2.1. Characterization of EC Derivatives
Four types of completely etherified EC derivatives such as EC-Pr, EC-Bu, EC-Pe, and EC-He were prepared by the Williamson ether synthesis according to our previously reported procedure in order to elucidate the side chain effect of etherified EC derivatives on the light reflection properties of lyotropic CLCs with MAA (
Figure 1A) [
27]. All of the EC derivatives were obtained as white powder, and did not exhibit thermotropic liquid crystallinity on their own unlike etherified hydroxypropyl cellulose derivatives [
28].
The etherified EC derivatives were characterized by measuring both Fourier transform infrared (FT-IR) and nuclear magnetic resonance (NMR) spectra to confirm that hydroxy groups of pristine EC are completely substituted with alkoxy groups.
Figure S1A in the Supplementary Materials shows the comparison of FT-IR spectra between pristine EC and etherified EC derivatives. As compared these spectra, the pristine EC showed an intense peak from the O-H stretching vibration of the hydroxy groups in a broad wavenumber range from 3000 cm
−1 to 3600 cm
−1. In contrast, the intense peaks around 3000–3600 cm
−1 were not observed for the completely etherified EC derivatives so as to be broad bands with weak intensities. The experimental results imply that the hydroxy groups of pristine EC are completely etherified regardless of the difference in the chain length of alkoxy groups.
Figure S1B in the Supplementary Materials represents the
1H-NMR spectra of etherified EC derivatives in CDCl
3. From the
1H-NMR spectra, the
DS values, that is, the degree of substitution with alkoxy groups, are quantitatively estimated according to the following equation [
27]:
where
A is the integrated value of the signal peak “
a” assigned to the protons of the terminal methyl group,
W is the sum of the integrated values of all protons in EC derivatives, and
x is the number of carbon atoms in alkoxy groups. For instance,
x corresponds to 3 in the case of EC-Pr. As will be mentioned in
Section 3.1, we adopted the
MS value of 2.50 from the
1H-NMR spectrum of pristine EC. By applying the experimental results to Equation (2), the
DS values of all etherified EC derivatives were estimated to be 0.50, which also assured that the hydroxy groups of pristine EC are completely etherified for EC-Pr, EC-Bu, EC-Pe, and EC-He.
2.2. Reflection Properties of Lyotropic CLCs of EC Derivatives with MAA
When the lyotropic CLCs of EC-Pr, EC-Bu, EC-Pe, and EC-He with MAA were prepared, the light reflection properties were found to be different behavior as the alkoxy side chains. Although we found that EC-Bu and EC-Pe are suitable for the preparation of lyotropic CLCs with visible Bragg reflection, the reflection color was not observed for the lyotropic CLCs of EC-Pr with MAA at the concentration of 35–60 wt%. As measuring the transmission spectra of the lyotropic CLCs, no peak was detected in the visible wavelength region of 400–800 nm, which was the detectable wavelength range of the spectroscopy used in this study. As observed using polarized optical microscope (POM) under cross-Nicols, the optical anisotropy appeared in the lyotropic CLCs of EC-Pr with MAA. It is plausible that the lyotropic CLC of EC-Pr with MAA might reflect ultraviolet light or infrared light. Similarly, the lyotropic CLCs of EC-He with MAA at the concentration of 60–75 wt% showed no reflection color even though they showed optical anisotropy by the POM observation. From the previous report on the thermotropic CLCs of etherified derivatives of hydroxypropyl cellulose, the reflection peak wavelength red-shifts with the increase in the chain length of alkoxy groups substituted to the hydroxy groups of hydroxypropyl cellulose [
28]. Therefore, it can be assumed that the lyotropic CLCs of EC-Pr and EC-He dissolved in MAA reflect ultraviolet and infrared light, respectively.
To investigate the temperature dependence of light reflection properties, six types of lyotropic CLCs were prepared from EC-Bu or EC-Pe. The preparation conditions and sample definition of lyotropic CLCs from EC-Bu or EC-Pe are summarized in
Table 1.
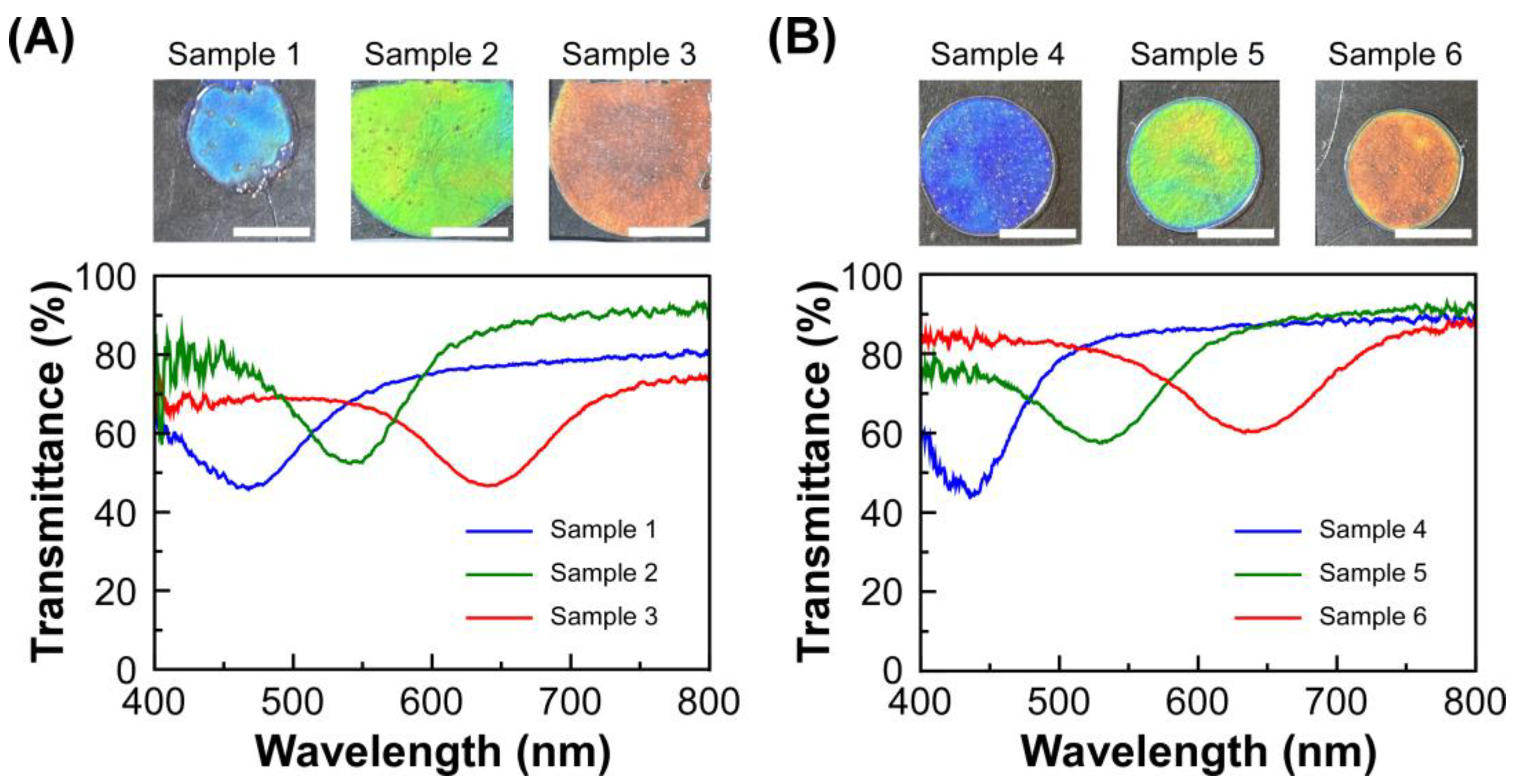
Figure 2 shows the transmission spectra of lyotropic CLCs of EC-Bu or EC-Pe, which were measured at 30 °C. When EC-Bu was completely dissolved in MAA at the concentration range of 59.2–61.5 wt%, the mixtures of EC-Bu and MAA showed reflection colors as blue, green, and red by the formation of the lyotropic CLC phase (
Figure 2A). The reflection wavelength continuously shifted to the longer wavelength side as diluting the concentration of EC-Bu in MAA, arising from the increase in the helical pitch length of CLC. Similar light reflection properties were observed for the lyotropic CLCs of EC-Pe with MAA at 67.4–69.4 wt% (
Figure 2B). The reflection color also changed to blue, green, and red in the descending order of the concentration of EC-Pe by the red-shift of reflection peak wavelength. As described in the Introduction part, the reflection wavelength of lyotropic CLCs of EC-Pe with MAA are unlikely to be changed upon the heating process. Since the detectable range of our transmission spectral measurement system is 400–800 nm, it is essential to prepare the lyotropic CLCs with visible Bragg reflection for the fair comparison of their reflection wavelength upon the heating process. From this reason, we focused on the lyotropic CLCs of EC-Bu or EC-Pe with MAA to explore the temperature-dependence of their reflection peak wavelength.
2.3. Temperature Dependence of the Reflection Properties of the Lyotropic CLCs from EC Derivatives and MAA
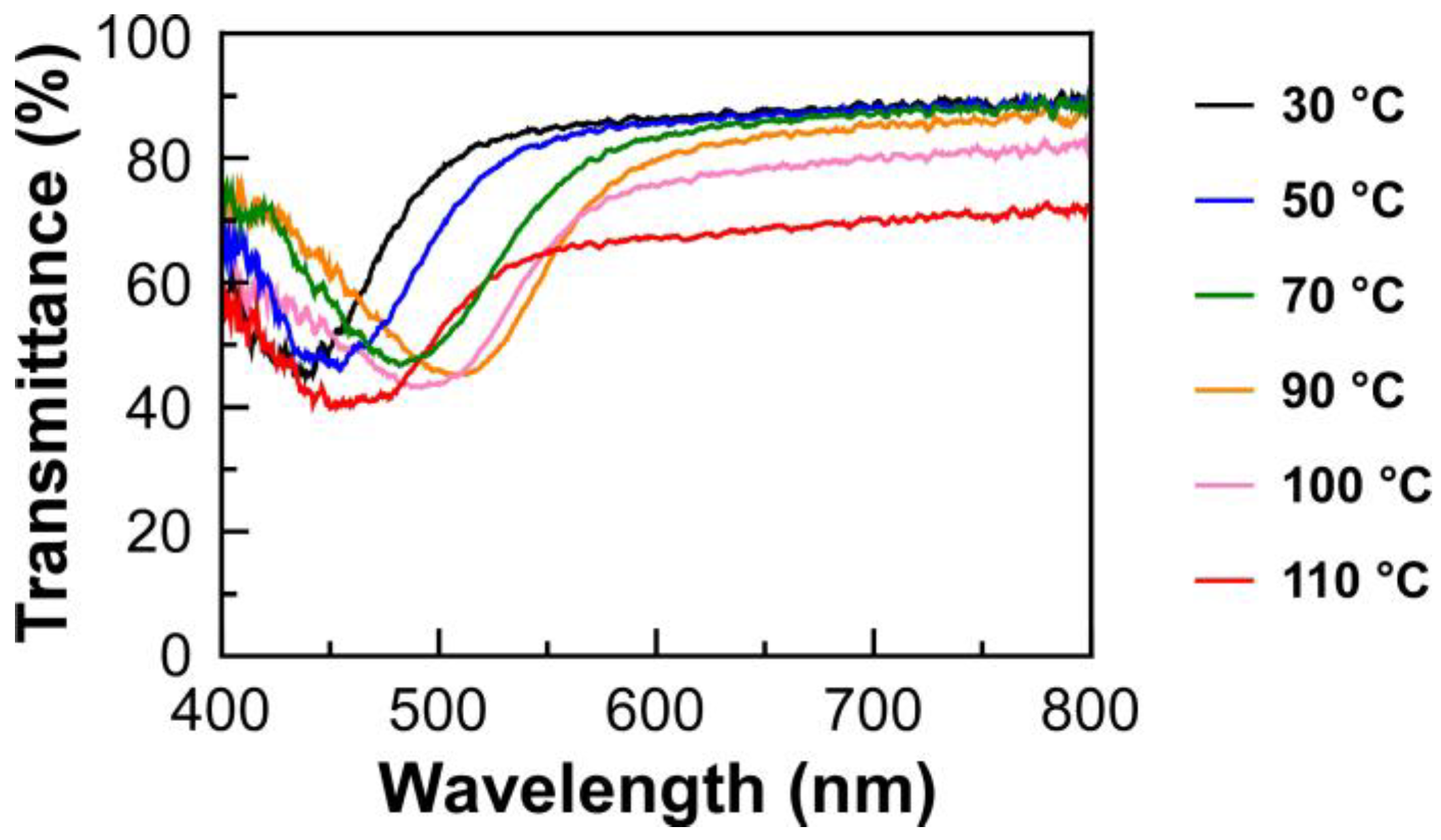
The transmission spectrum measurements were conducted for the lyotropic CLCs of EC-Bu or EC-Pe with MAA upon heating the process from 30 °C to 110 °C. The isotropic phase transition temperature was found to be ~150 °C for all samples. Furthermore, the result suggested that the lyotropic CLCs undergo no structural deterioration upon heating, as evidenced by the negligible change in their transmission spectra after the measurements. As an example,
Figure 3 shows the changes in transmission spectrum of Sample 4, which corresponds to the lyotropic CLC of EC-Pe in MAA at the EC-Pe concentration of 69.4 wt%, as a function of the temperature upon the heating process. The reflection peak wavelength red shifted upon heating from 30 °C up to 90 °C. However, interestingly, the reflection peak wavelength blue shifted as elevating the temperature over 90 °C. At this time, the baseline of the transmission spectrum became lower than those measured below 90 °C. For instance, the light transmittance at longer wavelength of 800 nm drastically decreased from ~90% to ~65% by rising the temperature from 90 °C to 110 °C. Although it is obvious that the helical molecular assemblage of lyotropic CLC is maintained because the reflection peak can be clearly observed even in this temperature range, the decrease in light transmittance upon elevating the temperature strongly suggested the structural change in the CLC helical molecular assemblage at the mesoscopic scale. Such tendencies were also observed for transmission spectra of the other lyotropic CLCs upon the heating process (
Supplementary Materials, Figure S2). The plausible mechanism for the changes in the CLC helical molecular assemblage will be discussed in the proceeding section.
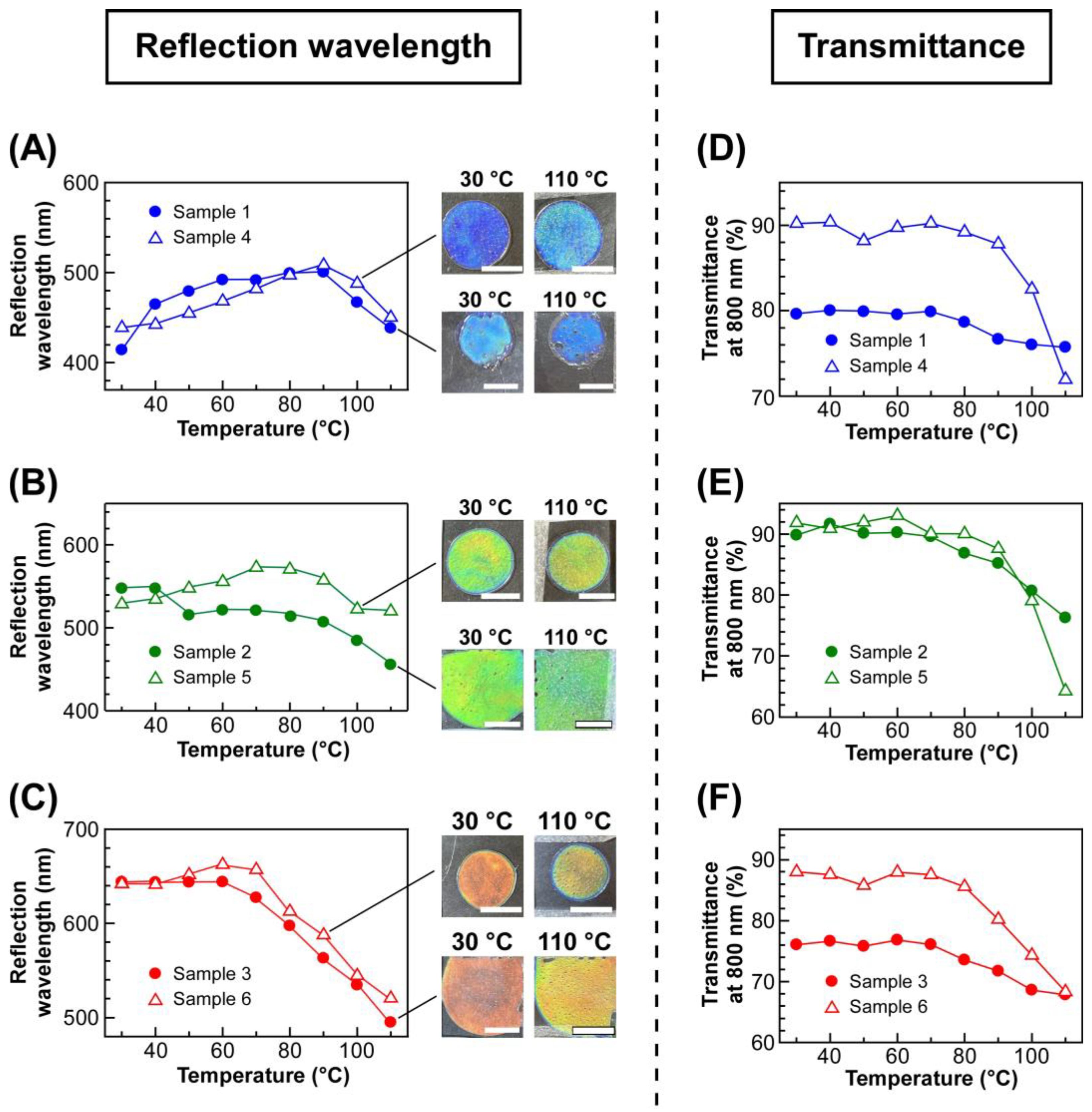
To quantitatively analyze the changes in transmission spectra of lyotropic CLCs by changing the temperature, the reflection wavelength and light transmittance at 800 nm of each CLC were plotted against the temperature (
Figure 4). The left panels of
Figure 4 show the plots of the reflection wavelength of lyotropic CLCs of EC-Bu or EC-Pe exhibiting a blue reflection color (
Figure 4A), green (
Figure 4B), and red (
Figure 4C), upon heating from 30 °C in a stepwise manner. As shown in
Figure 4A, although the reflection wavelength of Sample 1 and Sample 4 continuously shifted to the longer wavelength side, it began to shift to the shorter wavelength side with increasing the temperature from 90 °C to 110 °C. However, it should be noted that the reflection color can be recognized as blue by naked eyes because of the relatively small wavelength shift of reflection peak. Therefore, it was indicated that EC derivatives that can make lyotropic CLCs with thermally stable reflection colors is not only limited to EC-Pe but also to EC-Bu. In fact, the reflection wavelength of Sample 2 and Sample 5 was maintained especially in the temperature range of 30–90 °C. Although the reflection wavelength slightly blue shifted when heated above 90 °C, the reflection color change is hardly recognized by naked eyes (
Figure 4B). In the same manner, Sample 3 and Sample 6 showed exactly same tendency except that the blue-shift of reflection wavelength began at 70 °C, which was much lower than Sample 2 and Sample 5 (
Figure 4C). Among the lyotropic CLCs exhibiting blue and green colors, Sample 3 and Sample 6 displayed a relatively large shift of reflection peak wavelength. This can be ascribed to the increase in the mobility of CLC arising from the decrease in the concentration of etherified EC derivatives when preparing lyotropic CLCs. To quantitatively discuss the shift of reflection wavelength of the lyotropic CLCs, the following equation was adopted to calculate the average wavelength shift of the reflection peak at the temperature range of 10 °C (
λshift/10 °C).
where
λ30°C and
λ110°C are the reflection wavelength of lyotropic CLC at 30 °C and 110 °C, respectively [
27]. The
λshift/10 °C values are summarized in the fifth column of
Table 1. Because the λ
shift/10 °C values for lyotropic CLCs of EC-Bu with MAA (Samples 1–3) were larger than those of EC-Pe with MAA (Samples 4–6), it was indicated that EC-Pe is much more adequate to prepare lyotropic CLC with a thermally stable reflection color.
As mentioned in the
Section 2.2, the light transmittance of lyotropic CLCs decreased at elevated temperature. The right panels of
Figure 4 show the changes in light transmittances at 800 nm for the lyotropic CLCs of EC-Bu or EC-Pe with MAA, exhibiting a blue reflection color (
Figure 4D), green (
Figure 4E), and red (
Figure 4F), upon heating from 30 °C. The light transmittance of Sample 4 drastically decreased from ~90% to ~70% when the temperature exceeded 90 °C (
Figure 4D, closed circles). Notably, this temperature agreed well with the temperature at which the reflection wavelength became maximum (
Figure 4A, closed circles). Although the decrease in light transmittance was not clearly observed for Sample 1, the light transmittance at 90 °C was lower than that at 70 °C. These results indicated the temperature range can be divided into two regions; one is 30–90 °C, where the red-shift of reflection wavelength is observed, and the transmittance is maintained. The other is 90–110 °C, where the reflection wavelength blue-shifts and a decrease in light transmittance is observed. Similarly, the light transmittance of Samples 2 and 5 decreased above 90 °C, where the reflection wavelength began to blue-shift (
Figure 4E). Furthermore, Sample 3 and Sample 6 also exhibited a similar tendency except that the infection points of the plots shifted to 70 °C (
Figure 4F). These results strongly implied that internal structure of the lyotropic CLCs of etherified EC derivatives with MAA drastically changes throughout the temperature range of 30–110 °C. Therefore, the thermally stable reflection color of the lyotropic CLCs could not be ascribed to the stability of the internal structure upon heating.
2.4. USAXS and SAXS Measurements of UV-Cured Film from Lyotropic CLCs
To investigate the mechanism for the emergence of thermally stable Bragg reflection colors of CLCs from etherified EC derivatives and MAA, we conducted USAXS and SAXS measurements using the synchrotron radiation. This is because SAXS measurements have been widely used for the mesoscopic structure analysis of polymer-dispersed liquid crystals [
29], and phase separation behavior of polymer solutions [
30,
31]. Based on these precedents, we presumed that the difference in scattering length density between EC derivatives and MAA is large enough to obtain clear SAXS patterns.
However, when conducting SAXS measurements, another concern about the evaporation of MAA from the lyotropic CLC was raised. In general, the reflection peak wavelength of lyotropic CLCs are drastically affected by the evaporation of solvent, resulting in difference in the internal structure of CLCs. To solve this problem, we conducted USAXS and SAXS measurements at room temperature for CLC films prepared by irradiation with ultraviolet (UV) light at 365 nm to the lyotropic CLCs of EC derivatives with MAA at 30 °C, 70 °C, or 110 °C. These solid-state CLC films might maintain their internal helical molecular assemblages of lyotropic CLCs at each curing temperature because of the progress of photopolymerization of MAA and subsequent cross-linking via hydrogen bonds. This is also evident from the fact that the reflection peak wavelengths of lyotropic CLCs and the films prepared by UV-irradiation are almost identical.
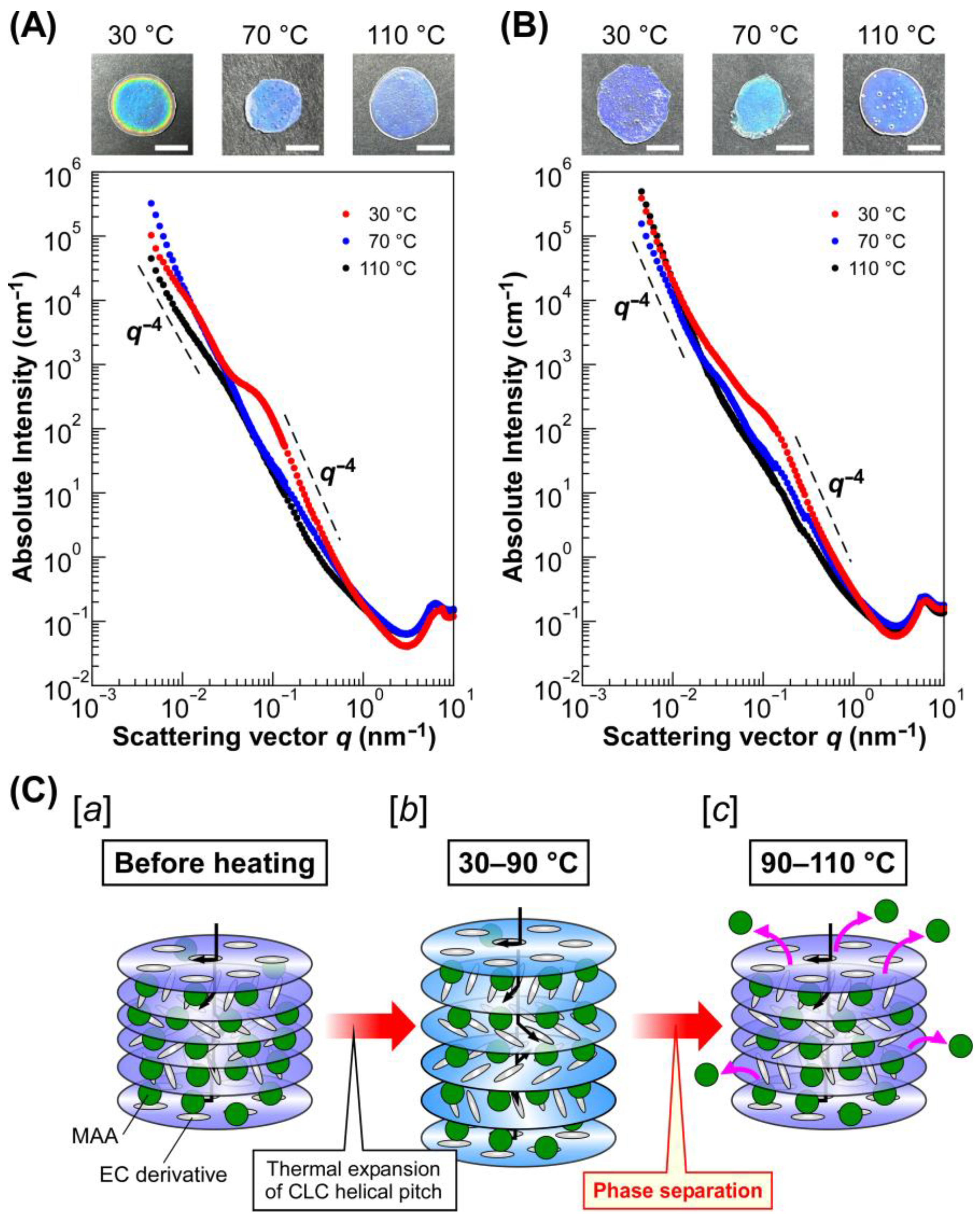
Figure 5 shows the intensity profiles of UV-cured CLC films exhibiting a blue refection color, which were prepared by using EC-Bu (
Figure 5A) and EC-Pe (
Figure 5B). These profiles were obtained by calculating the absolute intensity by using the standard of glassy carbon and plotting against the scattering vector (
q). A shoulder-like peak was observed at
q = ~0.03 nm
−1 in the profiles of the CLC film from Sample 1 cured at 30 °C (
Figure 5A, black circles). A similar shoulder-like peak was also observed at almost the same
q value for the intensity profiles of the film from Sample 1 cured at 70 °C (
Figure 5A, blue circles). Interestingly, this peak became apparent and shifted to
q = ~0.10 nm
−1 in the profile for the CLC film cured a 110 °C (
Figure 5A, red circles). Furthermore, the intensity became proportional to
q−4 in the
q range of 0.10–0.50 nm
−1 and below 0.02 nm
−1, suggesting the appearance of spherical scatterers. The size of spherical scatterers (
d) can be approximated by the following equation:
where
qs is the scattering vector at the shoulder-like peak. Therefore, it can be assumed that spherical scatterers with diameters of ~120 nm are formed in Sample 1 as cured at 110 °C. It should be noted that these scatterers are aggregated because the Guinier region was not clearly observed and the intensity continuously increased with a decrease in
q, suggesting the existence of extremely large scatterers that cannot detected by the USAXS measurements, similar to the intensity profiles of composite materials consisting of polymeric matrices with aggregated inorganic fillers [
32]. Such changes in USAXS-SAXS intensity profiles were also observed for the CLC films from EC-Pe. As shown in
Figure 5B, the intensity profiles of the CLC films of Sample 4 cured at 30 °C and 70 °C. However, a shoulder like peak appeared at
q = ~0.15 nm
−1 for the CLC film cured at 110 °C. Therefore, it was found that the scatterers with a size of ~80 nm exist in the CLC film. Furthermore, the emergence of a shoulder-like peak in USAXS-SAXS intensity profiles upon increasing the temperature were also observed for the CLC films fabricated from Samples 2, 3, 5, and 6 (
Supplementary Materials, Figure S3). Although the USAXS-SAXS intensity profiles were differed by curing temperatures and the reflection wavelength of the films due to changes in scattering length density difference or in the volume fraction of the scatterer, each profile had two regions where the intensity became proportional to
q−4. Therefore, it was indicated that the formation of aggregated spherical scatters upon increasing the temperature is irrelevant to the chemical structure of EC derivatives or the concentration of lyotropic CLCs with MAA. By considering the fact that X-ray is scattered by differences in electron density of the samples, we presumed that the internal helical molecular structure of lyotropic CLCs of EC derivatives with MAA is greatly transformed by changing the temperature.
This hypothesis can be also supported by the POM observation of lyotropic CLCs (
Supplementary Materials, Figure S4). As observing the POM images of lyotropic CLCs of EC-Bu or EC-PE with MAA exhibiting blue reflection colors at 30 °C, 70 °C, and 110 °C, all of the POM images were bright under cross-Nicols. Therefore, the lyotropic CLCs showed optical anisotropy arising from the liquid crystallinity [
26]. However, the POM images of EC-Bu with MAA taken at 110 °C (
Supplementary Materials, Figure S4C) and EC-Pe with MAA at 110 °C (
Supplementary Materials, Figure S4F) showed a different morphology with spherical domains, suggesting the drastic changes in CLC helical molecular assemblages [
33]. The size of spherical domains was estimated to be ~20 µm for the lyotropic CLC of EC-Bu with MAA, and ~30 µm for that of EC-Pe with MAA. Therefore, the lack of Guinier region in the USAXS-SAXS intensity profiles is reasonable because the size of scatterers, that is, domains observed by the POM, is extremely large to be measured by the USAXS.
Figure 5.
USAXS-SAXS intensity profiles of UV-cured films exhibiting blue reflection colors and plausible mechanism for the emergence of thermally stable Bragg reflection colors from lyotropic CLC of EC derivatives with MAA. (A,B) USAXS-SAXS intensity profiles of the solid-state CLC films from Sample 1 (A) and Sample 4 (B), which were cured with UV light at 30 °C (black circles), 70 °C (blue circles), and 110 °C (red circles). The insets represent the reflection images of UV-cured CLC films, and the white scale bars denote 5 mm. (C) Schematic illustration of structural changes in CLC from EC derivatives and MAA upon the heating process. [a] CLC structure before heating; [b] the CLC helical molecular assemblage at 30–90 °C, where reflection wavelength slightly red-shifts by thermal expansion of CLC helical pitch; [c] the CLC helical molecular assemblage at 90–110 °C, where MAA molecules expelled from the CLC helical molecular assemblage so as to induce the thermally induced phase separation at the mesoscopic scale.
Figure 5.
USAXS-SAXS intensity profiles of UV-cured films exhibiting blue reflection colors and plausible mechanism for the emergence of thermally stable Bragg reflection colors from lyotropic CLC of EC derivatives with MAA. (A,B) USAXS-SAXS intensity profiles of the solid-state CLC films from Sample 1 (A) and Sample 4 (B), which were cured with UV light at 30 °C (black circles), 70 °C (blue circles), and 110 °C (red circles). The insets represent the reflection images of UV-cured CLC films, and the white scale bars denote 5 mm. (C) Schematic illustration of structural changes in CLC from EC derivatives and MAA upon the heating process. [a] CLC structure before heating; [b] the CLC helical molecular assemblage at 30–90 °C, where reflection wavelength slightly red-shifts by thermal expansion of CLC helical pitch; [c] the CLC helical molecular assemblage at 90–110 °C, where MAA molecules expelled from the CLC helical molecular assemblage so as to induce the thermally induced phase separation at the mesoscopic scale.
![Molecules 30 02839 g005]()
2.5. Plausible Mechanism for the Emergence of Thermally Stable Bragg Reflection Colors from Lyotropic CLC of EC Derivatives and MAA
Based on the results mentioned in
Section 2.3 and
Section 2.4, we propose herein a mechanism for the emergence of thermally stable Bragg reflection colors from lyotropic CLCs of completely etherified EC derivatives with MAA. After heating the lyotropic CLCs of completely etherified EC derivatives with MAA, the CLC helical pitch length tends to expand by the thermodynamically induced increase in the mobility of CLC media (
Figure 5C, image
b). This is evident from the experimental fact that the red-shift of the reflection peak wavelength was observed from 30 °C to ~90 °C (Samples 1, 2, 4, and 5) or ~70 °C (Samples 3 and 6). At this stage, the absence of the change in the USAXS-SAXS profiles at 30 °C and 70 °C suggests that no significant structural change happens in the lyotropic CLCs. However, the phase separation was induced upon rising the temperature, resulting in the expulsion of MAA from the CLC helical molecular assemblage (
Figure 5C, image
c). This may be ascribed to the increase in the hydrophobicity of the EC derivatives arising from the complete etherification or increase in entropy because of the enhancement of the mobility of EC derivatives by their liquid crystallinity [
34]. Therefore, the reflection color of the lyotropic CLCs was unchanged because of the preservation of CLC helical pitch length by the expulsion of MAA from the CLC helical molecular assemblage.
Furthermore, the decrease in the transmittance upon heating can be attributed to the light scattering from the aggregated scatterers of MAA with a size of 40–100 nm, as confirmed from the USAXS-SAXS intensity profiles.