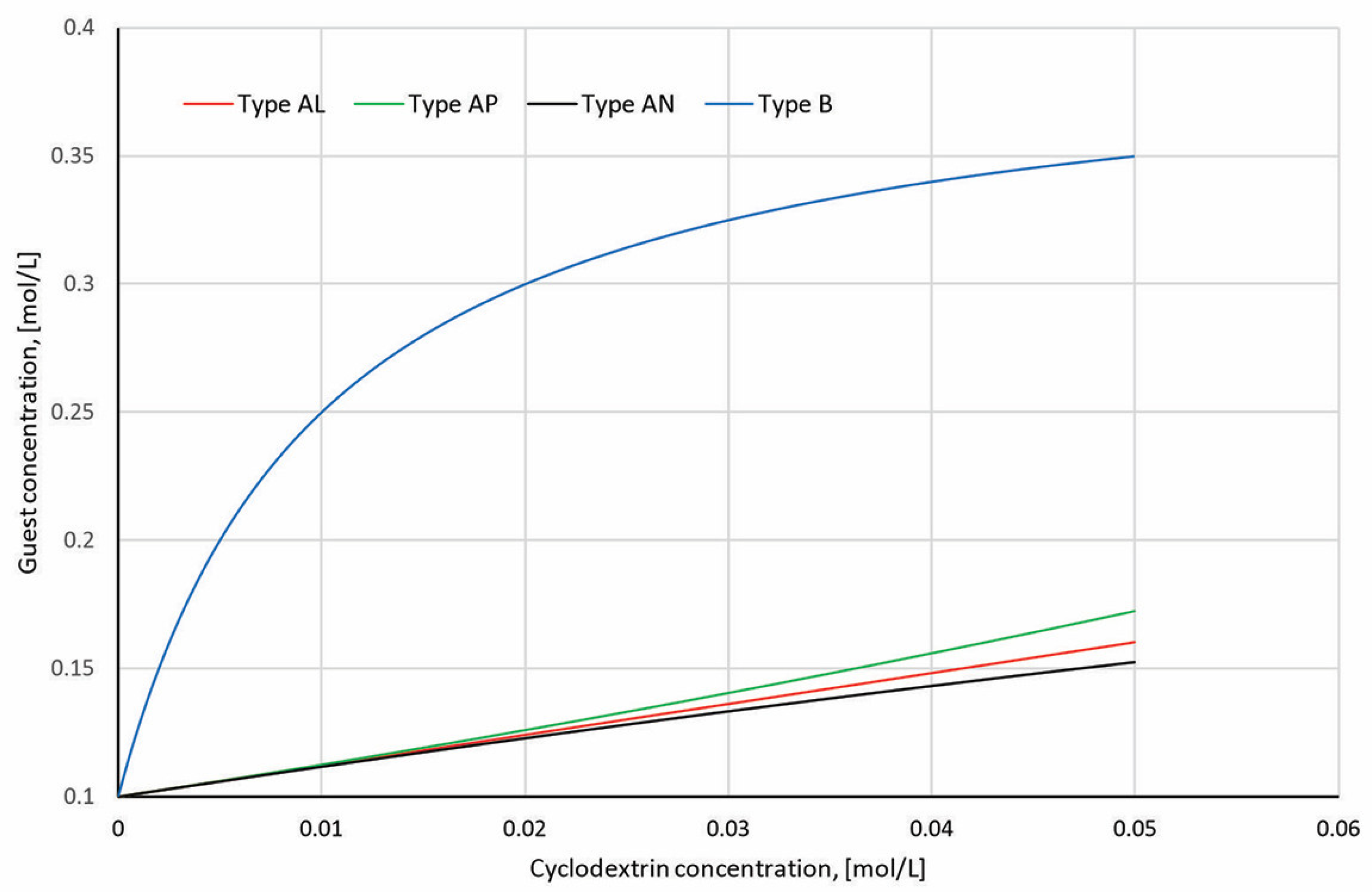

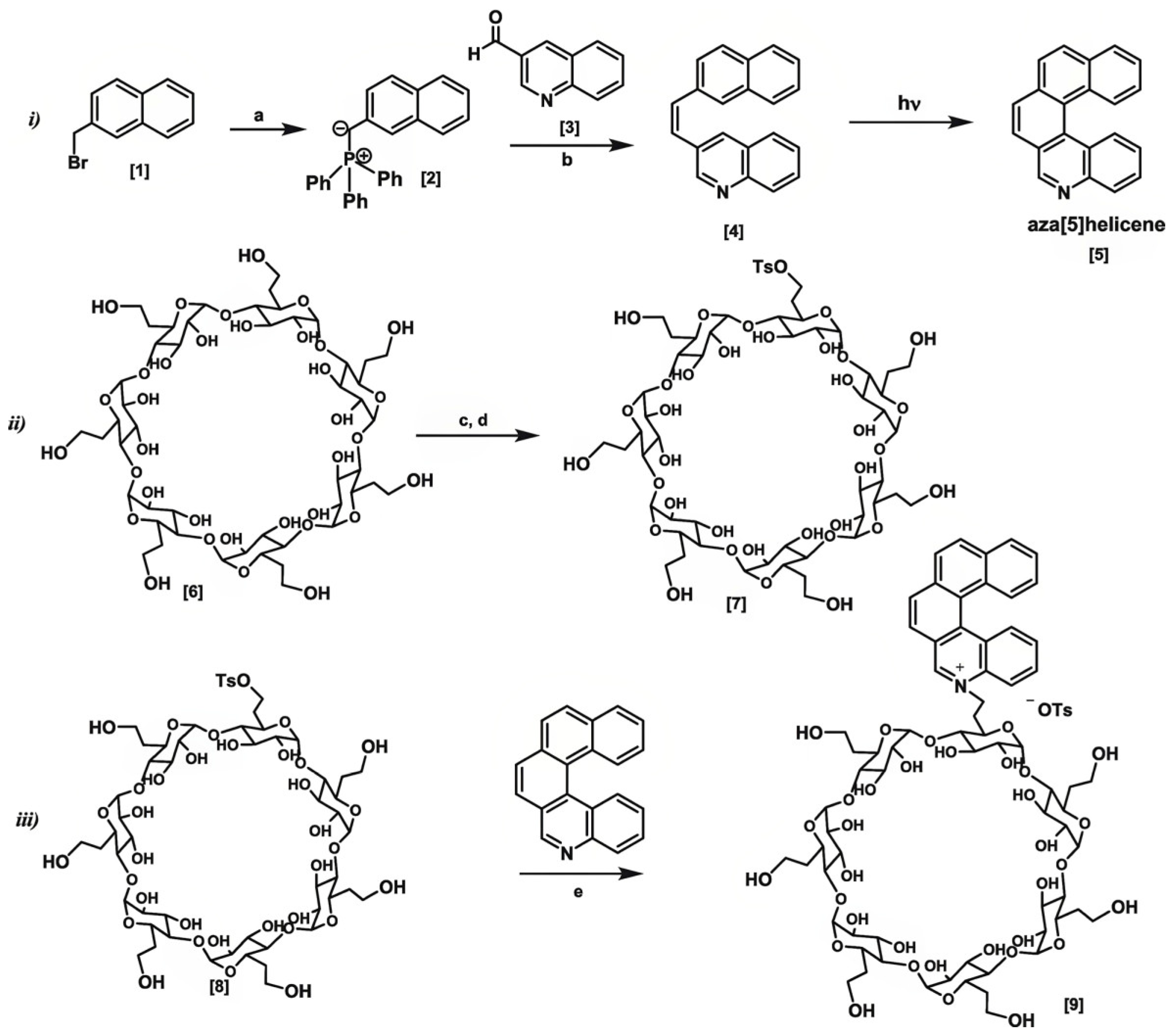
| Application Area | Key Functions and Benefits | References |
|---|---|---|
| Pharmaceuticals | Enhance drug solubility, stability, bioavailability; mask taste/smell; enable targeted and controlled drug delivery (oral, ocular, nasal, parenteral, topical); used in nanomedicine and cancer therapy | [64,65,66,67,68,69] |
| Food industry | Improve flavor, aroma, and shelf life; stabilize sensitive nutrients; remove cholesterol; act as food additives; extend storage period | [65,70,71,72] |
| Cosmetics and toiletries | Stabilize fragrances and active ingredients; improve product texture and shelf life | [68,70,71] |
| Environmental and industrial | Remove pollutants (e.g., pesticides, heavy metals); wastewater treatment; oilfield chemicals; used in nanomaterials and coatings | [71,73,74,75] |
| Agriculture | Enhance pesticide formulation and remediation; protect and deliver agrochemicals | [71,76] |
| Advanced materials | Used in nanotechnology (nanoparticles, nanofibers, nanomicelles); biomaterials; tissue engineering | [67,73,74] |
| Cyclodextrin Type | Drug/Compound | Solubility Increase | References |
|---|---|---|---|
| HP-β-CD | Myricetin | 31.45× | [96] |
| HP-β-CD | Kaempferol | 12.7× | [103] |
| SBE-β-CD (low-melting mix) | Fluticasone propionate | 4000× | [104] |
| SBE-β-CD | Pomalidomide | Highest among 9 CDs | [105] |
| Derivatization Pathway/System | Reaction Yield/Efficiency | Key Physicochemical Parameters | References |
|---|---|---|---|
| Cross-linking CD with epichlorohydrin (ECH) | Up to 67.1% (drug loading, α-CDNS) | Optimal: 6 h solubilization, ECH/CD molar ratio 8:1, NaOH 33%; affects drug loading and particle size | [152] |
| Mechanochemical synthesis of insoluble CD polymers | Not specified (focus on reproducibility) | CD/reagent ratio determines solubility; mechano-chemical methods yield insoluble polymers; high reproducibility | [153] |
| CD–metal organic frameworks (CD-MOFs) for enrichment and derivatization | 82.4–87.5% (relative recovery) | α-, β-, γ-CD-MOFs; RSD < 10.9%; LOD 0.934–2.77 ng/g; cavity size affects encapsulation and derivatization | [154] |
| Regioselective radical C–H trifluoromethylation (with CD) | Good yield (exact % not specified) | CD increases regioselectivity by stabilizing intermediates; metal salts increase yield | [155] |
| Factor | Effect on Stoichiometry | Example | References |
|---|---|---|---|
| Molecular size and shape | Small guests often form 1:1 complexes; larger or elongated guests may form 1:2 or 2:1 | Naphthalene forms 1:2 with α-CD, 2:1 and 2:2 with β-CD; vanillin forms 1:1 with β-CD | [202,227] |
| Type of cyclodextrin | α-, β-, and γ-cyclodextrins have different cavity sizes, affecting possible stoichiometry | Larger γ-CD can accommodate larger guests or multiple guests | [227,228,229] |
| Guest polarity and interactions | Hydrophobic and hydrogen bonding interactions stabilize complexes, influencing stoichiometry | Hydrophobic effects and electrostatic forces mediate 1:1 complexes with amino acids | [230,231,232] |
| Solvent environment | Solvent can alter complex stability and stoichiometry | Deep eutectic solvents can promote 2:1 complexes | [228,233] |
| Concentration | Higher concentrations can favor higher-order complexes (e.g., 1:2, 2:1) | 5-fluorouracil forms both 1:1 and 1:2 complexes with β-CD depending on conditions | [111,234] |
| Drug/Complex | Cyclodextrin Type | Solubility Increase | Bioavailability Increase | Half-Life/ Other Stability | References |
|---|---|---|---|---|---|
| Oxymetholone | Carboxymethyl-β-cyclodextrin | ~3-fold | Not specified | Improved stability | [243] |
| Progesterone | Sulfobutyl-ether-β-cyclodextrin | ~7000-fold | 5-fold (in rats) | Prevents precipitation | [118] |
| Ibuprofen | Porous carboxymethyl-β-cyclodextrin polymer | Not specified | ~4-fold (AUC), 3-fold (Cmax) | Enhanced release | [253] |
| Lisinopril | β-cyclodextrin | Not specified | 2-fold | Stable after 3 months | [254] |
| Flutamide | β-cyclodextrin | 5.7-fold | Implied by solubility | Rapid release | [255] |
| Altrenogest | Hydroxypropyl-β-cyclodextrin | 1026-fold | 1.14-fold (relative) | Improved dissolution | [256] |
| Curcumin | β-cyclodextrin/nanosponge | 2.3–2.95-fold | Not specified | Higher stability constant | [257] |
| Cyclolinopeptides | β-cyclodextrin (Pickering emulsion) | Not specified | 13.6-fold | Higher physical stability | [258] |
| Dexamethasone (ocular) | Thiolated hydroxypropyl-β-cyclodextrin | Not specified | 11.7-fold (AUC, ocular) | 4-fold longer residence | [146] |
| Guest Molecule | Cyclodextrin Type | Observed Effect | References |
|---|---|---|---|
| Curcumin | Hydroxypropyl-β-CD | Improved solubility and nasal/olfactory tissue permeation | [241,284] |
| Albendazole | Methyl-β-CD | Solubility increased by 150,000-fold; enhanced antitumor efficacy | [248,285] |
| Ibrutinib | Hydroxypropyl-β-CD (cross-linked) | Controlled release; improved solubility and bioavailability | [66,245] |
| Abiraterone acetate | 2,6-di-O-methyl-β-CD | Enhanced dissolution rate; potential in prostate cancer treatment | [286,287,288] |
| Oxymetholone | Carboxymethyl-β-CD | Improved encapsulation and prolonged release | [243] |
| Genistein | Amino-appended β-CD | 1000-fold solubility increase; enhanced antioxidant activity | [289,290] |
| Acyclovir | Pluronic127 + α/β-CD | Sustained release for topical application | [246,291,292] |
| Insulin | Various β-CD derivatives | Enhanced solubility, stability, and mucosal absorption | [268,293,294,295] |
| Doxorubicin | Modified β-CD conjugates | Targeted cancer cell delivery; increased cytotoxicity | [296,297,298] |
| Fenofibric acid | β-CD and methyl-β-CD | Controlled release; enhanced pharmacological profile | [273,299,300] |
| Product Name | Active Ingredient | CD Type | Formulation | Application Field | Manufacturer |
|---|---|---|---|---|---|
| Sporanox® IV | Itraconazole | HP-β-CD | Intravenous | Antifungal | Janssen Pharmaceuticals |
| Vfend® | Voriconazole | SBE-β-CD (Captisol®) | Intravenous | Antifungal | Pfizer |
| Geodon® | Ziprasidone | SBE-β-CD (Captisol®) | Intramuscular | Antipsychotic | Pfizer |
| Bridion® | Sugammadex | γ-CD derivative | Intravenous | Reversal of neuromuscular blockade | Merck & Co. |
| Ozurdex® | Dexamethasone | HP-β-CD | Ophthalmic implant | Macular edema | Allergan |
| Abilify® Injection | Aripiprazole | HP-β-CD | Intramuscular | Schizophrenia, bipolar disorder | Otsuka Pharmaceutical |
| Cerenia® | Maropitant citrate | SBE-β-CD (Captisol®) | Injectable (veterinary) | Antiemetic (dogs, cats) | Zoetis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spiridon, I.; Anghel, N. Cyclodextrins as Multifunctional Platforms in Drug Delivery and Beyond: Structural Features, Functional Applications, and Future Trends. Molecules 2025, 30, 3044. https://doi.org/10.3390/molecules30143044
Spiridon I, Anghel N. Cyclodextrins as Multifunctional Platforms in Drug Delivery and Beyond: Structural Features, Functional Applications, and Future Trends. Molecules. 2025; 30(14):3044. https://doi.org/10.3390/molecules30143044
Chicago/Turabian StyleSpiridon, Iuliana, and Narcis Anghel. 2025. "Cyclodextrins as Multifunctional Platforms in Drug Delivery and Beyond: Structural Features, Functional Applications, and Future Trends" Molecules 30, no. 14: 3044. https://doi.org/10.3390/molecules30143044
APA StyleSpiridon, I., & Anghel, N. (2025). Cyclodextrins as Multifunctional Platforms in Drug Delivery and Beyond: Structural Features, Functional Applications, and Future Trends. Molecules, 30(14), 3044. https://doi.org/10.3390/molecules30143044










