Comparison of High-Efficiency MgO/Na2CO3 and MgO/K2CO3 as Heterogeneous Solid Base Catalysts for Biodiesel Production from Soybean Oil
Abstract
1. Introduction
2. Results and Discussion
2.1. Phase and Surface Characterization
2.2. Catalyst Characterization and Performance Testing
2.3. Catalytic Performance Conditions
2.3.1. Effect of Loading Rate for MgO/Na2CO3
- Fixed parameters: Temperature = 60 °C; catalyst consumption = 4.0 wt%; methanol-to-oil ratio = 12:1; reaction time = 3 h.
- Variable parameter: Sodium carbonate content (10–50%).
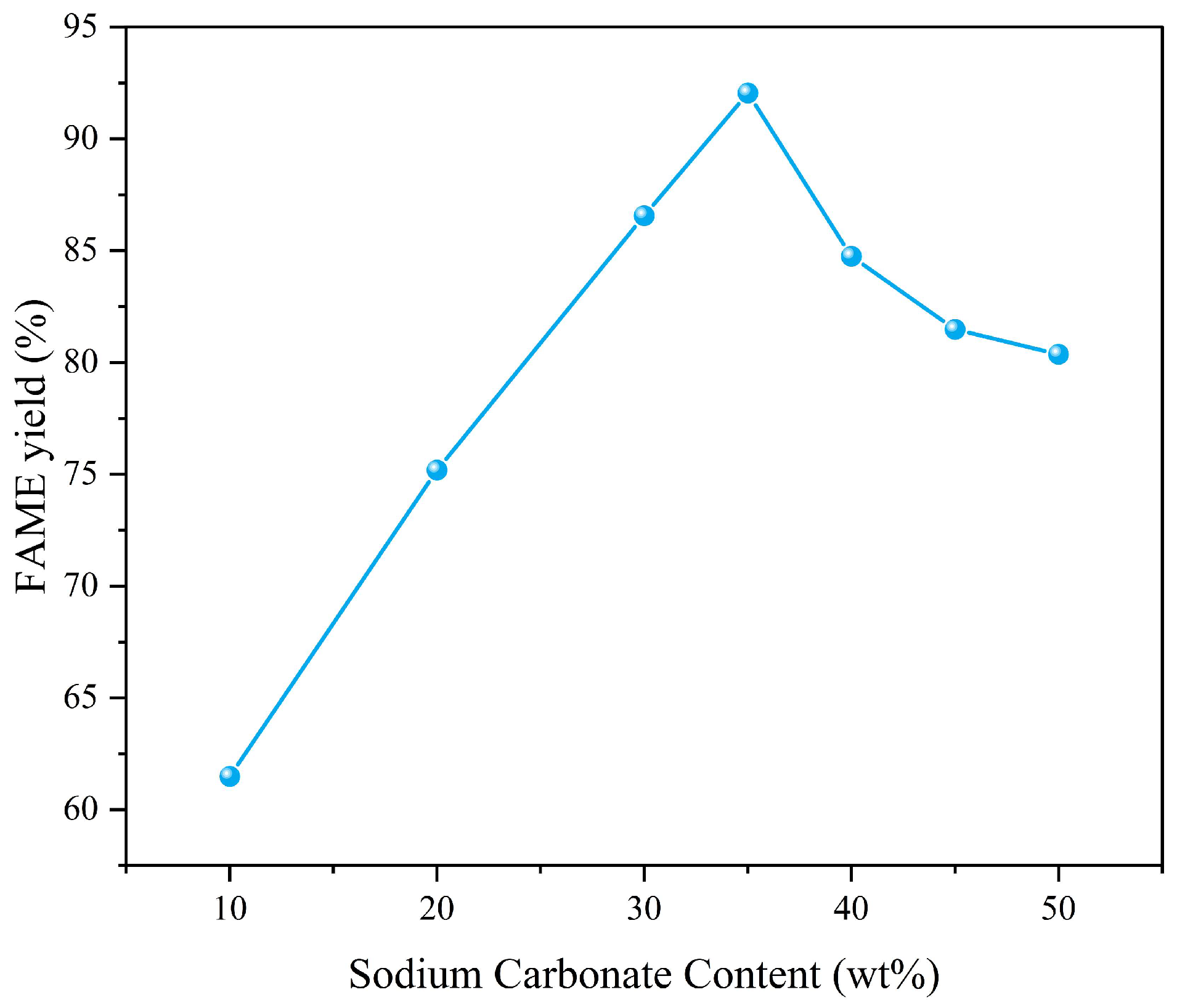
- Optimal range: Increasing the loading rate from 10% to 35% enhanced the FAME yield from 61.5% to 92.1% (Figure 7). This improvement can be attributed to the enhanced overall activity of the catalyst as the Na2CO3 content increases, further suggesting that MgO alone does not exhibit exceptionally strong catalytic properties.
- Decline at high sodium carbonate content: Further increasing the loading rate to 50% reduced the yield to 80.3%, which can be attributed to the excessively high loading rate that resulted in a reduction in the catalyst’s specific surface area.
- Considering that the increase in sodium carbonate content above 35% did not bring about an improvement in yield, we chose 35% as the optimal loading rate.
- The effect of the loading rate for Na2CO3-MgO on the FAME yield is shown in Figure 7.
2.3.2. Effect of Calcination Temperature for MgO/Na2CO3
- Fixed parameters: Temperature = 60 °C; catalyst consumption = 3.0 wt%; methanol-to-oil ratio = 12:1; reaction time = 2 h.
- Variable parameter: Calcination temperature for catalysts (500–700 °C).
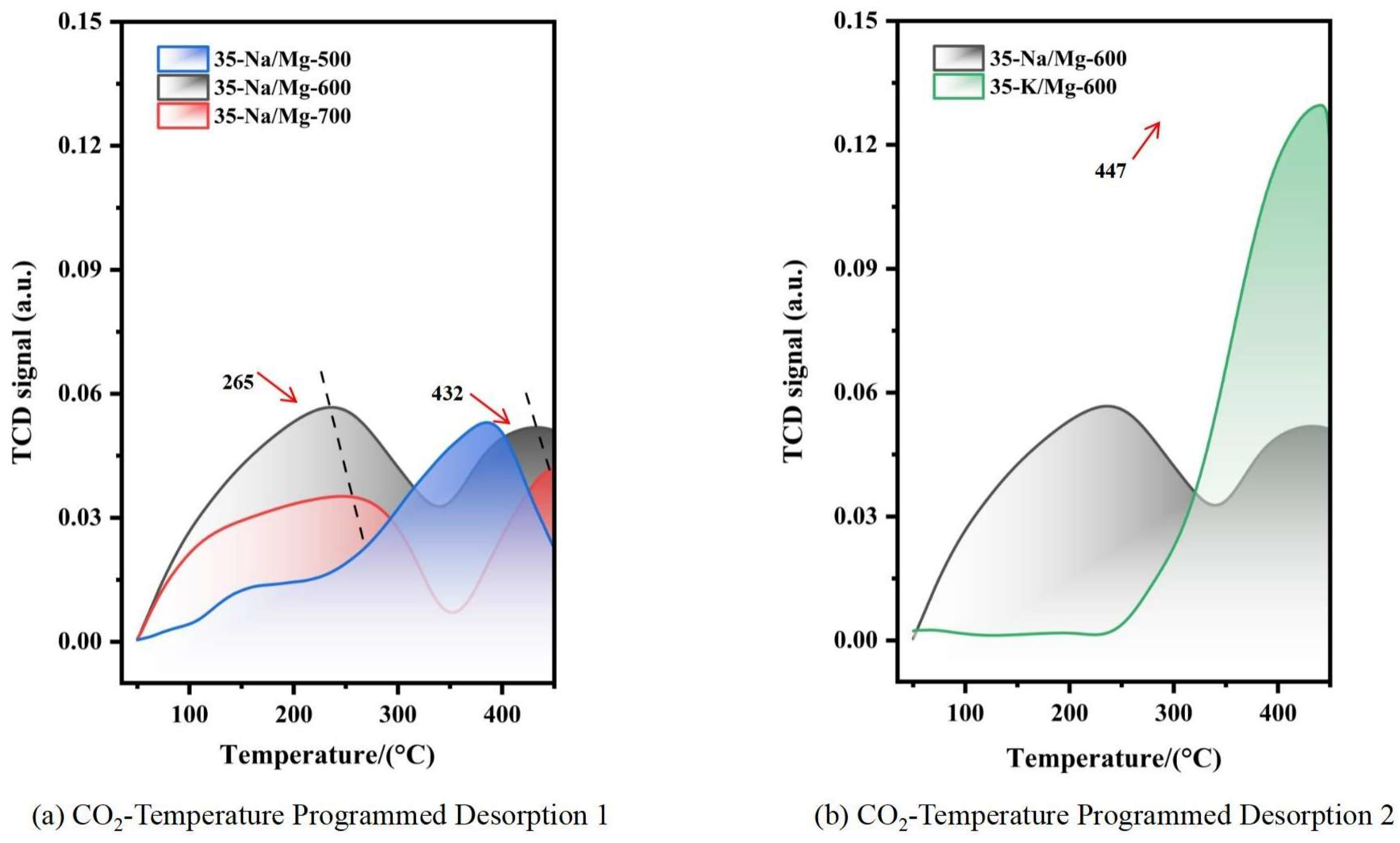
- Optimal range: When the calcination temperature increased from 500 to 600 °C, the FAME yield significantly rose from 64.8% to 84.2%. According to the BET and CO2-TPD characterization results in Figure 1a and Figure 2b, MgO/Na2CO3 calcined at 600 °C exhibited the highest catalytic activity and a relatively large specific surface area, which contributed to the substantial increase in yield. Due to the decreased amount of the catalyst and the shortened reaction time, the overall yield in this group was relatively low. Therefore, the corresponding yield changes of different catalysts can be clearly distinguished.
- Decline at high calcination temperature: Further increasing the calcination temperature to 700 °C reduced the yield to 69.8%. It is evident from the SEM in Figure 6 and BET in Figure 1b that an excessively high calcination temperature causes the agglomeration of the catalyst structure, thereby reducing the specific surface area and ultimately leading to a decrease in the FAME yield.
- The slightly higher specific surface area observed for the 500 °C sample (112.4 m2/g) compared to the 700 °C sample (98.5 m2/g) can be attributed to incomplete sintering at lower temperatures, preserving a more open pore structure. However, optimal catalytic activity was achieved at 600 °C due to balanced MgO crystal facet activation and uniform dispersion of carbonate species, maximizing strong alkaline site density, as evidenced by CO2-TPD. Calcination at 700 °C induced severe sintering, collapsing mesopores, and reducing accessibility. This temperature-dependent structural evolution aligns with findings for other mineral-based catalysts, where 600 °C represents the optimal window for achieving high surface area, developed porosity, and sufficient base strength critical for transesterification [16,17,18,19,20,21].
- Based on the experimental data and characterization results, it is reasonable to conclude that 600 °C represents the optimal calcination temperature.
- The effect of calcination temperature for MgO/Na2CO3 on the FAME yield is shown in Figure 8.
2.3.3. Effect of Reaction Temperature
- Fixed parameters: Catalyst consumption = 4.0 wt%; methanol-to-oil ratio = 12:1; reaction time = 4 h.
- Variable parameter: Temperature (30–80 °C).
- Optimal range: Increasing the temperature from 30 °C to 70 °C enhanced the FAME yield from 81.2% to 95.8% (Figure 9). This improvement is attributed to the accelerated mass transfer and reaction kinetics at elevated temperatures.
- Decline at high temperatures: Further increasing the temperature to 80 °C reduced the yield to 94.2%, likely due to methanol volatilization and partial catalyst sintering.
- Considering that the increase in temperature above 65 °C did not bring about a significant improvement in yield in terms of environmental protection and productivity, we chose 65 °C as the subsequent reaction condition.
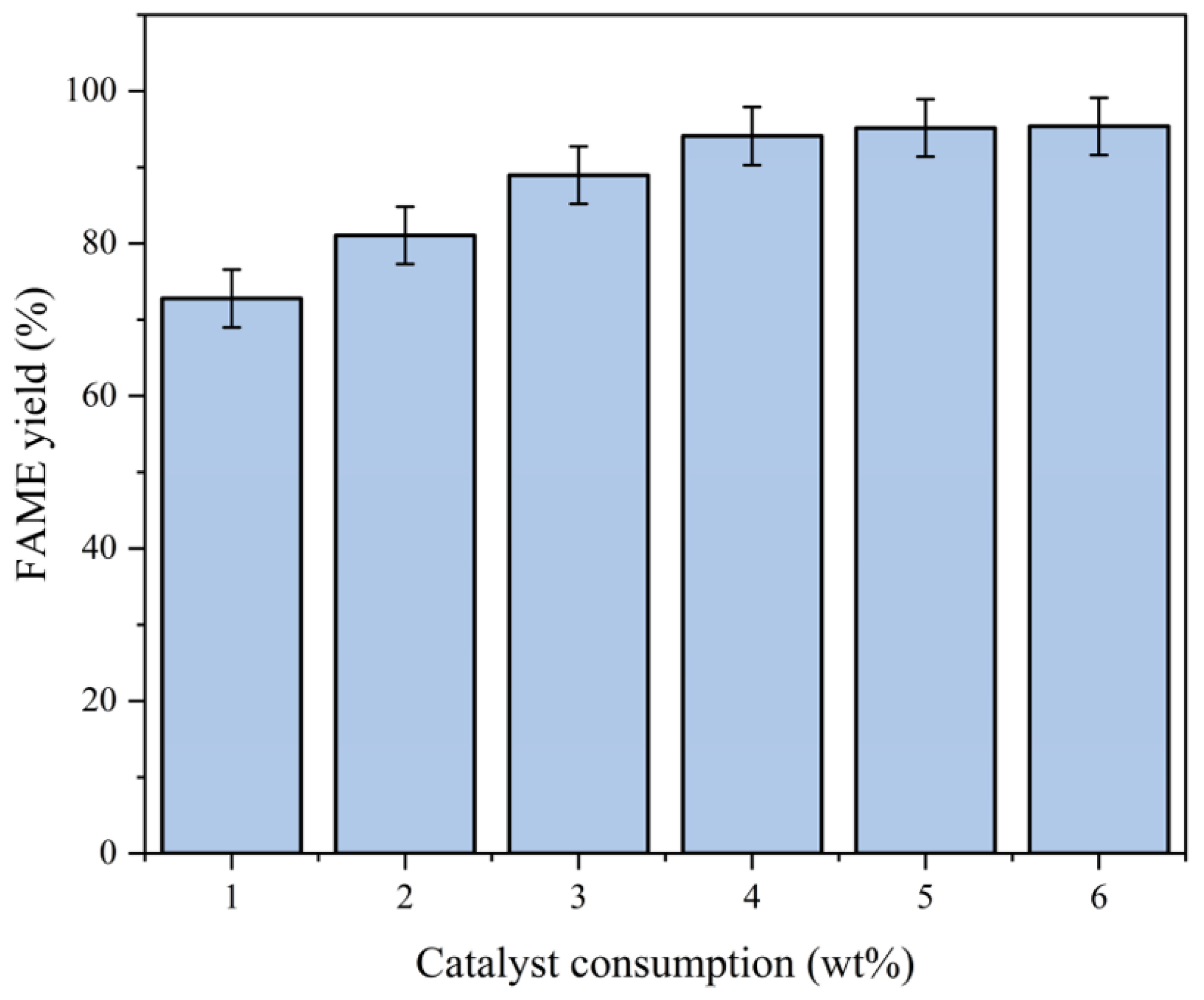
2.3.4. Effect of Catalyst Consumption
- Fixed parameters: Temperature = 65 °C; methanol-to-oil ratio = 12:1; reaction time = 3 h.
- Variable parameter: Catalyst consumption (1.0–6.0 wt%).
- Optimal loading: The FAME yield increased from 73.0% to 94.3% as the catalyst consumption rose from 1.0 wt% to 4.0 wt% (Figure 10).
- Overloading issues: Excessive loading (6.0 wt%) did not bring about a significant increase in yield, likely due to emulsion formation or active site shielding.
- Mechanism:
- Higher catalyst consumption provides more active sites for methanol activation.
- For the comprehensive consideration of economic efficiency and yield, we chose 4.0 wt% as the subsequent catalyst consumption.
- Overloading increases viscosity, hindering reactant diffusion to active sites.
- The effect of catalyst loading on FAME yield is shown in Figure 10.
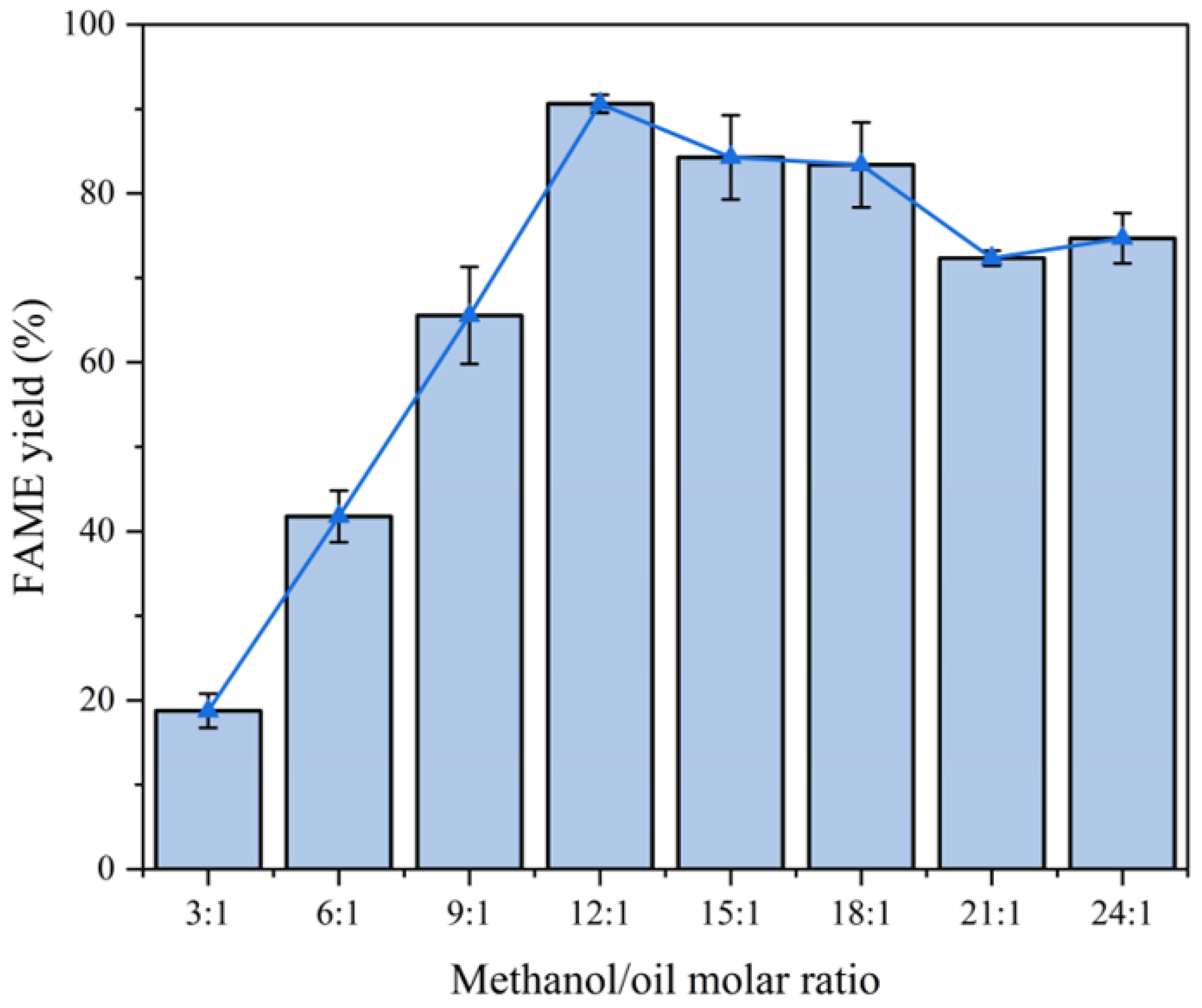
2.3.5. Effect of Methanol-to-Oil Ratio
- Fixed parameters: Temperature = 65 °C; catalyst consumption = 4.0 wt%; reaction time = 2 h.
- Variable parameter: Methanol-to-oil ratio (3:1–24:1).
- Optimal ratio: Increasing the ratio from 3:1 to 12:1 improved the yield by 72.8% (18.8% to 91.6%) (Figure 11).
- Dilution effect: A higher ratio (15:1) reduced efficiency due to reactant dilution.
- Mechanism:
- Excess methanol shifts the equilibrium toward ester formation but dilutes triglycerides at extreme ratios.
- For the comprehensive consideration of economic efficiency and yield, we chose 12:1 as the subsequent methanol/oil molar ratio.
- The relationship between methanol/oil molar ratio and FAME yield is shown in Figure 11.
2.3.6. Effect of Reaction Time
- Fixed parameters: Temperature = 65 °C; catalyst consumption = 4.0 wt%; methanol-to-oil ratio = 12:1.
- Variable parameter: Reaction time (1–4 h).
- Optimal duration: Extending the reaction time from 1 h to 3 h increased the yield from 79.3% to 97.4% (Figure 12).
- Reverse reactions: Prolonged time (>3 h) intensified reverse esterification, leading to no significant increase in the yield.
- Mechanism:
- Longer time ensures complete conversion but risks glycerol adsorption on active sites. Considering energy conservation and economic benefits, we chose 3 h as the optimal reaction time.
- The relationship between reaction time and FAME yield is shown in Figure 12.
2.4. Circulation and Regeneration
2.5. Comparative Literature
2.5.1. Structural Design of MgO-Based Composite Catalysts
2.5.2. Catalytic Performance in Biodiesel Production
2.5.3. Homogeneous vs. Heterogeneous Catalytic Systems
2.5.4. Research Gaps and Innovation
2.6. Specific Surface Area/Pore Structure, Alkaline Sites, Ionic Dissolution and Stability, Industrial Scale-Up Prospects
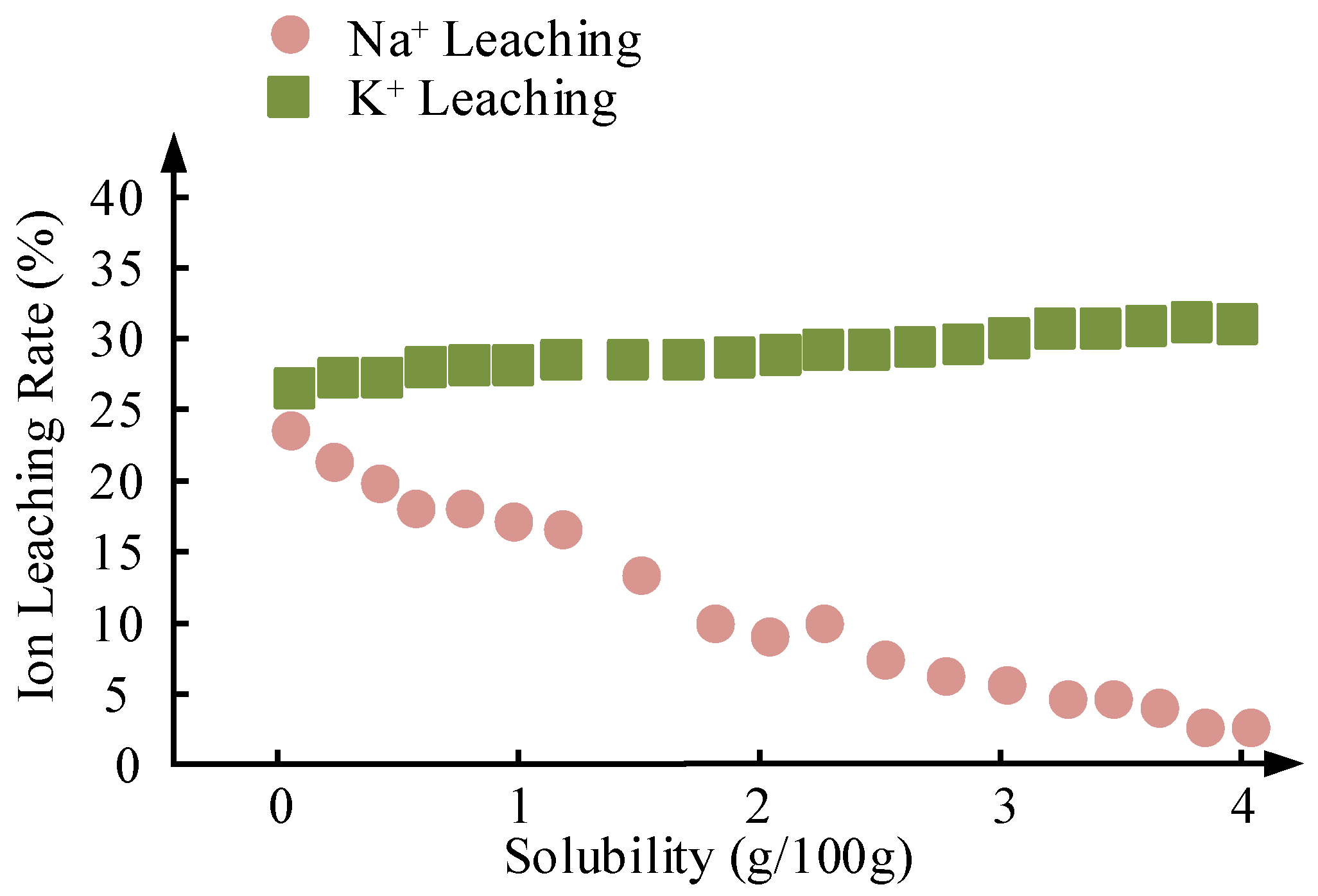
2.6.1. Solubility Differences in Methanol and Methanol/Glycerol Systems
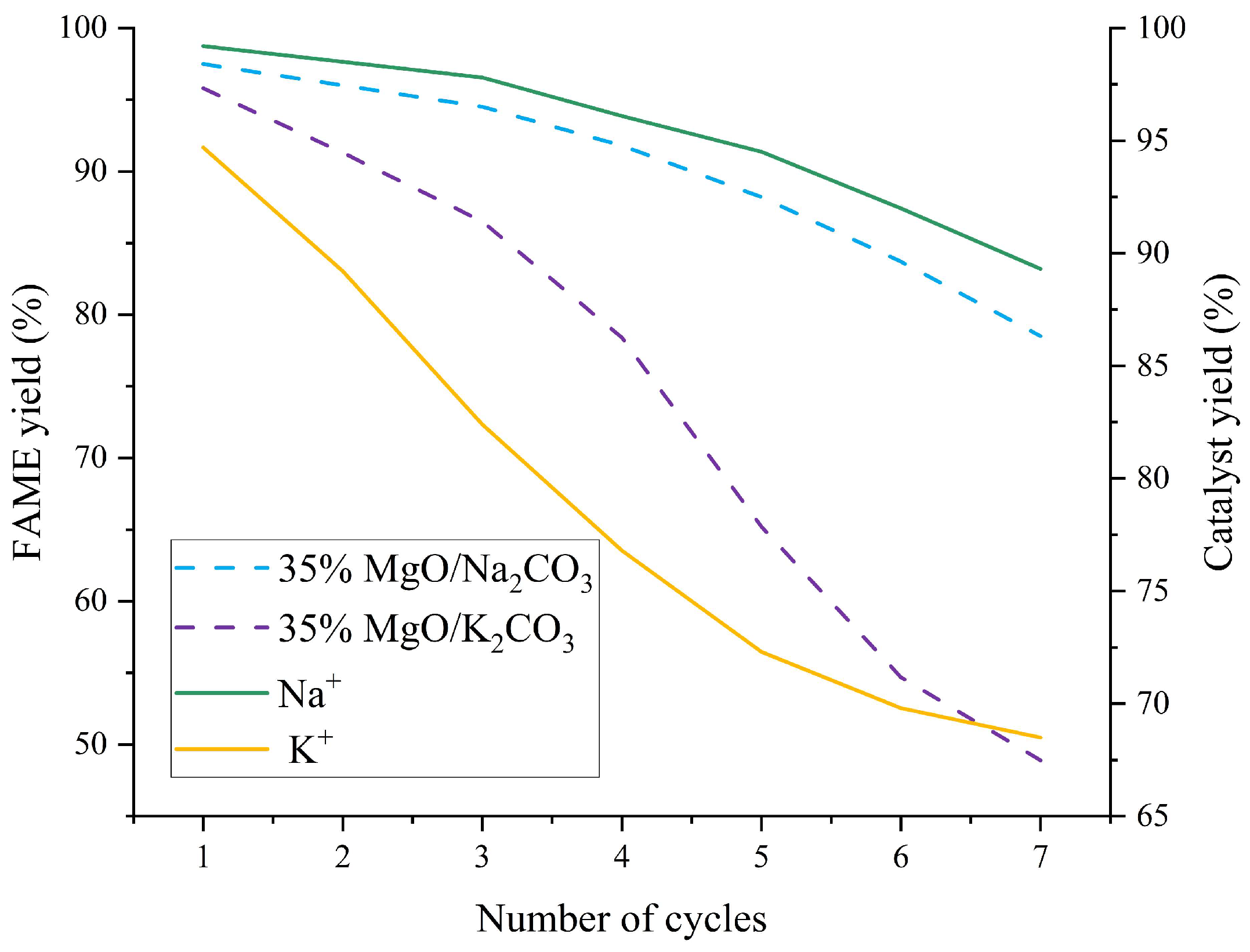
2.6.2. Reusability and Stability Analysis
2.6.3. Regeneration Methods and Performance Recovery
2.6.4. Comparative Analysis of Catalyst Performance
3. Materials and Methods
3.1. Experimental Reagents and Instruments
3.2. Experimental Methods
3.3. Quality Control and Safety Protocols
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FAME | Fatty Acid Methyl Ester |
| SEM | Scanning Electron Microscope |
| EDS | Energy Dispersive Spectroscopy |
| BET | Brunauer–Emmett–Teller |
| TPD | Temperature Programmed Desorption |
| FTIR | Fourier Transform Infrared spectroscopy |
| TG | Thermogravimetry Analysis |
| DTG | Derivative Thermogravimetry Analysis |
References
- Spanou, A.; Liakouli, N.C.; Fiotaki, C.; Pavlidis, I.V. Comparative Study of Immobilized Biolipasa-R for Second Generation Biodiesel Production from an Acid Oil. ChemBioChem 2024, 25, e202400514. [Google Scholar] [CrossRef] [PubMed]
- Hoe, B.C.; Arumugam, P.; Chew, I.M.L.; Tan, J.; Ooi, C.W. Extraction of Palm Carotene from Crude Palm Oil by Solvolytic Micellization: Economic Evaluation and Life Cycle Assessment. Chem. Eng. Commun. 2024, 211, 336–349. [Google Scholar] [CrossRef]
- Colaco, L.A.; Sousa, A.S.; Costa, A.C.F.M.; Farias, A.F.F.; Santos, I.M.G. Zinc Molybdate: A New Catalyst For Biodiesel Synthesis By Simultaneous Esterification/Transesterification Reaction. Fuel 2024, 371, 132066. [Google Scholar] [CrossRef]
- Kim, S.E.; Kim, J.H.; Kim, D.K.; Ham, H.C.; Lee, K.Y.; Kim, H.J. Na-Modified Carbon Nitride as a Leach-Resistant and Cost-Effective Solid Base Catalyst for Biodiesel Production. Fuel 2023, 341, 127548. [Google Scholar] [CrossRef]
- Winfield, D.M.D.; Cermak, S.C.; Evangelista, R.L.; Moser, B.R.; McKinney, J.; Pantalone, V. Evaluation of a High Oleic Soybean Oil Variety in Lubricant and Biodiesel Applications. J. Am. Oil Chem. Soc. 2024, 101, 493–499. [Google Scholar] [CrossRef]
- Agbulut, U.; Sathish, T.; Kiong, T.S.; Sambath, S.; Mahendran, G.; Kandavalli, S.R.; Sharma, P.; Gunasekar, T.; Kumar, P.S.; Saravanan, R. Production of Waste Soybean Oil Biodiesel with Various Catalysts, and the Catalyst Role on The CI Engine Behaviors. Energy 2024, 290, 130157. [Google Scholar] [CrossRef]
- Pariyan, K.; Ahmadi, A.; Hosseini, M.R. Effect of Thermal Pretreatment on the Two-Stage Extraction of Aluminum, Sulfur and Potassium from A Fine-Quartz Bearing Alunite. Miner. Eng. 2023, 204, 108430. [Google Scholar] [CrossRef]
- Sonnemberg, M.N.S.; Souza, E.F.; Ventura, M.; Simionatto, E.; Fiorucci, A.R. Investigation of Curcumin Antioxidant Efficiency on Oxidation Stability of Biodiesel from Soybean Oil and Beef Tallow, Contaminated with Metals: Kinetic and Storage Studies. Fuel 2024, 368, 131520. [Google Scholar] [CrossRef]
- Ying, A.; Bai, L.; Jiang, X.; Shen, R.; Liu, Y.; Liu, Z. Boosting Catalytic Efficiency of Lipase By Regulating Amphiphilic Microenvironment Through Reversible Addition-Fragmentation Chain Transfer Polymerized Modifications On Polyacrylonitrile Fiber. Int. J. Biol. Macromol. 2024, 277, 134196. [Google Scholar] [CrossRef]
- She, Q.; Qiu, M.; Li, K.; Liu, J.; Zhou, C. Acidic and Basic Sites on the Surface of Sodium Montmorillonite Active for Catalytic Transesterification of Glycerol to Glycerol Carbonate. Appl. Clay Sci. 2023, 238, 106916. [Google Scholar] [CrossRef]
- Rostamizadeh, M.; Oghabi, M.; Ghadimi, A. Highly Efficient and Reusable Mo/ZIF-8 Nanocatalyst In Esterification Reaction For Biodiesel Production. Res. Chem. Intermed. 2023, 49, 2053–2070. [Google Scholar] [CrossRef]
- Majedi, M.; Safaei, E. Molybdenum (VI) Complex of Resorcinol-Based Ligand Immobilized on Silica-Coated Magnetic Nanoparticles for Biodiesel Production. Appl. Organomet. Chem. 2023, 37, e7216. [Google Scholar] [CrossRef]
- Shao, X.B.; Liu, S.; Xing, Z.W.; Tang, J.X.; Li, P.; Liu, C.; Chi, R.Z.; Tan, P.; Sun, L.B. Atomically Dispersed Magnesium with Unusual Catalytic Activity For Transesterification Reaction. AIChE J. 2024, 70, e18567. [Google Scholar] [CrossRef]
- Visioli, L.J.; Nunes, A.L.B.; Wancura, J.H.C.; Enzweiler, H.; Vernier, L.J.; Castilhos, F. Batch and Continuous γ-Alumina-Catalyzed FAME Production from Soybean Oil Deodorizer Distillate by Interesterification. Fuel 2023, 351, 128954. [Google Scholar] [CrossRef]
- Krótki, A.; Spietz, T.; Dobras, S.; Chwola, T.; Tatarczuk, A.; Zorawski, D.; Skowron, K.; Skrzyniecki, D.; Hulisz, P. Carbon capture pilot study in Solvay soda ash process. Appl. Energy 2025, 380, 124995. [Google Scholar] [CrossRef]
- Wang, H.; Shi, Z.; Guo, K.; Wang, J.; Gong, C.; Xie, X.; Xue, Z. Boronic Ester Transesterification Accelerates Ion Conduction for Comb-Like Solid Polymer Electrolytes. Macromolecules 2023, 56, 2494–2504. [Google Scholar] [CrossRef]
- Ikeue, K.; Miyamoto, Y.; Ando, E. Metal-Substituted Layered Fe-Based Oxides as a Solid Base Catalyst. Chem. Phys. Lett. 2023, 830, 140816. [Google Scholar] [CrossRef]
- Bernard, E.; Yio, M.; Rentsch, D.; Chen, H.; Myers, R.J. Insights on the Effects of Carbonates and Phosphates on the Hydration of Magnesia (Alumino-)Silicate Cements. Appl. Geochem. 2024, 167, 106001. [Google Scholar] [CrossRef]
- Lobo, R.R.; Arce-Cordero, J.A.; So, S.; Soltis, M.; Marinho, M.N.; Agustinho, B.C.; Ravelo, A.D.; Vinyard, J.R.; Johnson, M.L.; Monteiro, H.F.; et al. Production, Physiological Response, and Calcium And Magnesium Balance of Lactating Holstein Cows Fed Different Sources of Supplemental Magnesium with or Without Ruminal Buffer. J. Dairy Sci. 2023, 106, 990–1001. [Google Scholar] [CrossRef]
- Kale, B.N.; Patle, S.D.; Kalambe, S.R.; Khawale, V.R. Comparative Analysis of Compression Ignition Engines Performance and Emission Characteristics Devouring Edible and Nonedible Oil Biodiesel. Environ. Prog. Sustain. Energy 2024, 43, e14363. [Google Scholar] [CrossRef]
- Vazquez-Garrido, I.; Guevara-Lara, A.; Lopez-Benitez, A. Hydroprocessing of New and Waste Soybean Oil for Obtaining Biodiesel: An Operational Conditions Study. Chem. Eng. J. 2023, 452, 139508. [Google Scholar] [CrossRef]
- Da Costa, G.B.; De Fernandes, D.D.S.; Veras, G.; Dias Gondim, P.H.G.; Gondim, A.D. Combining NIR Spectroscopy with DD-SIMCA for Authentication and iSPA-PLS-DA for Discrimination of Ethyl Route and Oil Feedstocks of Biodiesels in Biodiesel/Diesel Blends. J. Am. Oil Chem. Soc. 2024, 101, 187–196. [Google Scholar] [CrossRef]
- Emeji, I.C.; Patel, B. Box-Behnken Assisted RSM and ANN Modelling for Biodiesel Production over Titanium Supported Zinc-Oxide Catalyst. Energy 2024, 308, 132765. [Google Scholar] [CrossRef]
- Malins, K. The potential of K3PO4, K2CO3, Na3PO4 and Na2CO3 as reusable alkaline catalysts for practical application in biodiesel production. Fuel Process. Technol. 2018, 179, 302–312. [Google Scholar] [CrossRef]
- Santamaría, C.; Morales, E.; Río, C.; Herradón, B.; Amarilla, J.M. Studies on Sodium-Ion Batteries: Searching for the Proper Combination of the Cathode Material, the Electrolyte and the Working Voltage. the Role Of Magnesium Substitution in Layered Manganese-Rich Oxides, And Pyrrolidinium Ionic Liquid. Electrochim. Acta 2023, 439, 141654. [Google Scholar] [CrossRef]
- He, P.; Zhang, W.; Fu, G.; Xie, E.; Wang, W.; Zhang, Z.; Zhang, T.; Wu, G. Two-Dimensional Mo Catalysts Supported on the External Surface of Planar Silicalite-1 Zeolite for Biodiesel Production from Waste Cooking Soybean Oil: Transesterification Experiment and Kinetics. Fuel 2024, 360, 130610. [Google Scholar] [CrossRef]
- Hu, M.; Pu, J.; Qian, E.W.; Wang, H. Biodiesel production using MgO–CaO catalysts via transesterification of soybean oil: Effect of MgO addition and insights of catalyst deactivation. BioEnergy Res. 2023, 16, 2398–2410. [Google Scholar] [CrossRef]
- Margellou, A.G.; Koutsouki, A.A.; Petrakis, D.E.; Kontominas, M.G.; Pomonis, P.J. Catalysis and inhibition of transesterification of rapeseed oil over MgO–CaO. BioEnergy Res. 2022, 16, 528–538. [Google Scholar] [CrossRef]
- Xie, W.; Han, Y.; Tai, S. Biodiesel Production Using Biguanide-Functionalized Hydroxyapatite-Encapsulated-Gamma-Fe2O3 Nanoparticles. Fuel 2017, 210, 83–90. [Google Scholar] [CrossRef]
- Zheng, J.; Tao, S.; Yang, Y.; Tang, Y. Gel–sol synthesis of hierarchical CaO using pollen as biotemplate for biodiesel production. Comptes Rendus. Chim. 2024, 27, 1–14. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, X.; Ma, X.; Cao, T.; Xu, J.; Feng, H.; Diao, R.; Qi, F.; Huang, H.; Ma, P. Efficient Preparation of Biomass-Based Ultra-Thin 2D Porous Carbon Materials by In Situ Template-Activation and Its Application in Sodium Ion Capacitors. Adv. Funct. Mater. 2024, 34, 2310717. [Google Scholar] [CrossRef]
- Liu, W.; Liu, K.; McClements, D.J.; Jin, Z.; Chen, L. Fabrication and Characterization of Starch-Based Bigels Under Phase Control: Structural, Physicochemical and 3D Printing Properties. Food Hydrocoll. 2025, 159, 110623. [Google Scholar] [CrossRef]
- Zou, S.; Zhou, J.; Du, Y.; Cheng, J.; Wang, Y.; Zhang, Z. Texture and Volatile Profiles of Beef Tallow Substitute Produced by a Pilot-Scale Continuous Enzymatic Interesterification. Food Chem. 2023, 429, 136980. [Google Scholar] [CrossRef]
- Tahmasebi-Boldaji, R.; Rashidi, S.; Rajabi-Kuyakhi, H.; Tahmasebi-Boldaji, N.; Baghdadi, M.; Karbassi, A. Application of pharmaceutical waste as a heterogeneous catalyst for transesterification of waste cooking oil: Biofuel production and its modeling using predictive tools. Biofuels 2024, 15, 415–431. [Google Scholar] [CrossRef]
- Sajjad, N.; Orfali, R.; Perveen, S.; Rehman, S.; Sultan, A.; Akhtar, T.; Nazir, A.; Muhammad, G.; Mehmood, T.; Ghaffar, S.; et al. Biodiesel Production from Alkali-Catalyzed Transesterification of Tamarindus indica Seed Oil and Optimization of Process Conditions. Molecules 2022, 27, 3230. [Google Scholar] [CrossRef]
- Kelani, R.O.; Ahmad, Z.; Patle, D. Mechanistic model-based control of biodiesel production processes: A review of needs and scopes. Chem. Eng. Commun. 2023, 210, 274–290. [Google Scholar] [CrossRef]
- Karimi, K.; Saidi, M.; Moradi, P.; Najafabadi, A.T. Biodiesel production from Nannochloropsis microalgal biomass-derived oil: Experimental and theoretical study using RSM-CCD approach. Can. J. Chem. Eng. 2023, 101, 5600–5610. [Google Scholar] [CrossRef]
- Khalifa, A.; Faried, M.; Abdelsalam, E.M.; Samer, M.; Moselhy, M.A.; Elsayed, H.; Attia, Y.A. Photoactivation of nano MgO anchored g-C3N4 enhances biodiesel production in Chlorella sorokiniana: A sustainable approach. Environ. Prog. Sustain. Energy 2024, 43, e14470. [Google Scholar] [CrossRef]
- Mello, B.S.D.; Pozzi, A.; Rodrigues, B.C.G.; Costa, M.A.M.; Sarti, A. Anaerobic digestion of crude glycerol from biodiesel production for biogas generation: Process optimization and pilot scale operation. Environ. Res. 2024, 244, 117938. [Google Scholar] [CrossRef]
- Wang, F.; Li, Y.; Wang, Y.; Xu, X.; Cao, X.; Cao, T.; Zhang, W.; Liu, W. Engineering lipase TLL to improve its acid tolerance and its biosynthesis in Trichoderma reesei for biodiesel production from acidified oil. Bioresour. Technol. 2024, 413, 131521. [Google Scholar] [CrossRef]
- Siow, H.S.; Sudesh, K.; Ganesan, S. Insect oil to fuel: Optimizing biodiesel production from mealworm (Tenebrio molitor) oil using response surface methodology. Fuel 2024, 371, 132099. [Google Scholar] [CrossRef]
- Asif, M.; Javed, F.; Younas, M.; Gillani, M.A.; Zimmerman, W.B.; Rehman, F. Investigating biodiesel production from chicken fat oil using bi-functional catalysts and microbubble mediated mass transfer. Fuel 2024, 358, 130125. [Google Scholar] [CrossRef]
- Saidi, M.; Amirnia, R. Recent advances on application of metal-organic framework-based catalysts in biodiesel production process: A review of catalyst types and activity, challenges and opportunities. Fuel 2024, 363, 130905. [Google Scholar] [CrossRef]
- Atashkar, H.; Saidi, M. Green biodiesel production from Nannochloropsis microalgae-derived oil using ZnAl-LDH catalyst: Process optimization and kinetic study. Fuel 2024, 370, 131804. [Google Scholar] [CrossRef]
- Mansoorsamaei, Z.; Mowla, D.; Esmaeilzadeh, F.; Dashtian, K. Sustainable biodiesel production from waste cooking oil using banana peel biochar-Fe2O3/Fe2K6O5 magnetic catalyst. Fuel 2024, 357, 129821. [Google Scholar] [CrossRef]
- Annal, U.N.; Vaithiyanathan, R.; Natarajan, A.; Rajadurai, V.; Kumar, P.S.M.; Li, Y.Y. Electrolytic biodiesel production from spent coffee grounds: Optimization through response surface methodology and artificial neural network. J. Taiwan Inst. Chem. Eng. 2024, 165, 105697. [Google Scholar] [CrossRef]
- Wajeh, M.E.; Granderath, M.; Mitsos, A.; Mhamdi, A. Distributed economic nonlinear model predictive control for flexible electrified biodiesel production—Part II: Sequential and iterative architectures with computational delay compensation. Ind. Eng. Chem. Res. 2024, 63, 18013–18026. [Google Scholar] [CrossRef]
- Betiku, E.; Oraegbunam, J.C.; Falowo, O.A.; Ojumu, T.V.; Latinwo, L.M. Sustainable microwave-supported biodiesel production using sandbox oil and its waste shell as a nanoparticle green alkali heterogeneous catalyst. Process Biochem. 2024, 142, 1–12. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, J.; Tang, B.; Lu, Y.; Ho, S.H.; Wang, Y.; Chen, C.; Shen, L. Metabolic interpretation of NaCl stress-induced lipid accumulation in microalgae for promising biodiesel production with saline wastewater. Chem. Eng. Sci. 2024, 284, 119447. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, H.; Liu, C. Xie, J.; Han, J.; Dai, H. Synergistic fusion of physical modeling and data-driven approaches for parameter inference to enzymatic biodiesel production system. Appl. Energy 2024, 373, 123874. [Google Scholar] [CrossRef]
- Melchiorre, M.; Amoresano, A.; Budzelaar, P.H.M.; Cucciolito, M.E.; Mocerino, F.; Pinto, G.; Ruffo, F.; Tuzi, A.; Esposito, R. Parts-Per-Million (Salen)Fe(III) Homogeneous Catalysts for the Production of Biodiesel from Waste Cooking Oils. Catal. Lett. 2022, 152, 3785–3794. [Google Scholar] [CrossRef]
- Sun, C.; Hu, Y.; Sun, F.; Sun, Y.; Song, G.; Chang, H.; Lunprom, S. Comparison of biodiesel production using a novel porous Zn/Al/Co complex oxide prepared from different methods: Physicochemical properties, reaction kinetic and thermodynamic studies. Renew. Energy 2022, 181, 1419–1430. [Google Scholar] [CrossRef]
- Huda, M.S.; Odegaard, M.; Chandra Sarker, N.; Webster, D.C.; Monono, E. Enhancing Recovery Yield of Vegetable Oil Methyl Ester for Bioresin Production: A Comparison Study Using Acid Neutralization. ChemEngineering 2024, 8, 16. [Google Scholar] [CrossRef]
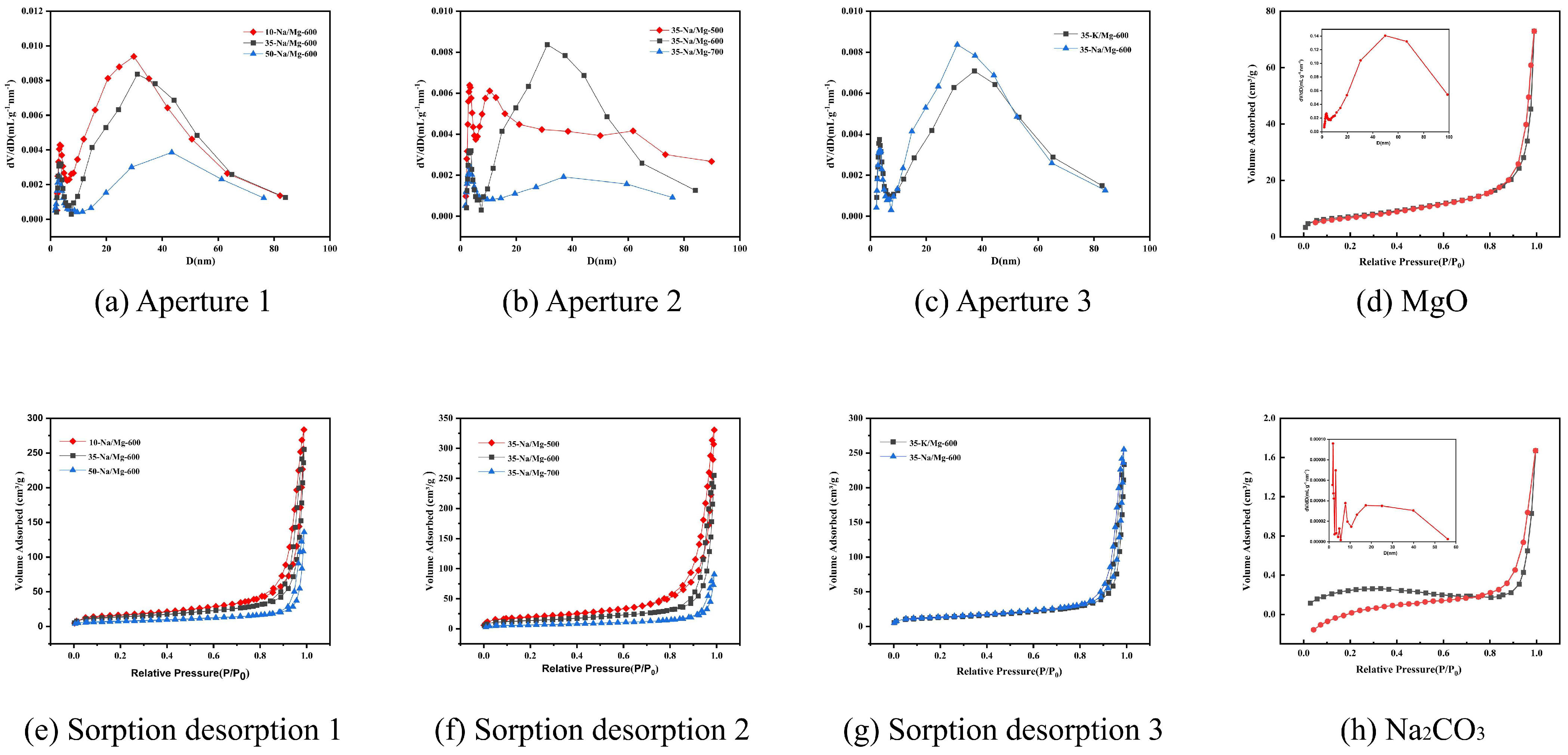



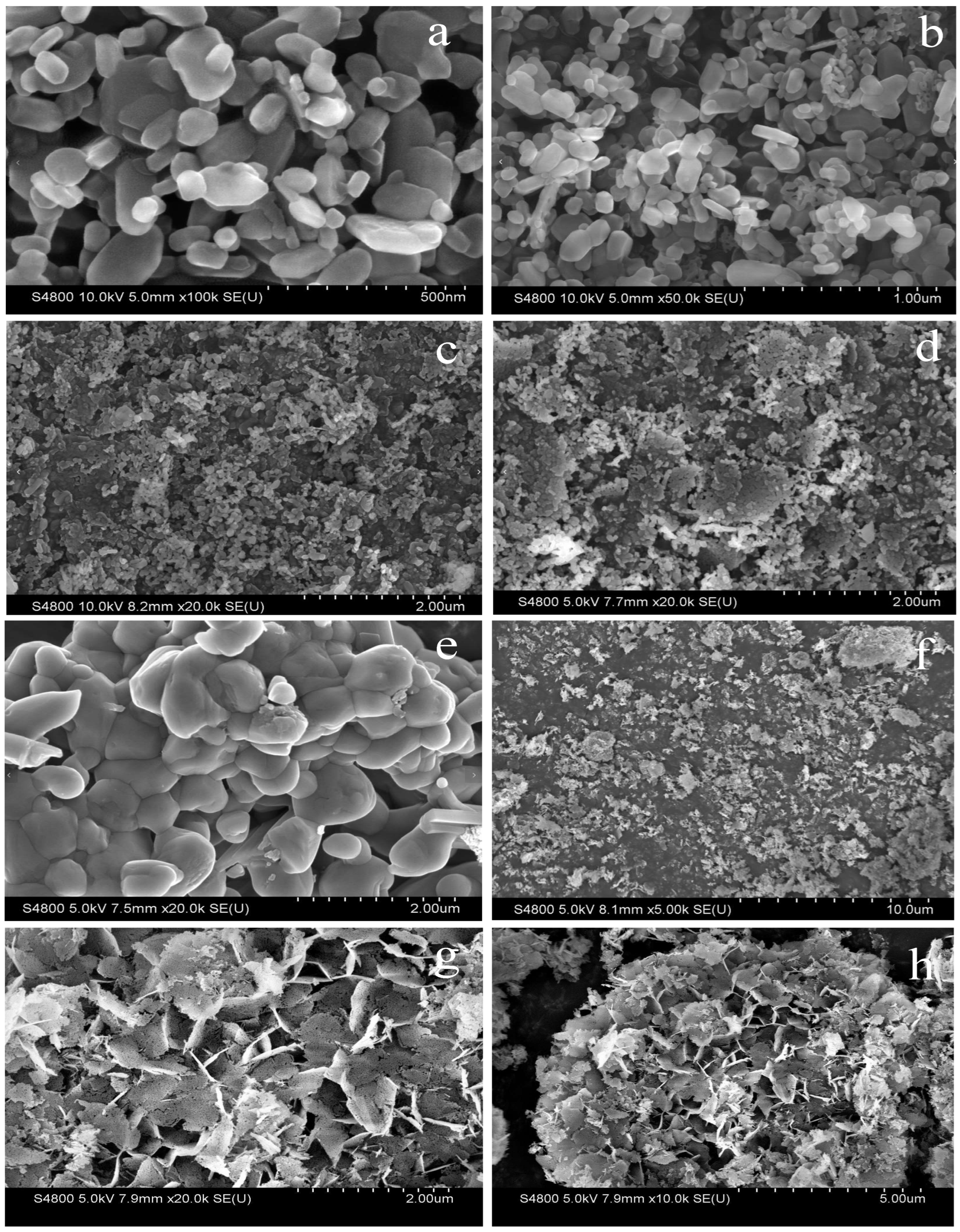




| Catalyst | Surface Area (m2/g) | Total Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| MgO-600 °C | 105.4 ± 3.5 | 0.51 ± 0.02 | 9.8 ± 0.3 |
| 35%-Na2CO3/MgO-600 °C | 148.6 ± 3.2 | 0.42 ± 0.02 | 8.7 ± 0.3 |
| 35%-K2CO3/MgO-600 °C | 126.3 ± 2.8 | 0.35 ± 0.01 | 9.2 ± 0.4 |
| 35%-Na2CO3/MgO-500 °C | 112.4 ± 2.5 | 0.28 ± 0.01 | 7.9 ± 0.2 |
| 35%-Na2CO3/MgO-700 °C | 98.5 ± 1.9 | 0.21 ± 0.01 | 6.5 ± 0.3 |
| Sample Code | Loading (wt%) | Calcination (°C) | Carbonate | Test Scope |
|---|---|---|---|---|
| 10-Na/Mg-600 | 10 | 600 | Na2CO3 | Loading screening |
| 20-Na/Mg-600 | 20 | 600 | Na2CO3 | Loading screening |
| 30-Na/Mg-600 | 30 | 600 | Na2CO3 | Loading screening |
| 35-Na/Mg-600 | 35 | 600 | Na2CO3 | All tests |
| 40-Na/Mg-600 | 40 | 600 | Na2CO3 | Loading screening |
| 50-Na/Mg-600 | 50 | 600 | Na2CO3 | Loading screening |
| 35-Na/Mg-500 | 35 | 500 | Na2CO3 | Temperature effect |
| 35-Na/Mg-550 | 35 | 550 | Na2CO3 | Temperature effect |
| 35-Na/Mg-650 | 35 | 650 | Na2CO3 | Temperature effect |
| 35-Na/Mg-700 | 35 | 700 | Na2CO3 | Temperature effect |
| 35-K/Mg-600 | 35 | 600 | K2CO3 | Ion comparison |
| Catalyst Type | Surface Area (m2/g) | FAME Yield (%) | Cycle Stability (5 Cycles) | Reference |
|---|---|---|---|---|
| Fe-Al-O (Al3+-doped) | 85 | 89 | 18% activity drop | [17] |
| Na-montmorillonite | 112 | 93 | 0.8 wt% Na loss | [10] |
| Mo/ZIF-8 | 980 | 97 | 91% retention | [11] |
| γ-Al2O3/Na | 125 → 78 * | 91 → 72 * | 62% less coking | [14] |
| MgO/Na2CO3 | 145 | 95 | 88% retention | [25] |
| Regeneration Method | MgO/Na2CO3 Yield Recovery (%) | MgO/K2CO3 Yield Recovery (%) | Surface Area Recovery (%) | Carbon Removal Rate (%) | Active Site Density Recovery (%) | Energy Consumption (MJ/kg) | Operational Complexity (1–5) |
|---|---|---|---|---|---|---|---|
| Calcination (600 °C, 2 h) | 82.3 ± 1.5 | 91.2 ± 1.2 | 88.5 ± 2.0 | 95.7 ± 1.8 | 78.4 ± 2.5 | 12.4 ± 0.8 | 2 |
| Acid Washing (HCl, 0.1 M) | 65.7 ± 2.0 | 73.8 ± 1.8 | 72.3 ± 2.5 | 85.2 ± 2.3 | 61.2 ± 3.0 | 8.2 ± 0.5 | 4 |
| Ultrasonic Cleaning (40 kHz) | 58.9 ± 2.5 | 67.5 ± 2.1 | 65.8 ± 3.0 | 76.4 ± 2.8 | 53.4 ± 3.5 | 5.6 ± 0.3 | 3 |
| Supercritical CO2 Treatment | 75.6 ± 1.8 | 84.3 ± 1.5 | 80.1 ± 2.3 | 90.1 ± 1.9 | 70.8 ± 2.8 | 18.9 ± 1.2 | 5 |
| Plasma Treatment (Ar) | 88.9 ± 1.2 | 93.5 ± 1.0 | 92.7 ± 1.5 | 97.3 ± 0.9 | 85.3 ± 2.0 | 24.7 ± 1.5 | 5 |
| Chemical Reduction (H2, 400 °C) | 71.2 ± 1.8 | 79.6 ± 1.6 | 76.3 ± 2.1 | 88.5 ± 2.0 | 68.9 ± 2.5 | 15.3 ± 1.0 | 4 |
| Enzyme Cleaning | 53.4 ± 3.0 | 62.1 ± 2.5 | 60.2 ± 3.2 | 70.3 ± 3.5 | 49.7 ± 3.8 | 3.8 ± 0.2 | 3 |
| Parameter | 35%-Na2CO3/MgO-600 °C | Key Points of Explanation | 35%-K2CO3/MgO-600 °C | Key Points of Explanation | Comparison of Regeneration Advantages |
|---|---|---|---|---|---|
| Initial Yield (%) | 97.5 ± 0.1 | High specific surface area (148.6 m2/g)+strong alkali site density (CO2-TPD 265 °C) | 95.8 ± 0.2 | Lower specific surface area (126.3 m2/g)+weak base site (CO2-TPD 447 °C) | / |
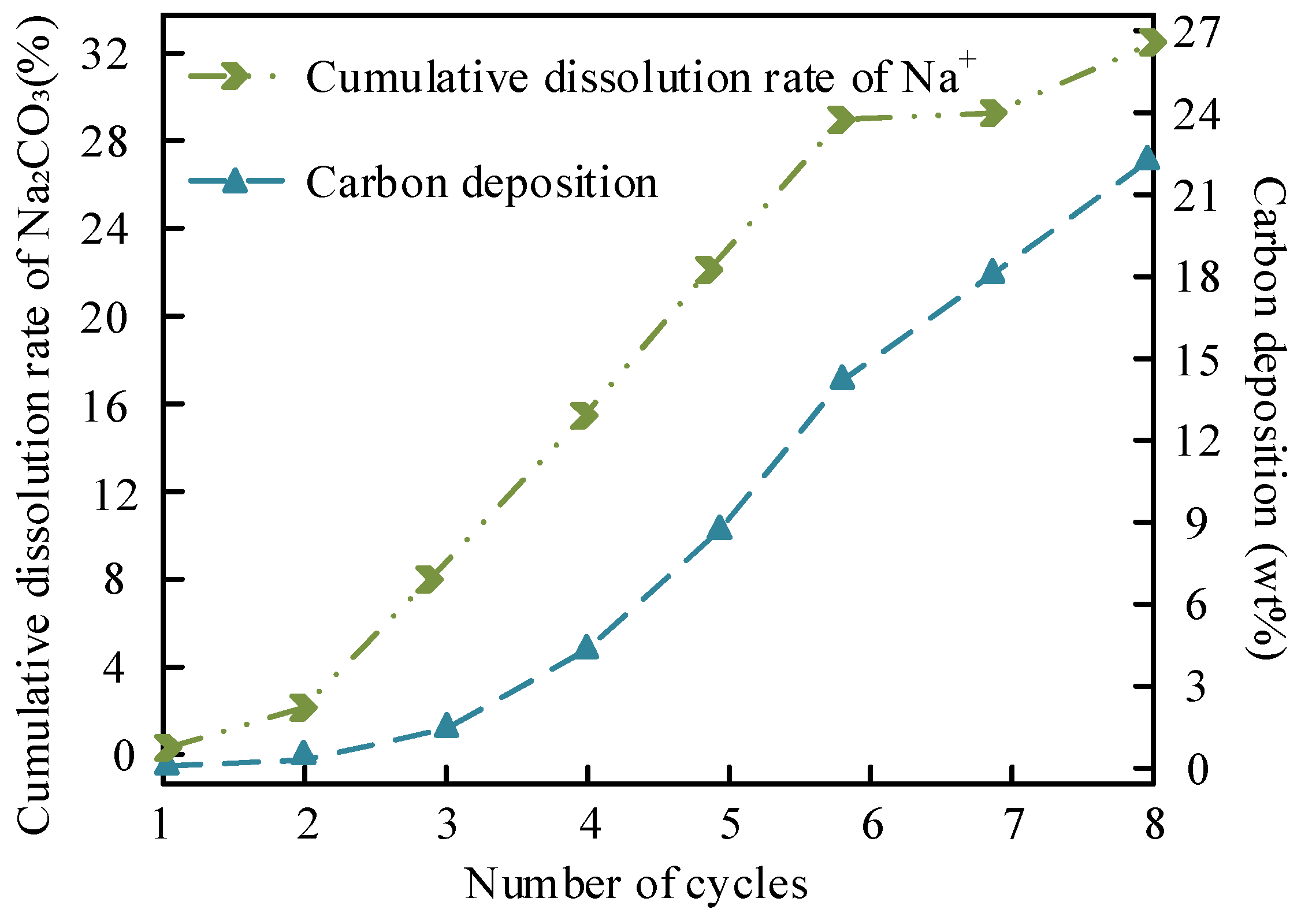
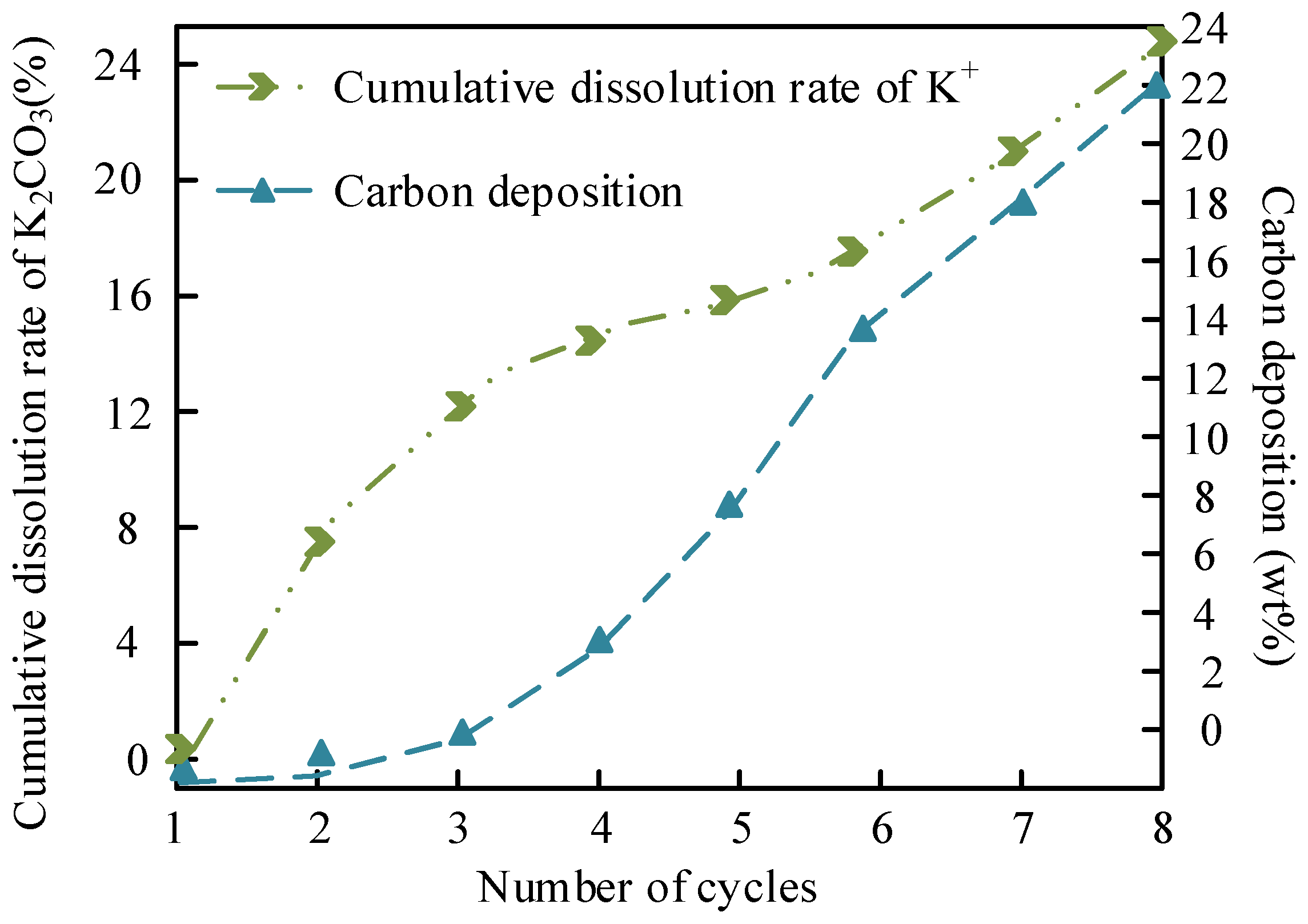
| Yield after 5th Cycle (%) | 88.2 ± 0.5 | Na+ dissolution is slow (18.9%) but carbon deposition is serious (22.3 wt%) → pore blockage | 65.2 ± 0.8 | High K+ dissolution (27.7%) + structural collapse → rapid loss of active sites | Better cycle stability of Na system |
| Specific Surface Area (m2/g) | 148.6 ± 3.2 | Na+ embedded in MgO lattice → homogeneous mesoporous | 126.3 ± 2.8 | K+ surface enrichment → pore blockage | / |
| Na+/K+ Leaching Rate (%) | 18.9 ± 0.1 | Low solubility of Na2CO3 methanol (1.15 g/100 g) | 27.7 ± 0.1 | High solubility of K2CO3 (3.25 g/100 g) | Better dissolution control of Na system |
| Yield Recovery after Regeneration (%) | 88.5 ± 1.5 | Na2CO3 is easy to sinter during regeneration → irreversible loss of active site | 94.2 ± 1.3 | K2CO3 crystal phase is stable + carbon deposit is easy to remove → active phase is recovered efficiently | Higher regeneration recovery rate of K series |
| Economic Cost (USD/ton) | 1200 | Na2CO3 raw material is cheap (40% lower than K2CO3 [17]) | 1350 | The price of K2CO3 is higher | Na series is economical |
| Reagent Name | Manufacturer | CAS Number | Concentration/Purity |
|---|---|---|---|
| Light Magnesium Oxide | Sinopharm Chemical Reagent Co., Ltd. Shanghai, China. | 1309-48-4 | Analytical Grade/AR |
| Anhydrous Sodium Carbonate | Sinopharm Chemical Reagent Co., Ltd. Shanghai, China | 497-19-8 | Analytical Grade/AR |
| Anhydrous Potassium Carbonate | Sinopharm Chemical Reagent Co., Ltd. Shanghai, China. | 584-08-7 | Analytical Grade/AR |
| Glycerol | Sinopharm Chemical Reagent Co., Ltd. Shanghai, China. | 56-81-5 | Analytical Grade/AR |
| Anhydrous Methanol | Sinopharm Chemical Reagent Co., Ltd. Shanghai, China. | 67-56-1 | Analytical Grade/AR |
| Soybean Oil | Nisshin Seifun Group, Inc., Suzhou, China. | N/A | First-Grade Edible Soybean Oil |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Jia, X.; Li, W.; Jia, S.; Zhang, S.; Song, J.; Wang, J. Comparison of High-Efficiency MgO/Na2CO3 and MgO/K2CO3 as Heterogeneous Solid Base Catalysts for Biodiesel Production from Soybean Oil. Molecules 2025, 30, 2876. https://doi.org/10.3390/molecules30132876
Li X, Jia X, Li W, Jia S, Zhang S, Song J, Wang J. Comparison of High-Efficiency MgO/Na2CO3 and MgO/K2CO3 as Heterogeneous Solid Base Catalysts for Biodiesel Production from Soybean Oil. Molecules. 2025; 30(13):2876. https://doi.org/10.3390/molecules30132876
Chicago/Turabian StyleLi, Xiangyang, Xunxiang Jia, Weiji Li, Shufan Jia, Siwei Zhang, Jiliang Song, and Jiao Wang. 2025. "Comparison of High-Efficiency MgO/Na2CO3 and MgO/K2CO3 as Heterogeneous Solid Base Catalysts for Biodiesel Production from Soybean Oil" Molecules 30, no. 13: 2876. https://doi.org/10.3390/molecules30132876
APA StyleLi, X., Jia, X., Li, W., Jia, S., Zhang, S., Song, J., & Wang, J. (2025). Comparison of High-Efficiency MgO/Na2CO3 and MgO/K2CO3 as Heterogeneous Solid Base Catalysts for Biodiesel Production from Soybean Oil. Molecules, 30(13), 2876. https://doi.org/10.3390/molecules30132876







