Urolithin A Attenuates Periodontitis in Mice via Dual Anti-Inflammatory and Osteoclastogenesis Inhibition: A Natural Metabolite-Based Therapeutic Strategy
Abstract
1. Introduction
2. Results
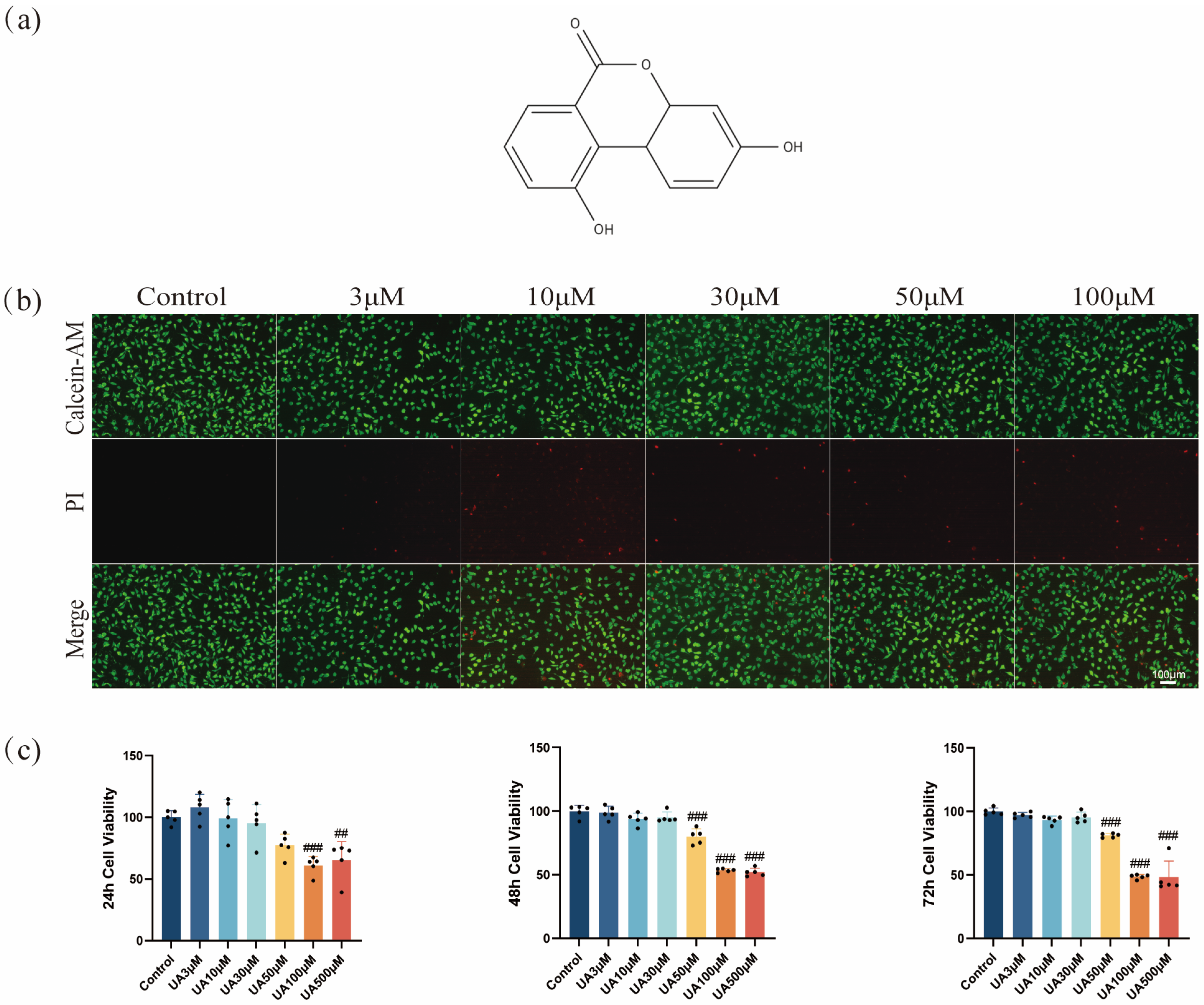
2.1. Effects of UA on L929 Viability
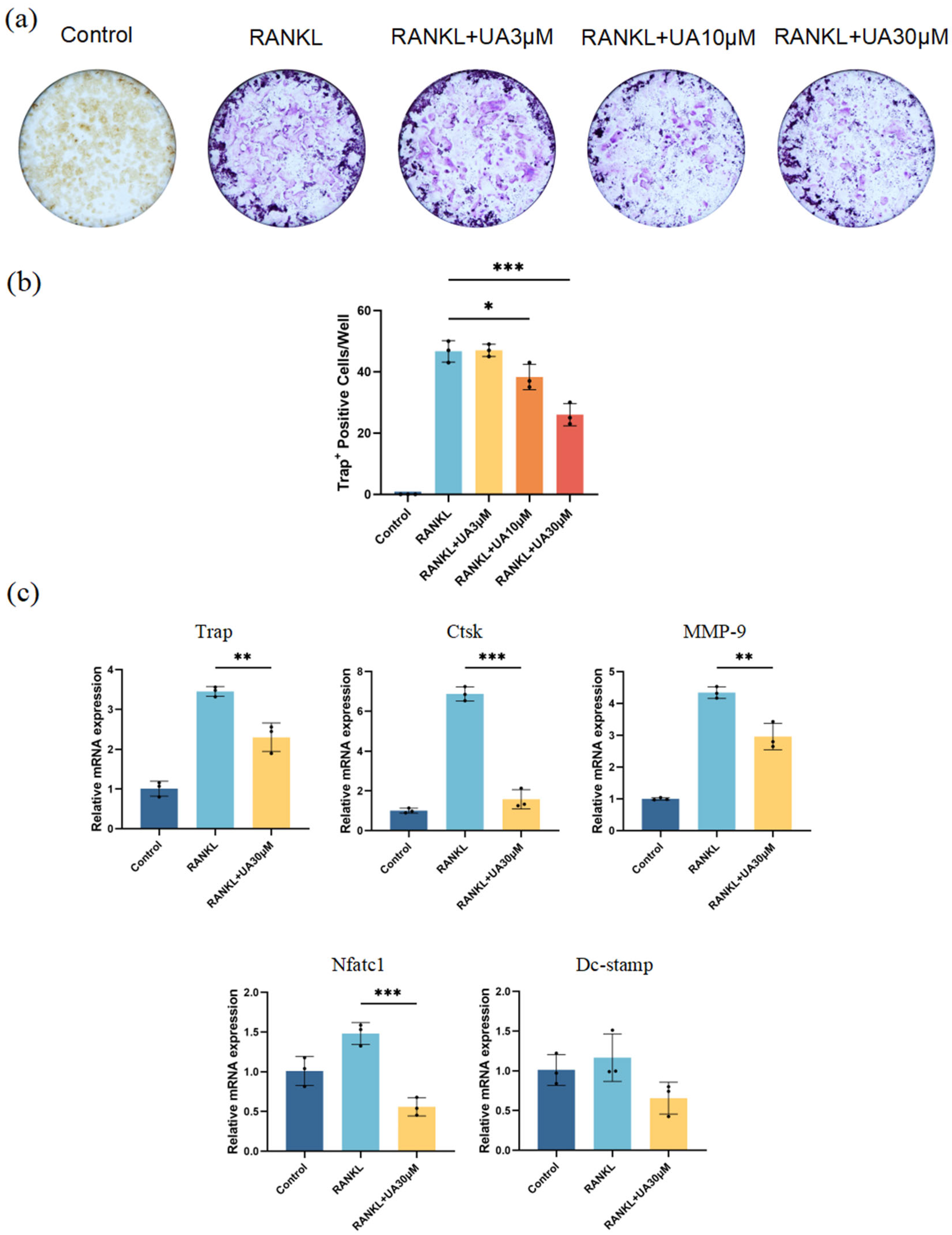
2.2. Effect of UA on Inflammation in a Periodontitis Model
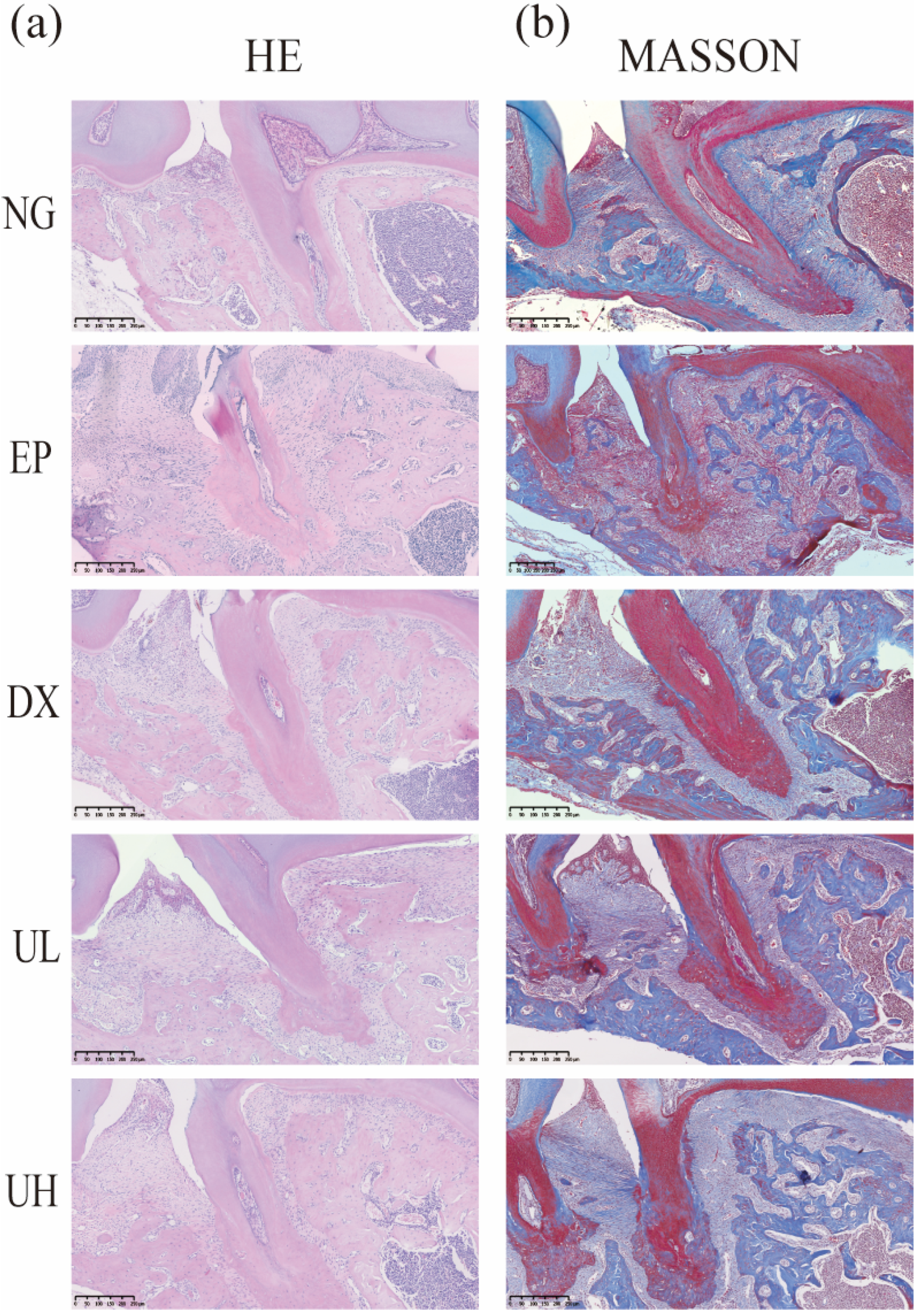
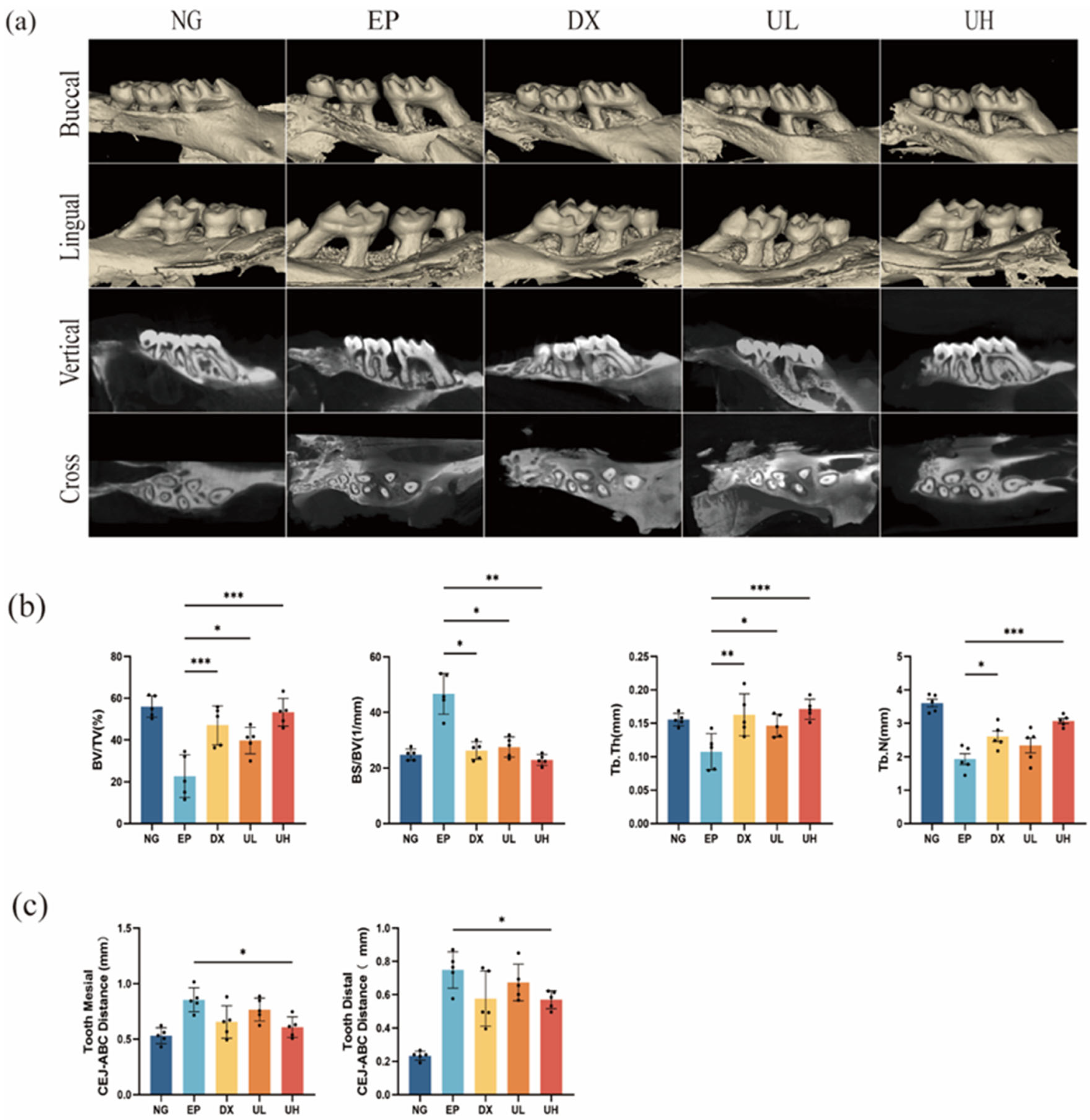
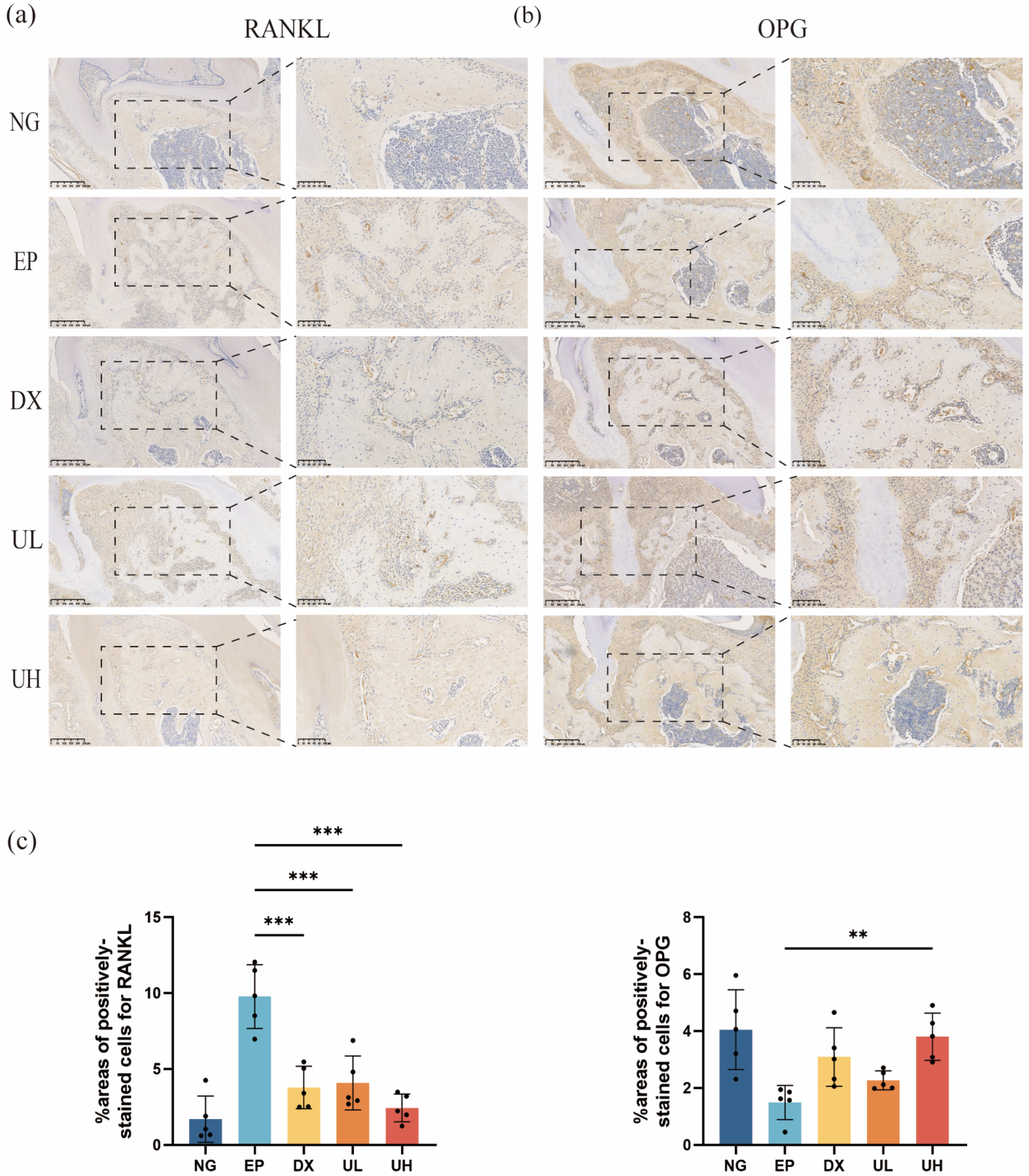
2.3. Effect of UA on Alveolar Bone Resorption in a Periodontitis Model
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Calcein-AM/Propidium Iodide(PI) Staining
4.5. Measurement of TNF-α and IL-6 Release by Enzyme-Linked Immunosorbent Assay (ELISA)
4.6. Osteoclast Differentiation
4.7. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.8. Animals
4.9. Micro-Computed Tomography
4.10. Haematoxylin and Eosin, Masson and Tartrate-Resistant Acid Phosphatase Staining
4.11. Immunohistochemistry
4.12. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| UA | Urolithin A |
| CCK-8 | Cell counting kit-8 |
| PBS | Phosphate buffered saline |
| IL-6 | Interleukin-6 |
| TNF-α | Tumor necrosis factor-α |
| IL-1β | Interleukin-1β |
| L929 | Mouse fibroblasts |
| RAW264.7 | Mouse macrophages |
| LPS | lipopolysaccharid |
| DMEM | Dulbecco’s modified eagle |
| ELISA | Enzyme linked immunosorbent assay |
| qRT-PCR | Quantitative real-time polymerase chain reaction |
| TRAP | Tartrate-resistant acid phosphatase stain |
| NFATc1 | nuclear factor of activated T cells 1 |
| Ctsk | cathepsin K |
| MMP-9 | matrix metalloproteinase-9 |
| DC-Stamp | dendritic cell-specific transmembrane protein |
| HE | Hematoxylin and eosin staining |
| MASSON | Masson’ trichrome stain |
| RANKL | Receptor activator of nuclear factor-κB ligand |
| OPG | osteoprotegerin |
| Micro-CT | Micro computed tomography |
| BV/TV | Bone volume/total volume |
| BS/BV | Bone surface area to bone volume ratio |
| Tb.N | Trabecular number |
| Tb.Th | Trabecular thickness |
| CEJ-ABC | Cemento-enamel junction to alveo bone crest |
References
- Kwon, T.; Lamster, I.B.; Levin, L. Current Concepts in the Management of Periodontitis. Int. Dent. J. 2021, 71, 462–476. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.X.; Zhong, Y.J.; Dong, Q.Q.; Wong, H.M.; Wen, Y.F. Global, Regional, and National Burden of Severe Periodontitis, 1990–2019: An Analysis of the Global Burden of Disease Study 2019. J. Clin. Periodontol. 2021, 48, 1165–1188. [Google Scholar] [CrossRef]
- Cobb, C.M. Lasers in Periodontics: A Review of the Literature. J. Periodontol. 2006, 77, 545–564. [Google Scholar] [CrossRef] [PubMed]
- Sethiya, K.R.; Dhadse, P.; Bajaj, P.; Durge, K.; Subhadarsanee, C.; Hassan, S. Platelet Rich Fibrin in Combination with Bioabsorbable Guided Tissue Regeneration (GTR) Membrane and GTR Membrane Alone Using Double Lateral Sliding Bridge Flap for Treatment of Multiple Gingival Recession Defects in Humans: A Randomized Controlled Clinical Trail. J. Indian Soc. Periodontol. 2022, 26, 245–253. [Google Scholar]
- de Molon, R.S.; Rodrigues, J.V.S.; Deroide, M.B.; da Silva Barbirato, D.; Garcia, V.G.; Theodoro, L.H. The Efficacy of Topical or Systemic Antibiotics as Adjuvants to Non-Surgical Periodontal Treatment in Diabetic Patients: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J. Clin. Med. 2024, 13, 4763. [Google Scholar] [CrossRef] [PubMed]
- Fayazi, M.; Rostami, M.; Amiri Moghaddam, M.; Nasiri, K.; Tadayonfard, A.; Roudsari, M.B.; Ahmad, H.M.; Parhizgar, Z.; Majbouri Yazdi, A. A State-of-the-Art Review of the Recent Advances in Drug Delivery Systems for Different Therapeutic Agents in Periodontitis. J. Drug Target. 2025, 33, 612–647. [Google Scholar] [CrossRef]
- Lecio, G.; Ribeiro, F.V.; Pimentel, S.P.; Reis, A.A.; da Silva, R.V.C.; Nociti, F., Jr.; Moura, L.; Duek, E.; Casati, M.; Casarin, R.C.V. Novel 20% Doxycycline-Loaded PLGA Nanospheres as Adjunctive Therapy in Chronic Periodontitis in Type-2 Diabetics: Randomized Clinical, Immune and Microbiological Trial. Clin. Oral Investig. 2020, 24, 1269–1279. [Google Scholar] [CrossRef]
- Buset, S.L.; Zitzmann, N.U.; Weiger, R.; Walter, C. Non-Surgical Periodontal Therapy Supplemented with Systemically Administered Azithromycin: A Systematic Review of RCTs. Clin. Oral Investig. 2015, 19, 1763–1775. [Google Scholar] [CrossRef]
- Mugri, M.H. Efficacy of Systemic Amoxicillin–Metronidazole in Periodontitis Patients with Diabetes Mellitus: A Systematic Review of Randomized Clinical Trials. Medicina 2022, 58, 1605. [Google Scholar] [CrossRef]
- Teughels, W.; Feres, M.; Oud, V.; Martín, C.; Matesanz, P.; Herrera, D. Adjunctive Effect of Systemic Antimicrobials in Periodontitis Therapy: A Systematic Review and Meta-analysis. J. Clin. Periodontol. 2020, 47, 257–281. [Google Scholar] [CrossRef]
- Feres, M.; Haffajee, A.D.; Allard, K.; Som, S.; Goodson, J.M.; Socransky, S.S. Antibiotic Resistance of Subgingival Species during and after Antibiotic Therapy. J. Clin. Periodontol. 2002, 29, 724–735. [Google Scholar] [CrossRef] [PubMed]
- Zaura, E.; Brandt, B.W.; Teixeira de Mattos, M.J.; Buijs, M.J.; Caspers, M.P.M.; Rashid, M.-U.; Weintraub, A.; Nord, C.E.; Savell, A.; Hu, Y.; et al. Same Exposure but Two Radically Different Responses to Antibiotics: Resilience of the Salivary Microbiome versus Long-Term Microbial Shifts in Feces. mBio 2015, 6, e01693-15. [Google Scholar] [CrossRef] [PubMed]
- van Winkelhoff, A.J.; Herrera, D.; Oteo, A.; Sanz, M. Antimicrobial Profiles of Periodontal Pathogens Isolated from Periodontitis Patients in The Netherlands and Spain. J. Clin. Periodontol. 2005, 32, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Selma, M.V.; Beltrán, D.; García-Villalba, R.; Espín, J.C.; Tomás-Barberán, F.A. Description of Urolithin Production Capacity from Ellagic Acid of Two Human Intestinal Gordonibacter Species. Food Funct. 2014, 5, 1779–1784. [Google Scholar] [CrossRef]
- Heilman, J.; Andreux, P.; Tran, N.; Rinsch, C.; Blanco-Bose, W. Safety Assessment of Urolithin A, a Metabolite Produced by the Human Gut Microbiota upon Dietary Intake of Plant Derived Ellagitannins and Ellagic Acid. Food Chem. Toxicol. 2017, 108, 289–297. [Google Scholar] [CrossRef]
- Andreux, P.A.; Blanco-Bose, W.; Ryu, D.; Burdet, F.; Ibberson, M.; Aebischer, P.; Auwerx, J.; Singh, A.; Rinsch, C. The Mitophagy Activator Urolithin A Is Safe and Induces a Molecular Signature of Improved Mitochondrial and Cellular Health in Humans. Nat. Metab. 2019, 1, 595–603. [Google Scholar] [CrossRef]
- Singh, R.; Chandrashekharappa, S.; Bodduluri, S.R.; Baby, B.V.; Hegde, B.; Kotla, N.G.; Hiwale, A.A.; Saiyed, T.; Patel, P.; Vijay-Kumar, M.; et al. Enhancement of the Gut Barrier Integrity by a Microbial Metabolite through the Nrf2 Pathway. Nat. Commun. 2019, 10, 89. [Google Scholar] [CrossRef]
- Giménez-Bastida, J.A.; González-Sarrías, A.; Larrosa, M.; Tomás-Barberán, F.; Espín, J.C.; García-Conesa, M.-T. Ellagitannin Metabolites, Urolithin A Glucuronide and Its Aglycone Urolithin A, Ameliorate TNF-α-Induced Inflammation and Associated Molecular Markers in Human Aortic Endothelial Cells. Mol. Nutr. Food Res. 2012, 56, 784–796. [Google Scholar] [CrossRef]
- Totiger, T.M.; Srinivasan, S.; Jala, V.R.; Lamichhane, P.; Dosch, A.R.; Gaidarski, A.A.; Joshi, C.; Rangappa, S.; Castellanos, J.; Vemula, P.K.; et al. Urolithin A, a Novel Natural Compound to Target PI3K/AKT/mTOR Pathway in Pancreatic Cancer. Mol. Cancer Ther. 2019, 18, 301–311. [Google Scholar] [CrossRef]
- Remadevi, V.; Jaikumar, V.S.; Vini, R.; Krishnendhu, B.; Azeez, J.M.; Sundaram, S.; Sreeja, S. Urolithin A, Induces Apoptosis and Autophagy Crosstalk in Oral Squamous Cell Carcinoma via mTOR /AKT/ERK1/2 Pathway. Phytomedicine 2024, 130, 155721. [Google Scholar] [CrossRef]
- Ryu, D.; Mouchiroud, L.; Andreux, P.A.; Katsyuba, E.; Moullan, N.; Nicolet-dit-Félix, A.A.; Williams, E.G.; Jha, P.; Sasso, G.L.; Huzard, D.; et al. Urolithin A Induces Mitophagy and Prolongs Lifespan in C. Elegans and Increases Muscle Func-tion in Rodents. Nat. Med. 2016, 22, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Nacarelli, T.; Lau, L.; Fukumoto, T.; Zundell, J.; Fatkhutdinov, N.; Wu, S.; Aird, K.M.; Iwasaki, O.; Kossenkov, A.V.; Schultz, D.; et al. NAD+ Metabolism Governs the Proin-flammatory Senescence-Associated Secretome. Nat. Cell Biol. 2019, 21, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Luan, P.; D’Amico, D.; Andreux, P.A.; Laurila, P.-P.; Wohlwend, M.; Li, H.; Imamura de Lima, T.; Place, N.; Rinsch, C.; Zanou, N.; et al. Urolithin A Improves Muscle Function by Inducing Mitophagy in Muscular Dystrophy. Sci. Transl. Med. 2021, 13, eabb0319. [Google Scholar] [CrossRef]
- Cinat, D.; Souza, A.L.D.; Soto-Gamez, A.; Jellema-de Bruin, A.L.; Coppes, R.P.; Barazzuol, L. Mitophagy Induction Improves Salivary Gland Stem/Progenitor Cell Function by Reducing Senescence after Irradiation. Radiother. Oncol. 2024, 190, 110028. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Li, J.; Jiang, L.; Li, H.; Lei, L. PINK1-Mediated Mitophagy Reduced Inflam-matory Responses to Porphyromonas Gingivalis in Macrophages. Oral Dis. 2023, 29, 3665–3676. [Google Scholar] [CrossRef]
- Lin, X.; Yuan, G.; Li, Z.; Zhou, M.; Hu, X.; Song, F.; Shao, S.; Fu, F.; Zhao, J.; Xu, J.; et al. Ellagic Acid Protects Ovariectomy-Induced Bone Loss in Mice by Inhibiting Osteoclast Differentiation and Bone Resorption. J. Cell Physiol. 2020, 235, 5951–5961. [Google Scholar] [CrossRef]
- Savi, M.; Bocchi, L.; Mena, P.; Dall’Asta, M.; Crozier, A.; Brighenti, F.; Del Rio, D. In Vivo Administration of Urolithin A and B Prevents the Occurrence of Cardiac Dysfunction in Streptozotocin-Induced Diabetic Rats. Cardiovasc. Diabetol. 2017, 16, 80. [Google Scholar] [CrossRef]
- Cui, G.-H.; Chen, W.-Q.; Shen, Z.-Y. Urolithin A Shows Anti-Atherosclerotic Activity via Activation of Class B Scavenger Receptor and Activation of Nef2 Signaling Pathway. Pharmacol. Rep. 2018, 70, 519–524. [Google Scholar] [CrossRef]
- Kim, K.B.; Lee, S.; Kim, J.H. Neuroprotective Effects of Urolithin A on H2O2-Induced Oxidative Stress-Mediated Apoptosis in SK-N-MC Cells. Nutr. Res. Pract. 2020, 14, 3. [Google Scholar] [CrossRef]
- Gong, Z.; Huang, J.; Xu, B.; Ou, Z.; Zhang, L.; Lin, X.; Ye, X.; Kong, X.; Long, D.; Sun, X.; et al. Urolithin A Attenuates Memory Impairment and Neuroinflammation in APP/PS1 Mice. J. Neuroinflamm. 2019, 16, 62. [Google Scholar] [CrossRef]
- Tuohetaerbaike, B.; Zhang, Y.; Tian, Y.; Zhang, N.N.; Kang, J.; Mao, X.; Zhang, Y.; Li, X. Pancreas Protective Effects of Urolithin A on Type 2 Diabetic Mice Induced by High Fat and Streptozotocin via Regulating Autophagy and AKT/mTOR Signaling Pathway. J. Ethnopharmacol. 2020, 250, 112479. [Google Scholar] [CrossRef] [PubMed]
- Toney, A.M.; Fan, R.; Xian, Y.; Chaidez, V.; Ramer-Tait, A.E.; Chung, S. Urolithin A, a Gut Metabolite, Improves Insulin Sensitivity Through Augmentation of Mitochondrial Function and Biogenesis. Obesity 2019, 27, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Z.; Gao, L.; Qi, Y.; Zhu, H.; Qin, X. The Regulation Effect of AMPK in Immune Related Diseases. Sci. China Life Sci. 2018, 61, 523–533. [Google Scholar] [CrossRef]
- Urban, J.F.; Schopf, L.; Morris, S.C.; Orekhova, T.; Madden, K.B.; Betts, C.J.; Gamble, H.R.; Byrd, C.; Donaldson, D.; Else, K.; et al. Stat6 Signaling Promotes Protective Immunity against Trichinella Spiralis through a Mast Cell- and T Cell-Dependent Mechanism. J. Immunol. 2000, 164, 2046–2052. [Google Scholar] [CrossRef] [PubMed]
- Kang, I.; Kim, Y.; Tomás-Barberán, F.A.; Espín, J.C.; Chung, S. Urolithin A, C, and D, but Not Iso-Urolithin A and Urolithin B, Attenuate Triglyceride Accumulation in Human Cultures of Adipocytes and Hepatocytes. Mol. Nutr. Food Res. 2016, 60, 1129–1138. [Google Scholar] [CrossRef]
- Jian, M.; Kwan, J.S.-C.; Bunting, M.; Ng, R.C.-L.; Chan, K.H. Adiponectin Suppresses Amyloid-β Oligomer (AβO)-Induced Inflammatory Response of Microglia via Adi-poR1-AMPK-NF-κB Signaling Pathway. J. Neuroinflamm. 2019, 16, 110. [Google Scholar] [CrossRef]
- Liu, J.; Ma, L.; Dong, W.; Du, G.; Dang, X. Effect of Urolithin A on Bone Repair in Mice with Bone Defects. Tissue Eng. Regen. Med. 2022, 19, 151–159. [Google Scholar] [CrossRef]
- Fu, X.; Gong, L.-F.; Wu, Y.-F.; Lin, Z.; Jiang, B.-J.; Wu, L.; Yu, K.-H. Urolithin A Targets the PI3K/Akt/NF-κB Pathways and Prevents IL-1β-Induced Inflammatory Response in Human Osteoarthritis: In Vitro and in Vivo Studies. Food Funct. 2019, 10, 6135–6146. [Google Scholar] [CrossRef]
- Wei, W.; Peng, C.; Gu, R.; Yan, X.; Ye, J.; Xu, Z.; Sheng, X.; Huang, G.; Guo, Y. Urolithin A Attenuates RANKL-Induced Osteoclastogenesis by Co-Regulating the P38 MAPK and Nrf2 Signaling Pathway. Eur. J. Pharmacol. 2022, 921, 174865. [Google Scholar] [CrossRef]
- Pan, W.; Wang, Q.; Chen, Q. The Cytokine Network Involved in the Host Immune Response to Periodontitis. Int. J. Oral Sci. 2019, 11, 30. [Google Scholar] [CrossRef]
- De Benedetti, F.; Rucci, N.; Del Fattore, A.; Peruzzi, B.; Paro, R.; Longo, M.; Vivarelli, M.; Muratori, F.; Berni, S.; Ballanti, P.; et al. Impaired Skeletal Development in Interleukin-6-Transgenic Mice: A Model for the Impact of Chronic Inflammation on the Growing Skeletal System. Arthritis Rheum. 2006, 54, 3551–3563. [Google Scholar] [CrossRef]
- Pan, W.; Yang, L.; Li, J.; Xue, L.; Wei, W.; Ding, H.; Deng, S.; Tian, Y.; Yue, Y.; Wang, M.; et al. Traumatic Occlusion Aggravates Bone Loss during Periodontitis and Activates Hippo-YAP Pathway. J. Clin. Periodontol. 2019, 46, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Takahashi, N.; Jimi, E.; Udagawa, N.; Takami, M.; Kotake, S.; Nakagawa, N.; Kinosaki, M.; Yamaguchi, K.; Shima, N.; et al. Tumor Necrosis Factor Alpha Stimulates Osteoclast Differentiation by a Mechanism Independent of the ODF/RANKL-RANK Interaction. J. Exp. Med. 2000, 191, 275–286. [Google Scholar] [CrossRef]
- Song, C.; Yang, X.; Lei, Y.; Zhang, Z.; Smith, W.; Yan, J.; Kong, L. Evaluation of Efficacy on RANKL Induced Osteoclast from RAW264.7 Cells. J. Cell Physiol. 2019, 234, 11969–11975. [Google Scholar] [CrossRef]
- Makihira, S.; Mine, Y.; Kosaka, E.; Nikawa, H. Titanium Surface Roughness Accelerates RANKL-Dependent Differentiation in the Osteoclast Precursor Cell Line, RAW264.7. Dent. Mater. J. 2007, 26, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Chaweewannakorn, W.; Ariyoshi, W.; Okinaga, T.; Fujita, Y.; Maki, K.; Nishihara, T. Ameloblastin Attenuates RANKL-Mediated Osteoclastogenesis by Suppressing Activation of Nuclear Factor of Activated T-Cell Cytoplasmic 1 (NFATc1). J. Cell Physiol. 2019, 234, 1745–1757. [Google Scholar] [CrossRef]
- Halleen, J.M.; Räisänen, S.; Salo, J.J.; Reddy, S.V.; Roodman, G.D.; Hentunen, T.A.; Le-henkari, P.P.; Kaija, H.; Vihko, P.; Väänänen, H.K. Intracellular Fragmentation of Bone Resorption Products by Reactive Oxygen Species Generated by Osteoclastic Tartrate-Resistant Acid Phosphatase. J. Biol. Chem. 1999, 274, 22907–22910. [Google Scholar] [CrossRef]
- Zou, N.; Liu, R.; Li, C. Cathepsin K+ Non-Osteoclast Cells in the Skeletal System: Function, Models, Identity, and Therapeutic Implications. Front. Cell Dev. Biol. 2022, 10, 818462. [Google Scholar] [CrossRef] [PubMed]
- Motyckova, G.; Fisher, D.E. Pycnodysostosis: Role and Regulation of Cathepsin K in Osteoclast Function and Human Disease. Curr. Mol. Med. 2002, 2, 407–421. [Google Scholar] [CrossRef]
- Reddy, R.A.; Varshini, M.S.; Kumar, R.S. Matrix Metalloproteinase-2 (MMP-2): As an Essential Factor in Cancer Progression. Recent Pat. Anticancer Drug Discov. 2025, 20, 26–44. [Google Scholar] [CrossRef]
- Yagi, M.; Miyamoto, T.; Sawatani, Y.; Iwamoto, K.; Hosogane, N.; Fujita, N.; Morita, K.; Ninomiya, K.; Suzuki, T.; Miyamoto, K.; et al. DC-STAMP Is Essential for Cell-Cell Fusion in Osteoclasts and Foreign Body Giant Cells. J. Exp. Med. 2005, 202, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Islam, R.; Bae, H.-S.; Yoon, W.-J.; Woo, K.-M.; Baek, J.-H.; Kim, H.-H.; Uchida, T.; Ryoo, H.-M. Pin1 Regulates Osteoclast Fusion through Suppression of the Master Regulator of Cell Fusion DC-STAMP. J. Cell. Physiol. 2014, 229, 2166–2174. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, X. Receptor Activator of Nuclear Factor-κB Ligand (RANKL)/RANK/Osteoprotegerin System in Bone and Other Tissues (Review). Mol. Med. Rep. 2015, 11, 3212–3218. [Google Scholar] [CrossRef] [PubMed]
- Sobacchi, C.; Frattini, A.; Guerrini, M.M.; Abinun, M.; Pangrazio, A.; Susani, L.; Bredius, R.; Mancini, G.; Cant, A.; Bishop, N.; et al. Osteoclast-Poor Human Osteopetrosis Due to Mutations in the Gene Encoding RANKL. Nat. Genet. 2007, 39, 960–962. [Google Scholar] [CrossRef]
- Amin, N.; Boccardi, V.; Taghizadeh, M.; Jafarnejad, S. Probiotics and Bone Disorders: The Role of RANKL/RANK/OPG Pathway. Aging Clin. Exp. Res. 2020, 32, 363–371. [Google Scholar] [CrossRef]
- Belibasakis, G.N.; Bostanci, N. The RANKL-OPG System in Clinical Periodontology. J. Clin. Periodontol. 2012, 39, 239–248. [Google Scholar] [CrossRef]
- Gomes, K.D.N.; Alves, A.P.N.N.; Dutra, P.G.P.; Viana, G.S.D.B. Doxycycline Induces Bone Repair and Changes in Wnt Signalling. Int. J. Oral Sci. 2017, 9, 158–166. [Google Scholar] [CrossRef]
- Yiğit, U.; Kırzıoğlu, F.Y.; Uğuz, A.C.; Nazıroğlu, M.; Özmen, Ö. Is Caffeic Acid Phenethyl Ester More Protective than Doxycycline in Experimental Periodontitis? Arch. Oral Biol. 2017, 81, 61–68. [Google Scholar] [CrossRef]


| Genes | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| Trap | CACTCCCACCCTGAGATTTGT | CATCGTCTGCACGGTTCTG |
| Ctsk | GAAGAAGACTCACCAGAA-GCAG | TCCAGGTTATGGGCAGAGATT |
| MMP-9 | CTG-GACAGCCAGACACTAAAG | CTCGCGGCAAGTCTTCAGAG |
| NFATc1 | GGAGCGGAGAAACTTTGCG | GTGACACTAGGG-GACACATAACT |
| DC-Stamp | TACGTGGAGAGAAGCAAGGAA | ACACTGAGACGTGGTTTAGGAAT |
| β-actin | CATCCGTAAA-GACCTCTATGCCAAC | ATGGAGCCACCGATCCACA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Y.; Wu, D.; Zhou, L.; Wu, X.; Chen, J. Urolithin A Attenuates Periodontitis in Mice via Dual Anti-Inflammatory and Osteoclastogenesis Inhibition: A Natural Metabolite-Based Therapeutic Strategy. Molecules 2025, 30, 2881. https://doi.org/10.3390/molecules30132881
Xia Y, Wu D, Zhou L, Wu X, Chen J. Urolithin A Attenuates Periodontitis in Mice via Dual Anti-Inflammatory and Osteoclastogenesis Inhibition: A Natural Metabolite-Based Therapeutic Strategy. Molecules. 2025; 30(13):2881. https://doi.org/10.3390/molecules30132881
Chicago/Turabian StyleXia, Yishu, Danni Wu, Linyi Zhou, Xinyu Wu, and Jianzhi Chen. 2025. "Urolithin A Attenuates Periodontitis in Mice via Dual Anti-Inflammatory and Osteoclastogenesis Inhibition: A Natural Metabolite-Based Therapeutic Strategy" Molecules 30, no. 13: 2881. https://doi.org/10.3390/molecules30132881
APA StyleXia, Y., Wu, D., Zhou, L., Wu, X., & Chen, J. (2025). Urolithin A Attenuates Periodontitis in Mice via Dual Anti-Inflammatory and Osteoclastogenesis Inhibition: A Natural Metabolite-Based Therapeutic Strategy. Molecules, 30(13), 2881. https://doi.org/10.3390/molecules30132881






