One-Pot Synthesis of Novel Pyrimidine Derivatives with Potential Antidiabetic Activity Through Dual α-Glucosidase and α-Amylase Inhibitors
Abstract
1. Introduction
2. Results and Discussion
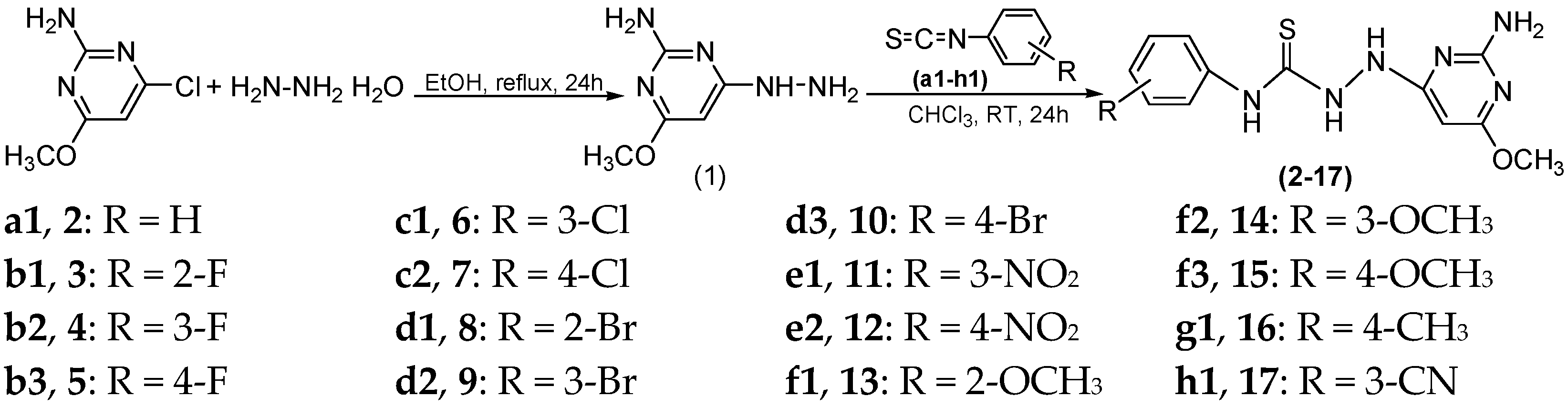
2.1. Chemistry
2.2. Biological Evaluation
Antidiabetic Activity
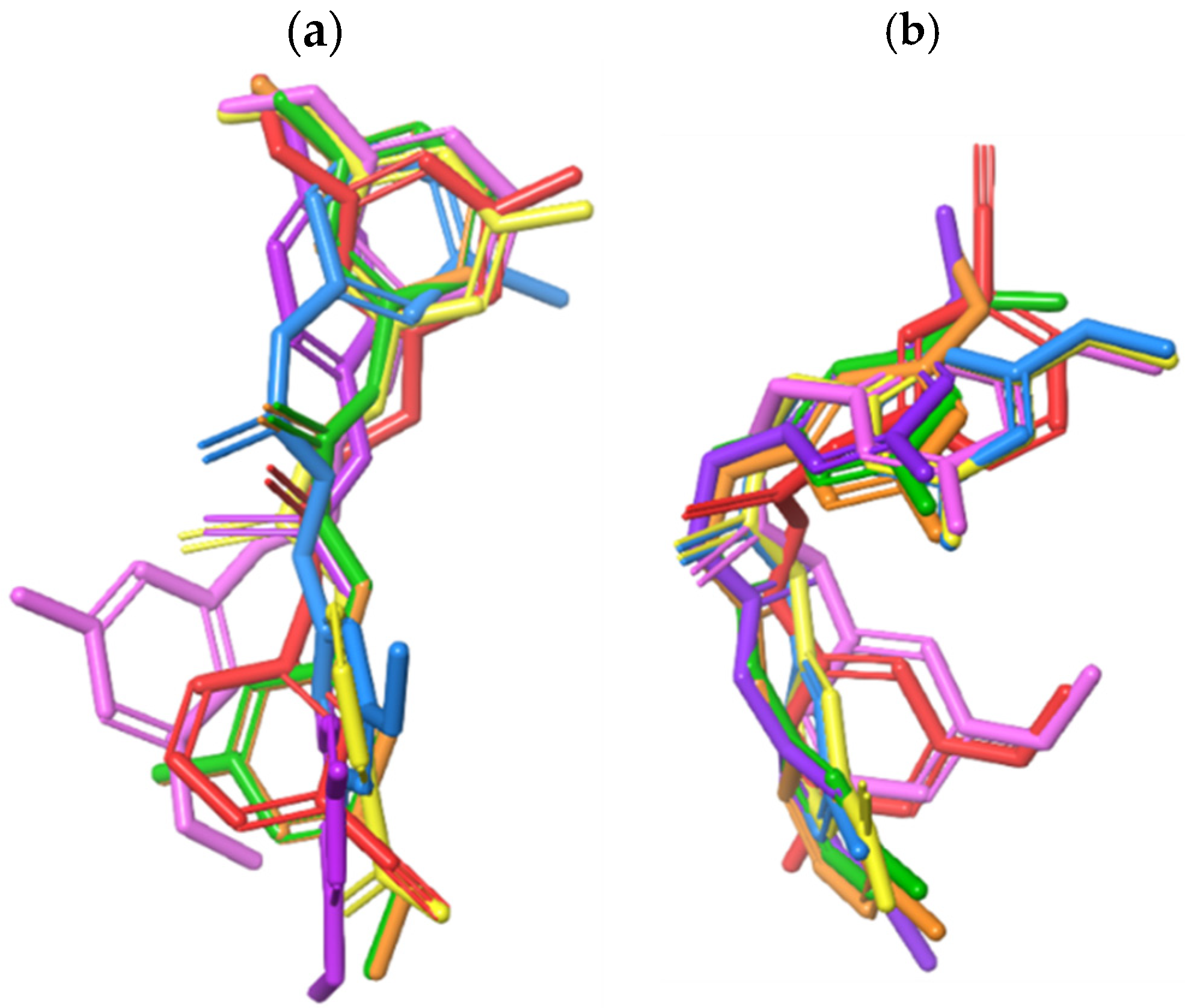
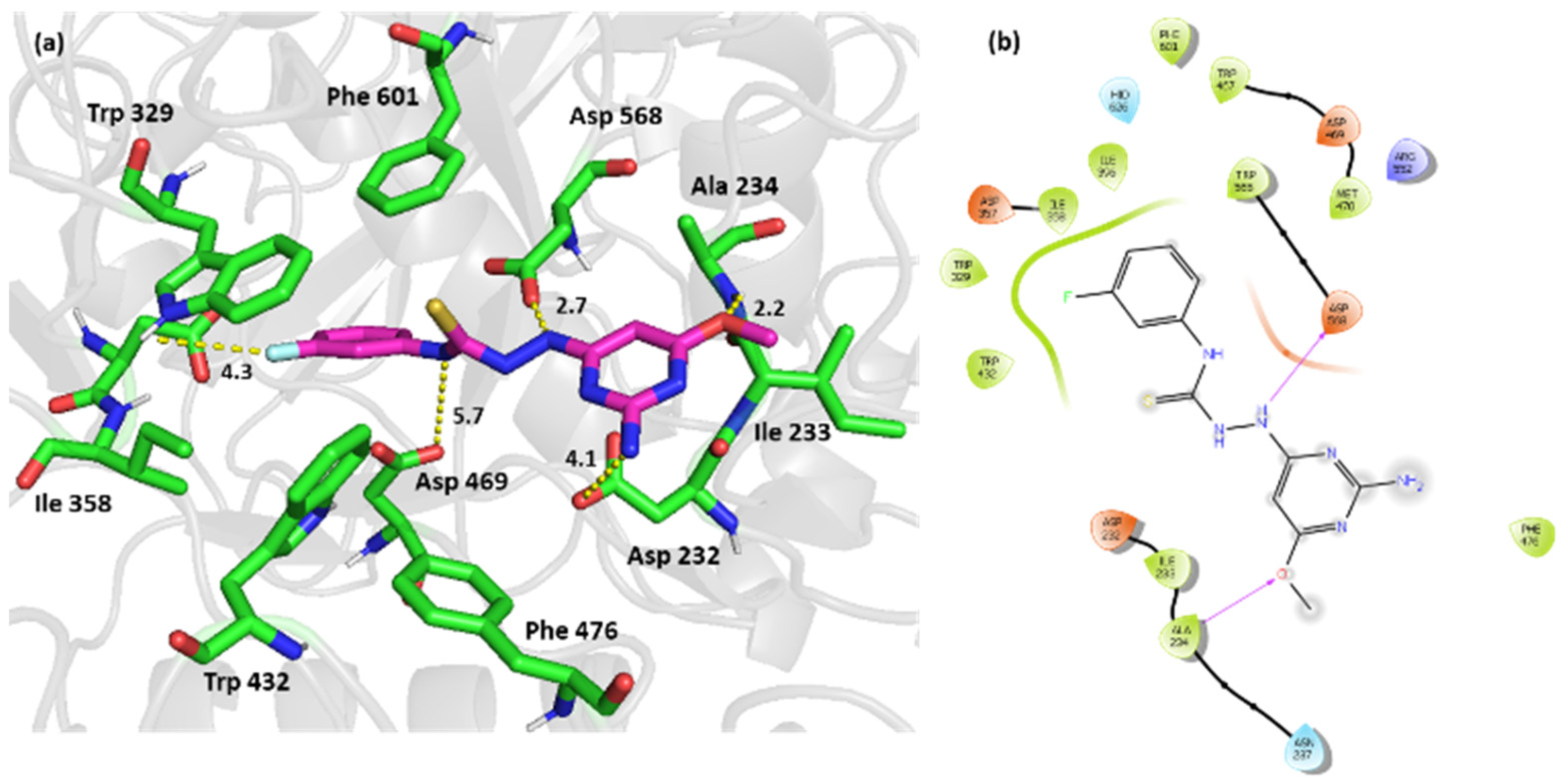
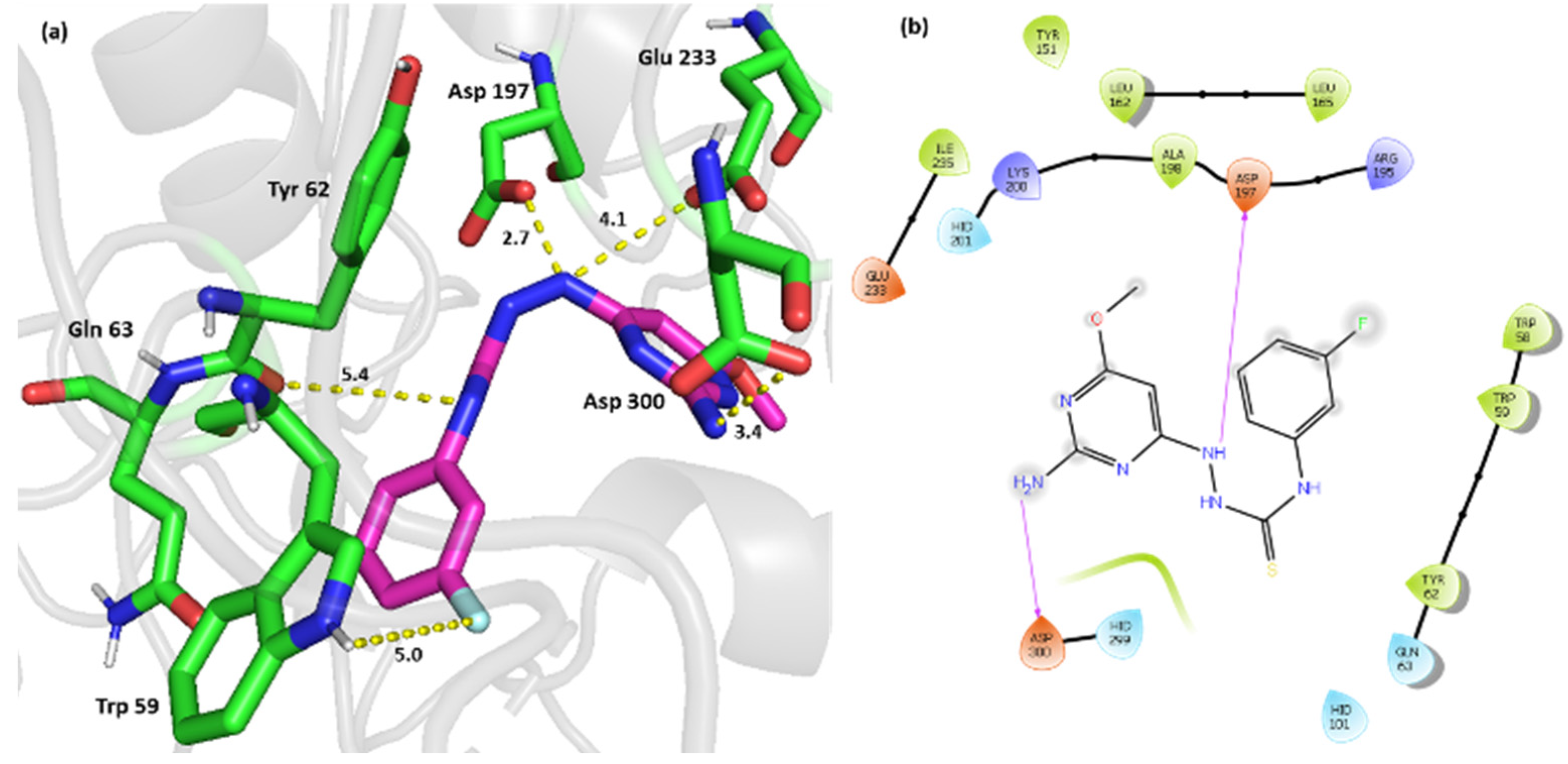
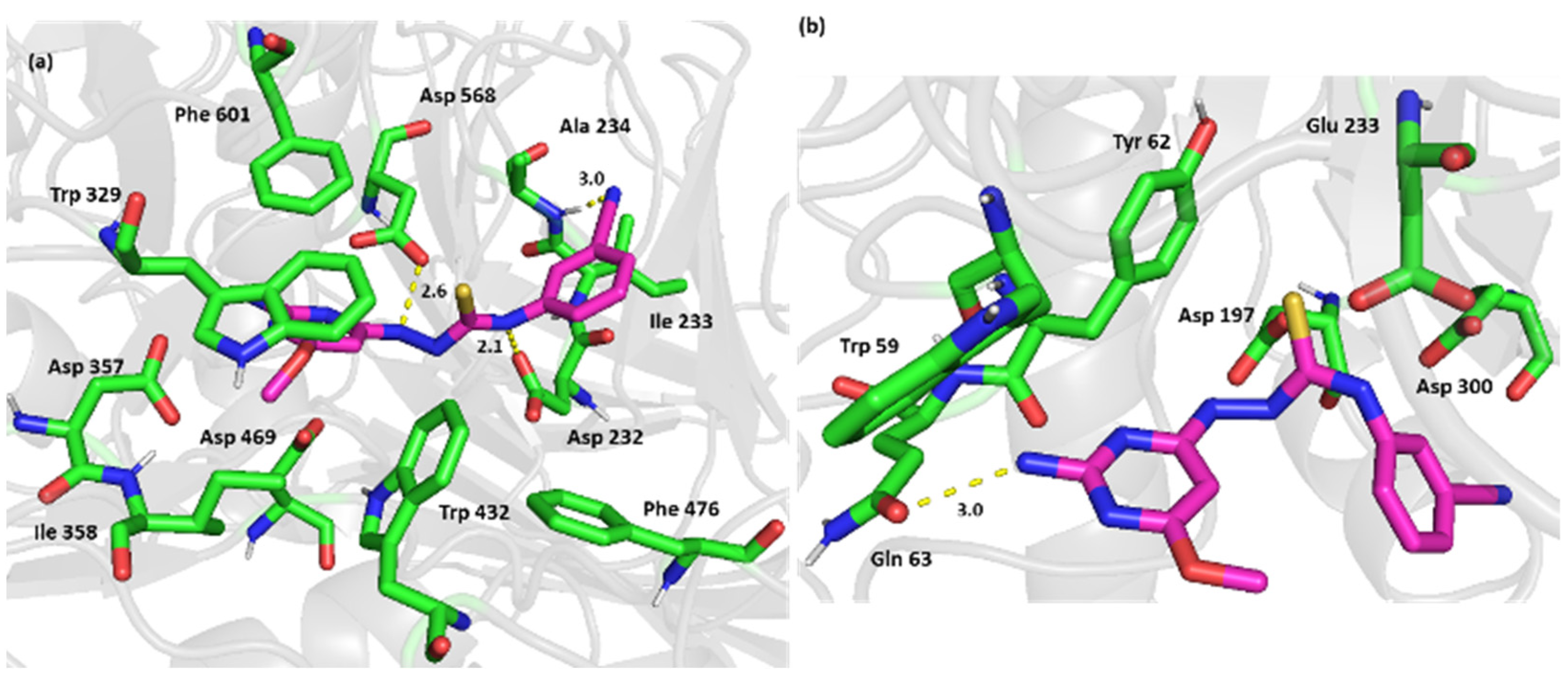
2.3. Computational Simulation Studies
3. Materials and Methods
3.1. General Methods
3.2. General Procedures of Synthesis New Compounds
3.2.1. Synthesis of 2-Amino-4-hydrazinyl-6-methoxypyrimidine (1)
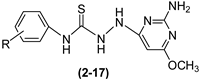
3.2.2. Reaction of 2-Amino-4-hydrazinyl-6-methoxypyrimidine with Phenyl Isothiocyanate Derivatives to Give Compounds (2–17)
2-(2-Amino-6-methoxypyrimidin-4-yl)-N-phenylhydrazine-carbothioamide (2)
2-(2-Amino-6-methoxypyrimidin-4-yl)-N-(2-fluorophenyl)-hydrazine Carbothioamide (3)
2-(2-Amino-6-methoxypyrimidin-4-yl)-N-(3-fluorophenyl)hydrazine Carbothioamide (4)
2-(2-Amino-6-Methoxypyrimidin-4-yl)-N-(4-Fluorophenyl)Hydrazine Carbothioamide (5)
2-(2-Amino-6-methoxypyrimidin-4-yl)-N-(3-chlorophenyl) Hydrazine Carbothioamide (6)
2-(2-Amino-6-methoxypyrimidin-4-yl)-N-(4-chlorophenyl)-hydrazine Carbothioamide (7)
2-(2-Amino-6-methoxypyrimidin-4-yl)-N-(2-bromophenyl)-hydrazine Carbothioamide (8)
2-(2-Amino-6-methoxypyrimidin-4-yl)-N-(3-bromophenyl)-hydrazine Carbothioamide (9)
2-(2-Amino-6-methoxypyrimidin-4-yl)-N-(4-bromophenyl)-hydrazine Carbothioamide (10)
2-(2-Amino-6-methoxypyrimidin-4-yl)-N-(3-nitrophenyl)hydrazine Carbothioamide (11)
2-(2-Amino-6-methoxypyrimidin-4-yl)-N-(4-nitrophenyl)hydrazine Carbothioamide (12)
2-(2-Amino-6-methoxypyrimidin-4-yl)-N-(2-methoxyphenyl)-hydrazine Carbothioamide (13)
2-(2-Amino-6-methoxypyrimidin-4-yl)-N-(3-methoxyphenyl)-hydrazine Carbothioamide (14)
2-(2-Amino-6-methoxypyrimidin-4-yl)-N-(4-methoxyphenyl) Hydrazine Carbothioamide (15)
2-(2-Amino-6-methoxypyrimidin-4-yl)-N-(p-tolyl)hydrazine Carbothioamide (16)
2-(2-Amino-6-methoxypyrimidin-4-yl)-N-(3-cyanophenyl)hydrazine Carbothioamide (17)
3.3. Biological Activity as Antidiabetic Activity of Most of Synthetic Compound of Pyrimidine Derivatives
3.3.1. α-Amylase Activity Assay
3.3.2. α-Glucosidase Activity Assay
3.4. Computational Simulation Studies
3.4.1. Crystal Structures
3.4.2. Protein Preparation
3.5. Receptor Grid Generation
3.5.1. Ligand Library Preparation
3.5.2. Validation of Molecular Docking
3.5.3. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joule, J.; Mills, K. Heterocyclic Chemistry at A Glance, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012; ISBN 978-1-118-38019-2. [Google Scholar]
- Kumar, S.; Narasimhan, B. Therapeutic Potential of Heterocyclic Pyrimidine Scaffolds. Chem. Cent. J. 2018, 12, 38. [Google Scholar] [CrossRef]
- Eicher, T.; Hauptmann, S.; Speicher, A. The Chemistry of Heterocycles: Structures, Reactions, Synthesis, and Applications, 3rd ed.; Wiley-VCH: Hoboken, NJ, USA, 2012; ISBN 978-3-527-32747-8. [Google Scholar]
- Becker, S.; Feldmann, J.; Wiedemann, S.; Okamura, H.; Schneider, C.; Iwan, K.; Crisp, A.; Rossa, M.; Amatov, T.; Carell, T. Unified Prebiotically Plausible Synthesis of Pyrimidine and Purine RNA Ribonucleotides. Science 2019, 366, 76–82. [Google Scholar] [CrossRef]
- Xu, J.; Tsanakopoulou, M.; Magnani, C.J.; Szabla, R.; Šponer, J.E.; Šponer, J.; Góra, R.W.; Sutherland, J.D. A Prebiotically Plausible Synthesis of Pyrimidine β-Ribonucleosides and Their Phosphate Derivatives Involving Photoanomerization. Nat. Chem. 2017, 9, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Singh, L.; Chibale, K.; Singh, K. Structure Elaboration of Isoniazid: Synthesis, in Silico Molecular Docking and Antimycobacterial Activity of Isoniazid–Pyrimidine Conjugates. Mol. Divers. 2020, 24, 949–955. [Google Scholar] [CrossRef]
- Pan, N.; Liu, C.; Wu, R.; Fei, Q.; Wu, W. Novel Pyrimidine Derivatives Bearing a 1,3,4-Thiadiazole Skeleton: Design, Synthesis, and Antifungal Activity. Front. Chem. 2022, 10, 922813. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Lan, W.; Wu, C.; Fei, Q. Synthesis and Antifungal Activity of Pyrimidine Derivatives Containing an Amide Moiety. Front. Chem. 2021, 9, 695628. [Google Scholar] [CrossRef]
- Tang, X.; Zhan, W.; Chen, S.; Zhou, R.; Hu, D.; Sun, N.; Fei, Q.; Wu, W.; Xue, W. Synthesis, Bioactivity and Preliminary Mechanism of Action of Novel Trifluoromethyl Pyrimidine Derivatives. Arab. J. Chem. 2022, 15, 104110. [Google Scholar] [CrossRef]
- Ren, Q.; Liu, X.; Luo, Z.; Li, J.; Wang, C.; Goldmann, S.; Zhang, J.; Zhang, Y. Discovery of Hepatitis B Virus Capsid Assembly Inhibitors Leading to a Heteroaryldihydropyrimidine Based Clinical Candidate (GLS4). Bioorg. Med. Chem. 2017, 25, 1042–1056. [Google Scholar] [CrossRef] [PubMed]
- Albratty, M.; Alhazmi, H.A. Novel Pyridine and Pyrimidine Derivatives as Promising Anticancer Agents: A Review. Arab. J. Chem. 2022, 15, 103846. [Google Scholar] [CrossRef]
- Gaber, A.A.; Sobhy, M.; Turky, A.; Abdulwahab, H.G.; Al-Karmalawy, A.A.; Elhendawy, M.A.; Radwan, M.M.; Elkaeed, E.B.; Ibrahim, I.M.; Elzahabi, H.S.A.; et al. Discovery of New 1 H-Pyrazolo[3,4-d ]Pyrimidine Derivatives as Anticancer Agents Targeting EGFRWT and EGFRT790M. J. Enzyme Inhib. Med. Chem. 2022, 37, 2283–2303. [Google Scholar] [CrossRef]
- Becan, L.; Pyra, A.; Rembiałkowska, N.; Bryndal, I. Synthesis, Structural Characterization and Anticancer Activity of New 5-Trifluoromethyl-2-Thioxo-Thiazolo[4,5-d]Pyrimidine Derivatives. Pharmaceuticals 2022, 15, 92. [Google Scholar] [CrossRef] [PubMed]
- Al-Masoudi, W.A.; Al-Masoudi, N.A.; Weibert, B.; Winter, R. Synthesis, X-Ray Structure, in Vitro HIV and Kinesin Eg5 Inhibition Activities of New Arene Ruthenium Complexes of Pyrimidine Analogs. J. Coord. Chem. 2017, 70, 2061–2073. [Google Scholar] [CrossRef]
- Al-Masoudi, W.A.; Al-Masoudi, N.A. A Ruthenium Complexes of Monastrol and Its Pyrimidine Analogues: Synthesis and Biological Properties. Phosphorus Sulfur Silicon Relat. Elem. 2019, 194, 1020–1027. [Google Scholar] [CrossRef]
- Sharaky, M.; Kamel, M.; Aziz, M.A.; Omran, M.; Rageh, M.M.; Abouzid, K.A.M.; Shouman, S.A. Design, Synthesis and Biological Evaluation of a New Thieno[2,3- d ]Pyrimidine-Based Urea Derivative with Potential Antitumor Activity against Tamoxifen Sensitive and Resistant Breast Cancer Cell Lines. J. Enzyme Inhib. Med. Chem. 2020, 35, 1641–1656. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Z.; Gao, C.; Dai, H.; Si, X.; Zhang, Y.; Meng, Y.; Zheng, J.; Ke, Y.; Liu, H.; et al. Design, Synthesis and Antitumor Activity Evaluation of Trifluoromethyl-Substituted Pyrimidine Derivatives. Bioorg. Med. Chem. Lett. 2021, 51, 128268. [Google Scholar] [CrossRef]
- Thangarasu, P.; Manikandan, A.; Thamaraiselvi, S. Discovery, Synthesis and Molecular Corroborations of Medicinally Important Novel Pyrazoles; Drug Efficacy Determinations through in Silico, in Vitro and Cytotoxicity Validations. Bioorg. Chem. 2019, 86, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Batista, D.; Schwarz, S.; Loesche, A.; Csuk, R.; Costa, P.J.; Oliveira, M.C.; Xavier, N.M. Synthesis of Glucopyranos-6′-Yl Purine and Pyrimidine Isonucleosides as Potential Cholinesterase Inhibitors. Access to Pyrimidine-Linked Pseudodisaccharides through Mitsunobu Reaction. Pure Appl. Chem. 2016, 88, 363–379. [Google Scholar] [CrossRef]
- Fatahala, S.S.; Mahgub, S.; Taha, H.; Abd-El Hameed, R.H. Synthesis and Evaluation of Novel Spiro Derivatives for Pyrrolopyrimidines as Anti-Hyperglycemia Promising Compounds. J. Enzyme Inhib. Med. Chem. 2018, 33, 809–817. [Google Scholar] [CrossRef]
- Peytam, F.; Adib, M.; Shourgeshty, R.; Firoozpour, L.; Rahmanian-Jazi, M.; Jahani, M.; Moghimi, S.; Divsalar, K.; Faramarzi, M.A.; Mojtabavi, S.; et al. An Efficient and Targeted Synthetic Approach towards New Highly Substituted 6-Amino-Pyrazolo[1,5-a]Pyrimidines with α-Glucosidase Inhibitory Activity. Sci. Rep. 2020, 10, 2595. [Google Scholar] [CrossRef]
- Rango, E.; Pastorino, F.; Brignole, C.; Mancini, A.; Poggialini, F.; Di Maria, S.; Zamperini, C.; Iovenitti, G.; Fallacara, A.L.; Sabetta, S.; et al. The Pyrazolo[3,4-d]Pyrimidine Derivative Si306 Encapsulated into Anti-GD2-Immunoliposomes as Therapeutic Treatment of Neuroblastoma. Biomedicines 2022, 10, 659. [Google Scholar] [CrossRef]
- Chen, L.; Magliano, D.J.; Zimmet, P.Z. The Worldwide Epidemiology of Type 2 Diabetes Mellitus—Present and Future Perspectives. Nat. Rev. Endocrinol. 2012, 8, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Munhoz, A.C.M.; Frode, T.S. Isolated Compounds from Natural Products with Potential Antidiabetic Activity—A Systematic Review. Curr. Diabetes Rev. 2018, 14, 36–106. [Google Scholar] [CrossRef]
- Kahn, S.E.; Cooper, M.E.; Del Prato, S. Pathophysiology and Treatment of Type 2 Diabetes: Perspectives on the Past, Present, and Future. Lancet 2014, 383, 1068–1083. [Google Scholar] [CrossRef]
- Nawaz, M.; Taha, M.; Qureshi, F.; Ullah, N.; Selvaraj, M.; Shahzad, S.; Chigurupati, S.; Waheed, A.; Almutairi, F.A. Structural Elucidation, Molecular Docking, α-Amylase and α-Glucosidase Inhibition Studies of 5-Amino-Nicotinic Acid Derivatives. BMC Chem. 2020, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Mittal, A.; Ramachandran, U. Design and Synthesis of 6-Methyl-2-Oxo-1,2,3,4-Tetrahydro-Pyrimidine-5-Carboxylic Acid Derivatives as PPARγ Activators. Bioorg. Med. Chem. Lett. 2007, 17, 4613–4618. [Google Scholar] [CrossRef]
- Bassyouni, F.; Tarek, M.; Salama, A.; Ibrahim, B.; Salah El Dine, S.; Yassin, N.; Hassanein, A.; Moharam, M.; Abdel-Rehim, M. Promising Antidiabetic and Antimicrobial Agents Based on Fused Pyrimidine Derivatives: Molecular Modeling and Biological Evaluation with Histopathological Effect. Molecules 2021, 26, 2370. [Google Scholar] [CrossRef]
- Becker, I. Preparation of Pyrimidine Derivatives as Potential Medicinal Agents by the Reaction of 2-Amino-4-Chloro-6-Methylpyrimidine with Primary and Secondary Amines. J. Heterocycl. Chem. 2005, 42, 1289–1295. [Google Scholar] [CrossRef]
- Selka, A.; Abidli, A.; Schiavo, L.; Jeanmart, L.; Hanquet, G.; Lubell, W.D. Recent Advances in Sustainable Total Synthesis and Chiral Pool Strategies with Emphasis on (−)-Sclareol in Natural Products Synthesis. Eur. J. Org. Chem 2025, 28, e202400983. [Google Scholar] [CrossRef]
- Nawaz, M.; Taha, M.; Qureshi, F.; Ullah, N.; Selvaraj, M.; Shahzad, S.; Chigurupati, S.; Abubshait, S.A.; Ahmad, T.; Chinnam, S.; et al. Synthesis, α-Amylase and α-Glucosidase Inhibition and Molecular Docking Studies of Indazole Derivatives. J. Biomol. Struct. Dyn. 2022, 40, 10730–10740. [Google Scholar] [CrossRef]
- Taha, M.; Khan, A.A.; Rahim, F.; Hayat, S.; Imran, S.; Iqbal, N.; Uddin, N.; Khan, K.M.; Anouar, E.H.; Farooq, R.K.; et al. Synthesis, in Vitro Evaluation and Molecular Docking Studies of Hybrid 4-Quinolinyl Bearing 1,3,4-Thiadiazole-2-Amine as a New Inhibitor of α-Amylase and α-Glucosidase. J. Mol. Struct. 2023, 1282, 135173. [Google Scholar] [CrossRef]
- Taha, M.; Salahuddin, M.; Almandil, N.B.; Farooq, R.K.; Rahim, F.; Uddin, N.; Nawaz, M.; Alhibshi, A.H.; Anouar, E.H.; Khan, K.M. In Vitro and in Vivo Anti Diabetics Study of New Oxadiazole Derivatives Along with Molecular Docking Study. Polycycl. Aromat. Compd. 2023, 43, 6911–6926. [Google Scholar] [CrossRef]
- Mekky, A.E.M.; Taha, N.A.S.; Mohammed, N.G.; Hussein, F.R.M.; Abdelfattah, E.H.; Gamal Eldin, A.A.; Abdelsalam, A.A.M.; Sanad, S.M.H. Development of Pyrazolo[1,5-a]Pyrimidine-Based Antibacterial Agents. Synth. Commun. 2023, 53, 1053–1068. [Google Scholar] [CrossRef]
- Elumalai, K.; Shanmugam, A.; Devaraji, M.; Srinivasan, S. Synthesis and Molecular Docking of Pyrimidine Derivatives as Antibacterial Agents. Carbon Resour. Convers. 2024, 7, 100222. [Google Scholar] [CrossRef]
- Alamshany, Z.M.; Tashkandi, N.Y.; Othman, I.M.M.; Ishak, E.A.; Gad-Elkareem, M.A.M. Synthesis, Antimicrobial and Antioxidant Activities of Some New Pyrazolo[1,5-a]Pyrimidine and Imidazo[1,2-b]Pyrazole Derivatives Based Isoxazole. Synth. Commun. 2023, 53, 1451–1467. [Google Scholar] [CrossRef]
- Teli, G.; Pal, R.; Maji, L.; Purawarga Matada, G.S.; Sengupta, S. Explanatory Review on Pyrimidine/Fused Pyrimidine Derivatives as Anticancer Agents Targeting Src Kinase. J. Biomol. Struct. Dyn. 2023, 42, 1582–1614. [Google Scholar] [CrossRef]
- Myriagkou, M.; Papakonstantinou, E.; Deligiannidou, G.-E.; Patsilinakos, A.; Kontogiorgis, C.; Pontiki, E. Novel Pyrimidine Derivatives as Antioxidant and Anticancer Agents: Design, Synthesis and Molecular Modeling Studies. Molecules 2023, 28, 3913. [Google Scholar] [CrossRef]
- Al Nasr, I.S.; Corona, A.; Koko, W.S.; Khan, T.A.; Ben Said, R.; Daoud, I.; Rahali, S.; Tramontano, E.; Schobert, R.; Amdouni, N.; et al. Versatile Anti-Infective Properties of Pyrido- and Dihydropyrido[2,3-d]Pyrimidine-Based Compounds. Bioorg. Med. Chem. 2023, 90, 117376. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, S.; Almarghalani, D.A.; James, A.W.; Raza, M.K.; Kausar, T.; Nayeem, S.M.; Hoda, N.; Shah, Z.A. Synthesis and Pharmacological Evaluation of Novel Triazole-Pyrimidine Hybrids as Potential Neuroprotective and Anti-Neuroinflammatory Agents. Pharm. Res. 2023, 40, 167–185. [Google Scholar] [CrossRef]
- Hassan, A.S.; Morsy, N.M.; Aboulthana, W.M.; Ragab, A. In Vitro Enzymatic Evaluation of Some Pyrazolo[1,5-a]Pyrimidine Derivatives: Design, Synthesis, Antioxidant, Anti-diabetic, anti-Alzheimer, and Anti-arthritic Activities with Molecular Modeling Simulation. Drug Dev. Res. 2023, 84, 3–24. [Google Scholar] [CrossRef]
- Amin, S.; Sheikh, K.A.; Iqubal, A.; Ahmed Khan, M.; Shaquiquzzaman, M.; Tasneem, S.; Khanna, S.; Najmi, A.K.; Akhter, M.; Haque, A.; et al. Synthesis, in-Silico Studies and Biological Evaluation of Pyrimidine Based Thiazolidinedione Derivatives as Potential Anti-Diabetic Agent. Bioorg. Chem. 2023, 134, 106449. [Google Scholar] [CrossRef]
- Mushtaq, A.; Azam, U.; Mehreen, S.; Naseer, M.M. Synthetic α-Glucosidase Inhibitors as Promising Anti-Diabetic Agents: Recent Developments and Future Challenges. Eur. J. Med. Chem. 2023, 249, 115119. [Google Scholar] [CrossRef] [PubMed]
- Finger, V.; Kufa, M.; Soukup, O.; Castagnolo, D.; Roh, J.; Korabecny, J. Pyrimidine Derivatives with Antitubercular Activity. Eur. J. Med. Chem. 2023, 246, 114946. [Google Scholar] [CrossRef]
- Sahoo, B.M.; Banik, B.K.; Kumar, B.V.V.R.; Panda, K.C.; Tiwari, A.; Tiwari, V.; Singh, S.; Kumar, M. Microwave Induced Green Synthesis: Sustainable Technology forEfficient Development of Bioactive Pyrimidine Scaffolds. Curr. Med. Chem. 2023, 30, 1029–1059. [Google Scholar] [CrossRef] [PubMed]
- Nammalwar, B.; Bunce, R.A. Recent Advances in Pyrimidine-Based Drugs. Pharmaceuticals 2024, 17, 104. [Google Scholar] [CrossRef]
- Patil, S.B. Recent Medicinal Approaches of Novel Pyrimidine Analogs: A Review. Heliyon 2023, 9, e16773. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Pemawat, G. Functionalized Pyrimidines: Synthetic Approaches and Biological Activities. A Review. Org. Prep. Proced. Int. 2024, 56, 1–18. [Google Scholar] [CrossRef]
- Acharya, P.T.; Bhavsar, Z.A.; Jethava, D.J.; Patel, D.B.; Patel, H.D. A Review on Development of Bio-Active Thiosemicarbazide Derivatives: Recent Advances. J. Mol. Struct. 2021, 1226, 129268. [Google Scholar] [CrossRef]
- Metwally, M.A.; Bondock, S.; El-Azap, H.; Kandeel, E.E.M. Thiosemicarbazides: Synthesis and Reactions. J. Sulfur Chem. 2011, 32, 489–519. [Google Scholar] [CrossRef]
- Kahvecioglu, D.; Ozguven, S.Y.; Sicak, Y.; Tok, F.; Öztürk, M.; Kocyigit-Kaymakcioglu, B. Synthesis and Molecular Docking Analysis of Novel Hydrazone and Thiosemicarbazide Derivatives Incorporating a Pyrimidine Ring: Exploring Neuroprotective Activity. J. Biomol. Struct. Dyn. 2024, 1–15. [Google Scholar] [CrossRef]
- Şenkardeş, S.; Bolat, İ.; Şahinbey, H.; Karakuş, S.; Erdoğan, Ö.; Cantürk, P.; Özgüven, S.Y.; Çevik, Ö. Design, Synthesis and Molecular Modeling Studies of Thiosemicarbazide & Thiazolyl-Hydrazone Derivatives as Potential Anticancer Agents and Topoisomerase Inhibitors. J. Mol. Struct. 2024, 1302, 137488. [Google Scholar] [CrossRef]
- Sherje, A.P.; Kulkarni, V.; Murahari, M.; Nayak, U.Y.; Bhat, P.; Suvarna, V.; Dravyakar, B. Inclusion Complexation of Etodolac with Hydroxypropyl-Beta-Cyclodextrin and Auxiliary Agents: Formulation Characterization and Molecular Modeling Studies. Mol. Pharm. 2017, 14, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Burley, S.K.; Bhikadiya, C.; Bi, C.; Bittrich, S.; Chen, L.; Crichlow, G.V.; Christie, C.H.; Dalenberg, K.; Di Costanzo, L.; Duarte, J.M.; et al. RCSB Protein Data Bank: Powerful New Tools for Exploring 3D Structures of Biological Macromolecules for Basic and Applied Research and Education in Fundamental Biology, Biomedicine, Biotechnology, Bioengineering and Energy Sciences. Nucleic Acids Res. 2021, 49, D437–D451. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Wu, C.; Ghoreishi, D.; Chen, W.; Wang, L.; Damm, W.; Ross, G.A.; Dahlgren, M.K.; Russell, E.; Von Bargen, C.D.; et al. OPLS4: Improving Force Field Accuracy on Challenging Regimes of Chemical Space. J. Chem. Theory Comput. 2021, 17, 4291–4300. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.; Froufe, H.; Costa, A.; Santos, A.; Oliveira, L.; Osório, S.; Abreu, R.; Pintado, M.; Ferreira, I. Docking Studies in Target Proteins Involved in Antibacterial Action Mechanisms: Extending the Knowledge on Standard Antibiotics to Antimicrobial Mushroom Compounds. Molecules 2014, 19, 1672–1684. [Google Scholar] [CrossRef]
- Kwofie, S.K.; Dankwa, B.; Odame, E.A.; Agamah, F.E.; Doe, L.P.A.; Teye, J.; Agyapong, O.; Miller, W.A.; Mosi, L.; Wilson, M.D. In Silico Screening of Isocitrate Lyase for Novel Anti-Buruli Ulcer Natural Products Originating from Africa. Molecules 2018, 23, 1550. [Google Scholar] [CrossRef]
- Jaundoo, R.; Bohmann, J.; Gutierrez, G.E.; Klimas, N.; Broderick, G.; Craddock, T.J.A. Using a Consensus Docking Approach to Predict Adverse Drug Reactions in Combination Drug Therapies for Gulf War Illness. Int. J. Mol. Sci. 2018, 19, 3355. [Google Scholar] [CrossRef]
 | |||
| Comp. No | R | α-Glucosidase (IC50, µM) | α-Amylase (IC50, µM) |
| 4 | 3-F | 12.16 ± 0.12 | 11.13 ± 0.12 |
| 6 | 3-Cl | 16.13 ± 0.11 | 15.17 ± 0.07 |
| 9 | 3-Br | 19.11 ± 0.11 | 18.16 ± 0.08 |
| 11 | 3-NO2 | 22.11 ± 0.13 | 21.14 ± 0.12 |
| 14 | 3-OCH3 | 30.12 ± 0.13 | 33.18 ± 0.14 |
| 16 | 4-CH3 | 36.14 ± 0.12 | 34.24 ± 0.16 |
| 17 | 3-CN | 42.19 ± 0.13 | 40.16 ± 0.11 |
| Acarbose | 10.60 ± 0.17 | 11.30 ± 0.12 | |
| Comp. No | α-Glucosidase | α-Amylase | ||||
|---|---|---|---|---|---|---|
| (IC50, µM) | XP Score | MM-GBSA dG Bind | (IC50, µM) | XP Score | MM-GBSA dG Bind | |
| 4 | 12.16 ± 0.12 | −4.1 | 5.3 | 11.13 ± 0.12 | −4.5 | −0.4 |
| 6 | 16.13 ± 0.11 | −5.0 | 13.6 | 15.17 ± 0.07 | −4.7 | −8.8 |
| 9 | 19.11 ± 0.11 | −4.8 | 12.7 | 18.16 ± 0.08 | −5.0 | 0.4 |
| 11 | 22.11 ± 0.13 | −3.9 | 29.2 | 21.14 ± 0.12 | −4.8 | −4.1 |
| 14 | 30.12 ± 0.13 | −2.9 | 17.3 | 33.18 ± 0.14 | −5.2 | 11.5 |
| 16 | 36.14 ± 0.12 | −4.8 | −2.7 | 34.24 ± 0.16 | −4.3 | −6.5 |
| 17 | 42.19 ± 0.13 | −2.4 | 16.0 | 40.16 ± 0.11 | −4.3 | −2.7 |
| Acarbose | 10.60 ± 0.17 | −11.5 | −29.9 | 11.30 ± 0.12 | −10.0 | −34.7 |
| Comp. No | α-Glucosidase | α-Amylase | ||
|---|---|---|---|---|
| (IC50, µM) | RMSD | (IC50, µM) | RMSD | |
| 4 | 12.16 ± 0.12 | 0.0 | 11.13 ± 0.12 | 0.0 |
| 6 | 16.13 ± 0.11 | 0.07 | 15.17 ± 0.07 | 0.6 |
| 9 | 19.11 ± 0.11 | 1.5 | 18.16 ± 0.08 | 1.7 |
| 11 | 22.11 ± 0.13 | 1.7 | 21.14 ± 0.12 | 1.7 |
| 14 | 30.12 ± 0.13 | 2.3 | 33.18 ± 0.14 | 1.8 |
| 16 | 36.14 ± 0.12 | 1.2 | 34.24 ± 0.16 | 1.2 |
| 17 | 42.19 ± 0.13 | 8.6 | 40.16 ± 0.11 | 6.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Shehri, O.; Abubshait, S.; Nawaz, M.; Gomaa, M.S.; Abubshait, H.A. One-Pot Synthesis of Novel Pyrimidine Derivatives with Potential Antidiabetic Activity Through Dual α-Glucosidase and α-Amylase Inhibitors. Molecules 2025, 30, 2857. https://doi.org/10.3390/molecules30132857
Al-Shehri O, Abubshait S, Nawaz M, Gomaa MS, Abubshait HA. One-Pot Synthesis of Novel Pyrimidine Derivatives with Potential Antidiabetic Activity Through Dual α-Glucosidase and α-Amylase Inhibitors. Molecules. 2025; 30(13):2857. https://doi.org/10.3390/molecules30132857
Chicago/Turabian StyleAl-Shehri, Ohood, Samar Abubshait, Muhammad Nawaz, Mohamed S. Gomaa, and Haya A. Abubshait. 2025. "One-Pot Synthesis of Novel Pyrimidine Derivatives with Potential Antidiabetic Activity Through Dual α-Glucosidase and α-Amylase Inhibitors" Molecules 30, no. 13: 2857. https://doi.org/10.3390/molecules30132857
APA StyleAl-Shehri, O., Abubshait, S., Nawaz, M., Gomaa, M. S., & Abubshait, H. A. (2025). One-Pot Synthesis of Novel Pyrimidine Derivatives with Potential Antidiabetic Activity Through Dual α-Glucosidase and α-Amylase Inhibitors. Molecules, 30(13), 2857. https://doi.org/10.3390/molecules30132857






