Abstract
Ischemic stroke, responsible for the majority of stroke cases worldwide, triggers profound neuroinflammatory responses largely mediated by microglia. Excessive activation of pro-inflammatory microglia exacerbates neuronal injury, highlighting the need for therapeutic strategies targeting microglial modulation. Propofol (2,6-diisopropylphenol), a widely used intravenous anesthetic, has emerged as a promising neuroprotective agent due to its potent anti-inflammatory properties. This review comprehensively explores the diverse cellular mechanisms by which propofol attenuates microglial activation and inflammation in ischemic stroke. By elucidating these molecular pathways, it underscores the therapeutic potential of propofol in mitigating ischemic brain injury and guiding future clinical interventions.
1. Introduction
Ischemic stroke is the most common type of stroke, accounting for approximately 87% of all stroke cases [1,2]. In 2021, there were approximately 69.9 million cases of ischemic stroke globally, reflecting a 101.8% increase since 1990 [3]. The total number of deaths due to ischemic stroke was approximately 3.59 million in 2021. Multiple approaches have been explored to facilitate the repair of damaged neural networks and prevent disability due to ischemic stroke [4]. Ischemic stroke is caused by the blockage of a blood vessel supplying oxygen and nutrients to the brain, resulting in an immediate loss of function. The surrounding tissue forms a penumbra, an area deprived of oxygen and glucose. Although restoration of blood flow is the first line of treatment, it can cause secondary damage to the brain tissue, i.e., cerebral ischemia–reperfusion (I/R) injury [5].
The cells in the penumbra produce a variety of damage-associated molecular patterns (DAMPs), which are recognized by microglial pattern recognition receptors (PRRs), resulting in a robust release of pro-inflammatory cytokines and hence cytotoxicity [6]. Upon ischemic stroke, microglial polarization to a pro-inflammatory (M1) or anti-inflammatory (M2) state can determine the prognosis of stroke [7]. The excessive activation of M1 microglia in ischemic stroke is the main factor in terms of creating an hyper-inflammatory environment in the affected brain area, leading to neuronal injury and neurodegeneration [8,9,10,11]. Therefore, modulating microglial activation is important for alleviating inflammation during ischemic stroke [9,10].
Propofol (2,6-diisopropylphenol) is an intravenous anesthetic widely used in clinical practice and is considered to have potentially anti-inflammatory properties in ischemic stroke via the modulation of microglial activation [11,12]. The main effect of propofol on the activation of microglia is supposed to be the inhibition of the release of proinflammatory cytokines throughout different mechanisms of action [13,14]. This review aims to describe the most important cellular mechanisms of action of propofol in ischemic stroke exerting neuroprotection. These molecular mechanisms are collectively illustrated in Figure 1, providing a visual summary of the pathways through which propofol modulates microglial activation and neuroinflammation in ischemic stroke.
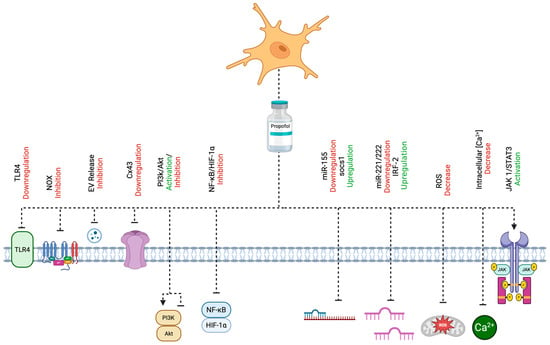
Figure 1.
A comprehensive depiction of the cellular mechanisms through which propofol attenuates microglial activation and neuroinflammation in the context of ischemic stroke. Propofol exerts its neuroprotective effects via multiple molecular targets and signaling pathways, including the modulation of the PI3K/Akt cascade, inhibition of NOX, downregulation of TLR4 and Cx43, and activation of the JAK1/STAT3 pathway. It also regulates miRNA-mediated signaling, particularly the miR-155/SOCS1 and miR-221/222–IRF2 axes. Additional mechanisms include the suppression of the NF-κB/HIF-1α pathways, reduction in EV release, attenuation of oxidative stress via decreased ROS, and maintenance of intracellular Ca2+ homeostasis. Created with BioRender.com (URL accessed on 25 April 2025).
2. Mechanisms of Action of Propofol
2.1. Target Receptors and Mechanisms Underlying Propofol’s Neuropharmacological Effects
The anesthetic effects of propofol are mainly mediated by the activation of GABAA receptors—the major inhibitory receptors in mammalian brains—composed of five subunits that form a central chloride-ion-selective channel gated by the ligand, γ-aminobutyric acid (GABA) [15,16]. Propofol-induced GABAA receptor activation results in an increase in the chloride influx current, contributing to the inhibition of glutamate release [17,18]. In addition, it has been found that propofol can inhibit glutamate release by blocking sodium currents through voltage-gated sodium channels [19].
The main anti-inflammatory propofol-related mechanisms of action are represented by the attenuation of the neurotoxic effects of excessive glutamate release through direct activation of the GABAA receptors and inhibition of the N-methyl-d-aspartate (NMDA) receptors [20]. NMDA receptors play key roles in fast excitatory synaptic transmission and the production of a variety of proinflammatory cytokines [21,22]. It has been reported that propofol can inhibit NMDA receptors, leading to the attenuation of intracellular Ca2+ accumulation and, ultimately, a decline in the production of pro-inflammatory cytokines by microglia [23].
Propofol also initiates its anti-inflammatory effects via its lipophilicity [20]. Propofol as a lipid formulation integrates into cellular membranes, altering membrane structure and function, as well as ion-channel flow, second-messenger generation, and the production of cytokines and eicosanoids [20].
2.2. PI3K/Akt Pathway Activation and Inhibition
2.2.1. PI3K/Akt Pathway Activation
Propofol has been shown to induce autophagy through PI3K/Akt pathway activation in a rat model of cerebral ischemia–reperfusion (I/R) injury and in oxygen–glucose deprivation (OGD)-activated primary microglia [24]. In general, the PI3K/Akt pathway has many vital biological functions for cells, including survival and growth under normal physiological conditions and in a variety of pathological disorders [24,25]. One of the essential outcomes by the PI3K/Akt pathway is the activation of autophagy in cells, which is crucial for recycling damaged organelles in injured cells and plays a key role in alleviating nerve injuries caused by stroke [24,26]. In a previous study, the effects of propofol on autophagy were investigated in rat models of cerebral ischemia–reperfusion (I/R) injury and in oxygen–glucose deprivation (OGD)-stimulated primary microglia derived from mouse brain cortex, with particular focus on the PI3K/Akt signaling pathway. To evaluate propofol-enhanced autophagy, the expression of LC3—a marker for autophagosome formation—was measured in rat brain tissue and primary microglial cells using immunofluorescence techniques. A dramatic increase in LC3 expression was observed upon propofol treatment [24]. To further confirm the enhancing effect of propofol on autophagy induction, they investigated the protein levels of the autophagy markers LC3II/I, Beclin-1, and Atg-7 using Western blot analysis; the results showed a significant increase in these protein levels in the I/R group treated with propofol compared to the untreated I/R group. They also measured the protein levels of OGD-activated primary microglia in vitro by Western blot and demonstrated that the levels of the LC3II/I, Beclin-1, and Atg-7 proteins were significantly increased in the propofol-treated primary microglia compared to the primary microglia that had not been treated with propofol [24]. This study also revealed a significant increase in the phosphorylation levels of PI3K, p-AKT, and p-mTOR proteins in both rat brain tissue with I/R injury and OGD-stimulated primary microglia, suggesting that propofol acts through the activation of the PI3K/Akt pathway [24]. Interestingly, in both rats with I/R injury and OGD-treated primary microglia, propofol caused the downregulation of a tumor suppressor gene PTEN, which inhibits the activation of the PI3K/Akt pathway [24]. Therefore, both the activation of the PI3K/Akt pathway and the downregulation of the PTEN gene are crucial for the protective role of propofol. Inhibition of the PI3K/Akt pathway with LY294002 prior to propofol treatment in I/R injury rats and OGD-stimulated primary microglia caused a significant reduction in autophagy-related markers, including LC3II/I ratio, Beclin-1, and Atg-7 [24]. This indicates that propofol upregulates autophagy through the PI3K/Akt pathway.
Another study showed the anti-inflammatory effects of delayed propofol delivery on the cytokine production of LPS-activated BV2 microglial cells through the PI3k/Akt pathway in different time intervals [27]. This study confirmed that activation of the PI3k/Akt pathway by propofol is key to reducing the production of inflammatory mediators, such as nitric oxide (NO), reactive oxygen species (ROS), and tumor necrosis factor (TNF) [27]. According to this study, BV2 cells treated with 50 μM propofol, showed a 2.1-fold increase in PKB phosphorylation at 30 min, rising to 2.3-fold after a 1 h incubation [27]. However, when the BV2 cell culture was treated with wortmannin—a PI3k/Akt pathway inhibitor—propofol’s effect on PKB phosphorylation was significantly reduced, simultaneously causing a significant elevation in the production of proinflammatory mediators NO, ROS, and TNF [27].
2.2.2. PI3K/Akt Pathway Inhibition
Although activation of the PI3K/Akt pathway is involved in increasing autophagy in neurological disorders, other studies have posited that overactivation of the PI3K/Akt pathway and autophagy can also contribute to inflammation and cell death [28]. Thus, studying and understanding the downregulation of the PI3K/Akt pathway remains another important aspect in neurological diseases [29]. In microglia exposed to LPS and treated with propofol, it was shown that the miR-106b/Pi3k/Akt axis is an important pathway for inhibiting the activation of the microglia, thereby reducing the production of proinflammatory cytokines [29]. In this pathway, propofol upregulates the expression of miR-106, which promotes anti-inflammatory effects by downregulating the PI3k/Akt pathway [29]. In the same study, to further confirm the anti-inflammatory effects of propofol via miR-106, a loss-of-function (LOF) approach using miR-106 inhibitors was carried out. The results showed that inhibiting miR-106 expression completely abolished the anti-inflammatory effects of propofol, resulting in a marked increase in the expression levels of TIR domain-containing adaptor molecule 1 (Ticam1), myeloid differentiation primary response 88 (Myd88), interferon regulatory factor 3 (Irf3), and nuclear factor kappa B (NF-κB) transcripts [29]. These transcripts serve as key inflammatory indicators for assessing how propofol suppresses activation of the inflammatory signaling cascade—particularly the TLR-NF-κB axis—via miR-106 in the microglia [29]. This indicates that propofol can effectively inhibit the production of inflammatory mediators regulated via the NF-κB pathway [29]. To study the role of PI3k/Akt as the downstream pathway mediated by miR-106, an experiment using a PI3k/Akt pathway-specific agonist, 740Y-P, and antagonist, Wortmannin, was performed using Western blot. The levels of p-Akt were significantly upregulated when the cells were treated with 740Y-P and downregulated when the cells were treated with Wortmannin. After an upregulation in p-Akt by 740Y-P, the miR-106b-mediated inhibition of TNF and Nos2 was significantly reduced, indicating PI3k/Akt signaling as a key part of the miR-106b/PI3k/Akt axis [29].
2.3. Inhibition of Nicotinamide Adenine Dinucleotide Phosphate Oxidase
Inhibition of Nicotinamide Adenine Dinucleotide Phosphate (NADPH) oxidase (NOX) in overactivated microglia ameliorates inflammation [30]. NOX is a membrane-bound protein complex composed of multiple subunits, all of which play important functions in phagocytic cells [31]. In phagocytic cells such as the microglia, NOX takes part in the production of extracellular and intracellular reactive oxygen species (ROS) [31]. The complex of NOX is composed of cytoplasmic (p47phox, p67phox, p40phox, and Rac2) and membrane-bound (gp91Phox and P22 Phox) subunits [31]. When phosphorylated by particular kinases, the cytoplasmic subunits form a complex, translocating to the membrane to dock with the membrane subunits, contributing to the production of superoxide anions, which are precursors for ROS [31,32]. ROS produced by NOX regulate intracellular signaling and are also responsible for cell damage, such as neuronal damage in pathological conditions [33]. After a brain injury, such as a stroke, microglia start an uncontrolled massive production of ROS and proinflammatory cytokines, leading to neuroinflammation [34]. Upon ischemic stroke, NOX can be overactivated, leading to the excessive production of ROS, such as superoxide anion (O2−) and hydrogen peroxide (H2O2). This causes damage to the proteins, lipids, and nucleic acids of neural cells, and eventually contributes to their death [35,36]. In another study, in order to detect the anti-inflammatory effects of propofol by NOX inhibition, Luo et al. measured the enzymatic activity of NOX in LPS-stimulated BV2 microglia with or without propofol pretreatment [36]. The results showed that pretreatment with propofol significantly decreased NOX activity. The decrease in enzymatic NOX activity is due to the inhibition in the expression of the gp91Phox and P22 Phox subunits, as shown by Western blot. Therefore, reduced assembly between the cytoplasmic and membrane subunits resulted in an inhibition of the production of proinflammatory factors in microglia [36]. In a parallel experiment, the authors found that the downregulation of gp91Phox and P22 Phox expression by propofol is dose-dependent [36]. Furthermore, to assess the individual roles of the gp91Phox and p22Phox subunits, the authors used siRNA-mediated silencing of the corresponding messenger RNAs in BV2 microglia and found that silencing p22phox reduced the anti-inflammatory effects of propofol, making it less effective at lowering nitric oxide and TNF production, whereas silencing gp91phox did not significantly alter propofol’s anti-inflammatory effects [36]. All together, these results illustrate the anti-inflammatory effects of propofol by microglia inactivation via the inhibition of NOX [36].
2.4. Blocking and Downregulation of Toll-like Receptor 4 Expression
Toll-like receptor 4 (TLR4) is a pattern recognition receptor (PRR) that, upon binding to PAMPs or DAMPs, leads to immune responses by producing proinflammatory and anti-inflammatory cytokines following the activation of various types of signaling cascades [37,38,39]. After a stroke, the injured cells produce DAMPs that can be detected by TLR4s presented on microglia, leading to the production of proinflammatory cytokines. The uncontrolled release of inflammatory cytokines can worsen ischemic brain injury [40,41]. Following recognition of either PAMPs or DAMPs, TLR4 utilizes its Toll/interleukin-1 receptor (TIR) domain to recruit key adaptor proteins—including MyD88, MyD88-adapter-like protein (MAL, also known as Toll/Interleukin-1 receptor domain-containing adaptor protein, TIRAP), TIR-domain-containing adapter-inducing interferon-β (TRIF, also known as TIR domain-containing adaptor molecule-1, TICAM-1), and TRIF-related adaptor molecule (TRAM, also known as TIR domain-containing adaptor molecule-2, TICAM-2)—thereby inducing the downstream activation of transcription factors NF-κB, AP-1, and IRFs, contributing to the production of a variety of proinflammatory cytokines and finally inflammation [42,43,44,45]. According to some studies, propofol has been shown to play a key role in mediating inflammation by downregulating TLR4 [46,47]. In an MCAO model, propofol reduced TLR4 expression in microglia, resulting in a significant decrease in the mRNA expression of the proinflammatory cytokines IL-6, IL-β, and TNF [14]. Hence, downregulation of TLR4 in microglia, supressed proinflammatory cytokine production, and reduced infarct volume together contribute to a significant attenuation in brain injury in ischemic stroke [14]. This significant decrease in proinflammatory cytokine production was not observed in TLR4 knockout mice, demonstrating the anti-inflammatory effect of propofol in microglia through the downregulation of TLR4 [14].
In one study, the protective effect of propofol on OGD/OGD/R BV2 microglia via inhibiting TLR4/MyD88/NF-κB pathway was investigated [48]. Once microglial TLR4 binds to DAMPs, it can sequentially recruit MyD88, the interleukin-1 (IL-1) receptor-associated kinase, TNF receptor-associated factor 6 (TRAF6), and transforming growth factor-beta-activated kinase 1 (TAK1), resulting in the activation of the IkappaB kinase (IKK) complex [48]. When the IKK complex is activated, it phosphorylates IkappaB-α at serine residues 32 and 36, triggering its degradation. NF-κB is then released, translocated to the nucleus, and finally induces the transcription of kappaB-dependent genes, such as IL-1, IL-6, and TNF-α [48]. According to the Western blotting analysis obtained by Qin et al., an increase in the expression of TLR4 and MyD88 protein levels was observed when BV2 cells were exposed to OGD/R [48]. However, the TLR4 and MyD88 protein levels dramatically declined when the BV2 cells had been pretreated with propofol [48]. Furthermore, in order to further investigate the downstream pathway of TLR4-mediated signal transduction, the levels of IkappaB-α phosphorylation and IkappaB-α were measured with and without propofol treatment by Western blotting analysis [48]. The results showed that propofol significantly decreased the phosphorylation of IkappaB-α for BV2 cells under OGD/R conditions. Also, the NF-κB protein levels increased in the nucleus of OGD/R BV2 cells, whereas this was significantly reversed upon propofol pretreatment of OGD/R BV2 cells [48]. This corresponded with propofol markedly reducing TNF release from the OGD/R BV2 cells, as confirmed using ELISA.
In the same study, Qin et al. suggested that the inactivation of glycogen synthase kinase-3β (GSK-3β) can be another protective effect of propofol during stroke. Essentially, GSK-3β is a constitutively active serine/threonine kinase that becomes inactivated in TLR4-stimulated cells through the PI3K pathway by phosphorylation at the regulatory serine residue of position 9 (Ser9), resulting in a decrease in the proinflammatory cytokines and an increase in the anti-inflammatory cytokine IL-10 [49,50]. GSK-3β and p-GSK-3β protein expression in LPS-stimulated BV-2 microglia cells was detected by Western blotting, showing an increase of 3.2 fold in the ratio of p-GSK-3β to total GSK-3β. Pretreatment with propofol further increased the ratio by 1.4 fold. Thus, in TLR4-stimulated microglia, propofol can help enhance the phosphorylation of GSK-3β while also downregulating TLR4, resulting in a decrease in the proinflammatory cytokines and an increase in the anti-inflammatory cytokine IL-10 [50,51].
2.5. Downregulation of Connexin 43
Promoting a significant downregulation of Connexin 43 (Cx43) in microglia is another unique protective effect displayed by propofol during stroke [52,53]. Cx43 is a vertebrate protein, forming gap junction channels that conduct direct signaling between the cytoplasmic compartments of adjacent cells [54]. Moreover, Cx43 is also capable of forming hemichannels, which enable the release of factors and molecules such as ATP, glutamate, ions (like Ca2+), and other small proinflammatory or neurotoxic molecules into the extracellular medium [55,56,57]. Connexin 43 can be phosphorylated at various sites, which affects its assembly and function in different ways [58]. Upon ischemia, microglia is activated by proinflammatory cytokines, such as TNF and INF-gamma, increasing Cx43 expression [55,59]. Activated microglia migrate to compromised neurons, physically exchanging ions, second messengers, and small molecules throughout gap junction channels formed by Cx43, to promote proinflammatory and neurodegenerative cellular functions such as apoptosis in either microglia or neurons [52,59,60,61]. Furthermore, the debris and proinflammatory cytokines produced by the cells that underwent apoptosis can further promote an overexpression of Cx43 in microglia, contributing to abnormal and massive apoptosis signals that may even kill healthy cells [55,62]. In a study, the anti-inflammatory effects of propofol in stroke through the downregulation of microglial Cx43 was confirmed [52]. In the in vitro model (hypoxia/reoxygenation (H/R) injury), a significant 20% increase in the Iba1+ cells was observed using immunofluorescence staining after H/R injury, indicating an increase in microglial activation [52]. An increase in the level of proinflammatory cytokines—such as IL-1β, IL-6, and TNF—and a decrease in the level of anti-inflammatory cytokines—such as IL-10—was also observed [52]. Importantly, according to the Western blotting results, upon H/R exposure, an increase in the level of Cx43 and phosphorylated Cx43 (p-Cx43) in microglia was observed [52]. Moreover, using TUNEL assay, a dramatic increase in apoptosis among microglia cells was observed [52]. Furthermore, in an ex vivo model designed to study the role of H/R-injured microglia on neurons, rat primary neurons were cultured in the supernatant of H/R-injured microglia (microglia-conditioned supernatant, MCS) for 24 h. The results obtained by MTT assay showed that the viability of the neurons decreased slightly due to H/R injury, but a significant decrease was observed after culture with MCS [52]. Using 40 μM propofol treatment, microglial viability improved, and Iba1, Cx43, Cx43, and p-Cx43 expression decreased, illustrating that propofol feasibly attenuates the expression and phosphorylation of Cx43 and decreases microglia activation [52]. Moreover, a noticeable decrease in the proinflammatory cytokines such as IL-1β, IL-6, and TNF, along with an increase in the anti-inflammatory cytokines such as IL-10 was observed, meaning propofol treatment could significantly protect the microglial cells from the effects of H/R injury [52]. Thus, the results confirmed that propofol has anti-inflammatory effects against I/R injury via the downregulation of Cx43 in microglial cells, thereby decreasing neuronal apoptosis [52]. In order to investigate the role of Cx43 in propofol rescue on H/R-induced neuronal impairment, they knocked down microglial Cx43. The silencing of Cx43 combined with propofol treatment resulted in a significant reduction in microtubule-associated protein 2 (Map2) expression and a higher morphological recovery among neurons treated with H/R-exposed MCS, compared with propofol treatment without Cx43 knock down [52]. Also, upon Cx43 knock down and propofol treatment, a more dramatic increase in the viability of microglial cells was also observed, along with a decrease and an increase in proinflammatory and anti-inflammatory cytokines, respectively, when compared to the H/R-injured cells. Finally, they studied the protective effect of propofol in I/R injury in an in vivo middle cerebral artery occlusion (MCAO) rat model of stroke [52]. The results were consistent with the data obtained from in vitro results, as MCAO animals with propofol treatment and the downregulation of Cx43 showed a significant decrease in cerebral infarct volume and neuronal apoptosis [52]. Through an overexpression of Cx43, the I/R injury conditions deteriorated, although propofol could still slightly attenuate cell death and microglial activation via downregulation of Cx43 expression [52].
2.6. JAK1/STAT3 Pathway Activation
The activation of specific Janus kinase-signal transducers and activators of transcription (JAK/STAT) signaling pathway such as JAK1/STAT3, by propofol has been reported to be neuroprotective in stroke [63,64]. The JAK/STAT pathway is engaged in both the immune response of a variety of cytokines and the actions of non-immune mediators such as growth factors and hormones [65]. In fact, the JAK family consists of four members (JAK1, JAK2, JAK3, and tyrosine kinase 2 (TYK2)) and the STAT family consist of seven members (STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b, and STAT6) [66]. JAK members all have different receptor affinities that are constitutively associated with their cytoplasmic portions [66,67]. When a specific ligand binds to its receptor, an active receptor complex is assembled, in which its cytoplasmic portion phosphorylates the receptor-associated JAKs. In turn, the phosphorylation of JAKs provides docking sites for STATs that as a result become phosphorylated on their tyrosine and serine residues [65,68]. Then, phosphorylated STATs are released from the receptor complex, form dimers, translocate to the nucleus to bind to the promoter regions of specific target genes, leading to the regulation of the transcription of these genes [65,67,68]. Among the JAK/STAT family members, activation of the JAK1/STAT3 pathway has shown anti-inflammatory effects in stroke [63,69]. Basically, the phosphorylation of JAK1 can regulate STAT3 activity via phosphorylating STAT3 on the tyrosine705 and serine727 residues, in which reduced phosphorylation at Ser727 residue is usually correlated with an increase in the phosphorylation of Tyr705 residue [63,70,71]. In a previous study, CoCl2-induced, hypoxia-injured BV2 cells were used to establish an in vitro hypoxia model to evaluate the protective effects of propofol in ischemic/hypoxic stroke [63]. According to the results obtained by Western blot, CoCl2 treatment decreased the expression and phosphorylation of JAK1 [63]. Moreover, CoCl2 treatment reduced the phosphorylation of STAT3 at Tyr-705, with no effect on the phosphorylation of STAT3 at Ser-727 [63]. However, propofol pretreatment diminished these CoCl2-modulated effects in CoCl2-induced hypoxic injured BV2 cells [63]. Additionally, in order to further investigate the anti-inflammatory effects of propofol via the activation of the JAK1/STAT3 pathway, Lu et al. pretreated BV2 cells with propofol and selective JAK1 inhibitor INCB039110, followed by CoCl2 treatment [63]. According to the results, INCB039110 abolished the effect of propofol, leading to an increase in the production of TNF [63]. Although it is evident that propofol’s anti-inflammatory effect in stroke involves activation of the JAK1/STAT3 pathway through increased JAK1 phosphorylation and the subsequent phosphorylation of STAT3 at Tyr-705, the exact molecular mechanisms by which propofol initiates or regulates this pathway remain unclear [63].
Furthermore, some studies suggest that STAT3 can function as both a proinflammatory and an anti-inflammatory factor, depending on its activation context and phosphorylation status. In the anti-inflammatory context, STAT3 promotes M2 microglial polarization, which is associated with tissue repair and anti-inflammatory responses. However, under certain conditions, STAT3 activation can also support proinflammatory processes, especially if the degree or pattern of phosphorylation is altered, for example, aberrant or excessive activation may promote inflammatory gene expression [72,73]. So, while the JAK/STAT3 pathway is involved in promoting M2 polarization and anti-inflammatory effects, the pathway itself is versatile because STAT3’s effects are context-dependent and can shift toward either anti-inflammatory or proinflammatory outcomes, depending on how precisely it is regulated [72,73].
2.7. miR-155/SOCS1 Pathway
Some anti-inflammatory effects of propofol have been associated with the downregulation of specific miRNAs such as miR-155 [74]—which is expressed upon the activation of TLR4 and represents one of the most important miRNAs regulating central nervous system (CNS) inflammatory responses—and is crucial for the robust induction of some proinflammatory cytokine genes such as IL-6 and TNF in microglia [75,76,77]. At the same time, upon microglia activation by LPS through TLR4, some negative feedback loops—such as the suppression of cytokine signaling-1 (SOCS1) expression—are initiated in order to prevent hyperresponsiveness and develop endotoxin tolerance [78]. It has been reported that miR-155 proinflammatory function comes by downregulating SOCS1 in microglia [79,80,81]. SOCS1 is a protein that inhibits cytokine signal translation by the direct inhibition of JAK/STAT activation, leading to an inhibition in the production of cytokines [79,82,83]. Thus, miR-155 post-transcriptionally regulates SOCS1 by targeting and degrading SOCS1 mRNA [79]. In another study, LPS-activated BV2 microglia were used in order to investigate the anti-inflammatory effects of propofol via the inhibition of miR-155/SOCS1 [74]. According to the results obtained by PCR assay, treating the BV2 cells with an increasing concentration of LPS contributed to a significant dose-dependant expression of miR-155 [74]. However, when the LPS-activated BV2 microglial were treated with propofol, a significant decrease in miR-155 expression was observed. To further evaluate the role of miR-155 in the anti-inflammatory effect of propofol, an miR-155 inhibitor was used to knock down miR-155. According to the results, in un-transfected BV2 cells, the production of proinflammatory mediators and cytokines such as NO, TNF, and IL-6 significantly decreased by propofol [74]. However, in the miR-155 knockdown BV2 cells, LPS induction of the proinflammatory cytokines were less robust, and propofol had very little effect on the production of these cytokines, indicating the critical role of miR-155 in the anti-inflammatory effect of propofol [74]. Moreover, the protein expression of SOCS1 in different treatments was measured [74]. In the cells transfected with a negative control inhibitor—a non-targeting inhibitor used as a baseline reference to compare against the specific inhibition of miR-155—the protein expression of SOCS1 was slightly increased upon LPS treatment [74]. However, the SOCS1 protein expression was significantly higher in the presence of propofol and LPS than LPS alone, confirming that propofol can upregulate SOCS1 [74]. Although propofol dramatically increased SOCS1 in negative control inhibitor-transfected cells upon LPS stimulation, it failed to induce SOCS1 in miR-155 knockdown cells [74]. Furthermore, in order to confirm the SOCS1 role in anti-inflammatory effects of propofol, the nitrite and cytokine levels in SOCS1downregulated microglial cells were measured [74]. BV2 microglial cells were transfected with either SOCS1 or control siRNA [74]. According to the results, in SOCS1 knockdown cells, the production levels of NO, TNF, and IL-6 were significantly increased in response to LPS compared to the control siRNA [74]. However, propofol markedly reduced the LPS-induced production of NO, TNF, and IL-6 in the control siRNA-treated cells—but not as significantly in SOCS1 knockdown cells—indicating that SOCS1 plays an essential role in the anti-inflammatory effects of propofol [74]. Overall, it can be concluded that the anti-inflammatory effects of propofol through the miR-155/SOCS1 pathway result from downregulating miR-155 and, consequently, upregulating SOCS1 expression—altogether contributing to a significant decrease in the production of proinflammatory mediators [74].
2.8. miR-221/222-IRF2 Pathway
The miR-221/222-IRF2 axis is considered as an anti-inflammatory pathway activated by propofol [84]. The miR-221/222 gene cluster is located on chromosome Xp11.3 [85,86]. The promoter region of miR-221/222 contains two canonical TATA boxes located 550 and 190 base pairs upstream of pre-miR-222, and three poly-A sequences downstream of pre-miR-221 [86,87]. Angiotensin II regulates the expression of this gene cluster along with a repressive complex, including estrogen receptor α and the nuclear receptors NCOR1 and NCOR2 [88,89]. miR-221 and miR-222 are encoded and transcribed together as pri-miR, with the two paralogous miRs separated by 726 bp and sharing the same seed nucleotide sequence [86,90]. Throughout nuclear processing mediated by Drosha and RNA-binding protein DiGeorge syndrome critical region gene 8 (DGCR8), the pri-miR transcription generate 110-nucleotide pre-miR-221 and pre-miR-222 [86]. The other component of the miR-221/222-IRF2 pathway, interferon regulatory factor 2 (IRF2), acts as a transcriptional repressor by binding to the same DNA sequence as IRF1—a pro-inflammatory transcription factor—does [91,92]. IRF2 exerts its transcriptional suppression effect by interacting with co-repressors, including IRF2 binding protein 2 (IRF2BP2), resulting in the activation of anti-inflammatory (M2) marker genes and the suppression of pro-inflammatory (M1) marker genes [91,93]. Some studies have shown that miR-221/222 reduces IRF2 protein levels by inhibiting IRF2 translation, thereby contributing to inflammation [91,93]. However, other studies have shown that propofol counteracts miR-221/222 function, which in turn increases IRF2 protein levels, leading to a decrease in the level of proinflammatory cytokines [84]. This study reported that the LPS-induced upregulation of miR-221/222 was markedly abolished by propofol treatment [84]. In order to investigate the role of miR-221/222 in microglia activation, they overexpressed miR-221/222 in BV2 cells by transfecting them with miR-221 and miR-222 mimics [84]. The results revealed a significant increase in the production of IL-1β, IL-6, and TNF-α in the cell culture supernatants [84]. However, when they silenced miR-221/222 expression in the LPS-primed BV2 cells via miR-221 and miR-222 inhibitors, they observed a dramatic decrease in the inflammatory cytokine levels, indicating the regulatory role of miR-221/222 in microglia activation [84]. In addition, using three publicly available algorithms (TargetScan, miRDB, and miRBase) it was found that IRF gene was the target of miR-221/222. In fact, ectopic expression in miR-221 or miR-222 caused a reduction in IRF2 protein expression in BV2 cells [84]. Finally, it was examined whether the miR-221/222-IRF2 axis mediates the anti-inflammatory role of propofol in microglia activation. Xiao et al. upregulated miR-221/222 in LPS-stimulated BV2 cells using propofol treatment [84]. The results showed a significant decrease in the expression of inflammatory genes (Il1b, IL6, Tnf, Ptgs2, and Nos2) and hence the level of cytokines due to propofol treatment, which could be restored by either miR-221 or miR-222 mimics [84]. Moreover, the genetically silenced IRF2 in LPS-primed BV2 cells with propofol treatment confirmed propofol’s failure to induce an inhibitory effect [84]. All these findings indicate that the miR-221/222–IRF2 axis is an essential functional mediator of propofol in suppressing microglia activation [84].
2.9. NF-κB/Hif-1α Signaling Pathway
Inhibition of the NF-κB/Hif-1α signaling pathway represents another mechanism by which propofol exerts its anti-inflammatory effects in neuroinflammation [94]. The NF-κB family of transcription factors include five members—p50, p52, p65 (also known as RelA), c-Rel, and RelB—that exist as either hetero- or homo-dimeric complexes [95,96]. These subunits are inactivated in the cytoplasm by the members of the IkB family [95]. Upon activation by certain compounds such as TNF, a kinase signaling cascade is induced, resulting in the IKK-mediated phosphorylation of IkB and its subsequent poly-ubiquitin-mediated proteasomal degradation. This contributes to the release of NF-kB, which translocates into the nucleus and binds to target gene promoters and enhancers, contributing to the production of a variety of proinflammatory cytokines [95,97,98,99]. Importantly, it has been reported that NF-κB subunit p65 plays a key role in the production of Hif-1α mRNA and protein [100,101]. Hypoxia inducible factor-1α (Hif-1α) is a subunit of HIF-1, a transcription factor that is initiated under low-oxygen conditions [95,102]. In fact, the stability of the Hif-1α subunit is improved during hypoxia, which causes Hif-1α to upregulate the transcription of proinflammatory genes, such as cytokines [103,104]. According to many studies, NF-kB plays a key role as the transcriptional activator of Hif-1α; in the absence of NF-kB, the Hif-1α gene is not transcribed, even during a lengthened hypoxia [101,105,106]. Propofol can supress the expression of Hif-1α by downregulating NF-κB p65 in CoCl2 hypoxic-induced BV2 cells, thereby inhibiting the production of proinflammatory cytokines TNF, IL-1β, and IL-6 [94]. In this study, in order to examine the anti-inflammatory effects of propofol via inhibiting the NF-κB/Hif-1α pathway, the authors measured the levels of the two proteins—NF-κB p65 and Hif-1α—that propofol potentially affects [94]. The cells were pretreated with propofol about 3 h prior to CoCl2 stimulation for 24 h, and then the levels of NF-κB p65 and Hif-1α production were measured by Western blotting [94]. The results revealed that propofol dramatically reduced the production of NF-κB p65 and Hif-1α compared to the CoCl2-treated group [94]. In order to further investigate the role of NF-κB in propofol-related anti-inflammatory mechanisms, the authors treated BV2 cells with siRNA against NF-κB p65, followed by exposure to hypoxia and incubation for 24 h [94]. According to the results, in NF-κB p65-silenced and CoCl2-treated cells, the levels of Hif-1α and IL-1β were downregulated compared to those in only CoCl2-treated cells [94]. Thus, propofol inhibits the upregulation of NF-κB p65, which in turn suppresses Hif-1α production, resulting in a decrease in the hypoxia-induced inflammation in BV2 cells [94].
2.10. Extracellular Vesicle Release
More recently, another anti-inflammatory mechanism of propofol has been reported, i.e., the inhibition of extracellular vesicle release [107]. Extracellular vesicles (EVs) are membrane-enclosed cargos—including exosomes and microvesicles—which are key players in intercellular signaling [108,109]. Upon brain damage, microglial cells release these vesicles to secrete proinflammatory cytokines, leading to inflammation [108,110,111]. EV release by microglia is generally stimulated by exposure to immune activators such as ROS, ATP, LPS, and TNF [107,111,112]. In a study performed using LPS-stimulated BV2 cells, the anti-inflammatory effects of propofol through the downregulation of immune-activated EV release was confirmed [107]. In this study, using Western blotting, the authors first measured EV release from LPS-stimulated microglia in the presence and absence of propofol by determining the levels of EV markers flotillin-2 and tissue transglutaminase (tTG) in protein lysates from the EV pellets [107]. According to the results, LPS alone significantly increased the protein levels of flotillin-2 and tTG; however, propofol treatment dramatically decreased flotillin-2 and tTG levels, indicating that propofol could decrease EV release from microglia [107]. Furthermore, in order to investigate the role of EVs on propofol-mediated anti-inflammatory response in microglia, the authors examined whether the anti-inflammatory effects of propofol could be reversed by the addition of EVs isolated from immune-activated microglia to the treatment groups, along with LPS and propofol [107]. The results revealed that the downregulation of the M1 marker genes and the upregulation of the M2 marker gene mediated by propofol was completely reversed upon EV treatment, confirming that propofol exerts its anti-inflammatory effects in activated microglia through a reduction in EV release [107]. In addition, to measure the reduction in microglia-mediated neurotoxicity through EV release inhibition by propofol, they collected EVs from LPS-stimulated BV2 cells and added them to the experimental groups [107]. According to the results, EV treatment did not markedly influence microglia-mediated neurotoxicity toward N2A cells in the absence of LPS [107]. However, EV treatment dramatically reversed propofol-mediated neuroprotection by LPS-stimulated microglia [107]. Finally, MAP2 ELISA was used in order to more specifically target MAP2-positive neurons in N2A cultures and quantitatively determine neuronal survival upon LPS and propofol treatment [107]. Propofol reversed the neurotoxicity caused by LPS-stimulated microglia in conditioned medium, although such protection was abolished by the addition of EVs to microglia cultures before collection of the conditioned medium [107]. LPS-activated microglia also induced TUNEL-positive cells in the cultures, which is a sign of apoptosis, and this effect was further exacerbated by the addition of EVs to microglia cultures before the collection of the conditioned medium. In summary, the results confirmed that propofol pretreatment decreased activated microglia-mediated neurotoxicity by inhibiting EV release [107].
2.11. Oxidative Stress and Increasing Antioxidant Activity
Mitochondria, as double-membrane subcompartments of cells, are a major source of ROS production, alongside their critical role in ATP production [113,114]. After stroke and IR injury, elevated levels of ROS produced by mitochondrial dysfunction contribute to oxidative stress, resulting in neurodegeneration [113,115,116,117]. Although the causes of mitochondrial ROS production during reperfusion remain unclear, it has been reported that elevated levels of ROS production by mitochondria during hypoxia-induced stroke and IR injury can be due to abnormally high levels of succinate, which is a citric acid cycle intermediate molecule in mitochondria [115,118]. Moreover, the overproduction of ROS during IR injury can also cause a decrease in mitochondrial membrane potential, thereby aggravating mitochondrial dysfunction [94]. In a study performed using CoCl2 hypoxic-induced BV2 cells, the protective effects of propofol through ameliorating oxidative stress and increasing antioxidant activity were confirmed [94]. According to the results, CoCl2 induced higher ROS production in BV2 cells compared to control cells, while propofol decreased ROS levels compared to CoCl2-treated cells [94]. At the same time, CoCl2 decreased superoxide dismutase (SOD) activity, and total antioxidant capacity (T-AOC), whereas propofol ameliorated this reduction [94]. The restoration of antioxidant activity SOD and T-AOC by propofol inhibits ROS overproduction and inflammatory responses [94]. SOD restoration by propofol can inhibit the production of inflammatory responses via the inhibition of NF-κB activation [94,119]. Moreover, ROS reduction by propofol can inhibit Fe2+ to Fe3+ oxidation, which in turn prevents Hif-1α protein stabilization, leading to a downregulation of inflammatory mediators [94]. Propofol also ameliorated the decrease in mitochondrial membrane potential in CoCl2-treated microglia [94].
2.12. Intracellular Ca2+ Homeostasis
An important anti-inflammatory mechanism of propofol in microglia has been reported to be the maintenance of intracellular Ca2+ homeostasis [63]. Many of the microglia’s physiological functions—such as cell proliferation, differentiation, migration, and the induction of intracellular enzymatic pathways involved in the transcriptional regulation of many genes—have been known to be linked to intracellular Ca2+ signaling [120,121]. According to some studies, an increase in the microglial intracellular Ca2+ concentration is related to the production of proinflammatory cytokines and ROS [122,123]. According to these studies, although an increase in the microglial intracellular Ca2+ concentration is required for the production of inflammatory cytokines, it is not sufficient on its own; it is also necessary to treat the microglia cells with LPS [122,123]. In nervous tissue, CaMKIIα is the major isoform of Ca2+/calmodulin-dependent protein kinase (CaMK), which is highly sensitive to intracellular Ca2+ levels, and its activation is associated with the production of proinflammatory cytokines, such as TNF and IL-1β [63,124,125]. Moreover, ERK 1/2 and NF-κB are also involved in Ca2+-mediated TNF-α release [63]. In a study performed using CoCl2 hypoxic-induced BV2 cells, the anti-inflammatory effects of propofol through maintaining intracellular Ca2+ homeostasis was studied [63]. According to the results, compared to the control, CoCl2 treatment increased the cytoplasmic Ca2+ concentration, along with the phosphorylation of the CAMKIIα, ERK, NF-κB proteins [63]. However, propofol pretreatment attenuated the increase in CoCl2-induced intracellular Ca2+ levels and downregulated the phosphorylation of CAM-KIIα, ERK, and NF-κB [63]. In order to investigate the role of Ca2+ homeostasis and the phosphorylation of CAMKIIα, ERK, and NF-κB in TNF production, BV2 cells were pretreated with calcium chelator BAPTA-AM, CAMKIIα inhibitor KN93, or ERK inhibitor U0126, followed by CoCl2 treatment. The results revealed that BAPTA-AM, KN93, and U0126 dramatically decreased TNF production, similar to the effect of propofol [63]. To confirm the role of the phosphorylation of CAMKIIα, ERK, and NF-κB in the protective effects of propofol against CoCl2 treatment, BV2 cells were pretreated with calcium chelator BAPTA-AM, CAMKIIα inhibitor KN93, or ERK inhibitor U0126, followed by CoCl2 treatment [63]. According to the results, similar to propofol treatment, BAPTA-AM and KN93 significantly decreased the phosphorylation of CAMKIIα [63]. Furthermore, BAPTA-AM, KN93, and U0126 markedly decreased the phosphorylation of ERK and NF-κB, also similar to propofol treatment [63]. In addition, they found that CoCl2 treatment increased apoptosis and the expression of cleaved caspase 3 in the cells; however, these effects were inhibited by propofol treatment, suggesting that apoptosis induced by CoCl2 could be inhibited by propofol [63]. Finally, BV2 cells were pretreated with calcium chelator BAPTA-AM, CAMKIIα inhibitor KN93, or ERK inhibitor U0126, followed by CoCl2 treatment, in order to investigate the role of Ca2+ homeostasis and the phosphorylation of the CAMKIIα, ERK, and NF-κB pathways in cell apoptosis [63]. According to the results, BAPTA, KN93, and U0126 could each reduce the percentage of apoptotic cells [63]. In addition, it was found that BAPTA, KN93, and U0126 could each inhibit the expression of cleaved caspase 3. However, propofol, BAPTA-AM, KN93, and U0126 pretreatment showed no effect on the expression of pro-caspase 3 [63]. Overall, the results revealed that one of the anti-inflammatory mechanisms of propofol is via limiting intracellular cellular Ca2+ overload, modulating the phosphorylation of CaMKIIα, ERK, and NF-κB, resulting in a decrease in the proinflammatory cytokine production and cell apoptosis.
All in all, a comprehensive summary of the experimental models, methodologies, and key findings supporting these mechanistic pathways is presented in Table 1.

Table 1.
Summary of experimental models and outcomes for the anti-inflammatory effects of propofol in microglia via different mechanisms of action.
3. Materials and Methods
A literature search was conducted to identify studies investigating the immunomodulatory and neuroprotective effects of propofol, with a focus on microglial activation and ischemic stroke. The search was performed using PubMed, employing the following keywords: “propofol,” “microglia,” “neuroinflammation,” “ischemic stroke”. Studies were included if they were original research articles published in English, investigated propofol’s effects on microglia or inflammation-related mechanisms, and used in vitro, in vivo, or ex vivo experimental models relevant to central nervous system inflammation. Exclusion criteria included studies not involving microglia or not focused on inflammation-related pathways, and articles without full-text access.
4. Conclusions
Ischemic stroke remains a major global health challenge, with neuroinflammation playing a critical role in secondary brain injury. Microglia, as central mediators of the inflammatory response, represent a crucial therapeutic target for limiting neuronal damage and promoting recovery. Propofol, beyond its well-established anesthetic properties, has demonstrated significant anti-inflammatory and neuroprotective effects in experimental models of stroke, primarily through its ability to modulate microglial activation. The evidence summarized in this review highlights the multifaceted ways in which propofol can attenuate inflammation and mitigate ischemic injury.
Numerous studies support the anti-inflammatory and neuroprotective properties of propofol in stroke models; however, a critical comparison reveals several gaps and inconsistencies. For instance, although PI3K/Akt activation is associated with autophagy-mediated neuroprotection, some reports indicate that excessive activation of this pathway may contribute to inflammation, suggesting that the context and degree of activation are crucial in determining its effects. Additionally, many in vitro studies rely on BV2 microglial cell lines or hypoxia mimetics, such as CoCl2, which may not accurately replicate the complexity of in vivo ischemic stroke environments. Few investigations employ standardized dosing protocols or the pharmacokinetic modeling of propofol, and many omit assessments of long-term behavioral or functional outcomes. Moreover, critical biological variables such as sex, age, and stroke severity are often not considered, despite their known influence on inflammatory responses and recovery trajectories. These limitations underscore the need for more rigorous, standardized, and translationally relevant experimental models to better evaluate the therapeutic potential of propofol and guide its clinical application in ischemic stroke.
Although the preclinical findings are promising, clinical studies are essential to validate the translational potential of propofol as an adjunct therapy in stroke management. This includes exploring optimized dosing, timing, and safety in human subjects. Bridging this gap will require rigorously designed clinical trials that incorporate stratification by patient age, sex, and stroke characteristics to determine whether the anti-inflammatory benefits observed in experimental models can be effectively and safely translated into human stroke therapy. Understanding and optimizing the therapeutic application of propofol could offer new avenues for improving outcomes in patients suffering from ischemic stroke.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yuan, Q.; Yuan, Y.; Zheng, Y.; Sheng, R.; Liu, L.; Xie, F.; Tan, J. Anti-Cerebral Ischemia Reperfusion Injury of Polysaccharides: A Review of the Mechanisms. Biomed. Pharmacother. 2021, 137, 111303. [Google Scholar] [CrossRef]
- Salvadori, E.; Papi, G.; Insalata, G.; Rinnoci, V.; Donnini, I.; Martini, M.; Falsini, C.; Hakiki, B.; Romoli, A.; Barbato, C.; et al. Comparison between Ischemic and Hemorrhagic Strokes in Functional Outcome at Discharge from an Intensive Rehabilitation Hospital. Diagnostics 2020, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Lv, J.; Guo, H.; Liu, X. Global, Regional, and National Burden of Ischemic Stroke, 1990–2021: An Analysis of Data from the Global Burden of Disease Study 2021. eClinicalMedicine 2024, 75, 102758. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Q.; Wang, W.; Lin, F.; Wang, S.; Zhao, J. Mesenchymal Stem Cell Therapy for Ischemic Stroke: A Look into Treatment Mechanism and Therapeutic Potential. J. Neurol. 2021, 268, 4095–4107. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, G.; He, S.; Liu, X.; Zhu, L.; Yang, X.; Zhang, Y.; Orgah, J.; Feng, Y.; Wang, X.; et al. Protection against Acute Cerebral Ischemia/Reperfusion Injury by QiShenYiQi via Neuroinflammatory Network Mobilization. Biomed. Pharmacother. 2020, 125, 109945. [Google Scholar] [CrossRef] [PubMed]
- DeLong, J.H.; Ohashi, S.N.; O’Connor, K.C.; Sansing, L.H. Inflammatory Responses After Ischemic Stroke. Semin. Immunopathol. 2022, 44, 625–648. [Google Scholar] [CrossRef]
- Dong, R.; Huang, R.; Wang, J.; Liu, H.; Xu, Z. Effects of Microglial Activation and Polarization on Brain Injury After Stroke. Front. Neurol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; He, D.; Bai, Y. Microglia-Mediated Inflammation and Neurodegenerative Disease. Mol. Neurobiol. 2016, 53, 6709–6715. [Google Scholar] [CrossRef]
- Yenari, M.A.; Xu, L.; Tang, X.N.; Qiao, Y.; Giffard, R.G. Microglia Potentiate Damage to Blood-Brain Barrier Constituents: Improvement by Minocycline in Vivo and in Vitro. Stroke 2006, 37, 1087–1093. [Google Scholar] [CrossRef]
- Cherry, J.D.; Olschowka, J.A.; O’Banion, M.K. Neuroinflammation and M2 Microglia: The Good, the Bad, and the Inflamed. J. Neuroinflammation 2014, 11, 98. [Google Scholar] [CrossRef]
- Yu, H.; Wang, X.; Kang, F.; Chen, Z.; Meng, Y.; Dai, M. Propofol Attenuates Inflammatory Damage on Neurons Following Cerebral Infarction by Inhibiting Excessive Activation of Microglia. Int. J. Mol. Med. 2019, 43, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Ye, J.-S.; Wang, Y.-L.; Chen, C.; Wang, C.-Y. Posttreatment with Propofol Attenuates Lipopolysaccharide-Induced up-Regulation of Inflammatory Molecules in Primary Microglia. Inflamm. Res. 2014, 63, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Yang, Z.; Tang, X.; Tan, Y.; Wu, X.; Liu, F. Propofol Protects Against Focal Cerebral Ischemia via Inhibition of Microglia-Mediated Proinflammatory Cytokines in a Rat Model of Experimental Stroke. PLoS ONE 2013, 8, e82729. [Google Scholar] [CrossRef]
- Mitsui, K.; Kotoda, M.; Hishiyama, S.; Takamino, A.; Morikawa, S.; Ishiyama, T.; Matsukawa, T. Propofol Ameliorates Ischemic Brain Injury by Blocking TLR4 Pathway in Mice. Transl Neurosci 2022, 13, 246–254. [Google Scholar] [CrossRef]
- Sigel, E.; Steinmann, M.E. Structure, Function, and Modulation of GABAA Receptors. J. Biol. Chem. 2012, 287, 40224. [Google Scholar] [CrossRef] [PubMed]
- Trapani, G.; Altomare, C.; Liso, G.; Sanna, E.; Biggio, G. Propofol in Anesthesia. Mechanism of Action, Structure-Activity Relationships, and Drug Delivery. Curr. Med. Chem. 2000, 7, 249–271. [Google Scholar] [CrossRef]
- Nelson, L.E.; Guo, T.Z.; Lu, J.; Saper, C.B.; Franks, N.P.; Maze, M. The Sedative Component of Anesthesia Is Mediated by GABAA Receptors in an Endogenous Sleep Pathway. Nat. Neurosci. 2002, 5, 979. [Google Scholar] [CrossRef]
- Buggy, D.J.; Nicol, B.; Rowbotham, D.J.; Lambert, D.G. Effects of Intravenous Anesthetic Agents on Glutamate Release: A Role for GABAA Receptor-Mediated Inhibition. Anesthesiology 2000, 92, 1067–1073. [Google Scholar] [CrossRef]
- Rehberg, B.; Duch, D.S. Suppression of Central Nervous System Sodium Channels by Propofol. Anesthesiology 1999, 91, 512–520. [Google Scholar] [CrossRef]
- Kotani, Y.; Shimazawa, M.; Yoshimura, S.; Iwama, T.; Hara, H. The Experimental and Clinical Pharmacology of Propofol, an Anesthetic Agent with Neuroprotective Properties. CNS Neurosci. Ther. 2008, 14, 95. [Google Scholar] [CrossRef]
- Mori, H.; Mishina, M. Structure and Function of the NMDA Receptor Channel. Neuropharmacology 1995, 34, 1219–1237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, D.; Zhang, B.; Zhu, J.; Zhou, Z.; Cui, L. Regulation of Microglia by Glutamate and Its Signal Pathway in Neurodegenerative Diseases. Drug Discov. Today 2020, 25, 1074–1085. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhao, Y.; Chen, X.; Zhu, M.; Miao, C. Propofol Attenuates BV2 Microglia Inflammation via NMDA Receptor Inhibition. Can. J. Physiol. Pharmacol. 2018, 96, 241–248. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Z. Protective Effects of Propofol on Rats with Cerebral Ischemia–Reperfusion Injury Via the PI3K/Akt Pathway. J. Mol. Neurosci. 2021, 71, 810–820. [Google Scholar] [CrossRef]
- Tewari, D.; Patni, P.; Bishayee, A.; Sah, A.N.; Bishayee, A. Natural Products Targeting the PI3K-Akt-mTOR Signaling Pathway in Cancer: A Novel Therapeutic Strategy. Semin. Cancer Biol. 2022, 80, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kirkin, V.; Rogov, V.V. A Diversity of Selective Autophagy Receptors Determines the Specificity of the Autophagy Pathway. Mol. Cell 2019, 76, 268–285. [Google Scholar] [CrossRef]
- Luo, J.; Huang, B.; Zhang, Z.; Liu, M.; Luo, T. Delayed Treatment of Propofol Inhibits Lipopolysaccharide-Induced Inflammation in Microglia through the PI3K/PKB Pathway. NeuroReport 2018, 29, 839–845. [Google Scholar] [CrossRef]
- Wang, P.; Shao, B.-Z.; Deng, Z.; Chen, S.; Yue, Z.; Miao, C.-Y. Autophagy in Ischemic Stroke. Prog. Neurobiol. 2018, 163–164, 98–117. [Google Scholar] [CrossRef]
- Liu, J.; Ai, P.; Sun, Y.; Yang, X.; Li, C.; Liu, Y.; Xia, X.; Zheng, J.C. Propofol Inhibits Microglial Activation via miR-106b/Pi3k/Akt Axis. Front. Cell. Neurosci. 2021, 15. [Google Scholar] [CrossRef]
- Guan, S.; Sun, L.; Wang, X.; Huang, X.; Luo, T. Propofol Inhibits Neuroinflammation and Metabolic Reprogramming in Microglia in Vitro and in Vivo. Front. Pharmacol. 2023, 14. [Google Scholar] [CrossRef]
- Choi, S.-H.; Aid, S.; Kim, H.-W.; Jackson, S.H.; Bosetti, F. Inhibition of NADPH Oxidase Promotes Alternative and Anti-Inflammatory Microglial Activation during Neuroinflammation. J. Neurochem. 2012, 120, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Kim, G.S.; Okami, N.; Narasimhan, P.; Chan, P.H. NADPH Oxidase Is Involved in Post-Ischemic Brain Inflammation. Neurobiol. Dis. 2011, 42, 341–348. [Google Scholar] [CrossRef]
- Chéret, C.; Gervais, A.; Lelli, A.; Colin, C.; Amar, L.; Ravassard, P.; Mallet, J.; Cumano, A.; Krause, K.-H.; Mallat, M. Neurotoxic Activation of Microglia Is Promoted by a Nox1-Dependent NADPH Oxidase. J. Neurosci. 2008, 28, 12039–12051. [Google Scholar] [CrossRef]
- Harting, M.T.; Jimenez, F.; Adams, S.D.; Mercer, D.W.; Cox, C.S. Acute, Regional Inflammatory Response after Traumatic Brain Injury: Implications for Cellular Therapy. Surgery 2008, 144, 803–813. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, X.; Yang, X.; Zhou, Z.; Lu, D.; Tang, Y.; Ling, Z.; Zhou, L.; Feng, X. Propofol Protects Against H2O2-Induced Oxidative Injury in Differentiated PC12 Cells via Inhibition of Ca2+-Dependent NADPH Oxidase. Cell Mol. Neurobiol. 2016, 36, 541–551. [Google Scholar] [CrossRef]
- Luo, T.; Wu, J.; Kabadi, S.V.; Sabirzhanov, B.; Guanciale, K.; Hanscom, M.; Faden, J.; Cardiff, K.; Bengson, C.J.; Faden, A.I. Propofol Limits Microglial Activation after Experimental Brain Trauma through Inhibition of Nicotinamide Adenine Dinucleotide Phosphate Oxidase. Anesthesiology 2013, 119, 1370–1388. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Lizarbe, S.; Pascual, M.; Guerri, C. Critical Role of TLR4 Response in the Activation of Microglia Induced by Ethanol1. J. Immunol. 2009, 183, 4733–4744. [Google Scholar] [CrossRef] [PubMed]
- Piccinini, A.M.; Midwood, K.S. DAMPening Inflammation by Modulating TLR Signalling. Mediat. Inflamm. 2010, 2010, 672395. [Google Scholar] [CrossRef]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 Trafficking and Its Influence on LPS-Induced pro-Inflammatory Signaling. Cell. Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef]
- Marsh, B.J.; Williams-Karnesky, R.L.; Stenzel-Poore, M.P. Toll-like Receptor Signaling in Endogenous Neuroprotection and Stroke. Neuroscience 2009, 158, 1007–1020. [Google Scholar] [CrossRef]
- Andresen, L.; Theodorou, K.; Grünewald, S.; Czech-Zechmeister, B.; Könnecke, B.; Lühder, F.; Trendelenburg, G. Evaluation of the Therapeutic Potential of Anti-TLR4-Antibody MTS510 in Experimental Stroke and Significance of Different Routes of Application. PLoS ONE 2016, 11, e0148428. [Google Scholar] [CrossRef] [PubMed]
- Ve, T.; Vajjhala, P.R.; Hedger, A.; Croll, T.; DiMaio, F.; Horsefield, S.; Yu, X.; Lavrencic, P.; Hassan, Z.; Morgan, G.P.; et al. Structural Basis of TIR-Domain-Assembly Formation in MAL- and MyD88-Dependent TLR4 Signaling. Nat. Struct. Mol. Biol. 2017, 24, 743–751. [Google Scholar] [CrossRef]
- Escoubet-Lozach, L.; Benner, C.; Kaikkonen, M.U.; Lozach, J.; Heinz, S.; Spann, N.J.; Crotti, A.; Stender, J.; Ghisletti, S.; Reichart, D.; et al. Mechanisms Establishing TLR4-Responsive Activation States of Inflammatory Response Genes. PLoS Genet 2011, 7, e1002401. [Google Scholar] [CrossRef] [PubMed]
- Valkov, E.; Stamp, A.; DiMaio, F.; Baker, D.; Verstak, B.; Roversi, P.; Kellie, S.; Sweet, M.J.; Mansell, A.; Gay, N.J.; et al. Crystal Structure of Toll-like Receptor Adaptor MAL/TIRAP Reveals the Molecular Basis for Signal Transduction and Disease Protection. Proc. Natl. Acad. Sci. USA 2011, 108, 14879–14884. [Google Scholar] [CrossRef] [PubMed]
- Gay, N.J.; Symmons, M.F.; Gangloff, M.; Bryant, C.E. Assembly and Localization of Toll-like Receptor Signalling Complexes. Nat. Rev. Immunol. 2014, 14, 546–558. [Google Scholar] [CrossRef]
- Wu, G.-J.; Lin, Y.-W.; Chuang, C.-Y.; Tsai, H.-C.; Chen, R.-M. Liver Nitrosation and Inflammation in Septic Rats Were Suppressed by Propofol via Downregulating TLR4/NF-κB-Mediated iNOS and IL-6 Gene Expressions. Life Sci. 2018, 195, 25–32. [Google Scholar] [CrossRef]
- Ye, H.-H.; Wu, K.-J.; Fei, S.-J.; Zhang, X.-W.; Liu, H.-X.; Zhang, J.-L.; Zhang, Y.-M. Propofol Participates in Gastric Mucosal Protection through Inhibiting the Toll-like Receptor-4/Nuclear Factor Kappa-B Signaling Pathway. Clin. Res. Hepatol. Gastroenterol. 2013, 37, e3–e15. [Google Scholar] [CrossRef]
- Qin, X.; Sun, Z.-Q.; Zhang, X.-W.; Dai, X.-J.; Mao, S.-S.; Zhang, Y.-M. TLR4 Signaling Is Involved in the Protective Effect of Propofol in BV2 Microglia against OGD/Reoxygenation. J. Physiol. Biochem. 2013, 69, 707–718. [Google Scholar] [CrossRef]
- Wang, H.; Garcia, C.A.; Rehani, K.; Cekic, C.; Alard, P.; Kinane, D.F.; Mitchell, T.; Martin, M. IFN-β Production by TLR4-Stimulated Innate Immune Cells Is Negatively Regulated by GSK3-Β1. J. Immunol. 2008, 181, 6797–6802. [Google Scholar] [CrossRef]
- Martin, M.; Rehani, K.; Jope, R.S.; Michalek, S.M. Toll-like Receptor–Mediated Cytokine Production Is Differentially Regulated by Glycogen Synthase Kinase 3. Nat. Immunol. 2005, 6, 777–784. [Google Scholar] [CrossRef]
- Gui, B.; Su, M.; Chen, J.; Jin, L.; Wan, R.; Qian, Y. Neuroprotective Effects of Pretreatment with Propofol in LPS-Induced BV-2 Microglia Cells: Role of TLR4 and GSK-3β. Inflammation 2012, 35, 1632–1640. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, Y.; Xia, Q.; Tu, Z.; Sun, J.; Jing, Q.; Chen, P.; Zhao, X. Propofol Mediated Protection of the Brain From Ischemia/Reperfusion Injury Through the Regulation of Microglial Connexin 43. Front. Cell Dev. Biol. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Wentlandt, K.; Samoilova, M.; Carlen, P.L.; Beheiry, H.E. General Anesthetics Inhibit Gap Junction Communication in Cultured Organotypic Hippocampal Slices. Anesth. Analg. 2006, 102, 1692. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Masaki, K.; Yamasaki, R.; Kawanokuchi, J.; Takeuchi, H.; Matsushita, T.; Suzumura, A.; Kira, J. Th1 Cells Downregulate Connexin 43 Gap Junctions in Astrocytes via Microglial Activation. Sci. Rep. 2016, 6, 38387. [Google Scholar] [CrossRef]
- Yang, Y.; Hang, W.; Li, J.; Liu, T.; Hu, Y.; Fang, F.; Yan, D.; McQuillan, P.M.; Wang, M.; Hu, Z. Effect of General Anesthetic Agents on Microglia. Aging Dis. 2024, 15, 1308–1328. [Google Scholar] [CrossRef]
- Harris, A.L. Connexin Channel Permeability to Cytoplasmic Molecules. Prog. Biophys. Mol. Biol. 2007, 94, 120–143. [Google Scholar] [CrossRef]
- Chever, O.; Lee, C.-Y.; Rouach, N. Astroglial Connexin43 Hemichannels Tune Basal Excitatory Synaptic Transmission. J. Neurosci. 2014, 34, 11228–11232. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Z.-Z.; Chen, N.-H. Connexin 43 Phosphorylation: Implications in Multiple Diseases. Molecules 2023, 28, 4914. [Google Scholar] [CrossRef]
- Eugenín, E.A.; Eckardt, D.; Theis, M.; Willecke, K.; Bennett, M.V.L.; Sáez, J.C. Microglia at Brain Stab Wounds Express Connexin 43 and in Vitro Form Functional Gap Junctions after Treatment with Interferon-γ and Tumor Necrosis Factor-α. Proc. Natl. Acad. Sci. USA 2001, 98, 4190–4195. [Google Scholar] [CrossRef]
- Bruzzone, R.; White, T.W.; Paul, D.L. Connections with Connexins: The Molecular Basis of Direct Intercellular Signaling. Eur. J. Biochem. 1996, 238, 1–27. [Google Scholar] [CrossRef]
- Simon, A.M.; Goodenough, D.A. Diverse Functions of Vertebrate Gap Junctions. Trends Cell Biol. 1998, 8, 477–483. [Google Scholar] [CrossRef]
- Frantseva, M.V.; Kokarovtseva, L.; Velazquez, J.L.P. Ischemia-Induced Brain Damage Depends on Specific Gap-Junctional Coupling. J. Cereb. Blood Flow Metab. 2002, 22, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Gu, Y.; Ding, X.; Wang, J.; Chen, J.; Miao, C. Intracellular Ca2+ Homeostasis and JAK1/STAT3 Pathway Are Involved in the Protective Effect of Propofol on BV2 Microglia against Hypoxia-Induced Inflammation and Apoptosis. PLoS ONE 2017, 12, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Xiao, X.; Han, Y.; Wang, P.; Zang, L.; Wang, L.; Zhao, Y.; Shi, P.; Yang, P.; Guo, C.; et al. Research Progress of Propofol in Alleviating Cerebral Ischemia/Reperfusion Injury. Pharmacol. Rep. 2024. [Google Scholar] [CrossRef]
- Kim, O.S.; Park, E.J.; Joe, E.; Jou, I. JAK-STAT Signaling Mediates Gangliosides-Induced Inflammatory Responses in Brain Microglial Cells. J. Biol. Chem. 2002, 277, 40594–40601. [Google Scholar] [CrossRef]
- Jia, L.; Wang, F.; Gu, X.; Weng, Y.; Sheng, M.; Wang, G.; Li, S.; Du, H.; Yu, W. Propofol Postconditioning Attenuates Hippocampus Ischemia-Reperfusion Injury via Modulating JAK2/STAT3 Pathway in Rats after Autogenous Orthotropic Liver Transplantation. Brain Res. 2017, 1657, 202–207. [Google Scholar] [CrossRef]
- Igaz, P.; Tóth, S.; Falus, A. Biological and Clinical Significance of the JAK-STAT Pathway; Lessons from Knockout Mice. Inflamm. Res. 2001, 50, 435–441. [Google Scholar] [CrossRef]
- Huang, C.; Ma, R.; Sun, S.; Wei, G.; Fang, Y.; Liu, R.; Li, G. JAK2-STAT3 Signaling Pathway Mediates Thrombin-Induced Proinflammatory Actions of Microglia in Vitro. J. Neuroimmunol. 2008, 204, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lin, W.; Zhang, Y.; Lin, L.; Chen, J.; Zeng, Y.; Zheng, M.; Zhuang, Z.; Du, H.; Chen, R.; et al. IL-10 Promotes Neurite Outgrowth and Synapse Formation in Cultured Cortical Neurons after the Oxygen-Glucose Deprivation via JAK1/STAT3 Pathway. Sci. Rep. 2016, 6, 30459. [Google Scholar] [CrossRef]
- Mandal, T.; Bhowmik, A.; Chatterjee, A.; Chatterjee, U.; Chatterjee, S.; Ghosh, M.K. Reduced Phosphorylation of Stat3 at Ser-727 Mediated by Casein Kinase 2 — Protein Phosphatase 2A Enhances Stat3 Tyr-705 Induced Tumorigenic Potential of Glioma Cells. Cell. Signal. 2014, 26, 1725–1734. [Google Scholar] [CrossRef]
- Breit, A.; Besik, V.; Solinski, H.J.; Muehlich, S.; Glas, E.; Yarwood, S.J.; Gudermann, T. Serine-727 Phosphorylation Activates Hypothalamic STAT-3 Independently From Tyrosine-705 Phosphorylation. Mol. Endocrinol. 2015, 29, 445–459. [Google Scholar] [CrossRef]
- Zhong, Y.; Gu, L.; Ye, Y.; Zhu, H.; Pu, B.; Wang, J.; Li, Y.; Qiu, S.; Xiong, X.; Jian, Z. JAK2/STAT3 Axis Intermediates Microglia/Macrophage Polarization During Cerebral Ischemia/Reperfusion Injury. Neuroscience 2022, 496, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Yin, B.; Ye, Y.; Dekhel, O.Y.A.T.; Xiong, X.; Jian, Z.; Gu, L. The Bidirectional Role of the JAK2/STAT3 Signaling Pathway and Related Mechanisms in Cerebral Ischemia-Reperfusion Injury. Exp. Neurol. 2021, 341, 113690. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Huang, H.; Liu, J.; Li, M.; Liu, M.; Luo, T. Propofol Attenuates Inflammatory Response in LPS-Activated Microglia by Regulating the miR-155/SOCS1 Pathway. Inflammation 2018, 41, 11–19. [Google Scholar] [CrossRef]
- Woodbury, M.E.; Freilich, R.W.; Cheng, C.J.; Asai, H.; Ikezu, S.; Boucher, J.D.; Slack, F.; Ikezu, T. miR-155 Is Essential for Inflammation-Induced Hippocampal Neurogenic Dysfunction. J. Neurosci. 2015, 35, 9764–9781. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-H.; Song, M.-F.; Song, H.-D.; Wang, Y.-W.; Luo, M.-J.; Yin, L.-M. miR-155 Mediates Inflammatory Injury of Hippocampal Neuronal Cells via the Activation of Microglia. Mol. Med. Rep. 2019, 19, 2627–2635. [Google Scholar] [CrossRef]
- Gao, Y.; Han, T.; Han, C.; Sun, H.; Yang, X.; Zhang, D.; Ni, X. Propofol Regulates the TLR4/NF-κB Pathway Through miRNA-155 to Protect Colorectal Cancer Intestinal Barrier. Inflammation 2021, 44, 2078–2090. [Google Scholar] [CrossRef]
- Androulidaki, A.; Iliopoulos, D.; Arranz, A.; Doxaki, C.; Schworer, S.; Zacharioudaki, V.; Margioris, A.N.; Tsichlis, P.N.; Tsatsanis, C. The Kinase Akt1 Controls Macrophage Response to Lipopolysaccharide by Regulating MicroRNAs. Immunity 2009, 31, 220–231. [Google Scholar] [CrossRef]
- Cardoso, A.L.; Guedes, J.R.; Pereira de Almeida, L.; Pedroso de Lima, M.C. miR-155 Modulates Microglia-Mediated Immune Response by down-Regulating SOCS-1 and Promoting Cytokine and Nitric Oxide Production. Immunology 2012, 135, 73–88. [Google Scholar] [CrossRef]
- Moore, C.S.; Rao, V.T.S.; Durafourt, B.A.; Bedell, B.J.; Ludwin, S.K.; Bar-Or, A.; Antel, J.P. miR-155 as a Multiple Sclerosis–Relevant Regulator of Myeloid Cell Polarization. Ann. Neurol. 2013, 74, 709–720. [Google Scholar] [CrossRef]
- Slota, J.A.; Booth, S.A. MicroRNAs in Neuroinflammation: Implications in Disease Pathogenesis, Biomarker Discovery and Therapeutic Applications. Noncoding RNA 2019, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Zingale, V.D.; Gugliandolo, A.; Mazzon, E. miR-155: An Important Regulator of Neuroinflammation. Int. J. Mol. Sci. 2021, 23, 90. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.J.; Akhtar, L.N.; Benveniste, E.N. SOCS1 and SOCS3 in the Control of CNS Immunity. Trends Immunol. 2009, 30, 392–400. [Google Scholar] [CrossRef]
- Xiao, X.; Hou, Y.; Yu, W.; Qi, S. Propofol Ameliorates Microglia Activation by Targeting MicroRNA-221/222-IRF2 Axis. J. Immunol. Res. 2021, 2021, 3101146. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.-Y.; Niu, J.-Z. miR-222 Regulates Brain Injury and Inflammation Following Intracerebral Hemorrhage by Targeting ITGB8. Mol. Med. Rep. 2020, 21, 1145–1153. [Google Scholar] [CrossRef]
- Martino, M.T.D.; Arbitrio, M.; Caracciolo, D.; Cordua, A.; Cuomo, O.; Grillone, K.; Riillo, C.; Caridà, G.; Scionti, F.; Labanca, C.; et al. miR-221/222 as Biomarkers and Targets for Therapeutic Intervention on Cancer and Other Diseases: A Systematic Review. Mol. Ther.-Nucleic Acids 2022, 27, 1191–1224. [Google Scholar] [CrossRef]
- Abak, A.; Amini, S.; Sakhinia, E.; Abhari, A. MicroRNA-221: Biogenesis, Function and Signatures in Human Cancers. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 3094–3117. [Google Scholar] [CrossRef]
- Leung, A.; Trac, C.; Jin, W.; Lanting, L.; Akbany, A.; Sætrom, P.; Schones, D.E.; Natarajan, R. Novel Long Noncoding RNAs Are Regulated by Angiotensin II in Vascular Smooth Muscle Cells. Circ. Res. 2013, 113, 266–278. [Google Scholar] [CrossRef]
- Di Leva, G.; Gasparini, P.; Piovan, C.; Ngankeu, A.; Garofalo, M.; Taccioli, C.; Iorio, M.V.; Li, M.; Volinia, S.; Alder, H.; et al. MicroRNA Cluster 221-222 and Estrogen Receptor α Interactions in Breast Cancer. JNCI J. Natl. Cancer Inst. 2010, 102, 706–721. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Sobenin, I.A.; Orekhov, A.N.; Bobryshev, Y.V. Human miR-221/222 in Physiological and Atherosclerotic Vascular Remodeling. Biomed Res. Int. 2015, 2015, 354517. [Google Scholar] [CrossRef]
- Cruz, S.A.; Hari, A.; Qin, Z.; Couture, P.; Huang, H.; Lagace, D.C.; Stewart, A.F.R.; Chen, H.-H. Loss of IRF2BP2 in Microglia Increases Inflammation and Functional Deficits after Focal Ischemic Brain Injury. Front. Cell. Neurosci. 2017, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Thomas, K.; Blanco, J.C.G.; Salkowski, C.A.; Vogel, S.N. The Role of the Interferon Regulatory Factors, IRF-1 and IRF-2, in LPS-Induced Cyclooxygenase-2 (COX-2) Expression in Vivo and in Vitro. J. Endotoxin Res. 2002, 8, 381–390. [Google Scholar] [CrossRef]
- Chen, H.-H.; Keyhanian, K.; Zhou, X.; Vilmundarson, R.O.; Almontashiri, N.A.M.; Cruz, S.A.; Pandey, N.R.; Lerma Yap, N.; Ho, T.; Stewart, C.A.; et al. IRF2BP2 Reduces Macrophage Inflammation and Susceptibility to Atherosclerosis. Circ. Res. 2015, 117, 671–683. [Google Scholar] [CrossRef]
- Peng, X.; Li, C.; Yu, W.; Liu, S.; Cong, Y.; Fan, G.; Qi, S. Propofol Attenuates Hypoxia-Induced Inflammation in BV2 Microglia by Inhibiting Oxidative Stress and NF-κB/Hif-1α Signaling. Biomed Res. Int. 2020, 2020, 8978704. [Google Scholar] [CrossRef] [PubMed]
- van Uden, P.; Kenneth, N.S.; Webster, R.; Müller, H.A.; Mudie, S.; Rocha, S. Evolutionary Conserved Regulation of HIF-1β by NF-κB. PLoS Genet. 2011, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. NF-κB in Immunobiology. Cell Res. 2011, 21, 223–244. [Google Scholar] [CrossRef]
- Perkins, N.D.; Gilmore, T.D. Good Cop, Bad Cop: The Different Faces of NF-κB. Cell Death Differ. 2006, 13, 759–772. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. Shared Principles in NF-κB Signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef]
- Bonizzi, G.; Karin, M. The Two NF-κB Activation Pathways and Their Role in Innate and Adaptive Immunity. Trends Immunol. 2004, 25, 280–288. [Google Scholar] [CrossRef]
- Liu, R.; Liao, X.-Y.; Pan, M.-X.; Tang, J.-C.; Chen, S.-F.; Zhang, Y.; Lu, P.-X.; Lu, L.J.; Zou, Y.-Y.; Qin, X.-P.; et al. Glycine Exhibits Neuroprotective Effects in Ischemic Stroke in Rats through the Inhibition of M1 Microglial Polarization via the NF-κB P65/Hif-1α Signaling Pathway. J. Immunol. 2019, 202, 1704–1714. [Google Scholar] [CrossRef]
- Azoitei, N.; Becher, A.; Steinestel, K.; Rouhi, A.; Diepold, K.; Genze, F.; Simmet, T.; Seufferlein, T. PKM2 Promotes Tumor Angiogenesis by Regulating HIF-1α through NF-κB Activation. Mol. Cancer 2016, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Oxygen Sensing, Homeostasis, and Disease. New Engl. J. Med. 2011, 365, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Walmsley, S.R.; Chilvers, E.R.; Thompson, A.A.; Vaughan, K.; Marriott, H.M.; Parker, L.C.; Shaw, G.; Parmar, S.; Schneider, M.; Sabroe, I.; et al. Prolyl Hydroxylase 3 (PHD3) Is Essential for Hypoxic Regulation of Neutrophilic Inflammation in Humans and Mice. J. Clin. Investig. 2011, 121, 1053–1063. [Google Scholar] [CrossRef]
- Palazon, A.; Goldrath, A.W.; Nizet, V.; Johnson, R.S. HIF Transcription Factors, Inflammation, and Immunity. Immunity 2014, 41, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Bonello, S.; Zähringer, C.; BelAiba, R.S.; Djordjevic, T.; Hess, J.; Michiels, C.; Kietzmann, T.; Görlach, A. Reactive Oxygen Species Activate the HIF-1α Promoter Via a Functional NFκB Site. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 755–761. [Google Scholar] [CrossRef]
- Rius, J.; Guma, M.; Schachtrup, C.; Akassoglou, K.; Zinkernagel, A.S.; Nizet, V.; Johnson, R.S.; Haddad, G.G.; Karin, M. NF-κB Links Innate Immunity to the Hypoxic Response through Transcriptional Regulation of HIF-1α. Nature 2008, 453, 807–811. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Xia, X.; Yang, X.; Zhao, R.; Peer, J.; Wang, H.; Tong, Z.; Gao, F.; Lin, H.; et al. Propofol Reduces Microglia Activation and Neurotoxicity through Inhibition of Extracellular Vesicle Release. J. Neuroimmunol. 2019, 333, 476962. [Google Scholar] [CrossRef]
- Frühbeis, C.; Fröhlich, D.; Kuo, W.P.; Krämer-Albers, E.-M. Extracellular Vesicles as Mediators of Neuron-Glia Communication. Front. Cell Neurosci. 2013, 7, 182. [Google Scholar] [CrossRef]
- Mathivanan, S.; Ji, H.; Simpson, R.J. Exosomes: Extracellular Organelles Important in Intercellular Communication. J. Proteom. 2010, 73, 1907–1920. [Google Scholar] [CrossRef]
- Joshi, P.; Turola, E.; Ruiz, A.; Bergami, A.; Libera, D.D.; Benussi, L.; Giussani, P.; Magnani, G.; Comi, G.; Legname, G.; et al. Microglia Convert Aggregated Amyloid-β into Neurotoxic Forms through the Shedding of Microvesicles. Cell Death Differ. 2014, 21, 582–593. [Google Scholar] [CrossRef]
- Bianco, F.; Pravettoni, E.; Colombo, A.; Schenk, U.; Möller, T.; Matteoli, M.; Verderio, C. Astrocyte-Derived ATP Induces Vesicle Shedding and IL-1β Release from Microglia1. J. Immunol. 2005, 174, 7268–7277. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ye, L.; Lu, H.; Chen, H.; Zhang, Y.; Huang, Y.; Zheng, J.C. TNF-α Promotes Extracellular Vesicle Release in Mouse Astrocytes through Glutaminase. J. Neuroinflammation 2017, 14, 87. [Google Scholar] [CrossRef]
- Votyakova, T.V.; Reynolds, I.J. ΔΨm-Dependent and -Independent Production of Reactive Oxygen Species by Rat Brain Mitochondria. J. Neurochem. 2001, 79, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Chinnery, P.F.; Schon, E.A. Mitochondria. J. Neurol. Neurosurg. Psychiatry 2003, 74, 1188–1199. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Pell, V.R.; James, A.M.; Work, L.M.; Saeb-Parsy, K.; Frezza, C.; Krieg, T.; Murphy, M.P. A Unifying Mechanism for Mitochondrial Superoxide Production during Ischemia-Reperfusion Injury. Cell Metab. 2016, 23, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Eltzschig, H.K.; Eckle, T. Ischemia and Reperfusion—from Mechanism to Translation. Nat. Med. 2011, 17, 1391–1401. [Google Scholar] [CrossRef]
- Suliman, H.B.; Piantadosi, C.A. Mitochondrial Quality Control as a Therapeutic Target. Pharmacol. Rev. 2016, 68, 20–48. [Google Scholar] [CrossRef]
- Yu, W.; Gao, D.; Jin, W.; Liu, S.; Qi, S. Propofol Prevents Oxidative Stress by Decreasing the Ischemic Accumulation of Succinate in Focal Cerebral Ischemia–Reperfusion Injury. Neurochem. Res. 2018, 43, 420–429. [Google Scholar] [CrossRef]
- Kang, J.; Park, E.J.; Jou, I.; Kim, J.-H.; Joe, E. Reactive Oxygen Species Mediate Aβ(25-35)-Induced Activation of BV-2 Microglia. NeuroReport 2001, 12, 1449. [Google Scholar] [CrossRef]
- Korvers, L.; de Andrade Costa, A.; Mersch, M.; Matyash, V.; Kettenmann, H.; Semtner, M. Spontaneous Ca2+ Transients in Mouse Microglia. Cell Calcium 2016, 60, 396–406. [Google Scholar] [CrossRef]
- Sharma, P.; Ping, L. Calcium Ion Influx in Microglial Cells: Physiological and Therapeutic Significance. J. Neurosci. Res. 2014, 92, 409–423. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.; Kann, O.; Ohlemeyer, C.; Hanisch, U.-K.; Kettenmann, H. Elevation of Basal Intracellular Calcium as a Central Element in the Activation of Brain Macrophages (Microglia): Suppression of Receptor-Evoked Calcium Signaling and Control of Release Function. J. Neurosci. 2003, 23, 4410–4419. [Google Scholar] [CrossRef] [PubMed]
- Färber, K.; Kettenmann, H. Functional Role of Calcium Signals for Microglial Function. Glia 2006, 54, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Rostas, J.A.P.; Skelding, K.A. Calcium/Calmodulin-Stimulated Protein Kinase II (CaMKII): Different Functional Outcomes from Activation, Depending on the Cellular Microenvironment. Cells 2023, 12, 401. [Google Scholar] [CrossRef]
- Bell, J.R.; Vila-Petroff, M.; Delbridge, L.M.D. CaMKII-Dependent Responses to Ischemia and Reperfusion Challenges in the Heart. Front. Pharmacol. 2014, 5. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).