1. Introduction
Contaminants of emerging concern (CECs) are natural or synthetic chemicals increasingly detected in water bodies but not consistently monitored in the environment [
1]. Recently, growing attention has been given to CECs due to their potential risks to ecosystems and human health. According to the United States Environmental Protection Agency (US EPA), CECs are newly identified chemicals with effects that remain poorly understood [
2]. These contaminants, believed to pose significant risks, originate from various sources and are found in concentrations ranging from micrograms to nanograms per liter [
1].
The list of CECs, including pharmaceuticals, personal care products, plasticizers, pesticides, industrial chemicals, and complexing agents, is extensive and continually expanding due to the introduction of new chemicals and changes in their use and disposal [
1,
3]. Pharmaceuticals are among the most critical environmental issues in industrialized countries due to their widespread use for treating human and veterinary ailments [
1]. Commonly detected pharmaceuticals in drinking water and wastewater include fluoxetine, lipid-lowering drugs, anti-acids, ciprofloxacin, diclofenac, steroids, beta-blockers, and analgesics [
4].
Wilkinson et al. [
5] identified active pharmaceutical ingredients in rivers across more than 50% of the world’s countries, with carbamazepine (CBZ) being the most frequently detected among the 61 compounds analyzed. CBZ, used to treat trigeminal neuralgia and bipolar disorders, is highly persistent in water due to its stability and resistance to biodegradation [
6]. Conventional wastewater treatment plants (WWTPs) are largely ineffective at removing pharmaceuticals, with only about 10% of CBZ being eliminated, resulting in considerable concentrations in effluents and surface waters [
1,
6]. For this reason, the Revised Urban Wastewater Directive 2024/3019 of the European Union imposed for WWTPs serving > 150,000 people equivalent a quaternary treatment aimed at the removal of 13 micropollutants, including CBZ [
7].
Various removal technologies, such as advanced oxidation processes and nanofiltration, have been proposed for micropollutants like CBZ. However, these often require high investment and maintenance costs and may lead to secondary pollution from oxidation by-products [
8]. One promising approach involves using an adsorption step after the water tertiary treatment [
9]. Commonly used adsorbents include commercial resins or activated carbons, which exhibit very high adsorption capacity but feature a complex and costly desorption process. In addition, the development of materials featuring a high selectivity for pharmaceuticals is of interest in specific applications, such as real-time sensors [
1,
6,
9].
In recent years, researchers have explored the potential of molecularly imprinted polymers (MIPs) as an alternative and more selective adsorbent for pharmaceutical removal. MIPs are synthetic polymers designed to have specific recognition sites for target molecules, enabling selective adsorption. While many studies focus on MIPs for non-steroidal anti-inflammatory drugs (NSAIDs), only a few address CBZ as a target. The adsorption capacity of these materials is due to a combination of steric effects and interactions with the polymer. MIPs could work with three types of interactions: covalent bond, semi-covalent bond, and non-covalent bond [
10,
11]. The first two interaction approaches require more drastic conditions for both preparation and desorption, as the formation and breaking of stronger bonds are necessary [
11,
12]. Most research, conducted in recent decades, has preferred to focus on an imprinting approach that exploits non-covalent bonds, thus allowing for the adsorption and desorption of pollutants based on the greater or lesser affinity between the polymer and the solvent used for washing. The non-covalent interactions used in molecularly imprinted technology are H-bond, ionic interactions, acid–basic interactions, and aromatic interactions like π-π stacking.
Non-covalent MIPs are synthesized through a process called molecular imprinting, which involves radical polymerization. During this process, monomers surround the target molecules (template) and are then fixed using a cross-linker to set the disposition. The target molecule is subsequently removed by a washing step, leaving behind a polymer with cavities that are complementary in size, shape, and functional groups to the target compound. These polymers can selectively retain target analytes from complex samples. A schematic representation of molecular imprinting is depicted in
Figure 1.
The shape of the cavities, the nature of the monomer, and the choice of porogen determine the nature and strength of the interactions between the target compounds and the imprinted polymer during extraction. Their stability under different pH, solvent, or temperature conditions makes MIPs excellent sorbents [
13].
The main classical polymerization methods reported in the literature include bulk, precipitation, emulsion, and suspension polymerizations [
10,
11,
12,
14].
The bulk method involves only a small amount of solvent, resulting in a monolithic compound that must be grinded into powder after polymerization. The precipitation method, in contrast, requires a larger amount of solvent, leading to the formation of finer particles with respect to the bulk method. This approach eliminates the need for a grin-ding step, ensuring that all bonding sites created during the polymerization remain untouched.
Emulsion polymerization takes place within small droplets of an organic phase, dispersed in an aqueous phase, and stabilized by surfactants. Finally, suspension polymerization, a heterogeneous polymerization, is based on the suspension of droplets of a pre-polymerization mixture in a continuous phase (water, mineral oil, or perfluorocarbon). Each droplet acts like a mini bulk reactor, allowing for the production of spherical beads with a broad size range, from a few micrometers to millimeters.
Recent studies have focused on producing MIPs using green chemistry principles to develop more sustainable materials [
15]. One example of these efforts is the testing of novel cross-linkers, including plant-based alternatives such as epoxidized soybean oil acrylate [
16]. Other approaches include the use of alternative solvents, such as ionic liquids or deep eutectic solvents, as well as modifying synthesis techniques by employing ultrasound or microwave-assisted methods [
17].
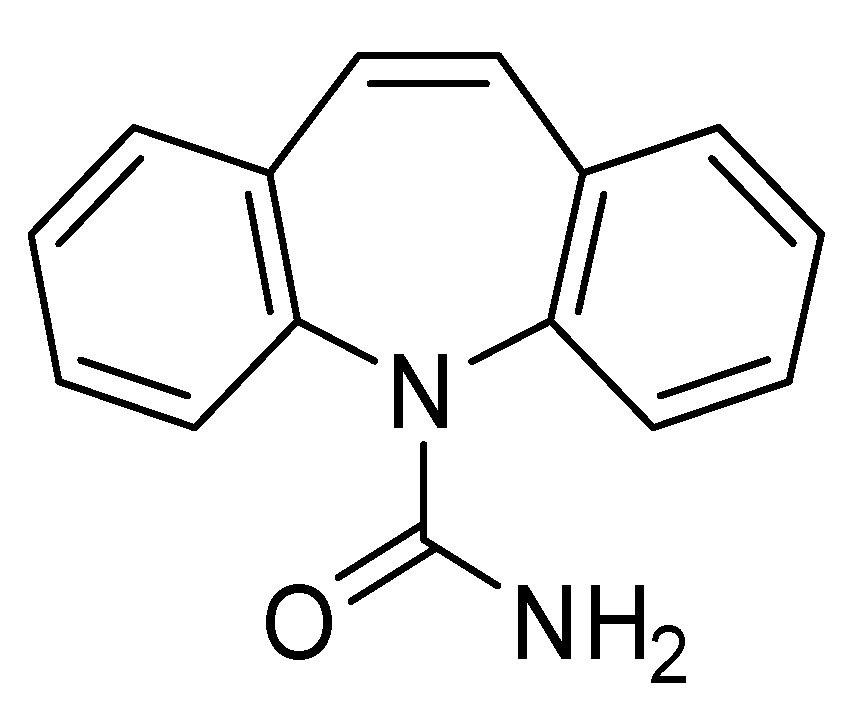
This study aims to synthesize and characterize a MIP for the selective adsorption of CBZ (
Figure 2) and to assess its adsorption performance. Common monomers used to adsorb CBZ are methacrylic acid (MAA) and 2-vinylpyridine (2VP) (
Figure 3a) [
13,
18,
19,
20]. This study seeks to replace these monomers, in particular toxic 2VP, with bio-based alternatives. The literature presents various hypotheses on replacing conventional monomers with bio-based alternatives, such as polysaccharides or biomolecules like peptides [
21]. In this study, compounds commonly used as templates have been re-evaluated and employed as functional monomers [
22]. To develop novel materials, six monomers with different types of interactions were selected. Fumaric acid (FUM) was chosen to assess whether the presence of two acid groups improves adsorption compared to MAA. Eugenol (EUG) was selected as a possible substitute for 2VP, given its aromatic ring. Additionally, three monomers, with both acidic and aromatic functionalities, were studied: coumaric acid (COU) and ferulic acid (FER), both containing an aromatic ring, and 3-(2-furyl) acrylic acid (FAA), which features a furan ring. Finally, 2-hydroxyethyl methacrylate (HEMA), a monomer with an ester functionality, was investigated to compare a different functional group from those commonly reported in the literature (
Figure 3a).
The cross-linking agent was chosen from either a difunctional or trifunctional option (ethylene glycol dimethacrylate (EGDMA) or trimethylolpropane triacrylate (TRIM)) based on the adsorption capacity of a MIP synthesized with 2VP as the monomer. The synthesis method adopted, although less technologically elaborate than microwave-assisted or ionic liquid-based approaches, distinguishes itself for its simplicity, cost-effectiveness, reproducibility, and, most importantly, scalability, making it a promising strategy for future applications beyond the laboratory scale, like in a quaternary step in a WWTP.
All synthesized materials were fully characterized using different techniques: FTIR, thermal analysis, and adsorption analysis. These analyses provide insight into the reaction status, polymer stability, and retention capacity for CBZ. For polymers prepared to optimize the reaction conditions, a reference material for each sample was also synthetized and compared with the corresponding MIP to verify the effectiveness of the imprinting.
This work presents two main novelties: (i) the development and optimization of MIPs for the adsorption of CBZ, an ubiquitous drug poorly investigated compared to NSAIDs such as ibuprofen, naproxen, diclofenac, or ketoprofen; (ii) no previous research has focused on the study of selective bio-based monomers targeting a pharmaceutical; instead, other studies have focused on the implementation of bio-based cross-linkers for the adsorption of micropollutants [
16].
2. Results
In this study, the preparation of molecularly imprinted polymer (MIP) materials for the capture of carbamazepine (CBZ) was addressed using a readily scalable bulk polymerization technology adapted from the one reported by Cantarella et al. [
23]. A pre-polymerization step was added, consisting of pre-mixing the monomer, the template, and the porogen for 20 min prior to the addition of the other polymerization components to promote the formation of the complex between the monomer and the target molecule [
14]. Regarding the porogen, among the most frequently used solvents in similar studies (chloroform, DMSO, toluene, and acetonitrile), we chose acetonitrile due to its lower toxicity, in accordance with green chemistry principles.
A preliminary study was conducted to evaluate which cross-linker provides the best adsorption performance. Subsequently, different bio-based monomers were tested with the selected cross-linker. The monomers used in the screening were chosen to represent different functional groups, enabling the investigation of a range of potential interactions with the template molecule. Finally, to assess whether a longer reaction time affects the adsorption capacity and morphology, several MIPs were prepared using different reaction times. Additionally, a different monomer-to-cross-linker ratio was also tested.
Details about the molar composition, reagents employed, reaction time, and polymerization temperature of all syntheses, together with sample codes, are reported in
Table 1. Acronyms are displayed in the acronym list.
2.1. Cross-Linker Selection
The first part of this study focused on the preliminary selection of two different cross-linkers: a trifunctional cross-linker, trimethylolpropane triacrylate (TRIM), and a bifunctional one, ethylene glycol dimethacrylate (EGDMA). These were evaluated by combining them with two commonly employed monomers, 2-vinylpyridine (2VP) and methacrylic acid (MAA), which are frequently reported in the literature [
9,
13,
18,
19,
20].
Figure 4 shows the FT-IR spectra of the synthesized MIPs together with the corresponding monomers and cross-linkers. The unsaturation of the double bonds (C=C), set at 1634–1638 cm
−1, for MAA and the cross-linkers, respectively, is disappearing or significantly diminishing, indicating the reaction evolution [
24,
25,
26,
27]. In the case of 2VP, the vinylic group unsaturation corresponds to the peaks at 1586 cm
−1 and 1471 cm
−1, while other absorptions at 1563 and 1463 cm
−1 are attributed to the aromatic pyridine ring [
28]. In general, the spectra exhibit the presence of important bands set at 1200–1145 cm
−1 (C-O and C-N stretch), 1728 cm
−1 (C=O stretch), and 2990–2930 cm
−1 (C-H stretch). Control NIPs were also synthesized, but no significant spectral differences were observed compared to the MIPs.
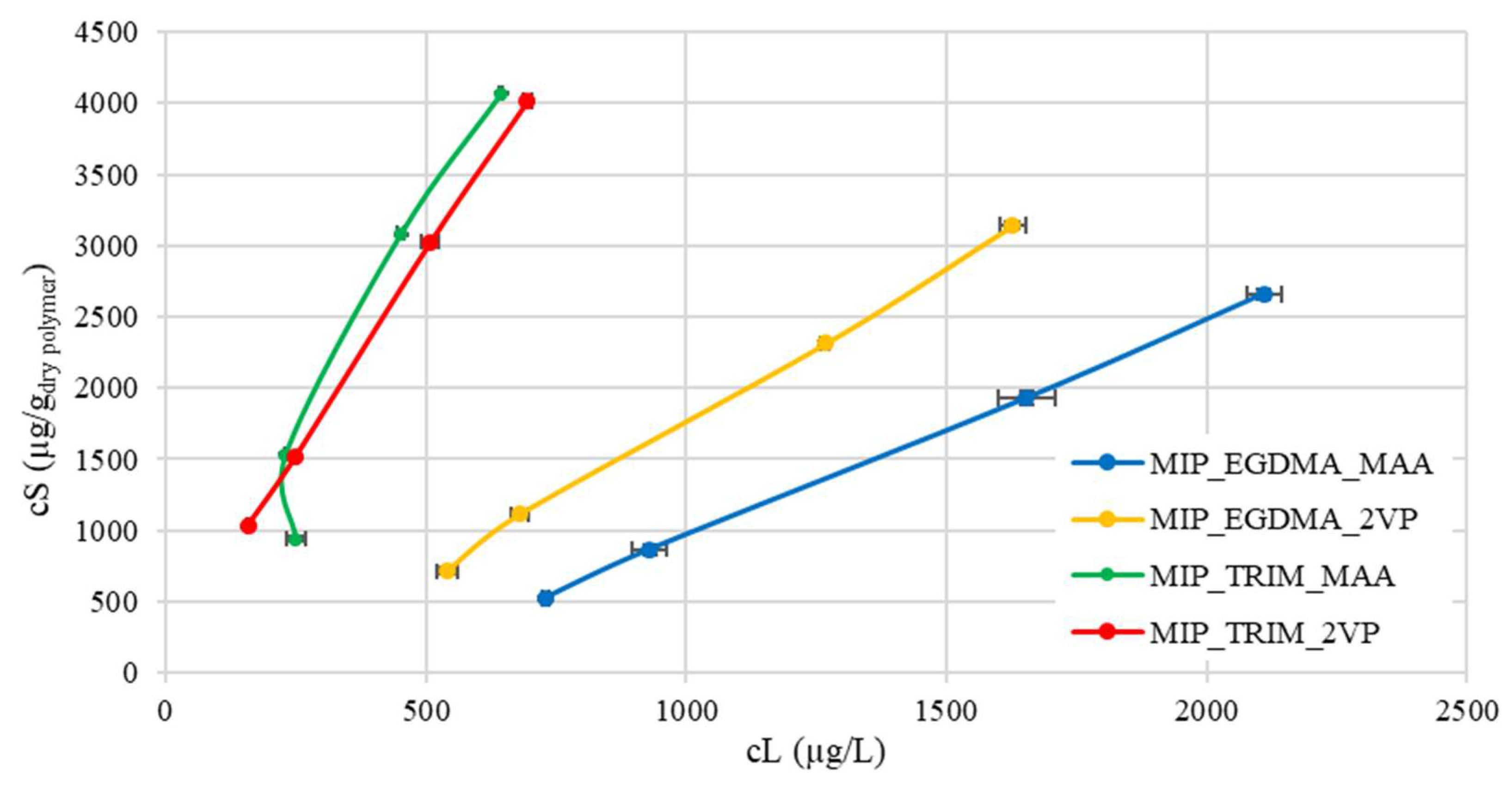
The adsorption capacity of the materials was evaluated in synthetic solutions prepared with deionized water. The adsorption data are presented as isotherms, where the concentration of CBZ adsorbed by the material (cS) is plotted against the concentration remaining in the solution (cL) once equilibrium is reached (
Figure 5). The CBZ adsorbed concentration was calculated according to Equation (1).
where cL0 is the initial concentration of the spiked solution (mg/L), V is the liquid volume (L), and m is the mass of the polymer (g).
The data obtained from the isotherms show that the trifunctional cross-linker offers the best results, with a higher ratio of adsorbed concentration to concentration remaining in solution. This trend can also be attributed to the greater surface area of these materials, as demonstrated by the BET analyses reported in
Table 2. The increased available surface area, due to the trifunctional cross-linker, appears to favor adsorption. The surface areas of MIP_EGDMA_2VP (270 m
2/g) and MIP_TRIM_MAA (440 m
2/g) are consistent with values reported by other authors [
16] but significantly higher than those reported by Dai et al. [
9] and Esfandyari-Manesh et al. [
29], equal to 136 m
2/g and 242 m
2/g, respectively. These values are also much higher than the surface area obtained using ESOA (epoxidized soybean oil acrylate) as the cross-linker, which was found to be close to 0 m
2/g [
16]. Notably, in the MIP reported by Dai et al. [
9], the monomer-to-cross-linker ratio was 1:5.4, while in the one reported by Esfandyari-Manesh [
29], it was 1:1. This suggests that the conditions employed in the synthesis enhance the porosity of the materials.
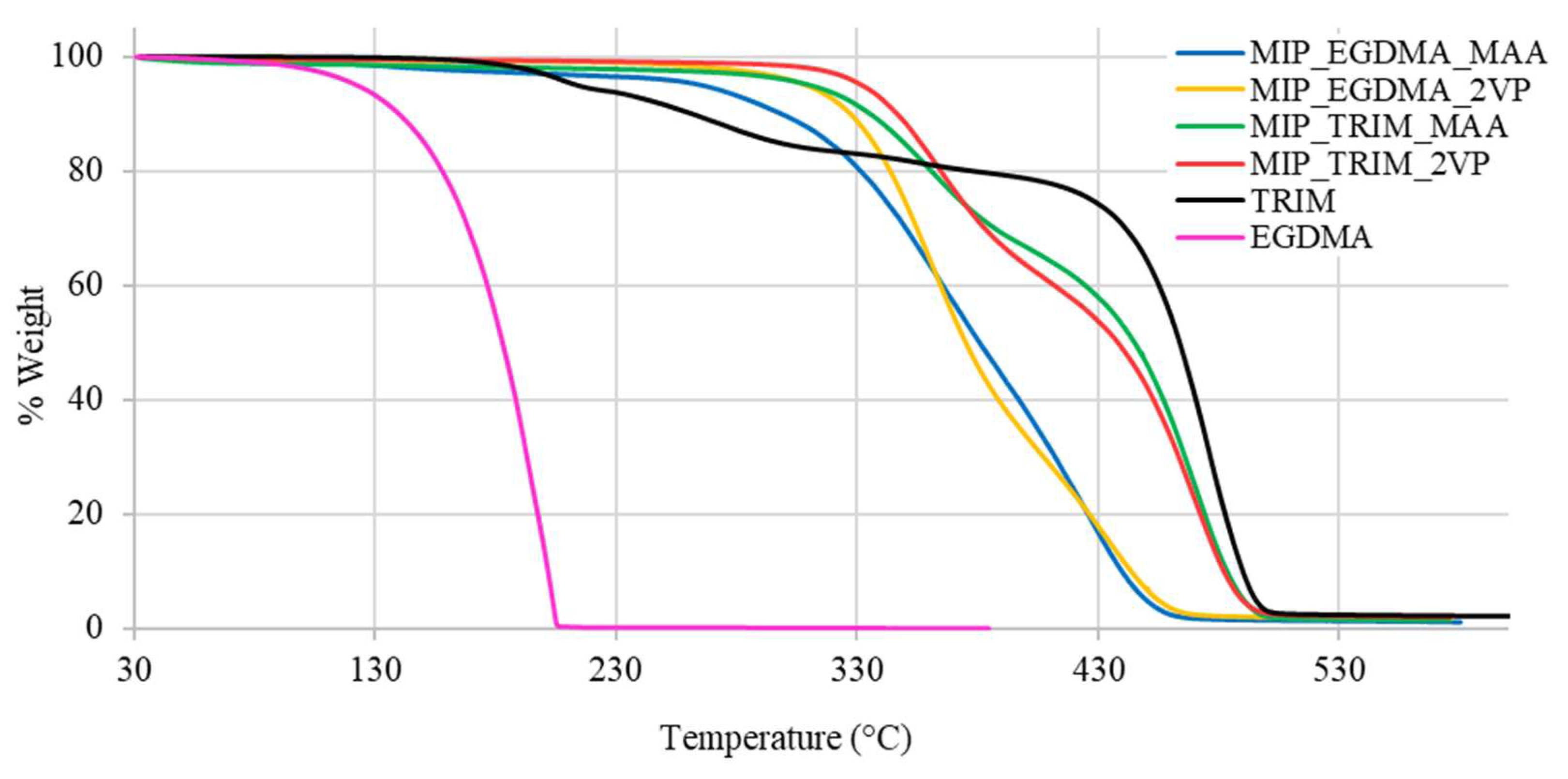
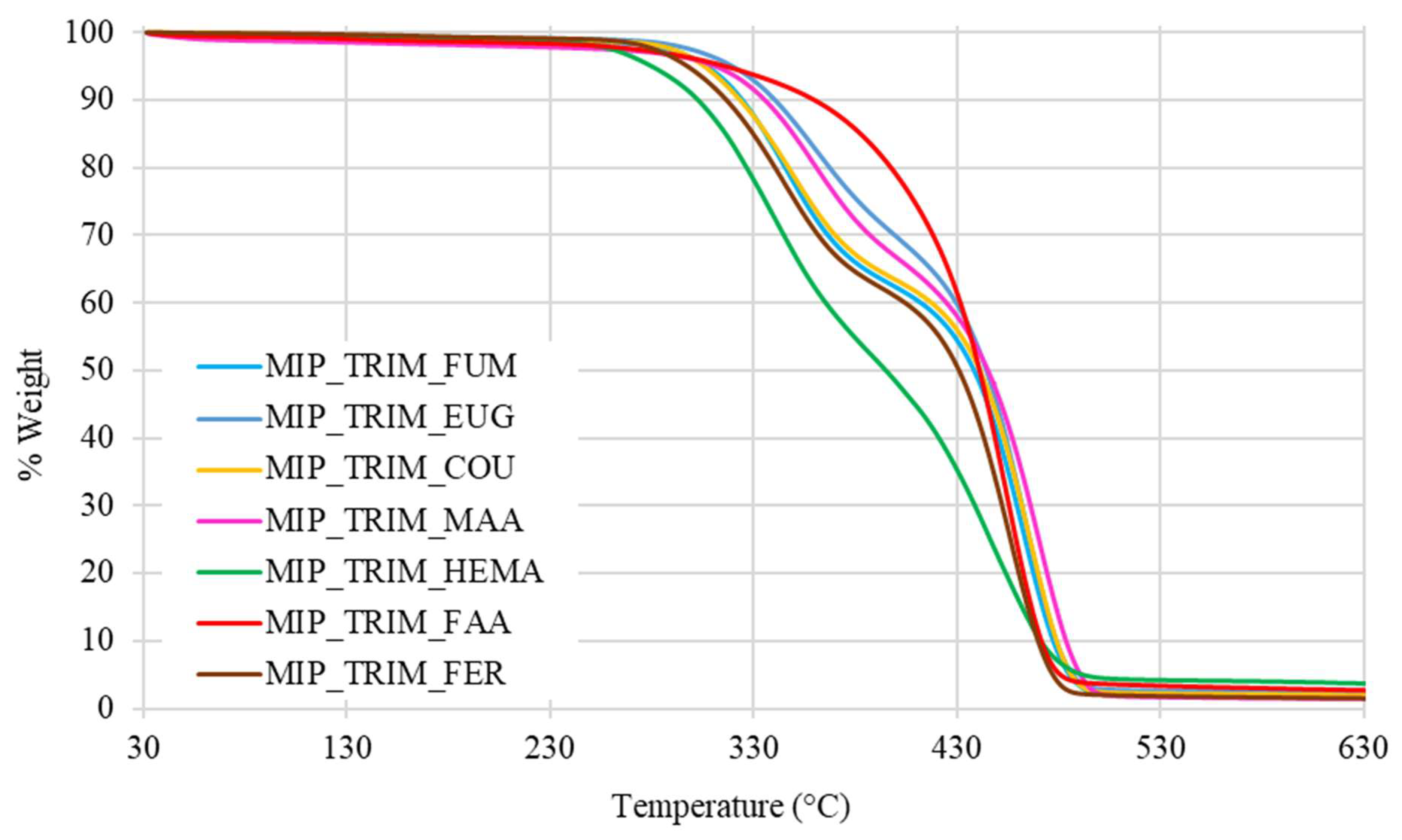
The effect of the trifunctional cross-linker is also reflected in the thermal stability of the materials determined by thermogravimetric analysis (TGA), which is a technique that monitors the mass of a sample continuously as a function of temperature under controlled atmosphere. This method provides detailed quantitative information on thermal degradation behavior, including decomposition onset temperature (T
onset) and the number and nature of distinct degradation steps, which are particularly significant in crosslinked polymer networks. As shown by the thermograms reported in
Figure 6, the MIPs synthesized with TRIM start degrading at higher temperatures and show two distinct degradation steps, reflecting the thermal profile of the starting reactants.
The analysis of the MIP T
onset shows the following order of thermal stability: MIP_TRIM_2VP > MIP_TRIM_MAA ≈ MIP_EGDMA_2VP > MIP_EGDMA_MAA (
Table 3). The presence of the trifunctional cross-linker promotes the formation of a more compact polymer network, resulting in higher thermal stability. In the case of the monomer, 2VP, possessing an aromatic ring, it starts degrading at higher temperatures with respect to MAA. Therefore, it is assumed that the presence of 2VP contributes to an even higher thermal stability of the corresponding MIP.
The variations in thermogram profiles further indicate that polymerization has taken place and that the choice of monomer affects the outcome.
2.2. Bio-Based Monomers Selection
The six selected monomers (2-hydroxyethyl methacrylate (HEMA), ferulic acid (FER), fumaric acid (FUM), eugenol (EUG), coumaric acid (COU), 3-(furyl)acrylic acid (FAA)) were used to produce MIPs, with the trifunctional cross-linker, which proved to be the best in the preliminary tests. All the results were also compared with MIP_TRIM_MAA previously prepared.
The FT-IR analyses, shown in
Figure 7, indicate the reaction evolution by the significant lowering of the signal corresponding to the C=C double bonds at 1638 cm
−1. Other significant transmittance bands, common to all the materials prepared, are the C=O double bond stretch at 1728 cm
−1 and the C-H stretching at 2997 cm
−1. The polymer’s carbonyl group is shifted to higher wavenumbers with respect to the carbonyl of the α,β-unsaturated ester present in TRIM (1716 cm
−1). This shift suggests that the double bond of the cross-linkers reacted, producing an aliphatic ester.
A semi-quantitative elaboration was also conducted evaluating the ratio between the C=C and C=O height. As reported in
Table 4, this ratio sensibly decreases with respect to the cross-linker.
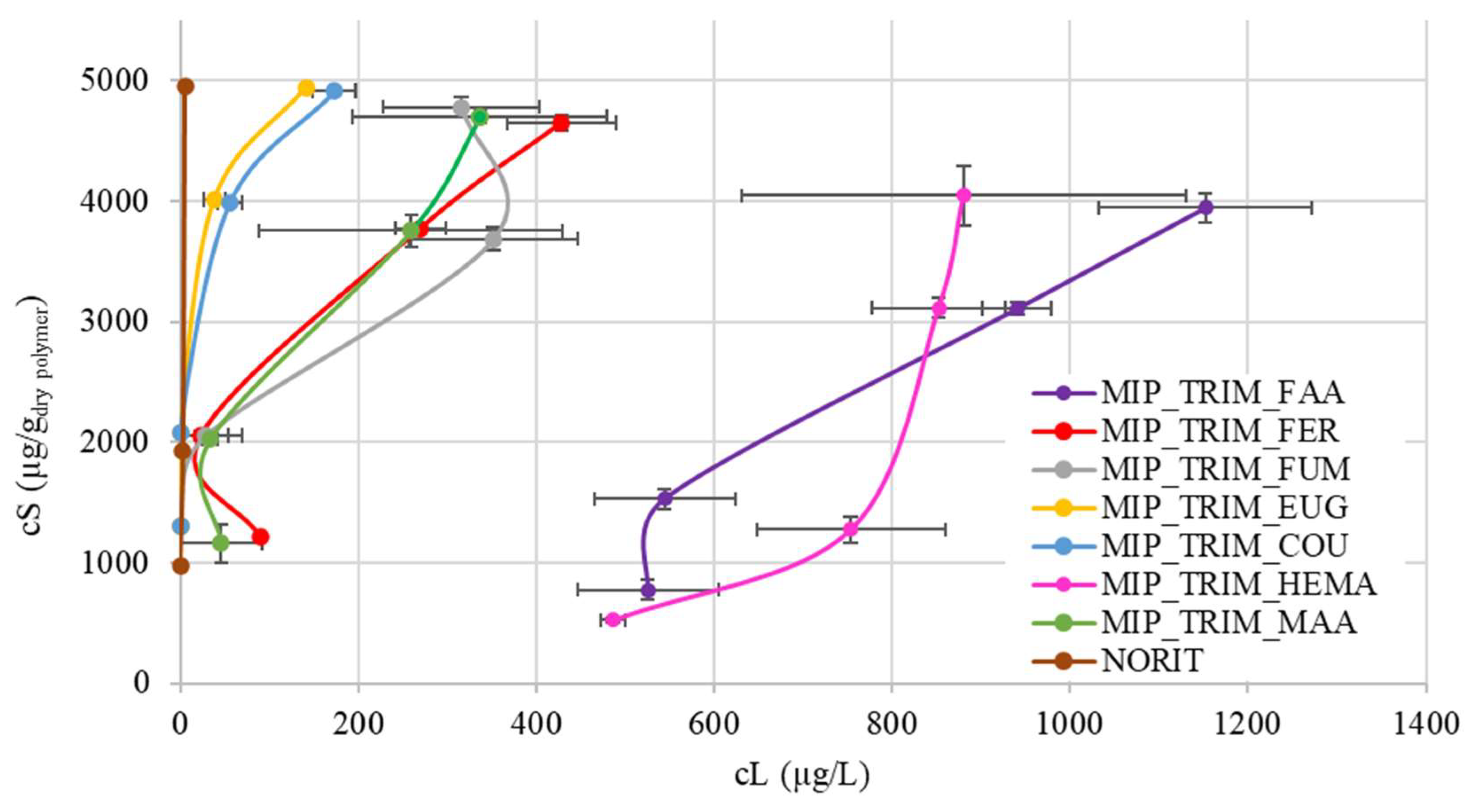
The CBZ adsorption capacity of the materials was evaluated by spiking the wastewater treatment plant (WWTP) effluent (
Table 5) with CBZ to consider the matrix effect. A commercial material, activated carbon Norit, is also reported as a reference. The adsorption data can be divided into three main groups (
Figure 8). An enlargement of
Figure 8 with detailed plots relative to the three groups of identified MIPs is reported in the
Supporting Information (Figures S1–S3).
The first group (
Figure S1) includes polymers with COU and EUG. Both materials provide the best results, likely due to the presence of the aromatic ring, which forms π-π stacking with CBZ. In the case of EUG, the aromatic ring has multiple substituents, and the unsaturation, which connects the monomer to the lattice, is one carbon away, allowing for greater movement of the ring. In the case of COU, the aromatic ring is sterically less hindered, with only one substituent, while the mobility of the chain connecting to the lattice is reduced since the double bond is one position away from the ring. Additionally, COU has an acidic group that may favor interaction with CBZ through hydrogen bonding. These two materials exhibit high adsorption capacity at low concentrations, comparable to activated carbon (Norit).
The second group (
Figure S2) includes MAA, FUM, and FER. FUM exhibits lower adsorption due to the lack of an aromatic ring. In the case of FER, despite its structure being quite similar to EUG, it is hypothesized that the presence of the methoxy group and the limited mobility of the aromatic ring may lead to a more challenging arrangement of the molecule around CBZ during adsorption.
Finally, the third group (
Figure S3) includes the MIPs synthesized with HEMA and FAA. HEMA shows the lowest adsorption, confirming that interaction with CBZ is due to acid group or π-π interaction. In the case of FAA, the low adsorption may be attributed to the poor wettability of the resulting material. Furthermore, FAA could inhibit radical polymerization or react with CBZ through a Diels–Alder reaction [
30,
31].
To further demonstrate the strong interaction between CBZ and EUG, a study on peak shifts due to their interaction was conducted using both FT-IR and UV analysis [
32]. In the FT-IR test, CBZ was solubilized in EUG, while in the UV test, both molecules were dissolved in water to confirm that the interaction also occurred in the presence of water (
Supporting Information, Text S1 and Figures S4–S7).
The thermal analysis confirms that the presence of bio-based monomers does not affect the materials’ stability, since they remain stable up to 300 °C, as shown in
Table 6.
The thermograms of MIPs with FUM, EUG, COU, MAA, and FER show a pattern like that of the MIPs previously synthesized with the same type of cross-linker, featuring a two-step degradation process. In the case of the MIP with FAA, the thermogram displays a single-step degradation pattern with a higher onset of degradation temperature due to interactions involving FAA during the polymerization process. Finally, for the MIP with HEMA, a degradation trend at lower temperatures is observed, probably ascribable to the absence of aromatic rings and acidic groups capable of forming hydrogen bonds and stabilizing the network (
Figure 9).
2.3. Reaction Conditions Optimization
Considering the results obtained from the monomer selection, we decided to further investigate the EUG-based MIPs.
As reported by Pratama et al. [
33], the ratio between the amount of monomer and cross-linker used in the synthesis of MIP is one of the factors influencing the material’s adsorptive capabilities. For this reason, the behavior of a MIP with the same reagents but a higher percentage of monomer was evaluated. Another factor examined was the reaction time, which was extended from 4 h to 24 h. For each material, a reference polymer (NIP, not imprinted polymer) synthesized without the addition of the template molecule (CBZ) was also prepared.
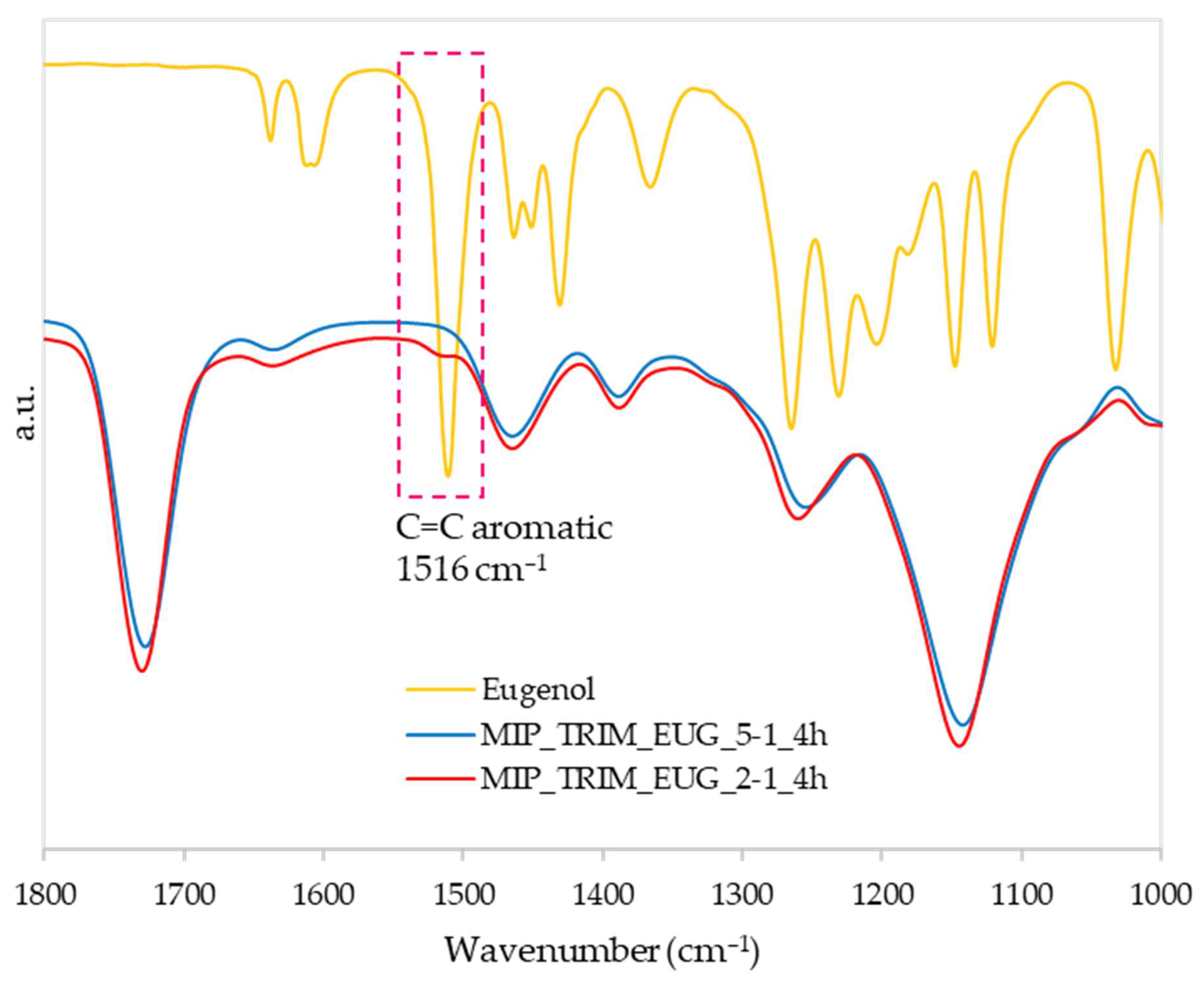
As in the previous test, FT-IR analyses confirmed the reaction evolution (spectra not reported), indicating no differences among the samples prepared with different reaction times. Conversely, in the case of the sample with a higher EUG concentration (MIP_TRIM_EUG_2-1_4h), a small but not negligible shoulder was detected at 1516 cm
−1, attributed to the stretching of the aromatic ring; this peak confirms the presence of the monomer (
Figure 10) [
34].
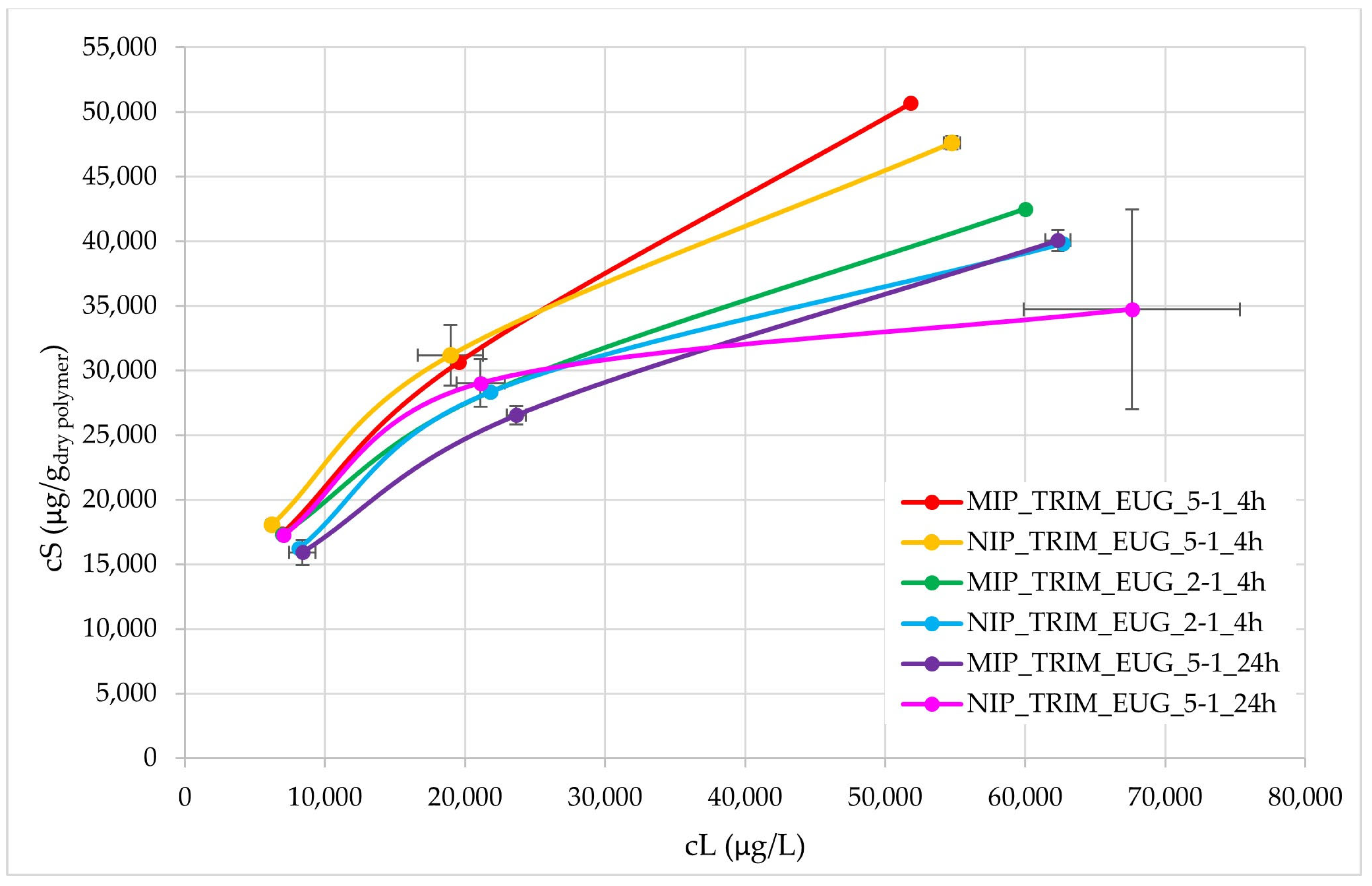
Further adsorption isotherms were conducted using the WWTP effluent enriched not only with CBZ but also with diclofenac (DCF) and ibuprofen (IBU) to evaluate both the CBZ adsorption capacity and the selectivity towards CBZ. CBZ, DCF, and IBU have similar structures with different steric hindrances. Higher initial pharmaceutical concentrations were tested (25, 50, 100 mg/L) in comparison to the previous tests, to verify more accurately MIP selectivity towards CBZ.
The resulting isotherms are shown in
Figure 11. In the initial part, up to adsorbed concentrations (
cS) of 3 mg/g, the isotherms are generally overlapping, except for MIP_TRIM_EUG_5-1_24h, which deviates and exhibits lower adsorption. At higher concentrations, the best performance is observed for MIP_TRIM_EUG_5-1_4h and NIP_TRIM_EUG_5-1_4h, whereas the material with the worst adsorption performance is NIP_TRIM_EUG_5-1_24h. In all cases, at high concentrations, a trend is observed that confirms the superior adsorption performance of the MIP compared to the corresponding NIP. It is also evident that the adsorption isotherm of MIP_TRIM_EUG_5-1_4h is higher than that of MIP_TRIM_EUG_2-1_4h. This result confirms that the molar ratio composition influences the performance of the final materials. Increasing the amount of monomer affects the adsorption capacity. A possible explanation could be the ambivalent effect of EUG, which shows an interaction with CBZ, thus favoring complex formation, but it may also slow down the polymerization process.
The difference between MIP and NIP was also assessed through the ratio of adsorption capacities, and no significant differences were observed at the two lowest concentrations.
This result can be attributed to the fact that the adsorption of CBZ in the NIP is primarily driven by a surface adsorption process, characterized by a strong interaction between the aromatic ring of eugenol and CBZ. Since no CBZ molecules were introduced during the synthesis of NIP to act as templates for the formation of selective cavities, the polymer lacks specific recognition sites. However, the presence of a monomer with a high affinity for CBZ still facilitates a generalized adsorption of the compound. In order to better investigate the differences between MIP and NIP, a kinetic test of CBZ adsorption on MIP_TRIM_EUG_5-1_4h and NIP_TRIM_EUG_5-1_4h was conducted, monitoring the CBZ concentration every minute for the first 10 min and then every 10 min, and the calculated solid-phase concentrations were interpolated with both a first-order and second-order kinetic model. As shown in
Figure S8 in the Supplementary Information, during the initial 60 min, CBZ adsorption on MIP_TRIM_EUG_5-1_4h was significantly faster than on NIP_TRIM_EUG_5-1_4h. This finding indicates that in the case of a scale-up process in continuous flow conditions, MIP_TRIM_EUG_5-1_4h would require a significantly shorter contact time than NIP_TRIM_EUG_5-1_4h, which implies a reduction both in the column investment cost and in the operational cost for the periodic sorbent replacement.
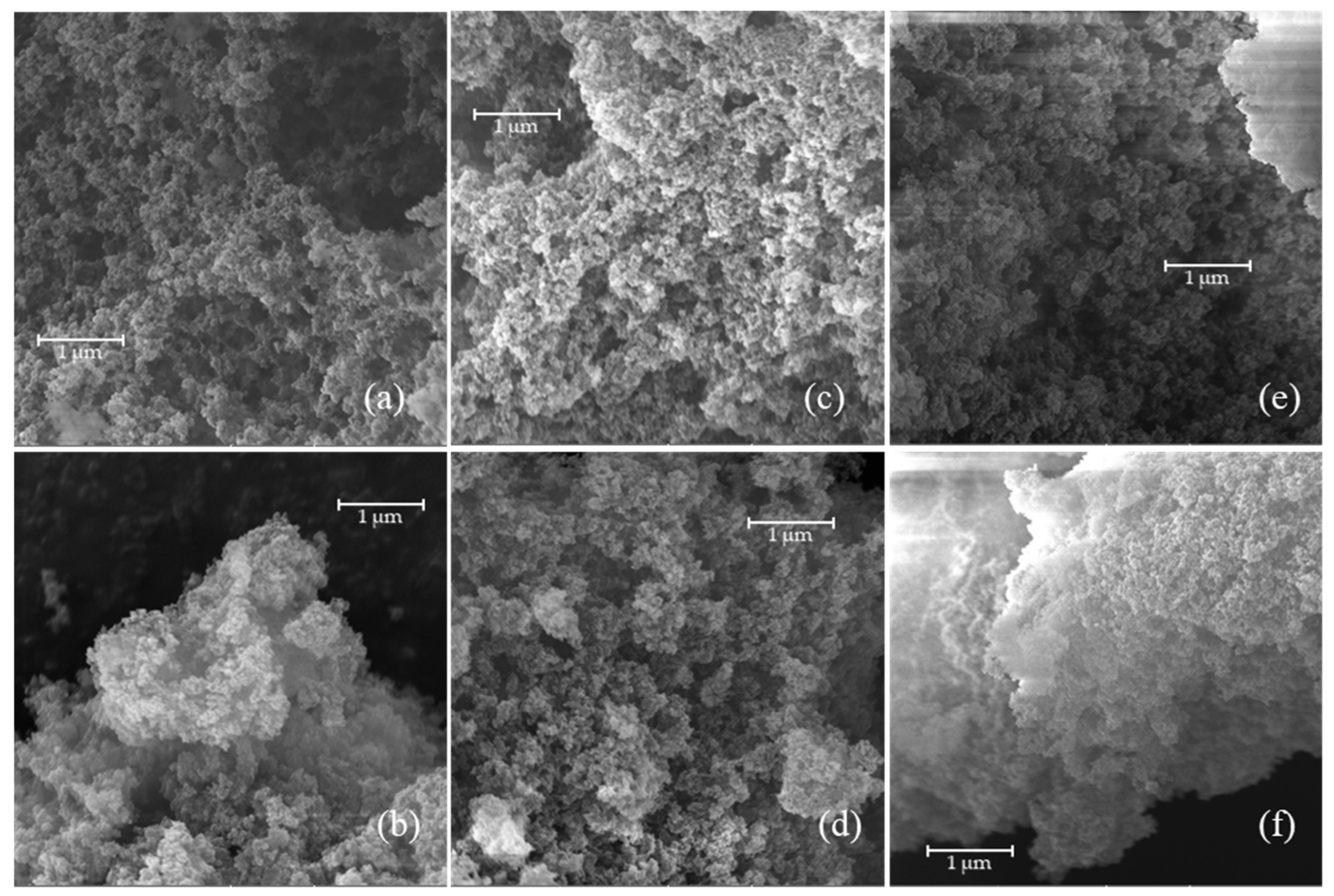
The MIPs prepared were also characterized in terms of morphology by scanning electron microscopy (
Figure 12), and the surface area was evaluated using a BET.
The morphology of MIPs primarily depends on the synthesis method used, followed by the choice of porogen, and finally the cross-linker [
16,
35]. Typically, polymers synthesized via precipitation polymerization exhibit homogeneous, spherical particles, while those prepared by bulk polymerization display more heterogeneous structures [
36,
37]. SEM images of MIPs prepared by bulk polymerization reveal typical morphologies characterized by agglomerates of irregularly shaped particles of varying sizes, often described as having a cauliflower-like appearance [
37].
The SEM analysis was conducted to assess whether increasing the monomer concentration and extending the reaction time would affect the polymer structure formation. The results show that the morphologies obtained are extremely porous and comparable, indicating that neither the monomer-to-cross-linker ratio nor the reaction duration significantly influence the MIP morphology. This conclusion is further supported by the similarities in surface area values reported in
Table 7, indicating that the investigated reaction conditions do not have a high impact on the sample morphology.
The MIP surface areas were slightly lower than those of NIPs, suggesting the possible presence of a residual template within the MIPs. Furthermore, the highest surface area was exhibited by the MIP synthesized with a lower EUG concentration and a longer reaction time. However, this sample did not demonstrate the best adsorption capacity; therefore, it can be concluded that the differences in adsorption capacity cannot be attributed to morphological variations. The MIP and NIP surface areas were equal to about 50% of the area of a typical activated carbon (Norit GAC 1240W).
The results of the test of MIP_TRIM_EUG selectivity for CBZ compared to other pharmaceuticals are presented in
Figure 13. The percentage of CBZ adsorbed by MIPs decreases with the rise in cL0, with adsorption values of 70%, 57%, and 43%, respectively, for initial concentrations of 25 mg/L, 50 mg/L, and 100 mg/L. In the literature, the adsorption capacity of MIPs for CBZ solutions in distilled water without interfering substances exceeds 90% [
13,
20]. However, in the case of solutions in distilled water containing multiple drugs, the adsorption capacity drops to values as low as 40% [
3]. These data suggest that multiple pollutants’ presence in the considered solution and the type of water used significantly affect the overall adsorption capacity of the materials.
In terms of adsorption yield (Yads) relative to CBZ, DCF and IBU were obtained at different initial concentrations in the 25–100 mg/L range. The Yads,CBZ/Yads,DCF ratio was equal to 1.5 ± 0.1, whereas the Yads,CBZ/Yads,IBU ratio was equal to 6 ± 3, indicating a high selectivity of the tested TRIM_EUG MIPs towards CBZ. The selectivity did not vary significantly among the three MIPs based on TRIM and EUG.
Considering the results, increasing the reaction time did not prove to be an effective choice. Since similar adsorption capacities and comparable morphological profiles were obtained, it is preferable to maintain a reaction time of 4 h with a view to future scale-up. This would also help to reduce energy consumption and production costs.
TGA revealed a comparable degradative behavior between the MIPs and NIPs prepared under identical conditions. This result indicates that the presence of the template during the synthesis process does not affect the thermal stability of the polymer network.
As illustrated in
Figure 14 and
Table 8, the materials synthesized with an increased monomer-to-cross-linker ratio exhibit a T
onset approximately 10 °C higher than that prepared with a lower one. This suggests that a higher monomer content enhances the material’s stability, likely due to the aromatic group present in EUG.
2.4. MIP Recyclability and Performances
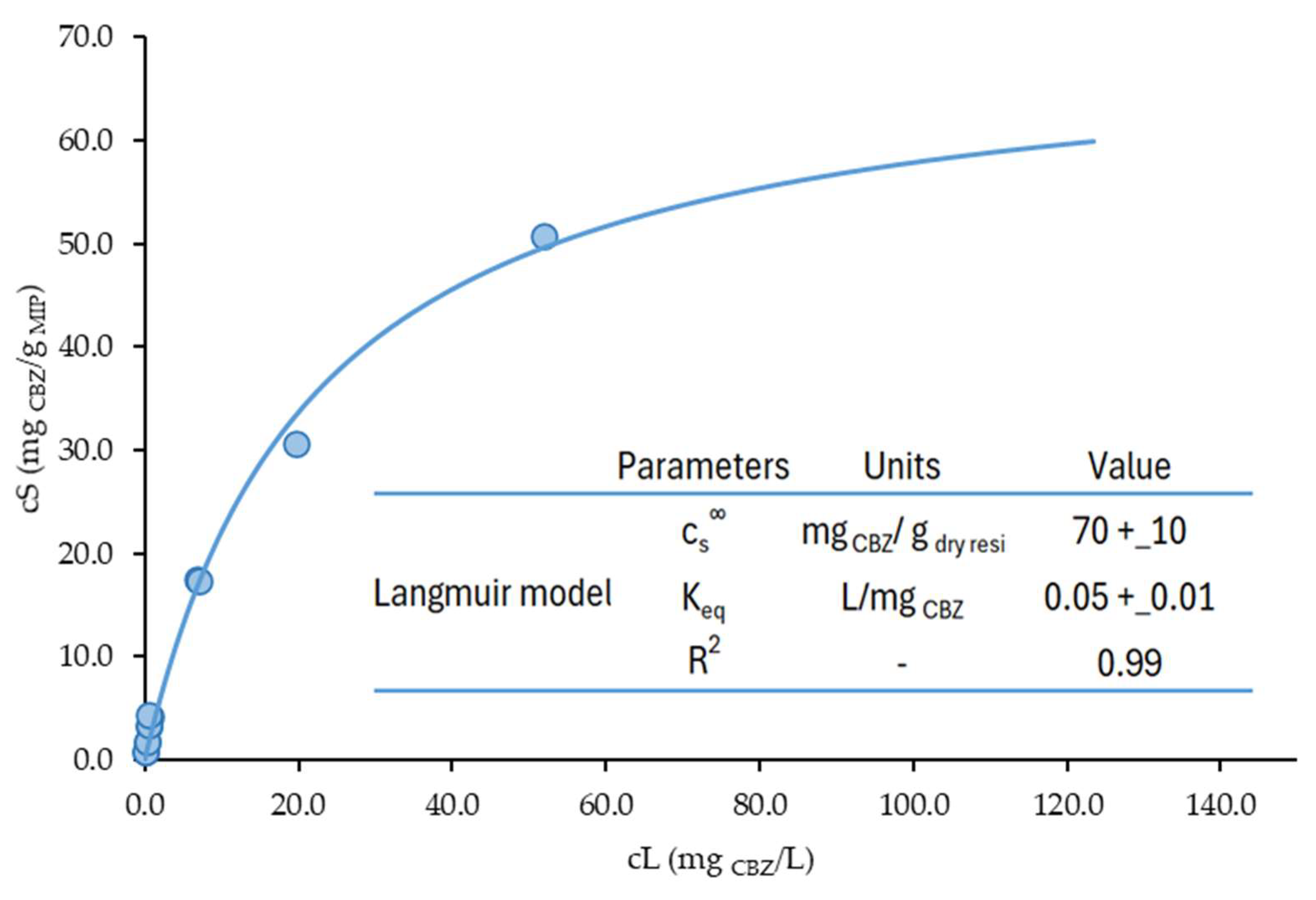
For the material that demonstrated the best performance, MIP_TRIM_EUG_5-1_4h, a more comprehensive study was carried out to evaluate its behavior at low concentrations of CBZ and to determine the saturation limit. The resulting isotherm is presented in
Figure 15, and the data were subsequently fitted using the Langmuir model, as expressed in Equation (2):
where
(mg
CBZ/g
dry resin) is the maximum amount sorbed per unit mass of adsorbent, corresponding to a complete monolayer on the adsorbent surface, and K
eq (L/mg
CBZ) is the constant related to the affinity between the binding sites and CBZ. The best-fit parameters obtained are reported in the box in
Figure 15 with their 95% confidence interval. The quality of the fitting was evaluated by calculating the coefficient of determination R
2, that resulted in being equal to 0.99.
As shown in
Figure 15, eight additional isotherm points were tested in the 70–740 μg/L liquid-phase concentration range at equilibrium to investigate the CBZ adsorption performances of MIP_TRIM_EUG_5-1_4h under conditions closer to those of real applications. The sorption performance of MIP_TRIM_EUG_5-1_4h at very low CBZ levels typical of WWTP (0.1–38 μg/L, average value 4.8 μg/L [
38]) can be safely estimated by extrapolating the strongly linear trend presented by these eight additional points tested in the low concentration range (R
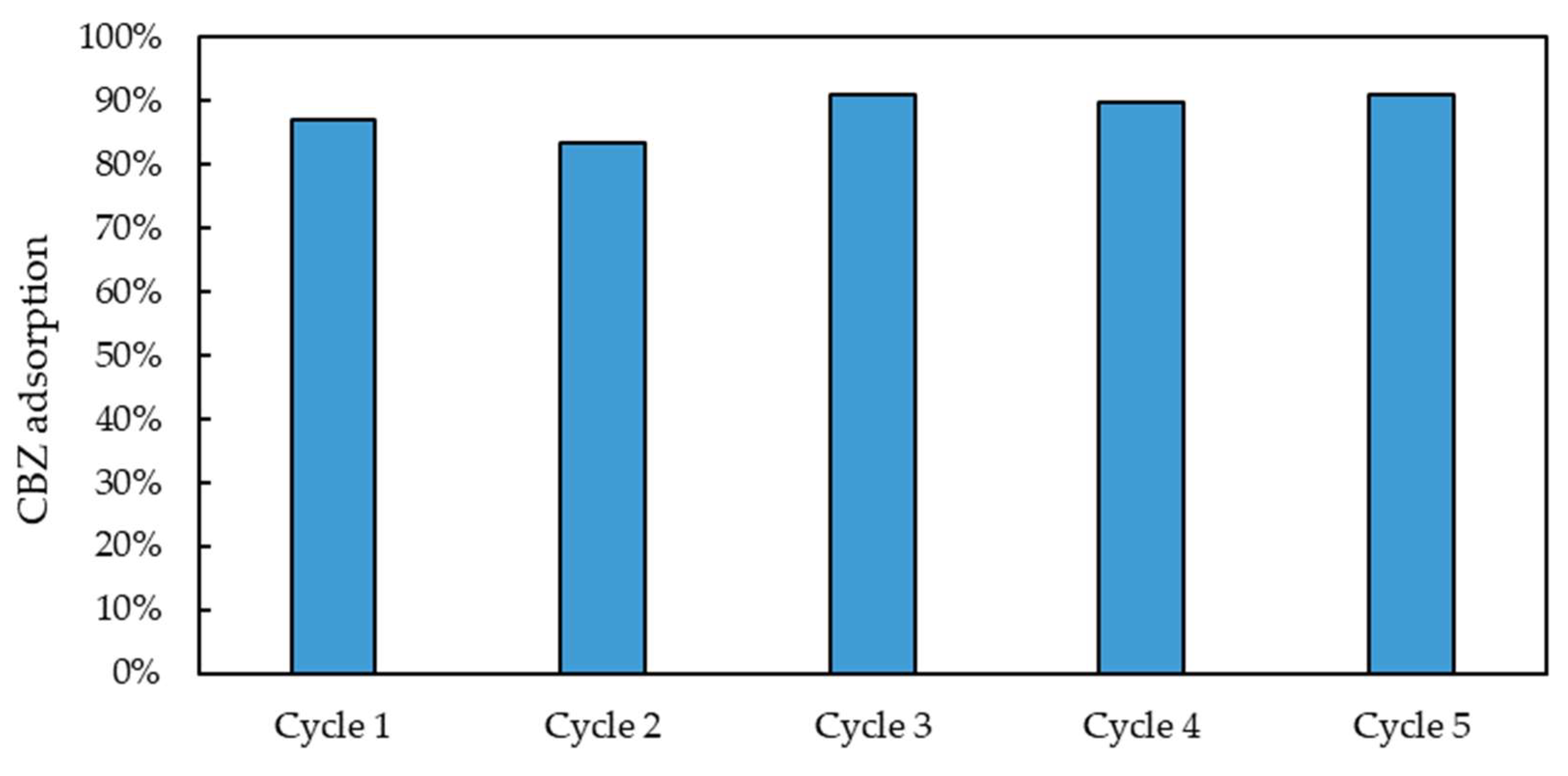
2 = 0.963). An additional study was carried out to assess the recyclability of the material. Five adsorption–desorption cycles were carried out using a WWTP effluent spiked with CBZ at 4 mg/L. For the desorption step, a methanol/acetic acid solution (9:1), also used for template removal post-polymerization, was employed. The set-up and procedure of the repeated adsorption–desorption tests are reported in
Section 3.3. As illustrated in
Figure 16, the material maintains a high adsorption capacity (about 90%, in line with MIPs synthesized for other pharmaceuticals [
39]) during the repeated cycles. In particular, no significant differences were observed in the adsorption capacities obtained during the five repeated cycles (
t test,
p = 0.05). Furthermore, the amount of desorption solution needed to completely regenerate the sorbent was very low (5–10 bed volumes), as reported in the
Supporting Information, Figure S9. The stability of the sorbent performances over multiple cycles and the low amount of regenerant required allowed for the achievement of a relevant reduction in the operating cost associated with the periodic regeneration and replacement of the sorbent.
The synthesized material demonstrates excellent reusability, showing a performance comparable to that of other CBZ-targeting MIPs described in the literature [
9,
19,
32].
Furthermore, the study of MIP_TRIM_EUG_5-1_4h over a broader concentration range allowed for a comparison with other materials reported in the literature, such as the resveratrol-selective MIP synthesized using a bio-based cross-linker [
16]. Notably, at the same liquid-phase equilibrium concentration (C
e = 28 mg/L), the MIP developed in this study is capable of adsorbing a significantly higher amount of analyte (35 mg/g of CBZ versus 3 mg/g of resveratrol).
Additionally, the slope of the initial portion of the CBZ adsorption isotherm obtained with MIP_TRIM_EUG_5-1_4h (0.007 L/mg) can be compared with that of other MIPs designed for CBZ, such as the materials synthesized via precipitation polymerization and deposited onto magnetic carriers (Fe
3O
4@SiO
2) [
32]. In that case, the slope varies depending on the monomer used, ranging from 0.08 to 0.002 L/mg. It should be noted, however, that the synthesis conditions differ between systems.
At the same equilibrium concentration (50 mg/L), the CBZ-selective MIP developed in this study adsorbs 50 mg/g, while MIPs prepared for IBU, under comparable conditions, reach only 7.2 mg/g [
36]. Although differences in target molecule properties may influence the adsorption behavior, these results clearly highlight the superior performance of the CBZ-specific MIP developed in this study in terms of adsorption capacity [
36].