Modulation of Human Colon Cell Activity by Synthetic Coumarin Derivatives Bearing a Phosphonate Group
Abstract
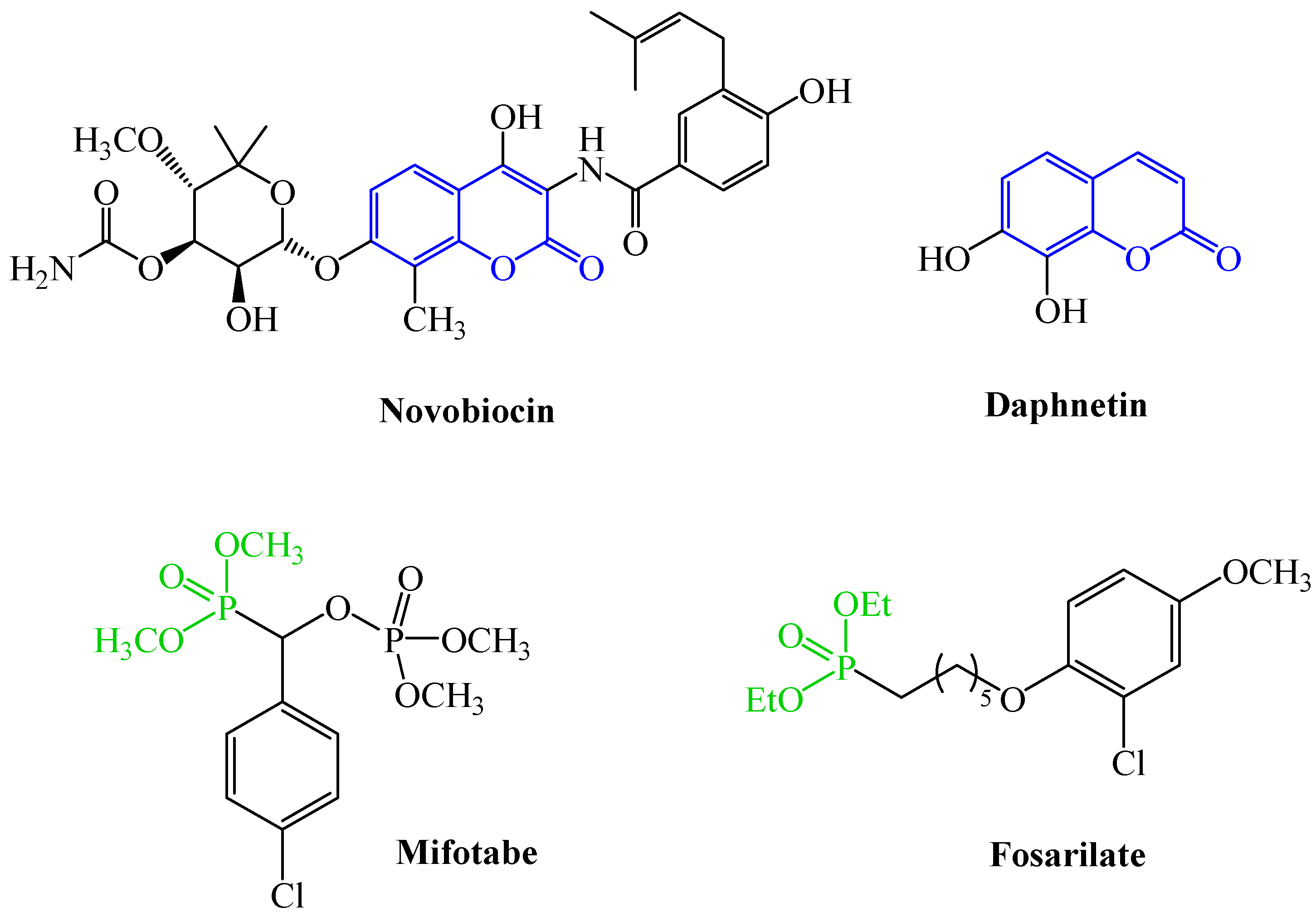
1. Introduction
2. Results and Discussion
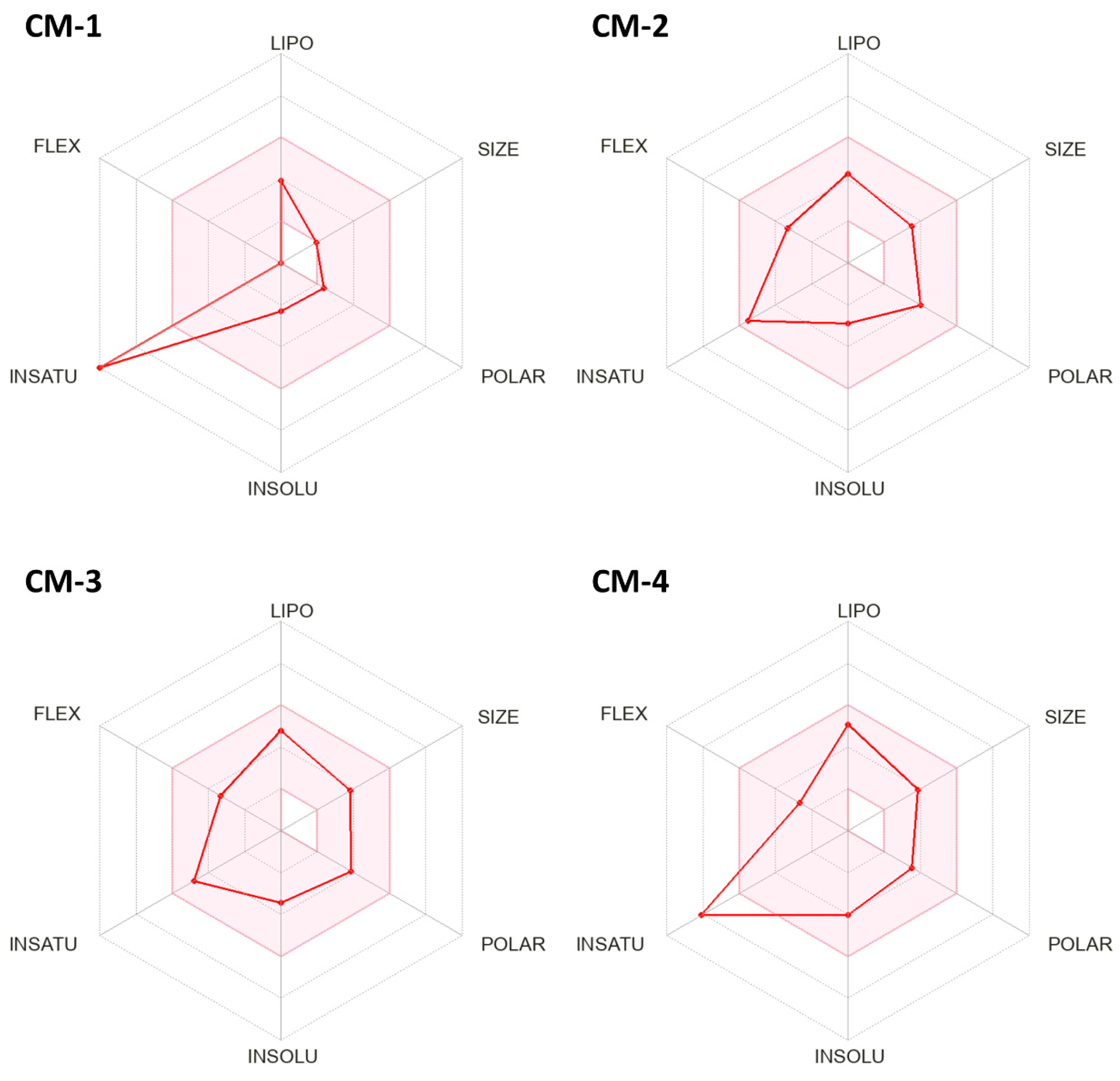
2.1. In Silico Prediction of Physicochemical and ADME Properties
- The topological polar surface area (TPSA) increased from 30.2 Å2 to 62.4–75.5 Å2, approaching an optimal range that ensures a better balance between lipophilicity and aqueous solubility, and improving the potential for polar interactions with molecular targets.
- The number of hydrogen bond acceptors rose from 2 to 4–5, potentially enabling more extensive hydrogen-bonding.
- Molar refractivity increased from 42.5 to 73.2–85.12, indicating a greater potential for hydrophobic interactions—an important feature that usually constitutes a significant contribution to the Gibbs energy of binding for optimized drug-like molecules [18].
- Average logP values increased from 1.8 to 2.1–3.2, with only a moderate decrease in logS. This balance still supports favorable oral absorption and adequate blood–brain barrier penetration (Table 2).
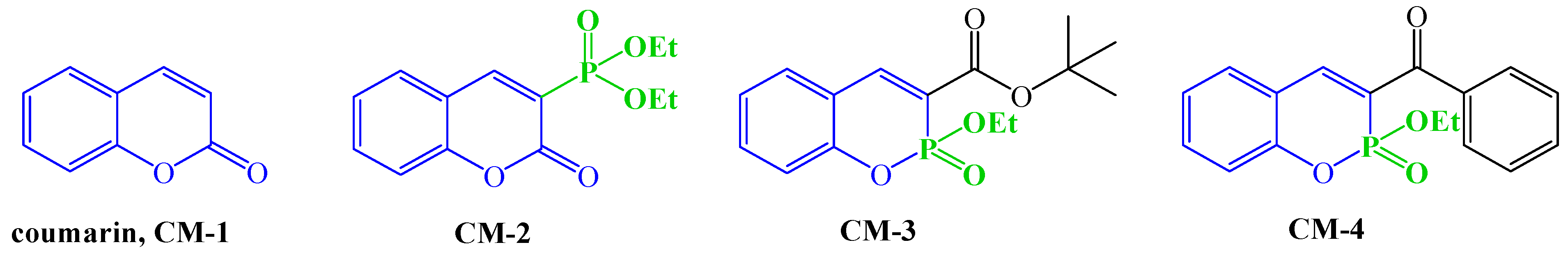
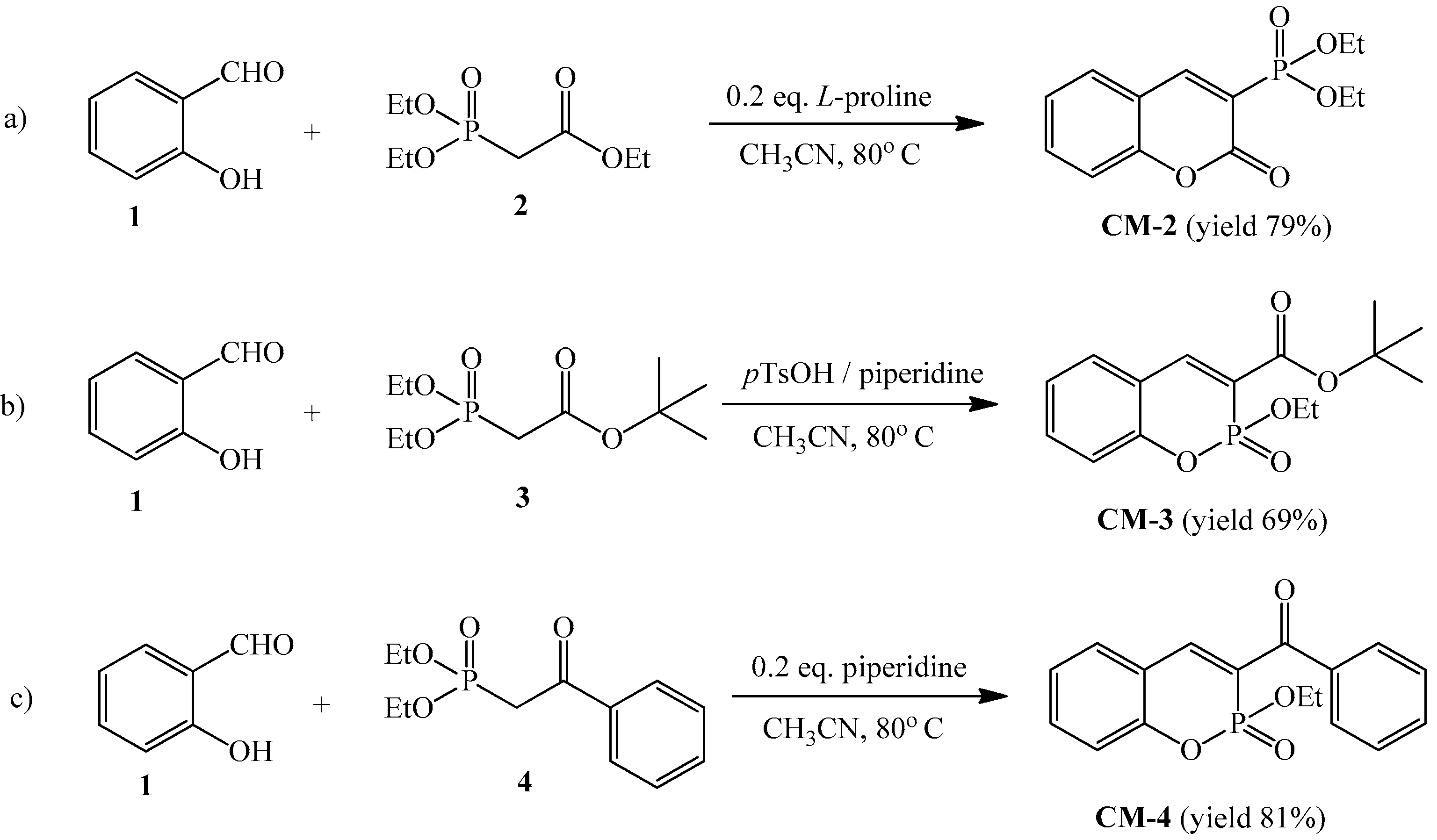
2.2. Synthesis of C-3 Phosphonate and Phosphacoumarin Derivatives
2.3. In Vitro Evaluation of Cytotoxicity, Apoptosis Induction, and Antioxidant Activity of CM-1–CM-4
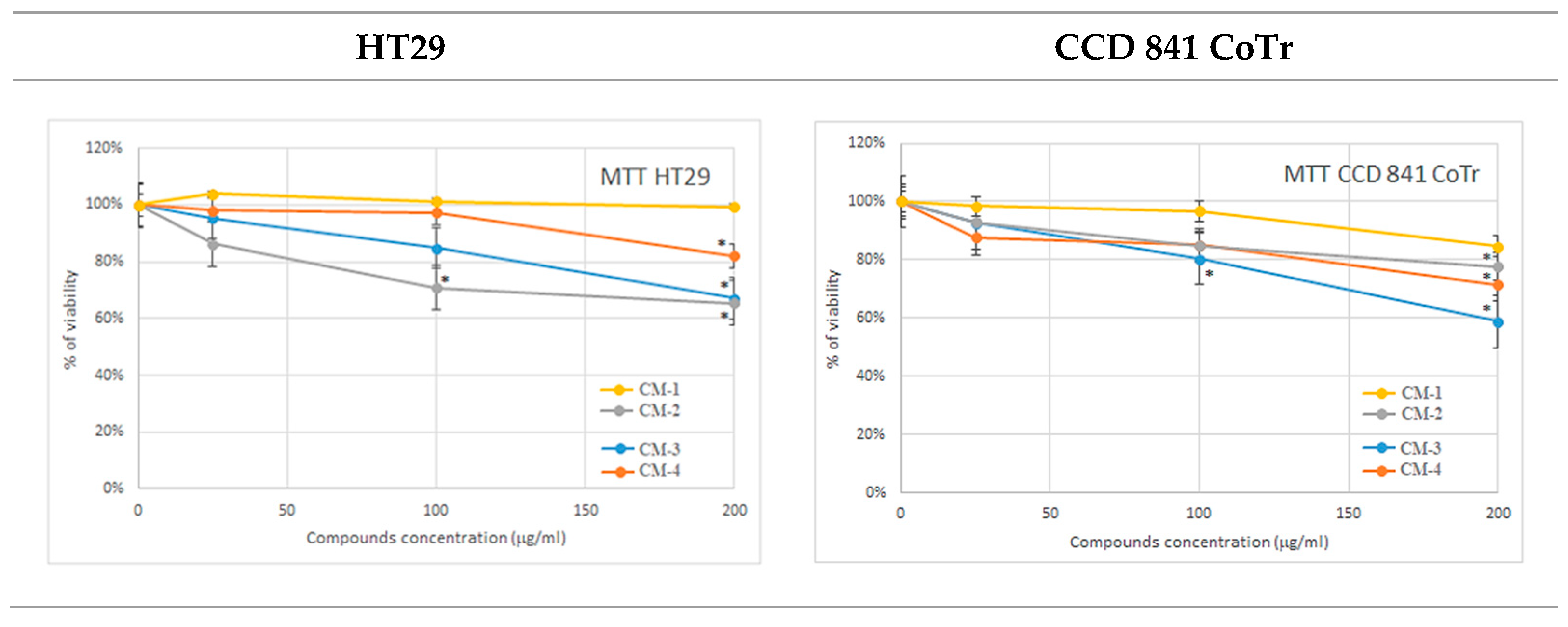
2.3.1. MTT Analysis
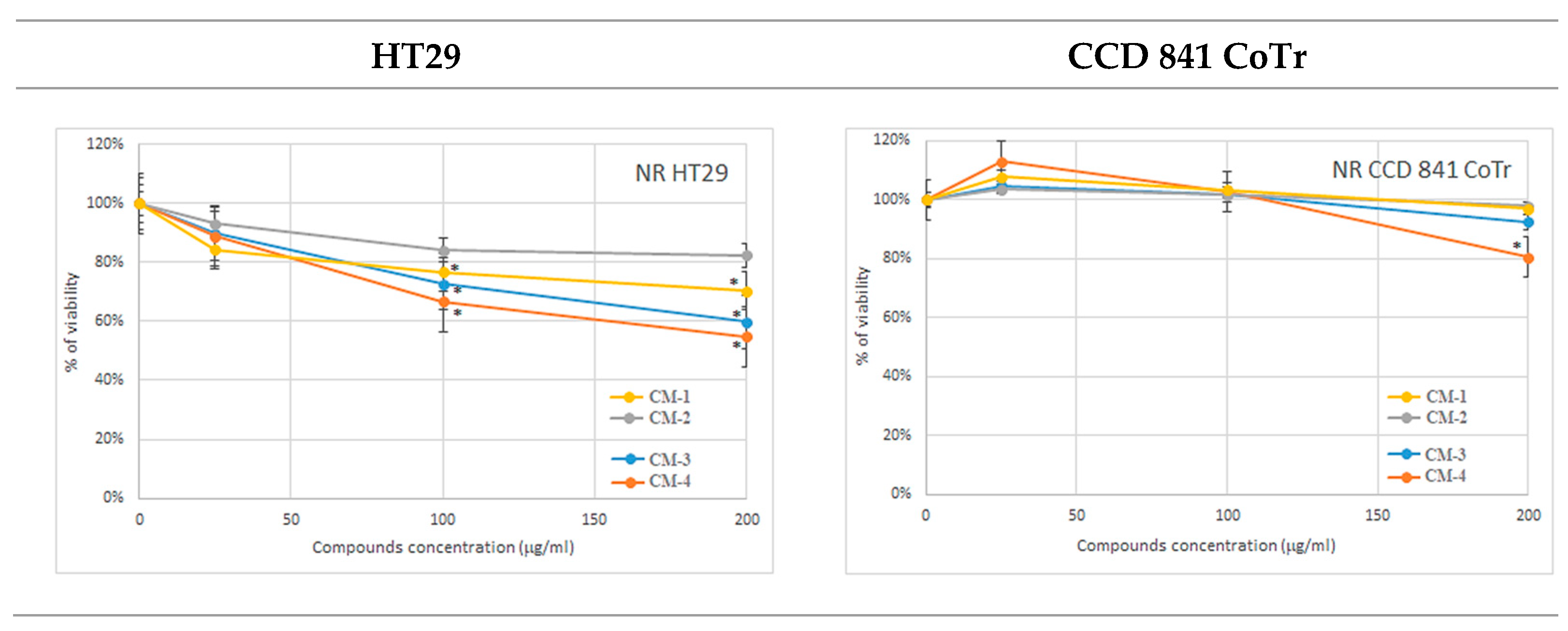
2.3.2. NR Uptake Assay
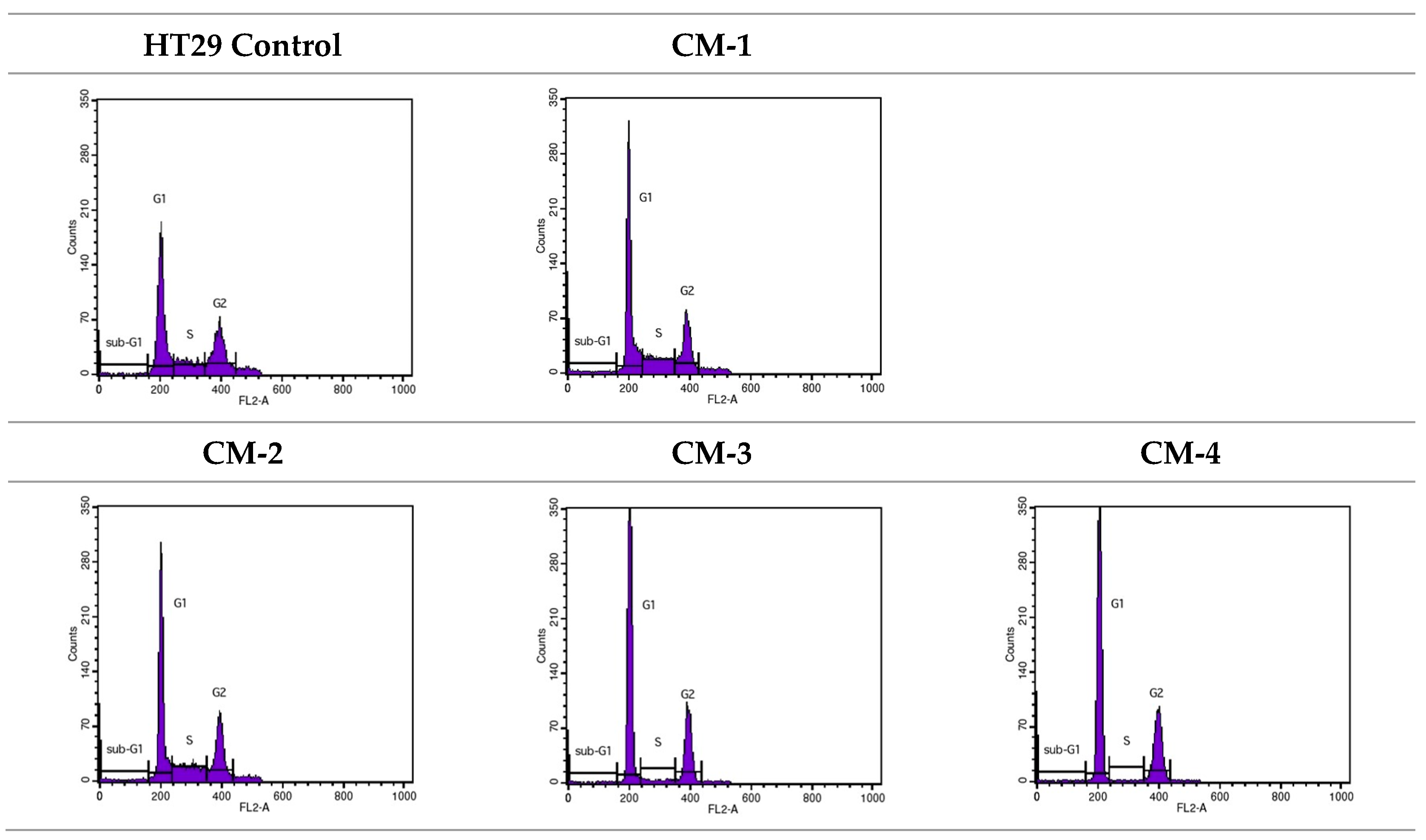
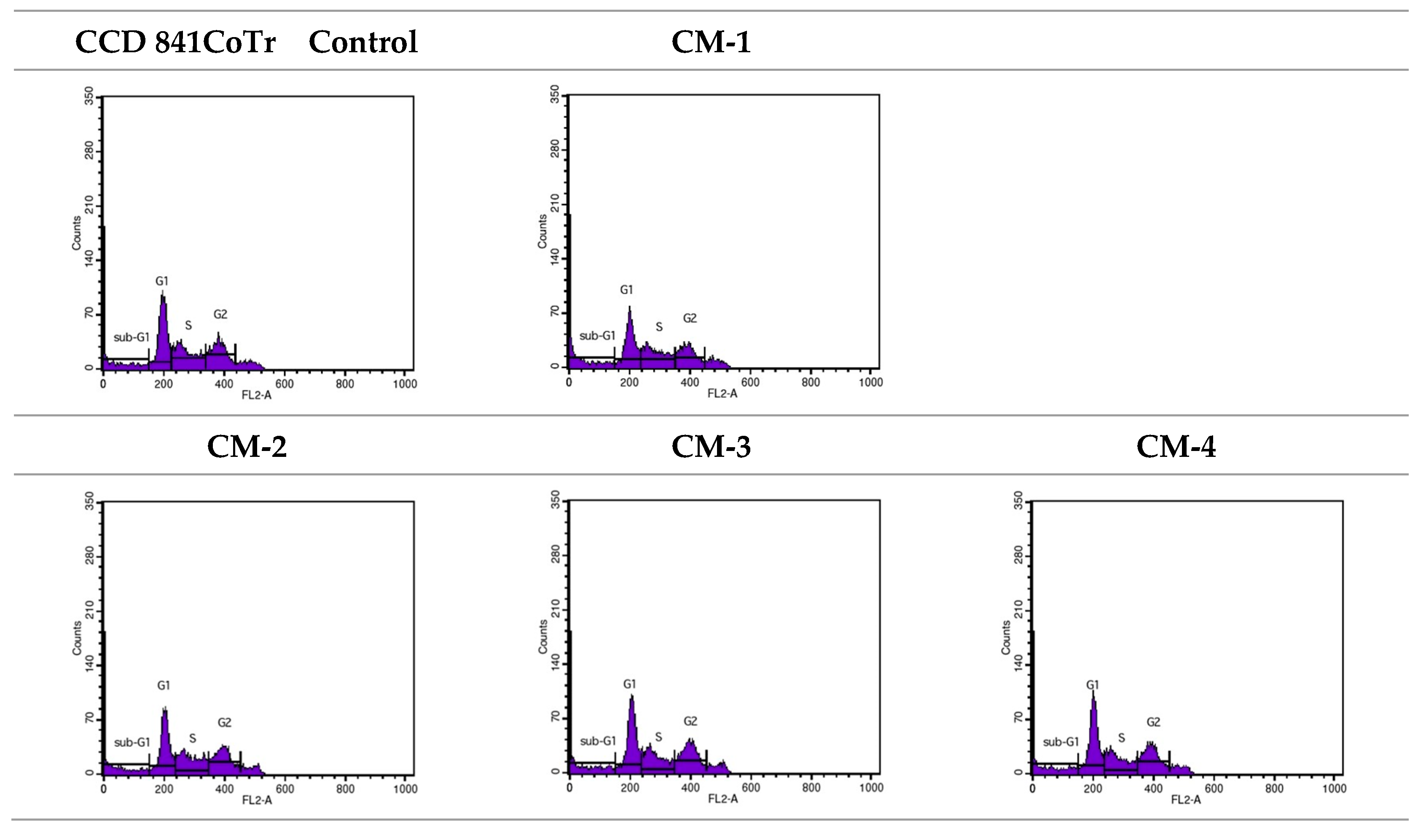
2.3.3. Cell Cycle Analysis
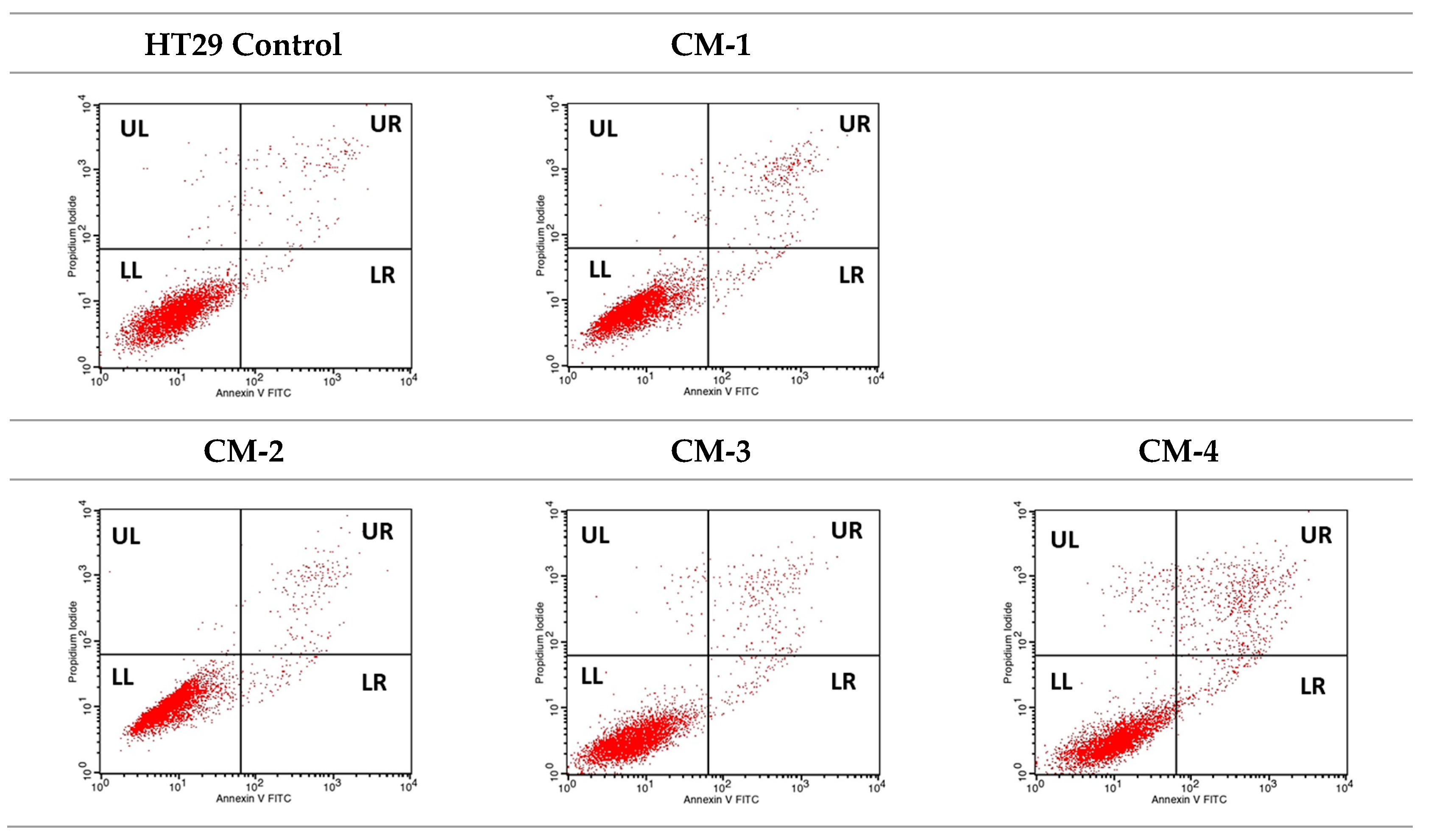
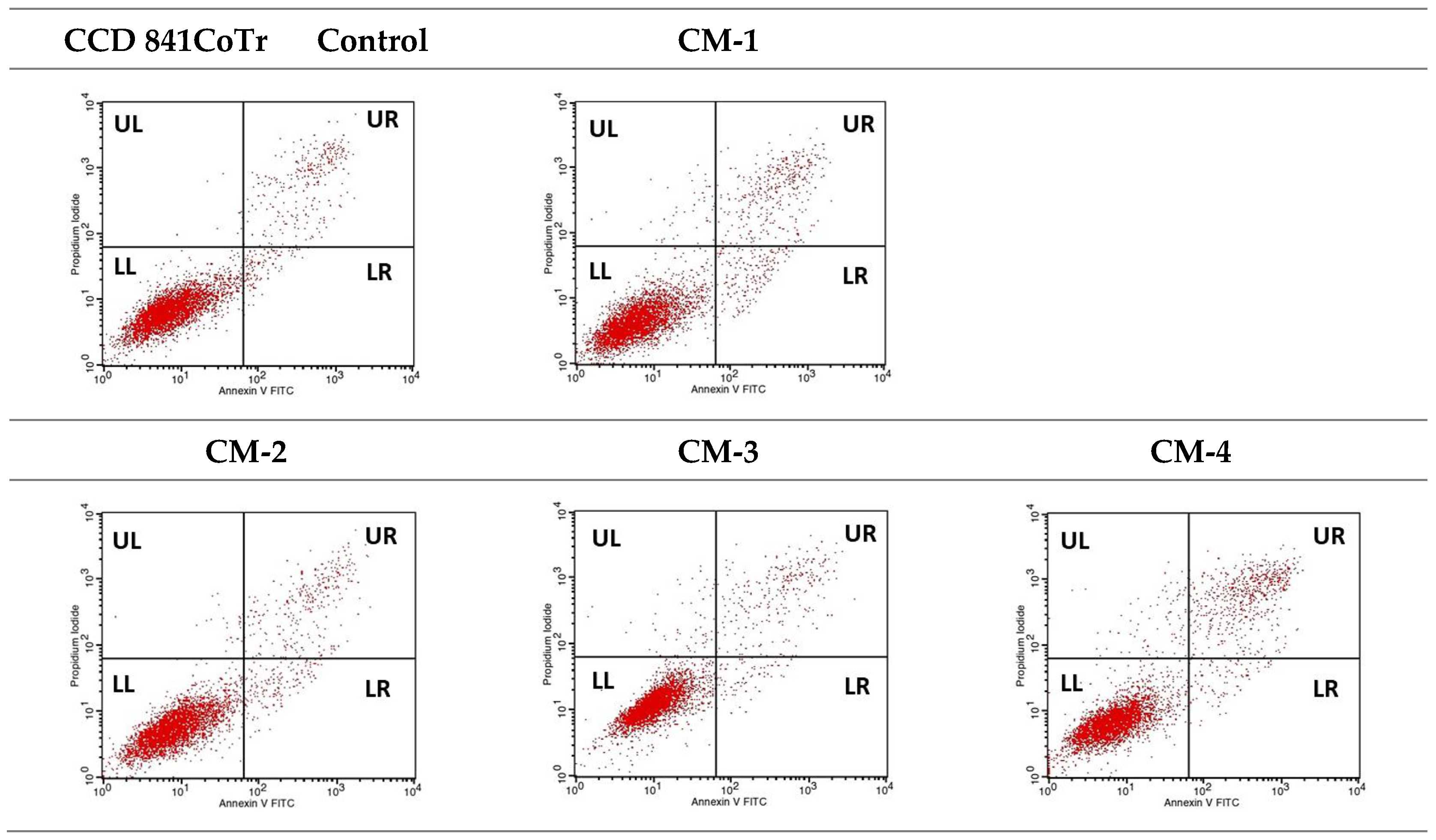
2.3.4. Quantitative Analysis of Cell Death Types
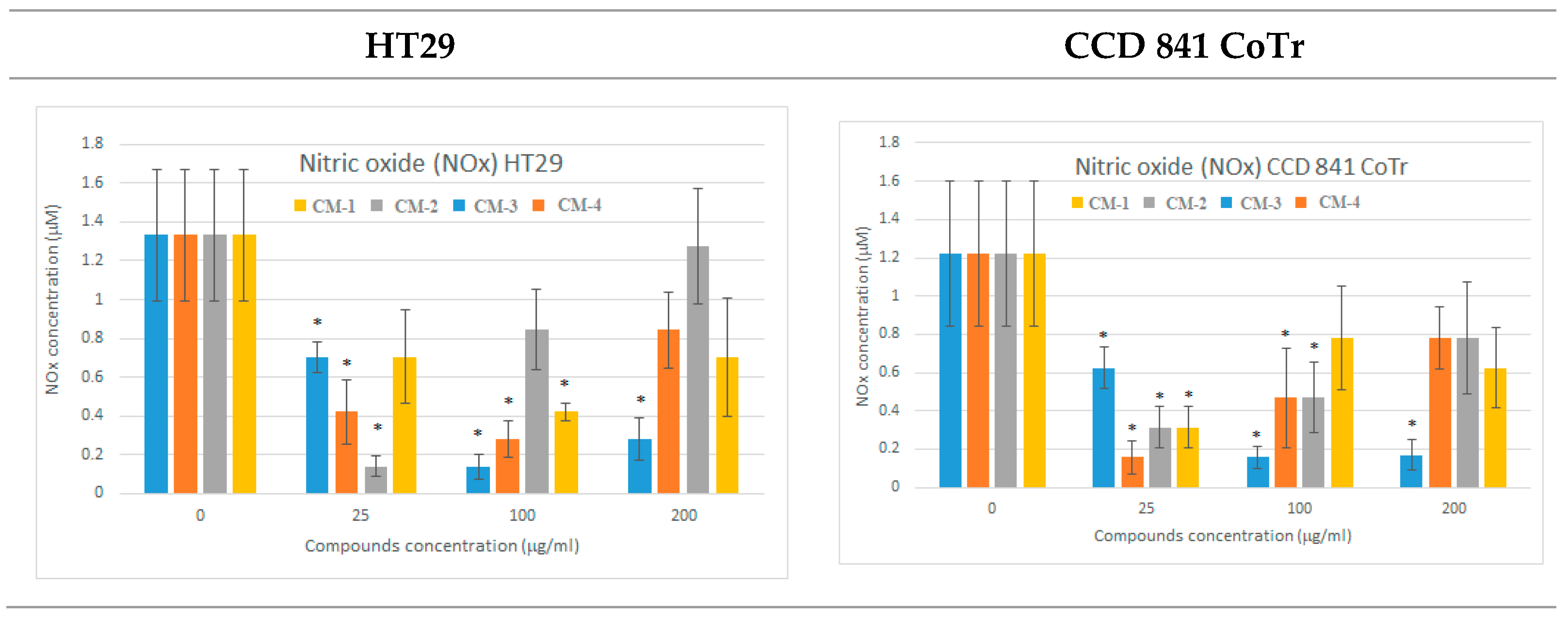
2.3.5. Nitric Oxide Level Analysis—Griess Method
2.3.6. DPPH Antioxidant Activity Analysis
2.3.7. FRAP Analysis
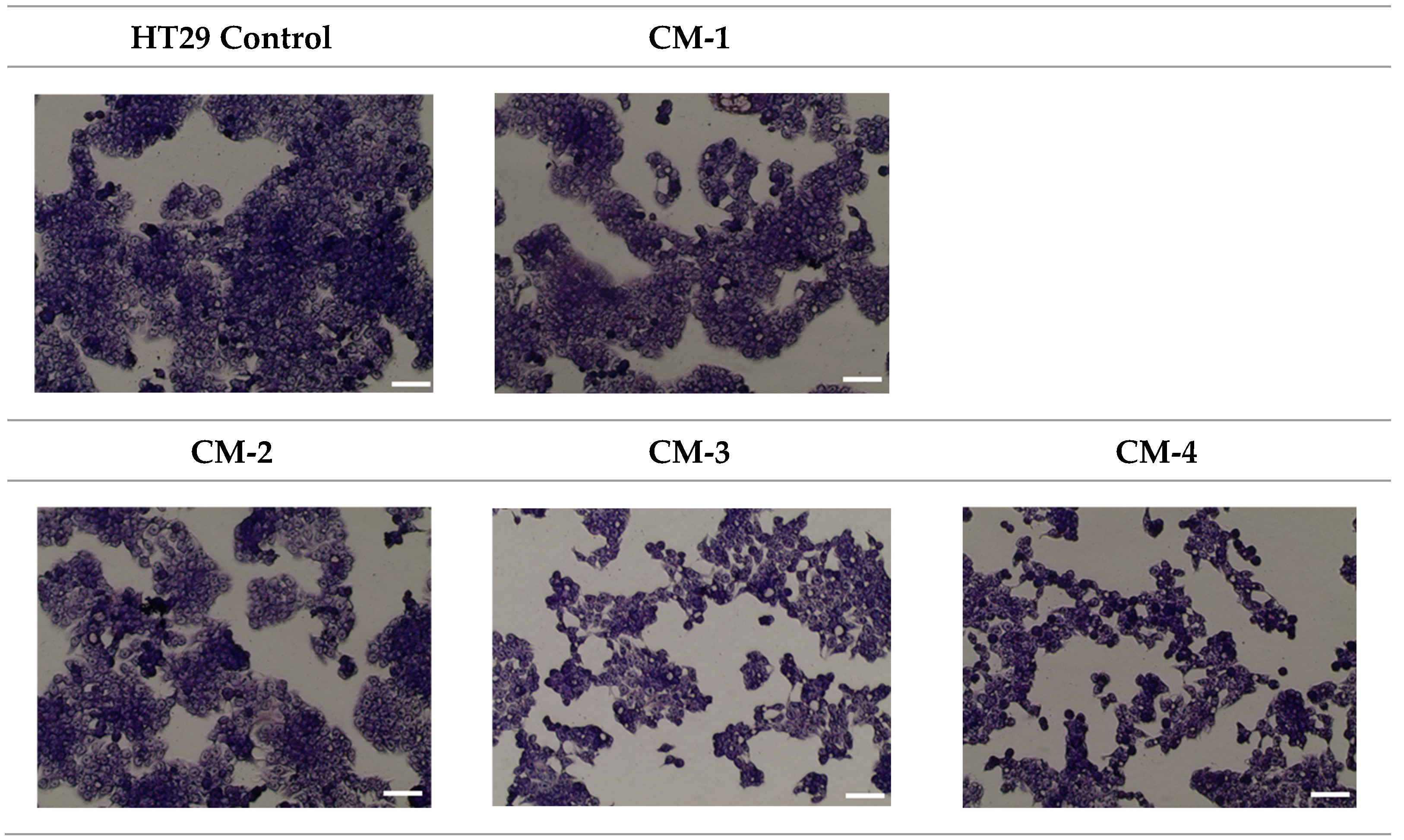
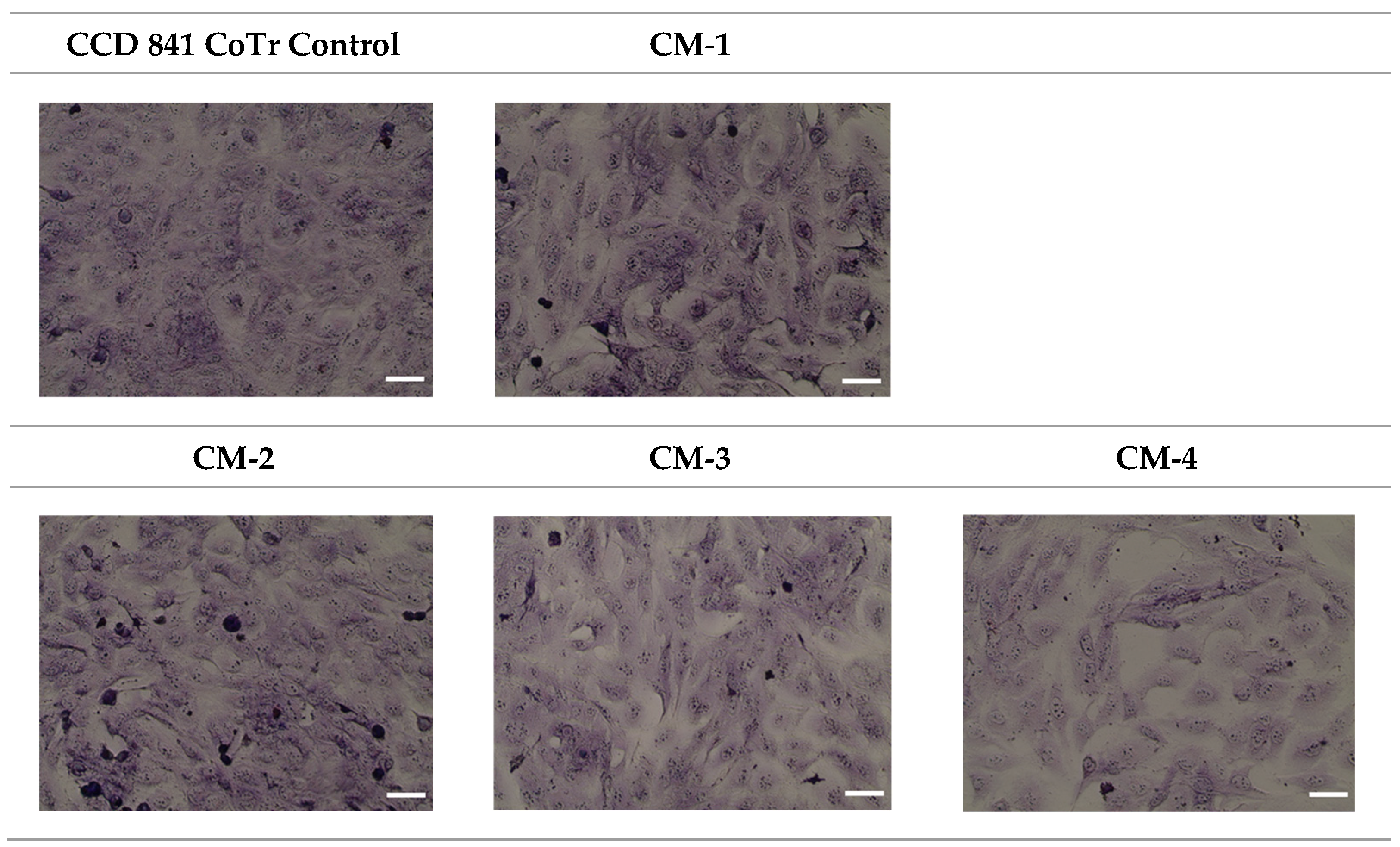
2.3.8. MGG Staining
3. Materials and Methods
3.1. Chemistry
3.1.1. Synthesis of Diethyl (2-Oxo-2H-chromen-3-yl)phosphonate (CM-2)
3.1.2. Synthesis of t-Butyl (2-Ethoxy-2-oxo-2H-1,2-benzoxaphosphorine)-3-carboxylate (CM-3)
3.1.3. Synthesis of 3-Benzoyl-(2-ethoxy-2-oxo-2H-1,2-benzoxaphosphorine) (CM-4)
3.2. Biology
3.2.1. Cell Cultures
3.2.2. MTT Assay
3.2.3. Neutral Red Uptake Assay
3.2.4. Cytometric Assessment of the Cell Cycle
3.2.5. Quantitative Analysis of Cell Death Types Using Flow Cytometry
3.2.6. Measurement of Nitric Oxide (NOx)
3.2.7. DPPH Free Radical Scavenging Assay
3.2.8. Ferric-Reducing Antioxidant Power Assay (FRAP)
3.2.9. May–Grünwald–Giemsa (MGG) Staining
3.3. Statistical Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rawat, A.; Reddy, A.V.B. Recent Advances on Anticancer Activity of Coumarin Derivatives. Eur. J. Med. Chem. Rep. 2022, 5, 100038. [Google Scholar] [CrossRef]
- Yildirim, M.; Poyraz, S.; Ersatir, M. Recent Advances on Biologically Active Coumarin-Based Hybrid Compounds. Med. Chem. Res. 2023, 32, 617–642. [Google Scholar] [CrossRef]
- Karatoprak, G.S.; Dumulpinar, B.; Celep, E.; Celep, I.K.; Akkol, E.K.; Sobarzo-Sánchez, E. A Comprehensive Review on the Potential of Coumarin and Related Derivatives as Multi-Target Therapeutic Agents in the Management of Gynecological Cancers. Front. Pharmacol. 2024, 15, 1423480. [Google Scholar] [CrossRef]
- Koley, M.; Han, J.; Soloshonok, V.A.; Mojumder, S.; Javahershenas, R.; Makarem, A. Latest Developments in Coumarin-Based Anticancer Agents: Mechanism of Action and Structure–Activity Relationship Studies. RSC Med. Chem. 2024, 15, 10–54. [Google Scholar] [CrossRef] [PubMed]
- Elshemy, H.A.H.; Zaki, M.A. Design and Synthesis of New Coumarin Hybrids and Insight into Their Mode of Antiproliferative Action. Bioorg. Med. Chem. 2017, 25, 1066–1075. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.Q.; Huang, C.; Jia, Y.M.; Song, B.A.; Li, J.; Liu, X.H. Novel Coumarin-Dihydropyrazole Thio-Ethanone Derivatives: Design, Synthesis and Anticancer Activity. Eur. J. Med. Chem. 2014, 74, 717–725. [Google Scholar] [CrossRef]
- Syed, A.; Filandr, F.; Patterson-Fortin, J.; Bacolla, A.; Ravindranathan, R.; Zhou, J.; McDonald, D.T.; Albuhluli, M.E.; Verway-Cohen, A.; Newman, J.A.; et al. Novobiocin blocks nucleic acid binding to Polθ and inhibits stimulation of its ATPase activity. Nucleic Acids Res. 2023, 51, 9920–9937. [Google Scholar] [CrossRef]
- Krečmerová, M.; Majer, P.; Rais, R.; Slusher, B.S. Phosphonates and Phosphonate Prodrugs in Medicinal Chemistry: Past Successes and Future Prospects. Front. Chem. 2022, 10, 889737. [Google Scholar] [CrossRef]
- Demkowicz, S.; Rachon, J.; Daśko, M.; Kozak, W. Selected Organophosphorus Compounds with Biological Activity: Applications in Medicine. RSC Adv. 2016, 6, 7101–7112. [Google Scholar] [CrossRef]
- Chamberlain, J.M.; Sortino, K.; Sethna, P.; Bae, A.; Lanier, R.; Bambara, R.A.; Dewhurst, S. Cidofovir Diphosphate Inhibits Adenovirus 5 DNA Polymerase via Both Nonobligate Chain Termination and Direct Inhibition, and Polymerase Mutations Confer Cidofovir Resistance on Intact Virus. Antimicrob. Agents Chemother. 2019, 63, e01925-18. [Google Scholar] [CrossRef]
- De Clercq, E. Potential of Acyclic Nucleoside Phosphonates in the Treatment of DNA Virus and Retrovirus Infections. Clin. Microbiol. Rev. 2003, 1, 21–43. [Google Scholar] [CrossRef]
- Yoshino, K.; Kohno, T.; Uno, T.; Morita, T.; Tsukamoto, G. Organic Phosphorus Compounds: 1.4-(Benzothiazol-2-yl) Benzylphosphonate as Potent Calcium Antagonistic Vasodilator. J. Med. Chem. 1986, 29, 820–825. [Google Scholar] [CrossRef]
- Tiwari, S.V.; Sharif, N.S.; Gajare, R.I.; Vazquez, J.A.S.; Sangshetti, J.N.; Damale, M.D.; Nikalje, A.P.G. New 2-Oxoindolin Phosphonates as Novel Agents to Treat Cancer: A Green Synthesis and Molecular Modeling. Molecules 2018, 23, 1981. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Iwasaki, M.; Murata-Hirai, K.; Matsumoto, K.; Hayashi, K.; Okamura, H.; Sugie, T.; Minato, N.; Morita, C.T.; Toi, M. Anti-Tumor Activity and Immunotherapeutic Potential of a Bisphosphonate Prodrug. Sci. Rep. 2017, 7, 5987. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Lipinski, C.A. Lead- and Drug-Like Compounds: The Rule-of-Five Revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Kralj, S.; Jukič, M.; Bren, U. Molecular Filters in Medicinal Chemistry. Encyclopedia 2023, 3, 501–511. [Google Scholar] [CrossRef]
- Ferreira de Freitas, R.; Schapira, M. A Systematic Analysis of Atomic Protein–Ligand Interactions in the PDB. Med. Chem. Commun. 2017, 8, 1970–1981. [Google Scholar] [CrossRef]
- Vekariya, R.H.; Patel, H.D. Recent Advances in the Synthesis of Coumarin Derivatives via Knoevenagel Condensation: A Review. Synth. Commun. 2014, 44, 2756–2788. [Google Scholar] [CrossRef]
- Bojilova, A.; Nikolova, R.; Ivanov, C.; Rodios, N.A.; Terzis, A.; Raptopoulou, C.P. A Comparative Study of the Interaction of Salicylaldehydes with Phosphonoacetates under Knoevenagel Reaction Conditions: Synthesis of 1,2-Benzoxaphosphorines and Their Dimers. Tetrahedron 1996, 52, 12597–12612. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.; Li, X.; Qu, C.; Qu, L.; Bi, W.; Sun, K.; Zhao, Y. Acetonitrile-Dependent Oxyphosphorylation: A Mild One-Pot Synthesis of β-Ketophosphonates from Alkenyl Acids or Alkenes. Tetrahedron 2017, 73, 2439–2446. [Google Scholar] [CrossRef]
- Al-Harbi, L.M.; Nassar, H.S.; Moustfa, A.; Alosaimi, A.M.; Mohamed, H.M.; Khowdiary, M.M.; El-Gazzar, M.A.; Elhenawy, A.A. Novel Coumarin Amino Acid Derivatives: Design, Synthesis, Docking, Absorption, Distribution, Metabolism, Elimination, Toxicity (ADMET), Quantitative Structure–Activity Relationship (QSAR) and Anticancer Studies. Mater. Express 2020, 10, 1375–1394. [Google Scholar] [CrossRef]
- Rahama, M.S.; Khairuddean, M.; Ismail, N.Z.; Al-Amin, M.; Salhimi, S.M. Synthesis, Characterization and In Silico Studies of Coumarin-Chalcone Derivatives and Their Cytotoxicity Activity Against Breast Cancer Cells. J. Mol. Struct. 2025, 1322, 140341. [Google Scholar] [CrossRef]
- Jevtić, M.; Pirković, M.S.; Komazec, T.; Mojić, M.; Mijatović, S.; Maksimović-Ivanić, D.; Dimić, D.; Marković, Z.; Simijonović, D.; Milenković, D.; et al. A Comprehensive Evaluation of a Coumarin Derivative and Its Corresponding Palladium Complex as Potential Therapeutic Agents in the Treatment of Gynecological Cancers: Synthesis, Characterization, and Cytotoxicity. Pharmaceutics 2024, 16, 1437. [Google Scholar] [CrossRef] [PubMed]
- Prateeptongkum, S.; Duangdee, N.; Mahavorasirikul, W. Evaluation of Cytotoxicity and Apoptosis Induced by Coumarin Hydrazide-Hydrazone Derivatives in Human Hepatocellular Carcinoma Cell Line. Trends Sci. 2024, 21, 7628. [Google Scholar] [CrossRef]
- Khosravifar, F.; Dehghan, G.; Bidoki, S.K.; Mahdavi, M. DNA-Binding Activity and Cytotoxic and Cell-Cycle Arrest Properties of Some New Coumarin Derivatives: A Multispectral and Computational Investigation. Luminescence 2020, 35, 98–106. [Google Scholar] [CrossRef]
- Choi, J.; Yoo, M.-J.; Park, S.-Y.; Seol, J.-W. Antitumor Effects of Esculetin, a Natural Coumarin Derivative, Against Canine Mammary Gland Tumor Cells by Inducing Cell Cycle Arrest and Apoptosis. Vet. Sci. 2023, 10, 84. [Google Scholar] [CrossRef]
- Umar, S.; Soni, R.; Durgapal, S.D.; Soman, S.; Balakrishnan, S. A Synthetic Coumarin Derivative (4-Fluorophenylacetamide-Acetyl Coumarin) Impedes Cell Cycle at G0/G1 Stage, Induces Apoptosis, and Inhibits Metastasis via ROS-Mediated p53 and AKT Signaling Pathways in A549 Cells. J. Biochem. Mol. Toxicol. 2020, 34, e22553. [Google Scholar] [CrossRef]
- Mohamed, T.K.; Batran, R.Z.; Elseginy, S.A.; Ali, M.M.; Mahmoud, A.E. Synthesis, Anticancer Effect and Molecular Modeling of New Thiazolylpyrazolyl Coumarin Derivatives Targeting VEGFR-2 Kinase and Inducing Cell Cycle Arrest and Apoptosis. Bioorg. Chem. 2019, 85, 253–273. [Google Scholar] [CrossRef]
- Abdel Ghany, L.M.A.; El-Dydamony, N.M.; Helwa, A.A.; Abdelraouf, S.M.; Abdelnaby, R.M. Coumarin-Acetohydrazide Derivatives as Novel Antiproliferative Agents via VEGFR-2/AKT Axis Inhibition and Apoptosis Triggering. New J. Chem. 2022, 46, 17394. [Google Scholar] [CrossRef]
- Dong, M.; Ye, T.; Bi, Y.; Wang, Q.; Kuerban, K.; Li, J.; Feng, M.; Wang, K.; Chen, Y.; Ye, L. A Novel Hybrid of 3-Benzyl Coumarin Seco-B-Ring Derivative and Phenylsulfonylfuroxan Induces Apoptosis and Autophagy in Non-Small-Cell Lung Cancer. Phytomedicine 2019, 52, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-H.; Cheng, C.-H.; Chen, C.-H.; Lee, W.-T.; Wang, Y.-F.; Xiao, C.-Q.; Lin, C.-W. Induction of ROS-Independent JNK-Activation-Mediated Apoptosis by a Novel Coumarin Derivative, DMAC, in Human Colon Cancer Cells. Chem. Biol. Interact. 2014, 218, 42–49. [Google Scholar] [CrossRef] [PubMed]
- El-Gamal, M.I.; Lee, W.-S.; Shin, J.-S.; Oh, C.-H.; Lee, K.-T.; Choi, J.; Myoung, N.; Baek, D. Synthesis of New Tricyclic and Tetracyclic Fused Coumarin Sulfonate Derivatives and Their Inhibitory Effects on LPS-Induced Nitric Oxide and PGE2 Productions in RAW 264.7 Macrophages: Part 2. Arch. Pharm. Chem. Life Sci. 2016, 349, 853–863. [Google Scholar] [CrossRef]
- Stefani, H.A.; Gueogjan, K.; Manarin, F.; Farsky, S.H.P.; Zukerman-Schpector, J.; Caracelli, I.; Rodrigues, S.R.P.; Muscará, M.N.; Teixeira, S.A.; Santin, J.R.; et al. Synthesis, Biological Evaluation and Molecular Docking Studies of 3-(Triazolyl)-Coumarin Derivatives: Effect on Inducible Nitric Oxide Synthase. Eur. J. Med. Chem. 2012, 58, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Popova, S.A.; Shevchenko, O.G.; Chukicheva, I.Y. Synthesis of New Coumarin[1,3]Oxazine Derivatives of 7-Hydroxy-6-Isobornyl-4-Methylcoumarin and Their Antioxidant Activity. Chem. Biol. Drug Des. 2022, 100, 994–1004. [Google Scholar] [CrossRef]
- Betti, N.; Shia, J.S.; Kadhum, A.A.H.; Al-Amiery, A.A. Harnessing Coumarin Chemistry: Antibacterial, Antifungal, and Antioxidant Profiling of Novel Coumarin Derivatives. J. Med. Pharm. Chem. Res. 2024, 6, 1530–1546. [Google Scholar] [CrossRef]
| CM | M.W. | Fsp3 | NRB | HBA | MR | TPSA | Av. LogP | XLogP3 | ESOL Log S | Ali Log S | Silicos-IT LogSw |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CM-1 | 146.14 | 0.00 | 0 | 2 | 42.48 | 30.21 | 1.82 | 1.39 | −2.29 | −1.63 | −3.59 |
| CM-2 | 282.23 | 0.31 | 5 | 5 | 73.24 | 75.55 | 2.13 | 1.96 | −2.88 | −3.17 | −4.69 |
| CM-3 | 310.28 | 0.40 | 5 | 5 | 80.82 | 71.64 | 2.84 | 2.84 | −3.43 | −4.00 | −3.87 |
| CM-4 | 314.27 | 0.12 | 4 | 4 | 85.18 | 62.41 | 3.19 | 3.34 | −4.03 | −4.33 | −5.44 |
| CM-1 | CM-2 | CM-3 | CM-4 | ||
|---|---|---|---|---|---|
| Pharmacokinetics predictions | GIT Absorption | High | High | High | High |
| BBB permeation | Yes | Yes | Yes | Yes | |
| P-glycoprotein substrate | No | No | No | No | |
| CYP450 inhibition | CYP3A4 | No | No | No | Yes |
| CYP2D6 | No | No | No | No | |
| Cyp1A2 | Yes | Yes | Yes | Yes | |
| Cyp2C19 | No | Yes | Yes | Yes | |
| Cyp 2C9 | No | No | Yes | Yes | |
| Drug-likeness filter violations | Lipiniski | 0 | 0 | 0 | 0 |
| Ghose | 2 | 0 | 0 | 0 | |
| Veber | 0 | 0 | 0 | 0 | |
| Egan | 0 | 0 | 0 | 0 | |
| Muegge | 1 | 0 | 0 | 0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szwaczko, K.; Paduch, R.; Dziuba, K.; Szafrański, K.; Wiater, A. Modulation of Human Colon Cell Activity by Synthetic Coumarin Derivatives Bearing a Phosphonate Group. Molecules 2025, 30, 2846. https://doi.org/10.3390/molecules30132846
Szwaczko K, Paduch R, Dziuba K, Szafrański K, Wiater A. Modulation of Human Colon Cell Activity by Synthetic Coumarin Derivatives Bearing a Phosphonate Group. Molecules. 2025; 30(13):2846. https://doi.org/10.3390/molecules30132846
Chicago/Turabian StyleSzwaczko, Katarzyna, Roman Paduch, Kamil Dziuba, Krzysztof Szafrański, and Adrian Wiater. 2025. "Modulation of Human Colon Cell Activity by Synthetic Coumarin Derivatives Bearing a Phosphonate Group" Molecules 30, no. 13: 2846. https://doi.org/10.3390/molecules30132846
APA StyleSzwaczko, K., Paduch, R., Dziuba, K., Szafrański, K., & Wiater, A. (2025). Modulation of Human Colon Cell Activity by Synthetic Coumarin Derivatives Bearing a Phosphonate Group. Molecules, 30(13), 2846. https://doi.org/10.3390/molecules30132846









