Ketamine—From an Anesthetic to a Psychiatric Drug: Mechanisms of Action, Clinical Applications and Potential Risks
Abstract
1. Basic Information
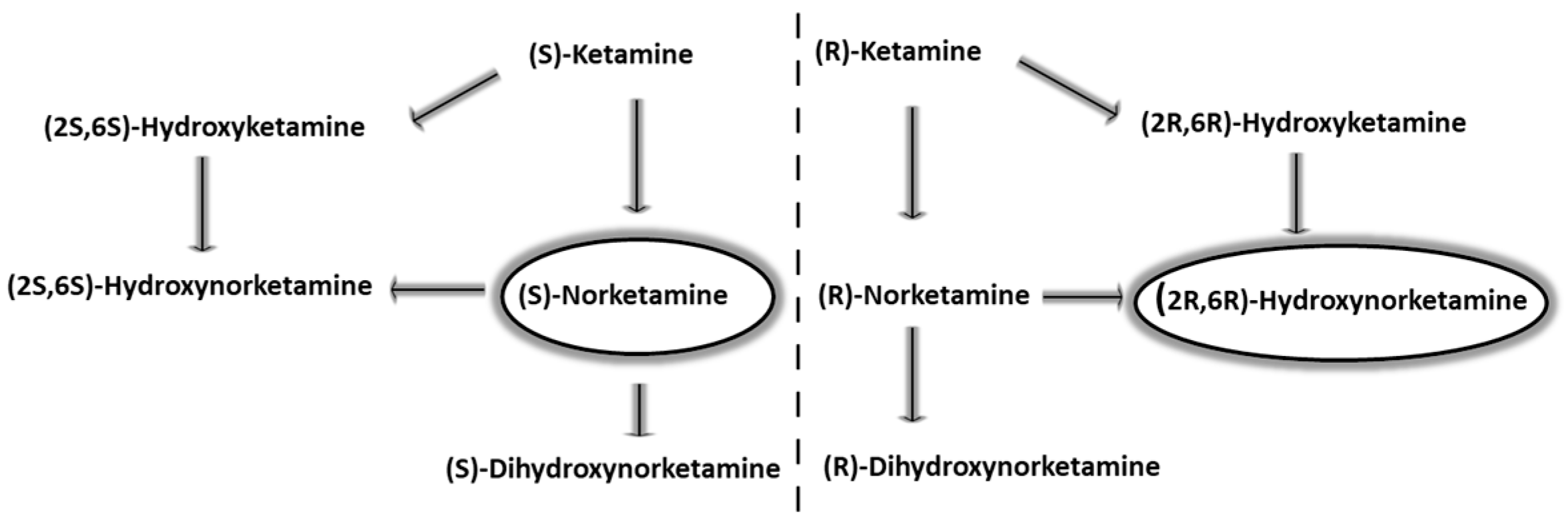
2. Pharmacokinetic Properties
3. Molecular Mechanism of Action of Ketamine and Its Enantiomers (S-, R- and Racemic)
4. Clinical Uses in Anesthesiology
5. Risks and Benefits
6. Ketamine in Pain Management
| Type of Pain | Administration Regimen/Key Features | Literature |
|---|---|---|
| Acute | Post-traumatic pain in prehospital settings fentanyl combined with low-dose ketamine (0.25–0.3 mg/kg) was administered, resulting in more effective and safer analgesia. | [88,89,90] |
| Postoperative pain in orthopedic surgery ketamine was administered as an i.v. bolus at a dose of 0.5 mg/kg, followed by a continuous i.v. infusion at 0.25 mg/kg/h, which resulted in a reduction in hyperalgesia areas without side effects. | [90,91,92] | |
| Pain in renal colic ketamine was administered intranasally at a dose of 1 mg/kg, which resulted in a reduction in pain intensity. | [91,92,93] | |
| Pain after third molar extraction a single intranasal dose of ketamine (50 mg) provided significant pain relief lasting for 3 h post-administration. | [43,88,94] | |
| Pain in acute traumatic conditions in children ketamine was administered intranasally 0.7 mg/kg, with the option of an additional 0.3 mg/kg bolus if pain exceeded 50 mm on the VAS scale; this regimen resulted in effective pain control with minimal and transient adverse effects. | [95,96,97] | |
| Chronic and neuropathic | Chronic pain—cancer-related (opioid-resistant) i.v. infusion of ketamine; dosage 0.25–0.6 mg/kg for 4–6 h daily for several days; reduction in pain in opioid-resistant patients and improvement in quality of life. | [98,99,100] |
| Chronic pain—CRPS (complex regional pain syndrome) daily infusions of ketamine 0.35 mg/kg i.v. for 4 h a day for 10 days results in long-lasting pain relief (lasting up to 12 weeks) and improved limb function. | [54,94,101] | |
| Neuropathic pain—chronic (e.g., diabetes, neuralgia) i.v. infusion of ketamine 0.1–0.5 mg/kg/h for 4–6 h; reduction in pain intensity, improved quality of life, possibility of reducing opioid dosage. | [102,103,104] | |
| Neuropathic pain—postherpetic neuralgia i.v. infusion of 0.1–0.5 mg/kg/h for 4–6 h; reduction in pain and improvement in patient functioning. | [97,103,104] | |
| Perioperative | Orthopedic surgery—arthroplasties i.v. bolus 0.5 mg/kg during induction of anesthesia, followed by continuous infusion at 0.25 mg/kg/h, which resulted in a reduction in pain management and reduced opioid therapy. | [69,105,106] |
| Spinal surgery i.v. bolus dose of 0.15–0.25 mg/kg prior to anesthesia induction, resulting in reduced pain. | [107,108,109] | |
| Laparoscopic procedures Low-dose ketamine—0.25 mg/kg, i.v. as a single bolus or by continuous infusion, resulting in improved pain control. | [110,111,112] | |
| Orthopedic limb surgery Subanalgesic doses of ketamine—i.v. bolus 0.3 mg/kg followed by i.v. infusion 0.2 mg/kg/h, which provides a strong analgesic effect. | [113,114,115] | |
| Cancer | Breakthrough cancer pain for sudden, severe pain, an additional dose of ketamine can be given sublingually or intranasally 10–50 mg; rapid pain relief, especially for patients who do not tolerate opioids well. | [116,117,118] |
| Advanced cancer pain—palliative therapy ketamine can be used as an add-on to opioids to help control pain: i.v., either as a continuous infusion or as a bolus (single dose): continuous infusion 0.1–0.3 mg/kg per hour or bolus 0.25 mg/kg every 8 h; pain relief and improved quality of life, especially in patients with opioid resistance. | [119,120,121] |
7. Ketamine as Potential Therapy for Addiction
- Some of the presented studies used only small populations of naïve individuals, lacked inactive placebo groups or were relatively homogeneous in terms of ethnicity, age and gender. Thus, the effects of ketamine administration early in life have not yet been clearly established, and it is possible that ketamine administration to adolescents for the treatment of depression may lead to an increased risk of addiction later in life.
- In the case of ethanol addiction, a very important element is the occurrence of potential interactions and co-dependency especially in that ketamine has become popular as a recreational drug, sometimes used with alcoholic beverages or stimulants.
- The issue of the addictive effect of ketamine and the possibility of its therapeutic use in controlled conditions without causing addiction also remains unresolved.
8. Present Primary Pharmacotherapy of Depression
9. The Mechanism of Antidepressant Activity of Ketamine
10. Current Position of Ketamine in the Treatment of Depression
11. The Antidepressant Effect of Ketamine in Animal Studies
12. Ketamine in Clinical Trials
13. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Domino, E.F.; Warner, D.S. Taming the ketamine tiger. Anesthesiology 2010, 113, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Ross, S. Ketamine and addiction. Prim. Psychiatry 2008, 15, 61–69. [Google Scholar]
- Radvansky, B.M.; Puri, S.; Sifonios, A.N.; Eloy, J.D.; Le, V. Ketamine-A narrative review of its uses in medicine. Am. J. Ther. 2016, 23, 1414–1426. [Google Scholar] [CrossRef]
- Adams, H.A. Wirkmechanismen von Ketamin Mechanisms of action of ketamine. Anaesthesiol. Reanim. 1998, 23, 60–63. [Google Scholar]
- White, P.F.; Schüttler, J.; Shafer, A.; Stanski, D.R.; Horai, Y.; Trevor, A.J. Comparative pharmacology of the ketamine isomers. Studies in volunteers. Br. J. Anaesth. 1985, 57, 197–203. [Google Scholar] [CrossRef]
- Ebert, B. Differential pharmacology of ketamine enantiomers. Anesth. Analg. 1997, 85, 119–124. [Google Scholar]
- Colla, M.; Scheerer, H.; Weidt, S.; Seifritz, E.; Kronenberg, G. Novel insights into the neurobiology of the antidepressant response from ketamine research: A mini review. Front. Behav. Neurosci. 2021, 15, 759466. [Google Scholar] [CrossRef] [PubMed]
- Zanos, P. Ketamine and ketamine metabolite pharmacology: Insights into therapeutic mechanisms. Trends Pharmacol. Sci. 2018, 39, 591–601. [Google Scholar] [CrossRef]
- Nichols, K.A.; Paciullo, C.A. Subdissociative Ketamine Use in the Emergency Department. Adv. Emerg. Nurs. J. 2019, 41, 15–22. [Google Scholar] [CrossRef]
- BinKharfi, M.; AlSagre, A. BET 2: Safety and efficacy of low-dose ketamine versus opioids for acute pain management in the ED. Emerg. Med. J. 2019, 36, 128–129. [Google Scholar] [CrossRef]
- Kapur, A.; Kapur, V. Conscious Sedation in Dentistry. Ann. Maxillofac. Surg. 2018, 8, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Doan, L.V.; Wang, J. An update on the basic and clinical science of ketamine analgesia. Clin. J. Pain 2018, 34, 1077–1088. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.K. Ketamine associated psychedelic effects and dependence. Singap. Med. 2003, 44, 31–34. [Google Scholar]
- Zou, L.; Tian, S.Y.; Quan, X.; Ye, T.H. Psychodelic effects of subanesthetic doses of ketamine. Zhongguo Yi XueKe Xue Yuan Xue Bao 2009, 31, 68–72. [Google Scholar]
- Sørensen, A.G.; Barnung, S.; Rasmussen, L.S. Ketamingenopdagetafbådelægerogmisbrugere [Ketamine is used again by both physicians and addicts]. Ugeskr. Laeger 2011, 173, 2123–2126. [Google Scholar] [PubMed]
- Sin, B.; Ternas, T.; Motov, S.M. The use of subdissociative-dose ketamine for acute pain in the emergency department. Acad. Med. 2015, 22, 251–257. [Google Scholar] [CrossRef]
- Lumanauw, D.D.; Youn, S.; Horeczko, T.; Yadav, K.; Tanen, D.A. Subdissociative-dose Ketamine Is Effective for Treating Acute Exacerbations of Chronic Pain. Acad. Med. 2019, 26, 1044–1051. [Google Scholar] [CrossRef]
- Puri, L.; Morgan, K.J.; Anghelescu, D.L. Ketamine and lidocaine infusions decrease opioid consumption during vaso-occlusive crisis in adolescents with sickle cell disease. Curr. Opin. Support. Palliat. Care 2019, 13, 402–407. [Google Scholar] [CrossRef]
- Mion, G. History of anaesthesia: The ketamine story—Past, present and future. Eur. J. Anaesthesiol. 2017, 34, 571–575. [Google Scholar] [CrossRef]
- Potter, D.E.; Choudhury, M. Ketamine: Repurposing and redefining a multifaceted drug. Drug Discov. Today 2014, 19, 1848–1854. [Google Scholar] [CrossRef]
- Desta, Z.; Moaddel, R.; Ogburn, E.T.; Xu, C.; Ramamoorthy, A.; Venkata, S.L.; Sanghvi, M.; Goldberg, M.E.; Torjman, M.C.; Wainer, I.W. Stereoselective and regiospecific hydroxylation of ketamine and norketamine. Xenobiotica 2012, 42, 1076–1087. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.K.; Flaker, A.M.; Friedel, C.C.; Kharasch, E.D. Role of Cytochrome P4502B6 Polymorphisms in Ketamine Metabolism and Clearance. Anesthesiology 2016, 125, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Andrade, C. Ketamine for Depression, 5: Potential Pharmacokinetic and Pharmacodynamic Drug Interactions. J. Clin. Psychiatry 2017, 78, 858–861. [Google Scholar] [CrossRef] [PubMed]
- ILangmia, M.; Just, K.S.; Yamoune, S.; Müller, J.P.; Stingl, J.C. Pharmacogenetic and drug interaction aspects on ketamine safety in its use as antidepressant—Implications for precision dosing in a global perspective. Br. J. Clin. Pharmacol. 2022, 88, 5149–5165. [Google Scholar] [CrossRef]
- Hijazi, Y.; Boulieu, R. Protein binding of ketamine and its active metabolites to human serum. Eur. J. Clin. Pharmacol. 2002, 58, 37–40. [Google Scholar] [CrossRef]
- Yanagihara, Y.; Kariya, S.; Ohtani, M.; Uchino, K.; Aoyama, T.; Yamamura, Y.; Iga, T. Involvement of CYP2B6 in n-demethylation of ketamine in human liver microsomes. Drug Metab. Dispos. 2001, 29, 887–890. [Google Scholar]
- Edwards, S.R.; Mather, L.E. Tissue uptake of ketamine and norketamine enantiomers in the rat: Indirect evidence for extrahepatic metabolic inversion. Life Sci. 2001, 69, 2051–2066. [Google Scholar] [CrossRef]
- Dinis-Oliveira, R.J. Metabolism and metabolomics of ketamine: A toxicological approach. Forensic Sci. Res. 2017, 2, 2–10. [Google Scholar] [CrossRef]
- Rosenbaum, S.B.; Gupta, V.; Patel, P.; Palacios, J.L. Ketamine. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024; Volume 30. [Google Scholar]
- Hashimoto, K. Molecular mechanisms of antidepressant actions of ketamine enantiomers and their metabolites. J. Pharmacol. 2019, 176, 1452–1456. [Google Scholar]
- Yang, C.; Kobayashi, S.; Nakao, K.; Dong, C.; Han, M.; Qu, Y.; Ren, Q.; Zhang, J.C.; Ma, M.; Toki, H.; et al. AMPA receptor activation-independent antidepressant actions of ketamine metabolite (S)-norketamine. Biol. Psychiatry 2018, 84, 591–600. [Google Scholar] [CrossRef]
- Craven, R. Ketamine. Anaesthesia 2007, 62, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Barrett, W.; Buxhoeveden, M.; Dhillon, S. Ketamine: A versatile tool for anesthesia and analgesia. Curr. Opin. Anaesthesiol. 2020, 33, 633–638. [Google Scholar] [CrossRef]
- Weber, F.; Wulf, H.; Gruber, M.; Biallas, R. S-ketamine and s-norketamine plasma concentrations after nasal and i.v. administration in anesthetized children. Paediatr. Anaesth. 2004, 14, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Clemens, J.; Nimmo, W.; Grant, I. Bioavailability, pharmacokinetics, and analgesic activity of ketamine in humans. Pharm. Sci. 1982, 71, 539–542. [Google Scholar] [CrossRef]
- Hasan, M.; Modess, C.; Roustom, T.; Dokter, A.; Grube, M.; Link, A.; Rey, H.; Adler, S.; Meissner, K.; Siegmund, W. Chiral Pharmacokinetics and Metabolite Profile of Prolonged-release Ketamine Tablets in Healthy Human Subjects. Anesthesiology 2021, 35, 326–339. [Google Scholar] [CrossRef]
- Chong, C.; Schug, S.A.; Page-Sharp, M.; Jenkins, B.; Ilett, K.F. Development of a sublingual/oral formulation of ketamine for use in neuropathic pain: Preliminary findings from a three-way randomized, crossover study. Clin. Drug Investig. 2019, 29, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Blonk, M.I.; Koder, B.G.; Bemt, P.M.; Huygen, F.J. Use of oral ketamine in chronic pain management: A review. Eur. J. Pain 2010, 14, 466–472. [Google Scholar] [CrossRef]
- Rolan, P.; Lim, S.; Sunderland, V.; Liu, Y.; Molnar, V. The absolute bioavailability of racemic ketamine from a novel sublingual formulation. Br. J. Clin. Pharmacol. 2014, 77, 1011–1016. [Google Scholar] [CrossRef]
- Yanagihara, Y.; Ohtani, M.; Kariya, S.; Uchino, K.; Hiraishi, T.; Ashizawa, N.; Aoyama, T.; Yamamura, Y.; Yamada, Y.; Iga, T. Plasma concentration profiles of ketamine and norketamine after administration of various ketamine preparations to healthy Japanese volunteers. Biopharm. Drug Dispos. 2003, 24, 37–43. [Google Scholar] [CrossRef]
- Malinovsky, J.M.; Servin, F.; Cozian, A.; Lepage, J.Y.; Pinaud, M. Ketamine and norketamine plasma concentrations after iv, nasal and rectal administration in children. Br. J. Anaesth. 1996, 77, 203–207. [Google Scholar] [CrossRef]
- Huge, V.; Lauchart, M.; Magerl, W.; Schelling, G.; Beyer, A.; Thieme, D.; Azad, S.C. Effects of low-dose intranasal (S)-ketamine in patients with neuropathic pain. Eur. J. Pain 2010, 14, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Andolfatto, G.; Willman, E.; Joo, D.; Miller, P.; Wong, W.B.; Koehn, M.; Dobson, R.; Angus, E.; Moadebi, S. Intranasal ketamine for analgesia in the emergency department: A prospective observational series. Acad. Emerg. Med. 2013, 20, 1050–1054. [Google Scholar] [CrossRef]
- Yeaman, F.; Meek, R.; Egerton-Warburton, D.; Rosengarten, P.; Graudins, A. Sub-dissociative-dose intranasal ketamine for moderate to severe pain in adult emergency department patients. Emerg. Med. 2014, 26, 237–242. [Google Scholar] [CrossRef]
- Fanta, S.; Kinnunen, M.; Backman, J.T.; Kalso, E. Population pharmacokinetics of S-ketamine and norketamine in healthy volunteers after intravenous and oral dosing. Eur. J. Clin. Pharmacol. 2015, 71, 441–447. [Google Scholar] [CrossRef]
- Bitter, C. Pharmacokinetics and Pharmacodynamics of Nasally Applied s-Ketamine. Ph.D. Thesis, The University of Basel, Basel, Switzerland, 2010. Available online: https://edoc.unibas.ch/1310/1/20110314_1408_DissCB_e_version.pdf (accessed on 16 December 2014).
- Peltoniemi, S.; Hagelberg, L.; Kurkinen, K.J.; Neuvonen, P.J.; Olkkola, K.T. Rifampicin has a profound effect on the pharmacokinetics of oral S-ketamine and less on intravenous S-ketamine. Basic Clin. Pharmacol. Toxicol. 2012, 111, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Perez-Ruixo, C.; Rossenu, S.; Zannikos, P.; Nandy, P.; Singh, J.; Drevets, W.C. Perez-Ruixo, J.J. Population Pharmacokinetics of Esketamine Nasal Spray and its Metabolite Noresketamine in Healthy Subjects and Patients with Treatment-Resistant Depression. Clin. Pharmacokinet. 2021, 60, 501–516. [Google Scholar] [CrossRef]
- Paoletti, P. NMDA receptor subunits: Function and pharmacology. Curr. Opin. Pharmacol. 2013, 13, 34–41. [Google Scholar]
- Hansen, K.B. Structural determinants of NMDA receptor function. Nat. Neurosci. 2016, 19, 1281–1291. [Google Scholar]
- Johnson, J.W. Mechanisms of NMDA receptor inhibition by ketamine and its metabolites. J. Neurosci. 2009, 29, 8563–8571. [Google Scholar]
- Abott, J.A.; Wen, H.; Liu, B.; Gupta, S.S.; Iacobucci, G.J.; Zheng, W.; Popescu, G.K. Allosteric inhibition of NMDA receptors by low dose ketamine. Mol. Psychiatry 2025, 30, 1009–1018. [Google Scholar] [CrossRef]
- Hayashi, T. Sigma-1 receptor chaperones regulate the ER-mitochondrion interface and mitochondrial function. Science 2007, 316, 585–589. [Google Scholar]
- Maurice, T.; Su, T.P. The pharmacology of sigma-1 receptors. Pharmacol. Rev. 2009, 61, 518–537. [Google Scholar] [CrossRef]
- Lepack, A.E.; Fuchikami, M.; Dwyer, J.M.; Banasr, M.; Duman, R.S. BDNF Release Is Required for the Behavioral Actions of Ketamine. Int. J. Neuropsychopharmacol. 2015, 18, pyu033. [Google Scholar] [CrossRef]
- Yamakura, T.; Chavez-Noriega, L.E.; Harris, R.A. Subunit-dependent inhibition of human neuronal nicotinic acetylcholine receptors and other ligand-gated ion channels by dissociative anesthetics ketamine and dizocilpine. Anesthesiology 2000, 92, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Laurence, E. The histamine-releasing effect of ketamine on mast cells: Mechanisms and clinical implications. Anesthesiology 2013, 119, 734–744. [Google Scholar]
- Loix, S. The anti-inflammatory and immunomodulatory effects of ketamine: A review. Acta Anaesthesiol. Belg. 2011, 62, 47–58. [Google Scholar]
- Chang, Y. Ketamine reduces lipopolysaccharide-induced neuroinflammation in the rat brain. Anesth. Analg. 2009, 109, 1544–1551. [Google Scholar]
- Moaddel, R. Ketamine and its metabolites: Effects on α7-nicotinic receptor inhibition. J. Med. Chem. 2013, 56, 8232–8236. [Google Scholar]
- Hirota, K. Interaction of ketamine with opioid receptors in the central nervous system: Mechanisms and implications. Anesth. Analg. 1999, 89, 1–5. [Google Scholar]
- Duman, R.S. Ketamine and rapid-acting antidepressants: A new era in the battle against depression. Nat. Rev. Drug Discov. 2016, 15, 285–295. [Google Scholar] [CrossRef]
- Yang, C. Comparison of the antidepressant effects of R-ketamine and S-ketamine in animal models of depression. Pharmacol. Biochem. Behav. 2015, 138, 22–26. [Google Scholar]
- Zanos, P.; Moaddel, R.; Morris, P.J.; Georgiou, P.; Fischell, J.; Elmer, G.I.; Alkondon, M.; Yuan, P.; Pribut, H.J.; Singh, N.S.; et al. NMDAR inhibition-independent antidepressant actions of 34 ketamine metabolites. Nature 2016, 533, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, A.; Santer, P.; Munoz-Acuna, R.; Hammer, M.; Schaefer, M.S.; Wachtendorf, L.J.; Rumyantsev, S.; Berra, L.; Chamadia, S.; Johnson-Akeju, O.; et al. Effects of Ketamine Infusion on Breathing and Encephalography in Spontaneously Breathing ICU Patients. J. Intensive Care Med. 2023, 38, 299–306. [Google Scholar] [CrossRef]
- Gales, A.; Maxwell, S.K. Recent evidence and current uses. ATOTW 2018, 381, 1–7. [Google Scholar]
- Świątkiewicz, G. Social sciences in “Alkoholizm i Narkomania”–25 years of experience. AiN 2013, 26, 221–230. [Google Scholar]
- Geisslinger, G.; Hering, W.; Thomann, P.; Knoll, R.; Kamp, H.D.; Brune, K. Pharmacokinetics and pharmacodynamics of ketamine enantiomers in surgical patients using a stereoselective analytical method. Br. J. Anaesth. 1993, 70, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, J.; Yang, S.; Cui, C.; Ye, L.; Wang, S.Y.; Yang, G.P.; Pei, Q. Pharmacokinetics and Safety of S-ketamine in Chinese Patients Undergoing Painless Gastroscopy in Comparison with Ketamine: A Randomized, Open-Label Clinical Study. Drug Dev. Ther. 2019, 13, 4135–4144. [Google Scholar] [CrossRef]
- Gao, M.; Rejaei, D.; Liu, H. Ketamine use in current clinical practice. Acta Pharmacol. Sin. 2016, 37, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Peltoniemi, M.A.; Hagelberg, N.M.; Olkkola, K.T.; Saari, T.I. Ketamine: A Review of Clinical Pharmacokinetics and Pharmacodynamics in Anesthesia and Pain Therapy. Clin. Pharmacokinet. 2016, 55, 1059–1077. [Google Scholar] [CrossRef] [PubMed]
- Trimmel, H.; Helbok, R.; Staudinger, T.; Jaksch, W.; Messerer, B.; Schöchl, H.; Likar, R. S(+)-ketamine: Current trends in emergency and intensive care medicine. Wien. Klin. Wochenschr. 2018, 130, 356–366. [Google Scholar] [CrossRef]
- Kung, J.; Meisner, R.C.; Berg, S.; Ellis, D.B. Ketamine: A review of an established yet often underappreciated medication. Newsl. J. APSF 2020, 35, 33–68. [Google Scholar]
- Stoicea, N.; Versteeg, G.; Florescu, D.; Joseph, N.; Fiorda-Diaz, J.; Navarrete, V.; Bergese, S.D. Ketamine-Based Anesthetic Protocols and Evoked Potential Monitoring: A Risk/Benefit Overview. Front. Neurosci. 2016, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Kurdi, M.S.; Theerth, K.A.; Deva, R.S. Ketamine: Current applications in anesthesia, pain, and critical care. Anesth. Essays Res. 2014, 8, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Woolf, C.J. Central sensitization: Implications for the diagnosis and treatment of pain. Pain 2011, 152, 2–15. [Google Scholar] [CrossRef]
- Petrenko, A.B.; Yamakura, T.; Baba, H.; Shimoji, K. The role of N-methyl-D-aspartate (NMDA) receptors in pain: A review. Anesth. Analg. 2003, 97, 1108–1116. [Google Scholar] [CrossRef]
- Feizerfan, A.; Sheh, G. Transition from acute to chronic pain. Contin. Educ. Anaesth. Crit. Care Pain 2015, 15, 98–102. [Google Scholar] [CrossRef]
- Tsui, P.Y.; Chu, M.C. Ketamine: An old drug revitalized in pain medicine. BJA Educ. 2017, 17, 84–87. [Google Scholar] [CrossRef]
- Niesters, M.; Martini, C.; Dahan, A. Ketamine for chronic pain: Risks and benefits. Br. J. Clin. Pharmacol. 2014, 77, 357–367. [Google Scholar] [CrossRef]
- Bell, R.F.; Dahl, J.B.; Moore, R.A.; Kalso, E. Perioperative ketamine for acute postoperative pain. Cochrane Database Syst. Rev. 2006, 5, CD004603. [Google Scholar]
- Choi, E.; Lee, H.; Park, H.S.; Lee, G.Y.; Kim, Y.J.; Baik, H.J. Effect of intraoperative infusion of ketamine on remifentanil-induced hyperalgesia. Korean J. Anesth. 2015, 68, 476–480. [Google Scholar] [CrossRef]
- Khan, Z.; Hameed, M.; Khan, F.A. Current role of perioperative intravenous ketamine: A narrative review. Anesthesiol. Perioper. Sci. 2023, 1, 36. [Google Scholar] [CrossRef]
- Martinez, M.R.; Garmon, E.H.; Starling, G.D.; Sheth, M.A. Ketamine as an Analgesic Adjunct for Opioid-Induced Hyperalgesia in a Patient With a Sickle Cell Pain Episode. Ochsner J. 2022, 22, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Cairns, J.; Feng, T.L.; Ong, M.C. Continuous ketamine infusion for the management of opioid-induced hyperalgesia following amputation. BMJ Case Rep. 2024, 17, 255333. [Google Scholar] [CrossRef]
- Żylicz, Z.; Krajnik, M. Hiperalgezja opioidowa jako problem w leczeniu bólu. Mechanizmy powstawania, rozpoznanie i leczenie. Med. Paliatywna W Praktyce 2007, 1, 16–23. [Google Scholar]
- Koppert, W. Opioid-inducedhyperalgesia–Pathophysiology and clinicalrelevance. Acute Pain 2007, 9, 21–34. [Google Scholar] [CrossRef]
- McMullan, J.T.; Droege, C.A.; Chard, K.M.; Otten, E.J.; Hart, K.W.; Lindsell, C.J. Out-of-Hospital Intranasal Ketamine as an Adjunct to Fentanyl for the Treatment of Acute Traumatic Pain: A Randomized Clinical Trial. Ann. Emerg. Med. 2024, 84, 363–373. [Google Scholar] [CrossRef] [PubMed]
- McMullan, J.; Droege, C.; Strilka, R.; Hart, K.; Lindsell, C. Intranasal Ketamine as an Adjunct to Fentanyl for the Prehospital Treatment of Acute Traumatic Pain: Design and Rationale of a Randomized Controlled Trial. Prehosp. Emerg. Care 2021, 25, 519–529. [Google Scholar] [CrossRef]
- Yousefifard, M. The Efficacy of Ketamine Administration in Prehospital Pain Management of Trauma Patients; a Systematic Review and Meta-Analysis. Arch. Acad. Emerg. Med. 2020, 8, 1. [Google Scholar] [CrossRef]
- Mozafari, J.; Verki, M.M.; Motamed, H.; Sabouhi, A.; Tirandaz, F. Intranasal ketamine versus intravenous morphine for pain management in patients with renal colic: A double-blind, randomized, controlled trial. Am. J. Emerg. Med. 2020, 38, 2132–2136. [Google Scholar] [CrossRef]
- Farnia, M.R.; Jalali, A.; Vahidi, E.; Momeni, M.; Seyedhosseini, J.; Saeedi, M. Comparison of intranasal ketamine versus IV morphine in reducing pain in patients with renal colic. Am. J. Emerg. Med. 2017, 35, 434–437. [Google Scholar] [CrossRef]
- Jalili, A.; Farnia, M.R.; Momeni, M.; Saeedi, M. Comparing intranasal ketamine with intravenous fentanyl in reducing pain in patients with renal colic: A double-blind randomized clinical trial. Am. J. Emerg. Med. 2020, 38, 549–553. [Google Scholar] [CrossRef]
- Schwartzman, S.A.; Alexander, M.M.; Kravitz, S.A.; Shah, S.A.; Kravitz, M.J. A dose-escalation clinical trial of intranasal ketamine for uncontrolled cancer-related pain. Pharmacotherapy 2022, 42, 321–329. [Google Scholar] [CrossRef]
- Shrestha, S.; Shrestha, R.; Shrestha, S.; Shrestha, B. Intranasal ketamine for the treatment of patients with acute pain in the emergency department. J. Nepal. Health Res. Counc. 2016, 14, 197–201. [Google Scholar] [CrossRef]
- Frey, T.M.; Florin, T.A.; Caruso, M.; Zhang, N.; Zhang, Y.; Mittiga, M.R. Effect of Intranasal Ketamine vs Fentanyl on Pain Reduction for Extremity Injuries in Children: The PRIME Randomized Clinical Trial. JAMA Pediatr. 2019, 173, 140–146. [Google Scholar] [CrossRef]
- Reynolds, S.L. Randomized Controlled Feasibility Trial of Intranasal Ketamine Compared to Intranasal Fentanyl for Analgesia in Children with Suspected Extremity Fractures. Acad. Emerg. Med. 2017, 24, 1430–1440. [Google Scholar] [CrossRef]
- Bell, R.F.; Eccleston, C.; Kalso, E.A. Ketamine as an adjuvant to opioids for cancer pain. Cochrane Database Syst. Rev. 2017, 6, CD003351. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Kim, J.J.; Oh, S.B.; Oh, S.Y.; Hong, Y.J.; Kim, S.J.; Park, E.J.; Choi, N.; Shin, S.H.; Kim, S.; et al. A Phase II Study About Efficacy and Safety of the Continuous IntraVenous Infusion of Ketamine as Adjuvant to Opioids in Terminally Ill Cancer Patients With Refractory Cancer Pain (CIVIK Trial). Am. J. Hosp. Palliat. Care 2025, 42, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Orhurhu, V.J.; Roberts, J.S.; Ly, N.; Cohen, S.P. Ketamine in Acute and Chronic Pain Management. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Chitneni, A.; Patil, A.; Dalal, S.; Ghorayeb, J.H.; Pham, Y.N.; Grigoropoulos, G. Use of Ketamine Infusions for Treatment of Complex Regional Pain Syndrome: A Systematic Review. Cureus 2021, 13, e18910. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.E.G.; Pereira, L.F.G.; Linhares, R.M.; Bersot, C.D.A.; Aslanidis, T.; Ashmawi, H.A. Efficacy and Safety of Ketamine in the Treatment of Neuropathic Pain: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Pain Res. 2022, 15, 1011–1037. [Google Scholar] [CrossRef]
- Bruna-Mejias, A.; Baeza, V.; Gamboa, J.; Baez Flores, B.; San Martin, J.; Astorga, C.; Leyton, J.; Nova-Baeza, P.; Orellana-Donoso, M.; Suazo-Santibañez, A.; et al. Use of Ketamine in Patients with Multifactorial Neuropathic Pain: A Systematic Review and Meta-Analysis. Pharmaceuticals 2024, 17, 1165. [Google Scholar] [CrossRef]
- Mitchell, A.C.; Fallon, M.T. A single infusion of intravenous ketamine improves pain relief in patients with critical limb ischaemia: Results of a double blind randomised controlled trial. Pain 2002, 10, 12044624. [Google Scholar] [CrossRef] [PubMed]
- Li, Q. S-Ketamine reduces the risk of rebound pain in patients following total knee arthroplasty: A randomized controlled trial. Drug Des. Dev. Ther. 2025, 19, 2315–2327. [Google Scholar] [CrossRef]
- Gumelar, W. The effectiveness of intraoperative ketamine and fentanyl as preemptive analgesia assessed with qNOX Score. J. Anestesi Indonesia. 2021, 13, 67–77. [Google Scholar] [CrossRef]
- Pendi, A.; Field, R.; Farhan, S.-D.; Eichler, M.; Bederman, S.S. Perioperative Ketamine for Analgesia in Spine Surgery: A Meta-analysis of Randomized Controlled Trials. Spine 2018, 43, 299–307. [Google Scholar] [CrossRef]
- Nielsen, R.V. Intraoperative Ketamine Reduces Immediate Postoperative Opioid Consumption After Spinal Fusion Surgery in Chronic Pain Patients with Opioid Dependency: A Randomized, Blinded Trial. Pain 2017, 158, 463–470. [Google Scholar] [CrossRef]
- Park, P.J.; Makhni, M.C.; Cerpa, M.; Lehman, R.A.; Lenke, L.G. Intravenous Ketamine for Postoperative Pain Control in Spine Surgery: A Randomized Controlled Trial. J. Spine Surg. 2020, 6, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, Y.; Wang, D. The Effect of Ketamine Infusion in the Treatment of Complex Regional Pain Syndrome: A Systemic Review and Meta-analysis. Curr. Pain. Headache Rep. 2018, 22, 12. [Google Scholar] [CrossRef]
- Jain, S.; Nazir, N.; Mustafi, S.M. Effect of Low-dose Ketamine on Inflammatory Markers, Perioperative Pain, and Chronic Pain After Laparoscopic Inguinal Hernia Surgery. Turk. J. Anaesthesiol. Reanim. 2024, 52, 200–207. [Google Scholar] [CrossRef]
- Xu, Y.; He, L.; Liu, S.; Zhang, C.; Ai, Y. Intraoperative Intravenous Low-dose Esketamine Improves Quality of Early Recovery After Laparoscopic Radical Resection of Colorectal Cancer: A Prospective, Randomized Controlled Trial. PLoS ONE 2023, 18, 0286590. [Google Scholar] [CrossRef]
- Zhao, L.; Li, Z.; Jin, B.; Hou, N.; Yang, H. Safety and efficacy of low-dose esketamine in laparoscopic cholecystectomy: A prospective, double-blind randomized controlled trial. BMC Anesthesiol. 2024, 24, 47. [Google Scholar] [CrossRef]
- Sukantarat, N.; Uthaiwan, K. Comparison of postoperative pain scores between intraoperative intravenous ketamine and placebo in patients undergoing unilateral total knee arthroplasty under general anesthesia: A double-blind randomized controlled trial. Thai J. Anesth. 2024, 50, 1–8. [Google Scholar]
- Chen, H.; Zhi, J.; Wang, L.; Jin, Z.; Xu, J.; Xing, F.; Wen, C.; Wang, Q.; Chen, C.; Li, W.; et al. Subanesthetic Dose of Esketamine Improves the Sedative and Analgesic Effects of Dexmedetomidine and Remifentanil in Liposuction Anesthesia: A Prospective, Double-Blinded, Randomized Controlled Trial. Drug Des. Dev. Ther. 2024, 20, 3645–3658. [Google Scholar] [CrossRef]
- Sutoh, K.; Sanuki, N.; Sakaki, T.; Imai, R. Specific induction of TaAAPT1, an ER- and Golgi-localized ECPT-type aminoalcoholphosphotransferase, results in preferential accumulation of the phosphatidylethanolamine membrane phospholipid during cold acclimation in wheat. Plant Mol. Biol. 2010, 72, 519–531. [Google Scholar] [CrossRef]
- Mercadante, S.; Villari, P.; Ferrera, P. Burst ketamine to reverse opioid tolerance in cancer pain. J. Pain Symptom Manag. 2023, 25, 302–305. [Google Scholar] [CrossRef]
- Singh, V.; Gillespie, T.W.; Harvey, R.D. Intranasal Ketamine and Its Potential Role in Cancer-Related Pain. Pharmacotherapy 2018, 38, 390–401. [Google Scholar] [CrossRef]
- Hocking, G.; Cousins, M.J. Ketamine in chronic pain management: An evidence-based review. Anesth. Analg. 2003, 97, 1730–1739. [Google Scholar] [CrossRef] [PubMed]
- Fine, P.G. Low-dose ketamine in the management of opioid nonresponsive terminal cancer pain. J. Pain Symptom Manag. 1999, 17, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Forth, N.; Nguyen, M.; Grech, A. A Case Report of Subanesthetic Ketamine Bolus and Infusion for Opioid Refractory Cancer Pain. J. Palliat. Med. 2022, 25, 1161–1165. [Google Scholar] [CrossRef]
- Hasin, D.S.; Goodwin, R.D.; Stinson, F.S.; Grant, B.F. Epidemiology of major depressive disorder: Results from the National Epidemiologic Survey on Alcoholism and Related Conditions. Arch. Gen. Psychiatry 2005, 62, 1097–1106. [Google Scholar] [CrossRef]
- Kessler, R.C.; Berglund, P.; Demler, O.; Jin, R.; Koretz, D.; Merikangas, K.R.; Rush, A.J.; Walters, E.E.; Wang, P.S. The epidemiology of major depressive disorder: Results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003, 289, 3095–3105. [Google Scholar] [CrossRef]
- Grant, B.F.; Stinson, F.S.; Dawson, D.A.; Chou, S.P.; Dufour, M.C.; Compton, W.; Pickering, R.P.; Kaplan, K. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: Results from the national epidemiologic survey on alcohol and related conditions. Arch. Gen. Psychiatry 2004, 61, 807–816. [Google Scholar] [CrossRef]
- Curran, H.V.; Freeman, T.P.; Mokrysz, C.; Lewis, D.A.; Morgan, C.J.; Parsons, L.H. Keep off the grass? Cannabis, cognition and addiction. Nat. Rev. Neurosci. 2016, 17, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Markou, A.; Kosten, T.R.; Koob, G.F. Neurobiological similarities in depression and drug dependence: A self-medication hypothesis. Neuropsychopharmacology 1998, 18, 135–174. [Google Scholar] [CrossRef]
- Quello, S.B.; Brady, K.T.; Sonne, S.C. Mood disorders and substance use disorder: A complex comorbidity. Sci. Pract. Perspect. 2005, 3, 13–21. [Google Scholar] [CrossRef]
- Greenfield, S.F.; Weiss, R.D.; Muenz, L.R. The effect of depression on return to drinking: A prospective study. Arch. Gen. Psychiatry 1998, 55, 259–265. [Google Scholar] [CrossRef] [PubMed]
- White, A.M.; Jordan, J.D.; Schroeder, K.M.; Acheson, S.K.; Georgi, B.D.; Sauls, G.; Ellington, R.R.; Swartzwelder, H.S. Predictors of Relapse During Treatment and Treatment Completion Among Marijuana-Dependent Adolescents in an Intensive Outpatient Substance Abuse Program Aaron. Subst. Abus. 2004, 25, 53–59. [Google Scholar] [CrossRef]
- Willinger, U.; Lenzinger, E.; Hornik, K.; Fischer, G.; Schönbeck, G.; Aschauer, H.N.; Meszaros, K. Anxiety as a predictor of relapse in detoxified alcohol dependent patients. Alcohol Alcohol. 2002, 37, 609–612. [Google Scholar] [CrossRef] [PubMed]
- Mason, B.J.; Kocsis, J.H.; Ritvo, E.C.; Cutler, R.B. A double blind, placebo controlled trial of desipramine for primary alcohol dependence stratified on the presence or absence of major depression. JAMA 1996, 275, 761–767. [Google Scholar] [CrossRef]
- Pettinati, H.M. Antidepressant treatment of co-occurring depression and 31 alcohol dependence. Biol. Psychiatry 2004, 56, 785–792. [Google Scholar] [CrossRef]
- Kalivas, P.W.; Kalivas, P.W.; Volkow, N.D. The glutamate homeostasis hypothesis of addiction. Nat. Rev. Neurosci. 2009, 10, 561–572. [Google Scholar] [CrossRef]
- Kalivas, P.W.; Volkow, N.D. The neural basis of addiction: A pathology of motivation and choice. Am. J. Psychiatry 2005, 162, 1403–1413. [Google Scholar] [CrossRef]
- Sabino, V.; Narayan, A.R.; Zeric, T.; Steardo, L.; Cottone, P. mTOR activation is required for the anti-alcohol effect of ketamine, but not memantine, in alcohol-preferring rats. Behav. Brain Res. 2013, 247, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Li, N. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 2010, 329, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Paul, R.K.; Singh, N.S.; Khadeer, M.; Moaddel, R.; Sanghvi, M.; Green, C.E.; O’Loughlin, K.; Torjman, M.C.; Bernier, M.; Wainer, I.W. (R,S)-Ketamine metabolites (R,S)-norketamine and (2S,6S)-hydroxynorketamine increase the mammalian target of rapamycin function. Anesthesiology 2014, 121, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Mandyam, C.D.; Koob, G.F. The addicted brain craves new neurons: Putative role for adult-born progenitors in promoting recovery. Trends Neurosci. 2012, 35, 250–260. [Google Scholar] [CrossRef]
- Noonan, M.A.; Bulin, S.E.; Fuller, D.C.; Eisch, A.J. Reduction of adult hippocampal neurogenesis confers vulnerability in an animal model of cocaine addiction. J. Neurosci. 2010, 30, 304–315. [Google Scholar] [CrossRef]
- Zanardini, R.; Fontana, A.; Pagano, R.; Mazzaro, E.; Bergamasco, F.; Romagnosi, G.; Gennarelli, M.; Bocchio-Chiavetto, L. Alterations of brain-derived neurotrophic factor serum levels in patients with alcohol dependence. Alcohol. Clin. Exp. Res. 2011, 35, 1529–1533. [Google Scholar] [CrossRef]
- Angelucci, F.; Ricci, V.; Pomponi, M.; Conte, G.; Mathé, A.A.; Attilio Tonali, P.; Bria, P. Chronic heroin and cocaine abuse is associated with decreased serum concentrations of the nerve growth factor and brain-derived neurotrophic factor. J. Psychopharmacol. 2007, 21, 820–825. [Google Scholar] [CrossRef]
- Corominas-Roso, M.; Roncero, C.; Eiroa-Orosa, F.J.; Gonzalvo, B.; Grau-Lopez, L.; Ribases, M.; Rodriguez-Cintas, L.; Sánchez-Mora, C.; Ramos-Quiroga, J.A.; Casas, M. Brain-derived neurotrophic factor serum levels in cocaine-dependent patients during early abstinence. Eur. Neuropsychopharmacol. 2013, 23, 1078–1084. [Google Scholar] [CrossRef]
- Sönmez, M.B.; Görgülü, Y.; Çınar, R.K.; Kılıç, E.K.; Ünal, A.; Vardar, M. Alterations of BDNF and GDNF serum levels in alcohol-addicted patients during alcohol withdrawal. Eur. J. Psychiatry 2016, 30, 109–118. [Google Scholar]
- Duncan, W.C., Jr.; Zarate, C.A., Jr. Ketamine, sleep, and depression: Current status and new questions. Curr. Psychiatry Rep. 2013, 15, 394. [Google Scholar] [CrossRef]
- Autry, A.E.; Adachi, M.; Nosyreva, E.; Na, E.S.; Los, M.F.; Cheng, P.F.; Kavalali, E.T.; Monteggia, L.M. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 2011, 475, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Ota, K.T.; Duman, R.S. Environmental and pharmacological modulations of cellular plasticity: Role in the pathophysiology and treatment of depression. Neurobiol. Dis. 2013, 57, 28–37. [Google Scholar] [CrossRef]
- Corlett, P.R.; Cambridge, V.; Gardner, J.M.; Piggot, J.S.; Turner, D.C.; Everitt, J.C.; Arana, F.S.; Morgan, H.L.; Milton, A.L.; Lee, J.L.; et al. Ketamine effects on memory reconsolidation favor a learning model of delusions. PLoS ONE 2013, 8, e65088. [Google Scholar] [CrossRef] [PubMed]
- Kolp, E.; Friedman, H.L.; Young, M.S. Ketamine enhanced psychotherapy: Preliminary clinical observations on its effectiveness in treating alcoholism. Humanist. Psychol. 2006, 34, 399–422. [Google Scholar] [CrossRef]
- Krupitsky, E.M.; Grinenko, A.Y. Ketamine psychedelic therapy (KPT): A review of the results of ten years of research. J. Psychoact. Drugs 1997, 29, 165–183. [Google Scholar] [CrossRef] [PubMed]
- Dakwar, E.; Anerella, C.; Hart, C.L. Therapeutic infusions of ketamine: Do the psychoactive effects matter? Drug Alcohol. Depend. 2014, 136, 153–157. [Google Scholar] [CrossRef]
- Kolp, E.; Friedman, H.L.; Krupitsky, E.; Jansen, K.; Sylvester, M.; Young, M.S.; Kolp, A. Ketamine psychedelic psychotherapy: Focus on its pharmacology, phenomenology, and clinical applications. Int. J. Transpers. Stud. 2014, 33, 84–140. [Google Scholar] [CrossRef]
- Kolp, E.; Krupitsky, E.; Young, M.S.; Jansen, K.; Friedman, H.; O’Connor, L.A. Ketamine-enhanced psychotherapy: Preliminary clinical observations on its effects in treating death anxiety. Int. J. Transpers. Stud. 2007, 26, 1–17. [Google Scholar] [CrossRef]
- Krupitsky, E.; Burakov, A.; Romanova, T. Ketamine psychotherapy for heroin addiction: Immediate effects and two-year follow-up. J. Subst. Abuse Treat. 2002, 23, 273–283. [Google Scholar] [CrossRef]
- Giese, K.P. Memory Mechanisms in Health and Disease: Mechanistic Basis of Memory; World Scientific: Singapore, 2012. [Google Scholar]
- Jansen, K.L.R. A Review of the Nonmedical Use of Ketamine: Use, Users and Consequences. J. Psychoact. Drugs 2000, 32, 419–433. [Google Scholar] [CrossRef]
- Jansen, K.L.; Darracot-Cankovic, R. The nonmedical use of ketamine, part two: A review of problem use and dependence. J. Psychoact. Drugs 2001, 33, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Ricuarte, G.; McCann, U. Recognition and management of complications of new recreational drug use. Lancet 2005, 365, 2137–2145. [Google Scholar] [CrossRef]
- Dakwar, E.; Levin, F.; Hart, C.L.; Basaraba, C.; Choi, J.; Pavlicova, M. A single ketamine infusion combined with motivational enhancement therapy for alcohol use disorder: A randomized midazolam-controlled pilot trial. Am. J. Psychiatry 2020, 177, 125–133. [Google Scholar] [CrossRef]
- Das, R.K.; Gale, G.; Walsh, K. Ketamine can reduce harmful drinking by pharmacologically rewriting drinking memories. Nat. Commun. 2019, 10, 5187. [Google Scholar] [CrossRef] [PubMed]
- Grabski, M.; McAndrew, A.; Lawn, W. Adjunctive ketamine with relapse prevention-based psychological therapy in the treatment of alcohol use disorder. Am. J. Psychiatry 2022, 179, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Krupitsky, E.M.; Grinenko, A.Y.; Berkaliev, T.N.; Paley, A.I.; Petrov, V.N.; Moshkov, K.A.; Borodkin, Y.S. The combination of psychedelic and aversive approaches in alcoholism treatment: The affective contra-attribution method. Alcohol. Treat. Q. 1992, 9, 99–105. [Google Scholar] [CrossRef]
- Pizon, A.F.; Lynch, M.J.; Benedict, N.J.; Yanta, J.H.; Frisch, A.; Menke, N.B.; Swartzentruber, G.S.; King, A.M.; Abesamis, M.G.; Kane-Gill, S.L. Adjunct ketamine use in the management of severe ethanol withdrawal. Crit. Care Med. 2018, 46, e768–e771. [Google Scholar] [CrossRef]
- Rothberg, R.L.; Azhari, N.; Haug, N.A.; Dakwar, E. Mystical-type experiences occasioned by ketamine mediate its impact on at-risk drinking: Results from a randomized, controlled trial. J. Psychopharmacol. 2021, 35, 150–158. [Google Scholar] [CrossRef]
- Shah, P.; McDowell, M.; Ebisu, R.; Hanif, T.; Toerne, T. Adjunctive use of ketamine for benzodiazepine-resistant severe alcohol withdrawal: A retrospective evaluation. J. Med. Toxicol. 2018, 14, 229–236. [Google Scholar] [CrossRef]
- Yoon, G.; Petrakis, I.L.; Krystal, J.H. Association of combined naltrexone and ketamine with depressive symptoms in a case series of patients with depression and alcohol use disorder. JAMA Psychiatry 2019, 76, 337–338. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.; Benedict, N.J.; Armahizer, M.J.; Kane-Gill, S.L. Evaluation of adjunctive ketamine to benzodiazepines for management of alcohol withdrawal syndrome. Ann. Pharmacother. 2015, 49, 14–19. [Google Scholar] [CrossRef]
- Dakwar, E.; Hart, C.L.; Levin, F.R. Cocaine self-administration disrupted by the N-methyl-D-aspartate receptor antagonist ketamine: A randomized, crossover trial. Mol. Psychiatry 2017, 22, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Dakwar, E.; Nunes, E.V.; Hart, C.L.; Foltin, R.W.; Mathew, S.J.; Carpenter, K.M.; Choi, C.J.J.; Basaraba, C.N.; Pavlicova, M.; Levin, F.R. A Single Ketamine Infusion Combined With Mindfulness-Based Behavioral Modification to Treat Cocaine Dependence: A Randomized Clinical Trial. Am. J. Psychiatry 2019, 176, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Jovaisa, T.; Laurinenas, G.; Vosylius, S.; Sipylaite, J.; Badaras, R.; Ivaskevicius, J. Effects of ketamine on precipitated opiate withdrawal. Medicina 2006, 42, 625–634. [Google Scholar]
- Lalanne, L.; Nicot, C.; Lang, J.P.; Bertschy, G.; Salvat, E. Experience of the use of Ketamine to manage opioid withdrawal in an addicted woman: A case report. BMC Psychiatry 2016, 16, 395. [Google Scholar] [CrossRef]
- Omoigui, S.; Hashmat, F.; Bernardo, Z. Use of Ketamine in Ameliorating Opioid Withdrawal Symptoms During an Induction Phase of Buprenorphine. Open Pain. J. 2011, 4, 1–3. [Google Scholar] [CrossRef]
- Pradhan, B.; Rossi, G. Combining Ketamine, Brain Stimulation (rTMS) and Mindfulness Therapy (TIMBER) for Opioid Addiction. Cureus 2020, 12, e11798. [Google Scholar] [CrossRef]
- Lapidus, K.A.; Levitch, C.F.; Perez, A.M.; Brallier, J.W.; Parides, M.K.; Soleimani, L.; Feder, A.; Iosifescu, D.V.; Charney, D.S.; Murrough, J.W. A randomized controlled trial of intranasal ketamine in major depressive disorder. Biol. Psychiatry 2014, 76, 970–976. [Google Scholar] [CrossRef]
- Stachowicz, K.; Sowa-Kućma, M. The treatment of depression—Searching for new ideas. Front. Pharmacol. 2022, 13, 988648. [Google Scholar] [CrossRef]
- Hashimoto, K. Molecular mechanisms of the rapid-acting and long-lasting antidepressant actions of (R)-ketamine. Biochem. Pharmacol. 2020, 177, 113935. [Google Scholar] [CrossRef] [PubMed]
- Amasi-Hartoonian, N.; Pariante, C.M.; Cattaneo, A.; Sforzini, L. Understanding treatment-resistant depression using «omics» techniques: A systematic review. J. Affect. Disord. 2022, 318, 423–455. [Google Scholar] [CrossRef]
- Bhatt, S.; Devadoss, T.; Jha, N.K.; Baidya, M.; Gupta, G.; Chellappan, D.K.; Singh, S.K.; Dua, K. Targeting inflammation: A potential approach for the treatment of depression. Metab. Brain Dis. 2023, 38, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Murdaca, G.; Paladin, F.; Casciaro, M.; Vicario, C.M.; Gangemi, S.; Martino, G. Neuro-Inflammaging and psychopathological distress. Biomedicines 2022, 10, 2133. [Google Scholar] [CrossRef] [PubMed]
- Baker, P.M.; Jhou, T.; Li, B.; Matsumoto, M.; Mizumori, S.J.; Stephenson-Jones, M.; Vicentic, A. The lateral habenula circuitry: Reward processing and cognitive control. J. Neurosci. 2016, 36, 11482–11488. [Google Scholar] [CrossRef]
- Strasburger, S.E.; Bhimani, P.M.; Kaabe, J.H.; Krysiak, J.T.; Nanchanatt, D.L.; Nguyen, T.N.; Pough, K.A.; Prince, T.A.; Ramsey, N.S.; Savsani, K.H.; et al. What is the mechanism of ketamine’s rapid-onset antidepressant effect? A concise overview of the surprisingly large number of possibilities. J. Clin. Pharm. Ther. 2017, 42, 147–154. [Google Scholar] [CrossRef]
- Zhang, J.C.; Yao, W.; Hashimoto, K. Arketamine, a new rapid-acting antidepressant: A historical review and future directions. Neuropharmacology 2022, 218, 109219. [Google Scholar] [CrossRef] [PubMed]
- Brendle, M.; Robison, R.; Malone, D.C. Cost-effectiveness of esketamine nasal spray compared to intravenous ketamine for patients with treatment-resistant depression in the US utilizing clinical trial efficacy and real-world effectiveness estimates. J. Affect. Disord. 2022, 319, 388–396. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Spravato: Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/spravato-epar-product-information_en.pdf (accessed on 18 May 2025).
- Available online: https://euromedis.pl/najnowszy-lek-przeciwdepresyjny-spravato-leczenie-dostepne-dla-pacjentow-w-centrum-medycznym-euromedis/ (accessed on 1 May 2025).
- Koike, H.; Iijima, M.; Chaki, S. Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models of depression. Behav. Brain Res. 2011, 224, 107–111. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, Y.; Sun, X.; Lian, B.; Sun, H.; Wang, G.; Du, Z.; Li, Q.; Sun, L. Short- and long-term antidepressant effects of ketamine in a rat chronic unpredictable stress model. Brain Behav. 2017, 7, 00749. [Google Scholar] [CrossRef]
- Bates, M.L.S.; Trujillo, K.A. Long-lasting effects of repeated ketamine administration in adult and adolescent rats. Behav. Brain Res. 2019, 369, 111928. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, P.J.; Yen, J.Y.; Watson, B.O. Stress-sensitive antidepressant-like effects of ketamine in the mouse forced swim test. PLoS ONE 2019, 14, 0215554. [Google Scholar] [CrossRef]
- Levinta, A.; Meshkat, S.; McIntyre, R.S.; Ho, C.; Lui, L.M.W.; Lee, Y.; Mansur, R.B.; Teopiz, K.M.; Rodrigues, N.B.; Di Vincenzo, J.D.; et al. The association between stage of treatment-resistant depression and clinical utility of ketamine/esketamine: A systematic review. J. Affect. Disord. 2022, 318, 139–149. [Google Scholar] [CrossRef]
- Daly, E.J.; Trivedi, M.H.; Janik, A.; Li, H.; Zhang, Y.; Li, X.; Lane, R.; Lim, P.; Duca, A.R.; Hough, D.; et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: A randomized clinical trial. JAMA Psychiatry 2019, 76, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Alario, A.A.; Niciu, M.J. (Es)Ketamine for suicidal ideation and behavior: Clinical efficacy. Chronic Stress 2022, 6, 24705470221128017. [Google Scholar] [CrossRef]
- Hassan, K.; Struthers, W.M.; Sankarabhotla, A.; Daid, P. Safety, effectiveness and tolerability of sublingual ketamine in depression and anxiety: A retrospective study of off-label, at-home use. Front. Psychiatry 2022, 13, 992624. [Google Scholar] [CrossRef]
- Khorassani, F.; Talreja, O. Intranasal esketamine: A novel drug for treatment-resistant depression. Am. J. Health Syst. Pharm. 2020, 77, 1382–1388. [Google Scholar] [CrossRef] [PubMed]
- Popova, V.; Daly, E.J.; Trivedi, M.; Cooper, K.; Lane, R.; Lim, P.; Mazzucco, C.; Hough, D.; Thase, M.E.; Shelton, R.C.; et al. Efficacy and safety of flexibly dosed esketamine nasal spray combined with a newly initiated oral antidepressant in treatment-resistant depression: A randomized double-blind active-controlled study. Am. J. Psychiatry 2019, 176, 428–438. [Google Scholar] [CrossRef]
- Fedgchin, M.; Trivedi, M.; Daly, E.J.; Melkote, R.; Lane, R.; Lim, P.; Vitagliano, D.; Blier, P.; Fava, M.; Liebowitz, M.; et al. Efficacy and safety of fixed-dose esketamine nasal spray combined with a new oral antidepressant in treatment-resistant depression: Results of a randomized, double-blind, active-controlled study TRANSFORM-1. Int. J. Neuropsychopharmacol. 2019, 22, 616–630. [Google Scholar] [CrossRef]
- Jha, M.K.; Williamson, D.J.; Magharehabed, G.; Turkoz, I.; Daly, E.J.; Trivedi, M.H. Intranasal esketamine effectively treats treatment-resistant depression in adults regardless of baseline irritability. J. Affect. Disord. 2022, 321, 153–160. [Google Scholar] [CrossRef]
- Ochs-Ross, R.; Daly, E.J.; Zhang, Y.; Lane, R.; Lim, P.; Morrison, R.L.; Hough, D.; Manji, H.; Drevets, W.C.; Sanacora, G.; et al. Efficacy and safety of esketamine nasal spray plus an oral antidepressant in elderly patients with treatment-resistant depression—TRANSFORM-3. Am. J. Geriatr. Psychiatry 2020, 28, 121–141. [Google Scholar] [CrossRef]
- Jeon, H.J.; Ju, P.C.; Sulaiman, A.H.; Aziz, S.A.; Paik, J.W.; Tan, W.; Bai, D.; Li, C.T. Long-term safety and efficacy of esketamine nasal spray plus an oral antidepressant in patients with treatment-resistant depression—An Asian sub-group analysis from the SUSTAIN-2 Study. Clin. Psychopharmacol. Neurosci. 2022, 20, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Martinotti, G.; Vita, A.; Fagiolini, A.; Maina, G.; Bertolino, A.; Dell’Osso, B.; Siracusano, A.; Clerici, M.; Bellomo, A.; Sani, G.; et al. Real-world experience of esketamine use to manage treatment-resistant depression: A multicentric study on safety and effectiveness REAL-ESK study. J. Affect. Disord. 2022, 319, 646–654. [Google Scholar] [CrossRef]
- Turkoz, I.; Lopena, O.; Salvadore, G.; Sanacora, G.; Shelton, R.; Fu, D.J. Treatment response to esketamine nasal spray in patients with major depressive disorder and acute suicidal ideation or behavior without evidence of early response: A pooled post hoc analysis of ASPIRE. CNS Spectr. 2022, 28, 482–488. [Google Scholar] [CrossRef]
- Wajs, E.; Aluisio, L.; Holder, R.; Daly, E.J.; Lane, R.; Lim, P.; George, J.E.; Morrison, R.L.; Sanacora, G.; Young, A.H.; et al. Esketamine nasal spray plus oral antidepressant in patients with treatment-resistant depression: Assessment of long-term safety in a phase 3, open-label study (SUSTAIN-2). J. Clin. Psychiatry 2020, 81, 19m12891. [Google Scholar] [CrossRef] [PubMed]
- Starr, H.L.; Abell, J.; Larish, A.; Lewis, S.; DeMuro, C.; Gogate, J.; Jamieson, C.; Daly, E.; Zaki, N.; Kramer, M. Self-reported review of the value of esketamine in patients with treatment-resistant depression: Understanding the patient experience in the STRIVE Study. Psychiatry Res. 2020, 293, 113376. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Q.; Dai, X.; Xiao, G.; Luo, H. Effect of low-dose esketamine on pain control and postpartum depression after cesarean section: A retrospective cohort study. Ann. Palliat. Med. 2022, 11, 45–57. [Google Scholar] [CrossRef]
- Leal, G.C.; Bandeira, I.D.; Correia-Melo, F.S.; Telles, M.; Mello, R.P.; Vieira, F.; Lima, C.S.; Jesus-Nunes, A.P.; Guerreiro-Costa, L.N.F.; Marback, R.F.; et al. Intravenous arketamine for treatment-resistant depression: Open-label pilot study. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 271, 577–582. [Google Scholar] [CrossRef]
- Masaki, Y.; Kashiwagi, Y.; Watabe, H.; Abe, K. (R)- and (S)-ketamine induce differential fMRI responses in conscious rats. Synapse 2019, 73, 22126. [Google Scholar] [CrossRef]


| Ketamine Enantiomer | Route of Administration | Dose (mg/kg) | Bioavailability (%) | Literature |
|---|---|---|---|---|
| (S-, R-)- ketamine | i.v. | 1–4.5 | 100 | [34] |
| i.m. | 8–10 | 93 | [32,33,35] | |
| p.o. | 0.25–0.5 | 17–29 | [36,37,38,39] | |
| intranasal | 0.3–9.0 | 45–50 | [8,40,41,42,43,44] | |
| S-ketamine | i.v. | 0.125–0.3 | 100 | [40,45,46,47,48] |
| i.m. | 0.27 | 100 | ||
| p.o. | 0.25–0.3 | 8–24 | ||
| intranasal | 0.33 | 70 | ||
| R-ketamine | i.v. | lack of data | [49,50,51] | |
| i.m. | 0.5 | 93 | ||
| p.o | lack of data | |||
| intranasal | lack of data |
| Indications | Contraindications | Additional Notes |
|---|---|---|
| Anesthesia: Sedation (mechanical ventilation) Procedural sedation Induction and maintenance of general anesthesia Anesthesia for patients with respiratory conditions Anesthesia where hemodynamic balance is required Subanesthesia | Psychosis Poorly controlled hypertension Severe liver dysfunction Epilepsy Cranial injuries (not fully studied) Schizophrenia | Required during administration and procedures: blood pressure, electrocardiogram and respiratory measurements |
| Analgesia: Acute pain Chronic pain Neuropathic pain Perioperative pain Cancer pain | Severe liver dysfunction Active substance abuse Ulcerative cystitis Significant coronary disease Psychosis | Simultaneous administration of benzodiazepines or α2-adrenoceptor agonists may be required Precaution against chronic postoperative pain—not effective |
| Author | Schedule of Ketamine Administration | Principal Conclusions |
|---|---|---|
| Ethanol addiction | ||
| Dakwar, E. et al., 2020 [158] | i.v. administration 0.71 mg/kg + motivational enhancement therapy/psychotherapy | increased likelihood of abstinence, delayed time to relapse and reduced likelihood of heavy drinking days compared to midazolam |
| Das et al., 2019 [159] | i.v. 0.5 mg/kg for 10 days | reduction in the reinforcing effects of ethanol, reduction in number of drinking days per week and volume of consumed alcohol |
| Grabski et al., 2022 [160] | i.v. 0.8 mg/kg + psychotherapy | increase the number of abstinent days |
| Kolp et al., 2006 [148] | i.m. administration 2–3 mg/kg + psychotherapy | extending the period of alcohol abstinence |
| Krupitsky et al., 1992 [161] | i.m. 3 mg/kg + psychotherapy | extending the period of alcohol abstinence |
| Krupitsky and Grinenko, 1997 [149] | i.m. 2.5 mg/kg + psychotherapy | extending the period of alcohol abstinence and reduced risk of relapse |
| Pizon et al., 2018 [162] | i.v. 0.15–0.3 mg/kg/h + bolus (0.3 mg/kg) + conventional withdrawal treatment | reduction in benzodiazepine requirements, decrease likelihood of intubation and a shorter length of stay in the intensive care unit (ICU) |
| Rothberg et al., 2021 [163] | i.v. 0.71 mg/kg + motivational enhancement therapy | increased probability of abstinence, delayed time to relapse, decreased likelihood of heavy drinking days compared to midazolam |
| Shah et al., 2018 [164] | i.v. 0.75 mg/kg + conventional withdrawal treatment | enhanced symptom control for benzodiazepine-refractory patients and reduced infusion requirements |
| Yoon et al., 2019 [165] | i.v. 0.5 mg/kg once a week for 4 weeks + naltrexone 380 mg | reduced alcohol craving and consumption |
| Wong et al., 2015 [166] | i.v. median infusion 0.20 mg/kg/h + conventional withdrawal treatment with a standardized treatment protocol (benzodiazepine + dexmedetomidine + phenobarbital + propofol ± antipsychotics + clonidine + intubation) | reduction in short-term benzodiazepine dose requirements in patients with alcohol withdrawal |
| Cocaine addiction | ||
| Dakwar et al., 2014 [150] | 3 × i.v. 0.41 mg/kg or 0.71 mg/kg | enhanced motivation to quit and dampened cue-induced craving |
| Dakwar et al., 2017 [167] | i.v. 0.11 mg/kg 2-min bolus + 0.60 mg/kg 50 min | decreased cocaine self-administration |
| Dakwar et al., 2018 [168] | i.v. 0.71 mg/kg | decreased cocaine self-administration, cocaine use and craving |
| Dakwar et al., 2019 [158] | i.v. 0.5 mg/kg | promoted abstinence, diminished craving and reduced risk of relapse |
| Opioid addiction | ||
| Jovaiša et al., 2006 [169] | i.v. 0.5 mg/kg | better control of withdrawal symptoms with no effects on treatment of opiate dependence after 4 months |
| Krupitsky et al., 2002 [153] | i.m. 0.2 or 2.0 mg/kg + psychotherapy | increased rate of abstinence within the first two years of follow-up, reduction in craving for heroin, positive change in nonverbal unconscious emotional attitudes |
| Lalanne et al., 2016 [170] | oral administration, 1 mg/kg | reduction in dosage of opioid painkillers without withdrawal symptoms |
| Omoigui et al., 2011 [171] | i.v. 5 mg/kg | effective treatment for the opioid withdrawal symptoms and pain during transition to buprenorphine |
| Pradhan and Rossi, 2020 [172] | i.v. 0.75 mg/kg | Combination therapy with ketamine, rTMS and TIMBER is feasible in patients with opioid addiction, reduces craving and promotes abstinence |
| TRANSFORM-1 | TRANSFORM-2 | TRANSFORM-3 | |
|---|---|---|---|
| Features of a clinical trial | Randomized, double-blinded and placebo controlled | ||
| Number of respondents included in the analysis | 324 | 223 | 137 |
| Age of respondents | 18–64 | 18–64 | ≥65 |
| Basic selection criterion | Recurrent MDD or an episode of MDD lasting ≥ 2 years without psychotic features | Recurrent MDD or an episode of MDD lasting ≥ 2 years without psychotic features | MDD without psychotic features and resistant to ≥2 different AD |
| Dosage in groups | 56 mg or 84 mg of S-ketamine + AD, placebo + AD | 56 mg or 84 mg of S-ketamine + AD, placebo + AD | 28 mg, 56 mg or 84 mg of S-ketamine + AD, placebo + AD |
| Duration of treatment phase | 28 days | 28 days | 28 days |
| TRANSFORM-1 | TRANSFORM-2 | TRANSFORM-3 | ||
|---|---|---|---|---|
| Initial average score on the MADRS | 37.55 | 37.15 | 35.2 | |
| Mean change in MADRS score | S-ketamine | −18.9 | −21.4 | −10 |
| Placebo | −14.8 | −17 | −6.3 | |
| Mean change in irritability on the 7-GAD scale | S-ketamine | −7.4 | −7.9 | - |
| Placebo | −6 | −6.8 | ||
| Summary response and remission by day 28 | S-ketamine | 53.6% | 69.3% | 44.4% |
| Placebo | 38.9% | 52% | 20% | |
| SUSTAIN-1 | |
|---|---|
| Main objective of the clinical trial | Long-term effectiveness |
| Features of the clinical trial | Randomized, double-blinded, placebo controlled |
| Number of respondents | 705 |
| Age of respondents | 18–64 |
| Basic selection criterion | Recurrent MDD or an episode of MDD lasting ≥ 2 years without psychotic features. No suicidal thoughts or behavior. |
| Duration analyzed | Induction phase (4 weeks) Optimization phase (12 weeks) Sustaining phase (variable duration) |
| Dosage | Variable dosage 56 mg or 84 mg S-ketamine with an AD. Induction phase—twice a week Optimization phase—one or two times a week Sustaining phase—AD only |
| Relapse | S-ketamine + AD | Placebo + AD |
|---|---|---|
| Patients with stable remission [%] | 26.7 | 45.3 |
| Mean time to relapse in stable remission in days | 635 | 88 |
| Patients with stable response [%] | 25.8 | 57.6 |
| SUSTAIN-2 | |
|---|---|
| Main objective of the clinical trial | Long-term safety and effectiveness |
| Features of the clinical trial | Non-randomized, no placebo |
| Number of respondents | 802 |
| Age of respondents | ≥18 |
| Basic selection criterion | MDD without psychotic features and resistant to ≥2 different antidepressants. No suicidal thoughts or behavior. |
| Duration analyzed | Induction phase (4 weeks) Sustaining phase (48 weeks) Observation phase (up to one year) |
| Dosage | Flexible dosage of 28 mg (in age of ≥65), 56 mg or 84 mg S-ketamine with an AD Induction phase—twice a week Sustaining phase—one or two times a week Observation phase—AD only |
| Result | ||
|---|---|---|
| Mean baseline MADRS score | 31.4 ± 5.39 | |
| Mean change in the MADRS score | −16.4 | |
| Clinical response of respondents (↓MADRS ≥ 50%) | Induction phase | 78.4% |
| Optimization/observation phase | 76.5% | |
| Remission of respondents (MADRS ≤ 12) | Induction phase | 47.2% |
| Optimization/observation phase | 58.2% | |
| Mean change in 7-GAD score | Induction phase | −5.9 |
| Optimization/observation phase | 0.2 | |
| Total S-Ketamine + AD (343 Participants) | Total Placebo + AD (222 Participants) | |
|---|---|---|
| Incidence of Adverse Event [%] | ||
| Adverse event | 27.8 | 8.5 |
| Nausea | 26.5 | 3.6 |
| Dissociation | 22.6 | 6.7 |
| Dizziness | 20.2 | 17.1 |
| Headache | 16.3 | 9 |
| Somnolence | 20.2 | 13.5 |
| Dysgeusia | 8.9 | 2.2 |
| Blood pressure increased | 27.8 | 8.5 |
| Adverse Effects | [%] |
|---|---|
| Prevalence TEAE | 90.1 |
| ≥1 serious TEAE | 14.7 |
| Serious TEAE associated with increased pressure | 12.8 |
| TEAE suggesting abuse | 53.5 |
| Overdose | Not reported |
| Abuse of S-ketamine | |
| Request of increased dosage | |
| Attempting to get the drug |
| [%] | |
|---|---|
| Dizziness | 32.9 |
| Headache | 24.9 |
| Cognitive impairment | Not reported |
| Somnolence | 16.7 |
| Dysgeusia | 11.8 |
| Dissociation | 27.6 |
| Nausea | 25.1 |
| Vomiting | 10.8 |
| Urinary tract infection | 8.1 |
| Bladder inflammation | Not reported |
| Increase in blood pressure | 9.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gibuła-Tarłowska, E.; Wiszniewska, A.; Turyk, M.; Szymczyk, P.; Kotlińska, J.H.; Kędzierska, E. Ketamine—From an Anesthetic to a Psychiatric Drug: Mechanisms of Action, Clinical Applications and Potential Risks. Molecules 2025, 30, 2824. https://doi.org/10.3390/molecules30132824
Gibuła-Tarłowska E, Wiszniewska A, Turyk M, Szymczyk P, Kotlińska JH, Kędzierska E. Ketamine—From an Anesthetic to a Psychiatric Drug: Mechanisms of Action, Clinical Applications and Potential Risks. Molecules. 2025; 30(13):2824. https://doi.org/10.3390/molecules30132824
Chicago/Turabian StyleGibuła-Tarłowska, Ewa, Anna Wiszniewska, Magdalena Turyk, Paulina Szymczyk, Jolanta H. Kotlińska, and Ewa Kędzierska. 2025. "Ketamine—From an Anesthetic to a Psychiatric Drug: Mechanisms of Action, Clinical Applications and Potential Risks" Molecules 30, no. 13: 2824. https://doi.org/10.3390/molecules30132824
APA StyleGibuła-Tarłowska, E., Wiszniewska, A., Turyk, M., Szymczyk, P., Kotlińska, J. H., & Kędzierska, E. (2025). Ketamine—From an Anesthetic to a Psychiatric Drug: Mechanisms of Action, Clinical Applications and Potential Risks. Molecules, 30(13), 2824. https://doi.org/10.3390/molecules30132824







