The Role of Nitric Oxide in Cancer Treatment: Ally or Foe?
Abstract
1. Introduction
2. Materials
2.1. Literature Search Strategy
2.2. Eligibility and Exclusion Criteria
2.3. Data Extraction Process and Quality Assessment
3. Results
3.1. Nitric Oxide in Different Types of Cancer
3.2. NO and PDT
3.3. Role of iNOS Inhibitors in Cancer Therapy
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oszajca, K.; Szemraj, J.; Bartkowiak, J. Udział tlenku azotu w regulacji ekspresji genów [Regulation of gene expression by nitric oxide]. Postepy Biochem. 2007, 53, 254–262. [Google Scholar] [PubMed]
- Kowalczyk, E.; Kopff, A.; Kopff, M.; Błaszczyk, J.; Fijałkowski, P.; Kowalski, J. Metabolizm tlenku azotu [Nitric oxide metabolism]. Wiad. Lek. 2006, 59, 889–893. [Google Scholar] [PubMed]
- Luo, Y.; Zhu, Y.; Basang, W.; Wang, X.; Li, C.; Zhou, X. Roles of Nitric Oxide in the Regulation of Reproduction: A Review. Front. Endocrinol. 2021, 12, 752410. [Google Scholar] [CrossRef]
- Kaplish, D.; Vagha, J.D.; Meshram, R.J.; Lohiya, S. A Comprehensive Review of Inhaled Nitric Oxide Therapy: Current Trends, Challenges, and Future Directions. Cureus 2024, 16, e53558. [Google Scholar] [CrossRef]
- Prast, H.; Philippu, A. Nitric Oxide as Modulator of Neuronal Function. Prog. Neurobiol. 2001, 64, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Sanders, D.B.; Kelley, T.; Larson, D. The Role of Nitric Oxide Synthase/Nitric Oxide in Vascular Smooth Muscle Control. Perfusion 2000, 15, 97–104. [Google Scholar] [CrossRef]
- Zaragoza, C.; Soria, E.; López, E.; Browning, D.; Balbín, M.; López-Otín, C.; Lamas, S. Activation of the Mitogen Activated Protein Kinase Extracellular Signal-Regulated Kinase 1 and 2 by the Nitric Oxide-cGMP-cGMP-Dependent Protein Kinase Axis Regulates the Expression of Matrix Metalloproteinase 13 in Vascular Endothelial Cells. Mol. Pharmacol. 2002, 62, 927–935. [Google Scholar] [CrossRef]
- Muenster, S.; Zarragoikoetxea, I.; Moscatelli, A.; Balcells, J.; Gaudard, P.; Pouard, P.; Marczin, N.; Janssens, S.P. Inhaled NO at a Crossroads in Cardiac Surgery: Current Need to Improve Mechanistic Understanding, Clinical Trial Design and Scientific Evidence. Front. Cardiovasc. Med. 2024, 11, 1374635. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Wilcox, J.; Webb, A.J.; O’Gallagher, K. Dysfunctional and Dysregulated Nitric Oxide Synthases in Cardiovascular Disease: Mechanisms and Therapeutic Potential. Int. J. Mol. Sci. 2023, 24, 15200. [Google Scholar] [CrossRef]
- Elshaer, S.L.; Lemtalsi, T.; El-Remessy, A.B. High Glucose-Mediated Tyrosine Nitration of PI3-Kinase: A Molecular Switch of Survival and Apoptosis in Endothelial Cells. Antioxidants 2018, 7, 47. [Google Scholar] [CrossRef]
- Radi, R. Protein Tyrosine Nitration: Biochemical Mechanisms and Structural Basis of Functional Effects. Acc. Chem. Res. 2013, 46, 550–559. [Google Scholar] [CrossRef]
- Livramento, J.B.; Rodrigues, G.S.; Faber, J.; de Souza Filho, L.A.; Moura, F.V.; Barros, C.D.S.; Pinto, W.B.V.R.; Schmidt, B.; Oliveira, A.S.B.; Kiyomoto, B.H.; et al. Protein Nitration in Patients with Mitochondrial Diseases. Antioxidants 2025, 14, 211. [Google Scholar] [CrossRef] [PubMed]
- Corpas, F.J.; Palma, J.M.; Del Río, L.A.; Barroso, J.B. Protein tyrosine nitration in higher plants grown under natural and stress conditions. Front. Plant Sci. 2013, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, F.; Ponderato, N.; Basini, G.; Tamanini, C. Nitric oxide synthase expression and nitric oxide/cyclic GMP pathway in swine granulosa cells. Domest. Anim. Endocrinol. 2001, 20, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Mueller, B.J.; Roberts, M.D.; Mobley, C.B.; Judd, R.L.; Kavazis, A.N. Nitric oxide in exercise physiology: Past and present perspectives. Front. Physiol. 2025, 15, 1504978. [Google Scholar] [CrossRef]
- Tang, H.; Vanderpool, R.R.; Wang, J.; Yuan, J.X. Targeting L-arginine–nitric oxide–cGMP pathway in pulmonary arterial hypertension. Pulm. Circ. 2017, 7, 569–571. [Google Scholar] [CrossRef]
- Durán, W.N.; Beuve, A.V.; Sánchez, F.A. Nitric oxide, S-nitrosation, and endothelial permeability. IUBMB Life 2013, 65, 819–826. [Google Scholar] [CrossRef]
- Winn, N.C.; Cappel, D.A.; Pollock, E.D.; Lantier, L.; Riveros, J.K.; Debrow, P.; Bracy, D.P.; Beckman, J.A.; Wasserman, D.H. Increased cGMP improves microvascular exercise training adaptations independent of endothelial nitric oxide synthase. bioRxiv 2024. [Google Scholar] [CrossRef]
- Cuong, D.V.; Kim, N.; Youm, J.B.; Joo, H.; Warda, M.; Lee, J.W.; Park, W.S.; Kim, T.; Kang, S.; Kim, H.; et al. Nitric oxide–cGMP–protein kinase G signaling pathway induces anoxic preconditioning through activation of ATP-sensitive K+ channels in rat hearts. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H1808–H1817. [Google Scholar] [CrossRef]
- Watanabe, H. [Role of NO–cGMP–PKG axis in pulmonary arterial hypertension]. Nihon Yakurigaku Zasshi 2022, 157, 221–225. [Google Scholar] [CrossRef]
- Singh, J.; Lee, Y.; Kellum, J.A. A new perspective on NO pathway in sepsis and ADMA lowering as a potential therapeutic approach. Crit. Care 2022, 26, 246. [Google Scholar] [CrossRef] [PubMed]
- Cinelli, M.A.; Do, H.T.; Miley, G.P.; Silverman, R.B. Inducible nitric oxide synthase: Regulation, structure, and inhibition. Med. Res. Rev. 2020, 40, 158–189. [Google Scholar] [CrossRef] [PubMed]
- Divakaran, S.; Loscalzo, J. The role of nitroglycerin and other nitrogen oxides in cardiovascular therapeutics. J. Am. Coll. Cardiol. 2017, 70, 2393–2410. [Google Scholar] [CrossRef]
- Alderton, W.K.; Cooper, C.E.; Knowles, R.G. Nitric oxide synthases: Structure, function and inhibition. Biochem. J. 2001, 357, 593–615. [Google Scholar] [CrossRef] [PubMed]
- Farahani, A.; Farahani, A.; Kashfi, K.; Ghasemi, A. Inducible nitric oxide synthase (iNOS): More than an inducible enzyme? Rethinking the classification of NOS isoforms. Pharmacol. Res. 2025, 216, 107781. [Google Scholar] [CrossRef]
- Jenkins, D.C.; Charles, I.G.; Thomsen, L.L.; Moss, D.W.; Holmes, L.S.; Baylis, S.A.; Rhodes, P.; Westmore, K.; Emson, P.C.; Moncada, S. Roles of nitric oxide in tumor growth. Proc. Natl. Acad. Sci. USA 1995, 92, 4392–4396. [Google Scholar] [CrossRef]
- Ridnour, L.A.; Thomas, D.D.; Donzelli, S.; Espey, M.G.; Roberts, D.D.; Wink, D.A.; Isenberg, J.S. The biphasic nature of nitric oxide responses in tumor biology. Antioxid. Redox Signal. 2006, 8, 1329–1337. [Google Scholar] [CrossRef]
- Matsunaga, T.; Yamaji, Y.; Tomokuni, T.; Morita, H.; Morikawa, Y.; Suzuki, A.; Yonezawa, A.; Endo, S.; Ikari, A.; Iguchi, K.; et al. Nitric oxide confers cisplatin resistance in human lung cancer cells through upregulation of aldo-keto reductase 1B10 and proteasome. Free Radic. Res. 2014, 48, 1371–1385. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Bakrim, S.; Fessikh, M.E.; Elhrech, H.; Omari, N.E.; Amanullah, M.; Ming, L.C.; Moshawih, S.; Bouyahya, A. Targeting inflammation in cancer therapy: From mechanistic insights to emerging therapeutic approaches. J. Transl. Med. 2025, 23, 588. [Google Scholar] [CrossRef]
- Chen, T. Unveiling the significance of inducible nitric oxide synthase: Its impact on cancer progression and clinical implications. Cancer Lett. 2024, 592, 216931. [Google Scholar] [CrossRef] [PubMed]
- Bui, I.; Baritaki, S.; Libra, M.; Zaravinos, A.; Bonavida, B. Cancer resistance is mediated by the upregulation of several anti-apoptotic gene products via the inducible nitric oxide synthase/nitric oxide pathway: Therapeutic implications. Antioxid. Redox Signal. 2023, 39, 853–889. [Google Scholar] [CrossRef]
- Sharma, S.; Saini, A.; Nehru, B. Neuroprotective effects of carbenoxolone against amyloid-beta 1–42 oligomer-induced neuroinflammation and cognitive decline in rats. Neurotoxicology 2021, 83, 89–105. [Google Scholar] [CrossRef]
- San Martín, A.; Arce-Molina, R.; Galaz, A.; Pérez-Guerra, G.; Barros, L.F. Nanomolar nitric oxide concentrations quickly and reversibly modulate astrocytic energy metabolism. J. Biol. Chem. 2017, 292, 9432–9438. [Google Scholar] [CrossRef]
- Markiewicz, R.; Litowczenko, J.; Gapiński, J.; Woźniak, A.; Jurga, S.; Patkowski, A. Nanomolar nitric oxide concentrations in living cells measured by means of fluorescence correlation spectroscopy. Molecules 2022, 27, 1010. [Google Scholar] [CrossRef]
- Hall, C.N.; Attwell, D. Assessing the physiological concentration and targets of nitric oxide in brain tissue. J. Physiol. 2008, 586, 3597–3615. [Google Scholar] [CrossRef]
- Hall, C.N.; Garthwaite, J. What is the real physiological NO concentration in vivo? Nitric Oxide 2009, 21, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.D.; Ridnour, L.A.; Isenberg, J.S.; Flores-Santana, W.; Switzer, C.H.; Donzelli, S.; Hussain, P.; Vecoli, C.; Paolocci, N.; Ambs, S.; et al. The chemical biology of nitric oxide: Implications in cellular signaling. Free Radic. Biol. Med. 2008, 45, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Gupta, A.K. Nitric oxide: Role in tumour biology and iNOS/NO-based anticancer therapies. Cancer Chemother. Pharmacol. 2011, 67, 1211–1224. [Google Scholar] [CrossRef]
- Vannini, F.; Kashfi, K.; Nath, N. The dual role of iNOS in cancer. Redox Biol. 2015, 6, 334–343. [Google Scholar] [CrossRef]
- Khan, F.H.; Dervan, E.; Bhattacharyya, D.D.; McAuliffe, J.D.; Miranda, K.M.; Glynn, S.A. The role of nitric oxide in cancer: Master regulator or NOt? Int. J. Mol. Sci. 2020, 21, 9393. [Google Scholar] [CrossRef]
- Ambs, S.; Merriam, W.G.; Bennett, W.P.; Felley-Bosco, E.; Ogunfusika, M.O.; Oser, S.M.; Klein, S.; Shields, P.G.; Billiar, T.R.; Harris, C.C. Frequent nitric oxide synthase-2 expression in human colon adenomas: Implication for tumor angiogenesis and colon cancer progression. Cancer Res. 1998, 58, 334–341. [Google Scholar] [PubMed]
- Azad, N.; Vallyathan, V.; Wang, L.; Tantishaiyakul, V.; Stehlik, C.; Leonard, S.S.; Rojanasakul, Y. S-nitrosylation of Bcl-2 inhibits its ubiquitin-proteasomal degradation. A novel antiapoptotic mechanism that suppresses apoptosis. J. Biol. Chem. 2006, 281, 34124–34134. [Google Scholar] [CrossRef]
- Fukumura, D.; Kashiwagi, S.; Jain, R.K. The role of nitric oxide in tumour progression. Nat. Rev. Cancer 2006, 6, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, T.A.; da Silva, R.S.; Miranda, K.M.; Switzer, C.H.; Wink, D.A.; Fukuto, J.M. Biological nitric oxide signalling: Chemistry and terminology. Br. J. Pharmacol. 2013, 169, 1417–1430. [Google Scholar] [CrossRef] [PubMed]
- de la Cruz-Ojeda, P.; Flores-Campos, R.; Dios-Barbeito, S.; Navarro-Villarán, E.; Muntané, J. Role of nitric oxide in gene expression regulation during cancer: Epigenetic modifications and non-coding RNAs. Int. J. Mol. Sci. 2021, 22, 6264. [Google Scholar] [CrossRef]
- Kwak, Y.D.; Ma, T.; Diao, S.; Zhang, X.; Chen, Y.; Hsu, J.; Lipton, S.A.; Masliah, E.; Xu, H.; Liao, F.F. NO signaling and S-nitrosylation regulate PTEN inhibition in neurodegeneration. Mol. Neurodegener. 2010, 5, 49. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, M.S.; Huh, S.H.; Park, J.; Chung, J.; Choi, E.J. Nitric oxide negatively regulates c-Jun N-terminal kinase/stress-activated protein kinase by means of S-nitrosylation. Proc. Natl. Acad. Sci. USA 2000, 97, 14382–14387. [Google Scholar] [CrossRef]
- Ridnour, L.A.; Isenberg, J.S.; Espey, M.G.; Thomas, D.D.; Roberts, D.D.; Wink, D.A. Nitric oxide regulates angiogenesis through a functional switch involving thrombospondin-1. Proc. Natl. Acad. Sci. USA 2005, 102, 13147–13152. [Google Scholar] [CrossRef]
- Girotti, A.W. Nitric Oxide-Mediated Resistance to Antitumor Photodynamic Therapy. Photochem. Photobiol. 2020, 96, 500–505. [Google Scholar] [CrossRef]
- Abu-Soud, H.; Gachhui, R.; Raushel, F.M.; Stuehr, D.J. The ferrous-dioxy complex of neuronal nitric oxide synthase: Divergent effects of L-arginine and tetrahydrobiopterin on its stability. Jpn. J. Pharmacol. 1997, 75, 24. [Google Scholar] [CrossRef]
- Kone, B.C.; Kuncewicz, T.; Zhang, W.; Yu, Z.Y. Protein interactions with nitric oxide synthases: Controlling the right time, the right place, and the right amount of nitric oxide. Am. J. Physiol. Renal Physiol. 2003, 285, F178–F190. [Google Scholar] [CrossRef]
- Kim, H.Y. Biological Role of Nitric Oxide. Acute Crit. Care 1998, 13, 139–146. [Google Scholar]
- McAdam, E.; Haboubi, H.N.; Forrester, G.; Eltahir, Z.; Spencer-Harty, S.; Davies, C.; Griffiths, A.P.; Baxter, J.N.; Jenkins, G.J. Inducible nitric oxide synthase (iNOS) and nitric oxide (NO) are important mediators of reflux-induced cell signalling in esophageal cells. Carcinogenesis 2012, 33, 2035–2043. [Google Scholar] [CrossRef]
- Girotti, A.W.; Fahey, J.F.; Korytowski, W. Role of nitric oxide in hyper-aggressiveness of tumor cells that survive various anti-cancer therapies. Crit. Rev. Oncol. Hematol. 2022, 179, 103805. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.M.; Girotti, A.W. Nitric oxide-mediated resistance to photodynamic therapy in a human breast tumor xenograft model: Improved outcomes with NOS2 inhibitors. Nitric Oxide 2017, 62, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, R.; Girotti, A.W. Pro-survival and pro-growth effects of stress-induced nitric oxide in a prostate cancer photodynamic therapy model. Cancer Lett. 2014, 343, 115–122. [Google Scholar] [CrossRef]
- Fahey, J.M.; Girotti, A.W. Accelerated migration and invasion of prostate cancer cells after a photodynamic therapy-like challenge: Role of nitric oxide. Nitric Oxide 2015, 49, 47–55. [Google Scholar] [CrossRef]
- Fahey, J.M.; Emmer, J.V.; Korytowski, W.; Hogg, N.; Girotti, A.W. Antagonistic effects of endogenous nitric oxide in a glioblastoma photodynamic therapy model. Photochem. Photobiol. 2016, 92, 842–853. [Google Scholar] [CrossRef]
- Bazak, J.; Korytowski, W.; Girotti, A.W. Hyper-Aggressiveness of Bystander Cells in an Anti-Tumor Photodynamic Therapy Model: Role of Nitric Oxide Produced by Targeted Cells. Crit. Rev. Oncog. 2023, 28, 15–25. [Google Scholar] [CrossRef]
- Hussain, S.P.; He, P.; Subleski, J.; Hofseth, L.J.; Trivers, G.E.; Mechanic, L.; Hofseth, A.B.; Bernard, M.; Schwank, J.; Nguyen, G.; et al. Nitric oxide is a key component in inflammation-accelerated tumorigenesis. Cancer Res. 2008, 68, 7130–7136. [Google Scholar] [CrossRef]
- Beyer, M.; Schultze, J.L. Regulatory T cells in cancer. Blood 2006, 108, 804–811. [Google Scholar] [CrossRef]
- Kruglyakov, D.; Ojha, S.K.; Kartawy, M.; Tripathi, M.K.; Hamoudi, W.; Bazbaz, W.; Khaliulin, I.; Amal, H. Nitric Oxide Synthase Inhibition Prevents Cell Proliferation in Glioblastoma. J. Mol. Neurosci. 2023, 73, 875–883. [Google Scholar] [CrossRef]
- Sahoo, D.R.; Babu, S.K.; Naik, B.B.; Hota, S.S.; Bhoi, N.; Sarkar, B.; Ali, S.K.M.; Naik, P.K. UPLC-QToF-MS/MS screening and characterization of Symphorema polyandrum and in vitro assessment of its antioxidant, anticancer, and anti-inflammatory potential. 3 Biotech 2024, 14, 298. [Google Scholar] [CrossRef]
- Merenzon, M.A.; Hincapie Arias, E.; Bhatia, S.; Shah, A.H.; Higgins, D.M.O.; Villaverde, M.; Belgorosky, D.; Eijan, A.M. Nitric oxide synthase inhibitors as potential therapeutic agents for gliomas: A systematic review. Nitric Oxide 2023, 138–139, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, R.; Girotti, A.W. Rapid upregulation of cytoprotective nitric oxide in breast tumor cells subjected to a photodynamic therapy-like oxidative challenge. Photochem. Photobiol. 2011, 87, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, R.; Girotti, A.W. Cytoprotective signaling associated with nitric oxide upregulation in tumor cells subjected to photodynamic therapy-like oxidative stress. Free Radic. Biol. Med. 2013, 57, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, S.; Zhao, Z.; East, A.K.; Hernandez, R.T.; Forzano, J.A.; Shapiro, B.A.; Yadav, A.K.; Swartchick, C.B.; Chan, J. Activity-Based Nitric Oxide-Responsive Porphyrin for Site-Selective and Nascent Cancer Ablation. ACS Appl. Mater. Interfaces 2024, 16, 9680–9689. [Google Scholar] [CrossRef]
- Qin, Y.; Gao, H.; Yin, Y.; Li, J.; He, X.; Gao, M.; Sun, L.; Yuan, Y.; Tian, Y.; Zhou, Y.; et al. Photo-Facilitated Nitric Oxide-Triggered Turn-on Photodynamic Therapy for Precise Antitumor Application. Adv. Healthc. Mater. 2025, 14, e2404265. [Google Scholar] [CrossRef]
- Chen, L.; Wang, W.; Tian, J.; Bu, F.; Zhao, T.; Liu, M.; Lin, R.; Zhang, F.; Lee, M.; Zhao, D.; et al. Imparting Multi-Functionality to Covalent Organic Framework Nanoparticles by the Dual-Ligand Assistant Encapsulation Strategy. Nat. Commun. 2021, 12, 4556. [Google Scholar] [CrossRef]
- Aikelamu, K.; Bai, J.; Zhang, Q.; Huang, J.; Wang, M.; Zhong, C. Self-Assembled Nanoparticles of Silicon (IV)-NO Donor Phthalocyanine Conjugate for Tumor Photodynamic Therapy in Red Light. Pharmaceutics 2024, 16, 1166. [Google Scholar] [CrossRef]
- Sarı, C.; Değirmencioğlu, İ.; Eyüpoğlu, F.C. Synthesis and Characterization of Novel Schiff Base-Silicon (IV) Phthalocyanine Complex for Photodynamic Therapy of Breast Cancer Cell Lines. Photodiagnosis Photodyn. Ther. 2023, 42, 103504. [Google Scholar] [CrossRef] [PubMed]
- Edmonds, C.; Hagan, S.; Gallagher-Colombo, S.M.; Busch, T.M.; Cengel, K.A. Photodynamic Therapy Activated Signaling from Epidermal Growth Factor Receptor and STAT3: Targeting Survival Pathways to Increase PDT Efficacy in Ovarian and Lung Cancer. Cancer Biol. Ther. 2012, 13, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Weijer, R.; Clavier, S.; Zaal, E.A.; Pijls, M.M.; van Kooten, R.T.; Vermaas, K.; Leen, R.; Jongejan, A.; Moerland, P.D.; van Kampen, A.H.; et al. Multi-OMIC Profiling of Survival and Metabolic Signaling Networks in Cells Subjected to Photodynamic Therapy. Cell. Mol. Life Sci. 2017, 74, 1133–1151. [Google Scholar] [CrossRef]
- Papa, V.; Furci, F.; Minciullo, P.L.; Casciaro, M.; Allegra, A.; Gangemi, S. Photodynamic Therapy in Cancer: Insights into Cellular and Molecular Pathways. Curr. Issues Mol. Biol. 2025, 47, 69. [Google Scholar] [CrossRef]
- Basudhar, D.; Glynn, S.A.; Greer, M.; Somasundaram, V.; No, J.H.; Scheiblin, D.A.; Garrido, P.; Heinz, W.F.; Ryan, A.E.; Weiss, J.M.; et al. Co-Expression of NOS2 and COX2 Accelerates Tumor Growth and Reduces Survival in Estrogen Receptor-Negative Breast Cancer. Proc. Natl. Acad. Sci. USA 2017, 114, 13030–13035. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.Y.S.; Ridnour, L.A.; Wink, A.L.; Gonzalez, A.L.; Femino, E.L.; Rittscher, H.; Somasundaram, V.; Heinz, W.F.; Coutinho, L.; Rangel, M.C.; et al. Interferon-Gamma Is Quintessential for NOS2 and COX2 Expression in ER- Breast Tumors That Lead to Poor Outcome. Cell Death Dis. 2023, 14, 319. [Google Scholar] [CrossRef]
- Granados-Principal, S.; Liu, Y.; Guevara, M.L.; Blanco, E.; Choi, D.S.; Qian, W.; Patel, T.; Rodriguez, A.A.; Cusimano, J.; Weiss, H.L.; et al. Inhibition of iNOS as a Novel Effective Targeted Therapy against Triple-Negative Breast Cancer. Breast Cancer Res. 2015, 17, 25. [Google Scholar] [CrossRef]
- Dávila-González, D.; Choi, D.S.; Rosato, R.R.; Granados-Principal, S.M.; Kuhn, J.G.; Li, W.F.; Qian, W.; Chen, W.; Kozielski, A.J.; Wong, H.; et al. Pharmacological Inhibition of NOS Activates ASK1/JNK Pathway Augmenting Docetaxel-Mediated Apoptosis in Triple-Negative Breast Cancer. Clin. Cancer Res. 2018, 24, 1152–1162. [Google Scholar] [CrossRef]
- Putra, M.; Sharma, S.; Gage, M.; Gasser, G.; Hinojo-Perez, A.; Olson, A.; Gregory-Flores, A.; Puttachary, S.; Wang, C.; Anantharam, V.; et al. Inducible Nitric Oxide Synthase Inhibitor, 1400W, Mitigates DFP-Induced Long-Term Neurotoxicity in the Rat Model. Neurobiol. Dis. 2020, 133, 104443. [Google Scholar] [CrossRef]
- Jafarian-Tehrani, M.; Louin, G.; Royo, N.C.; Besson, V.C.; Bohme, G.A.; Plotkine, M.; Marchand-Verrecchia, C. 1400W, a Potent Selective Inducible NOS Inhibitor, Improves Histopathological Outcome Following Traumatic Brain Injury in Rats. Nitric Oxide 2005, 12, 61–69. [Google Scholar] [CrossRef]
- Mintz, J.; Vedenko, A.; Rosete, O.; Shah, K.; Goldstein, G.; Hare, J.M.; Ramasamy, R.; Arora, H. Current Advances of Nitric Oxide in Cancer and Anticancer Therapeutics. Vaccines 2021, 9, 94. [Google Scholar] [CrossRef]
- Wang, X. Highlights the Recent Important Findings in Cancer Heterogeneity. Holist. Integr. Oncol. 2023, 2, 15. [Google Scholar] [CrossRef]
- Wang, Z.; Jin, A.; Yang, Z.; Huang, W. Advanced Nitric Oxide Generating Nanomedicine for Therapeutic Applications. ACS Nano 2023, 17, 8935–8965. [Google Scholar] [CrossRef]
- Ma, C.; Cheng, Z.; Tan, H.; Wang, Y.; Sun, S.; Zhang, M.; Wang, J. Nanomaterials: Leading Immunogenic Cell Death-Based Cancer Therapies. Front. Immunol. 2024, 15, 1447817. [Google Scholar] [CrossRef]
- Abbasi, K.; Siddiqui, K.; Bano, S.; Iqbal, S.; Shaikh, S.A. Future Trends and Innovation in Nano Drug Delivery for Cancer Therapy, Application of siRNA (Nanoparticle-Based RNA) Therapy, Ultrasound Linked Nano-Cancer Therapeutics, and Application of Exosomes-Based Cancer Therapy. In Nano Drug Delivery for Cancer Therapy; Khan, F.A., Ed.; Springer: Singapore, 2023. [Google Scholar]
- Girotti, A.W.; Korytowski, W. Upregulation of iNOS/NO in Cancer Cells That Survive a Photodynamic Challenge: Role of NO in Accelerated Cell Migration and Invasion. Int. J. Mol. Sci. 2024, 25, 5697. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.M.; Girotti, A.W. Nitric Oxide Antagonism to Anti-Glioblastoma Photodynamic Therapy: Mitigation by Inhibitors of Nitric Oxide Generation. Cancers 2019, 11, 231. [Google Scholar] [CrossRef] [PubMed]
- Girotti, A.W.; Fahey, J.M.; Korytowski, W. Multiple Means by Which Nitric Oxide can Antagonize Photodynamic Therapy. Curr. Med. Chem. 2016, 23, 2754–2769. [Google Scholar] [CrossRef]
- Girotti, A.W.; Fahey, J.M. Upregulation of Pro-Tumor Nitric Oxide by Anti-Tumor Photodynamic Therapy. Biochem. Pharmacol. 2020, 176, 113750. [Google Scholar] [CrossRef]
- Girotti, A.W. Nitric Oxide-Elicited Resistance to Antitumor Photodynamic Therapy via Inhibition of Membrane Free Radical-Mediated Lipid Peroxidation. Photochem. Photobiol. 2021, 97, 653–663. [Google Scholar] [CrossRef]
- Girotti, A.W.; Bazak, J.; Korytowski, W. Pro-Tumor Activity of Endogenous Nitric Oxide in Anti-Tumor Photodynamic Therapy: Recently Recognized Bystander Effects. Int. J. Mol. Sci. 2023, 24, 11559. [Google Scholar] [CrossRef] [PubMed]
- Girotti, A.W.; Fahey, J.M.; Korytowski, W. Negative Effects of Tumor Cell Nitric Oxide on Anti-Glioblastoma Photodynamic Therapy. J. Cancer Metastasis Treat. 2020, 6, 52. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Shan, X.; Zhang, H.; Shi, X.; Huang, P.; Sun, J.; He, Z.; Luo, C.; Zhang, S. Nitric Oxide-Driven Nanotherapeutics for Cancer Treatment. J. Control. Release 2023, 362, 151–169. [Google Scholar] [CrossRef]
- Osaki, T.; Kunisue, N.; Ota, U.; Imazato, H.; Ishii, T.; Takahashi, K.; Ishizuka, M.; Tanaka, T.; Okamoto, Y. Mechanism of Differential Susceptibility of Two (Canine Lung Adenocarcinoma) Cell Lines to 5-Aminolevulinic Acid-Mediated Photodynamic Therapy. Cancers 2021, 13, 4174. [Google Scholar] [CrossRef]
- Tian, D.; Teng, C.; Lv, Y.; Zhang, T.; Zhang, W. Photodynamic Therapy with Intratumoral Injection of Photosensitizers Combined with Immunotherapy Brings Hope to Patients with Refractory Cutaneous Malignant Ulcers. Discov. Oncol. 2024, 15, 522. [Google Scholar] [CrossRef]
- Liu, X.; Lu, Y.; Li, X.; Luo, L.; You, J. Nanoplatform-Enhanced Photodynamic Therapy for the Induction of Immunogenic Cell Death. J. Control. Release 2024, 365, 1058–1073. [Google Scholar] [CrossRef] [PubMed]
- Alzeibak, R.; Mishchenko, T.A.; Shilyagina, N.Y.; Balalaeva, I.V.; Vedunova, M.V.; Krysko, D.V. Targeting Immunogenic Cancer Cell Death by Photodynamic Therapy: Past, Present and Future. J. Immunother. Cancer 2021, 9, e001926. [Google Scholar] [CrossRef]
- Xu, F.; Wang, M.; Dotse, E.; Chow, K.T.; Lo, P.C. Inducing Immunogenic Cancer Cell Death through Oxygen-Economized Photodynamic Therapy with Nitric Oxide-Releasing Photosensitizers. Angew. Chem. Int. Ed. 2024, 63, e202404561. [Google Scholar] [CrossRef]
- Xu, S.; Xie, X.; He, P.; Zhu, S.; Li, X.; Chen, Q.; Ma, X.; Liang, X. Nitric Oxide-Producing Multiple Functional Nanoparticle Remodeling Tumor Microenvironment for Synergistic Photodynamic Immunotherapy against Hypoxic Tumor. ACS Nano 2025, 19, 6371–6387. [Google Scholar] [CrossRef]
- Zhang, S.; Li, M.; Wang, J.; Zhou, Y.; Dai, P.; Zhao, M.; Lv, W.; Liu, S.; Zhao, Q. NIR-Triggered On-Demand Nitric Oxide Release for Enhanced Synergistic Phototherapy of Hypoxic Tumor. Bioconjug. Chem. 2023, 34, 1327–1335. [Google Scholar] [CrossRef]
- Xia, M.; Yan, Y.; Pu, H.; Du, X.; Liang, J.; Sun, Y.; Zheng, J.; Yuan, Y. Glutathione Responsive Nitric Oxide Release for Enhanced Photodynamic Therapy by a Porphyrinic MOF Nanosystem. Chem. Eng. J. 2022, 442, 136295. [Google Scholar] [CrossRef]
- Liang, S.; Liu, Y.; Zhu, H.; Liao, G.; Zhu, W.; Zhang, L. Emerging Nitric Oxide Gas-Assisted Cancer Photothermal Treatment. Exploration 2024, 4, 20230163. [Google Scholar] [CrossRef] [PubMed]


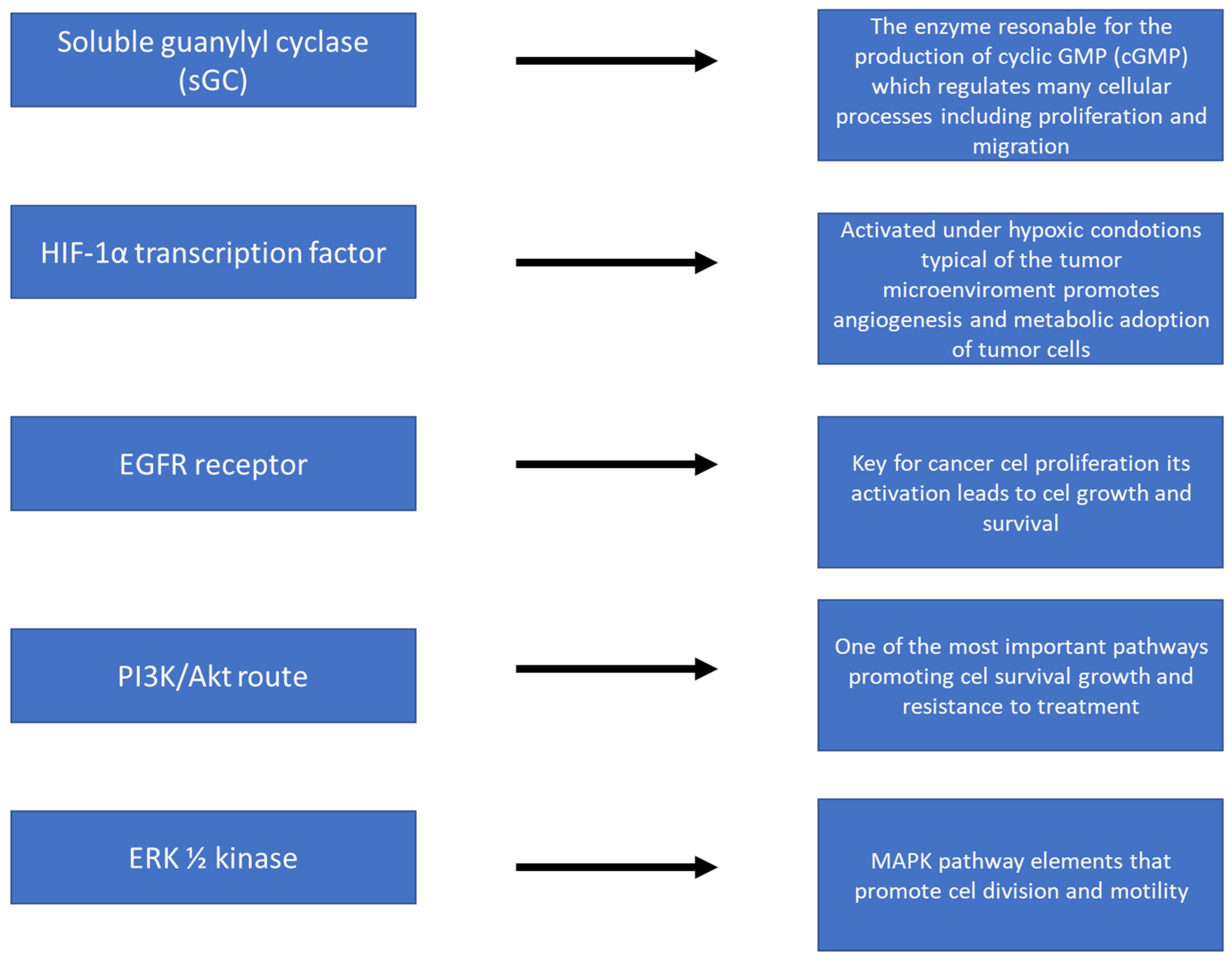

| Concentration | Description | Biological Function |
|---|---|---|
| ~100 nM | Very low; typical resting levels in many cell types | Basic vasodilation, immune surveillance, basic neurotransmission |
| 300–500 nM | Slight increase during cell signaling | Endothelial NO production (eNOS)—regulation of vascular tone, weak immune activation |
| >1 µM | High concentrations | Associated with inflammation, immune system activation, potential cytotoxicity |
| Cancer Type | Level No (INOS) | Biological Effect | Related Glasses/molecules |
|---|---|---|---|
| BREAST CANCER | Low–medium (nanomolar) | Promotion of growth and migration | EGFR, PI3K/Akt, ERK1/2, S-nitrosation of PTEN, Bcl-2 |
| PROSTATE CANCER | Low–Medium | Supporting tumor survival and progression | S-nitrosation of Bcl-2, inactivation of PTEN |
| BLADDER CANCER | Low–Medium | Activation of pro-growth pathways | HIF-1α, sGC, PI3K/Akt |
| CERVICAL CANCER | Low–Medium | Increased angiogenesis and cell survival | HIF-1α, S-nitrosation of JNK/ASK1 |
| GLEJAK (BRAIN) | Low–Medium | Growth, migration, resistance | EGFR, PI3K/Akt, ERK1/2, S-nitrosation |
| CANCERS WITH P53 MUTATED | High (micromolar) | Increased aggressiveness and migration | Overexpression of iNOS, lack of growth inhibition by p53 |
| MACROPHAGES (INOS) IN TME | High | Cytotoxicity against cancer cells | NO as an effector of the immune response (micromolar level) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Myśliwiec, A.; Bartusik-Aebisher, D.; Aebisher, D. The Role of Nitric Oxide in Cancer Treatment: Ally or Foe? Molecules 2025, 30, 2802. https://doi.org/10.3390/molecules30132802
Myśliwiec A, Bartusik-Aebisher D, Aebisher D. The Role of Nitric Oxide in Cancer Treatment: Ally or Foe? Molecules. 2025; 30(13):2802. https://doi.org/10.3390/molecules30132802
Chicago/Turabian StyleMyśliwiec, Angelika, Dorota Bartusik-Aebisher, and David Aebisher. 2025. "The Role of Nitric Oxide in Cancer Treatment: Ally or Foe?" Molecules 30, no. 13: 2802. https://doi.org/10.3390/molecules30132802
APA StyleMyśliwiec, A., Bartusik-Aebisher, D., & Aebisher, D. (2025). The Role of Nitric Oxide in Cancer Treatment: Ally or Foe? Molecules, 30(13), 2802. https://doi.org/10.3390/molecules30132802








