Abstract
To date, various species of Erigeron genus have been used both in the ethnopharmacology of numerous nations across the world and in contemporary herbal practices. The objective of this study is to revise the phytochemical data on the essential oils (EOs) of various fleabanes species and to evaluate the variability of their biological activities. Up to June 2025, this review provides an updated overview of 105 literature sources (published during last 25 years) related to 14 Erigeron sp. (native, naturalized, or invasive) which have been investigated extensively and are of the greatest significance. It summarizes the compositional variability of the EOs and their pharmacological and toxic effects, such as anti-inflammatory, anticancer, antiproliferative, skin regeneration, antioxidant, antifungal, antibacterial, insecticidal, larvicidal, repellent, and allelopathic activity. The EOs of each Erigeron species were characterized, and a chemical structure of 43 major constituents is presented herein. The most characteristic and prevalent compounds were found to be limonene, δ-3-carene, matricaria ester, lachnophyllum ester, germacrene D, β-caryophyllene, β-farnesene, α-bergamotene, allo-aromadendrene, etc., in the EOs from the E. acris, E. annuus, E. bonariensis, E. canadensis, E. floribundus E. mucronatus, and E. speciosus plants. Major constituents, such as borneol, bornyl acetate, modhephen-8-β-ol, cis-arteannuic alcohol, β-caryophyllene, and τ-cadinol, were found in the oils of E. graveolens (Inula graveolens). A paucity of data concerning E. incanus EOs was revealed, with the prevalence of 3-hydroxy-4-methoxy cinammic acid and thymol acetate noted in the oils. The EOs from E. multiradiatus and E. sublyratus were comprised mainly of matricaria and lachnophyllum esters. The available data on EOs of E. ramosus is limited, but the main constituents are known to be α-humulene, 1,8-cineole, eugenol, and globulol. The EOs containing appreciable amounts of matricaria and lachnophyllum esters exhibited strong anticancer, anti-inflammatory, antimicrobial, larvicidal, and repellent activities. Repellence is also related to borneol, bornyl acetate, caryophyllene derivatives, τ-cadinol, modhephen-8-β-ol, and cis-arteannuic alcohol. Cytotoxicity was determined due to the presence of limonene, δ-3-carene, α- and β-farnesene, (E)-β-ocimene, ledene oxide, sesquiphellandrene, and dendrolasin in the fleabanes EOs. Skin regeneration and antifungal properties were related to germacrene D; and anti-inflammatory effects were determined due to high amounts of limonene (E)-β-ocimene, lachnophyllum ester, and germacrene D. The antimicrobial properties of the oils were conditioned by appreciable quantities of limonene, β-pinene, 1,8-cineole, carvacrol, thymol acetae, β-eudesmol, 2,6,7,7α-tetrahydro-1,5-dimethyl-1H-indene-3-carboxaldehyde, caryophyllene and its oxide, allo-aromadendrene, α-humulene, farnesene, carvacrol, and eugenol. This review provides a foundation for further studies on volatile secondary metabolites to explore the potential sources of new biologically active compounds in Erigeron sp.
Keywords:
Erigeron; f. Asteraceae; essential oils; limonene; β-ocimene matricaria ester; lachnophyllum ester; β-farnesene; α-bergamotene; modhephen-8-β-ol; dendrolasin; ledene oxide; antimicrobial activity; anticancer properties; insecticidal; larvicidal and repellent activities; allelopathic effects 1. Introduction
The genus name Erigeron (first published in 1753) (Asteraceae) was accepted by World Flora Online (WFO, Plant List), and a number of accepted species names in the genus is approximately 450 [1]. Additionally, up to 480 species of plants in the genus Erigeron are listed on Plants of the World Online (POWO) as of June 2025 [2]. The genus comprises over 200 annual, biennial, and perennial species native to North and Central America, as well as 390 species distributed worldwide [3]. Plants of the genus have been classified within the tribe Astereae (f. Asteraceae), exhibiting a close relationship with both asters and the true daisies (bellis). It is important to note that fleabanes have been frequently misidentified as a species of asters, particularly within alpine environments. The taxonomic classification of plants into this genus has been a complicated process that has undergone modification over time.
The current global distribution, preferred climatic conditions, biological information, and morphological features of Erigenon have been well documented [1,2,3,4,5]. The plants (subshrubs, shrubs, or trees) are distinguished by its well-branched structure, with erect stems (glabrous or hairy) that bear plenty of white, lavender, or pink ray flowers, complemented by yellow disk flowers [4].
Erigeron has a long history of traditional uses in various ethno-cultures, and occupies an important place in traditional medicines all over the world. In the folk medicine of European countries, the roots of E. acris are applied to alleviate a range of ailments, including toothaches, arthritic pains, bruises, digestive disorders, and enteritis [6,7]. In Traditional Chinese Medicine, the plants of E. acris and E. annuus are widely used in various formulations to treat indigestion, enteritis, epidemic hepatitis, diabetes, hematuria, malaria, and obesity [8,9]. E. bonariensis is a well-known therapeutic herb in many cultures. Its properties have been utilized in ethnomedical practices for a variety of ailments, including cancer, diabetes, the management of age-related changes, antimicrobial properties, wound healing, diuretic effects, and the treatment of diarrhea and hemorrhoids [10,11,12,13]. Formulations of E. breviscapus are effective ethnopharmacological remedies in many Asian countries for a wide range of diseases, including cardiovascular, gastrointestinal, respiratory, and metabolic diseases [14,15]. The plants of E. canadensis are used for the treatment of acute toothache, otitis media, conjunctivitis, stomatitis, allergic diarrhea, wounds, swellings, and pain caused by arthritis in Chinese folk medicine [16,17]. The utilization of Canadian fleabanes in the folk medicine in the Northern regions of Pakistan is a long-standing practice [18,19]. The plant is employed in the treatment of a wide range of ailments, including pain, inflammation, fever, and, most notably, microbial infections such as urinary tract infections, respiratory tract infections, diarrhea, and dysentery [18]. In African folk medicine, E. canadensis was used to treat granuloma annulare, ringworm, eczema, sore throats, and urinary tract infections, as well as in medicinal baths [18,19]. E. floribundus, in addition to its established antimicrobial, analgesic, and anti-inflammatory properties, is a traditional remedy utilized in the treatment of malaria, a globally significant tropical parasitic disease, within the folk medicinal traditions of Cameroon and Uganda [20,21]. Moreover, the plant has multiple traditional uses in African countries, including healing stomach disorders, rheumatism, gout, cystitis, nephritis, dysmenorrhea, dental pains, skin problems, and headaches [21,22]. E. graveolens is another species that has been employed in both folk and modern medicine due to its antifungal, antibacterial, anti-inflammatory, and sedative properties [23]. E. incana plants are traditionally used as a tonic herb and for treatment of wounds in Yemen [24]. In India, the tribes of the Nilgiri Hills use E. mucronatus as a folk remedy for the treatment of a range of ailments, including diarrhea, dysentery, epilepsy, hemorrhages, paralysis, and diabetes, among others [25]. Preparation of E. multiradiatus plants are used by traditional healers and native people to cure hepatitis, meningitis, hemiparalysis, enteritis, diarrhea, rheumatism, and polyneuritis in Traditional Tibetan Medicine [26].
A considerable number of Erigeron species (native, casual, naturalized, or invasive) are employed as remedies in the ethnopharmacology of numerous countries; and the pivotal role of the essential oils (EOs) must be emphasized. EOs are defined as mixtures of fragrant compounds or as mixtures of fragrant and odorless substances [27]. Usually, EOs are aromatic volatile liquids, obtained mainly using steam distillation (hydro-distillation) of volatile organic compounds from plant material, which are produced by glandular trichomes and other secretory organs in different parts of plants. These secondary metabolites have been shown to play a multipurpose role in plant physiology, contributing to processes such as growth, defense, communication, reproduction, etc. However, volatile organic compounds have been found to have a significant impact on the bioactivity of herbal material.
The aim of the present study is to revise and summarize phytochemical data on the EOs of various Erigeron species that have been published during the last twenty-five years; to make comparisons between them; and to evaluate the variability in their biological activities. The objective of this review is to stimulate further studies in order to establish the molecular pathways in the observed activities and to explore potential sources of new biologically active compounds of the Erigeron genus.
2. Methodology
This review comprises data published between the years 2000 and 2025 concerning the chemical composition of EOs and their bioactivity of the Erigeron species which were predominantly investigated and are of the greatest significance, such as E. acris, E. annuus, E. bonariensis, E. breviscapus, E. canadensis, E. floribundus, E. graveolens, E. incanus, E. mucronatus, E. multiradiatus, E. philadelphicus, E. strigosus, E. speciosus, and E. sublyratus. This study examines compositional variability on the basis of major compounds, representing >5% of the total EO content. Furthermore, the origin of the Erigeron plant specimens is introduced, along with details concerning the oil preparation process.
Relevant information was gathered and synthesized using an online search engine of scientific databases such as Google Scholar (https://scholar.google.com (accessed on 5 May 2025)), PubMed (https://pubmed.ncbi.nlm.nih.gov (accessed on 5 May 2025)), Scopus (https://www.scopus.com/home.uri (accessed on 5 May 2025)), ScienceDirect (https://www.sciencedirect.com), Wiley Online Library (https://onlinelibrary.wiley.com/advanced/search (accessed on 5 May 2025)), and ACS Publications (https://pubs.acs.org (accessed on 5 May 2025)). The keywords, such as “Erigeron essential oil”, “Conyza canadensis essential oil”, “Conyza graveolens essential oil”, “Erigeron multiradiatus essential oil”, etc., were applied for this purpose.
A Principal Component Analysis (PCA) of major components and various biological activities of E. bonariensis and E. canadensis EOs was performed using Microsoft Excel Add-in Analyse-it 5.30 Standard Edition (Analyse-it Software, Ltd., Leeds, UK) (Supplementary Material, Figures S1 and S2).
3. Results
3.1. Erigeron acris L.
Erigeron acris (L.) (common names blue fleabane, bitter fleabane) is an annual, biennial, or rarely perennial plant, 15–50 cm in height. It is characterized by the following features: a taproot and a woody rhizome, a stem ascending, covered with bristles, oblong hairy leaves, and flowers, which are typically blue or blue-purple in color [28]. The plant is known by various other names, the most popular being Aster erigeron or Erigeron alpinus Lam. (illeg.) [1]. The species is native to Canada, northern parts of the United States, Asia, and most of Europe [2].
3.1.1. Compositional Data of Essential Oils (EOs) from Erigeron acris L.
As demonstrated in Table 1, the available data concerning E. acris EOs is extremely limited during last quarter of a century [28,29]. Plant material was collected in Poland, where E. acris is a native taxon [30]. The optimal growing locations for this species are dry grasslands, fields, wastelands, and roadsides, where it can thrive in full-sun conditions [28,29].

Table 1.
Data on Erigeron acris (L.) EOs published during the period 2000–2025.
Major constituents in the EOs derived from the herbal material of E. acris were determined to be some monoterpenes, namely limonene (1), β-pinene (2), and β-ocimene (3), and a sesquiterpene α-murolene (4), while the root oils were found to be rich in matricaria ester (5) and lachnophyllum ester (6) (Figure 1).
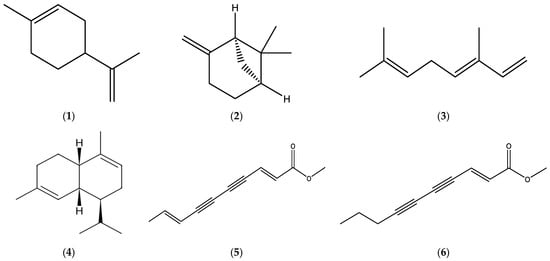
Figure 1.
Chemical structures of limonene (1), β-pinene (2), β-ocimene (3), α-murolene (4), matricaria ester (5) (https://pubchem.ncbi.nlm.nih.gov/compound/Matricaria-ester (accessed on 6 May 2025)), and lachnophyllum ester (6) (https://pubchem.ncbi.nlm.nih.gov/compound/Lachnophyllum-ester (accessed on 6 May 2025)). Chemical structures of compounds were drawn using the ChemDraw (version 16.0) molecule editor.
3.1.2. Bioactivity (Anticancer and Antifungal Properties) of E. acris EOs
The antiproliferative and antifungal activities of the EOs from Polish E. acris roots and herbs were investigated by Nazaruk et al. [6]. A cell viability assay was performed in cultured fibroblasts, cancer cell lines (MCF-7 and MDA-MBA-231), endometrial adenocarcinoma (Ishikawa), and colon adenocarcinoma (DLD-1) cells. The root oils demonstrated the highest antiproliferative activity in the MCF-7 cells (IC50 = 14.5 μg/mL) and could be applied for breast cancer treatment [6,31]. Furthermore, the remarkable antifungal properties of the bitter fleabane EOs were estimated against various strains of five Candida species (C. albicans, C. glabrata, C. tropicalis, C. krusei, and C. parapsilosis) using the microdilution method (the MICs ranged from 30 to 0.4 µL/mL) [6].
3.2. Erigeron annuus L.
Erigeron annuus (L.) (commonly known as the annual fleabane or daisy fleabane) is usually an annual herb, but sometimes grows as a biennial, blooming with white petals and yellow middles in the flowers. Previously, this species was named most frequently as Aster annuus.
E. annuus has many synonyms [1]. It is an indigenous weed from eastern North America. The native distribution of E. annuus extends from eastern Canada to central USA and the eastern United States, including Florida, Louisiana, Mississippi, and other states [2]. The species was introduced in many European countries (including the Baltic states), some states of USA (California, Oregon, and Washington), Asia (India, Japan, Kazakhstan, Kirgizstan, Korea, Nepal, Vietnam, etc.), and some islands such as Kuril, Newfoundland, Corse, Réunion, Sicilia, Ireland, etc. [2]. E. annuus possesses a number of biological properties that facilitate its ability to invade and adapt to a wide range of environmental conditions. Annual fleabane is a harmful invasive weed to the natural flora of numerous countries and represents a considerable risk to agriculture.
3.2.1. Compositional data of EOs of Erigeron annuus L.
Data of Erigeron annuus L. EOs (published during the period 2000–2025) is presented in Table 2 [29,32,33,34,35]. The plant material for the studies was collected in Poland, India, and Korea. E. annuus is an invasive species in Poland [6], cultivated or naturalized in India [36], and widely naturalized throughout Korea [37]. In Poland, A. annuus often grows at the same places as A. acris [29].

Table 2.
Main data on Erigeron annuus (L.) EOs published during the period 2000–2025.
The annual fleabane EOs derived from the aerial parts have been shown to contain significant quantities of lachnophyllum ester (6) (Figure 1) and some sesquiterpenes, such as germacrene D (7) and β-caryophyllene (8), and its oxide (9) (Figure 2).
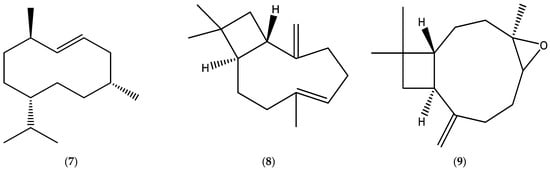
Figure 2.
Chemical structures of germacrene D (7), β-caryophyllene (8), and caryophyllene oxide (9).
It should be mentioned that four new sesquiterpenoids, (7R*) opposit-4(15)-ene-1β,7-diol, 11-methoxyopposit-4(15)-en-1β-ol, 15-methoxyisodauc-3-ene-1β,5α-diol, and 10α-hydroxycadin-4-en-15-al, have been isolated from the areal parts of E. annuus [38].
3.2.2. Bioactivity (Skin Regeneration and Antifungal Properties) and Toxicity (Allelopathic Effects) of EOs of E. annuus (L.)
The impact of the annual fleabane EO on skin-regeneration-associated responses, especially proliferation and migration, using human epidermal-keratinocytes (HaCats) was investigated by Kim et al. [35]. Results of the above research indicate that the oils stimulated proliferation and migration in HaCats and increased the phosphorylation of serine/threonine-specific protein kinase (AKT) and extracellular signal-regulated kinase (ERK) 1/2; and furthermore, the EO promoted sprout outgrowth in HaCats [35]. Additionally, the EOs from the E. annuus herb demonstrated a moderate antifungal activity against various strains of five Candida fungi (MIC values varied from 30 to 1.8 μL/mL) [6]. However, significant antifungal effects of annual fleabane EOs were documented by Kumar et al. against Fusarium oxysporum, Helminthosporium maydis, Rhizoctonia solani, Alternaria solani, and Sclerotinia sclerotiorum (inhibition varied from 37.6 ± 1.8 to 85.5 ± 1.4%, and IC50 values ranged from 660.0 to 153.2 to μg/mL). Moreover, remarkable effects of the spore germination were observed for F. oxysporum, Curvularia lunata, and Albugo candida (the strongest activity was assessed against F. oxysporum for, IC50 = 120.7) in the above study [34].
A recent article written by Rana et al. (published in 2023) provides a comprehensive review of the phytochemistry and biological activity of various extracts (and individual constituents) from annual fleabane [9]. It was reported herein that ten particular sesquiterpenoids of eudesmane, oppositene, cadinene, and isodaucene types of skeletons were identified in this plant. Moreover, the review [9] presents the chemical structures of twenty monoterpenoids, fifty-nine sesquiterpenoids, and eleven various polyacetylenic compounds identified in different parts of E. annuus.
3.3. Erigeron bonariensis (L.)
Erigeron bonariensis (L.) (commonly called as the flax-leaf fleabane or hairy fleabane) is also referred to by many alternative names, including Conyza bonariensis (L.) Cronquist, which is the most widely recognized [1]. It is an annual or perennial herbaceous plant that can reach a height of 1–2 m; the leaves are covered with hairs, including long hairs near the apex of the bracts. Its flowers have white ray florets and yellow-center floret disk. The native geographical distribution of the species extends from Mexico to the tropical regions of South America [2]. The species has been first described in Argentina, but it is now widely distributed across the warmer regions of Europe (predominantly in the Mediterranean basin), Africa, the Caribbean and Central America, Asia, New Zealand, and all states of Australia [39,40]. Confusion between the taxonomic classifications of C. bonariensis, C. canadensis, C. sumatrensis, and other Conyza species has been identified, especially during the initial phases of seedling development [40,41,42]. Due to the distinctive reproductive system and the high efficiency of the seed dispersal mechanism, the flax-leaf fleabane spreads rapidly. The detrimental allelopathic impact of this invasive species on plant communities and soil parameters is related to the phytotoxicity of flax-leaf fleabane [43]. It is estimated that this harmful weed has the potential to cause 28–68% yield loss in key field crops, including soybean and cotton, on an annual basis [44]. One of the effective and environmentally sustainable methods of controlling the noxious weed may be the use of other EOs [45].
3.3.1. Compositional Data of EOs of Erigeron bonariensis L.
Data of the Erigeron bonariensis L. EOs (published during the period 2000–2025) are presented in Table 3 [13,39,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62]. The plant material for these studies was collected from the Amazon regions in Brazil, and Argentina, Venezuela, Greece, Sardinia (Italy), Tunisia, Egypt, Kenya, Pakistan, India (Uttar Pradesh and Udham Singh Nagar regions), and Vietnam.

Table 3.
Main compositional data of EOs of Erigeron bonariensis L. from various geographical origins.
E. bonariensis species is native to S. America continent, while all taxa of Erigeron sect. Conyza (including E. bonariensis) are invasive to the Mediterranean basin. Moreover, the species was introduced in African and Asian countries [2].
The EOs obtained from E. bonariensis (L.) were comprised of significant amounts of monoterpenes, limonene (1), β-pinene (2), and β-ocimene (3) (Figure 1)); oxygenated monoterpenes, matricaria (5) and lachnophyllum (6) esters (Figure 1), carvone (10), carvacrol (11), thymol (12), and dendrolasin (13) (Figure 3); sesquiterpenes, germacrene D (7), β-caryophyllene (8) (Figure 2), α-bergamotene (14), bicyclogermacrene (15), allo-aromadendrene (16), α-curcumene (17), β-farnesene (18), and sesquisabinene (19) (Figure 3); and oxygenated sesquiterpenes, caryophyllene oxide (9) (Figure 2), sesquicineole (20), β-eudesmol (21), spathulenol (22), and ledene oxide (23) (Figure 3).
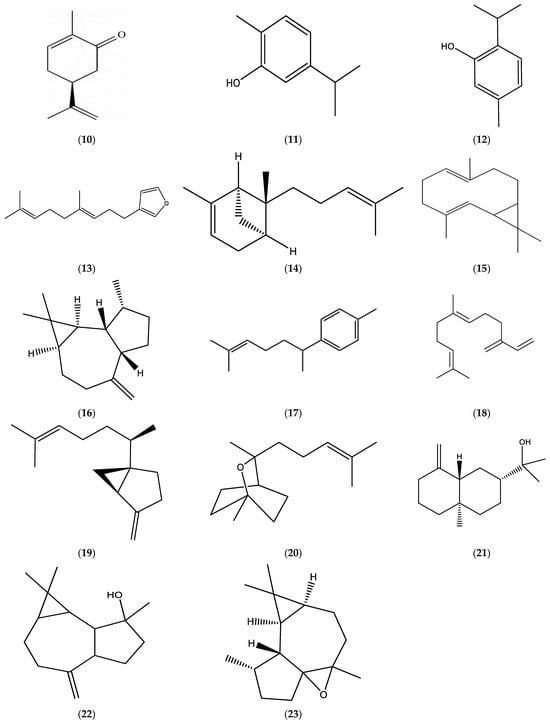
Figure 3.
Chemical structures of carvone (10), carvacrol (11), thymol (12), dendrolasin (13), α-bergamotene (14), bicyclogermacrene (15), allo-aromadendrene (16), α-curcumene (17), β-farnesene (18), sesquisabinene (19), sesquicineole (20), β-eudesmol (21), spathulenol (22), and ledene oxide (23).
3.3.2. Bioactivity and Toxicity of Erigeron bonariensis EOs
A number of the oils derived from E. bonariensis were examined for their biological activities, including anti-inflammatory, anticancer, anti-aging, and antimicrobial activities, and toxically (insecticidal, larvicidal, nematicidal, repellent, etc.) effects [13,39,52,53,54,55,56,58,59,60,61,62,63,64,65].
An investigation was conducted by Souza et al. in which the EOs obtained from Brazilian C. bonariensis (L.) Cronq. were screened for anti-inflammatory activity in a mouse model of pleurisy induced by zymosan and lipopolysaccharide (LPS). The results of the study demonstrate that when the oils were administered orally, they were able to inhibit the LPS-induced inflammation, including cell migration [13]. Moreover, the EOs of the E. bonariensis from Egypt [59] showed a strong cytotoxicity against HepG2 (IC50 = 25.6 μM), and remarkable inhibitory effects of the collagenase, elastase, hyaluronidase, and tyrosinase, compared to epigallocatechin gallate (as a reference). Ferreira et al. investigated anticancer activity against human tumor cell lines (melanoma, cervical, colorectal, and leukemias) and non-tumor keratinocyte lines using an MTT assay, toxic effects using the zebrafish model, and anti-aging properties (antioxidant potential using a DCFH-DA assay, and a protection assay using the antioxidant N-acetyl-L-cysteine) [61]. In addition, cytotoxicity activity tests were performed by Araujo et al. against HeLa (cervix carcinoma), A-459 (lung carcinoma), and MCF-7 (breast adenocarcinoma) human cell lines and against normal Vero cells (African green monkey kidney); obtained IC50 ranged from 1.4 to 45.8 µg/mL [52]. Furthermore, the EOs demonstrated significant antimicrobial effects against Bacillus cereus (MIC 25–50 µg/mL) and a moderate activity against Staphylococcus epidermidis and Candida albicans (MIC 100–200 µg/mL) in the above study [52]. Antibacterial effects of the EOs from C. bonariensis of Indian origin were evaluated against Gram-negative bacteria Erwinia herbicola (Lohnis) (syn. Pantoea agglomerans) and Pseudomonas putida (Kris Hamilton) (1.42 ± 0.23 and 2.47 ± 0.9 mm of inhibition zones of these two phytopathogenic bacteria, respectively) [53]. Additionally, another study related to the antibacterial activity of the oils of Indian E. bonariensis was conducted by Kushwaha et al. [55]. The oils demonstrated a significant degree of activity against Salmonella enterica, Pseudomonas aeruginosa, and Escherichia coli, but comparatively less activity against Staphylococcus aureus and Klebsiella pneumoniae [55]. The antibacterial activity of EOs obtained from Kenyan C. bonariensis (L.) Cronq. was revealed using the disk diffusion method against the pathogenic Escherichia coli and Salmonella typhi strains; the MICs were determined to be 12.5% and 6.25%, respectively [56]. Antifungal effects of C. bonariensis EO against pathogenic Colletotrichum musae were investigated, and its potential application was suggested for reducing the anthracnose development in banana fruits during storage [60]. However, antimicrobial activity of the EOs of Colombian C. bonariensis showed activity against yeasts (Candida parapsilosis and C. krusei) and fungi (Aspergillus flavus and A. fumigatus), with MIC values of ˃ 500 µg/mL, and cytotoxic effects on the Vero cell line using an MTT assay (IC50 = 70 ± 16.1 μg/mL) [63]. In another study [54], antimicrobial and insecticidal properties were assessed; it was determined that the EOs exhibited moderate effects against B. subtilis (MIC = 125 μg/mL) and a strong larvicidal activity against the adults of C. pipiens mosquitoes (EC50 = 2.455 ppm) and against the same larvae (EC50 = 9.307 ppm) [54]. Furthermore, mosquito larvicidal activity was revealed against Aedes aegypti, Ae. albopictus, and Culex quinquefasciatus in the study by Hoi et al. [58]. However, insecticidal and nematicidal activities of the EOs from C. bonariensis plants collected in Togo were tested on cowpea weevil Callosobruchus maculatus adults (LC50/24h = 1.75 μL oil/L air and 100% mortality at 3.91 μL oil/L air) and on freshly hatched second-stage juveniles of root-knot nematode Meloidogyne incognita (EO from the whole plant was the most effective, EC50/72h = 1817 mg/L) [39]. Results of larvicidal and repellent activity tests against adult female mosquitos and the larvae of yellow fever mosquitos, Aedes aegypti, show that the flax-leaf fleabane EOs exhibited 40.2% repellency against mosquitoes at a tested dose of 33.3 μg/cm2, more than 40% repellency for 60 min at a tested dose of 330 μg/cm2 in time-span bioassays, and that the larvae were very susceptible to the EOs upon 48 h. exposure (LC50 = 26.0 mg/L) [62].
Recently, two review articles were published by Opiyo et al. and Mahanur et al., which examined the chemical composition and biological activity of the EOs of C. bonariensis [64] and the red listed South African plant Erigeron bonariensis: var. microcephala Cabrera (EB), respectively [65].
Given the sufficient quantity of published data, a statistical analysis was conducted to examine the relationships between the compositional variability and bioactivity of C. bonariensis EOs. Refer to the illustration presented in the Supplementary Material (Figure S1).
3.4. Erigeron breviscapus (Vaniot) Hand.-Mazz.
Erigeron breviscapus (also known as Herba Erigerontis or Lamp Chrysanthemum) has several synonyms, including Erigeron dielsii and Erigeron praecox. It is a perennial herb growing primarily in the temperate regions in Asian, and is a native species in Tibet and South-Central and South-East parts of China (Yunnan, Hunan, Guangdong, Guangxi, Guizhou, and Sichuan provinces) [2]. E. breviscapus is 5–50 cm in height, has woody and thick rhizomes, the stem is upright with a few branches in the middle, and the whole plant is covered with bristles or mixed with glandular hairs; the leaves are mainly concentrated at the base and are in the shape of a rosette; the flower head is solitary [66]. Usually, this species grows at an altitude of between 1,700 and 3,000 m in open hillside grassland and at the edges of forests.
Compositional Data of EOs of Erigeron breviscapus and Their Bioactivity
To date, there is a paucity of literature concerning EOs of Erigeron breviscapus. A detailed review of its phytochemistry, pharmacology, clinical applications, and toxicology was published by Wu et al. [66]. The whole plant has been used in Chinese medicine for more than 600 years due to its broad range of pharmacological effects, including cerebrovascular, cardiovascular, anti-diabetic, anti-inflammatory, antioxidant, neuroprotective, etc., activities [14,15,66]. β-Pinene, 1,8-cineole, thymol, α-terpineol, borneol, eugenol, and nerolidol were found to be the main characteristic terpenoids, and the chemical structures of ten volatiles are presented herein [66].
3.5. Erigeron canadensis L.
Erigeron canadensis has numerous synonyms (at least 35, with Conyza canadensis (L.) being the most common), and is commonly called as a horseweed or Canadian fleabane. The species is considered to be an annual or perennial weed, indigenous to both American continents and widespread around the world [1,2]. It can grow to be 1.5 m tall, has hairy stems, the leaves are long (2–10 cm) with toothed margins and dense inflorescences, and each individual flower has white or light purple florets and yellow-center disks. E. canadensis is an invasive weed in many countries and exhibits resistance to multiple herbicides, thereby causing considerable damage to agricultural land [45].
3.5.1. Compositional Data of EOs of Erigeron canadensis L.
The main data of E. canadensis EOs (published during the period 2000–2025) are presented in Table 4 [48,53,58,62,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83]. The plant material for these studies was collected in Brazil (where E. canadensis is a native species), in many European countries (Poland, France, Italy, Spain, Greece, Hungary, Belgium, Bulgaria, and Lithuania), and in Ethiopia, Turkey, Iran, Jordan, Pakistan, India, China, Korea, and Vietnam, where the species is invasive [2].

Table 4.
Main compositional data of the EOs of Erigeron canadensis L. (Conyza canadensis (L.) Cronq.).
Monoterpenes, limonene (1), β-pinene (2), β-ocimene (3) (Figure 1), myrcene (24), and δ-3-carene (25) (Figure 4); oxygenated monoterpenes, matricaria (5) and lachnophyllum (6) esters (Figure 1); sesquiterpene hydrocarbons, germacrene D (7), β-caryophyllene (8) (Figure 2), α-bergamotene (14), β-farnesene (18) (Figure 3), ar-curcumene (26), zingiberene (27), and epi-bicyclosesquiphellandrene (28) (Figure 4); oxygenated sesquiterpenes, caryophyllene oxide (9) (Figure 2) and spathulenol (22) (Figure 3); and a diterpenoid, phytol (29) (Figure 4) were determined among the major constituents present in the EOs of Erigeron canadensis L. (Conyza canadensis (L.) Cronq.).
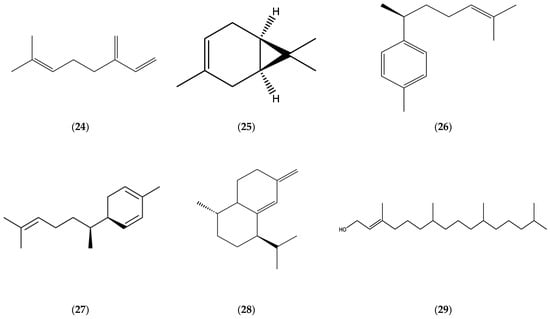
Figure 4.
Chemical structures of myrcene (24), δ-3-carene (25), ar-curcumene (26), zingiberene (27), epi-bicyclosesquiphellandrene (28), and phytol (29).
3.5.2. Bioactivity and Toxicity of Erigeron canadensis (L.) EOs
Strong cytotoxic activity was revealed for the EOs obtained from the aerial part of Korean E. canadensis L. in an MTT assay using HaCaT keratinocyte cells (IC50 = 0.027 µg/mg) [72]. Furthermore, its substantial antitumoral potential was noticed for horseweed leaf EOs containing high amounts of limonene against neoplastic cell lines K562 (leukemia) and NCI-ADR/RES (ovary with multidrug resistance phenotype), with TGI values of 16.8 and 19.0 mg/mL, respectively [81]. Moreover, under the impact of the oils (rich in limonene, up 65.7%) of Chinese origin, the cell viability of the normal liver cell lines L02 and the human cervical cancer cell lines HeLa dramatically declined [84].
The horseweed EOs were demonstrated to possess a weak capacity to inhibit the growth of some phytopathogenic fungi, such as Rhizoctonia solani Kuhn, Fusarium solani (Mart.) Sacc., and Colletotrichum lindemuthianum (Sacc. & Magn.) Briosi & Cav. [44]. On the other hand, the Canadian fleabane EOs, comprised of more than 50% of limonene, exhibited a significant broad spectrum of antimicrobial effects against some food-borne pathogens and fungi, especially on Alternaria panax Whetz, Rhizoctonia solani, and Shigella dysenteriae [85]. Furthermore, the above-mentioned oils possessed inhibition properties on germination and the seedling growth of plants, and can enhance the length of the Daucus carota var. sativa L. roots. Moderate to strong fungicidal effects against the reference fungal strains, and fungal strains isolated from patients (Aspergillus, Candida, Cryptococcus, Rhodotorula, and Trichophyton), except against A. fumigatus, were revealed by Veres et al. [75]. The highest zones of inhibition were observed on Cryptococcus neoformans and Trichophyton interdigitalis in the above study. However, the antimicrobial activity of horseweed EOs was assayed against three bacteria (Salmonella enteritidis, Staphylococcus aureus, and Pseudomonas aeruginosa) and three fungi (Alternaria alternata, Aspergillus niger, and Penicillium digitatum) applying the modified disk volatilization method [76]. Moreover, the antibacterial tests of Indian horseweed EOs showed 1.50 ± 0.70 and 8.17 ± 0.88 mm of inhibition zones of two Gram-negative bacteria Erwinia herbicola (Lohnis) and Pseudomonas putida (Kris Hamilton), respectively [53]. Screening of the EOs against both Gram-positive and Gram-negative bacteria and one strain of fungus using the broth microdilution method showed the highest antibacterial activity of the oils from aerial parts against Escherichia coli RSKK 234 (MIC = 0.039 μg/mL) and Candida albicans ATCC 10231 (MIC = 0.078 μg/mL) [79]. The EOs from C. canadensis growing wild in the Kashmir valley (India) exhibited significant antibacterial activity against the tested Gram-positive and Gram-negative food-borne bacteria (MIC values ranged from 12.00 to 40.00 μg/mL), and the strongest inhibitory effects were determined against Candida albicans and Candida parapsilosis, with MICs of 2.50 and 1.80 μg/mL, respectively [80]. Also, the EO investigated above showed an effective antioxidant potential using DPPH and hydroxyl radical scavenging assays [80].
Furthermore, the allelopathic properties of horseweed EOs on seed germination of the receptor plants (Brassica chinensis Linn., B. pekinensis Rupr., Triticum sestivum Linn., and Sorghum bicolor (Linn.) Moench) were assessed in the studies by Liu et al. [73,74]. The significant insecticidal potential of the fleabane EOs against the first–fourth instar larvae and pupae of Aedes albopictus and Culex quinquefasciatus was revealed using the immersion and fumigation methods [78]. Horseweed EOs demonstrated repellent and larvicidal activity against Aedes aegypti, Ae. albopictus, and Culex quinque [58,82]. The EOs of E. canadensis from Pakistan exhibited 41.7% repellence against mosquitoes (at a tested dose of 33.3 μg/cm2), and the larvae of Ae. aegypti were susceptible to the tested oils (LC50 = 35.7 mg/L, upon 48 h exposure) in time-span bioassays [62].
A statistical analysis was conducted on the available published data in order to examine the compositional variability and bioactivity of E. canadensis EOs. Refer to the visual depiction of a biplot provided in the Supplementary Material (Figure S2).
3.6. Erigeron floribundus (Kunth) Sch.Bip. (Erigeron sumatrensis)
Erigeron floribundus (commonly known as Bilbao fleabane, Asthma weed, tall fleabane, many-flowered fleabane, and Bilbao’s Erigeron) has many synonyms, and the most frequent is Conyza floribunda Kunth, Conyza sumatrensis var. floribunda (Kunth), and Conyza albida Willd. ex Spreng. [2]. C. albida and C. floribunda are treated as synonyms of C. sumatrensis. The species of Conyza showed differentiation in the inflorescence topology and the structural features of the capitulum. The stem and leaf cover’s trichomes are heterogeneous in C. sumatrensis var. sumatrensis, and it has one set of uniform abundant short hair on both faces of the leaves. C. sumatrensis var. floribunda possesses a hirsute pubescence with few trichomes above the rib of the stem and margin of the leaf, few trichomes in the central nervation of the phyllary, and biseriate glandular hair in the corolla apical region of the flowers. C. sumatrensis var. floribunda presents short hair on both faces of the leaf [49].
E. floribundus is an herbaceous plant, up to 1.5 m in height, with pubescent, lanceolate leaves and flowers in yellowish panicles. It is an annual or biennial herb of American origin, a persistent invader spreading throughout the world, and a difficult weed to control.
3.6.1. Compositional Data of EOs of Erigeron floribundus
The main data of Erigeron floribundus (E. sumatrensis) EOs, published during the period 2000–2025, are presented in Table 5 [48,49,86,87,88,89,90,91,92]. Specific constituent, lachnophyllumlactone (30) was identified the EOs (Figure 5). In order to avoid any confusion due to the taxonomic attribution of the Erigeron species, identification of the species’ names performed by the authors is included in Table 5. The plant material utilized in these studies was collected in Argentina (where it is a native species), Greece, Tunisia, Cameroon, Côte d’Ivoire, India, and Pakistan, where the species was introduced [2].

Table 5.
Main compositional data of EOs of Erigeron floribundus (H.B. et K.) Sch. Bip. (Conyza floribunda H.B. et K.).

Figure 5.
Chemical structure of lachnophyllumlactone (30).
Two new sesquiterpenoids, (7R*) opposit-4(15)-ene-1β,7-diol and 15-methoxyisodauc-3-ene-1β,5α-diol, have been isolated from E. sumatrensis plants for the first time [12].
3.6.2. Bioactivity and Toxicity of Erigeron floribundus (L.) EOs
The EOs obtained from E. floribundus growing in Cameroon exhibited a broad spectrum of fungicidal effects against Trichophyton rubrum, Trichophyton mentagrophytes, Candida albicans, and Cryptococcus neoformans [87]. The flower’s EO was found to be more active than the leaf’s oil. It was revealed that C. albicans was the most sensitive fungus (MIC = 2.25 µL/mL for both oils), and the MIC values for other fungi ranged from 12.5 to 8.5 µL/mL [87]. In addition, the EOs of aerial parts of Bilbao fleabane from Cameroon showed a good inhibition activity against Staphylococcus aureus (MIC = 512 µg/mL), against the NadD enzyme from S. aureus (IC50 = 98 µg/mL, and with no effects on mammalian orthologue enzymes), and on T. brucei proliferation (IC50 = 33.5 µg/mL) in another study [91]. However, the above-mentioned oils demonstrated a strong cytotoxicity on HCT 116 colon carcinoma cells (IC50 = 14.89 µg/mL) and remarkable antioxidant properties (tocopherol-equivalent antioxidant capacity, TEAC = 411.9 μmol·TE/g) [91].
The E. floribundus EO was screened against ten human pathogenic bacteria and fungi [89]. The oil was found more active against the tested fungal strains, with MIC values of 0.41 ± 0.18, 0.7 2± 0.47, 0.36 ± 0.23, 0.45 ± 0.28, 0.57 ± 0.59, and 0.88 ± 0.63 mg/mL against Staphylococcus aureus, Escherichia coli, Candida albicans, Aspergillus niger, Saccharomyces cerevisiae, and Penicillium chrysogenum, respectively [89]. The oils were evaluated for antibacterial, antifungal, and allelopathic activities [90]. The results indicate that the leaf’s oil exhibited significant antibacterial activity against Enterococcus faecalis, Staphylococcus aureus, and Proteus mirabilis; and that the C. sumatrensis oils isolated from the aerial parts presented high mycelia-growth inhibition of Candida albicans and the filamentous fungi tested. Moreover, the EOs of the different plant parts inhibited the shoot and root growth of Raphanus sativus (radish) seedlings. Indeed, the inhibition of the hypocotyl growth varied from 28.6 to 90.1% and that of the radicle from 42.3 to 96.2%. Furthermore, the allelopathic potential of the oil and its fractions was evaluated using two sensitive indicators, Avena sativa and Spirodella polyrhiza [86]. Furthermore, Azeem et al. assessed a pesticidal of potential of C. sumatrensis EOs against grain pests, the red flour beetle Tribolium castaneum (toxicity on adult population: LD50 = 33.91 mg/10 g rice and LD90 = 126.9 mg/10 g rice; and fumigation toxicity at 72 h. exposure: LD50 = 6.620 mg/10 g rice and LD90 = 15.83 mg/10 g rice;) and the mold Aspergillus flavus (16.5 mm zones of inhibition) [92].
Guetchueng et al. conducted in 2023 an extensive review of the phytochemistry, pharmacology, toxicology and traditional uses of E. floribundus [93].
3.7. Erigeron graveolens L.
Erigeron graveolens is a basionym for a number of other names, the most common of which are listed below. These include Dittrichia graveolens (L.) Greuter, Inula graveolens (L.) Desf., Solidago graveolens (L.) Lam., Paniopsis graveolens Raf., Cupularia graveolens (L.), and Pulicaria graveolens (L.) [2,94]. It is an annual plant, which is commonly known as stinkwort or stinking fleabane, a branched subshrub that grows up to 1.30 m tall and possesses an aromatic camphor-like odor. The native geographical distribution of this species extends from Southern Europe (the Mediterranean region) through to North Africa and as far as Western Asia and the Western Himalayas in Pakistan.
3.7.1. Compositional Data of Erigeron graveolens (L.) EOs
A detailed and very informative review concerning the chemistry and bioactivity of Dittrichia graveolens (L.) Greuter was published in 2022 [23]. Consequently, the subsequent data of EOs are presented in Table 6 [95]. E. graveolens is an indigenous species in Tunisia [2].

Table 6.
Compositional data of EOs of Inula graveolens published after 2022.
The main constituents of the EOs from aerial parts of E. graveolens (identified as Inula graveolens (L.) or Dittrichia graveolens (L.) Greuter)) were determined to be borneol (31), bornyl acetate (32), β-caryophyllene (8), caryophyllene oxide (9), and τ-cadinol (33) (Table 2, Figure 6) [23,95,96]. The root oils under consideration were characterized by the following components: sesquiterpenoids, modhephen-8-β-ol (34) and cis-arteannuic alcohol (35); and monoterpene esters, neryl isovalerate (36) and thymol isobutyrate (37) (Table 6, Figure 6).
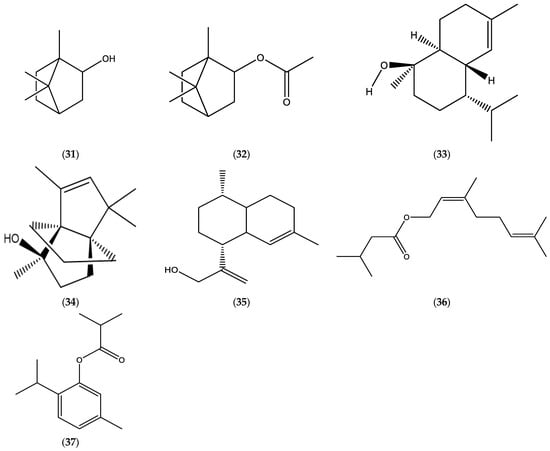
Figure 6.
Chemical structures of borneol (31), bornyl acetate (32), τ-cadinol (33), modhephen-8-β-ol (34), cis-arteannuic alcohol (35), neryl isovalerate (36), and thymol isobutyrate (37).
3.7.2. Bioactivity and toxicity of E. graveolens (Inula graveolens (L.)) EOs
To the best our knowledge, there is an absence of available data concerning the biological properties of E. graveolens EOs. For the first time, in 2023, Mustapha et al. evaluated the repellence and contact toxicity properties of EOs from aerial parts and roots of Inula graveolens against adults of stored-product beetle Tribolium castaneum. The oils displayed strong repellence effects after 2 h of exposure (up to 90.0 % at 0.12 μL/cm2); and the LD50 values were found to be 7.44% and 4.88%, respectively, for the root and aerial parts EOs [95].
3.8. Erigeron incanus Vahl (Erigeron leucophyllus (Sch. Bip. ex A. Rich.) Schweinf.)
Erigeron incanus Vahl (called by common names such as wooly daisy, wooly fleabane or, wooly erigeron) is known by several synonyms (the most common being Erigeron leucophyllus and Conyza incana Willd.) [1]. E. incanus is a native species to Eritrea, Ethiopia, Somalia, Yemen, and Saudi Arabia, where it is found in deserts and dry shrublands [2]. It is a distinctive, branched, dense, gray-tomentose undershrub up to 50 cm tall with very leafy stems. It is widespread and frequent on steep, bare rocky hillsides from 1600 to 3100 m on the escarpment and high plateau in Yemen [24].
3.8.1. Compositional Data of EOs of Erigeron incanus Vahl
Limited data of E. incanus EOs (published during the period 2000–2025) are documented in Table 7 [24]; and a chemical structure of the main constituents, 3-hydroxy-4-methoxy cinammic acid (38) and thymol acetate (39), in the EOs of E. incanus are presented in Figure 7.

Table 7.
Compositional data of EOs of Erigeron incanus Vahl L. (published 2000–2025).
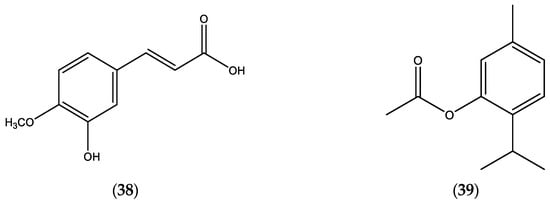
Figure 7.
Chemical structures of 3-hydroxy-4-methoxy cinammic acid (38) and thymol acetate (39).
3.8.2. Bioactivity of EOs of Erigeron incanus
Antimicrobial activity of the EO of E. incanus from Yemen was evaluated against two Gram-positive (Staphylococcus aureus and Bacillus subtilis) and two Gram-negative (Escherichia coli, Pseudomonas aeruginosa) bacteria and on Candida albicans using the disk diffusion method [24]. The oil demonstrated strong fungicidal effects (MIC = 40 μg/mL against C. albicans) and bactericidal activity against B. subtilus and S. aureus, with MIC(s) values of 150 μg/mL and 234 μg/mL, respectively. Moreover, the oil showed a strong antioxidant activity in a DPPH radicals scavenging assay (IC50 = 5.2 μg/mL) [24]. EOs from the seeds of wild E. incanus, collected in Yemen (Taiz-Hojariah region), exhibited antimicrobial (moderate effects against Proteus vulgaris bacterial and Fusariumoxy sporium fungal strains) and antioxidant properties [97,98].
3.9. Erigeron mucronatus DC
E. mucronatus (common name Mexican fleabane) is a synonym of Erigeron karvinskianus. However, the perennial species is known by other synonyms, the most frequent being Erigeron karvinskianus var. mucronatus (DC.) Asch. or Erigeron trilobus Sond. [2]. The native range of this species is the subtropical regions in Central America, Colombia, Mexico, and Venezuela. It is naturalized in many other world parts of Africa, Europe, Australia, Chile, and the west coast of the USA. Moreover, it is also cultivated for the purpose of its distinctive daisy-like blooms. It is an annual herb reaching about 3 m in height with either white- or pink-colored flowers [25].
3.9.1. Compositional Data of Erigeron mucronatus (Erigeron karvinskianus) EOs
The main chemical composition of the EOs of Erigeron mucronatus are presented in Table 8 [25,34]. The plant material utilized in these investigations was collected in India, where the species is not indigenous [2].

Table 8.
Compositional data of EOs of Erigeron mucronatus DC published during the period 2000–2025.
3.9.2. Bioactivity of Erigeron mucronatus (Erigeron karvinskianus) EOs
Awen et al. reported that E. mucronatus EOs exhibited a strong antibacterial activity against Staphylococcus aureus and Escherichia coli strains and a moderate activity against Pseudomonas aeruginosa. Moreover, the genotoxicity of the oil was evaluated in AB blood serum in the above study [25]. Antifungal effects of the EOs from the Himalayan Erigeron species (E. mucronatus and E. karwinskianus) against the tested fungi in the growth inhibition range of 40.4–83.9% (with IC50 values ranging from 88.8 to 602.7 μg/mL) were revealed, and the most significant inhibition of spore germination was determined for Fusarium oxysporum, Curvularia lunata, and Albugo candida [34].
3.10. Erigeron multiradiatus (Lindl. ex DC.) Benth
Erigeron multiradiatus (commonly known as Himalayan fleabane) is a perennial or annual Asian species with violet or dark-purple flowers native to the eastern and western parts of the Himalayas (Pakistan, Afghanistan, Nepal, Tibet, Bhutan, etc.). The species has several synonyms. It grows primarily in the subalpine or subarctic regions of South–Central Asia (Iran), Afghanistan, and South–Central China [2]. It is a beautiful plant with an erect hairy stem up to 40 cm; the leaves are ovate or lance-like, pointed or blunt, and dented at the tip. Flower-heads vary in size 1.5–5 cm across; the central disk is yellow. Distribution of this species is from 2600 to 4400 m in the Himalayas [36].
Compositional Data of Erigeron multiradiatus EOs and Their Activity
It was reported that the EO obtained from the aerial parts of Erigeron multiradiatus (Lindl. ex DC.) Benth growing wild in the central Himalayan region (Uttarakhand, India, where the species is native) and comprised by the predominant constituents, trans-2-cis-8-matricaria-ester (77.8%) and cis-lachnophyllum ester (11.0%), exhibited remarkable leishmanicidal effects against Leishmania donovani promastigotes and intracellular amastigotes [99].
3.11. Erigeron philadelphicus L.
Erigeron philadelphicus (commonly known as the Philadelphia fleabane or fleabane daisy) is a perennial herb that is native to the subarctic regions of North America and the USA [2]. However, this species was introduced in Europe (France, Germany, Great Britain, Italy, etc.), Asia (China, Japan, Korea, etc.), and some islands (Corse, Newfoundland, and Mauritius). The optimal climatic conditions of the plants are temperate regions. E. philadelphicus has been identified as an ecologically destructive invasive species in numerous countries. The fleabane daisy grows along roadsides and in fields and woodlands. Its yellow center with a large number of very fine white ray flowers is its best identifier [100].
Compositional Data of Erigeron philadelphicus EOs and Their Activity
A sesquiterpenoid, 6β,14-epoxyeudesm-4(15)-en-1β-ol, and a diterpenoid, philadelphinone, were isolated for the first time from the aerial parts of E. philadelphicus plants [38]. Moreover, several other new terpenoids and related compounds were identified in E. philadelphicus by Yaoita et al. [101]. No available date concerning the biological properties of E. philadelphicus EOs has been reported over the past 25 years.
3.12. Erigeron strigosus Muhl. ex Willd (Erigeron ramosus)
The species has many synonyms (known by the common names daisy fleabane, prairie fleabane, vergerette rude, or common eastern fleabane), at least 22, including Erigeron annuus var. ramosus (Walter) Hyl. [2,4]. E. strigosus is a native species to Canada and the USA, growing in temperate regions. Additionally, the species has become an invasive plant in Newfoundland Island, many parts of Asia (China, Tibet, Japan, Korea, and Sakhalin), and Europe (Germany) [2]. This species is categorized as an annual, biennial, or short-lived perennial, producing a multitude of flower heads. It is 30–70 cm tall, fibrous-rooted; the stems are erect or ascending, eglandular; the leaves are basal and cauline. Ray florets are of corollas white, pinkish, or bluish [4].
Two species, namely E. strigosus Muhl. ex Willd and E. annuus (L.) Pers. were documented as invasive weeds in Lithuania (in 2004) [102].
3.12.1. Compositional Data of Erigeron strigosus (Erigeron ramosus) EOs
There is a lack of available data related to the investigations of Erigeron strigosus (Erigeron ramosus) EOs and their properties. Limited data of E. ramosus EOs (published during the period 2000–2025) are documented in Table 9 [103], and a chemical structure of the main constituents, α-humulene (40), 1,8-cineole (41), eugenol (42), and globulol (43), in the EOs are presented in Figure 8.

Table 9.
Compositional data of EOs of Erigeron ramosus published during the period 2000–2025.
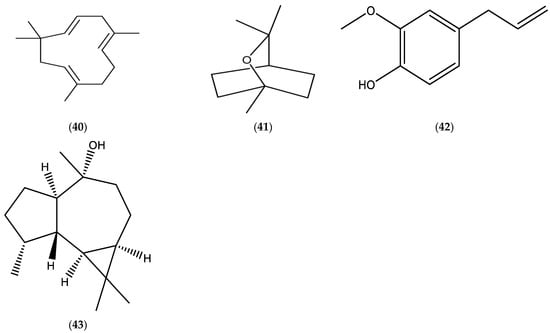
Figure 8.
Chemical structures of α-humulene (40), 1,8-cineole (41), eugenol (42), and globulol (43).
3.12.2. Biological Activity of Erigeron strigosus (Erigeron ramosus) EOs
The antibacterial activity of the EO from E. ramosus (WALT.) B.S.P. plants growing in Korea was determined against fourteen (seven g-positive and seven g-negative) foodborne bacteria, and the strongest activity was revealed against seven Gram-positive bacteria stains of Staphylococcus aureus, Listeria monocytogenes, and Bacillus subtilis, and four Gram-negative bacteria lines (Pseudomonas aeruginosa, Enterobacter aerogenes, and Escherichia coli) with MIC values ranging from 62.5 to 500 µg/mL [103].
3.13. Erigeron speciosus (Lindl) DC.
Erigeron speciosus (commonly known as aspen fleabane, garden fleabane, or Oregon fleabane) has many synonyms (at least 16). It is a perennial plant with a native population in the western parts of Canada to the northwest of Mexico. The species was introduced in some European countries (Great Britain, Sweden, Baltic States, France, Germany, Ukraine, etc.), Uzbekistan, and Indonesia (Jawa island) [2]. It is perennial, usually 30–80 cm tall, rhizomatous, fibrous-rooted, stems erect, leaves basal (usually withering by flowering), and cauline. Ray florets range from a blue to lavender color. Preferring growing locations are gravelly or loamy soil, prairies, and pine, pine–fir, spruce–fir, aspen–spruce forests and their edges [4].
Compositional Data of Erigeron speciosus EOs and Their Activity
Steam distillate from the aerial parts of E. speciosus, containing appreciable amounts of matricaria esters, was tested against strawberry plant pathogenic fungi Botrytis cinerea, Colletotrichum acutatum, Colletotrichum_fragariae, and C. gloeosporioides. Additionally, its molluscicidal activity was assessed against the intermediate host snail Planobdella trivolvis in a study [104].
3.14. Erigeron sublyratus Roxb. ex DC.
Erigeron sublyratus has several synonyms (including Erigeron hirsutus Wall., Conyza graveolens Wall. ex DC.) [2]. It is an annual herb native to the Indian Subcontinent (the Eastern Himalayan region, Nepal, India, and Sri Lanka) and to the Indo-China region (Myanmar, Thailand, and Vietnam). The plant is an erect, aromatic, branchlets hirsute, annual herb. Its leaves alternate from oblanceolate to spathulate. Ray florets are numerous, of a purplish color, and disk florets are yellowish. Preferred growing site is plains [36].
3.14.1. Compositional Data of Erigeron sublyratus EOs
As demonstrated in Table 10, the available data concerning E. sublyratus EOs is extremely limited during last quarter of a century [105].

Table 10.
Compositional data of EOs of Erigeron sublyratus (published in 2000–2025).
3.14.2. Biological Activity of Erigeron sublyratus EOs
In a paper published in 2025, the composition of E. sublyratus EOs and their anti-inflammatory and cytotoxic properties were investigated for the first time [105]. The EOs from aerial parts inhibited nitric oxide (NO) production on LPS-induced RAW 264.7 cells (IC50 = 1.41 ± 0.10 μg/mL). In addition, both the root and aerial EOs were found to display cytotoxic activity against MCF-7, SK-LU-1, and HepG2 cell lines [105].
4. Discussion
A compositional revision of various EOs obtained from fourteen Erigeron sp. from various countries worldwide revealed significant interspecies as well as intraspecies variability. Chemical variability (both quantitative and qualitative) was determined between different plant organs and at various phenological stages of revised Erigeron sp. [25,28,29,33,39,50,51,68,75,79,83,95]. Furthermore, the preparation method, technique, and duration employed in the extraction process are all of paramount importance to the composition of the final product.
A sufficiently extensive list of the most prevalent characteristic compounds was established. Limonene, δ-3-carene, matricaria ester, lachnophyllum ester, germacrene D, β-caryophyllene, β-farnesene, α-bergamotene, allo-aromadendrene, etc., were determined in the oils from the E. acris, E. annuus, E. bonariensis, E. canadensis, E. floribundus, E. mucronatus, and E. speciosus plants. Specific constituents, such as monoterpenoid, dendrolasin; diterpenene, neophytadiene and manool (a labdane type diterpenoid); 2,6,7,7α-tetrahydro-1,5-dimethyl-1H-indene-3-carboxaldehyde; and sesquiterpenoid, ledene oxide were identified in the EOs of E. bonariensis L [47,51,56,59]. Appreciable quantities of the rare constituents, 2,3-dimethyl-4(3H)-quinazolinone and lachnophyllumlactone, were identified in the oils of E. canadensis and E. sumatrensis, respectively [74,86]. Other predominant characteristic constituents, such as borneol, bornyl acetate, modhephen-8-β-ol, cis-arteannuic alcohol, neryl isovalerate, thymol isobutyrate, and τ-cadinol, were determined in the E. graveolens (Inula graveolens) oils [95]. A paucity of data concerning E. incanus EOs was revealed, and 3-hydroxy-4-methoxy cinammic acid and thymol acetate were found to be the major ones [24]. The EOs from E. multiradiatus and E. sublyratus are characterized mostly by matricaria and lachnophyllum esters [99,105]. The available data on EOs of E. ramosus is limited, but the main constituents are known to be α-humulene, 1,8-cineole, eugenol, and globulol [103]. The species E. breviscapus has a very long history in Chinese ethnopharmacology and is cultivated in significant amounts; therefore, a section regarding its EOs was included, despite the fact that available data regarding volatile secondary metabolites of E. breviscapus is very limited [14,15,66].
The different classes of compounds present in the EOs are responsible for their numerous pharmacological and toxic activities. EOs are comprised of a mixture of numerous different compounds with different mechanisms of actions that may work in synergy. It is erroneous to assert that the responsibility for the biological activity lies exclusively with the main constituents. It is evident that the impact of other compounds present in the oils in quantities that may be negligible must not be overlooked. However, in order to simplify a verification of the correlation between EO composition and activity, it is necessary to consider the factors below, and I will highlight the most promising pharmacological and toxic effects of the EOs and correlate these properties with the principal constituents only. The antimicrobial properties of the EOs of various Erigeron species were mostly investigated. Antifungal and antibacterial effects against various strains were exhibited by the oils of E. acris, E. annuus, E. bonariensis, E. canadensis, E. floribundus, E. incanus, E. mucronatus, E. speciosus, and E. ramosus. These oils were characterized by appreciable quantities of limonene, β-pinene, 1,8-cineole, carvacrol, thymol acetae, β-eudesmol, 2,6,7,7α-tetrahydro-1,5-dimethyl-1H-indene-3-carboxaldehyde, caryophyllene and its oxide, allo-aromadendrene, α-humulene, farnesene isomers, carvacrol, globulol, or eugenol. The EOs of E. acris, E. annuus, E. bonariensis, E. canadensis, E. floribundus, E. mucronatus, E. multiradiatus, E. sublyratus, and E. speciosus containing appreciable amounts of matricaria and lachnophyllum esters exhibited strong anticancer, antimicrobial, anti-inflammatory, leishmanicidal, or larvicidal and repellent activities [6,9,25,29,34,39,61,62,64,65,75,79,80,86,87,90,92,93,99,104]. Moreover, repellence and contact toxicity against adults of T. castaneum was related to the high content of borneol, bornyl acetate, caryophyllene derivatives, τ-cadinol, modhephen-8-β-ol, and cis-arteannuic alcohol in I. graveolens oils [95]. Cytotoxicity was determined due to the presence of limonene, δ-3-carene, α- and β-farnesene, (E)-β-ocimene, ledene oxide, sesquiphellandrene, and dendrolasin in the fleabanes (E. acris, E. bonariensis, E. canadensis, E. floribundus, and E. sublyratus) EOs [6,52,59,61,63,72,81,84,91,105]. Skin-regeneration and antifungal properties were related to germacrene D in E. annuus oils [35]. The anti-inflammatory effects were exhibited because of the high amounts of limonene and (E)-β-ocimene in E. bonariensis oils [13], and, owing to lachnophyllum ester, germacrene D and trans-β-ocimene in E. sublyratus EOs [105].
A sufficient amount of published data concerning C. bonariensis and E. canadensis EOs allowed us to apply a statistical data analysis and examine the relations between compositional variability and their activity. PCA and biplots demonstrate the correlations between major components of the EOs E. bonariensis and E. canadensis their biological activities, respectively (Supplementary Material, Figures S1 and S2). Cytotoxicity of E. bonariensis EOs against HepG2 and the inhibitory effects of collagenase, elastase, hyaluronidase, and tyrosinase was related to α-farnesene, α-maaliene, dendrolasin, and ledene oxide. β-Ocimene, sesquiphellandrene, and β-farnesene were responsible mostly for the cytotoxic effects of the oils against HeLa, A-459, and MCF-7 human cell lines, as well as against normal Vero cells; and antimicrobial effects against B. cereus, S. epidermidis, and C. albicans. Matricaria and lachnophyllum esters impacted larvicidal activity against Aedes aegypti, Ae. albopictus, and Culex quinquefasciatus, insecticidal and nematicidal activities against Callosobruchus maculatus and Meloidogyne incognita, and larvicidal and repellent activity against adults and larvae of yellow fever mosquitos, Aedes aegypti (Supplementary Material, Figure S1).
In case of E. canadensis EOs, matricaria and lachnophyllum esters were responsible for the antitumoral potential against neoplastic cells K562 and NCI-ADR/RES, fungicidal effects against many fungal strains (Aspergillus, Candida, Cryptococcus, Rhodotorula, Trichophyton, etc.), and antimicrobial activity against E. coli and C. albican. Insecticidal potential against larvae and pupae of Ae. albopictus and C. quinquefasciatus related to the epi-bicyclophellandrene present in the oils (Supplementary Material, Figure S2).
5. Conclusions
This article provides a comprehensive overview of the subject matter. This review evaluated interspecies and intraspecies compositional variability of the essential oils (EOs) of fourteen Erigeron species (native, naturalized, or invasive), such as E. acris, E. annuus, E. bonariensis, E. breviscapus, E. canadensis, E. floribundus, E. graveolens, E. incanus, E. mucronatus, E. multiradiatus, E. philadelphicus, E. strigosus, E. speciosus, and E. sublyratus. Chemical variability (both quantitative and qualitative) has been demonstrated between different plant organs and at various phenological stages of Erigeron sp. As the most common characteristic compounds, limonene, δ-3-carene, matricaria ester, lachnophyllum ester, germacrene D, β-caryophyllene, β-farnesene, α-bergamotene, allo-aromadendrene, etc., were determined in the oils of the E. acris, E. annuus, E. bonariensis, E. canadensis, E. floribundus, E. mucronatus, and E. speciosus plants. Specific constituents, such as monoterpenoid, dendrolasin, diterpenene, neophytadiene, and manool (a labdane type diterpenoid), 2,6,7,7a-tetrahydro-1,5-dimethyl-1H-indene-3-carboxaldehyde, and sesquiterpenoid ledene oxide, were identified in the EOs of E. bonariensis L. Appreciable quantities of the rare constituents, 2,3-dimethyl-4(3H)-quinazolinone, and lachnophyllumlactone were identified in the oils of E. canadensis and E. sumatrensis, respectively. Other prevalent characteristic constituents, such as borneol, bornyl acetate, modhephen-8-β-ol, cis-arteannuic alcohol, neryl isovalerate, thymol isobutyrate, and τ-cadinol, were determined in the E. graveolens (Inula graveolens) oils. A paucity of data concerning E. incanus EOs was revealed, and 3-hydroxy-4-methoxy cinammic acid and thymol acetate were found to be the major ones. The EOs from E. multiradiatus and E. sublyratus were characterized by matricaria and lachnophyllum esters. The available data on EOs of E. ramosus is limited, and the main constituents are known to be α-humulene, 1,8-cineole, eugenol, and globulol.
Moreover, the presence of certain significant characteristic compounds in the oils has the potential to function as chemomarkers for the Erigeron taxonomic classification.
The different classes of compounds present in the EOs are responsible for various pharmacological and toxic activities, such as anti-inflammatory, anticancer, antiproliferative, skin regeneration, antioxidant, antifungal, antibacterial, insecticidal, larvicidal, repellent, and allelopathic properties, as summarized in this review. The EOs containing appreciable amounts of matricaria and lachnophyllum esters exhibited strong anticancer, anti-inflammatory, antimicrobial, larvicidal, and repellent activities. Repellence also related to borneol, bornyl acetate, caryophyllene derivatives, τ-cadinol, modhephen-8-β-ol, and cis-arteannuic alcohol. Cytotoxicity was determined due to the presence of limonene, δ-3-carene, α- and β-farnesene, (E)-β-ocimene, ledene oxide, sesquiphellandrene, and dendrolasin in the fleabanes EOs. Skin regeneration and antifungal properties were related to germacrene D, and anti-inflammatory effects were determined due to high amounts of limonene, (E)-β-ocimene, lachnophyllum ester, and germacrene D. The antimicrobial properties of the oils were conditioned by appreciable quantities of limonene, β-pinene, 1,8-cineole, carvacrol, thymol acetae, β-eudesmol, 2,6,7,7α-tetrahydro-1,5-dimethyl-1H-indene-3-carboxaldehyde, caryophyllene and its oxide, allo-aromadendrene, α-humulene, farnesene, carvacrol, and eugenol.
This review establishes a foundation for subsequent studies on the secondary metabolites (both volatile and non-volatile) and studies that explore potential sources of new biologically active compounds from other Erigeron species. A further review article could be focused on the phytochemistry and biological properties of the plant extracts or individual components from the Erigeron genus.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30142989/s1, Figure S1: Principal component biplot of PC1 and PC2 scores demonstrates the relationships between major components of E. bonariensis EOs and various biological activities; Figure S2: Principal Component Analysis (PCA) of Erigeron canadensis EOs, biplot illustrates the relationships between major components of the oils and various biological activities.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
The author thanks Jurga Būdienė (Department of Organic Chemistry, Center for Physical Sciences and Technology, Vilnius, Lithuania) for her assistance in the statistical analysis.
Conflicts of Interest
The author declares no conflicts of interest.
References
- World Flora Online. WFO Plant List. Available online: https://www.worldfloraonline.org (accessed on 8 May 2025).
- Plants of the World Online. Kew, Royal Botanical Gardens. Available online: https://powo.science.kew.org (accessed on 12 May 2025).
- Nesom, G.L. Classification of Subtribe Conyzinae (Asteraceae: Astereae). Lundellia 2008, 11, 8–38. [Google Scholar] [CrossRef]
- Nesom, G.L. Erigeron (Astereae). In Flora of North America (FNA); Editorial Committee, Ed.; Oxford University Press: New York, NY, USA; Oxford, UK, 2006; Volume 20, pp. 3, 9, 11, 12, 14, 17, 36, 204, 256, 257, 334. Available online: http://www.efloras.org/florataxon.aspx?flora_id=1&taxon_id=112000 (accessed on 10 July 2025).
- Florentine, S.; Humphries, T.; Chauhan, B.S. Chapter 7-Erigeron bonariensis, Erigeron canadensis, and Erigeron sumatrensis. In Biology and Management of Problematic Crop Weed Species; Chauhan, B.S., Ed.; Academic Press: London, UK; San Diego, CA, USA; Cambridge, MA, USA; Oxford, UK, 2021; pp. 131–149. [Google Scholar] [CrossRef]
- Nazaruk, J.; Karna, E.; Wieczorek, P.; Sacha, P.; Tryniszewska, E. In vitro antiproliferative and antifungal activity of essential oils from Erigeron acris L. and Erigeron annuus (L.) Pers. Z. Naturforsch. C 2010, 65, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Nalewajko-Sieliwoniuk, E.; Nazaruk, J.; Antypiuk, E.; Kojło, A. Determination of phenolic compounds and their antioxidant activity in Erigeron acris L. extracts and pharmaceutical formulation by flow injection analysis with inhibited chemiluminescent detection. J. Pharm. Biomed. Anal. 2008, 48, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Fei, D.; Gao, K. Aromadendrane-type sesquiterpene derivatives and other constituents from Erigeron acer. Die Pharm. 2007, 62, 312–315. [Google Scholar] [CrossRef]
- Rana, R.; Pundir, S.; Lal, U.R.; Chauhan, R.; Upadhyay, S.K.; Kumar, D. Phytochemistry and biological activity of Erigeron annuus (L.) Pers. Naunyn-Schmiedeb. Arch. Pharmacol. 2023, 396, 2331–2346. [Google Scholar] [CrossRef] [PubMed]
- Aijaz, F.; Shadab, M.; Akhtar, N.; Parveen, U.; Siddiqui, M.B. Erigeron bonariensis: Phytochemistry, allelopathy, and potential for sustainable pest management and healthcare. Vegetos 2025. [Google Scholar] [CrossRef]
- Sharma, K.S.; Alam, A. Phytochemical characterization, antioxidant and antimicrobial activity of Erigeron bonariensis L.: A therapeutic weed. IJSM 2025, 12, 188–203. [Google Scholar] [CrossRef]
- Ibrahim, W.W.; Sayed, R.H.; Abdelhameed, M.F.; Omara, E.A.; Nassar, M.I.; Abdelkader, N.F.; Farag, M.A.; Elshamy, A.I.; Afifi, S.M. Neuroprotective potential of Erigeron bonariensis ethanolic extract against ovariectomized/D-galactose-induced memory impairments in female rats in relation to its metabolite fingerprint as revealed using UPLC/MS. Inflammopharmacology 2024, 32, 1091–1112. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.C.; Siani, A.C.; Ramos, M.F.S.; Menezes-de-lima, O.J.; Henriques, M.G.M.O. Evaluation of anti-inflammatory activity of essential oils from two Asteraceae species. Pharmazie 2003, 58, 582–586. [Google Scholar] [PubMed]
- Jo, H.-G.; Baek, C.Y.; Lee, J.; Hwang, Y.; Baek, E.; Hwang, J.H.; Lee, D. Anti-Inflammatory, analgesic, functional improvement, and chondroprotective effects of Erigeron breviscapus (Vant.) Hand.-Mazz. extract in osteoarthritis: An in vivo and in vitro study. Nutrients 2024, 16, 1035. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Lin, P.; Kang, Q.; Zhao, Z.-L.; Wang, J.; Cheng, J.-Y. Metabolism and pharmacological mechanisms of active ingredients in Erigeron breviscapus. Curr. Drug Metab. 2021, 22, 24–39. [Google Scholar] [CrossRef] [PubMed]
- Li, T.S.C. Chinese and related North American herbs. In Phytopharmacology and Therapeutic Values; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar] [CrossRef]
- Yan, M.M.; Li, T.Y.; Zhao, D.Q.; Shao, S.; Bi, S.N. A new derivative of triterpene with anti-melanoma B16 activity from Conyza canadensis. Chin. Chem. Lett. 2010, 21, 834–837. [Google Scholar] [CrossRef]
- Shakirullah, M.; Ahmad, H.; Shah, M.R.; Ahmad, I.; Ishaq, M.; Khan, N.; Badshah, A.; Khan, I. Antimicrobial activities of Conyzolide and Conyzoflavone from Conyza canadensis. J. Enzym. Inhib. Med. Chem. 2011, 26, 468–471. [Google Scholar] [CrossRef] [PubMed]
- Al-Snafi, A.E. Pharmacological and Therapeutic Importance of Erigeron canadensis. Indo Am. J. Pharm. Sci. 2017, 4, 248–256. [Google Scholar]
- Boniface, P.K.; Pal, A. Substantiation of the ethnopharmacological use of Coniza sumatrensis (Retz) E.H. Walker in the treatment of malaria through in-vivo evaluation in Plasmodium berghei infected mice. J. Ethnopharm. 2013, 145, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Asongalem, E.A.; Foyet, H.S.; Ngogang, J.; Folefoc, G.N.; Dimo, T.; Kamtchouing, P. Analgesic and antiinflammatory activities of Erigeron floribundus. J. Ethnopharm. 2004, 91, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Jack, I.R.; Okorosaye-Orubite, K. Phytochemical analysis and antimicrobial activity of the extract of leaves of fleabane (Conyza sumatrensis). J. Appl. Sci. Environ. Manag. 2008, 12, 63–65. Available online: www.bioline.org.br/ja (accessed on 10 July 2025).
- Ponticelli, M.; Lela, L.; Russo, D.; Faraone, I.; Sinisgalli, C.; Mustapha, M.B.; Esposito, G.; Jannet, H.B.; Costantino, V.; Milella, L. Dittrichia graveolens (L.) Greuter, a rapidly spreading invasive plant: Chemistry and bioactivity. Molecules 2022, 27, 895. [Google Scholar] [CrossRef] [PubMed]
- Zabin, S.A. Antimicrobial, antiradical capacity and chemical analysis of Conyza incana essential oil extracted from aerial parts. J. Essent. Oil-Bear. Plants 2018, 21, 502–510. [Google Scholar] [CrossRef]
- Awen, B.Z.; Unnithan, C.R.; Ravi, S.; Lakshmanan, A.J. GC-MS Analysis, antibacterial activity and genotoxic property of Erigeron mucronatus essential oil. Nat. Prod. Commun. 2010, 5, 621–624. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Luo, P.; Li, J.; Yi, T.; Wang, J.; An, J.; Zhang, H. Comparison of the anti-inflammatory activities of three medicinal plants known as “meiduoluomi” in Tibetan folk medicine. Yakugaku Zasshi 2008, 128, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Ríos, J.L. Chapter 1—Essential Oils: What They Are and How the Terms Are Used and Define. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 3–10. [Google Scholar] [CrossRef]
- Nazaruk, J.; Gudej, J.; Majda, T.; Góra, J. Investigation of the essential oil of Erigeron acris L. herb. J. Essent. Oil Res. 2006, 18, 88–90. [Google Scholar] [CrossRef]
- Nazaruk, J.; Kalemba, D. Chemical composition of the essential oils from the roots of Erigeron acris L. and Erigeron annuus (L.) Pers. Molecules 2009, 14, 2458–2465. [Google Scholar] [CrossRef] [PubMed]
- Pliszko, A.; Łazarski, G.; Kalinowski, P.; Musiał, K. New records on chromosome numbers in non-native Erigeron L. taxa (Asteraceae) from Poland. BioInvasions Rec. 2024, 13, 843–853. [Google Scholar] [CrossRef]
- Magalhães, F.B.I.; Tellis, C.J.M.; Calabrese, K.S.; Abreu-Silva, A.L.; Almeida-Souza, F. Essential oils’ potential in breast cancer treatment: An Overview. In Essential Oils-Bioactive Compounds, New Perspectives and Applications; Oliveira, M.S., Almeida da Costa, W., Silva, S.G., Eds.; IntechOpen: London, UK, 2020. [Google Scholar]
- Lis, A.; Mielczarek, J.; Kalemba, D.; Nazaruk, J. Chemical composition of the essential oil from the herb of Erigeron annuus (L.) Pers. J. Essent. Oil Res. 2008, 20, 229–232. [Google Scholar] [CrossRef]
- Lis, A.; Nazaruk, J.; Mielczarek, J.; Kalemba, D. Comparative study of chemical composition of essential oils from different organs of Erigeron annuus (L.) Pers. J. Essent. Oil-Bear. Plants 2008, 11, 17–21. [Google Scholar] [CrossRef]
- Kumar, V.; Mathela, C.S.; Tewari, G.; Singh, D.; Tewari, A.K.; Bisht, K.S. Chemical composition and antifungal activity of essential oils from three Himalayan Erigeron species. LWT-Food Sci. Technol. 2014, 56, 278–283. [Google Scholar] [CrossRef]
- Kim, D.Y.; Won, K.J.; Hwang, D.I.; Park, S.M.; Kim, H.B.; Lee, H.M. Chemical composition of essential oil from Erigeron annuus (L.) Pers. flower and its effect on migration and proliferation in keratinocyte. J. Essent. Oil-Bear. Plants 2018, 21, 1146–1154. [Google Scholar] [CrossRef]
- Database of Plants of Indian Subcontinent. eFloraofIndia. Available online: https://efloraofindia.com/ (accessed on 8 July 2025).
- Park, J.H.; Choi, Y.-J.; Choi, I.-Y.; Shin, H.-D. First report of powdery mildew caused by Golovinomyces ambrosiae on Erigeron annuus in Korea. Plant Dis. 2023, 107, 2257. [Google Scholar] [CrossRef]
- Iijima, T.; Yaoita, Y.; Kikuchi, M. Five new sesquiterpenoids and a new diterpenoid from Erigeron annuus (L.) PERS., Erigeron philadelphicus L. and Erigeron sumatrensis RETZ. Chem. Pharm. Bull. 2003, 51, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Adande, K.; Eloh, K.; Simalou, O.; Bakaï, M.; Caboni, P. Chemical composition of different extracts of Conyza bonariensis: Insecticidal and nematicidal activities. AJAC 2023, 14, 95–120. [Google Scholar] [CrossRef]
- Wu, H. Conyza bonariensis (L.) Cronquist. The Biology of Australian Weeds. Plant Prot. Q. 2007, 22, 122–131. Available online: https://api.semanticscholar.org/CorpusID:84053843 (accessed on 8 May 2025).
- Marochio, C.A.; Bevilaqua, M.R.R.; Takano, H.K.; Mangolim, C.A.; Oliveira Junior, R.S.; Machado, M.F.P.S. Genetic admixture in species of Conyza (Asteraceae) as revealed by microsatellite markers. Acta Sci. Agron. 2017, 39, 437–445. [Google Scholar] [CrossRef]
- Wang, A.; Wu, H.; Zhu, X.; Lin, J. Species identification of Conyza bonariensis assisted by chloroplast genome sequencing. Front. Genet. 2018, 9, 374. [Google Scholar] [CrossRef] [PubMed]
- Balah, M.A.; Al-Andal, A.; Radwan, A.M.; Donia, A.E.M. Unveiling allelopathic dynamics and impacts of invasive Erigeron bonariensis and Bidens pilosa on plant communities and soil parameters. Sci. Rep. 2024, 14, 10159. [Google Scholar] [CrossRef] [PubMed]
- Amaro-Blanco, I.; Fernández-Moreno, P.T.; Osuna-Ruiz, M.D.; Bastida, F.; De Prado, R. Mechanisms of glyphosate resistance and response to alternative herbicide-based management in populations of the three Conyza species introduced in southern Spain. Pest Manag. Sci. 2018, 74, 1925–1937. [Google Scholar] [CrossRef] [PubMed]
- Verdeguer, M.; Castañeda, L.G.; Torres-Pagan, N.; Llorens-Molina, J.A.; Carrubba, A. Control of Erigeron bonariensis with Thymbra capitata, Mentha piperita, Eucalyptus camaldulensis, and Santolina chamaecyparissus essential oils. Molecules 2020, 25, 562. [Google Scholar] [CrossRef] [PubMed]
- Maia, J.G.S.; da Silva, M.H.L.; Zoghbi, M.G.B.; Andrade, E.H.A. Composition of the essential oils of Conyza bonariensis (L.) Cronquist. J. Essent. Oil Res. 2002, 14, 325–326. [Google Scholar] [CrossRef]
- Barbosa, L.C.A.; Paula, V.F.; Azevedo, A.S.; Silva, E.A.M.; Nascimento, E.A. Essential oil composition from some plant parts of Conyza bonariensis (L.) Cronquist. Flavour Fragr. J. 2005, 20, 39–41. [Google Scholar] [CrossRef]
- Tzakou, O.; Vagias, C.; Gani, A.; Yannitsaros, A. Volatile constituents of essential oils isolated at different growth stages from three Conyza species growing in Greece. Flavour Fragr. J. 2005, 20, 425–428. [Google Scholar] [CrossRef]
- Urdampilleta, J.D.; Amat, A.G.; Bidau, C.J.; Koslobsky, N.K. Biosystematic and chemosystematic studies in five South American species of Conyza (Asteraceae). Bol. Soc. Argent. Bot. 2005, 40, 101–107. Available online: https://www.researchgate.net/publication/262704198_Biosystematic_and_Chemosystematic_studies_in_five_south_american_species_of_Conyza_Asteraceae (accessed on 15 May 2025).
- Mabrouk, S.; Elaissi, A.; Ben Jannet, H.; Harzallah-Skhiri, F. Chemical composition of essential oils from leaves, stems, flower heads and roots of Conyza bonariensis L. from Tunisia. Nat. Prod. Res. 2011, 25, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Benzarti, A.; Hammami, S.; Piras, A.; Falconieri, D.; El Mokni, R.; M’Henni, M.F.; Marongiu, B.; Mighri, Z. Effects of different ecological conditions and extraction techniques on the quality of volatile oils from flaxleaf fleabane (Erigeron bonariensis L.). J. Med. Plant Res. 2013, 7, 3059–3065. [Google Scholar]
- Araujo, L.; Moujir, L.M.; Rojas, J.; Carmona, J.; Rondón, M. Chemical composition and biological activity of Conyza bonariensis essential oil collected in Mérida, Venezuela. Nat. Prod. Commun. 2013, 8, 1175–1178. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Mohan, M.; Singh, P.; Palni, U.T.; Tripathi, N.N. Chemical composition, antibacterial and antioxidant activity of essential oil of Eupatorium adenophorum Spreng. from Eastern Uttar Pradesh, India. Food Biosci. 2014, 7, 80–87. [Google Scholar] [CrossRef]
- Harraz, F.M.; Hammoda, H.M.; El Ghazouly, M.G.; Farag, M.A.; El-Aswad, A.F.; Bassam, S.M. Chemical composition, antimicrobial and insecticidal activities of the essential oils of Conyza linifolia and Chenopodium ambrosioides. Nat. Prod. Res. 2015, 29, 879–882. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, P.; Singh, D.; Singh, G.B. Chemical composition of volatile extract of Erigeron bonariensis and its antimicrobial activity. Int. J. Res. Med. Basic Sci. 2016, 2, 1–11. Available online: https://www.researchgate.net/publication/317731557_Chemical_Composition_of_volatile_extract_of_Erigeron_bonariensis_and_its_anti_microbial_activity (accessed on 6 May 2025).
- Musembei, R.; Joyce, K.J. Chemical composition and antibacterial activity of essential oil from Kenyan Conyza bonariensis (L.) Cronquist. Sci. Lett. 2017, 5, 180–185. [Google Scholar]
- do Amaral, W.; Deschamps, C.; Biasi, L.A.; Bizzo, H.R.; Machado, M.P.; da Silva, L.E. Yield and chemical composition of the essential oil of species of the Asteraceae family from Atlantic Forest, South of Brazil. J. Essent. Oil Res. 2018, 30, 278–284. [Google Scholar] [CrossRef]
- Hoi, T.M.; Huong, L.T.; Chinh, H.V.; Hau, D.V.; Satyal, P.; Tai, T.A.; Dai, D.N.; Hung, N.H.; Hien, V.T.; Setzer, W.N. Essential oil compositions of three invasive Conyza species collected in Vietnam and their larvicidal activities against Aedes aegypti, Aedes albopictus, and Culex quinquefasciatus. Molecules 2020, 25, 4576. [Google Scholar] [CrossRef] [PubMed]
- Elgamal, A.M.; Ahmed, R.F.; Abd-El Gawad, A.M.; El Gendy, A.E.-N.G.; Elshamy, A.I.; Nassar, M.I. Chemical profiles, anticancer, and anti-aging activities of essential oils of Pluchea dioscoridis (L.) DC. and Erigeron bonariensis L. Plants 2021, 10, 667. [Google Scholar] [CrossRef]
- Lundgren, G.A.; Braga, S.D.P.; de Albuquerque, T.M.R.; Árabe Rimá de Oliveira, K.; Tavares, J.F.; Vieira, W.A.D.S.; Câmara, M.P.S.; de Souza, E.L. Antifungal effects of Conyza bonariensis (L.) Cronquist essential oil against pathogenic Colletotrichum musae and its incorporation in gum Arabic coating to reduce anthracnose development in banana during storage. J. Appl. Microbiol. 2022, 132, 547–561. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.C.; do Nascimento, Y.M.; de Araújo Loureiro, P.B.; Martins, R.X.; de Souza Maia, M.E.; Farias, D.F.; Tavares, J.F.; Gonçalves, J.C.R.; da Silva, M.S.; Sobral, M.V. Chemical composition, in vitro antitumor effect, and toxicity in Zebrafish of the essential oil from Conyza bonariensis (L.) Cronquist (Asteraceae). Biomolecules 2023, 13, 1439. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.G.; Haris, A.; Binyameen, M.; Nazir, A.; Mozūratis, R.; Azeem, M. Chemical composition, larvicidal and repellent activities of wild plant essential oils against Aedes aegypti. Biology 2023, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Zapata, B.; Duran, C.; Stashenko, E.; Betancur-Galvis, L.; Mesa-Arango, A.C. Actividad antimicótica y citotoxica de aceites esenciales de plantas de la familia Asteraceae. Rev. Iberoam. Micol. 2010, 2, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Opiyo, S.A.; Njoroge, P.W.; Kuria, K.M. Chemical composition and biological activity of extracts from Conyza species. J. Appl. Chem. (IOSR-JAC) 2023, 16, 61–71. [Google Scholar]
- Mahanur, V.B.; Rajge, R.R.; Pal, R.S.; Chaitanya, M.V.N.L.; Vishwas, S.; Gupta, S.; Gupta, G.; Kumar, D.; Oguntibeju, O.O.; Rehman, Z.; et al. Harnessing unexplored medicinal values of the red listed South African weed Erigeron bonariensis: From ethnobotany to biomedicine. S. Afr. J. Bot. 2023, 160, 535–546. [Google Scholar] [CrossRef]
- Wu, R.; Liang, Y.; Xu, M.; Ke, F.; Zhang, Y.; Wu, L.; Wang, Z. Advances in chemical constituents, clinical applications, pharmacology, pharmacokinetics and toxicology of Erigeron breviscapus. Front. Pharmacol. 2021, 12, 656335. [Google Scholar] [CrossRef] [PubMed]
- Lis, A.; Góra, J. Essential oil of Conyza canadensis (L.) Cronq. J. Essent. Oil Res. 2000, 12, 781–783. [Google Scholar] [CrossRef]
- Lis, A.; Piggott, J.R.; Góra, J. Chemical composition variability of the essential oil of Conyza canadensis Cronq. Flav. Fragr. J. 2003, 18, 364–367. [Google Scholar] [CrossRef]
- Curini, M.; Bianchi, A.; Epifano, F.; Bruni, R.; Torta, L.; Zambonelli, A. Composition and in vitro antifungal activity of essential oils of Erigeron canadensis and Myrtus communis from France. Chem. Nat. Comp. 2003, 39, 191–194. [Google Scholar] [CrossRef]
- Stoyanova, S.; Georgiev, E.; Kermedchieva, D.; Lis, A.; Góra, J. Changes in the essential oil of Conyza canadensis (L.) Cronquist. during its vegetation. J. Essent. Oil Res. 2003, 15, 44–45. [Google Scholar] [CrossRef]
- Rustaiyan, A.; Azar, P.A.; Moradalizadeh, M.; Masoudi, S.; Ameri, N. Volatile constituents of three Compositae herbs: Anthemis altissima L. var. altissima, Conyza canadensis (L.) Cronq. and Grantina aucheri Boiss. growing wild in Iran. J. Essent. Oil Res. 2004, 16, 579–781. [Google Scholar] [CrossRef]
- Choi, H.-J.; Wang, H.-Y.; Kim, Y.-N.; Heo, S.-J.; Kim, N.-K.; Jeong, M.-S.; Park, Y.-H.; Kim, S. Composition and cytotoxicity of essential oil extracted by steam distillation from horseweed (Erigeron canadensis L.) in Korea. J. Korean Soc. Appl. Biol. Chem. 2008, 51, 55–59. [Google Scholar]
- Liu, Z.M.; Wang, H.G.; Liu, S.S.; Jiang, N.X. Comparative study of volatile components of essential oil from Conyza canadensis between hydrodistillation and steam distillation. Adv. Mat. Res. 2010, 113–116, 1644–1647. [Google Scholar] [CrossRef]
- Liu, Z.M.; Wang, H.Y.; Liu, S.S.; Jiang, N.X.; Li, L. Analysis of active components of essential oil from Conyza canadensis. Biomass Chem. Eng. 2010, 44, 22–26. Available online: https://journals.caf.ac.cn/en/swzhxgc/article/id/swzhxgc_372?viewType=HTML (accessed on 28 May 2025).
- Veres, K.; Csupor-Löffler, B.; Lázár, A.; Hohmann, J. Antifungal activity and composition of essential oils of Conyza canadensis herbs and roots. Sci. World J. 2012, 2012, 489646. [Google Scholar] [CrossRef] [PubMed]
- Kloucek, P.; Smid, J.; Frankova, A.; Kokoska, L.; Valterovaand, I.; Pavela, R. Fast screening method for assessment of antimicrobial activity of essential oils in vapor phase. Food Res. Int. 2012, 47, 161–165. [Google Scholar] [CrossRef]
- Unnithan, C.R.; Muuz, M.; Woldu, A.; Reddy, D.N.; Gebremariam, G.; Menasbo, B.; Hilawie, M.; Teklu, T. Chemical analysis of the essential oil of Erigeron canadensis. UJPBS 2014, 2, 8–10. Available online: https://www.academia.edu/113976306/Chemical_Analysis_of_the_Essential_Oil_of_Erigeron_Canadensis_L (accessed on 28 May 2025).
- Zeng, D.-Q.; Peng, Y.-H.; Chen, F.-F.; Zhang, Y.; Liu, M. Insecticidal activity of essential oil derived from horseweed Conyza canadensis (L.) Cronq. against two mosquitoes and its volatile components. Acta Entomol. Sin. 2014, 57, 204–211. [Google Scholar]
- Ayaz, F.; Küçükboyaci, N.; Demirci, B. Chemical composition and antimicrobial activity of the essential oil of Conyza canadensis (L.) Cronquist from Turkey. J. Essent. Oil Res. 2017, 29, 336–343. [Google Scholar] [CrossRef]
- Lateef, R.; Bhat, K.A.; Chandra, S.; Banday, G.A. Chemical composition, antimicrobial and antioxidant activities of the essential oil of Conyza canadensis growing wild in Kashmir valley. Am. J. Essent. Oils Nat. Prod. 2018, 6, 35–41. Available online: https://api.semanticscholar.org/CorpusID:55770726 (accessed on 12 June 2025).
- Aragão, L.W.; Fernandes, S.S.L.; Mallmann, V.; Facco, J.T.; Matos, M.d.F.C.; Cabral, M.R.P.; de Carvalho, J.E.; Ruiz, A.L.T.G.; Cardoso, C.A.L.; da Silva, R.C.L.; et al. Chemical composition and evaluation of antitumoral activity of leaf and root essential oils of Conyza canadensis (Asteraceae). Orbital Electron. J. Chem. 2019, 11, 284–291. [Google Scholar] [CrossRef]
- Azeem, M.; Zaman, T.; Tahir, M.; Haris, A.; Iqbal, Z.; Binyameen, M.; Nazir, A.; Shad, S.A.; Majeed, S.; Mozūraitis, R. Chemical composition and repellent activity of native plants essential oils against dengue mosquito, Aedes aegypti. Ind. Crops Prod. 2019, 140, 111609. [Google Scholar] [CrossRef]
- Barhoumi, L.M.; Shakya, A.K.; Al-Fawares, O.; Al-Jaber, H.I. Conyza canadensis from Jordan: Phytochemical profiling, antioxidant, and antimicrobial activity evaluation. Molecules 2024, 29, 2403. [Google Scholar] [CrossRef] [PubMed]
- Si, C.; Ou, Y.; Ma, D.; Hei, L.; Wang, X.; Du, R.; Yang, H.; Liao, Y.; Zhao, J. Cytotoxic effect of the essential oils from Erigeron canadensis L. on human cervical cancer HeLa cells in itro. Chem. Biodivers. 2022, 19, e202200436. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, C.; Han, M.; Xiao, C.; Wang, H.; Shen, W.; Yang, L. Biological activities of the volatile oil from Conyza canadensis (L.) Cronq. on fungi, bacteria and plants and its chemical constituents. Nongyao 2010, 49, 801. Available online: https://api.semanticscholar.org/CorpusID:88061422 (accessed on 8 May 2025).
- Tzakou, O.; Gani, A.; Economou, G.; Yannitsaros, A. Chemical composition and allelopathic activity of oil and volatile fractions of Conyza albida Willd. ex Sprengel from Greece. J. Essent. Oil Res. 2004, 16, 425–428. [Google Scholar] [CrossRef]
- Kuiate, J.-R.; Tsona, A.A.; Foko, J.; Bessiere, J.M.; Menut, C.; Zollo, P.-H.A. Chemical composition and in vitro antifungal properties of essential oils from leaves and flowers of Erigeron floribundus (H.B. et K.) Sch. Bip. from Cameroon. J. Essent. Oil Res. 2005, 17, 261–264. [Google Scholar] [CrossRef]
- Boti, J.B.; Koukola, G.; Guessan, T.Y.; Casanova, J. Chemical variablity of Conyza sumatrensis and Microglossa pyrifolia from Côte d’Ivoire. Flavour Fragr. J. 2007, 22, 27–31. [Google Scholar] [CrossRef]
- Joshi, R.K.; Mujawar, M.H.K.; Badakar, V.M.; Khatib, N.A. In vitro antimicrobial activity of the essential oils Erigeron floribundus. IJRAP 2011, 2, 236–238. [Google Scholar]
- Mabrouk, S.; Salah, K.B.H.; Elaissi, A.; Jlaiel, L.; Jannet, H.B.; Aouni, M.; Harzallah-Skhiri, F. Chemical composition and antimicro bial and allelopathic activity of Tunisian Conyza sumatrensis (Retz.) E. Walker essential oils. Chem. Biodivers. 2013, 10, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, R.; Orsomando, G.; Sorci, L.; Maggi, F.; Ranjbarian, F.; Biapa Nya, P.C.; Petrelli, D.; Vitali, L.A.; Lupidi, G.; Quassinti, L.; et al. Biological activities of the essential oil from Erigeron floribundus. Molecules 2016, 21, 1065. [Google Scholar] [CrossRef] [PubMed]
- Azeem, M.; Zaman, T.; Abbasi, A.M.; Abid, M.; Mozuratis, R.; Alwahibif, M.S.; Elshikh, M.S. Pesticidal potential of some wild plant essential oils against grain pests Tribolium castaneum (Herbst, 1797) and Aspergillus flavus (Link, 1809). Arab. J. Chem. 2022, 15, 103482. [Google Scholar] [CrossRef]
- Guetchueng, S.T.; Lontsi, A.T.; Kowa, T.K.; Tchamgoue, J.; Tsabang, N.; Nnanga, E.N. Traditional uses, phytochemistry, pharmacology, and toxicology of Erigeron floribundus (Kunth) Sch. Bip.: A Review. Nat. Prod. J. 2023, 13, 115–121. [Google Scholar] [CrossRef]
- National Library of Medicine. NCBI (National Center for Biotechnology Information). Available online: https://www.ncbi.nlm.nih.gov/Taxonomy (accessed on 10 June 2025).
- Mustapha, M.B.; Algethami, F.K.; Elamin, M.R.; Abdulkhair, B.Y.; Chaieb, I.; Jannet, H.B. Chemical composition, toxicity and repellency of Inula graveolens essential oils from roots and aerial parts against stored-product beetle Tribolium castaneum (Herbst). Chem. Biodivers. 2023, 20, e202200978. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. Chemical constituents and pharmacological effect of Inula graveolens (syn: Dittrichia graveolens): A review. Indo Am. J. Pharm. Sci. (IAJPS) 2018, 5, 2183–2190. [Google Scholar] [CrossRef]
- Al-Zubiri, A.S.; Al-Mamary, M.A.; Al-Ghassani, E.A. The antibacterial, antifungal and antioxidant activities of essential oil from different aromatic plants. Glob. Adv. Res. J. Med. Sci. 2017, 6, 224–233. Available online: http://garj.org/garjmms (accessed on 8 July 2025).
- Al-Mamary, M.; Abdelwahab, S.I.; Al-Ghalibi, S.; Al-Ghasani, E. The antioxidant and tyrosinase inhibitory activities of some essential oils obtained from aromatic plants grown and used in Yemen. Scien. Res. Essays (SRE) 2011, 6, 6840–6845. [Google Scholar]
- Chandra Pandey, S.; Dhami, D.S.; Jha, A.; Chandra Shah, G.; Kumar, A.; Samant, M. Identification of trans-2-cis-8-Matricaria-ester from the essential oil of Erigeron multiradiatus and evaluation of its antileishmanial potential by in vitro and in silico approaches. ACS Omega 2019, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Plant Database of Lady B. Jonson Wildflower Center. Available online: https://www.wildflower.org/plants/index.php (accessed on 9 July 2025).
- Yaoita, Y.; Kikuchi, M.; Machida, K. Terpenoids and related compounds from plants of the family Compositae (Asteraceae). Nat. Prod. Commun. 2012, 7, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Regarding the Amendment of the Minister of the Environment of the Republic of Lithuania “Regarding the Approval of the List of Species of Invasive Organisms in Lithuania and the Recognition of Some Orders of the Minister of the Environment as Having Lost Their Validity”. Available online: https://e-seimas.lrs.lt/portal/legalAct/lt/TAD/TAIS.239692/asr (accessed on 8 June 2025).
- Rahman, A.; Kang, S.C. Inhibition of foodborne pathogens and spoiling bacteria by essential oil and extracts of Erigeron ramosus (WALT.) B.S.P. J. Food Saf. 2009, 29, 176–189. [Google Scholar] [CrossRef]
- Meepagala, K.M.; Sturtz, G.; Wise, D.; Wedge, D.E. Molluscicidal and antifungal activity of Erigeron speciosus steam distillate. Pest Manag. Sci. 2002, 58, 1043–1047. [Google Scholar] [CrossRef] [PubMed]
- Giang An, N.T.; Duc Giang, L.; Tran Trung, H.; Xuan Duc, D.; Thi Thu, N.; Thu Hien, N.T.; Xuan Ha, N.; Khoa Nguyen, D.; Sy Vo, V. Chemical constituents, biological activities and molecular docking studies of root and aerial part essential oils from Erigeron sublyratus Roxb. ex DC. (Asteraceae). Chem. Biodivers. 2025, 22, e202401356. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).