Creation of Crystalline Orientation of Tin(II) Oxide Polycrystals with High Photocatalytic Activity
Abstract
1. Introduction
2. Results and Discussion
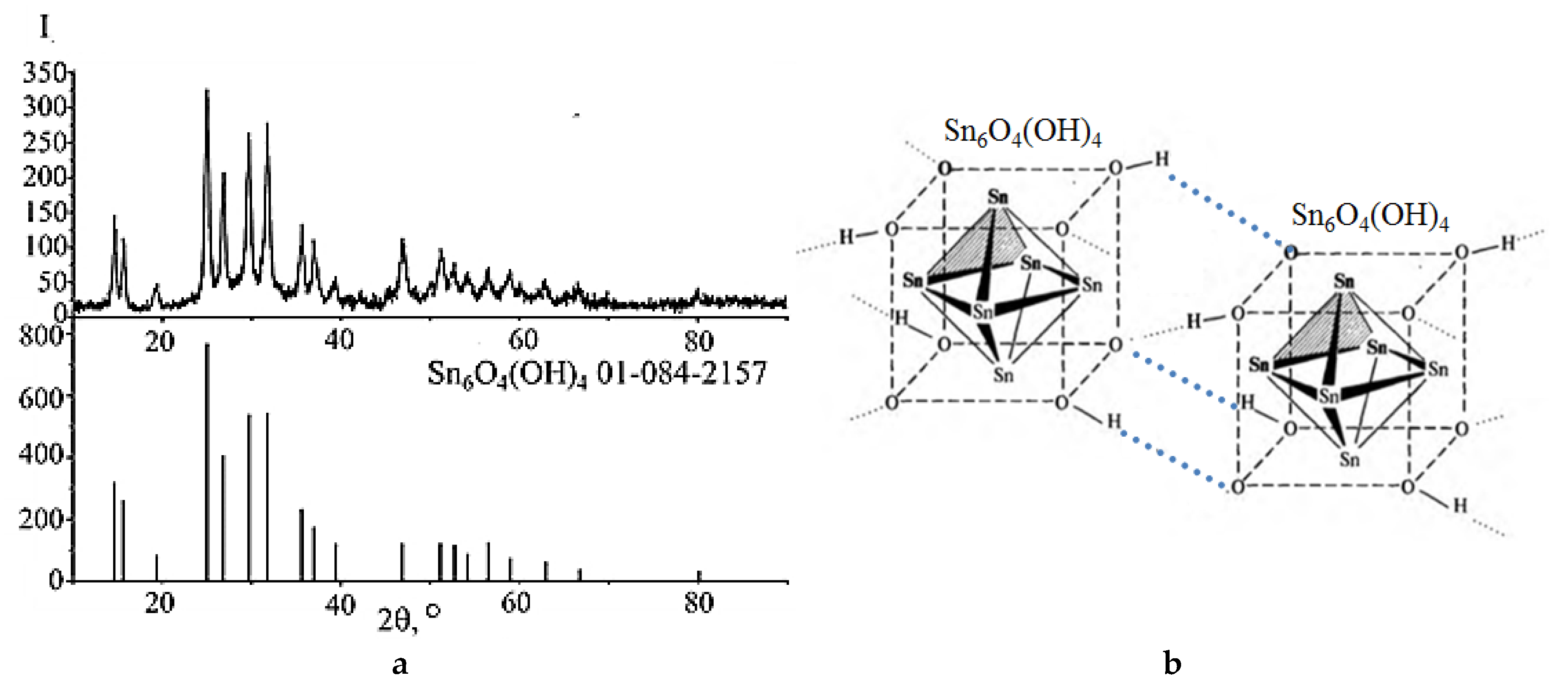
2.1. Preparation of Polycrystalline SnO from Sn6O4(OH)4 Suspensions by Microwave and Hydrothermal–Microwave Treatments
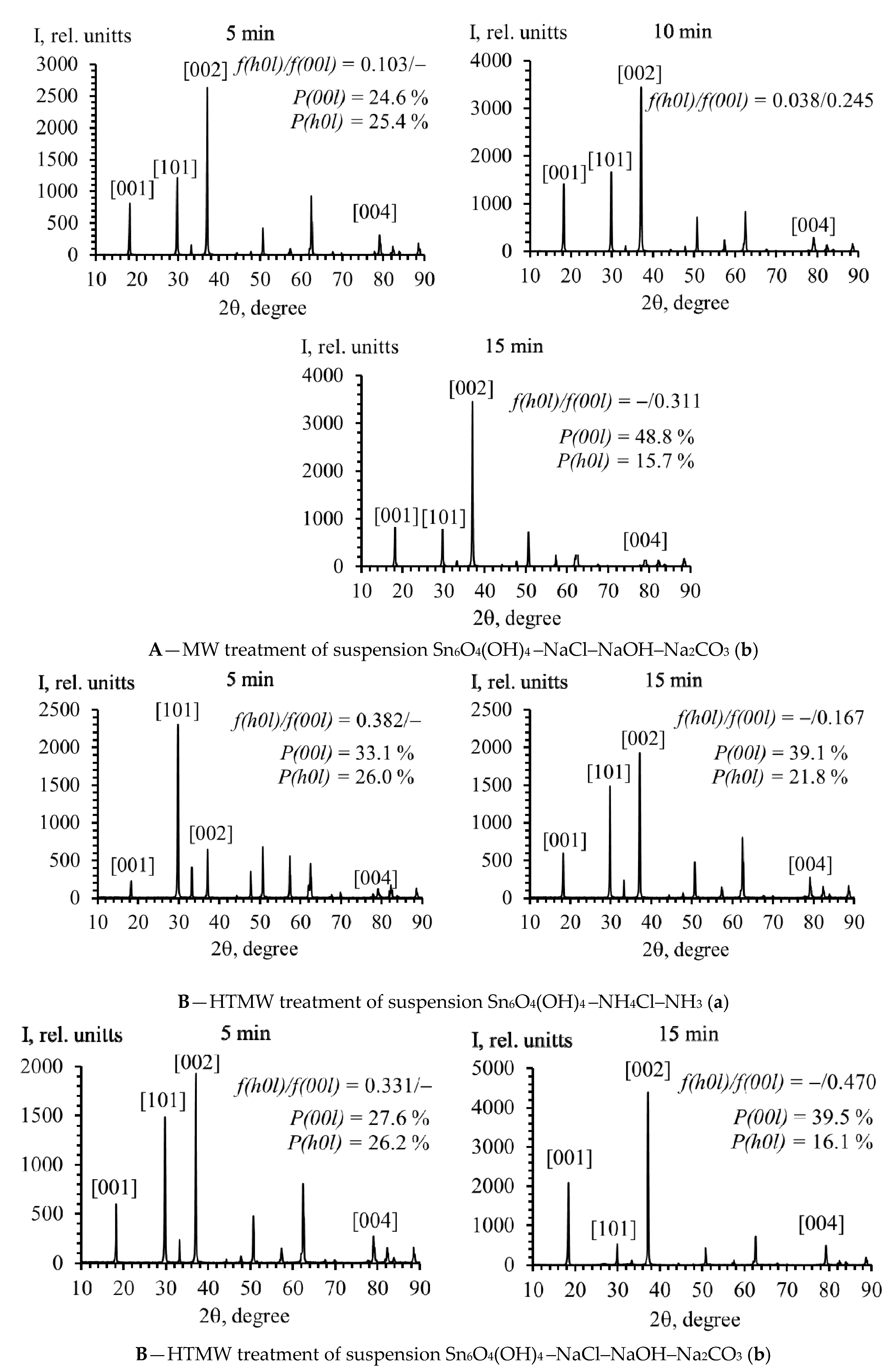
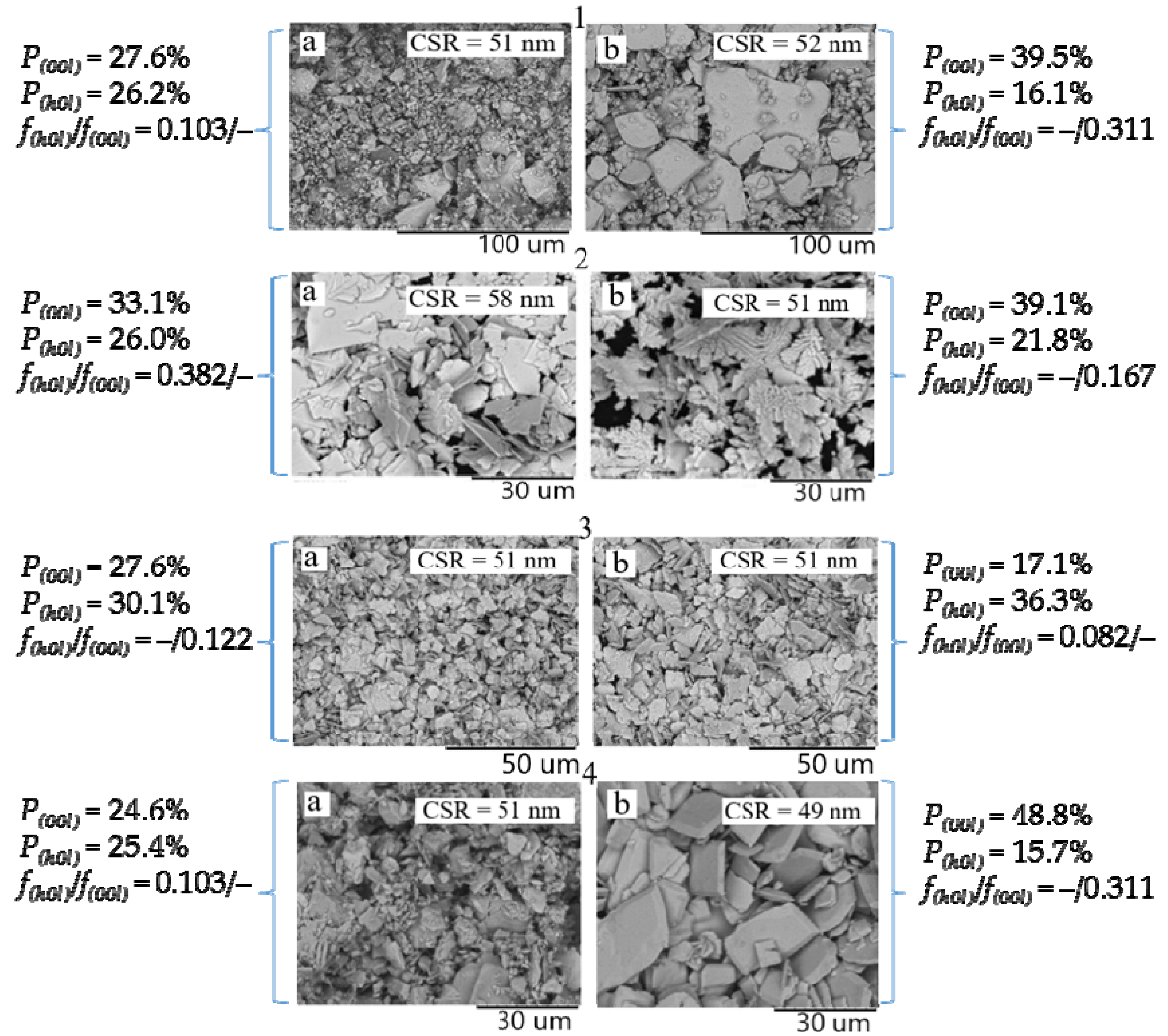
2.2. Phase Composition, Structure, Crystallographic Texture, and Surface Morphology of Polycrystalline SnO
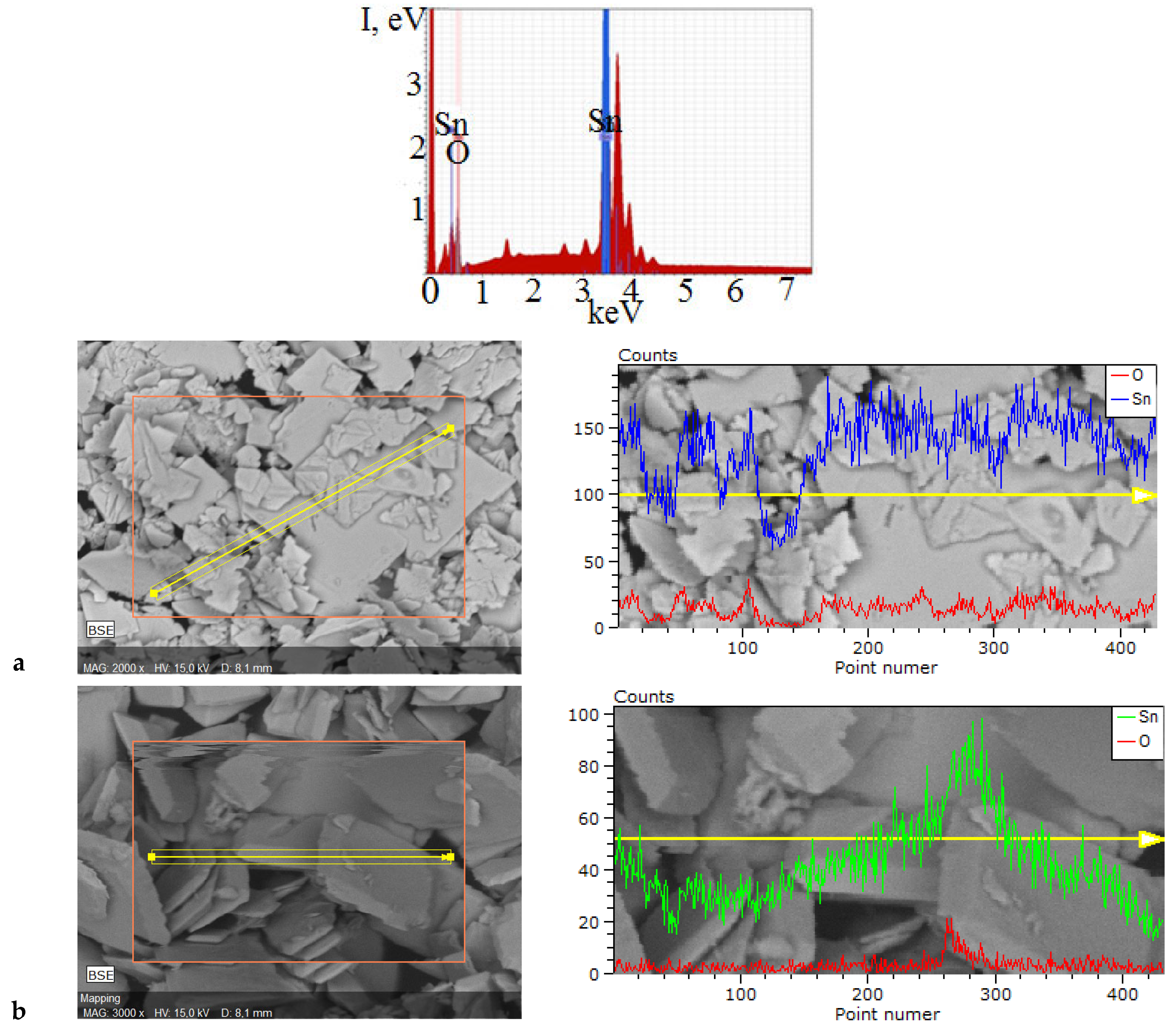
2.3. Surface Properties of Polycrystalline SnO
2.4. Photocatalytic Properties of Polycrystalline SnO with Different Crystallographic Textures
3. Materials and Methods
4. Conclusions
- -
- Recrystallization of SnO crystallites in the ammonia solution occurs due to the growth of crystallites in the (h0l) plane, and in the presence of sodium carbonate in the (00l) plane;
- -
- The pressure in the reaction system promotes recrystallization, leading to an increase in the number of SnO crystallites that are oriented in the (00l) plane, regardless of the composition of the dispersion medium.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shahvelayati, A.S.; Sheshmani, S.; Siminghad, M. Preparation and characterization of SnO nanoflowers with controllable thicknesses using imidazolium-based ionic liquids as green media: Visible light photocatalytic degradation of Acid Blue 19. Mater. Chem. Phys. 2022, 278, 125442. [Google Scholar] [CrossRef]
- Zubair Iqbal, M.; Wang, F.; Zhao, H.; Yasir Rafique, M.; Wang, J.; Li, Q. Structural and electrochemical properties of SnO nanoflowers as an anode material for lithium ion batteries. Scr. Mater. 2012, 67, 665–668. [Google Scholar] [CrossRef]
- Hiroyuki, Yamaguchi, Shinji, Nakanishi, Hideki, Iba, Takahito, Itoh Amorphous polymeric anode materials from poly(acrylic acid) and tin(II) oxide for lithium ion batteries. J. Power Source. 2025, 275, 1−5. [CrossRef]
- Jiang, Y.H.; Kao, P.K.; He, J.C.; Chiu, I.C.; Yang, Y.J.; Wu, Y.H.; Hsu, C.C.; Cheng, I.C.; Chen, J.Z. Optoelectronic properties of infrared rapid-thermal-annealed SnOx thin films. Ceram. Int. 2015, 41, 13502–13508. [Google Scholar] [CrossRef]
- Kim, Y.J.; Park, M.S.; Sohn, H.J.; Lee, H. Electrochemical behaviors of SnO and Sn anodes for lithium rechargeable batteries. J. Alloys Compd. 2011, 509, 4367–4371. [Google Scholar] [CrossRef]
- Kumar, R.; Kushwaha, N.; Mittal, J. Superior, rapid and reversible sensing activity of graphene-SnO hybrid film for low concentration of ammonia at room temperature. Sens. Actuators B Chem. 2017, 244, 243–251. [Google Scholar] [CrossRef]
- Martin, S.B.; Pedro, H.S.; Jae, J.K.; Harry, L.T.; José, A.V.; Marcelo, O.O. Gas sensor properties of Ag- and Pd-decorated SnO micro-disks to NO2, H2 and CO: Catalyst enhanced sensor response and selectivity. Sens. Actuators B Chem. 2017, 239, 253–261. [Google Scholar] [CrossRef]
- Kumar Rawat, R.; Chauhan, P. A novel approach to achieve the tin (II) oxide based proton conductive sensor for ammonia detection at room temperature. Mater. Lett. 2021, 285, 129049. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, J.; Zhang, D.; Schwingenschlögl, U.; Alshareef, H.N. Two-dimensional SnO anodes with a tunable number of atomic layers for sodium ion batteries. Nano Lett. 2017, 17, 1302–1311. [Google Scholar] [CrossRef]
- Kim, K.C.; Lee, D.H.; Maeng, S. Synthesis of novel pure SnO nanostructures by thermal evaporation. Mater. Lett. 2012, 86, 119–121. [Google Scholar] [CrossRef]
- Kaizra, S.; Bellal, B.; Louafi, Y.; Trari, M. Improved activity of SnO for the photocatalytic oxygen evolution. J. Saudi Chem. Soc. 2018, 22, 76–83. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, F.; Zubair Iqbal, M.; Wang, Z.; Li, Y.; Tu, J. Synthesis of novel 3D SnO flower-like hierarchical architectures self-assembled by nano-leaves and its photocatalysis. Mater. Res. Bull. 2015, 70, 784–788. [Google Scholar] [CrossRef]
- Ogo, Y.; Hiramatsu, H.; Nomura, K.; Yanagi, H.; Kamiya, T.; Hirano, M.; Hosono, H. p-channel thin-film transistor using p-type oxide semiconductor SnO. Appl. Phys. Lett. 2008, 93, 032113. [Google Scholar] [CrossRef]
- Haspulat, B.; Sarıbel, M.; Kamıs, H. Surfactant assisted hydrothermal synthesis of SnO nanoparticles with enhanced photocatalytic activity. Arab. J. Chem. 2020, 13, 96–108. [Google Scholar] [CrossRef]
- Nyiko, M.C.; Mpfunzeni, C.R. Tin oxide materials for industrial wastewater treatment: Promising adsorbents and catalyst. In Book Wastewater Treatment and Sludge Management Systems—The Gutter-to-Good Approaches; Taşeli, B.K., Jacob-Lopes, E., Deprá, C.M., Zepka, L.Q., Eds.; [Working Title]; IntechOpen: London, UK, 2024; 376p. [Google Scholar] [CrossRef]
- Sarala Devia, G.; Manasaa, M.V.; Vanaja, A. Evaluation of optical and structural properties of SnO nanoparticles synthesized via versatile hydrothermal protocol. Mater. Sci. Eng. B 2022, 261, 114629. [Google Scholar] [CrossRef]
- Liang, B.; Zhang, W.; Zhang, Y.; Zhang, R.; Liu, Y. Efficient visible-light photocatalyst synthesized by modifying SnO with activated carbon. Mater. Res. Express 2019, 6, 015603. [Google Scholar] [CrossRef]
- Lianga, B.; Hana, D.; Suna, C.; Zhanga, W.; Zhang, Y.; Zhang, R. Carbon black-modified SnO nanocomposites with improved photocatalytic activity. Fuller. Nanotub. Carbon Nanostructures 2018, 26, 751–755. [Google Scholar] [CrossRef]
- Huda, A.; Ichwani, R.; Tri Handoko, C.; Yudono, B.; Djoni Bustan, M.; Gulo, F. Enhancing the visible-light photoresponse of SnO and SnO2 through the heterostructure formation using one-step hydrothermal route. Mater. Lett. 2018, 238, 264–266. [Google Scholar] [CrossRef]
- Lianga, B.; Hana, D.; Suna, C.; Zhanga, W.; Qin, Q. Synthesis of SnO/g-C3N4 visible light driven photocatalysts via grinding assisted ultrasonic route. Ceram. Int. 2018, 44, 7315–7318. [Google Scholar] [CrossRef]
- Jaśkaniec, S.; Kavanagh, S.R.; Coelho, J.; Ryan, S.; Hobbs, C.; Walsh, A.; Scanlon, D.O.; Nicolosi, V. Solvent engineered synthesis of layered SnO for high-performance anodes. NPJ 2D Mater. Appl. 2021, 5, 27. [Google Scholar] [CrossRef]
- Wang, Z.L. Transmission electron microscopy of shape-controlled nanocrystals and their assemblies. J. Phys. Chem. B 2000, 104, 1153–1175. [Google Scholar] [CrossRef]
- Uchlyama, H.; Hosono, E.; Honma, I.; Zhou, H.; Imai, H. A nanoscale meshed electrode of single-crystalline SnO for lithium-ion rechargeable batteries. Electrochem. Commun. 2008, 10, 52–55. [Google Scholar] [CrossRef]
- Zhao, Z.-Y. Theoretical Study of Pt Cocatalyst Loading on Anatase TiO2(101) Surface: From Surface Doping to Interface Forming. J. Phys. Chem. C 2014, 118, 24591–24602. [Google Scholar] [CrossRef]
- Hejduk, P.; Witko, M.; Hermann, K. Electronic sructure of unsaturated V2O5(001) and (100) surfaces: Ab initio density functional theory studies. Top. Catal. 2009, 52, 1105–1115. [Google Scholar] [CrossRef]
- Abbasi, M.; Rozati, S.M.; Irani, R.; Beke, S. Synthesis and gas sensing behavior of nanostructured V2O5 thin films prepared by spray pyrolysis. Mater. Sci. Semicond. Process. 2015, 29, 132–138. [Google Scholar] [CrossRef]
- Hermann, K.; Witko, M.; Michalak, A. Density functional studies of the electronic structure and adsorption at molybdenum oxide surfaces. Catal. Today 1999, 50, 567–577. [Google Scholar] [CrossRef]
- Zhang, C.; Uchikoshi, T.; Ishigaki, T. Effect of crystalline orientation on photocatalytic performance for Nb-doped TiO2 nanoparticles. Adv. Powder Technol. 2021, 32, 4149–4154. [Google Scholar] [CrossRef]
- Santhi, K.; Rani, C.; Karuppuchamy, S. Synthesis and characterization of a novel SnO/SnO2 hybrid photocatalyst. J. Alloys Compd. 2016, 662, 102–107. [Google Scholar] [CrossRef]
- Sakaushi, K.; Oaki, Y.; Uchiyama, H.; Hosono, E.; Zhou, H.; Imai, H. Synthesis and applications of SnO nanosheets: Parallel control of oxidation state and nanostructure through an aqueous solution route. Small 2010, 6, 776–781. [Google Scholar] [CrossRef]
- Vázquez-López, A.; Martínez-Casado, R.; Cremades, A.; Maestre, D. Effect of Li-doping on the optoelectronic properties and stability of tin(II) oxide (SnO) nanostructures. J. Alloys Compd. 2023, 959, 170490. [Google Scholar] [CrossRef]
- Ning, J.J.; Dai, Q.Q.; Jiang, T.; Men, K.K.; Liu, D.H.; Xiao, N.R.; Li, C.Y.; Li, D.M.; Liu, B.B.; Zou, B.; et al. Facile synthesis of tin oxide nanoflowers: A potential high-capacity lithium-ion-storage material. Langmuir 2009, 25, 1818–1821. [Google Scholar] [CrossRef]
- Ning, J.J.; Jiang, T.; Men, K.K.; Dai, Q.Q.; Li, D.M.; Wei, Y.J.; Liu, B.B.; Chen, G.; Zou, B.; Zou, G.T. Syntheses, characterizations, and applications in lithium ion batteries of hierarchical SnO nanocrystals. J. Phys. Chem. C 2009, 113, 14140–14144. [Google Scholar] [CrossRef]
- Han, C.-Y.; Wang, S.-Y.; Wu, N.-L.; Rusakova, I.A. Microwave-assisted solution synthesis of SnO nanocristallites. Mater. Lett. 2002, 53, 155–159. [Google Scholar]
- Pires, F.I.; Joanni, E.; Savu, R.; Zaghete, M.A.; Longo, E.; Varela, J.A. Microwave-assisted hydrothermal synthesis of nanocristalline SnO powders. Mater. Lett. 2008, 26, 239–242. [Google Scholar] [CrossRef]
- Zubair Iqbal, M.; Wang, F.; Quarat u lain, J.; Rafique, M.Y.; Qiu, H.; Ghulam, N. Synthesis of novel nano-flowers assembled with nano-petals array of stannous oxide. Mater. Lett. 2012, 75, 236–239. [Google Scholar] [CrossRef]
- Jia, Z.-j.; Zhu, L.-p.; Liao, G.-h.; Yu, Y.; Tang, Y.-w. Preparation and characterization of SnO nanowhiskers. Solid State Commun. 2004, 132, 79–82. [Google Scholar] [CrossRef]
- Liu, B.; Ma, J.H.; Zhao, H.; Chen, Y.; Yang, H.Q. Room-temperature synthesis, photoluminescence and photocatalytic properties of SnO nanosheet-based flowerlike architectures. Appl. Phys. A 2012, 107, 437–443. [Google Scholar] [CrossRef]
- Sakaushi, K.; Oaki, Y.; Uchiyama, H.; Hosono, E.; Zhou, H.S.; Imai, H. Aqueous solution synthesis of SnO nanostructures with tuned optical absorption behavior and photoelectrochemical properties through morphological evolution. Nanoscale 2010, 2, 2424–2430. [Google Scholar] [CrossRef]
- Uchiyama, H.; Imai, H. Tin oxide meshes consisting of nanoribbons prepared through an intermediate phase in an aqueous solution. Cryst. Growth Des. 2007, 7, 841–843. [Google Scholar] [CrossRef]
- Kim, J.H.; Jeon, K.M.; Park, J.S.; Kang, Y.C. Excellent Li-ion storage performances of hierarchical SnO–SnO2 composite powders and SnO nanoplates prepared by one-pot spray pyrolysis. J. Power Sources 2017, 359, 363–370. [Google Scholar] [CrossRef]
- Xu, X.B.; Ge, M.Y.; Stahl, K.; Jiang, J.Z. Growth mechanism of cross-like SnO structure synthesized by thermal decomposition. Chem. Phys. Lett. 2009, 482, 287–290. [Google Scholar] [CrossRef]
- Chen, M.H.; Huang, Z.C.; Wu, G.T.; Zhu, G.M.; You, J.K.; Lin, Z.G. Synthesis and characterization of SnO-carbon nanotube composite as anode material for lithium-ion batteries. Mater. Res. Bull. 2003, 38, 831–836. [Google Scholar] [CrossRef]
- Chen, M.H.; Huang, Z.C.; Wu, G.T.; Zhu, G.M.; You, J.K.; Lin, Z.G. Solvothermal preparation and morphological evolution of stannous oxide powders. Mater. Lett. 2001, 48, 99–103. [Google Scholar] [CrossRef]
- Zubair Iqbal, M.; Wang, F.; Feng, T.; Zhao, H.; Rafique, M.Y.; ud Din, R.; ul ain Javed, Q. Facile synthesis of self-assembled SnO nano-square sheets and hydrogen absorption characteristics. Mater. Res. Bull. 2012, 47, 3902–3907. [Google Scholar] [CrossRef]
- Kuznetsova, S.A.; Lisitsa, K.V.; Malchik, A.G.; Kozik, V.V. Oxygen solubility in a suspension of tin (II) oxyhydroxide, a precursor in the synthesis of tin (II) and (IV) oxides. Her. Bauman Mosc. State Tech. Univ. Ser. Nat. Sci. 2018, 5, 112–124. [Google Scholar]
- Arshia, M.H.; Sheshmani, S.; Shahvelayati, A.S.; Hajiaghababaei, L.; Allahgholi Ghasri, M.R. Intercalated chitosan-ionic liquid ionogel in SnO nanoplate: Band gap narrow and adsorption-photodegradation process. Int. J. Biol. Macromol. 2023, 234, 123660. [Google Scholar] [CrossRef]
- Mishra, S.R.; Ahmaruzzaman, M. Tin oxide based nanostructured materials: Synthesis and potential applications. Nanoscale 2022, 14, 1566–1605. [Google Scholar] [CrossRef]
- Dias, J.S.; Batista, F.R.M.; Bacani, R.; Tribon, E.R. Structural characterization of SnO nanoparticles synthesized by the hydrothermal and microwave routes. Sci. Rep. 2020, 10, 9446. [Google Scholar] [CrossRef]
- Wang, L.; Ji, H.; Zhu, F.; Chen, Z.; Yang, Y.; Jiang, X.; Pintoc, J.; Yang, G. Large-scale preparation of shape-controlled SnO and improved capacitance for supercapacitors: From nanoclusters to square microplates. Nanoscale 2013, 5, 7613–7621. [Google Scholar] [CrossRef]
- Ren, Q.; Zhang, X.; Guo, Y.; Xu, M.; Zhu, H.; Yun, J.; Zhao, W.; Zhang, Z.; Wang, Y. Shape-controlled SnO and their improved properties in the field of gas sensor, photocatalysis and lithium-ion battery. Sens. Actuators B. Chem. 2022, 372, 132622. [Google Scholar] [CrossRef]
- Liang, Y.; Zheng, H.; Fang, B. Synthesis and characterization of SnO with controlled flowerlike microstructures. Mater. Lett. 2013, 108, 235–238. [Google Scholar] [CrossRef]
- Domashevskaya, É.P.; Ryabtsev, S.V.; Turishchev, S.Y.; Kashkarov, V.M.; Yurakov, Y.A.; Chuvenkova, O.A.; Shchukarev, A.V. XPS and xanes studies of SnOx nanolayers. J. Struct. Chem. 2008, 49, S80. [Google Scholar] [CrossRef]
- Fondella, M.; Gorgoib, M.; Bomana, M.; Lindblada, A. An HAXPES study of Sn, SnS, SnO and SnO2. J. Electron Spectrosc. Relat. Phenom. 2014, 195, 195–199. [Google Scholar] [CrossRef]
- Reyes-Gonzalez, J.; Garcia-Gutierrez, D.; Garza-Navarro, M.; Garcia-Gutierrez, D. Structure composition and morphology of self-assembled 2d nanostructures based on SnO2 nanoparticles observed in unannealed Mn doped hydrated form of tin oxide (II) or (IV) synthesized by Co-precipitation method. Curr. Nanomater. 2023, 8, 385–396. [Google Scholar] [CrossRef]
- Furushima, R.; Tanaka, S.; Kato, Z.; Uematsu, K. Orientation distribution–Lotgering factor relationship in a polycrystalline material–as an example of bismuth titanate prepared by a magnetic field. J. Ceram. Soc. Jpn. 2010, 118, 921–926. [Google Scholar] [CrossRef]
- Batzill, M.; Diebold, U. The surface and materials science of tin oxide. Prog. Surf. Sci. 2005, 79, 47–154. [Google Scholar] [CrossRef]
- Damoyi, N.E.; Friedrich, H.B.; Kruger, G.H.; Willock, D.J. A DFT study of the catalytic ODH of n-hexane over a cluster model of vanadium oxide. Mol. Catal. 2023, 541, 113078. [Google Scholar] [CrossRef]




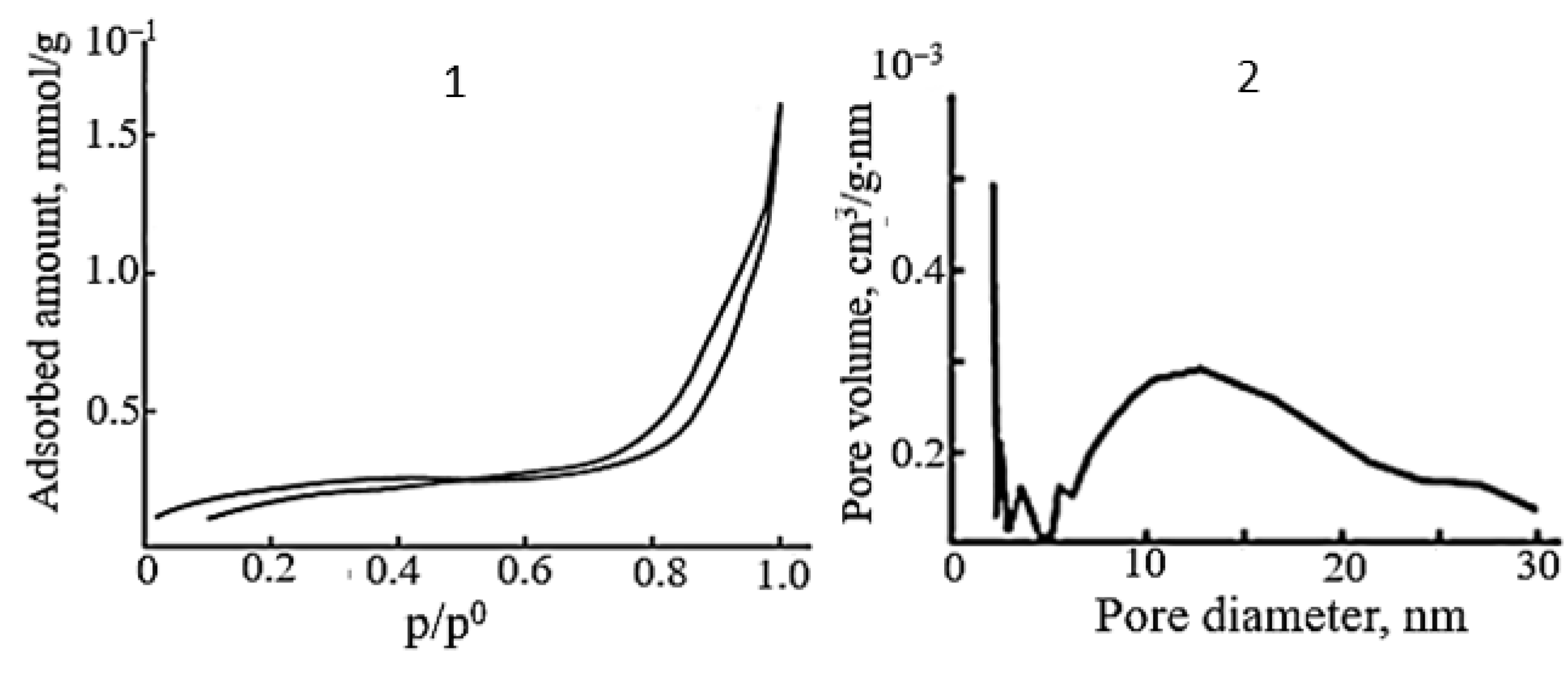

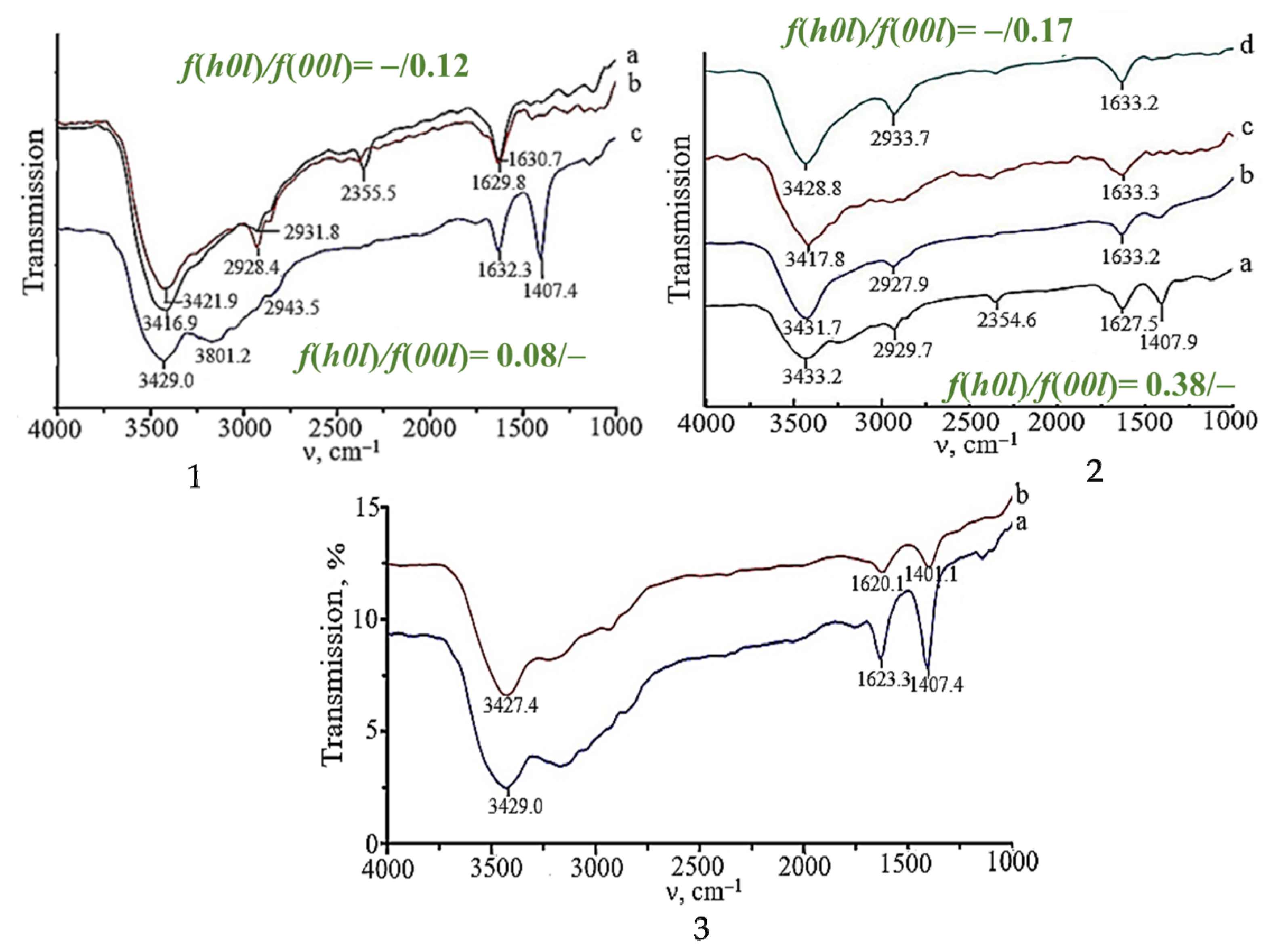
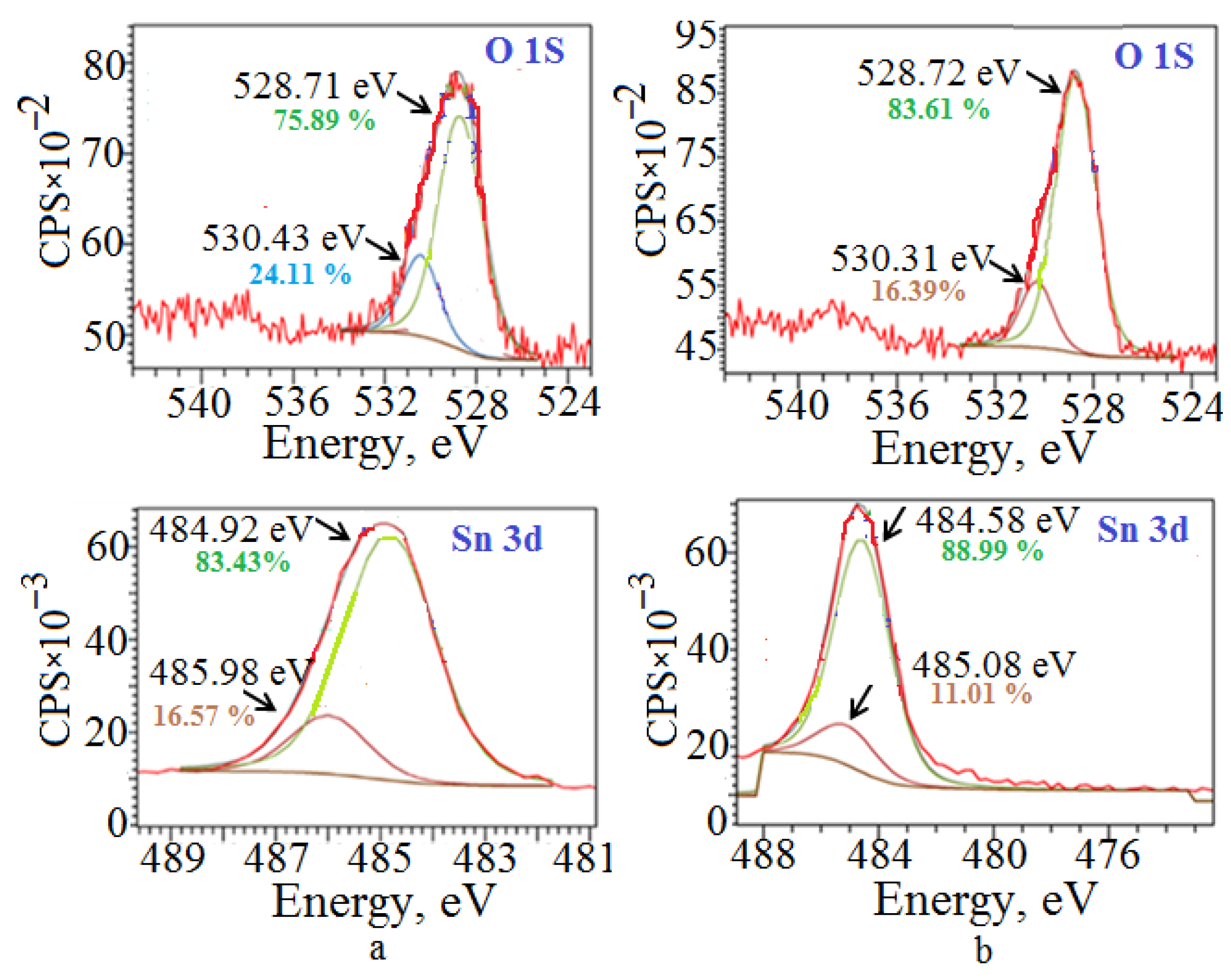


| Form of Agglomerates | Nanoplate Size, Length/Width/Thickness, nm | S, m2/g | ΔE, eV | Dye */kυ, 10−2 min−1 | Reference |
|---|---|---|---|---|---|
| Flower | 800/400/25 | 7.59 | 2.59 | MO/0.996 | [12] |
| Square | 100–200/–/– | – | 2.99 | MB/1.126 RdB/0.593 | [14] |
| Sphere | 10–50/–/– | – | 1.12 | – | [19] |
| Sphere | 22.6/–/– 21.7/–/– 19.3/–/– | 73.46 108.44 157.45 | 3.70 4.00 4.20 | – | [16] |
| Flower | 10–30/–/– | 52.90 | 2.65 | MO/– | [17] |
| Flower | –/–/48 | – | 2.60 | KG/0.390 | [1] |
| Flower | –/–/20–30 | – | 2.75 | MO/– | [18] |
| Flower | 500/–/– | – | – | MO/0.013 | [20] |
| Flower | – | – | 1.46 | – | [11] |
| The Composition of Suspension Medium | Time of MW Treatment | |||||||
|---|---|---|---|---|---|---|---|---|
| 5 min | 7 min | 10 min | 15 min | |||||
| Element, ±5 at. % | ||||||||
| Sn | O | Sn | O | Sn | O | Sn | O | |
| NH4Cl–NH3 | 51.6 | 48.4 | 51.2 | 48.8 | 50.9 | 49.1 | 51.1 | 48.9 |
| NaCl–NaOH– Na2CO3 | 50.9 | 49.1 | 51.5 | 48.5 | 50.3 | 49.7 | 50.9 | 49.1 |
| Group A | Group B | ||
|---|---|---|---|
| Sample, AP00l | Lotgering coefficients f (±0.002) and repeatability factor P (±0.2%) | Sample, БP00l | Lotgering coefficients f (±0.002) and repeatability factor P (±0.2%) |
| 1A17.1 | f(h0l) = 0.082; f(00l) = – P(00l) = 17.1% | 1B27.6 | f(h0l) = –; f(00l) = 0.122 P(00l) = 27.6% |
| 2A29.8 | f(h0l) = 0.330; f(00l) = – P(00l) = 29.8% | 2B39.1 | f(h0l) = –; f(00l) = 0.167 P(00l) = 39.1% |
| 3A33.1 | f(h0l) = 0.382; f(00l) = – P(00l) = 33.1% | 3B48.8 | f(h0l) = –; f(00l) = 0.311 P(00l) = 48.8% |
| Photocatalyst | Sorption of MO, ±1 wt. % | α, ±0.5 wt. % | kν·10−2, ±0.1 min−1 |
|---|---|---|---|
| 1A17.1 | 49 | 95.0 | 1.3 |
| 2A29.8 | 36 | 96.2 | 2.8 |
| 3A33.1 | 35 | 96.0 | 2.7 |
| 1B27.6 | 44 | 65.0 | 0.9 |
| 2B39.1 | 46 | 54.1 | 0.5 |
| 3B48.8 | 4 | – | – |
| P25 (TiO2) | 27 | 43.9 | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuznetsova, S.A.; Khalipova, O.S.; Chen, Y.-W. Creation of Crystalline Orientation of Tin(II) Oxide Polycrystals with High Photocatalytic Activity. Molecules 2025, 30, 2870. https://doi.org/10.3390/molecules30132870
Kuznetsova SA, Khalipova OS, Chen Y-W. Creation of Crystalline Orientation of Tin(II) Oxide Polycrystals with High Photocatalytic Activity. Molecules. 2025; 30(13):2870. https://doi.org/10.3390/molecules30132870
Chicago/Turabian StyleKuznetsova, Svetlana A., Olga S. Khalipova, and Yu-Wen Chen. 2025. "Creation of Crystalline Orientation of Tin(II) Oxide Polycrystals with High Photocatalytic Activity" Molecules 30, no. 13: 2870. https://doi.org/10.3390/molecules30132870
APA StyleKuznetsova, S. A., Khalipova, O. S., & Chen, Y.-W. (2025). Creation of Crystalline Orientation of Tin(II) Oxide Polycrystals with High Photocatalytic Activity. Molecules, 30(13), 2870. https://doi.org/10.3390/molecules30132870








