Abstract
New conjugated carbazolyl-substituted azulenes, such as 1,2,3-tris(carbazolyl)azulene and 1,2,3,6-tetrakis(carbazolyl)azulene, were synthesized via cross-coupling reactions in high yields. The resulting compounds exhibit a significant ability to absorb and emit light in the visible region, in the range of 400 to 600 nanometers. Studies have shown that azulene with carbazolyl substituents at positions 1, 2, 3, and 6 possesses unique photophysical properties, manifested as intense emission in the blue photoluminescence region (λPL at 444 and 490 nm), which is not observed in the original azulene. This feature arises due to the donor properties of carbazolyl substituents, which have a strong effect on the electronic structure of azulene, creating the conditions for a permitted HOMO-LUMO electronic transition.
1. Introduction
The widespread application of aromatic compounds possessing elongated π-electronic systems is due to their crucial role as functional materials in the field of organic optoelectronics.
In recent years, increasing attention has been focused on aromatic and heteroaromatic molecules, especially those containing aryl groups or substituents with electron-donor properties. For example, phenylamine naphthalenes and phenylamine anthracenes, due to their pronounced electron donor properties, attract considerable attention from scientists, since they have great potential for use in various fields of organic electronics [1,2,3,4].
Their application is considered in such promising areas as the development of organic semiconductors, catalysts for redox reactions, metal-organic frameworks (MOFs), and organic emitting diodes (OLEDs) [5,6,7,8,9,10].
One of the main advantages of these aromatic systems is their ability to precisely control the electronic structure of materials, which in turn leads to improved performance and optimized morphology.
In this regard, it is necessary to emphasize the importance of the structural isomer of naphthalene–azulene [11,12,13,14,15,16,17,18,19,20]. Its peculiarity lies in its unique aromatic structure, which determines its unique electronic and optical characteristics. Among them, a polarized structure with a dipole moment of 1.08 D [21] and anomalous fluorescence is particularly noteworthy. This fluorescence deviates from traditional concepts based on Kasha’s rule and manifests in a transition between the S2 and S0 energy levels [22,23,24].
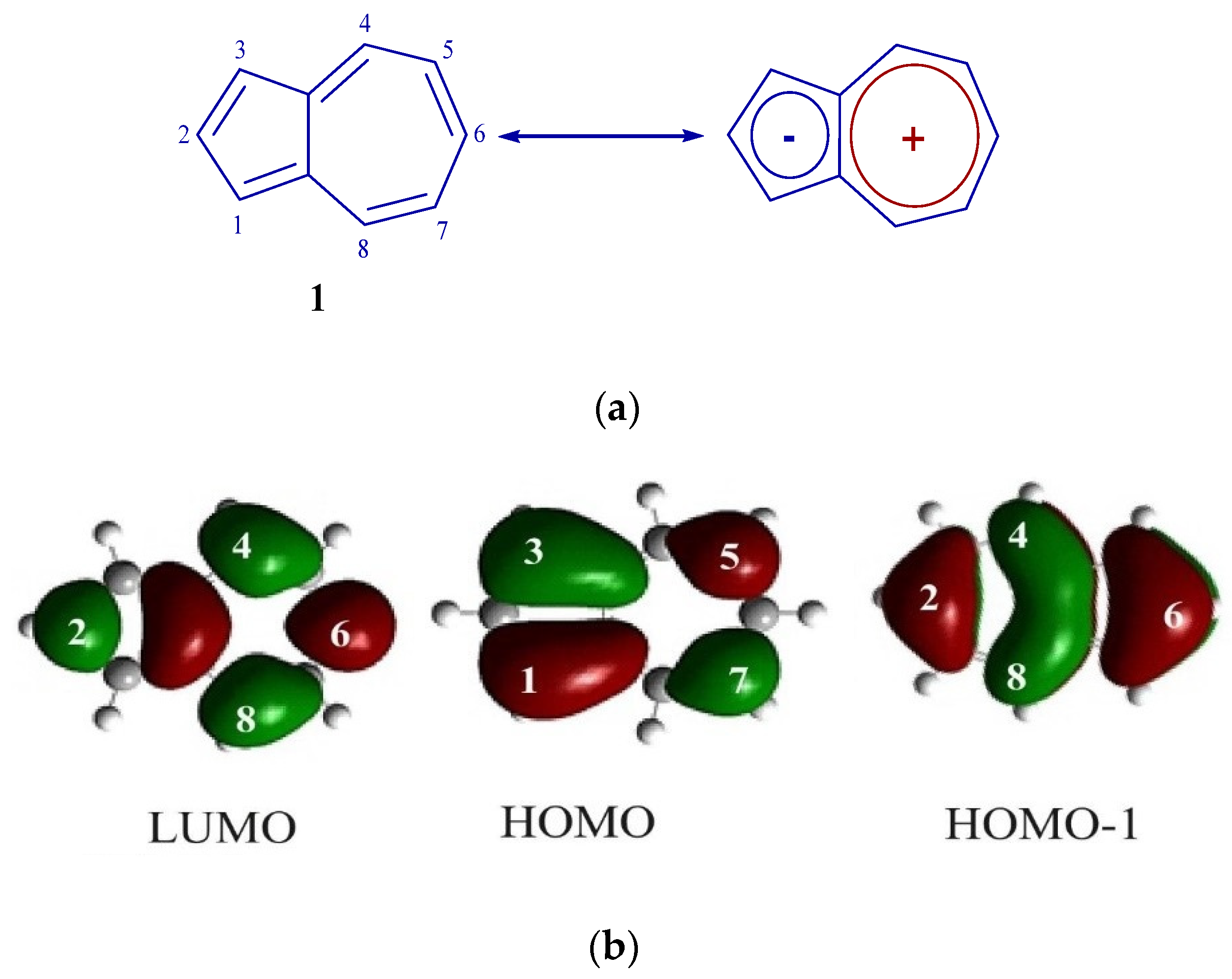
Azulene 1 can be represented as a molecule formed by the condensation of the tropylium cation and the cyclopentadiene anion, which is clearly demonstrated in Figure 1a. It is precisely this unique molecular architecture that causes to the deviation of the HOMO and LUMO energy levels from the characteristics typical of standard aromatic hydrocarbons. In the azulene molecule, atoms located at positions 1 and 3, possess large HOMO coefficients, and carbon atoms C2 and C6 have large HOMO-1/LUMO (see Figure 1b).
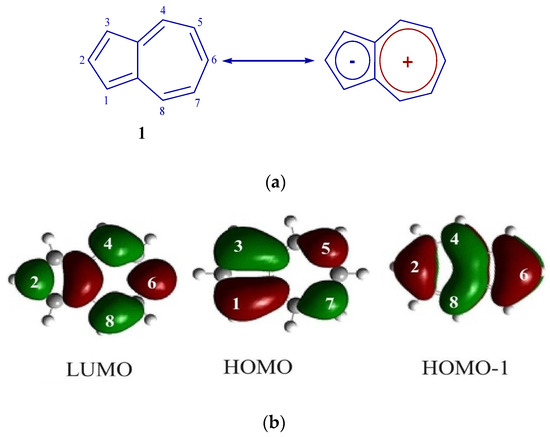
Figure 1.
(a) Structure 1. (b) FMO 1.
A distinctive feature of azulene 1 compared to naphthalene is its saturated blue color, which indicates the absorption of light corresponding to the transition S0 → S1 (λ max is in the long-wave region of the spectrum at 580 nanometers). However, the molar absorption coefficient has a small value (350 M−1 cm−1), which indicates that it does not reach the level expected for the permitted π–π* transition [25].
Thus, modification of the structure of azulene by introducing functional groups, especially electron donor groups, can significantly change its electronic properties. This opens up ample opportunities for creating unique materials with unusual optical and electronic characteristics.
In this work, we demonstrate the synthesis of new carbazolyl-substituted azulenes. An effective cross-coupling method yielded 1,2,3-tris(carbazolyl)azulene 6 and 1,2,3,6-tetrakis(carbazolyl)azulene 10 in high yields. The study of photophysical properties of these molecular structures has shown that they lead to an allowed HOMO → LUMO electronic transition. This causes the appearance of intense absorption and luminescence spectra in the visible range.
2. Results and Discussions
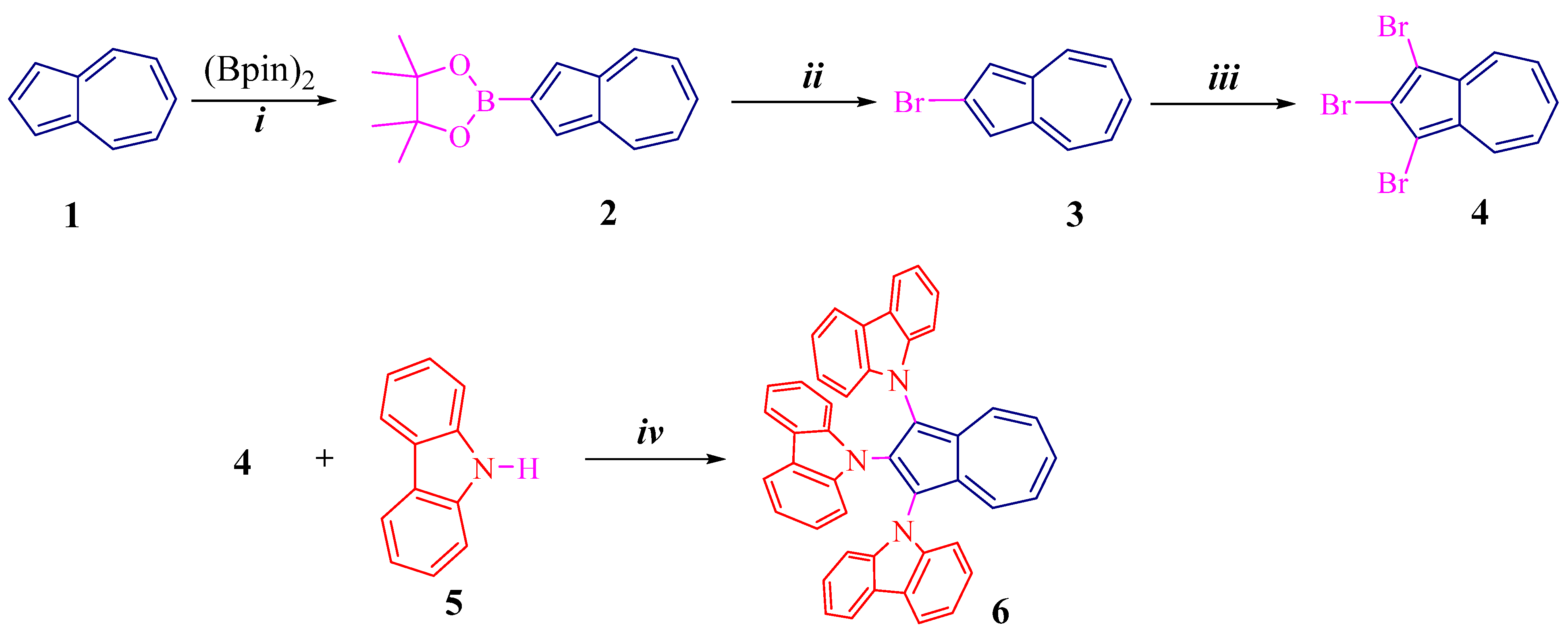
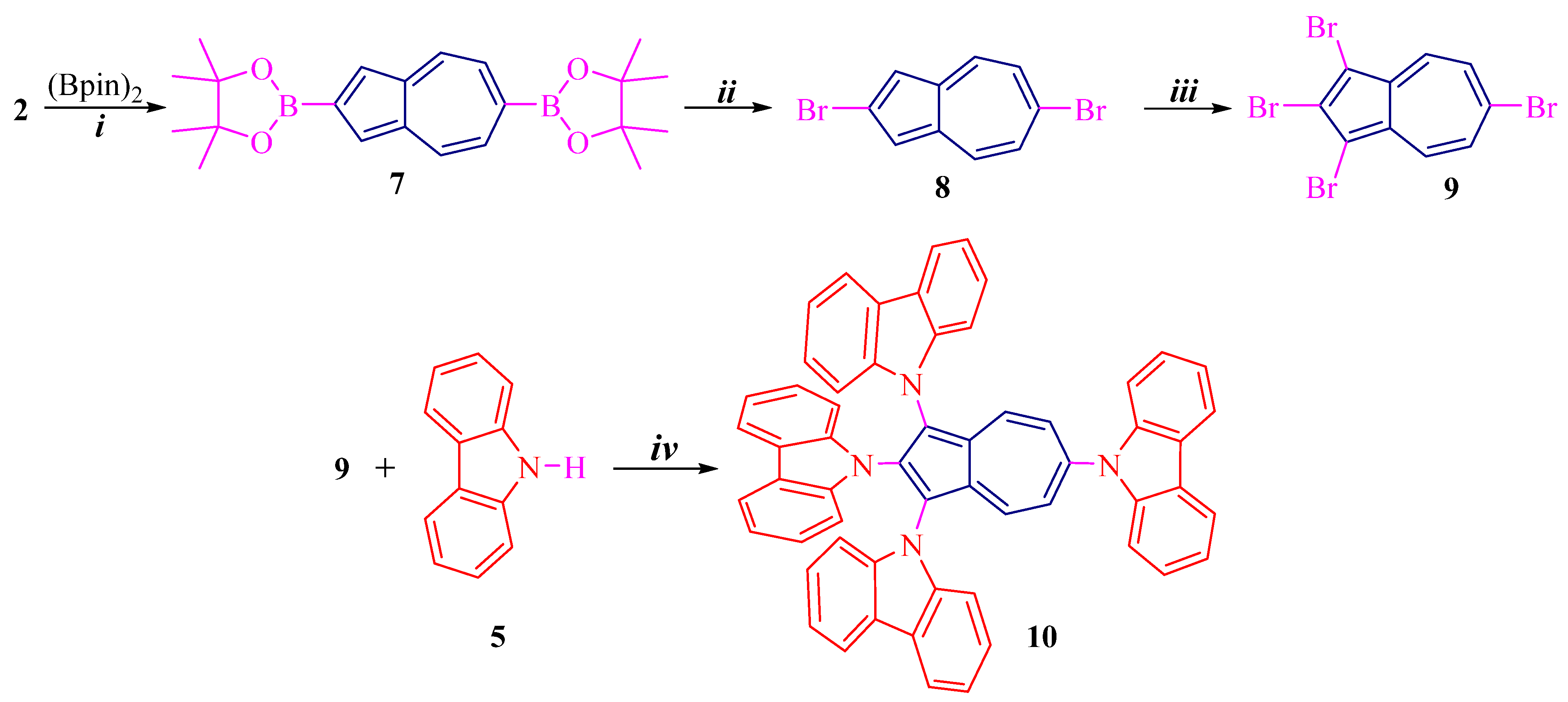
Scheme 1 and Scheme 2 illustrate synthetic routes for azulene conjugated compounds: 1,2,3-tri(carbazolyl)azulene 6 and 1,2,3,6-tetra(carbazolyl)azulene 10.
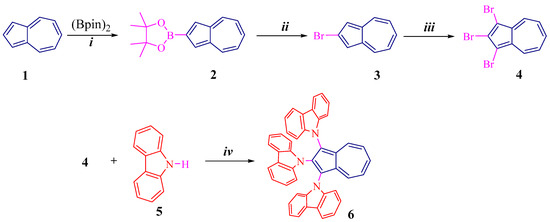
Scheme 1.
Preparation of 6: (i) [Ir(cod)Cl]2, 2,2′-bpy, cyclohexane, reflux, 70%; (ii) CuBr, DMF, 90 °C, 72%; (iii) NBS, CH2Cl2, −20 °C, 92%; (iv) Pd(OAc)2, t-BuOK, t-Bu3PHBF4, toluene, reflux, 78%.
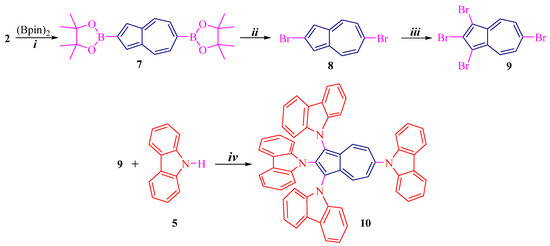
Scheme 2.
Preparation of 10: (i) [Ir(cod)Cl]2, 2,2′-bpy, cyclohexane, reflux, 50%; (ii) CuBr, DMF, 90 °C, 70%; (iii) NBS, CH2Cl2, −20 °C, 90%; (iv) Pd(OAc)2, t-BuOK, t-Bu3PHBF4, toluene, reflux, 75%.
As shown (Scheme 1), key molecule 2 was synthesized by direct borylation at the C2 position of 1 in the presence of an Ir-catalyst, according to the methodology described in the literature [26]. This compound is then successfully reacted with copper bromide to give bromoazulene 3 in 72% yield [27].
Next, the bromide 3 reacts with NBS (N-bromosuccinimide) to form tribromoazulene 4 in a high yield of 92%. The reaction proceeds regioselectively at the C1 and C3 positions.
Finally, the cross-coupling of tribromoazulene 4 and carbazole 5 in toluene, in the presence of a palladium catalyst, gives the final product, tris(carbazolyl)azulene 6, in a high yield of 78%.
Another key molecule, 7, was synthesized by selective C6 borylation of 2 under the above conditions, according to [28] (Scheme 2). Further reaction of diborylazulene 7 with copper bromide gives 2,6-dibromoazulene 8 in 70% yield [27,29]. The resulting dibromide 8 is then also reacted regioselectively at positions 1 and 3 with NBS to give tetrabromoazulene 9 in high yield of 90%. In a similar way, by cross-coupling reaction of tetrabromide 9 and 5, the final product, tetrakis(carbazolyl)azulene 10, was obtained. The yield is high, amounting to 75%.
The obtained tris(carbazolyl)azulene 6 and tetrakis(carbazolyl)azulene 10 are colored brown in contrast to the original 1, which has a blue tint. These compounds are stable solids (Supplementary Materials, Section 3, Figure S13) that are easily soluble in organic solvents (CH2Cl2, CHCl3, C6H5CH3, and C6H5Cl) at ambient temperature.
The structure and purity of synthesized compounds 6 and 10 were proved by NMR (1H and 13C), IR, and Mass spectrometry (Supplementary Materials).
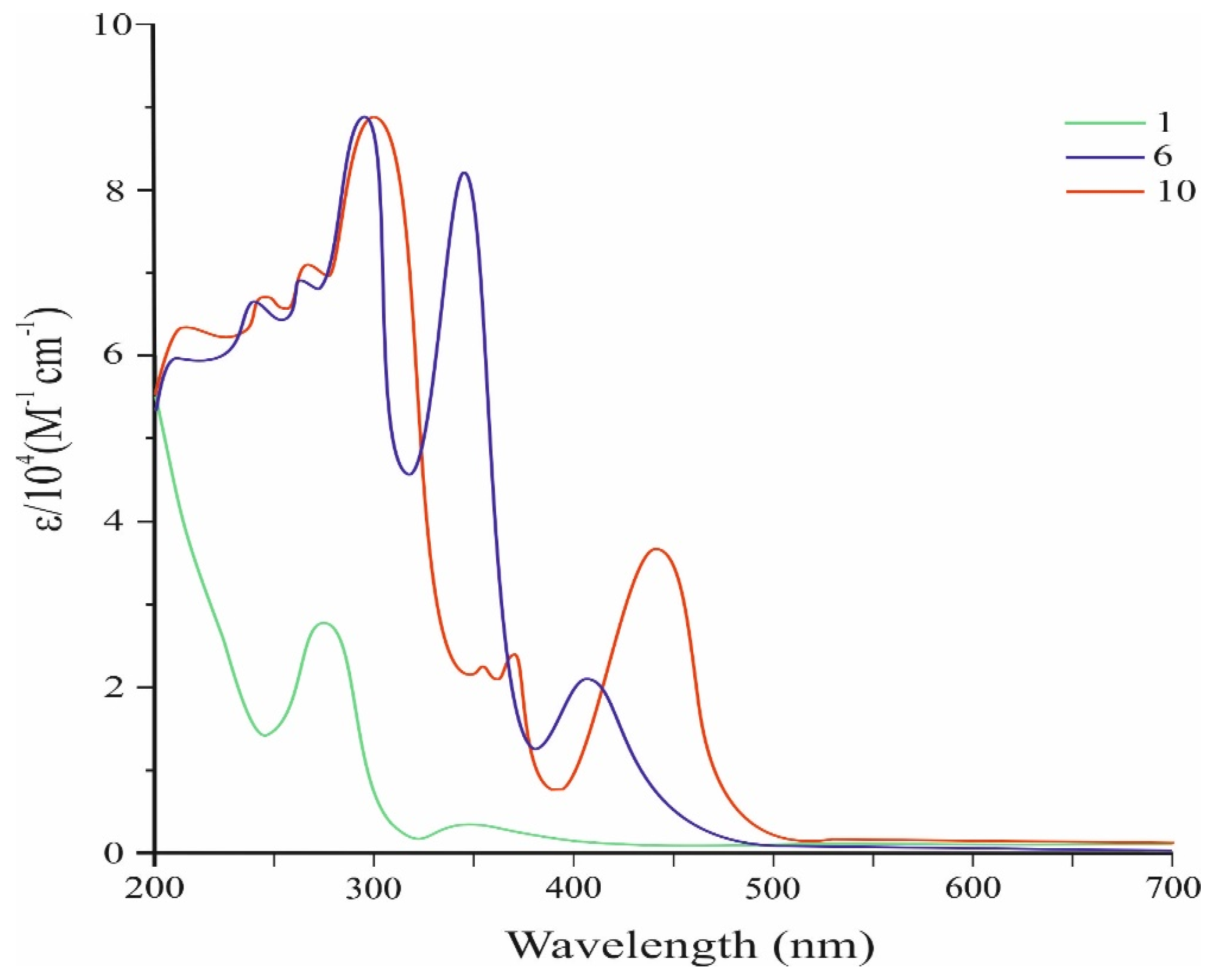
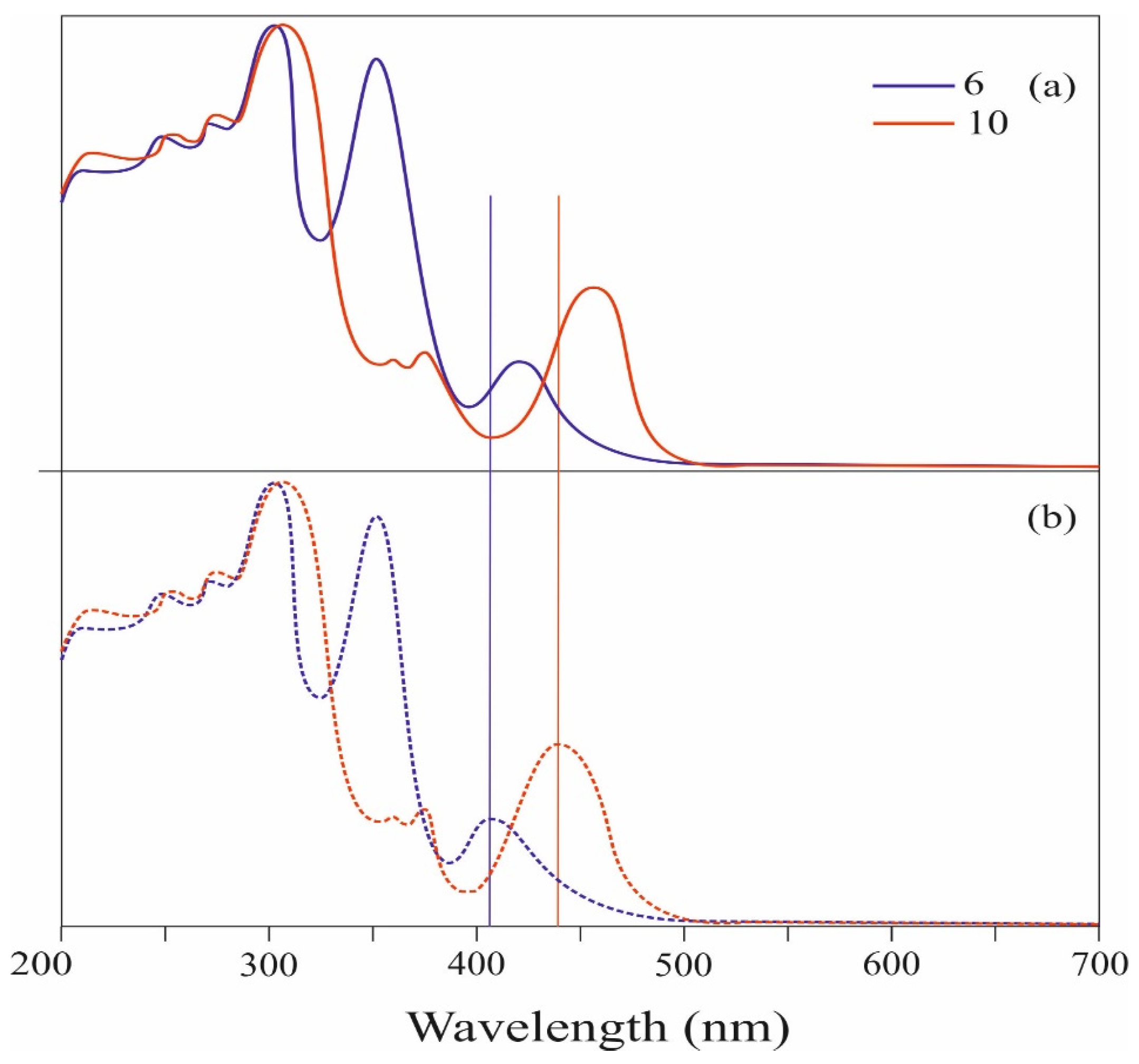
Figure 2 shows the UV-Vis spectra of conjugated tris- and tetrakis(carbazolyl)azulenes 6 and 10, while Table 1 presents their characteristics.
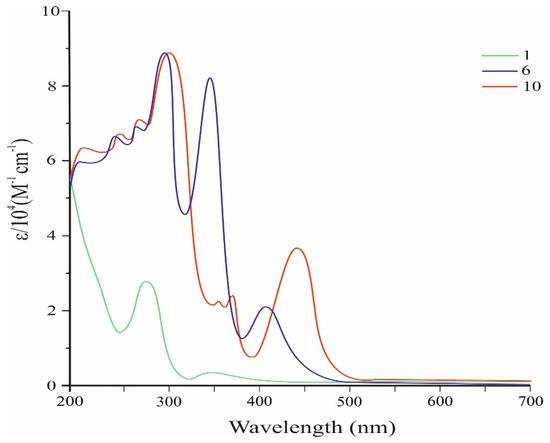
Figure 2.
UV–Vis spectra of compounds 6, 10, and the original 1.

Table 1.
Photophysical properties of compounds 6 and 10.
The absorption spectra of these compounds were analyzed against the spectrum of the initial molecule 1 (Figure 2).
As shown in Figure 2, the absorption spectrum of the compound 6 in dichloromethane solution shows the appearance of a new visible band with λmax at 407 nm (ɛ = 21.132 M−1 cm−1). The compound 10 also exhibits a strong visible absorption corresponding to the permitted π–π* electron transition with a maximum at 441 nm (ɛ = 36.666 M−1 cm−1).
The effective absorption of visible light in the compounds 6 and 10 is due to the strong electron donor effect of the carbazolyl groups. It should be noted that visible absorbances of 6 and 10 are significantly stronger than that of the original azulene 1, which has a molar absorption coefficient in the visible region of only 350 M−1 cm−1 [25].
It is also seen from the spectrum that the visible absorption maximum of 10 is significantly (by 34 nm) shifted to the long-wave region with an increase in intensity compared to the absorption maximum of 6. These changes may be due to a significant increase in π-conjugation and de-localization of electrons, which leads to narrowing of the HOMO–LUMO gap of molecule 10 (Figure 5).
The study of the electronic absorbances of the compounds 6 and 10 was also carried out in the form of thin films (Supplementary Materials). Analysis of the spectra presented in Figure 3 shows that the absorption pattern of the compounds 6 and 10 in thin films is similar to their absorption in solution (see Figure 3b or Figure 2). However, the visible absorption maxima (λmax at 407 and 441 nm, respectively) for the compounds 6 and 10 are shifted to the long wavelength region by about 13 and 15 nanometers, respectively, compared to the solution. Redshift of spectral bands may be due to intermolecular interaction of compounds in the solid state.
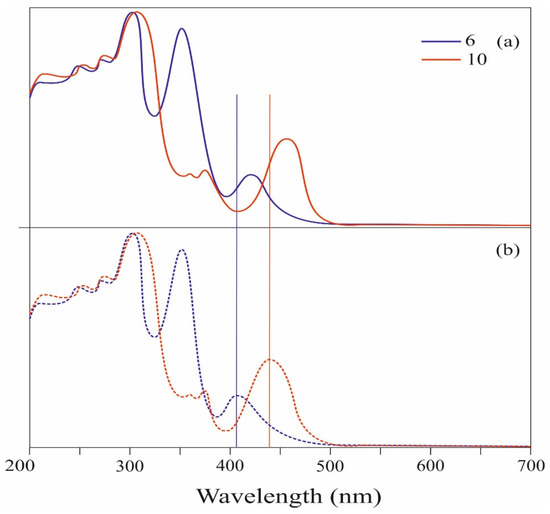
Figure 3.
UV–Vis absorption spectra of 6 and 10: (a) in film; (b) in CH2Cl2.
It is worth noting that the absorption bands of compounds 6 and 10 observed in the spectrum coincide with the wavelength range (380–560 nm) characteristic of commercially available functional materials used in organic solar cells and organic dyes [30,31,32,33,34,35,36].
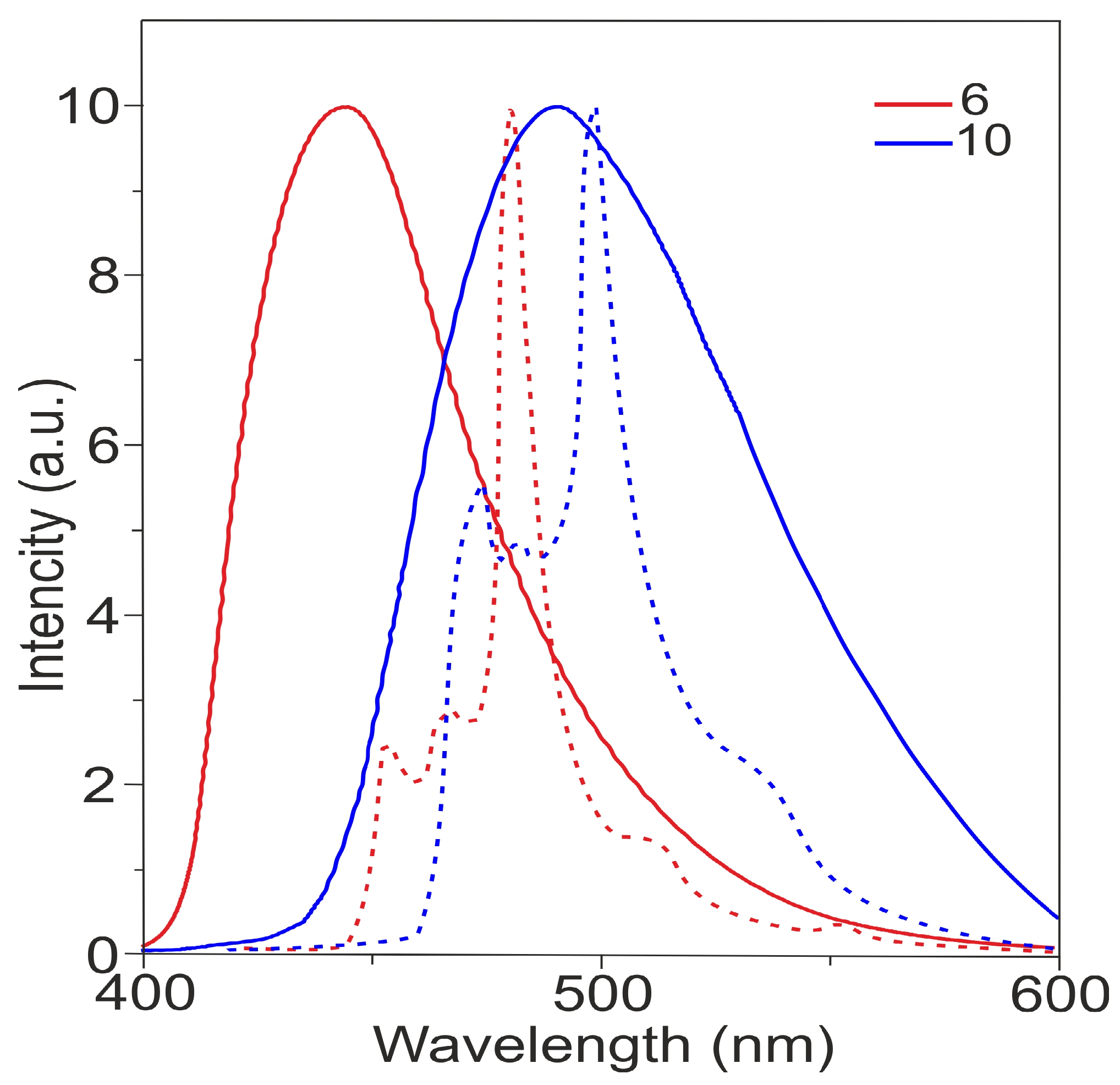
Figure 4 shows the photoluminescence (PL) spectra of tris- and tetrakis (carbazolyl)azulenes 6 and 10 studied in solution, and Table 1 shows their characteristics.
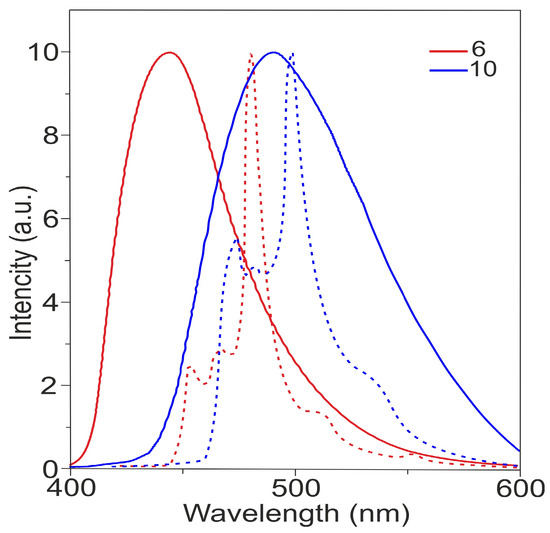
Figure 4.
Photoluminescence (PL) spectra of compounds 6 and 10 in dichloromethane. Fluorescence spectra–solid lines, phosphorescence spectra–dashed lines.
The spectrum shows that compounds 6 and 10 exhibit new photoluminescence bands in the visible region with maxima at 411 and 437 nm. The quantum yields (ΦPL) are 12% and 25%, respectively (Table 1).
The fluorescence spectra of compounds 6 and 10 at room temperature are broad and structureless, while the phosphorescence spectra measured at 77 K show vibrational structures.
The analysis of Table 1 demonstrates that the most donor tetrasubstituted azulene 10 possesses lower S1 (2.87 eV) and T1 (2.63 eV) energies compared to the trisubstituted compound 6. Thus, the S1 of compound 10 is significantly shifted towards lower energies by 0.19 eV. The T1 energy also has a shift to lower energies by 0.16 eV. As a result, the ΔEST of molecule 10 reaches 0.24 eV.
It is also observed that the lifetime (τp/τd) and the quantum yield of the fluorescence of compound 10 are superior to those of molecule 6 (Table 1).
So, the synthesized carbazolylazulenes 6 and 10 have a significant feature–intense photoluminescence in the visible part of the spectrum, which is not observed in the original azulene 1 [24,25].
Thus, studies of photophysical properties have shown that the introduction of electron-donor carbazolyl groups at positions 1,2,3, and 6 of azulene leads to the appearance of intense electronic absorptions (ε 21.132 M−1 cm−1 and ε 36.666 M−1 cm−1) and unique photoluminescent emissions (ΦPL 12% and 25%) in the visible part of the spectrum.
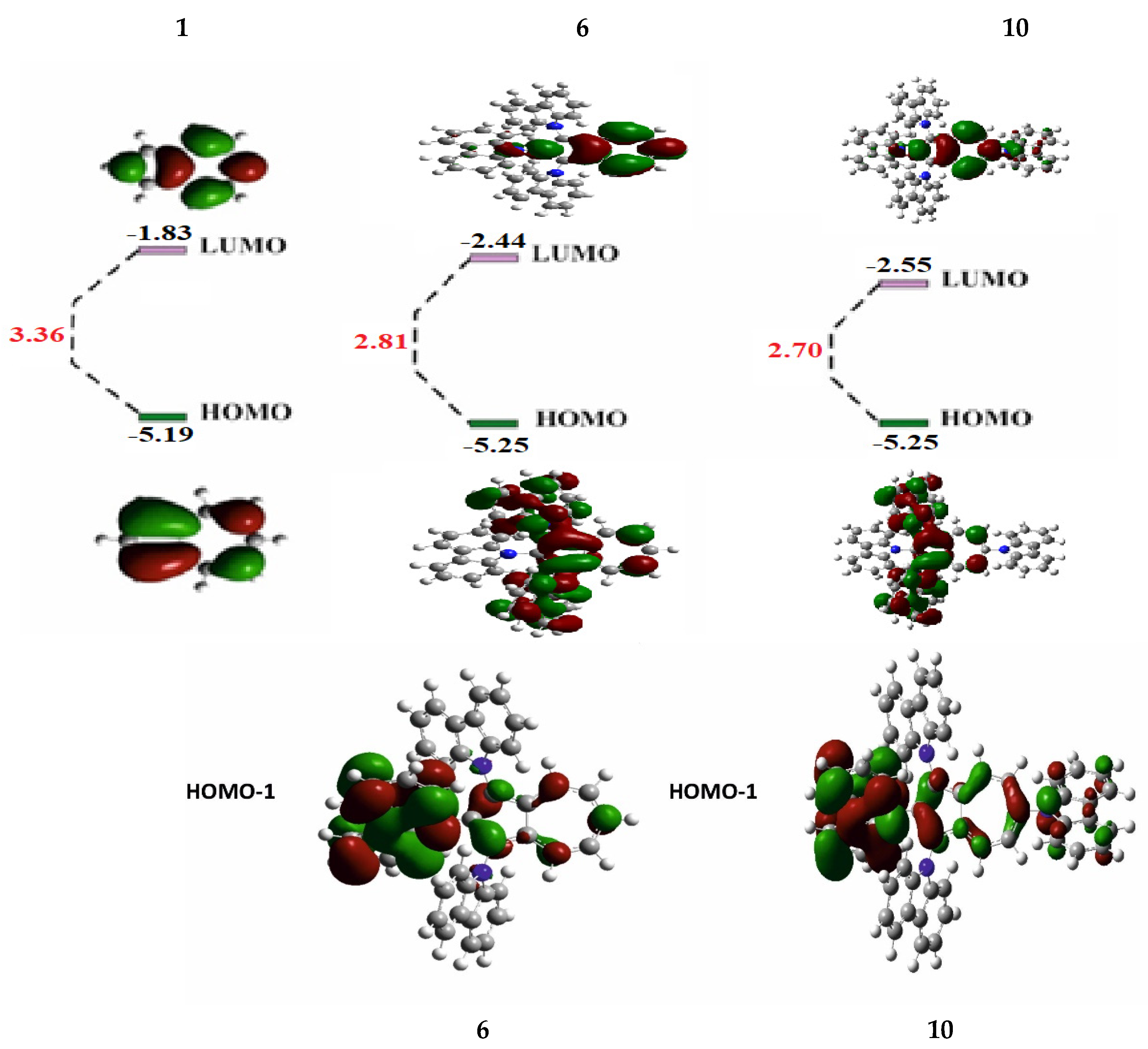
To understand the electronic properties of the synthesized tris- and tetrakis(carbazolyl)azulenes 6 and 10 and the relationship between their structural configuration and optical behavior, DFT calculations (method B3LYP/6-31G*) were performed (Figure 5, Supplementary Materials).
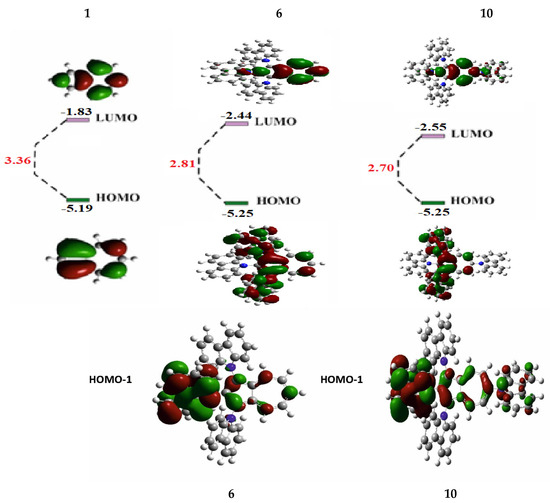
Figure 5.
Distribution of frontal MO 6 and 10 versus azulene 1.
Figure 5 shows that HOMO (and HOMO-1) of the compounds 6 and 10 are distributed throughout both the azulene structure and at the carbazolyl substituents.
This distribution of orbitals can occur as a result of interaction of HOMO-1 of azulene 1 with HOMO of carbazole 5 [29], because in HOMO of the original azulene C2 and C6 are nodal, while in HOMO-1 these atoms are not included in the nodal plane and have large atomic-orbital coefficients (Figure 1b).
Studies also revealed that HOMO (5.25 eV each) and LUMO (−2.44 eV and −2.55 eV) levels of the compounds 6 and 10 occupy lower energy positions compared to similar levels of the original azulene 1.
At the same time, LUMO levels decrease significantly by 0.61 and 0.72 eV, respectively. As a result, the energy gaps between the frontal orbitals of these compounds narrow by 0.55 eV and 0.66 eV.
Probably, the decrease in FMO causes the permitted π–π* electronic transition in molecules 6 and 10, in contrast to azulene 1, where this transition is prohibited [25]. As a result, intense absorption and emission are observed in the visible spectrum (Figure 2 and Figure 4, data in Table 1), which indicates significant differences in the electronic structure of these compounds.
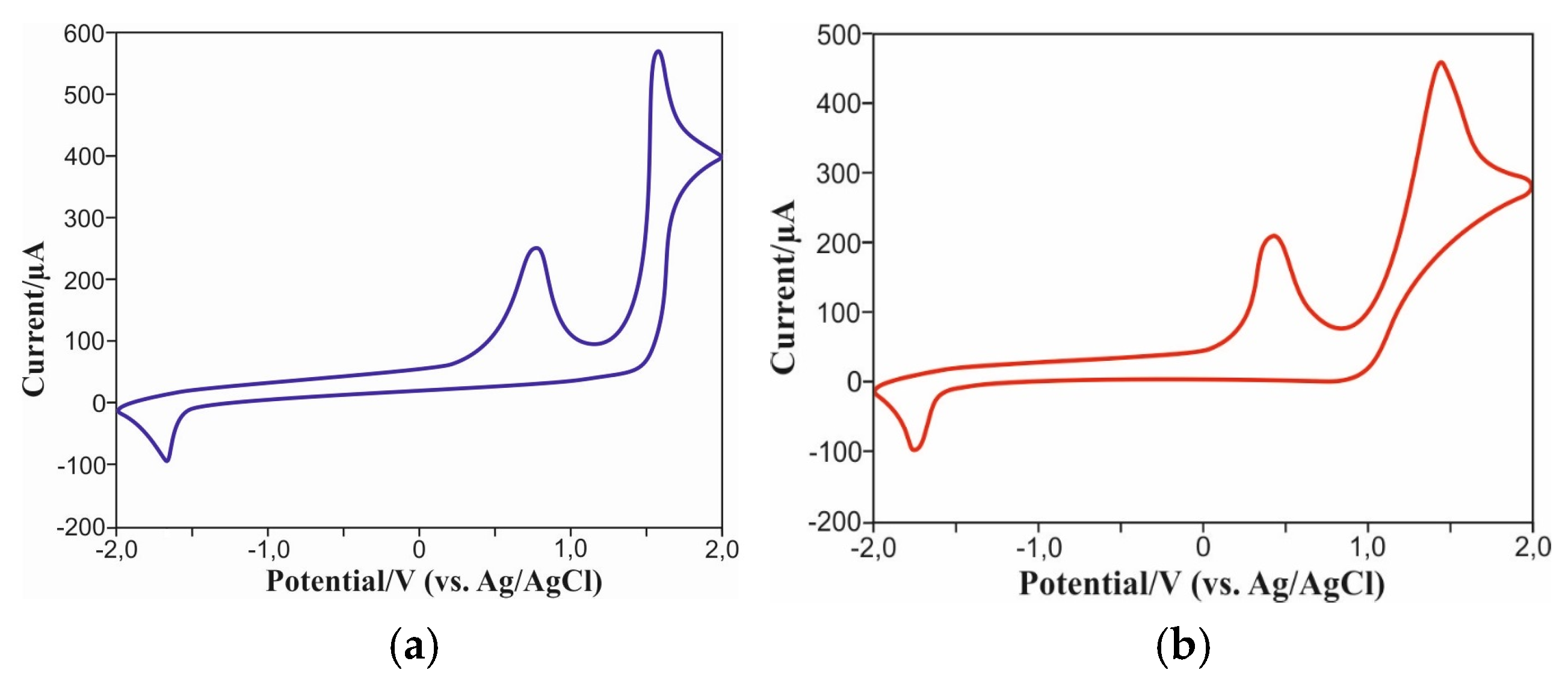
To determine the energy levels of the frontal MO of the carbazolylazulenes 6 and 10, a CV (cyclic voltammetry) analysis of their redox behavior was carried out (Figure 6, Supplementary Materials).
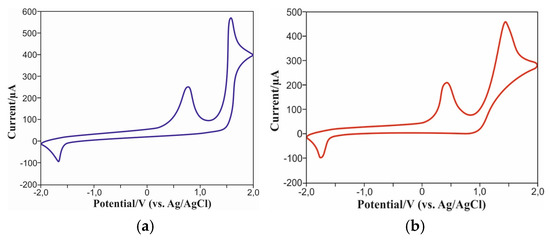
Figure 6.
Cyclic voltammogram of 6 (a) and 10 (b).
Analysis of CV curves revealed irreversible double oxidation peaks of the compound 6 at potentials of 0.77 V and 1.58 V. In addition, an irreversible single reduction peak was recorded at a potential of −1.67 V. The compound 10 also exhibited similar behavior, with irreversible oxidation at potentials of 0.43 V and 1.44 V, as well as an irreversible reduction wave observed at −1.75 V. Determining the initial oxidation (0.1 V for Eoxonset) and reduction (−1.57 V for Eredonset) potentials, we calculated the energy values of the frontal orbitals for the compound 6: −5.10 eV for HOMO and −2.83 eV for LUMO. Taking into account the initial oxidation (0.08 V for Eoxonset) and reduction (–1.50 V for Eredonset) potentials, the HOMO and LUMO energy levels for molecule 10 were determined to be −5.12 eV and −2.90 eV, respectively. The calculation of these values was performed on the basis of the formula set forth in [37].
Thus, experimentally determined values of HOMO energy levels for the compounds 6 and 10 demonstrate good consistency with the results obtained using the DFT method. The difference between theoretically calculated and experimentally measured values of LUMO energy levels is insignificant, being approximately 0.39 V and 0.35 V for the compounds 6 and 10, respectively.
3. Materials and Methods
1,2,3-Tribromoazulene 4. To a solution of monobromazulene 3 (207 mg, 1.00 mmol) in dichloromethane (5 mL) was slowly poured a solution of NBS (389 mg, 2.2 mmol) in dichloromethane (25 mL) at −20 °C under argon and stirred for 1.5 h. Then the mixture was treated with NaHCO3 solution, the organic phase was washed with brine and dried with Na2SO4. The resulting solution was filtered through alumina. After concentration on a rotary evaporator and recrystallization from dichloromethane, 337 mg (92%) of a dark green solid was obtained. m.p. 132–133 °C. IR spectrum (ν, cm−1): 2920, 2851, 1581, 1462, 1385, 1269, 725. 1H NMR: δ 7.94 (d, J = 11.4 Hz, 2H4,8Az), 7.62–7.59 (m, 2H5,7Az), 7.33 (t, J = 10.2 Hz, 1H6Az). 13C NMR: δ 139.6, 137.5, 136.3, 135.5, 134.3, 134.2, 128.9, 125.6, 107.1. HRMS (ESI): calcd. for C10H5 79Br3 [M]+: 361,7898, found: 361,7891.
1,2,3-Tris(carbazolyl)azulene 6. Tribromoazulene 4 (336 mg, 1.00 mmol) and carbazole 5 (554 mg, 3.6 mmol) were mixed in dry toluene (35 mL) under argon. Palladium (II) acetate (12 mg, 0.05 mmol), t-BuOK (941 mg, 16.8 mmol), and t-Bu3PHBF4 (3.0 mg, 0.01 mmol) were then added. The mixture was then refluxed for 24 h, filtered through Celite and dried with Na2SO4. The solvent was removed by rotary evaporation. The pure compound was obtained by purification on silica gel using a 1:9 solution of dichloromethane and hexane (CC with SiO2) as eluent. After purification, the compound was recrystallized from CH2CI2. 487 mg (78%) of a brown solid were obtained. Melting point: 142–143 °C. IR spectrum (ν, cm−1): 2920, 2850, 1583, 1462, 1380, 1269, 724. 1H NMR: δ 8.40 (d, J = 9.6 Hz, 2H4,8Az), 8.09–8.03 (m, 6H1,8Cz), 7.91–7.85 (m, 2H5,7Az), 7.62–7.53 (m, 6H4,5Cz,1H6Az), 7.40–7.33 (m, 12H2,3,6,7Cz). 13C NMR: δ 138.2, 134.8, 134.2, 131.7, 130.8, 126.6, 124.3, 123.6, 123.1, 122.7, 122.2, 118.2, 117.1, 114.9, 112.5. HRMS (ESI): calcd. for C46H29N3 [M]+: 623,0720, found: 623,0712.
1,2,3,6-Tetrabromoazulene 9. To a solution of dibromoazulene 8 (286 mg, 1.00 mmol) in dichloromethane (5 mL) was slowly poured a solution of NBS (389 mg, 2.2 mmol) in dichloromethane (25 mL) at −20 °C under argon. The mixture was then stirred for 2 h. A saturated aqueous solution of sodium hydrogen carbonate was then added, after which the organic layer was separated and washed with brine until neutral. It was then dried over sodium sulfate. The resulting solution was filtered through alumina. Concentration on a rotary evaporator and recrystallization from dichloromethane gave 399 mg (90%) of a dark green solid. Batch 177–178 °C. IR spectrum (ν, cm−1): 2920, 2850, 1580, 1465, 1385, 1269, 725. 1H NMR: δ 8.29 (d, J = 9.7 Hz, 2H4,8Az), 7.33 (d, J = 9.9 Hz, 2H5,7Az).13C NMR: δ 139.6, 136.3, 135.5, 134,2, 130,9, 129.0, 125.6, 104.7. HRMS (ESI): calcd. for C10H4 79Br4 [M]+: 440,0570, found: 440,0563.
1,2,3,6-Tetrakis(carbazolyl)azulene 10. Tetrabromazulene 9 (444 mg, 1.00 mmol) and carbazole 5 (738 mg, 4.8 mmol) were mixed in dry toluene (45 mL). The catalyst palladium (II) acetate (12 mg, 0.05 mmol), t-BuOK (1.254 g, 22.4 mmol), and t-Bu3PHBF4 (3.0 mg, 0.01 mmol) were then added to the mixture under continuous argon purge at ambient conditions. The mixture was then refluxed for 24 h, filtered through Celite and dried over sodium sulfate. The solvent was removed by rotary evaporation. The pure compound was obtained by purification on silica gel using a 1:9 solution of DCM and hexane (CC with SiO2) as eluent. After purification, the compound was recrystallized from CH2Cl2. 590 mg (75%) of a dark brown solid was obtained. Melting point: 146–148 °C. IR spectrum (ν, cm−1): 2920, 2854, 1580, 1461, 1384, 1268, 725. 1H NMR: δ 8.40 (d, J = 11.6 Hz, 2H4,8Az), 8.22–8.20 (m, 8H1,8Cz), 7.63–7.57 (m, 2H5,7Az),7.37–7.31 (m, 8H4,5Cz), 6.92–6.90 (m, 16H2,3,6,7Cz). 13C NMR: δ 139.9, 135.4, 134.4, 126,7, 126,6, 124.5, 124.3, 122.09, 122.06, 122.05, 121.4, 121.3, 120.7, 120.7, 110.1, 109.2, 106.6. HRMS (ESI): calcd. for C58H36N4 [M]+: 788,0685, found: 788,0678.
4. Conclusions
The synthesis of tris- and tetrakis(carbazolyl)azulenes 6 and 10 in high yields and their subsequent careful analysis showed that these compounds possess special optical characteristics. The inclusion of carbazolyl groups at positions 1,2,3, and 6 of azulene leads to pronounced absorbing and emitting properties in the visible spectrum (400–600 nm), in particular to intense emission in the region in the blue region, which is not demonstrated by the compound 1. DFT calculations support these results by showing changes in the electronic structure that make possible π → π * electronic transitions previously qualified as prohibited. These conjugated compounds have great potential for applications in organic optoelectronics and materials science.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30132797/s1, Figure S1: 1H NMR spectra of 1,2,3-tribromoazulene 4.; Figure S2: 13C NMR spectra of 1,2,3-tribromoazulene 4; Figure S3: HRMS spectra of 1,2,3-tribromoazulene 4; Figure S4: 1H NMR spectra of 1,2,3-tris(carbazolyl)azulene 6; Figure S5: 13C NMR spectra of 1,2,3-tris(carbazolyl)azulene 6; Figure S6: 13C NMR spectra of 1,2,3,6-tetrabromoazulene 9; Figure S7: 1H NMR spectra of 1,2,3,6-tetrakis(carbazolyl)azulene 10; Figure S8: 13C NMR spectra of 1,2,3,6-tetrakis(carbazolyl)azulene 10; Figure S9: HRMS spectra of 1,2,3,6-tetrabromoazulene 9; Figure S10: 1H NMR spectra of 1,2,3,6-tetrakis(carbazolyl)azulene 10; Figure S11: 13C NMR spectra of 1,2,3,6-tetrakis(carbazolyl)azulene 10; Figure S12: HRMS spectra of 1,2,3,6-tetrakis(carbazolyl)azulene 10; Figure S13: Thermogravimetric (TGA) analysis of carbazolylazulene 6 and 10; Table S1: Atomic coordinates of optimized geometry of 6; Table S2: Atomic coordinates of optimized geometry of 10.
Author Contributions
Conceptualization, N.M.; methodology, N.M.; software, A.I. (Amantay Iskanderov) and A.I. (Ablaykhan Iskanderov); validation, N.M., A.I. (Amantay Iskanderov), A.I. (Ablaykhan Iskanderov) and S.A.; formal analysis, A.I. (Amantay Iskanderov) and A.I. (Ablaykhan Iskanderov); investigation, N.M., A.I. (Amantay Iskanderov) and A.I. (Ablaykhan Iskanderov); resources, N.M.; data curation, N.M., A.I. (Amantay Iskanderov) and A.I. (Ablaykhan Iskanderov), S.A. and P.V.; writing—original draft preparation, N.M.; writing—review and editing, N.M.; visualization, A.I. (Amantay Iskanderov), A.I. (Ablaykhan Iskanderov), S.A. and P.V.; supervision, N.M. and S.A.; project administration, N.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research is funded by the Committee of Science of the Ministry of Science and Higher Education of the Republic of Kazakhstan (Grant No. BR21882309).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wu, C.; Djurovich, P.I.; Thompson, M.E. Study of energy transfer and triplet exciton diffusion in hole-transporting host materials. Adv. Funct. Mater. 2009, 19, 3157–3164. [Google Scholar] [CrossRef]
- Taniguchi, R.; Noto, N.; Tanaka, S.; Takahashi, K.; Sarkar, S.K.; Oyama, R.; Abe, M.; Koike, T.; Akita, M. Simple generation of various α-monofluoroalkyl radicals by organic photoredox catalysis: Modular synthesis of β-monofluoroketones. Chem. Commun. 2021, 57, 2609–2612. [Google Scholar] [CrossRef]
- Noto, N.; Koike, T.; Akita, M. Visible-Light-Triggered Monofluoromethylation of Alkenes by Strongly Reducing 1,4-Bis(diphenylamino)naphthalene Photoredox Catalysis. ACS Catal. 2019, 9, 4382–4387. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Song, Y.; Su, Y.; Wang, X. Bis(phenothiazine)arene diradicaloids: Isolation, characterization and crystal structures. Chem. Commun. 2015, 51, 11822–11825. [Google Scholar] [CrossRef]
- Freudenberg, J.; Jänsch, D.; Hinkel, F.; Bunz, U.H.F. Immobilization Strategies for Organic Semiconducting Conjugated Polymers. Chem. Rev. 2018, 118, 5598–5689. [Google Scholar] [CrossRef]
- Roy, M.; Walton, J.H.; Fettinger, J.C.; Balch, A.L. Direct Crystallization of Diamine Radical Cations: Carbon-Nitrogen Bond Formation from the Reaction of Triphenylamine with TiCl4, TiBr4,or SnCl4 versus Carbon-Carbon Bond Formation with SbCl5. Chem.–Eur. J. 2022, 28, e202104631. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Z.; Yang, L.; Pan, F.-F.; Yu, G.-A.; Yin, J.; Liu, S.H. Elaborately Tuning Intramolecular Electron Transfer Through Varying Oligoacene Linkers in the Bis(diarylamino) Systems. Sci. Rep. 2016, 6, 36310. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Dmitrieva, E.; Kohn, B.; Scheler, U.; Liu, Y.; Tkachova, V.; Yang, L.; Fu, Y.; Ma, J.; Zhang, P. An Efficient Rechargeable Aluminum–Amine Battery Working Under Quaternization Chemistry. Angew. Chem. Int. Ed. 2022, 61, e202116194. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, X.; Johnson, L.R.; Bruce, P.G. Kinetics of lithium peroxide oxidation by redox mediators and consequences for the lithium–oxygen cell. Nat. Commun. 2018, 9, 767. [Google Scholar] [CrossRef]
- Mayer, D.C.; Manzi, A.; Medishetty, R.; Winkler, B.; Schneider, C.; Kieslich, G.; Pöthig, A.; Feldmann, J.; Fischer, R.A. Controlling Multiphoton Absorption Efficiency by Chromophore Packing in Metal-Organic Frameworks. J. Am. Chem. Soc. 2019, 141, 11594–11602. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Higashibeppu, M.; Mazaki, Y. Synthesis and Properties of Twisted and Helical Azulene Oligomers and Azulene-Based Polycyclic Hydrocarbons. ChemistryOpen 2023, 12, e202100298. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, T.; Katsuoka, Y.; Yoza, K.; Sato, H.; Mazaki, Y. Stereochemistry, Stereodynamics, and Redox and Complexation Behaviors of 2,2′-Diaryl-1,1′-Biazulenes. ChemPlusChem 2019, 84, 1659–1667. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Umemura, R.; Kaminaga, M.; Kushida, S.; Ohkubo, K.; Noro, S.I.; Mazaki, Y. Paddlewheel Complexes with Azulenes:Electronic Interaction between Metal Centers and Equatorial Ligands. ChemPlusChem 2019, 84, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Konishi, A.; Yasuda, M. Breathing new life into nonalternant hydrocarbon chemistry: Syntheses and properties of polycyclic hydrocarbons containing azulene, pentalene, and heptalene frameworks. Chem. Lett. 2021, 50, 195–212. [Google Scholar] [CrossRef]
- Xin, H.; Hou, B.; Gao, X. Azulene-Based π-Functional Materials: Design, Synthesis, and Applications. Acc. Chem. Res. 2021, 54, 1737–1753. [Google Scholar] [CrossRef]
- Elwahy, A.H.; Hafner, K. Alkynylazulenes as Building Blocks for Highly Unsaturated Scaffolds. Asian J. Org. Chem. 2021, 10, 2010–2083. [Google Scholar] [CrossRef]
- Bakun, P.; Czarczynska-Goslinska, B.; Goslinski, T.; Lijewski, S. In vitro and in vivo biological activities of azulene derivatives with potential applications in medicine. Med. Chem. Res. 2021, 30, 834–846. [Google Scholar] [CrossRef]
- Lvov, A.G.; Bredihhin, A. Azulene as an ingredient for visible-light- and stimuli-responsive photo switches. Org. Biomol. Chem. 2021, 19, 4460–4468. [Google Scholar] [CrossRef]
- Murfin, L.C.; Lewis, S.E. Azulene—A Bright Core for Sensing and Imaging. Molecules 2021, 26, 353. [Google Scholar] [CrossRef]
- Shoji, T.; Ito, S.; Yasunami, M. Synthesis of Azulene Derivatives from 2H-Cyclohepta[b]furan-2-ones as Starting Materials: Their Reactivity and Properties. Int. J. Mol. Sci. 2021, 22, 10686. [Google Scholar] [CrossRef]
- Anderson, A.G.; Steckler, B.M. Azulene. VIII. A Study of the Visible Absorption Spectra and Dipole Moments of Some 1- and 1,3-Substituted Azulenes. J. Am. Chem. Soc. 1959, 81, 4941–4946. [Google Scholar] [CrossRef]
- Del Valle, J.C.; Catalán, J. A Reappraisal of Kasha’s Rule. Phys. Chem. Chem. Phys. 2019, 21, 10061–10069. [Google Scholar] [CrossRef]
- Behera, S.K.; Park, S.Y.; Gierschner, J. Dual Emission: Classes, Mechanisms, and Conditions. Angew. Chem. Int. Ed. 2021, 60, 22624–22638. [Google Scholar] [CrossRef]
- Dunlop, D.; Ludvíková, L.; Banerjee, A.; Ottosson, H.; Slanina, T. Excited-State (Anti)Aromaticity Explains Why Azulene Disobeys Kasha’s Rule. J. Am. Chem. Soc. 2023, 145, 21569–21575. [Google Scholar] [CrossRef]
- Shevyakov, S.V.; Li, H.; Muthyala, R.; Asato, A.E.; Croney, J.C.; Jameson, D.M.; Liu, R.S. Orbital control of the color and excited state properties of formylated and fluorinated derivatives of azulene. J. Phys. Chem. A 2003, 107, 3295–3299. [Google Scholar] [CrossRef]
- Kurotobi, K.; Miyauchi, M.; Takakura, K.; Murafuji, T.; Sugihara, Y. Direct Introduction of a Boryl Substituent into the 2-Position of Azulene: Application of the Miyaura and Smith Methods to Azulene. Eur. J. Org. Chem. 2003, 2003, 3663–3665. [Google Scholar] [CrossRef]
- Narita, M.; Murafuji, T.; Yamashita, S.; Fujinaga, M.; Hiyama, K.; Oka, Y.; Tani, F.; Kamijo, S.; Ishiguro, K. Synthesis of 2-Iodoazulenes by the Iododeboronation of Azulen-2-ylboronic Acid Pinacol Esters with Copper(I) Iodide. J. Org. Chem. 2018, 83, 1298–1303. [Google Scholar] [CrossRef] [PubMed]
- Fujinaga, M.; Murafuji, T.; Kurotobi, K.; Sugihara, Y. Polyborylation of azulenes. Tetrahedron 2009, 65, 7115–7121. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Hamano, T.; Inoue, M.; Nakamura, T.; Wakamiya, A.; Mazaki, Y. Intense absorption of azulene realized by molecular orbital inversion. Chem. Commun. 2023, 59, 10604–10607. [Google Scholar] [CrossRef]
- Rahimi, K.; Botiz, I.; Agumba, J.O.; Motamen, S.; Stingelin, N.; Reiter, G. Light absorption of poly(3-hexylthiophene) single crystals. RSC Adv. 2014, 4, 11121–11123. [Google Scholar] [CrossRef]
- Wang, Z.-S.; Koumura, N.; Cui, Y.; Takahashi, M.; Sekiguchi, H.; Mori, A.; Kubo, T.; Furube, A.; Hara, K. Hexylthiophene-Functionalized Carbazole Dyes for Efficient Molecular Photovoltaics: Tuning of Solar-Cell Performance by Structural Modification. Chem. Mater. 2008, 20, 3993–4003. [Google Scholar] [CrossRef]
- Shirota, Y.; Kageyama, H. Charge Carrier Transporting Molecular Materials and Their Applications in Devices. Chem. Rev. 2007, 107, 953–1010. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Habibi, A.; Ni, P.; Nahdi, H.; Bouanis, F.Z.; Bourcier, S.; Clavier, G.; Frigoli, M.; Yassar, A. Synthesis and characterization of solution-processed indophenine derivatives for function as a hole transport layer for perovskite solar cells. Dye. Pigment. 2023, 213, 111136. [Google Scholar] [CrossRef]
- Ren, S.; Wang, Z.; Zhang, W.; Ding, Y.; Yi, Z. Donor-acceptor-based organic polymer semiconductor materials to achieve high hole mobility in organic field-effect transistors. Polymers 2023, 15, 3713. [Google Scholar] [CrossRef]
- Murphy, A.R.; Fréchet, J.M.J. Organic Semiconducting Oligomers for Use in Thin Film Transistors. Chem. Rev. 2007, 107, 1066–1096. [Google Scholar] [CrossRef]
- Zaumseil, J.; Sirringhaus, H. Electron and Ambipolar Transport in Organic Field-Effect Transistors. Chem. Rev. 2007, 107, 1296–1323. [Google Scholar] [CrossRef]
- Ren, S.; Wang, Z.; Zhang, W.; Yassar, A.; Chen, J.; Wang, S. Incorporation of diketopyrrolopyrrole into polythiophene for the preparation of organic polymer transistors. Molecules 2024, 29, 260. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).