Role of Substitution Patterns in Four Regioisomeric Tetraphenylethylene–Thiophene Derivatives
Abstract
1. Introduction
2. Results and Discussion
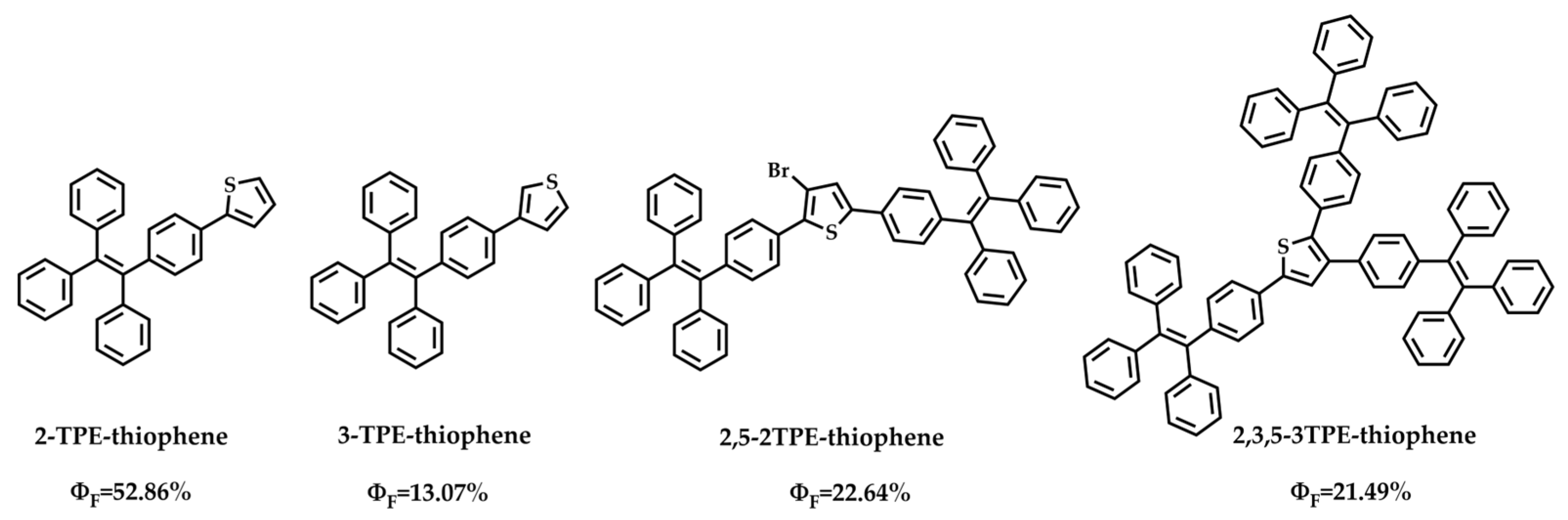
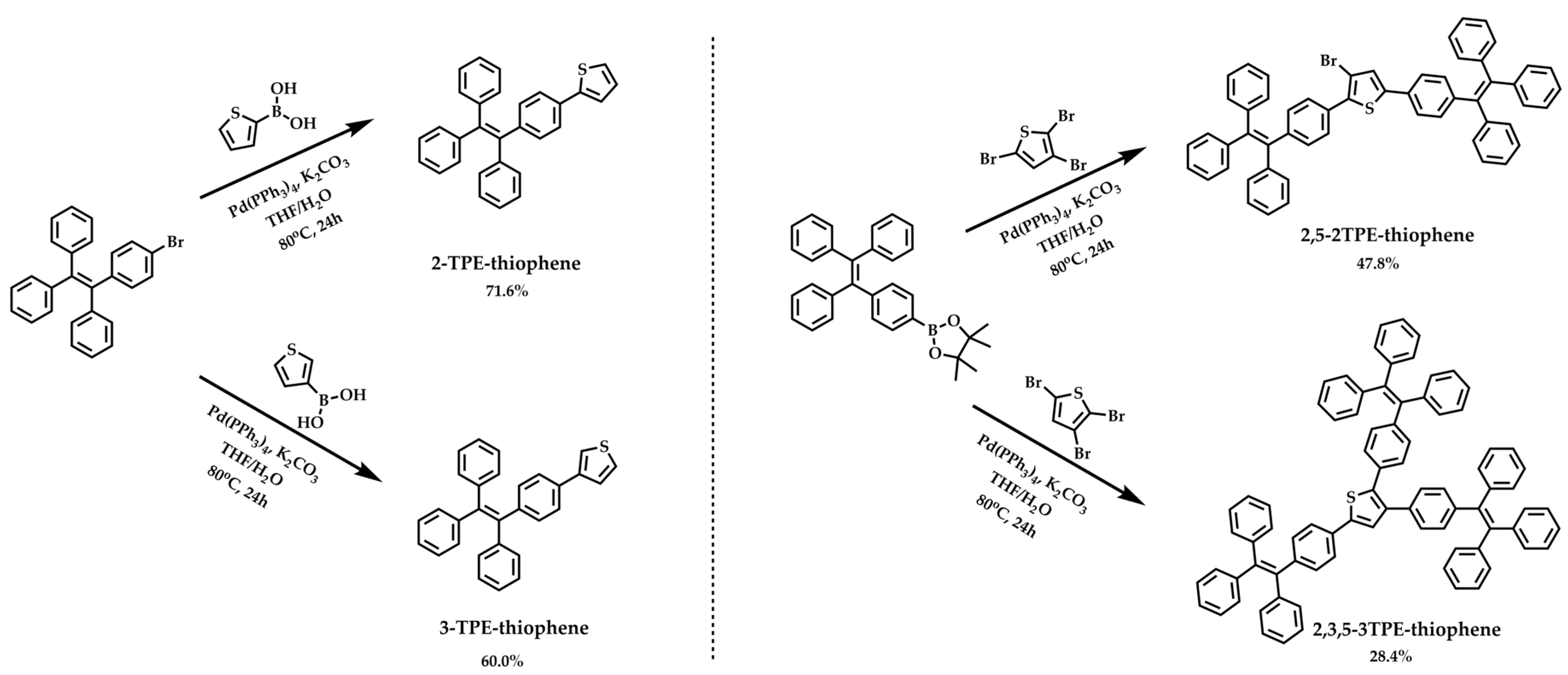
2.1. Synthesis and Characteristics
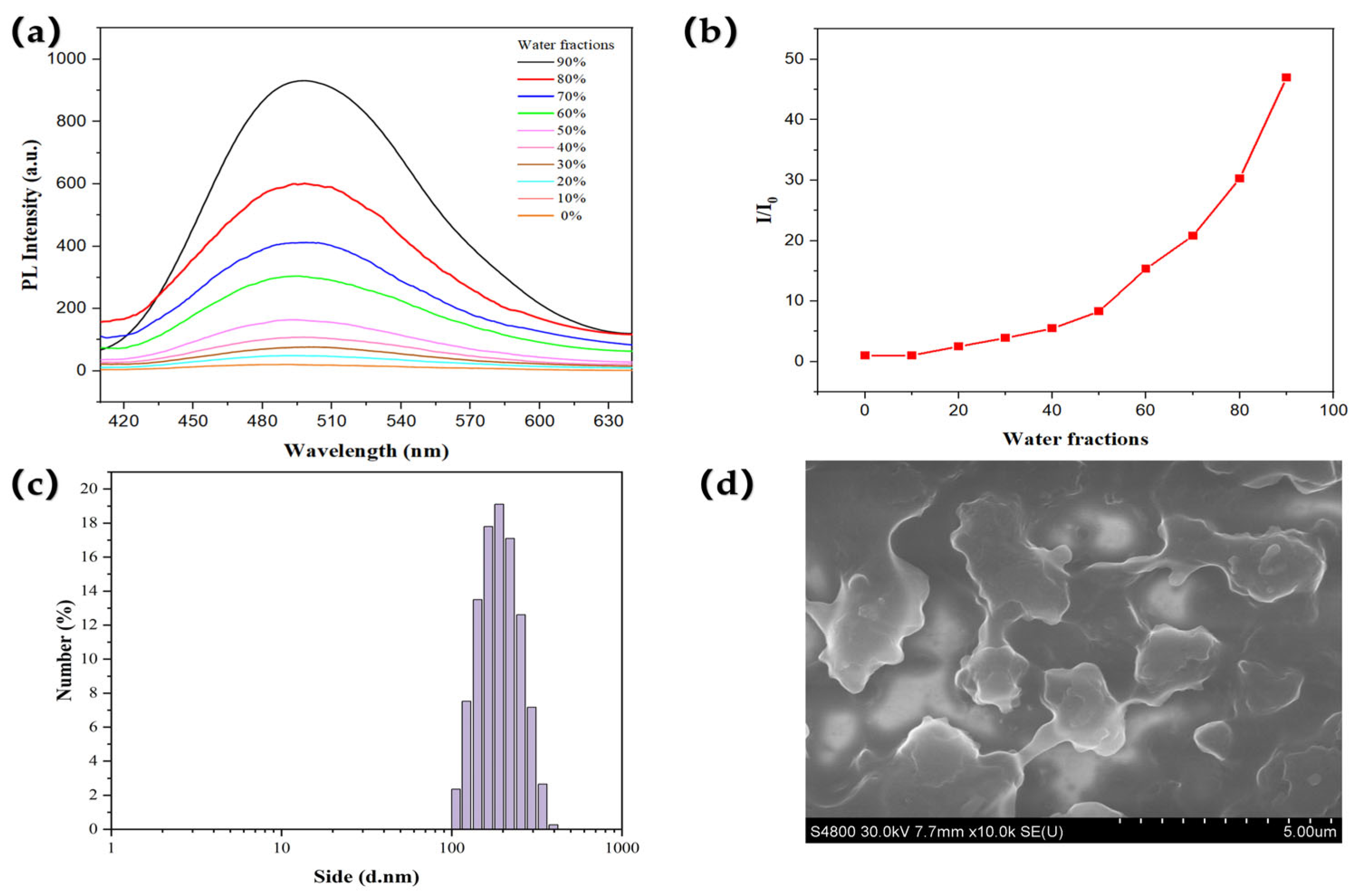
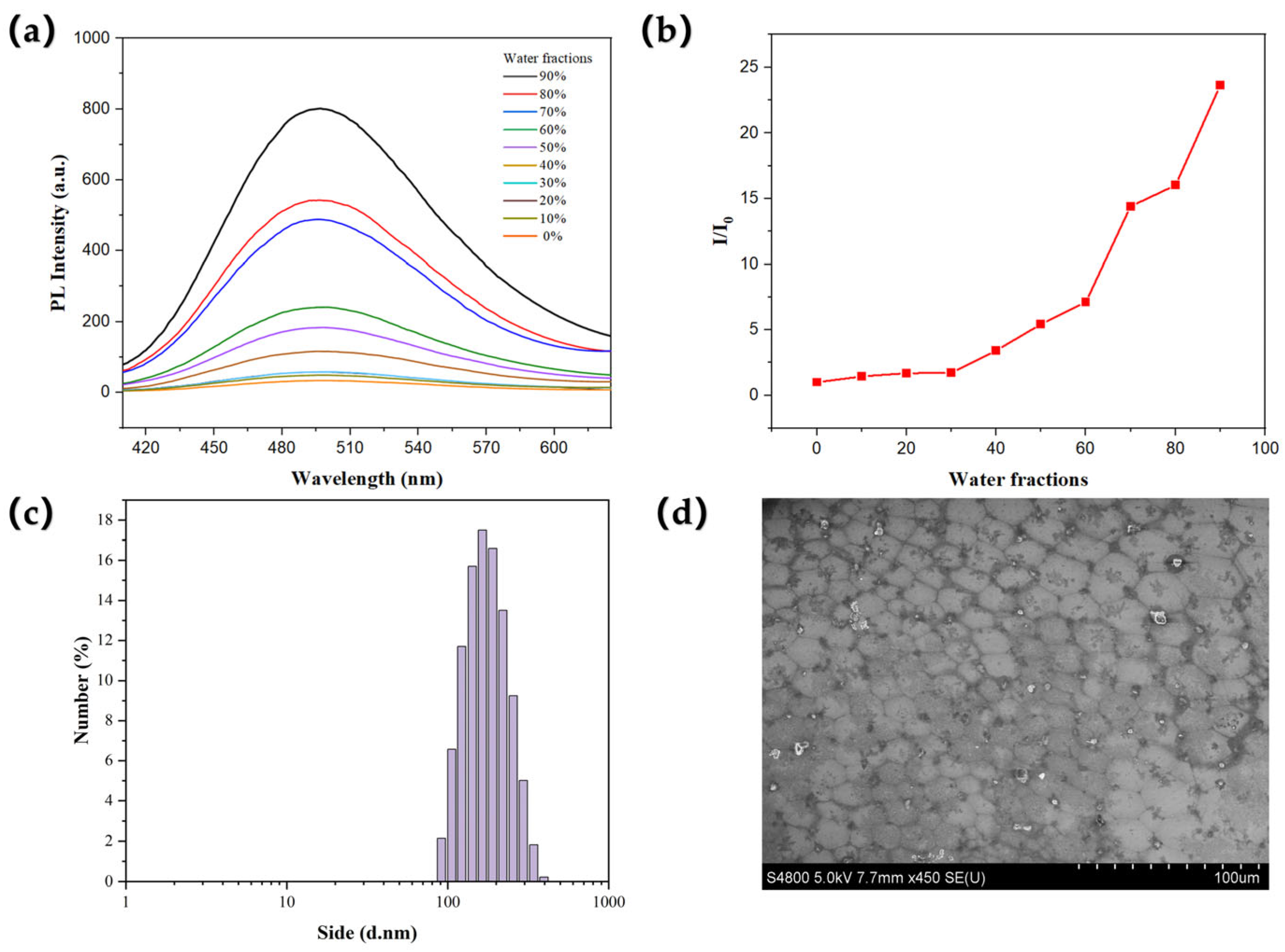
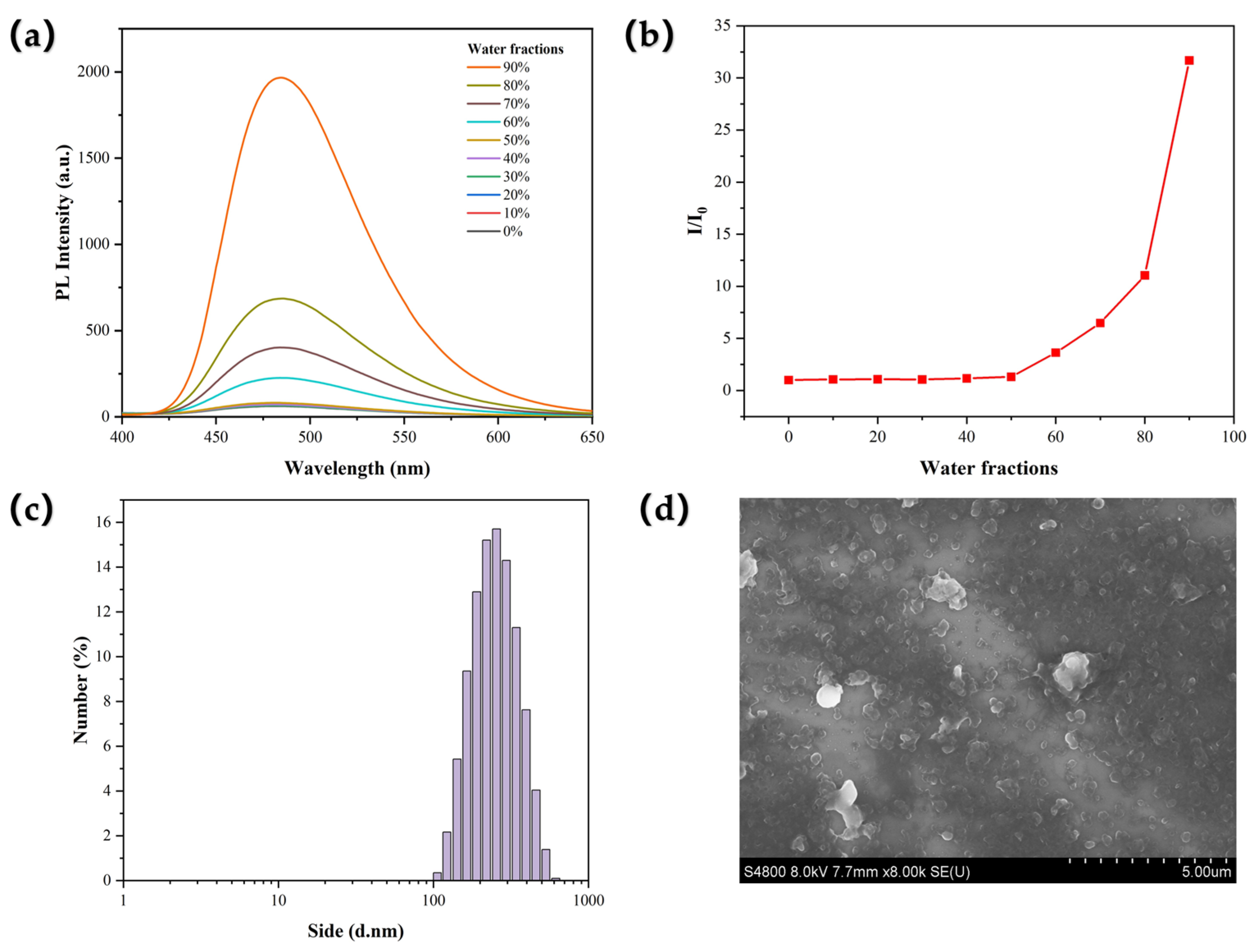
2.2. AIE Properties in Solution
2.3. Thermal Properties
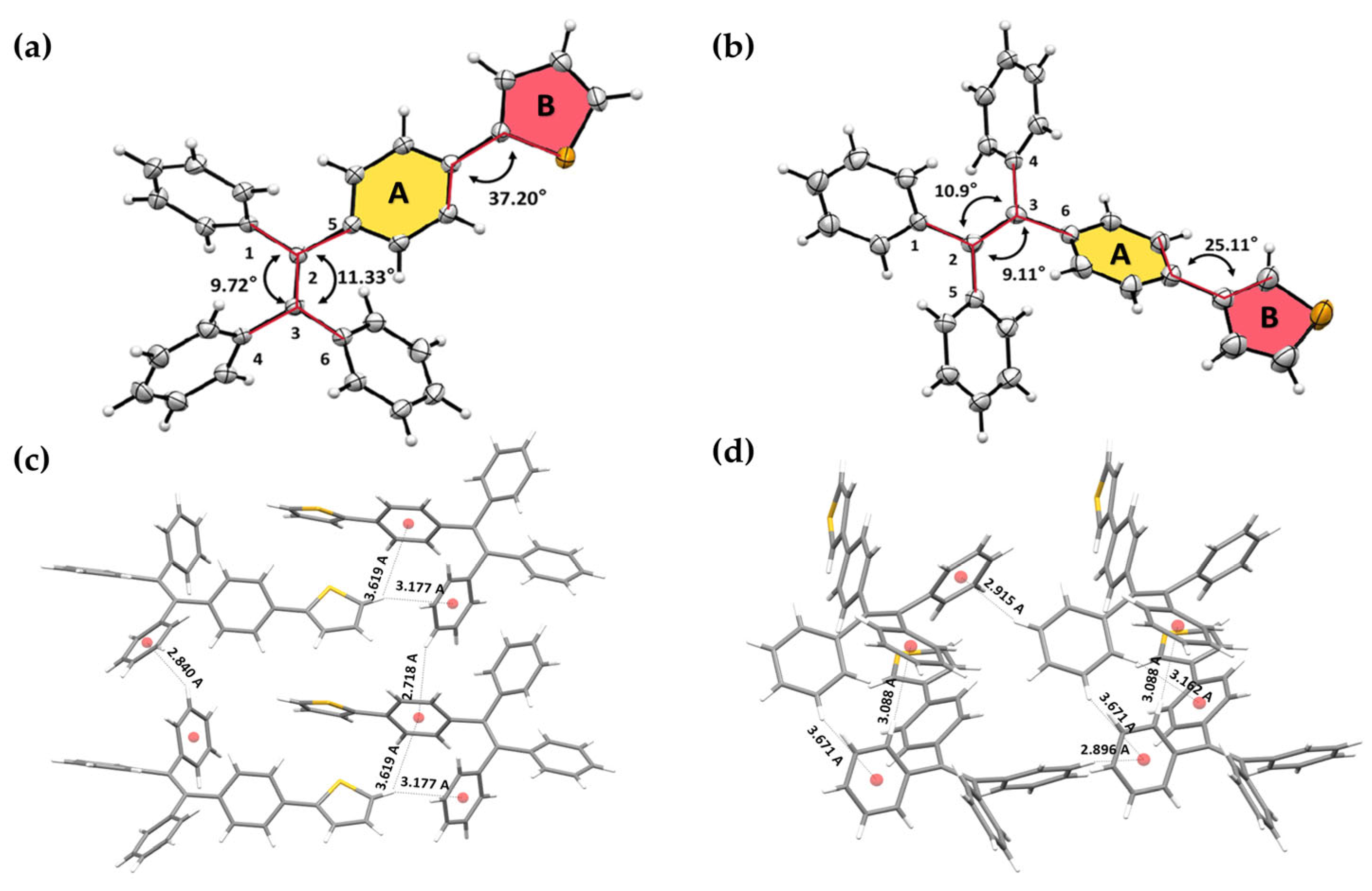
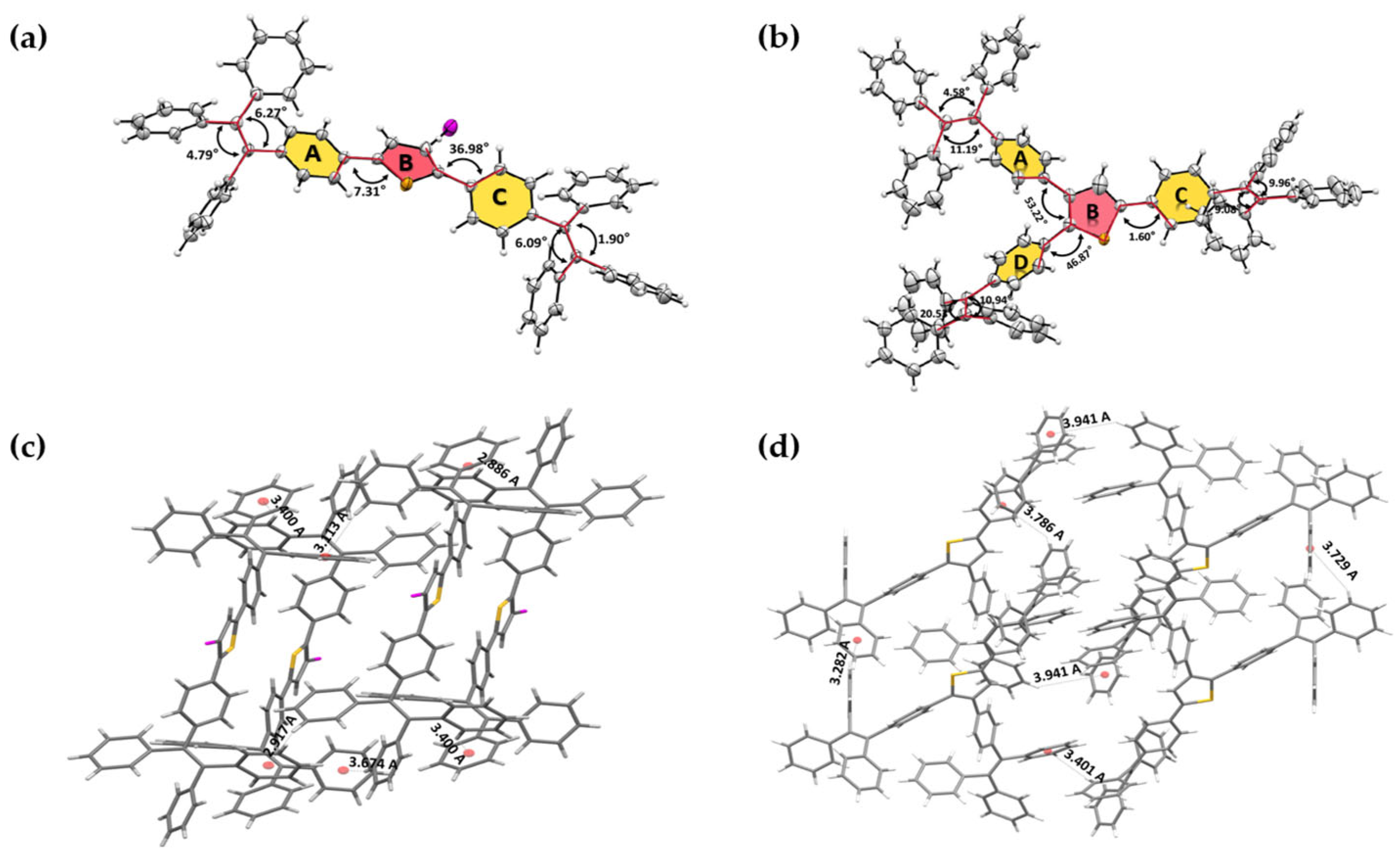
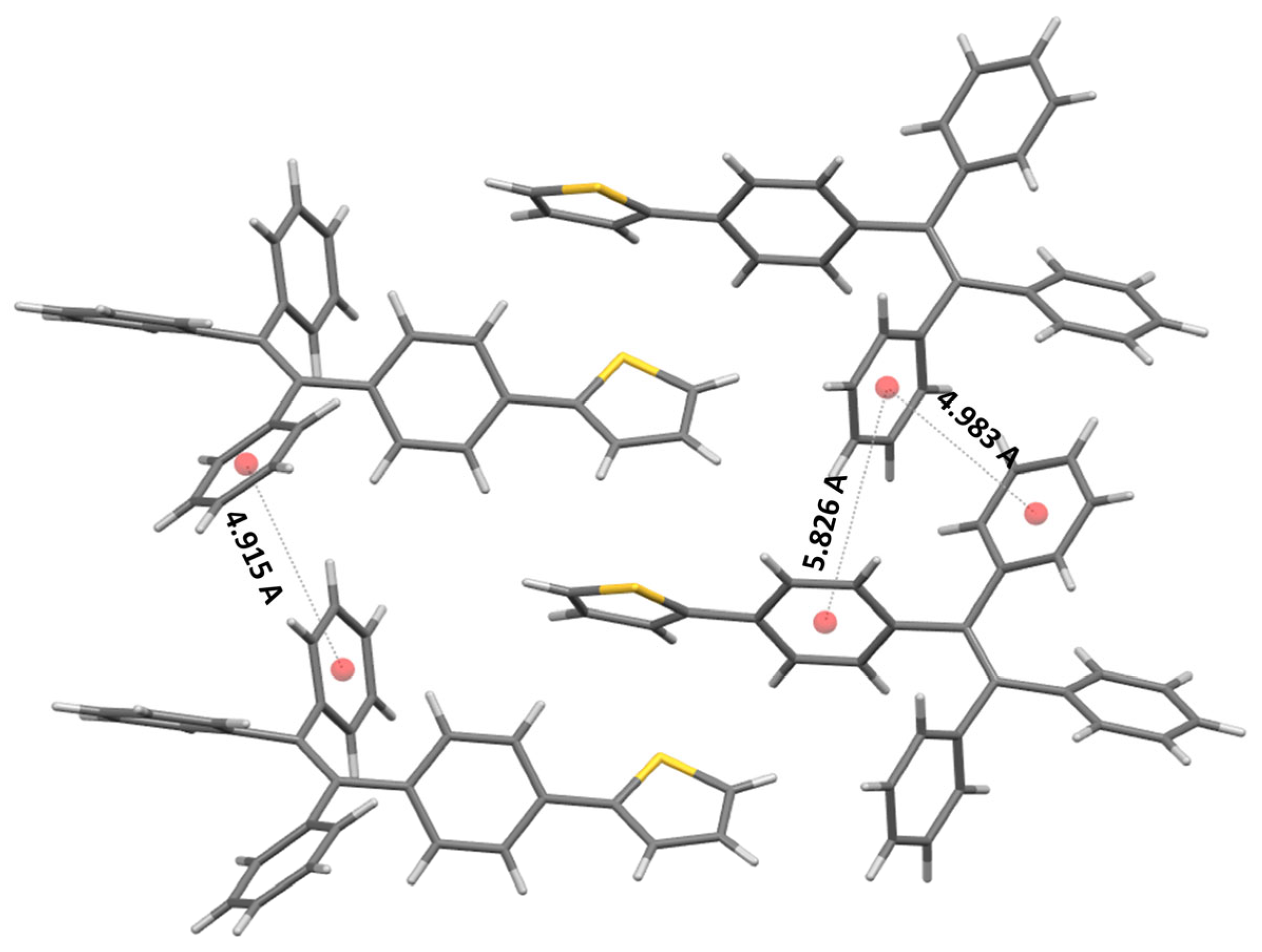
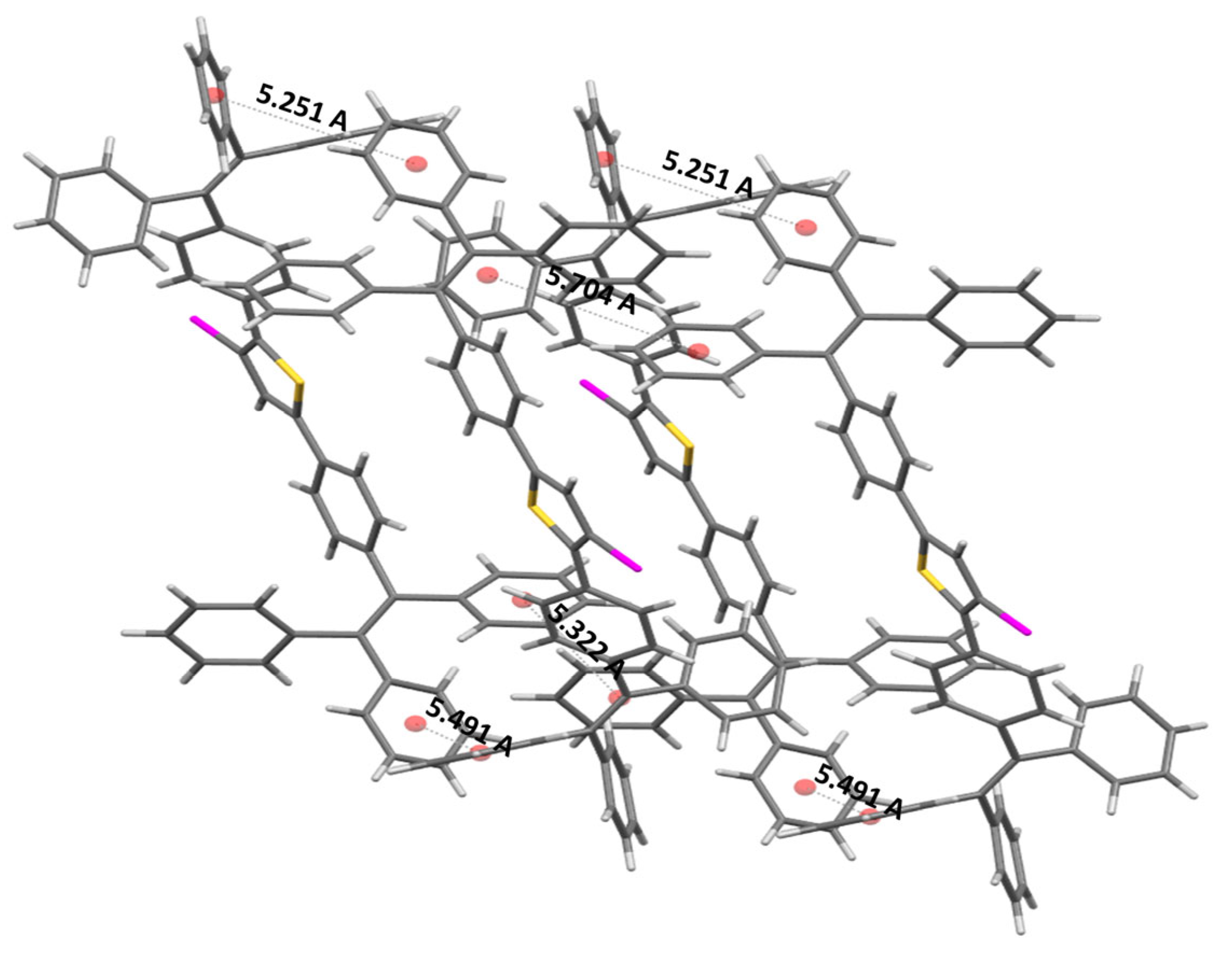
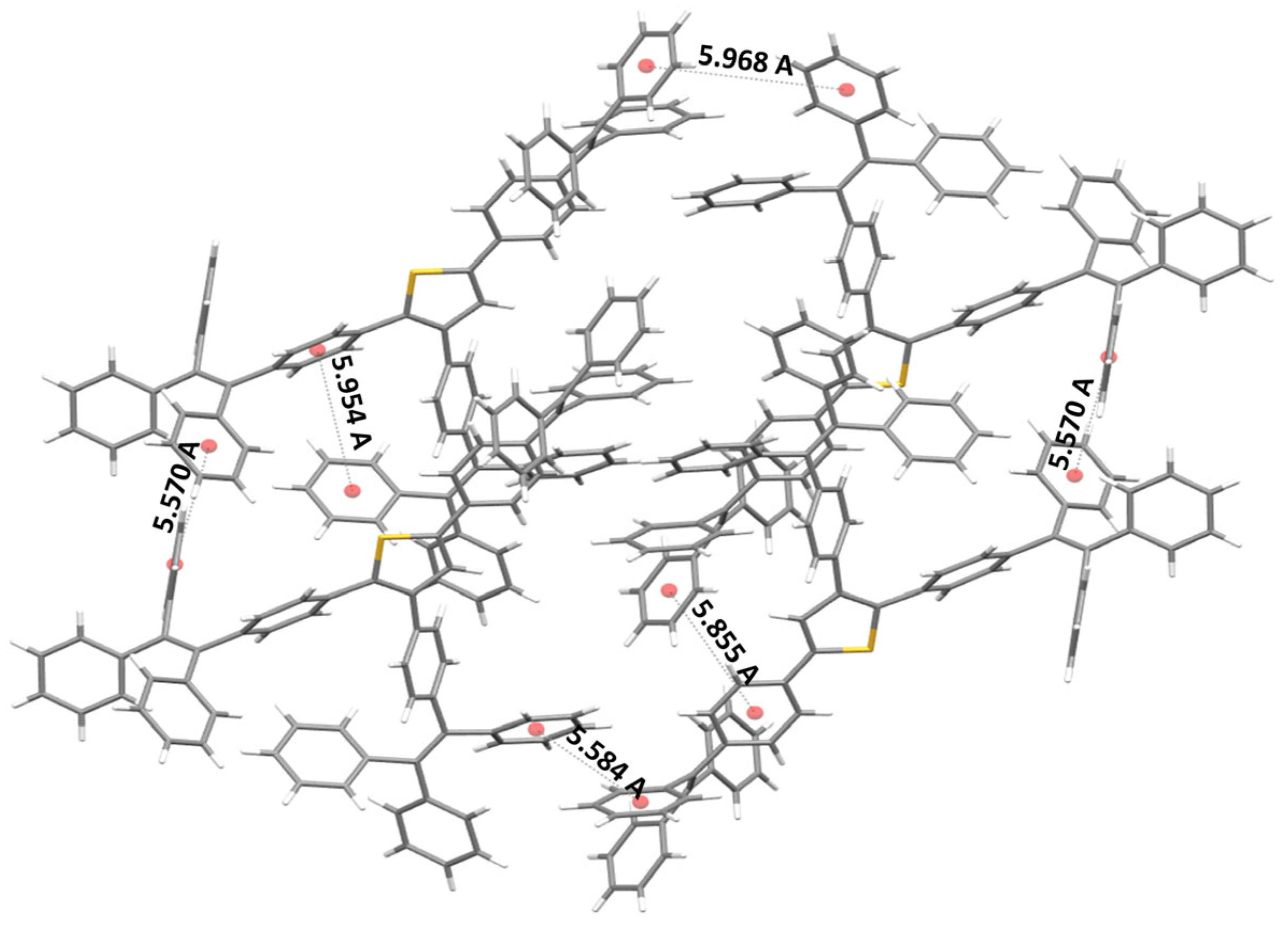
2.4. Single-Crystal Structure Analysis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Synthesis of 2-TPE-Thiophene
4.2.2. Synthesis of 3-TPE-Thiophene
4.2.3. Synthesis of 2,5-2TPE-Thiophene
4.2.4. Synthesis of 2,3,5-3TPE-Thiophene
4.2.5. Computational Details
4.3. Characterization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Isci, R.; Tekin, E.; Kaya, K.; Piravadili Mucur, S.; Gorkem, S.F.; Ozturk, T. Tetraphenylethylene substituted thienothiophene and dithienothiophene derivatives: Synthesis, optical properties and OLED applications. J. Mater. Chem. C 2020, 8, 7908–7915. [Google Scholar] [CrossRef]
- Jin, R.; Zhang, X.; Xiao, W.; Irfan, A. Rational design of diketopyrrolopyrrole-based multifunctional materials for organic light-emitting diodes and organic solar cells. Theor. Chem. Acc. 2018, 137, 145. [Google Scholar] [CrossRef]
- Esen, C.; Antonietti, M.; Kumru, B. Oxidative Photopolymerization of 3,4-Ethylenedioxythiophene (EDOT) via Graphitic Carbon Nitride: A Modular Toolbox for Attaining PEDOT. ChemPhotoChem 2021, 5, 857–862. [Google Scholar] [CrossRef]
- Tao, T.; Qian, H.-F.; Zhang, K.; Geng, J.; Huang, W. Functionalized oligothiophene-based heterocyclic aromatic fluorescent compounds with various donor–acceptor spacers and adjustable electronic properties: A theoretical and experimental perspective. Tetrahedron 2013, 69, 7290–7299. [Google Scholar] [CrossRef]
- Topal, S.; Suna, G.; Ulukan, P.; Sezer, E.; Ozturk, T. Synthesis and optoelectronic and charge storage characterizations of conducting polymers based on tetraphenylethylene and thienothiophenes. Electrochim. Acta 2021, 392, 139020. [Google Scholar] [CrossRef]
- Lv, Z.; Li, W.; Yang, L.; Loh, X.J.; Chen, X. Custom-Made Electrochemical Energy Storage Devices. ACS Energy Lett. 2019, 4, 606–614. [Google Scholar] [CrossRef]
- Mackanic, D.G.; Chang, T.-H.; Huang, Z.; Cui, Y.; Bao, Z. Stretchable electrochemical energy storage devices. Chem. Soc. Rev. 2020, 49, 4466–4495. [Google Scholar] [CrossRef]
- Wang, R.; Li, J.; Gao, L.; Yu, J. One-step electropolymerized thieno[3,2-b]thiophene-based bifunctional electrode with controlled color conversion for electrochromic energy storage application. Chem. Eng. J. 2022, 445, 136731. [Google Scholar] [CrossRef]
- Zhang, L.; Dou, S.X.; Liu, H.K.; Huang, Y.; Hu, X. Symmetric Electrodes for Electrochemical Energy-Storage Devices. Adv. Sci. 2016, 3, 119–121. [Google Scholar] [CrossRef]
- Li, J.; Shan, T.; Yao, M.; Gao, Y.; Han, X.; Yang, B.; Lu, P. The effect of different binding sites on the optical and electronic properties of tetraphenylethylene-substituted thiophene isomers. J. Mater. Chem. C 2017, 5, 2552–2558. [Google Scholar] [CrossRef]
- Kuo, C.-W.; Chang, J.-C.; Huang, W.-C.; Lee, P.-Y.; Wu, T.-Y. Electrochromic polymers based on 2,5-di(thiophen-2-yl)thieno[3,2-b]thiophene and thiophene derivatives as potential anodic layers for high performance electrochromic devices. J. Taiwan Inst. Chem. Eng. 2021, 125, 41–57. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, W.; Chen, H.; Han, Y.; Liu, J. Electropolymerization of D-A-D type monomers consisting of thiophene and quionaxline moieties for electrochromic devices and supercapacitors. J. Solid State Chem. 2022, 307, 122739. [Google Scholar] [CrossRef]
- Choi, K.-S.; Han, J.W.; Kim, M.H.; Yoon, M.; Kim, Y.-R.; Kim, I.T. Electropolymerization of thiazole derivative bearing thiophene and its application in capacitors and electrochromic devices. J. Electroanal. Chem. 2019, 848, 113329. [Google Scholar] [CrossRef]
- Liang, M.; Liu, L.; Sun, Y.; Li, J.; Zhang, L.E.; Jiang, X.; Wu, W. Furan-modified thiadiazolo quinoxaline as an electron acceptor for constructing second near-infrared aggregation-induced emission fluorophores for beyond 1300 nm fluorescence/photoacoustic imaging and photothermal therapy. Aggregate 2023, 5, e458. [Google Scholar] [CrossRef]
- Cinar, M.E.; Ozturk, T. Thienothiophenes, Dithienothiophenes, and Thienoacenes: Syntheses, Oligomers, Polymers, and Properties. Chem. Rev. 2015, 115, 3036–3140. [Google Scholar] [CrossRef]
- Wang, H.; Xu, X.; Cao, L.; Zhang, Z.; Li, J.; Liu, X.; Tao, X.; Zhu, G. Enhanced photocatalytic performance of tetraphenylethylene-based porous aromatic frameworks by bandgap adjustment for the synthesis of benzimidazoles. EES Catal. 2024, 2, 1100–1110. [Google Scholar] [CrossRef]
- Pan, Y.; Zhu, R.; Qi, C.; Wang, A.; Jia, Q.; Lin, C.; Qi, H.; Zhou, Q.; Xu, J.; Xiao, S. Convergence of tetraphenylethene towards a series of large size AIE compounds. Dye. Pigment. 2022, 204, 110429. [Google Scholar] [CrossRef]
- Ooyama, Y.; Sugino, M.; EnoKi, T.; Yamamoto, K.; Tsunoji, N.; Ohshita, J. Aggregation-induced emission (AIE) characteristic of water-soluble tetraphenylethene (TPE) bearing four sulfonate salts. New J. Chem. 2017, 41, 4747–4749. [Google Scholar] [CrossRef]
- Zhuang, W.; Ma, B.; Liu, G.; Li, G.; Wang, Y. TPE-conjugated biomimetic and biodegradable polymeric micelle for AIE active cell imaging and cancer therapy. J. Appl. Polym. Sci. 2017, 135, 45651. [Google Scholar] [CrossRef]
- Huang, J.; Chen, P.; Yang, X.; Tang, R.; Wang, L.; Qin, J.; Li, Z. Construction of deep-blue AIE luminogens with TPE and oxadiazole units. Sci. China Chem. 2013, 56, 1213–1220. [Google Scholar] [CrossRef]
- Feng, H.-T.; Yuan, Y.-X.; Xiong, J.-B.; Zheng, Y.-S.; Tang, B.Z. Macrocycles and cages based on tetraphenylethylene with aggregation-induced emission effect. Chem. Soc. Rev. 2018, 47, 7452–7476. [Google Scholar] [CrossRef] [PubMed]
- Tao, T.; Fang, D.; Chen, Y.; Fan, Y.; Lu, H. The length effect and color tuning of tetraphenylethylene functionalized oligothiophenes for effective detection of explosives. Dye. Pigment. 2021, 195, 109673. [Google Scholar] [CrossRef]
- Lu, H.; Gai, L.; Zhou, C.; Xie, W.; Zhou, Z.; Yu, F. Structure and Photophysical Properties of Silane Bridged Tetraphenylethylene-Oligothiophene Derivatives. Chin. J. Org. Chem. 2023, 43, 3652. [Google Scholar] [CrossRef]
- Yin, Y.; Chen, Z.; Yang, Y.; Liu, G.; Fan, C.; Pu, S. Thiophene-containing tetraphenylethene derivatives with different aggregation-induced emission (AIE) and mechanofluorochromic characteristics. RSC Adv. 2019, 9, 24338–24343. [Google Scholar] [CrossRef] [PubMed]
- Isci, R.; Rahimi Varzeghani, A.; Kaya, K.; Sütay, B.; Tekin, E.; Ozturk, T. Triphenylamine/Tetraphenylethylene Substituted 4-Thieno[3,2-b]thiophen-3-ylbenzonitriles: Synthesis, Photophysical-Electronic Properties, and Applications. ACS Sustain. Chem. Eng. 2022, 10, 1605–1615. [Google Scholar] [CrossRef]
- Zhu, R.; Pan, Y.; Yu, H.; Huang, C.; Tian, H.; Wang, T.; Xu, J.; Xiao, S. Three Isomeric Tetraphenylethylene-pyridine Compounds: Synthesis, Crystal Structures, and Photophysical Properties. Chem.—Asian J. 2023, 18, e202300600. [Google Scholar] [CrossRef]
- Xie, Y.; Husband, J.T.; Torrent-Sucarrat, M.; Yang, H.; Liu, W.; O’Reilly, R.K. Rational design of substituted maleimide dyes with tunable fluorescence and solvafluorochromism. Chem. Commun. 2018, 54, 3339–3342. [Google Scholar] [CrossRef]
- Sato, R.; Iso, Y.; Isobe, T. Fluorescence Solvatochromism of Carbon Dot Dispersions Prepared from Phenylenediamine and Optimization of Red Emission. Langmuir 2019, 35, 15257–15266. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, B.; Zhang, Y.; Guo, J.; Wu, X.; Ouyang, D.; Chen, S.; Chen, Y.; Wang, S.; Xing, G.; et al. Perylenedioic Acid-Derived Carbon Dots with Near 100% Quantum Yield in Aqueous Solution for Lasing and Lighting. Adv. Funct. Mater. 2024, 34, 2401353. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, Z.; Pu, J.; Guo, D.; Li, G.; Chen, Z.; Su, S.J.; Deng, H.; Zhao, J.; Chi, Z. Multiple-resonance thermally activated delayed emitters through multiple peripheral modulation to enable efficient blue OLEDs at high doping levels. Aggregate 2024, 5, e585. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Rev. B.01; Gaussian Inc.: Wallingford, CT, USA, 2016.
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView, Version 6.1; Semichem Inc.: Shawnee Mission, KS, USA, 2016.


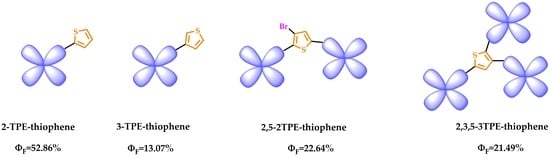
| Sample | λabs 1 (nm) | λem 1 (nm) | ε 2 (L/mol·cm) | ΦF 3 (%) | Td 4 (oC) |
|---|---|---|---|---|---|
| 2-TPE-thiophene | 331 | 480 | 27,510 | 52.86 | 207 |
| 3-TPE-thiophene | 321 | 482 | 21,380 | 13.07 | 208 |
| 2,5-2TPE-thiophene | 365 | 477 | 25,272 | 22.64 | 400 |
| 2,3,5-3TPE-thiophene | 338 | 481 | 52,548 | 21.49 | 431 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, S.; Tian, H.; Li, R.; Huang, Z.; Zhu, D.; Xiao, F.; Zhao, Y.; Xu, J. Role of Substitution Patterns in Four Regioisomeric Tetraphenylethylene–Thiophene Derivatives. Molecules 2025, 30, 2953. https://doi.org/10.3390/molecules30142953
Hou S, Tian H, Li R, Huang Z, Zhu D, Xiao F, Zhao Y, Xu J. Role of Substitution Patterns in Four Regioisomeric Tetraphenylethylene–Thiophene Derivatives. Molecules. 2025; 30(14):2953. https://doi.org/10.3390/molecules30142953
Chicago/Turabian StyleHou, Shuai, Hanxiao Tian, Ruiyao Li, Zishuai Huang, Dongyuan Zhu, Fan Xiao, Yunmeng Zhao, and Jingjing Xu. 2025. "Role of Substitution Patterns in Four Regioisomeric Tetraphenylethylene–Thiophene Derivatives" Molecules 30, no. 14: 2953. https://doi.org/10.3390/molecules30142953
APA StyleHou, S., Tian, H., Li, R., Huang, Z., Zhu, D., Xiao, F., Zhao, Y., & Xu, J. (2025). Role of Substitution Patterns in Four Regioisomeric Tetraphenylethylene–Thiophene Derivatives. Molecules, 30(14), 2953. https://doi.org/10.3390/molecules30142953