Ultraporous Amine-Functionalized Organosilicas: Tuning Morphology and Surface Chemistry for Adsorption Applications
Abstract
1. Introduction and Aim of Work
2. Results and Discussion
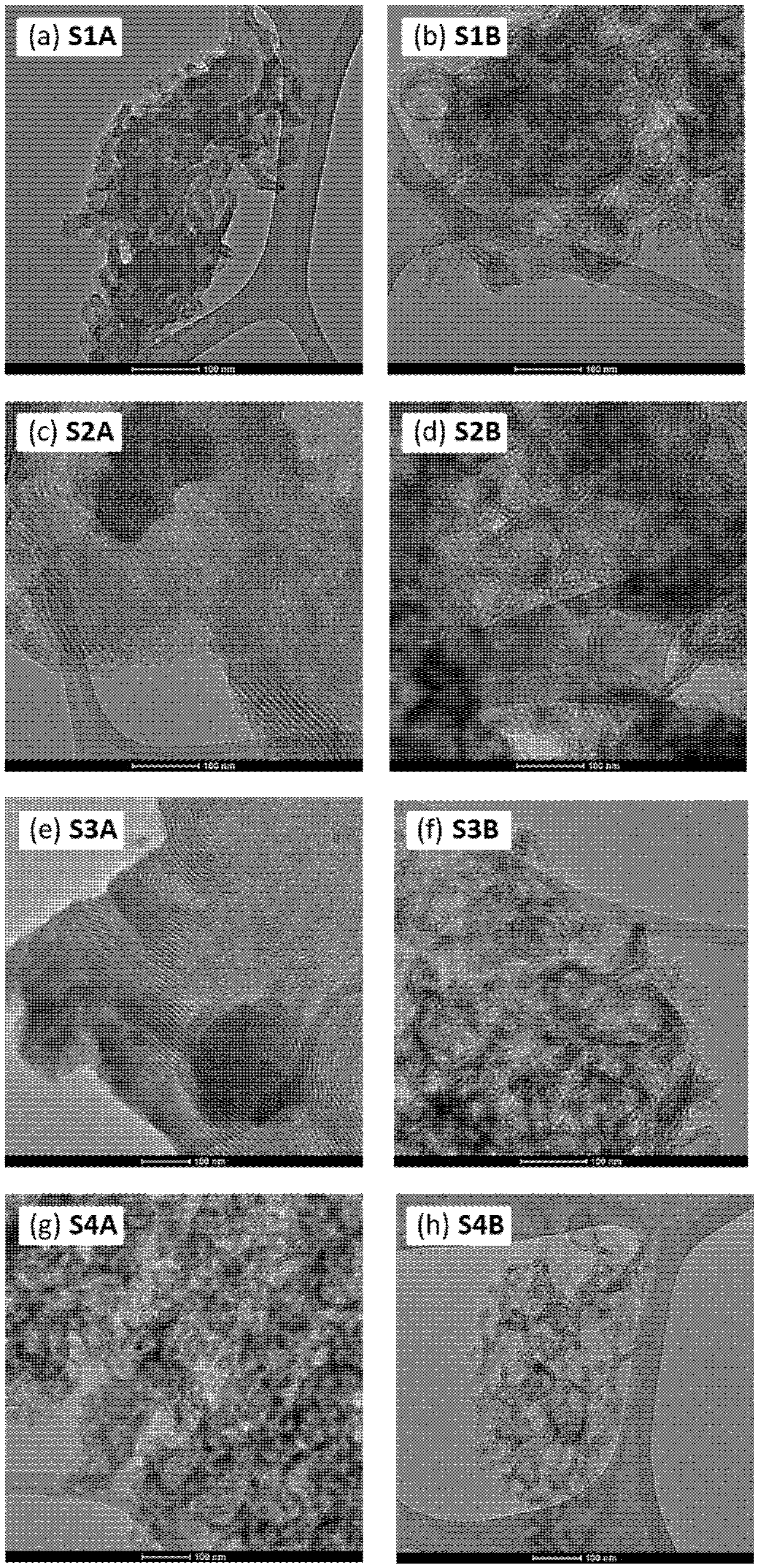
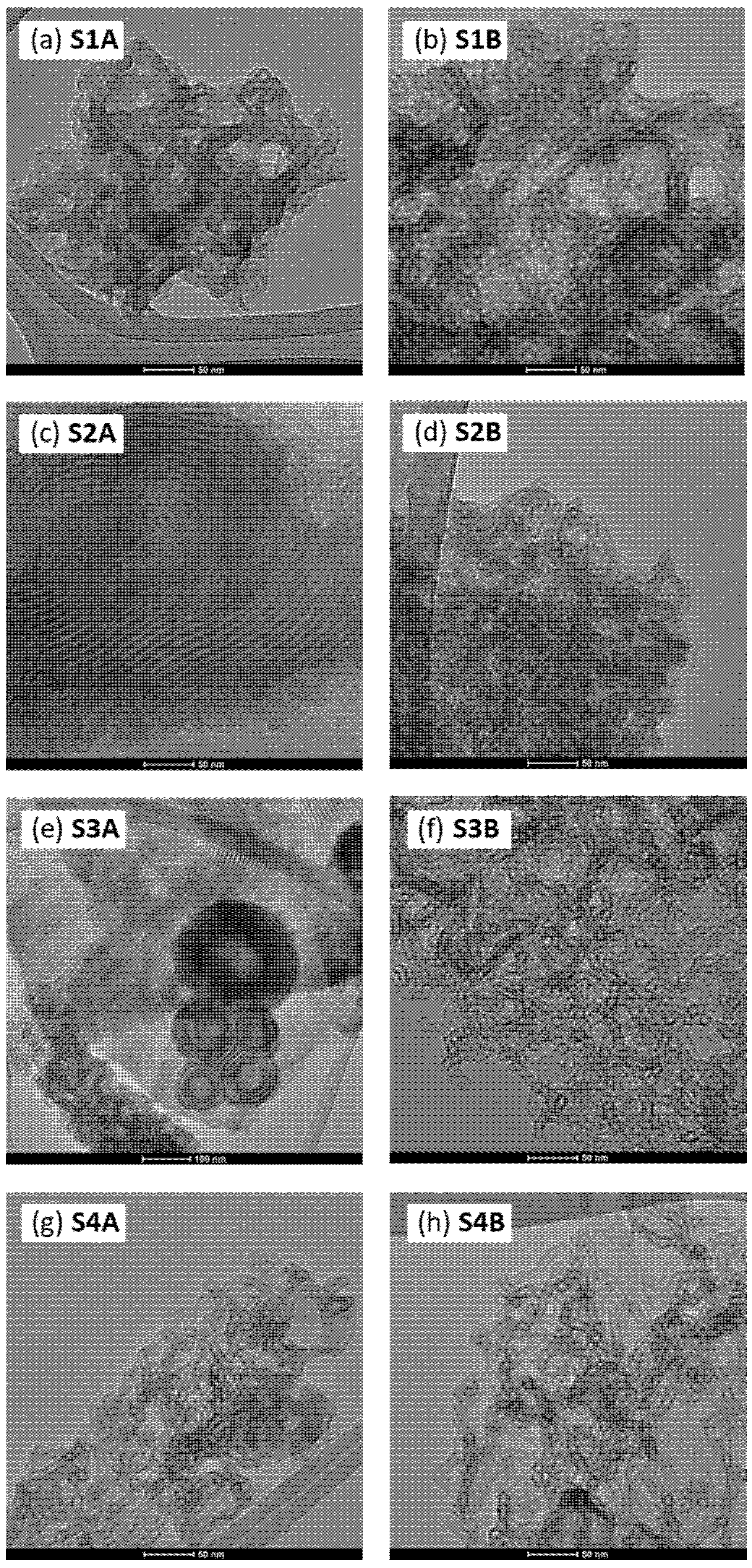
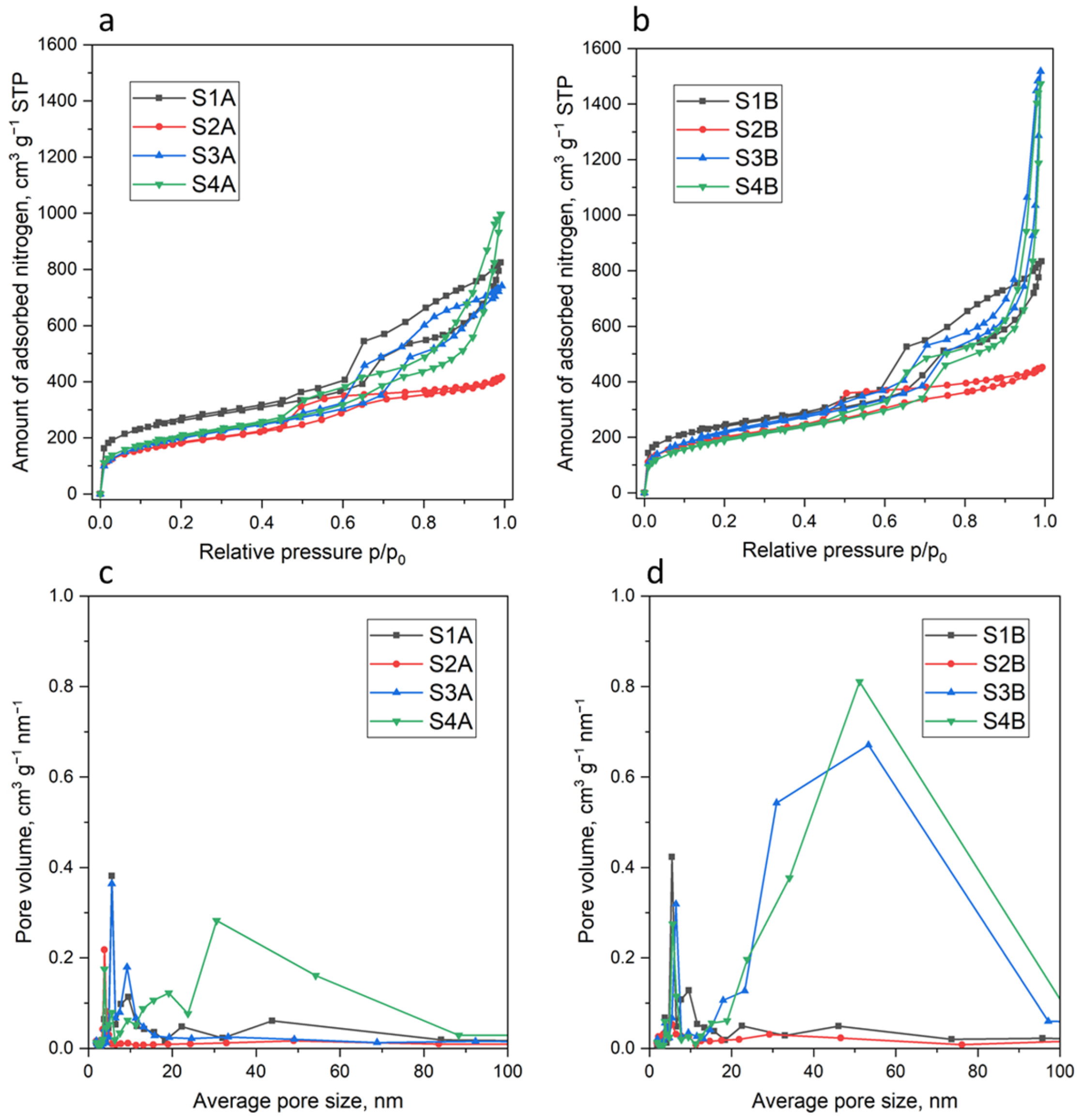
3. Materials and Methods
3.1. Reagents
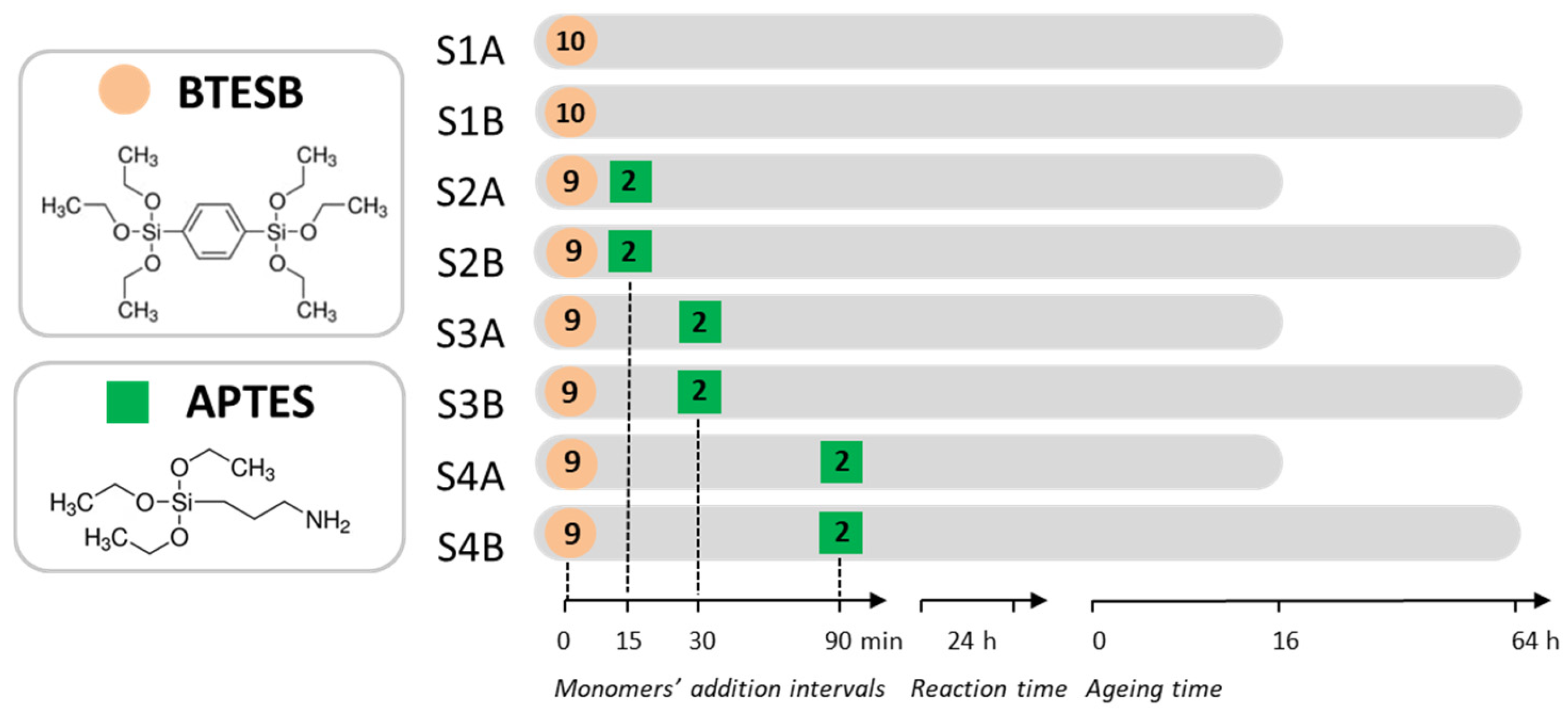
3.2. Synthesis of the Samples
3.3. Instrumental Characterization
3.4. Sorption and Release of Diclofenac
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Verma, P.; Kuwahara, Y.; Mori, K.; Raja, R.; Yamashita, H. Functionalized Mesoporous SBA-15 Silica: Recent Trends and Catalytic Applications. Nanoscale 2020, 12, 11333–11363. [Google Scholar] [CrossRef]
- Yu, X.; Williams, C.T. Recent Advances in the Applications of Mesoporous Silica in Heterogeneous Catalysis. Catal. Sci. Technol. 2022, 12, 5765–5794. [Google Scholar] [CrossRef]
- Wei, Y.; Yang, W.; Yang, Z. An Excellent Universal Catalyst Support-Mesoporous Silica: Preparation, Modification and Applications in Energy-Related Reactions. Int. J. Hydrogen Energy 2022, 47, 9537–9565. [Google Scholar] [CrossRef]
- Fatimah, I.; Fadillah, G.; Sagadevan, S.; Oh, W.C.; Ameta, K.L. Mesoporous Silica-Based Catalysts for Biodiesel Production: A Review. ChemEngineering 2023, 7, 56. [Google Scholar] [CrossRef]
- Larki, A.; Saghanezhad, S.J.; Ghomi, M. Recent Advances of Functionalized SBA-15 in the Separation/Preconcentration of Various Analytes: A Review. Microchem. J. 2021, 169, 106601. [Google Scholar] [CrossRef]
- Lee, S.L.; Thomas, J.; Mou, C.Y.; Liu, C.L.; Tung, K.L. High-Performance Separation for Ultra-Low Concentration Nanoparticles with Mesoporous Silica Thin Membrane. Sep. Purif. Technol. 2024, 350, 127918. [Google Scholar] [CrossRef]
- Brady, R.; Woonton, B.; Gee, M.L.; O’Connor, A.J. Hierarchical Mesoporous Silica Materials for Separation of Functional Food Ingredients—A Review. Innov. Food Sci. Emerg. Technol. 2008, 9, 243–248. [Google Scholar] [CrossRef]
- Ren, Y.; Yang, R.; Shao, L.; Tang, H.; Wang, S.; Zhao, J.; Zhong, J.; Kong, C. The Removal of Aqueous Uranium by SBA-15 Modified with Phosphoramide: A Combined Experimental and DFT Study. RSC Adv. 2016, 6, 68695–68704. [Google Scholar] [CrossRef]
- Cashin, V.B.; Eldridge, D.S.; Yu, A.; Zhao, D. Surface Functionalization and Manipulation of Mesoporous Silica Adsorbents for Improved Removal of Pollutants: A Review. Environ. Sci. Water Res. Technol. 2018, 4, 110–128. [Google Scholar] [CrossRef]
- Diagboya, P.N.E.; Dikio, E.D. Silica-Based Mesoporous Materials; Emerging Designer Adsorbents for Aqueous Pollutants Removal and Water Treatment. Microporous Mesoporous Mater. 2018, 266, 252–267. [Google Scholar] [CrossRef]
- Melde, B.J.; Johnson, B.J.; Charles, P.T. Mesoporous Silicate Materials in Sensing. Sensors 2008, 8, 5202–5228. [Google Scholar] [CrossRef] [PubMed]
- Parra, M.; Gil, S.; Gaviña, P.; Costero, A.M. Mesoporous Silica Nanoparticles in Chemical Detection: From Small Species to Large Bio-Molecules. Sensors 2021, 22, 261. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.H.; Lee, C.H.; Yang, C.S.; Tseng, F.G.; Mou, C.Y.; Lo, L.W. Mesoporous Silica Nanoparticles Functionalized with an Oxygen-Sensing Probe for Cell Photodynamic Therapy: Potential Cancer Theranostics. J. Mater. Chem. 2009, 19, 1252–1257. [Google Scholar] [CrossRef]
- Tan, S.Y.; Teh, C.; Ang, C.Y.; Li, M.; Li, P.; Korzh, V.; Zhao, Y. Responsive Mesoporous Silica Nanoparticles for Sensing of Hydrogen Peroxide and Simultaneous Treatment toward Heart Failure. Nanoscale 2017, 9, 2253–2261. [Google Scholar] [CrossRef]
- Jahandar Lashaki, M.; Ziaei-Azad, H.; Sayari, A. Insights into the Hydrothermal Stability of Triamine-Functionalized SBA-15 Silica for CO2 Adsorption. ChemSusChem 2017, 10, 4037–4045. [Google Scholar] [CrossRef]
- Shin, H.J.; Ryoo, R.; Kruk, M.; Jaroniec, M.; Ozin, G.A.; Ko, C.H.; Shin, H.J.; Ryoo, R. Modification of SBA-15 Pore Connectivity by High-Temperature Calcination Investigated by Carbon Inverse Replication. Chem. Commun. 2001, 103, 349–350. [Google Scholar] [CrossRef]
- Mercier, L.; Pinnavaia, T.J. Heavy Metal Ion Adsorbents Formed by the Grafting of a Thiol Functionality to Mesoporous Silica Molecular Sieves: Factors Affecting Hg(II) Uptake. Environ. Sci. Technol. 1998, 32, 2749–2754. [Google Scholar] [CrossRef]
- Hiyoshi, N.; Yogo, K.; Yashima, T. Adsorption Characteristics of Carbon Dioxide on Organically Functionalized SBA-15. Microporous Mesoporous Mater. 2005, 84, 357–365. [Google Scholar] [CrossRef]
- Aguado, J.; Arsuaga, J.M.; Arencibia, A.; Lindo, M.; Gascón, V. Aqueous Heavy Metals Removal by Adsorption on Amine-Functionalized Mesoporous Silica. J. Hazard. Mater. 2009, 163, 213–221. [Google Scholar] [CrossRef]
- Barczak, M. Synthesis and Structure of Pyridine-Functionalized Mesoporous SBA-15 Organosilicas and Their Application for Sorption of Diclofenac. J. Solid State Chem. 2018, 258, 232–242. [Google Scholar] [CrossRef]
- Barczak, M.; Da̧browski, A.; Pikus, S.; Ryczkowski, J.; Borowski, P.; Kozak, M. Studies of the Structure and Chemistry of SBA-15 Organosilicas Functionalized with Amine, Thiol, Vinyl and Phenyl Groups. Adsorption 2010, 16, 457–463. [Google Scholar] [CrossRef]
- Barczak, M. Amine-Modified Mesoporous Silicas: Morphology-Controlled Synthesis toward Efficient Removal of Pharmaceuticals. Microporous Mesoporous Mater. 2019, 278, 354–365. [Google Scholar] [CrossRef]
- Estevão, B.M.; Miletto, I.; Hioka, N.; Marchese, L.; Gianotti, E. Mesoporous Silica Nanoparticles Functionalized with Amino Groups for Biomedical Applications. ChemistryOpen 2021, 10, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.Y.; Jang, S.G.; Cho, J.; Kang, M. Amine-Functionalized Mesoporous Silica for Efficient CO2 Capture: Stability, Performance, and Industrial Feasibility. Int. J. Mol. Sci. 2025, 26, 4313. [Google Scholar] [CrossRef] [PubMed]
- Dobrowolski, R.; Oszust-Cieniuch, M.; Dobrzyńska, J.; Barczak, M. Amino-Functionalized SBA-15 Mesoporous Silicas as Sorbents of Platinum (IV) Ions. Colloids Surfaces A Physicochem. Eng. Asp. 2013, 435, 63–70. [Google Scholar] [CrossRef]
- Dudarko, O.; Budnyak, T.; Tkachenko, O.; Agback, T.; Agback, P.; Bonnet, B.; Ahrens, L.; Daniel, G.; Seisenbaeva, G. Removal of Poly- and Perfluoroalkyl Substances from Natural and Wastewater by Tailored Silica-Based Adsorbents. ACS EST Water 2024, 4, 1303–1314. [Google Scholar] [CrossRef]
- Chong, A.S.M.; Zhao, X.S.; Kustedjo, A.T.; Qiao, S.Z. Functionalization of Large-Pore Mesoporous Silicas with Organosilanes by Direct Synthesis. Microporous Mesoporous Mater. 2004, 72, 33–42. [Google Scholar] [CrossRef]
- Dral, A.P.; Lievens, C.; Ten Elshof, J.E. Influence of Monomer Connectivity, Network Flexibility, and Hydrophobicity on the Hydrothermal Stability of Organosilicas. Langmuir 2017, 33, 5527–5536. [Google Scholar] [CrossRef]
- Barczak, M.; Gil, M.; Terpiłowski, K.; Kamiński, D.; Borowski, P. Influence of Bridged Monomer on Porosity and Sorption Properties of Mesoporous Silicas Functionalized with Diethylenetriamine Groups. Adsorption 2019, 25, 575–589. [Google Scholar] [CrossRef]
- Pal, N.; Sim, S.; Cho, E.B. Multifunctional Periodic Mesoporous Benzene-Silicas for Evaluation of CO2 Adsorption at Standard Temperature and Pressure. Microporous Mesoporous Mater. 2020, 293, 109816. [Google Scholar] [CrossRef]
- Sim, K.; Lee, N.; Kim, J.; Cho, E.B.; Gunathilake, C.; Jaroniec, M. CO2 Adsorption on Amine-Functionalized Periodic Mesoporous Benzenesilicas. ACS Appl. Mater. Interfaces 2015, 7, 6792–6802. [Google Scholar] [CrossRef] [PubMed]
- Erans, M.; Arencibia, A.; Sanz-Pérez, E.S.; Sanz, R. Amine-Bridged Periodic Mesoporous Organosilica Adsorbents for CO2 Capture. J. Environ. Chem. Eng. 2023, 11, 111590. [Google Scholar] [CrossRef]
- Tomina, V.; Stolyarchuk, N.; Semeshko, O.; Barczak, M.; Melnyk, I. Diamine Groups on the Surface of Silica Particles as Complex-Forming Linkers for Metal Cations. Molecules 2023, 28, 430. [Google Scholar] [CrossRef] [PubMed]
- Barczak, M.; Pietras-Ożga, D.; Seliem, M.K.; de Falco, G.; Giannakoudakis, D.A.; Triantafyllidis, K. Mesoporous Silicas Obtained by Time-Controlled Co-Condensation: A Strategy for Tuning Structure and Sorption Properties. Nanomaterials 2023, 13, 2065. [Google Scholar] [CrossRef]
- Rouquerol, F. Adsorption by Powders and Porous Solids: Principles, Methodology and Applications; Academic Press: Cambridge, MA, USA, 2013; ISBN 0080970362. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. IUPAC Technical Report Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Laird, M.; Carcel, C.; Oliviero, E.; Toquer, G.; Trens, P.; Bartlett, J.R.; Wong Chi Man, M. Single-Template Periodic Mesoporous Organosilica with Organized Bimodal Mesoporosity. Microporous Mesoporous Mater. 2020, 297, 110042. [Google Scholar] [CrossRef]
- Wei, Y.; Li, X.; Zhang, R.; Liu, Y.; Wang, W.; Ling, Y.; El-Toni, A.M.; Zhao, D. Periodic Mesoporous Organosilica Nanocubes with Ultrahigh Surface Areas for Efficient CO2 Adsorption. Sci. Rep. 2016, 6, 20769. [Google Scholar] [CrossRef]
- Ma, J.; Liu, Q.; Chen, D.; Wen, S.; Wang, T. Synthesis and Characterisation of Pore-Expanded Mesoporous Silica Materials. Micro Nano Lett. 2015, 10, 140–144. [Google Scholar] [CrossRef]
- Agudelo, N.A.; Escobar, S.; Tejada, J.C.; López, B.L. Understanding of the Formation of Mesocellular-like Silica Foam Particles of Nano Size and Its Chemical Surface to Immobilization of Thermomyces Lanuginosus Lipase. Microporous Mesoporous Mater. 2020, 294, 109948. [Google Scholar] [CrossRef]
- Barczak, M.; Borowski, P. Silica Xerogels Modified with Amine Groups: Influence of Synthesis Parameters on Porous Structure and Sorption Properties. Microporous Mesoporous Mater. 2019, 281, 32–43. [Google Scholar] [CrossRef]
- Barczak, M.; Dobrowolski, R.; Dobrzyńska, J.; Zięba, E.; Dąbrowski, A.; Ziȩba, E.; Da̧browski, A.; Zięba, E.; Dąbrowski, A. Amorphous and Ordered Organosilicas Functionalized with Amine Groups as Sorbents of Platinum (II) Ions. Adsorption 2013, 19, 733–744. [Google Scholar] [CrossRef]
- Barczak, M.; Pikus, S.; Skrzydło-Radomańska, B.; Da̧browski, A. Synthesis, Structure and Adsorption Properties of Nanoporous SBA-15 Materials with Framework and Surface Functionalities. Adsorption 2009, 15, 278–286. [Google Scholar] [CrossRef]
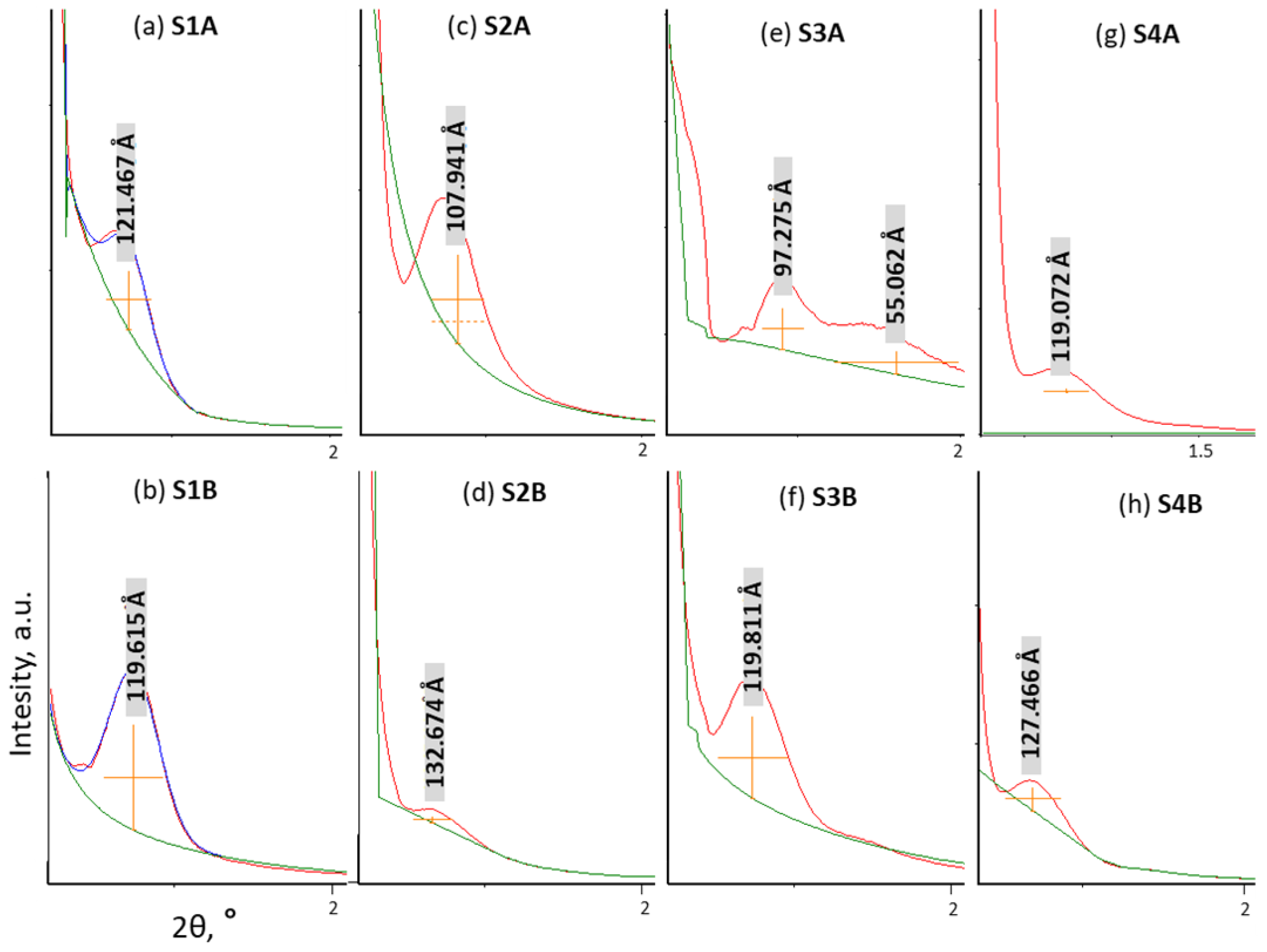
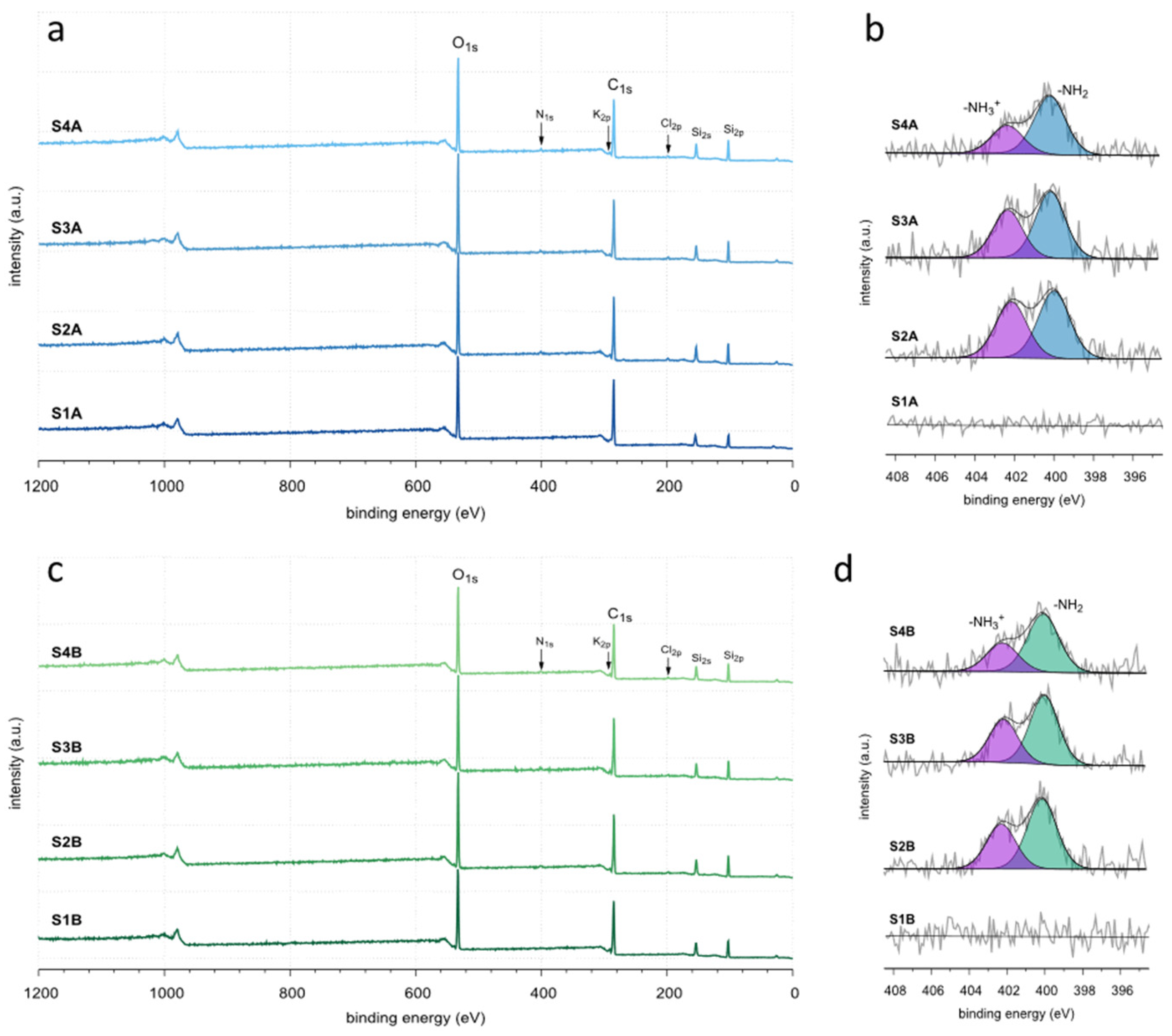
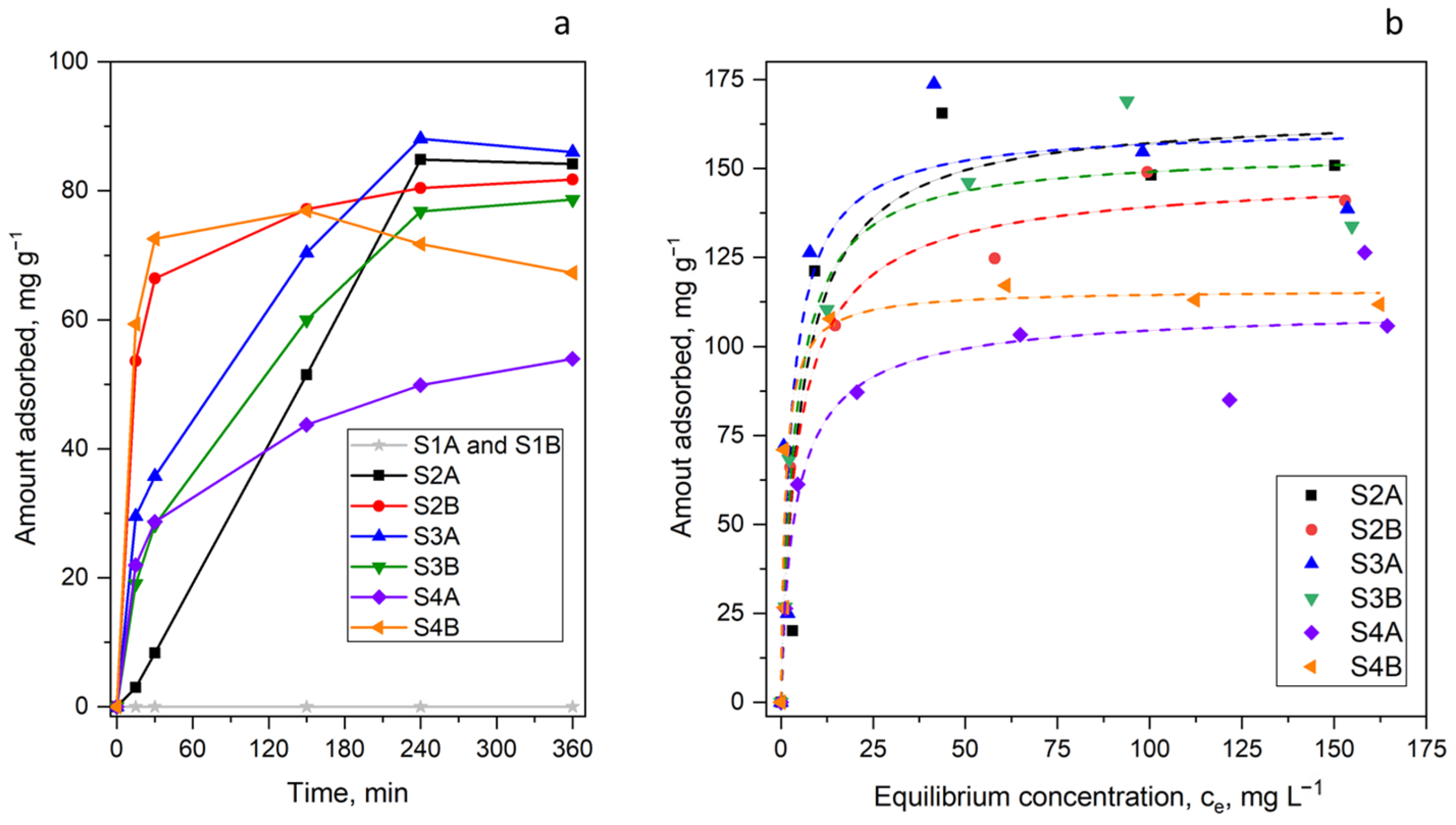
| Sample | Porous Structure Parameters | CHN Elemental Analysis (wt.%) | XPS Elemental Analysis (at.%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SBET (m2/g) | Sext (m2/g) | Smic (m2/g) | Vt (cm3/g) | C | H | N | Si | C | O | N | |
| S1A | 908 | 664 | 244 | 1.28 | 37.76 | 4.58 | --- | 14.9 | 57.9 | 26.4 | --- |
| S1B | 834 | 631 | 203 | 1.29 | 39.34 | 5.30 | --- | 18.9 | 53.5 | 26.7 | --- |
| S2A | 639 | 545 | 95 | 0.64 | 37.64 | 5.40 | 0.97 | 18.8 | 51.2 | 26.2 | 2.1 |
| S2B | 700 | 605 | 95 | 0.70 | 36.67 | 5.31 | 0.97 | 19.4 | 51.2 | 25.8 | 2.1 |
| S3A | 717 | 691 | 25 | 1.15 | 38.75 | 5.41 | 0.96 | 18.6 | 51.9 | 26.3 | 1.8 |
| S3B | 785 | 772 | 13 | 2.35 | 38.87 | 5.69 | 0.95 | 18.4 | 52.8 | 25.7 | 1.6 |
| S4A | 727 | 649 | 79 | 1.54 | 38.82 | 5.56 | 0.76 | 18.5 | 53.1 | 26.1 | 1.1 |
| S4B | 682 | 670 | 12 | 2.28 | 38.87 | 5.60 | 0.82 | 18.0 | 53.3 | 26.4 | 1.1 |
| Sample | Sips Fitting | SSC (mg/g) | |||
|---|---|---|---|---|---|
| qm | KL | n | R2 | ||
| S1A | --- | --- | --- | --- | 0 |
| S1B | --- | --- | --- | --- | 0 |
| S2A | 166 | 0.185 | 1.000 | 0.886 | 166 |
| S2B | 150 | 0.256 | 0.861 | 0.980 | 149 |
| S3A | 162 | 0.355 | 0.970 | 0.858 | 155 |
| S3B | 155 | 0.259 | 1.000 | 0.959 | 168 |
| S4A | 111 | 0.290 | 0.855 | 0.935 | 126 |
| S4B | 116 | 0.805 | 0.988 | 0.917 | 117 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bytniewska, M.; Latusek, K.; Powęzka, M.; Kuśmierz, M.; Kapusta, O.; Barczak, M. Ultraporous Amine-Functionalized Organosilicas: Tuning Morphology and Surface Chemistry for Adsorption Applications. Molecules 2025, 30, 2990. https://doi.org/10.3390/molecules30142990
Bytniewska M, Latusek K, Powęzka M, Kuśmierz M, Kapusta O, Barczak M. Ultraporous Amine-Functionalized Organosilicas: Tuning Morphology and Surface Chemistry for Adsorption Applications. Molecules. 2025; 30(14):2990. https://doi.org/10.3390/molecules30142990
Chicago/Turabian StyleBytniewska, Marlena, Kacper Latusek, Maria Powęzka, Marcin Kuśmierz, Oliwia Kapusta, and Mariusz Barczak. 2025. "Ultraporous Amine-Functionalized Organosilicas: Tuning Morphology and Surface Chemistry for Adsorption Applications" Molecules 30, no. 14: 2990. https://doi.org/10.3390/molecules30142990
APA StyleBytniewska, M., Latusek, K., Powęzka, M., Kuśmierz, M., Kapusta, O., & Barczak, M. (2025). Ultraporous Amine-Functionalized Organosilicas: Tuning Morphology and Surface Chemistry for Adsorption Applications. Molecules, 30(14), 2990. https://doi.org/10.3390/molecules30142990








