Cascade Reactions of Indigo with an Allenylic Reactant
Abstract
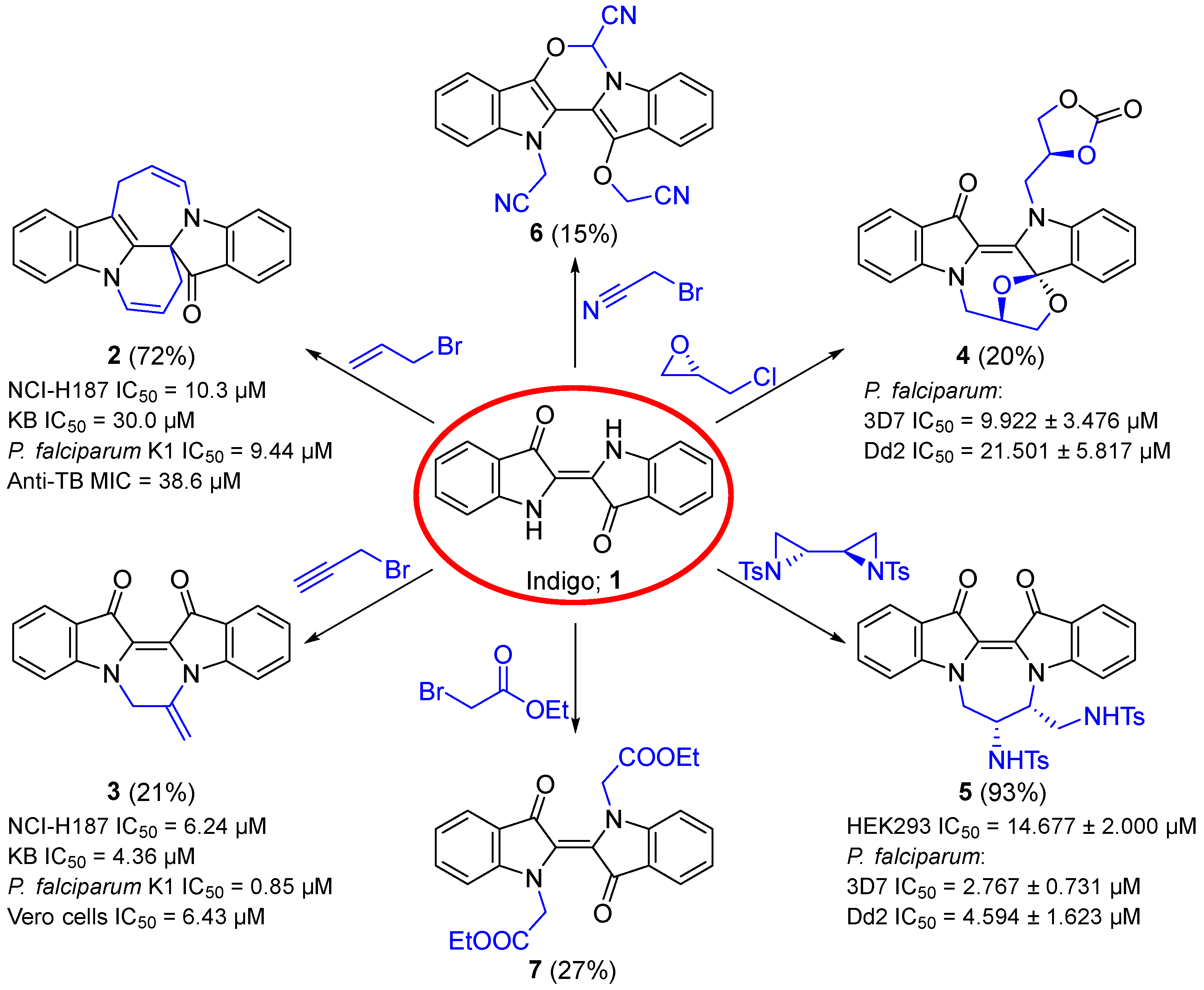
1. Introduction
2. Results and Discussion
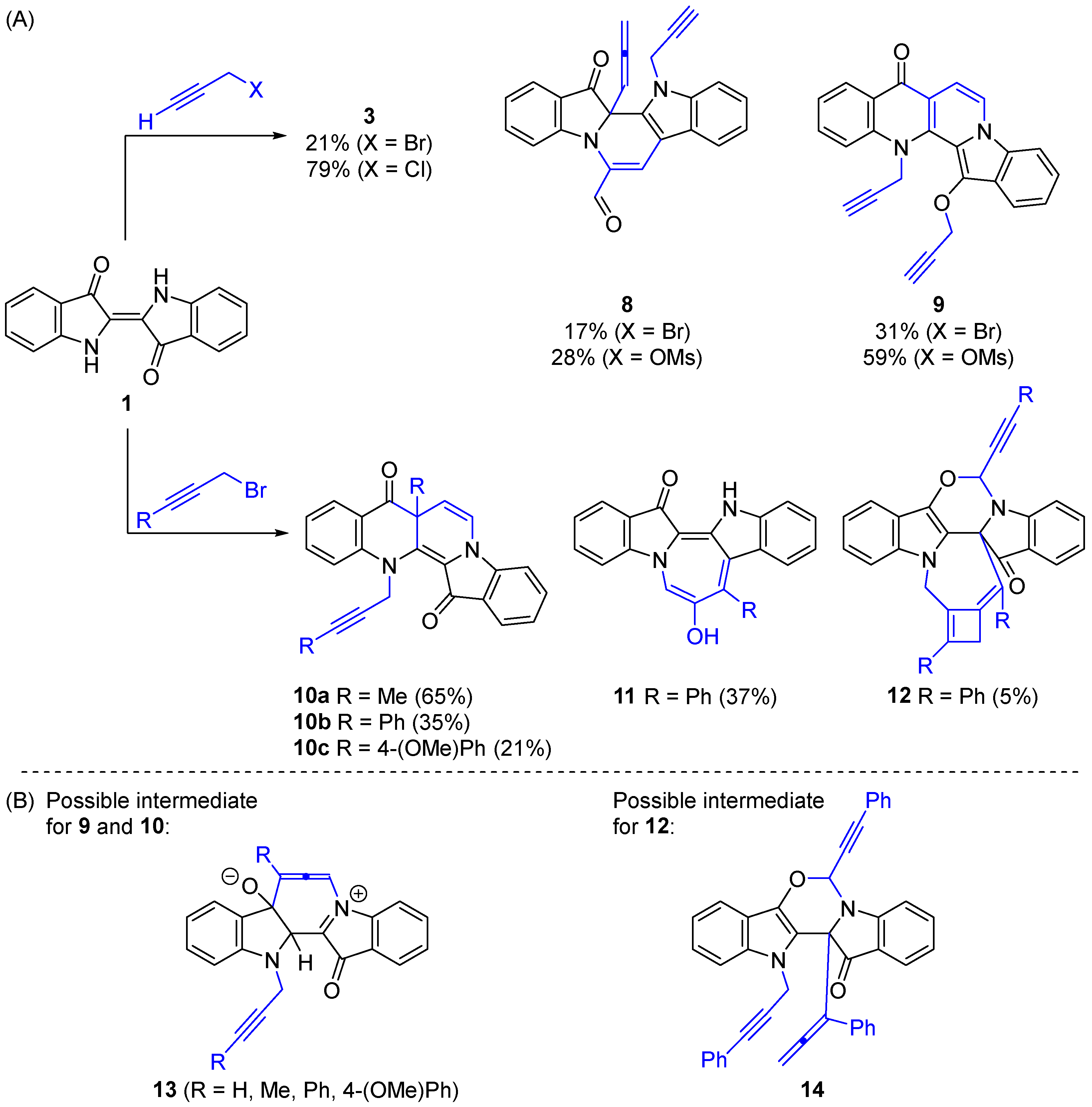
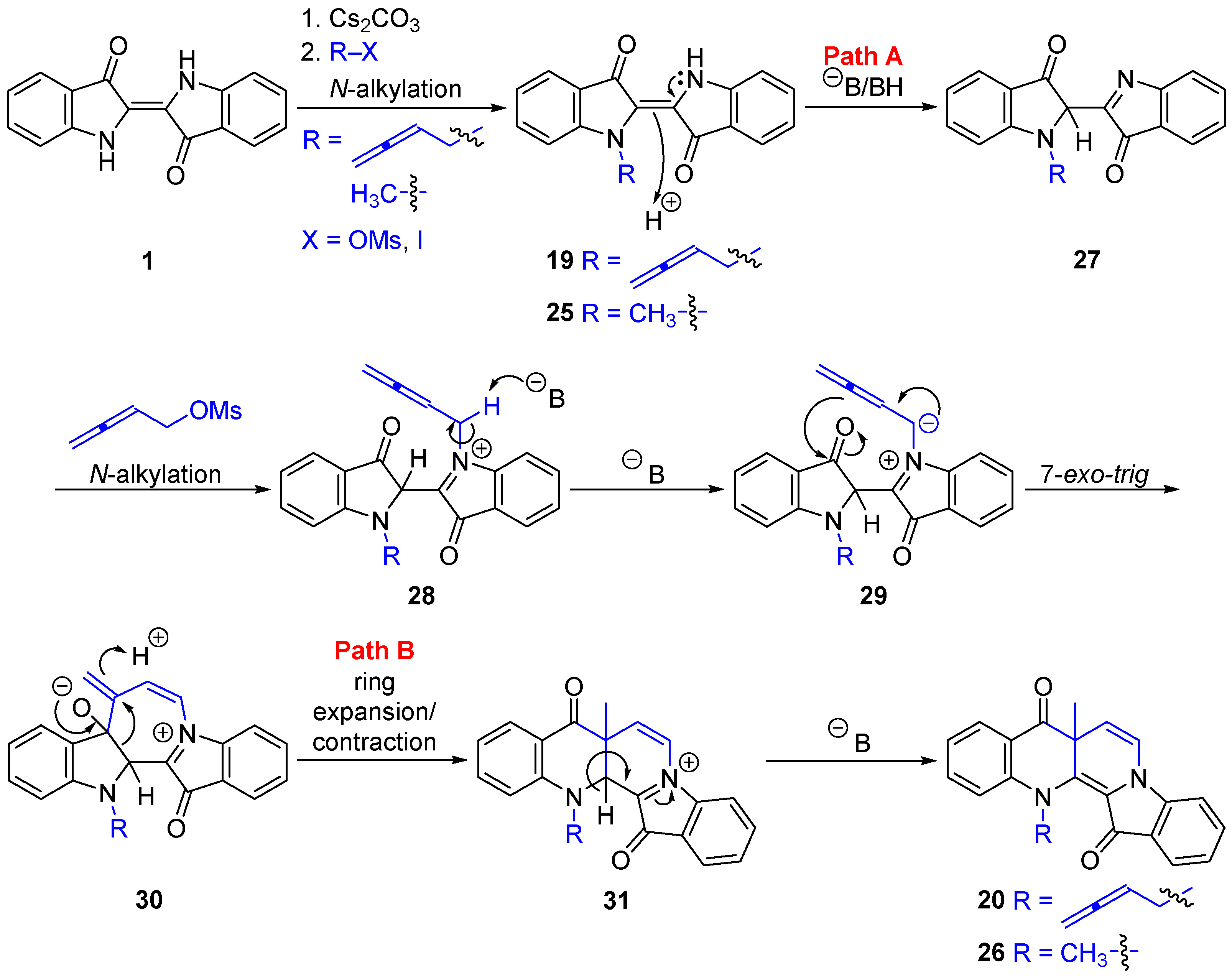
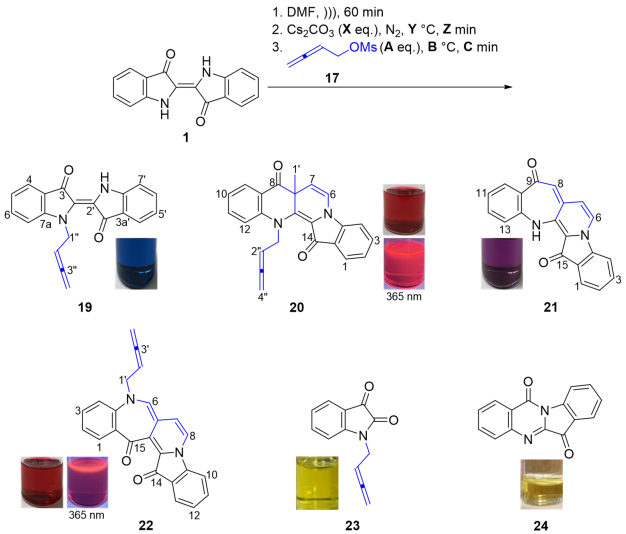
2.1. The Buta-2,3-dien-1-ylation of Indigo
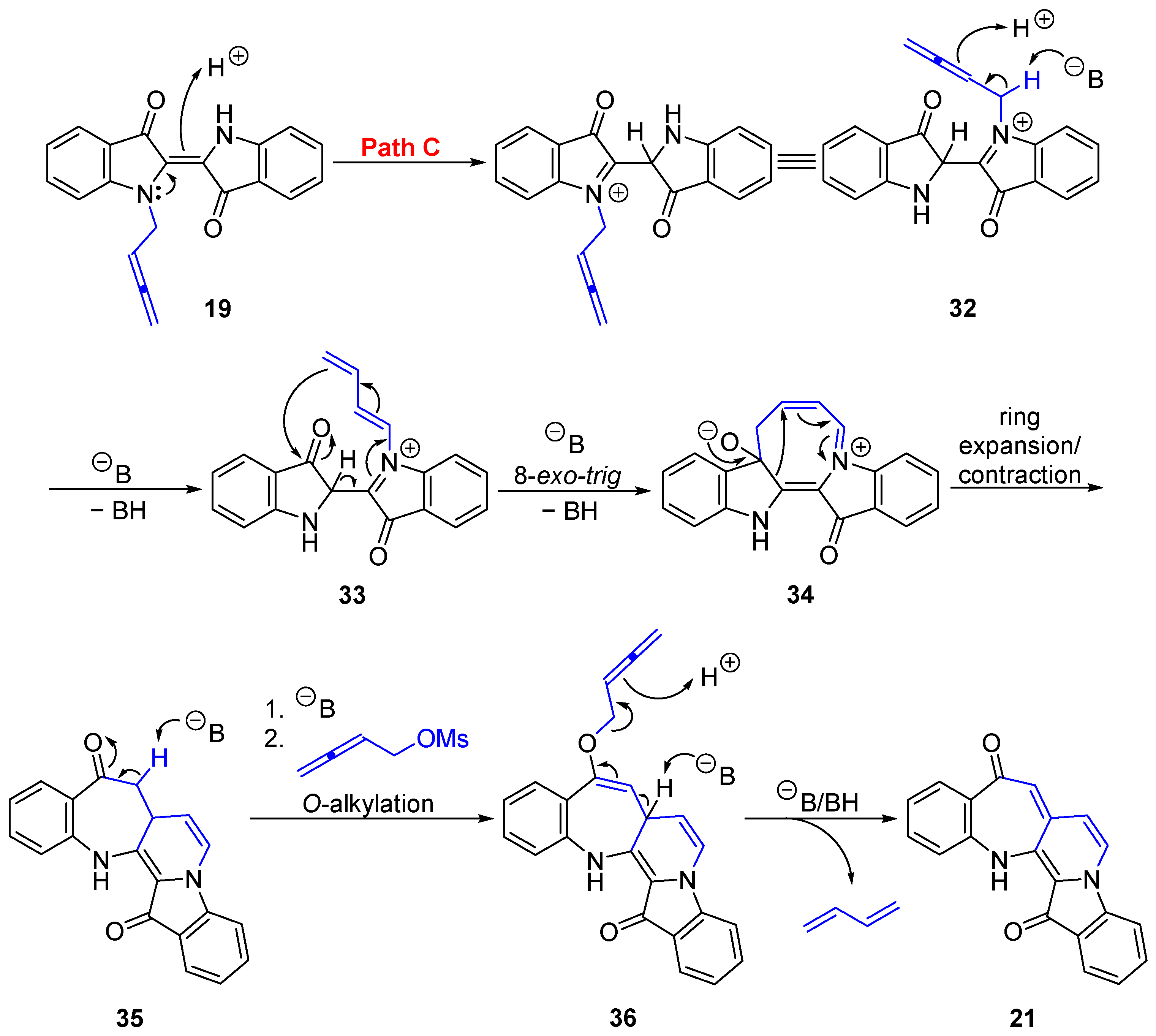
2.2. Mechanistic Proposals for the Indigo Cascade Reactions
3. Materials and Methods
3.1. Chemistry
3.2. Synthesis of the Electrophile
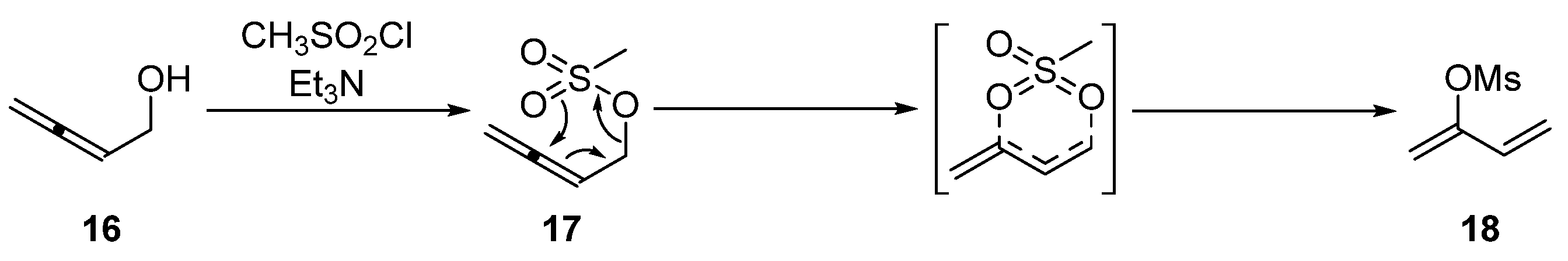
3.2.1. Synthesis of Buta-2,3-dien-1-ol 16

3.2.2. Synthesis of Buta-2,3-dien-1-yl Methanesulfonate 17
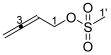
3.2.3. Synthesis of Buta-1,3-dien-2-yl Methanesulfonate 18
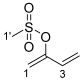
3.3. General Procedure for the Reaction of Indigo with Buta-2,3-dien-1-yl Methanesulfonate

- (E)-1-(Buta-2,3-dien-1-yl)-[2,2′-biindolinylidene]-3,3′-dione 19
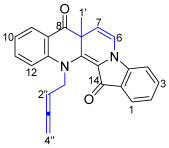
- 13-(Buta-2,3-dien-1-yl)-7a-methylbenzo[b]indolo[1,2-h][1,7]naphthyridine-8,14(7aH,13H)-dione 20
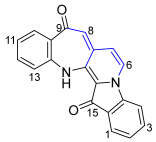
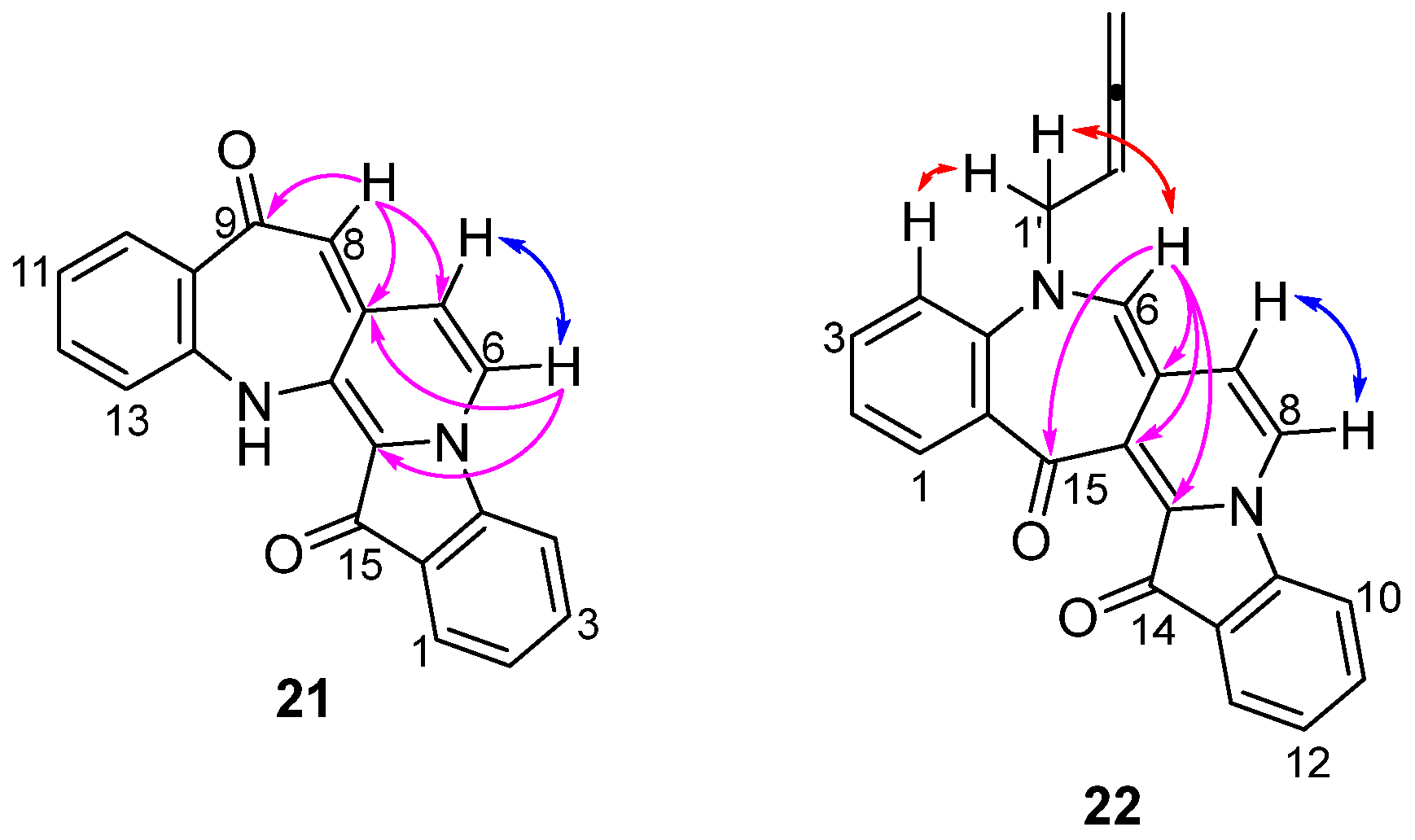
- 9H-Benzo[6′,7′]azepino[2′,3′:3,4]pyrido[1,2-a]indole-9,15(14H)-dione 21
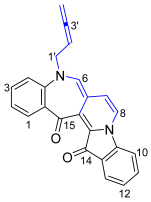
- 5-(Buta-2,3-dien-1-yl)-5H-benzo[6′,7′]azepino[4′,3′:3,4]pyrido[1,2-a]indole-14,15-dione 22

- 1-(Buta-2,3-dien-1-yl)indoline-2,3-dione 23: (a) structure; (b) X-ray structure

- Indolo[2,1-b]quinazoline-6,12-dione 24 (Tryptanthrin)
3.4. Preparation of N-Methylindigo

3.5. General Procedure for the Reaction of N-Methylindigo with Buta-2,3-dien-1-yl Methanesulfonate
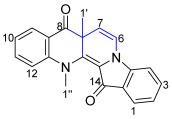
- 7a,13-Dimethylbenzo[b]indolo[1,2-h][1,7]naphthyridine-8,14(7aH,13H)-dione 26
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clark, R.J.H.; Cooksey, C.J.; Daniels, M.A.M.; Withnall, R. Indigo, woad, and Tyrian Purple: Important vat dyes from antiquity to the present. Endeavour 1993, 17, 191–199. [Google Scholar] [CrossRef]
- Stasiak, N.; Kukuła-Koch, W.; Głowniak, K. Modern industrial and pharmacological applications of indigo dye and its derivatives—A review. Acta Pol. Pharm. 2014, 71, 215–221. [Google Scholar] [PubMed]
- Huang, C.-Y.; Hecht, S. A blueprint for transforming indigos to photoresponsive molecular tools. Chem. Eur. J. 2023, 29, e202300981. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.; Seferoğlu, Z.; Berdnikova, D.V. Photochromic derivatives of indigo: Historical overview of development, challenges and applications. Beilstein J. Org. Chem. 2024, 20, 228–242. [Google Scholar] [CrossRef]
- Shakoori, A.; Bremner, J.B.; Willis, A.C.; Haritakun, R.; Keller, P.A. Rapid cascade synthesis of poly-heterocyclic architectures from indigo. J. Org. Chem. 2013, 78, 7639–7647. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Bonasera, A.; Hristov, L.; Garmshausen, Y.; Schmidt, B.M.; Jacquemin, D.; Hecht, S. N,N′-Disubstituted indigos as readily available red-light photoswitches with tunable thermal half-lives. J. Am. Chem. Soc. 2017, 139, 15205–15211. [Google Scholar] [CrossRef]
- Pinheiro, D.; Pineiro, M.; Galvão, A.M.; Seixas de Melo, J.S. Deep in blue with green chemistry: Influence of solvent and chain length on the behaviour of N- and N,N′-alkyl indigo derivatives. Chem. Sci. 2021, 12, 303–313. [Google Scholar] [CrossRef]
- Abdel-Hamid, M.K.; Bremner, J.B.; Coates, J.; Keller, P.A.; Miländer, C.; Torkamani, Y.S.; Skelton, B.W.; White, A.H.; Willis, A.C. Novel spiro and fused heterocycles from the allylation of indigo. Tetrahedron Lett. 2009, 50, 6947–6950. [Google Scholar] [CrossRef]
- Shakoori, A.; Bremner, J.B.; Abdel-Hamid, M.K.; Willis, A.C.; Haritakun, R.; Keller, P.A. Further exploration of the heterocyclic diversity accessible from the allylation chemistry of indigo. Beilstein J. Org. Chem. 2015, 11, 481–492. [Google Scholar] [CrossRef]
- McCosker, P.M.; Butler, N.M.; Shakoori, A.; Volland, M.K.; Perry, M.J.; Mullen, J.W.; Willis, A.C.; Clark, T.; Bremner, J.B.; Guldi, D.M.; et al. The cascade reactions of indigo with propargyl substrates for heterocyclic and photophysical diversity. Chem. Eur. J. 2021, 27, 3708–3721. [Google Scholar] [CrossRef]
- Butler, N.M.; Hendra, R.; Bremner, J.B.; Willis, A.C.; Lucantoni, L.; Avery, V.M.; Keller, P.A. Cascade reactions of indigo with oxiranes and aziridines: Efficient access to dihydropyrazinodiindoles and spiro-oxazocinodiindoles. Org. Biomol. Chem. 2018, 16, 6006–6016. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; McCosker, P.M.; Willis, A.C.; Pyne, S.G.; Richardson, C.; Bremner, J.B.; Keller, P.A. Mapping of some further alkylation-initiated pathways to polyheterocyclic compounds from indigo and indirubin. Molecules 2024, 29, 4242. [Google Scholar] [CrossRef] [PubMed]
- Kanda, J.; Egami, N.; Sasamori, T.; Imayoshi, A.; Hosoya, T.; Tsubaki, K. Synthesis of bridged indigos and their thermoisomerization and photoisomerization behaviors. J. Org. Chem. 2021, 86, 17620–17628. [Google Scholar] [CrossRef]
- Yu, S.; Ma, S. Allenes in catalytic asymmetric synthesis and natural product syntheses. Angew. Chem. Int. Ed. 2012, 51, 3074–3112. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Z.; Shan, Y.; Ma, Y.; Li, T.; Yuan, C.; Guo, H.; Mao, B. Palladium-catalyzed asymmetric [4 + 3] cycloaddition of methylene-trimethylenemethane: Access to seven-membered exocyclic axially chiral allenes. Chem. Sci. 2024, 15, 9703–9708. [Google Scholar] [CrossRef]
- Ma, S. Some typical advances in the synthetic applications of allenes. Chem. Rev. 2005, 105, 2829–2872. [Google Scholar] [CrossRef]
- Ma, S. Electrophilic addition and cyclization reactions of allenes. Acc. Chem. Res. 2009, 42, 1679–1688. [Google Scholar] [CrossRef]
- Sikandar, S.; Zahoor, A.F.; Ghaffar, A.; Anjum, M.N.; Noreen, R.; Irfan, A.; Munir, B.; Kotwica-Mojzych, K.; Mojzych, M. Unveiling the chemistry and synthetic potential of catalytic cycloaddition reaction of allenes: A review. Molecules 2023, 28, 704. [Google Scholar] [CrossRef]
- Chan, W.-L.; Tang, X.; Zhang, F.; Quek, G.; Mei, G.-J.; Lu, Y. Phosphine-Catalyzed (3+2) Annulation of isoindigos with allenes: Enantioselective formation of two vicinal quaternary stereogenic centers. Angew. Chem. Int. Ed. 2019, 58, 6260–6264. [Google Scholar] [CrossRef]
- Osano, M.; Jhaveri, D.P.; Wipf, P. Formation of 6-azaindoles by intramolecular Diels–Alder reaction of oxazoles and total synthesis of marinoquinoline A. Org. Lett. 2020, 22, 2215–2219. [Google Scholar] [CrossRef]
- Doutheau, A.; Gore, J.; Malacria, M. Preparation et epoxydation de trienes-1,2,4 ynes-6 (allenenynes). Tetrahedron 1977, 33, 2393–2398. [Google Scholar] [CrossRef]
- Alcaide, B.; Almendros, P.; Aragoncillo, C.; Redondo, M.C. New domino transposition/intramolecular Diels–Alder reaction in monocyclic allenols: A general strategy for tricyclic compounds. Chem. Commun. 2002, 1472–1473. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M.; Jonasson, C.; Wuchrer, M. Atom economy: Aldol-type products by vanadium-catalyzed additions of allenic alcohols and aldehydes. J. Am. Chem. Soc. 2001, 123, 12736–12737. [Google Scholar] [CrossRef]
- Alonso, J.M.; Almendros, P. Deciphering the chameleonic chemistry of allenols: Breaking the taboo of a onetime esoteric functionality. Chem. Rev. 2021, 121, 4193–4252. [Google Scholar] [CrossRef]
- Ogasawara, M.; Ge, Y.; Uetake, K.; Takahashi, T. Vinyl ketones to allenes: Preparation of 1,3-dien-2-yl triflates and their application in Pd-catalyzed reactions with soft nucleophiles. Org. Lett. 2005, 7, 5697–5700. [Google Scholar] [CrossRef]
- McCammant, M.S.; Shigeta, T.; Sigman, M.S. Palladium-catalyzed 1,3-difunctionalization using terminal alkenes with alkenyl nonaflates and aryl boronic acids. Org. Lett. 2016, 18, 1792–1795. [Google Scholar] [CrossRef]
- Zha, T.; Rui, J.; Zhang, Z.; Zhang, D.; Yang, Z.; Yu, P.; Wang, Y.; Peng, F.; Shao, Z. Direct catalytic asymmetric and regiodivergent N1- and C3-allenylic alkylation of indoles. Angew. Chem. Int. Ed. 2023, 62, e202300844. [Google Scholar] [CrossRef]
- Brandão, P.; Pinheiro, D.; Sérgio Seixas de Melo, J.; Pineiro, M. I2/NaH/DMF as oxidant trio for the synthesis of tryptanthrin from indigo or isatin. Dyes. Pigments. 2020, 173, 107935. [Google Scholar] [CrossRef]
- Pinheiro, D.; Pineiro, M.; Pina, J.; Brandão, P.; Galvão, A.M.; Seixas de Melo, J.S. Tryptanthrin from indigo: Synthesis, excited state deactivation routes and efficient singlet oxygen sensitization. Dyes. Pigments. 2020, 175, 108125. [Google Scholar] [CrossRef]
- Gandra, N.; Frank, A.T.; Le Gendre, O.; Sawwan, N.; Aebisher, D.; Liebman, J.F.; Houk, K.N.; Greer, A.; Gao, R. Possible singlet oxygen generation from the photolysis of indigo dyes in methanol, DMSO, water, and ionic liquid, 1-butyl-3-methylimidazolium tetrafluoroborate. Tetrahedron 2006, 62, 10771–10776. [Google Scholar] [CrossRef]
- Solís Correa, H.; Ortiz, E.; Uc, V.H.; Barceló Quintal, I.D.; Hernández Avila, J.L. Indigo stability: An ab initio study. Mol. Simul. 2011, 37, 1085–1090. [Google Scholar] [CrossRef]
- Iuga, C.; Ortíz, E.; Alvarez-Idaboy, J.R.; Vivier-Bunge, A. Molecular description of indigo oxidation mechanisms initiated by OH and OOH radicals. J. Phys. Chem. A. 2012, 116, 3643–3651. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, S.; Bureš, F.; Lee, R.; Ye, X.; Jiang, Z. Visible light photocatalytic aerobic oxygenation of indoles and pH as a chemoselective switch. ACS Catal. 2016, 6, 6853–6860. [Google Scholar] [CrossRef]
- Pattarawarapan, M.; Wiriya, N.; Hongsibsong, S.; Phakhodee, W. Divergent synthesis of methylisatoid and tryptanthrin derivatives by Ph3P–I2-mediated reaction of isatins with and without alcohols. J. Org. Chem. 2020, 85, 15743–15751. [Google Scholar] [CrossRef]
- Pummerer, R.; Meininger, F. Über eine neue methode zur darstellung von N,N′-alkylierten indigofarbstoffen mittels technischer indigosole 4. Mitteilung über indigofarbstoffe. Justus Liebigs Ann. Chem. 1954, 590, 173–194. [Google Scholar] [CrossRef]



 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Entry | Reaction Conditions | Compounds Yield (%) | ||||||||||
| X eq. | Y °C | Z min | A eq. | B °C | C min | 19 | 20 | 21 | 22 | 23 | 24 | |
| 1 | 2 | 85–87 | 60 | 5 | 85–87 | 7 | 16 | 3 | 6 | <1 | – | – |
| 2 | 2 | 85–87 | 60 | 5 | 85–87 | 1 | 2 | – | – | – | 24 | 15 |
| 3 | 4 | 85–87 | 60 | 5 | 85–87 | 1 | 1 | – | – | – | – | 4 |
| 4 | 4 | 85–87 | 60 | 5 | 85–87 | 7 | 16 | 4 | 6 | <1 | <1 | – |
| 5 a | 4 | 90 | 60 | 8 | 85–87 | 15 | 2 | 3 | 6 | 3 | 2 | 4 |
| 6 | 4 | 90 | 60 | 5 | 70 | 55 | <1 | – | 4 | – | – | – |
| 7 | 4 | 90 | 90 | 5 | 75 | 10 | 4 | 9 | 5 | – | – | – |
| 8 | 4 | 90 | 90 | 5 | 75 | 7 | 9 | 3 | 3 | – | – | – |
| 9 | 4 | 90 | 90 | 5 | 80 | 7 | 6 | 3 | 8 | – | – | – |
| 10 b | 1.2 | 85 | 30 | 2.4 | 85 | 7 | 8 | 1 | 4 | – | – | – |
 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Entry | Reactions Conditions | Compound Yield (%) | ||||||||
| Solvent | Base | X eq. | Y °C | Z min | A eq. | B °C | C | 25 | 26 * | |
| 1 | DMF | Cs2CO3 | 2 | 85–87 | 30 | 3 | 85–87 | 7 min | 58 | 8 |
| 2 a | DMF | Cs2CO3 | 2 | 85–87 | 30 | 5 | 75–80 | 24 h | 41 | 4 |
| 3 b | DMF | Cs2CO3 | 1 | 85–87 | 60 | 7 | 85–87 | 75 min | 24 | 3 |
| 4 c | DMF | Cs2CO3 | 1 | 85–87 | 60 | 7 | 85–87 | 90 min | NP | NP |
| 5 d | THF | Cs2CO3 | 1 | 65–68 | 60 | 8 | 65–68 | 21 h | NP | NP |
| 6 e | DMF | Cs2CO3 | 2 | 85–87 | 30 | 6 | 85–87 | 40 min | NP | NP |
| 7 f | THF | KHMDS | 2 | 65–68 | 30 | 6 | 65–68 | 40 min | NP | NP |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahayu, D.U.C.; Richardson, C.; Bremner, J.B.; Keller, P.A. Cascade Reactions of Indigo with an Allenylic Reactant. Molecules 2025, 30, 2899. https://doi.org/10.3390/molecules30142899
Rahayu DUC, Richardson C, Bremner JB, Keller PA. Cascade Reactions of Indigo with an Allenylic Reactant. Molecules. 2025; 30(14):2899. https://doi.org/10.3390/molecules30142899
Chicago/Turabian StyleRahayu, Dyah U. C., Christopher Richardson, John B. Bremner, and Paul A. Keller. 2025. "Cascade Reactions of Indigo with an Allenylic Reactant" Molecules 30, no. 14: 2899. https://doi.org/10.3390/molecules30142899
APA StyleRahayu, D. U. C., Richardson, C., Bremner, J. B., & Keller, P. A. (2025). Cascade Reactions of Indigo with an Allenylic Reactant. Molecules, 30(14), 2899. https://doi.org/10.3390/molecules30142899










