Synthesis of Novel 7-Phenyl-2,3-Dihydropyrrolo[2,1-b]Quinazolin-9(1H)-ones as Cholinesterase Inhibitors Targeting Alzheimer’s Disease Through Suzuki–Miyaura Cross-Coupling Reaction
Abstract
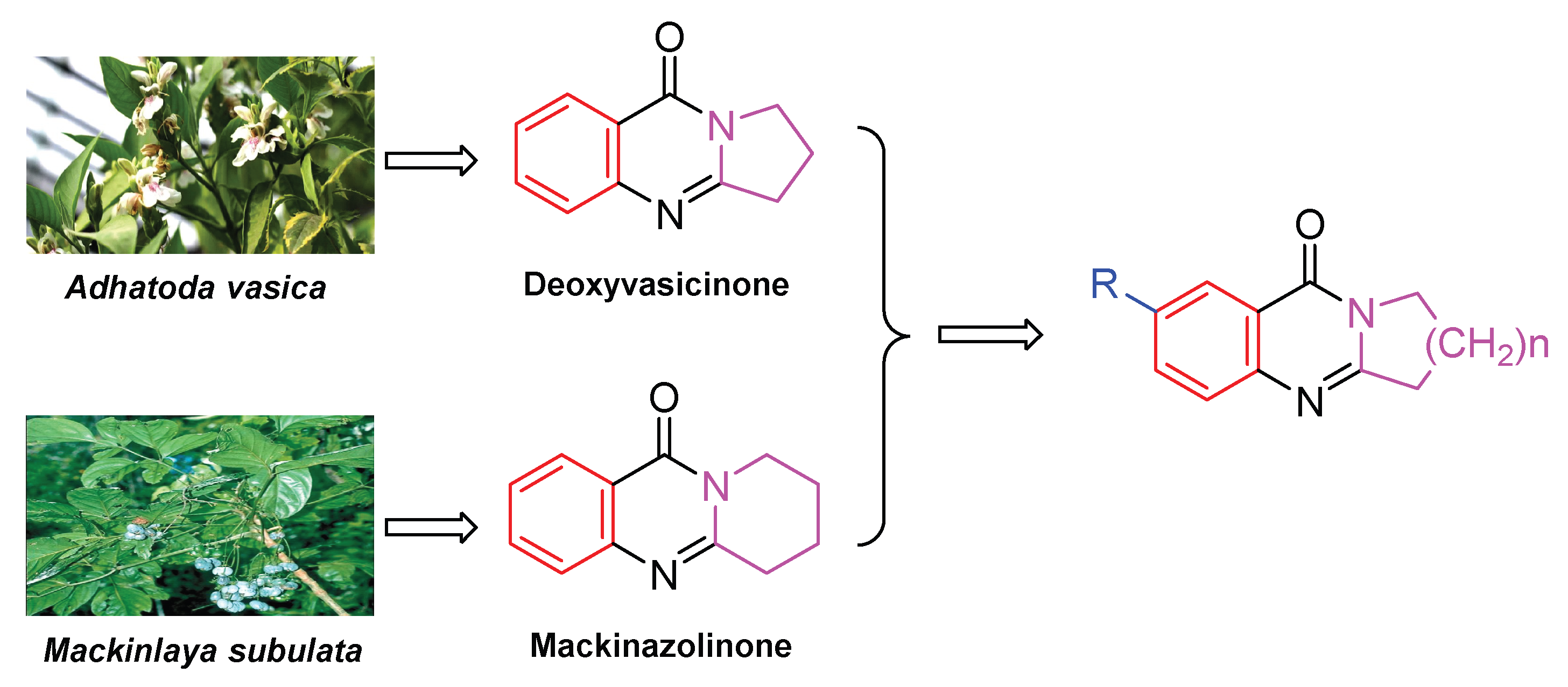
1. Introduction
2. Results and Discussion
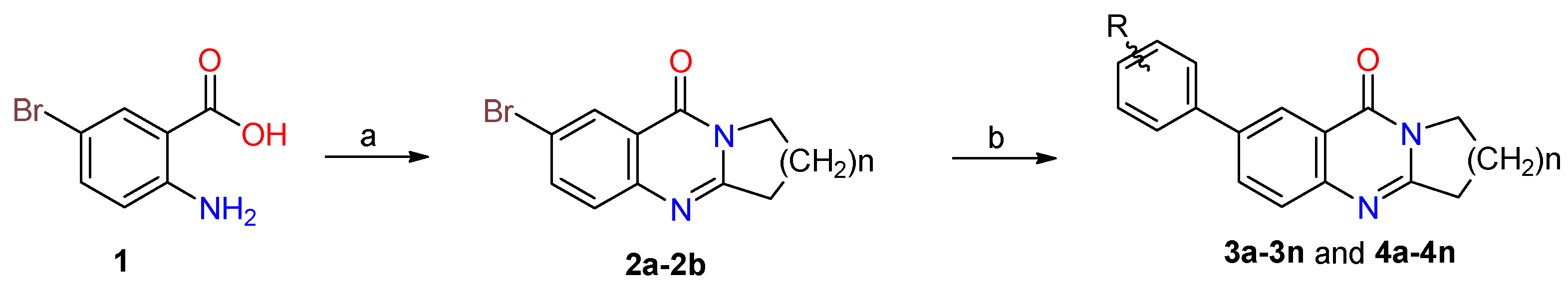
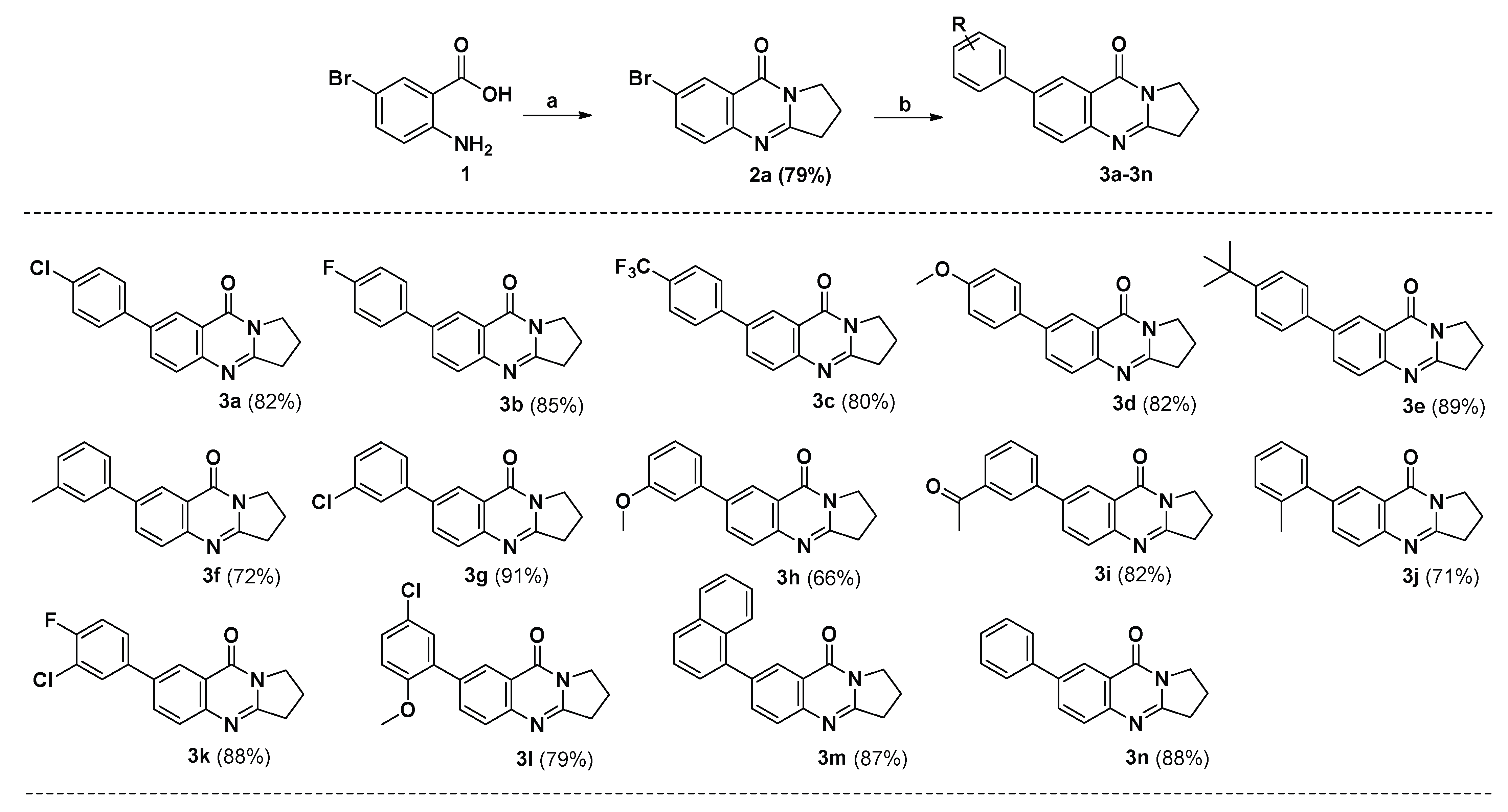
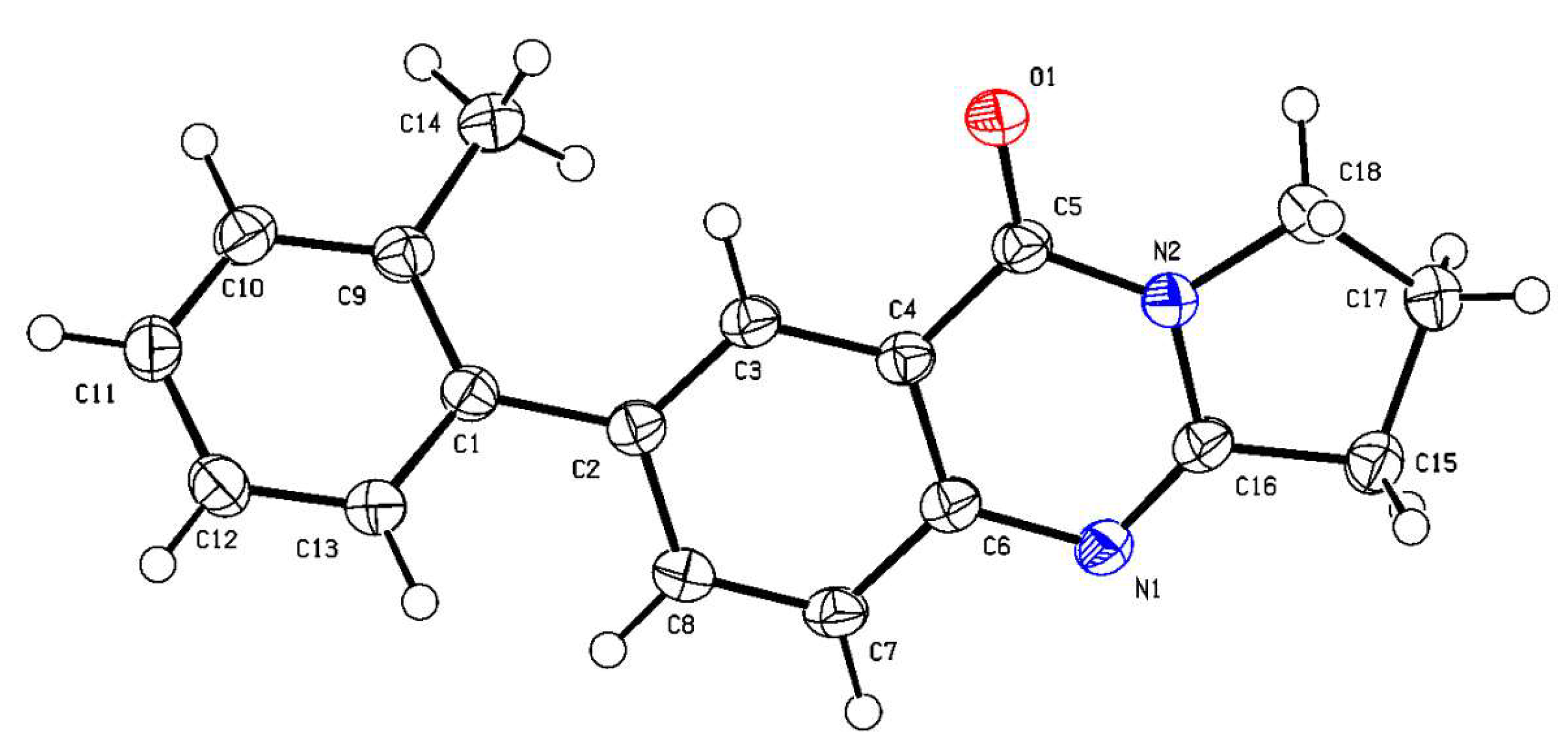
2.1. Synthesis
2.2. Inhibitory Activities of AChE and BChE for 3a–3n and 4a–4n
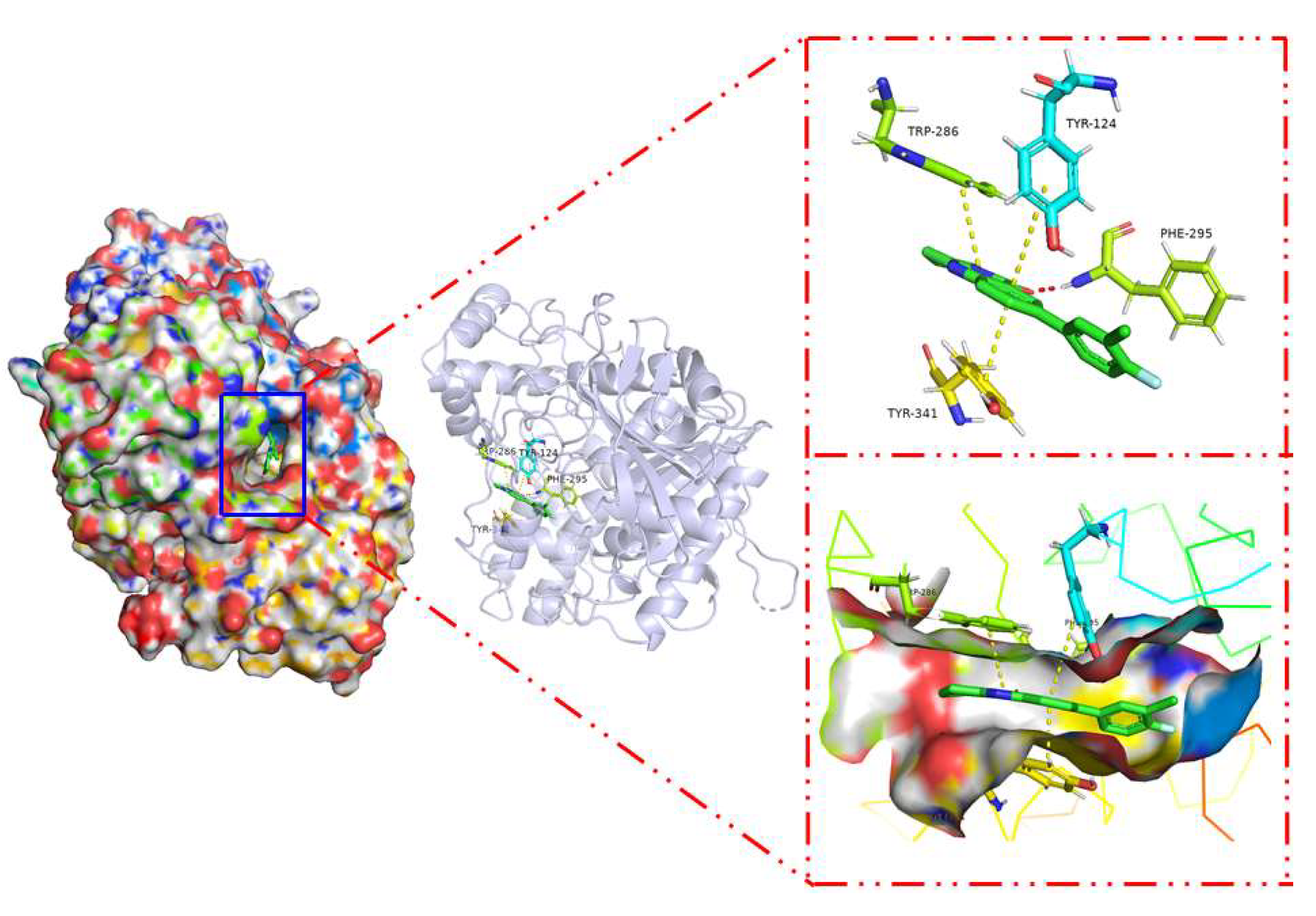
2.3. Docking Study
2.4. Molecular Dynamics
2.4.1. Root Mean Square Deviation (RMSD)
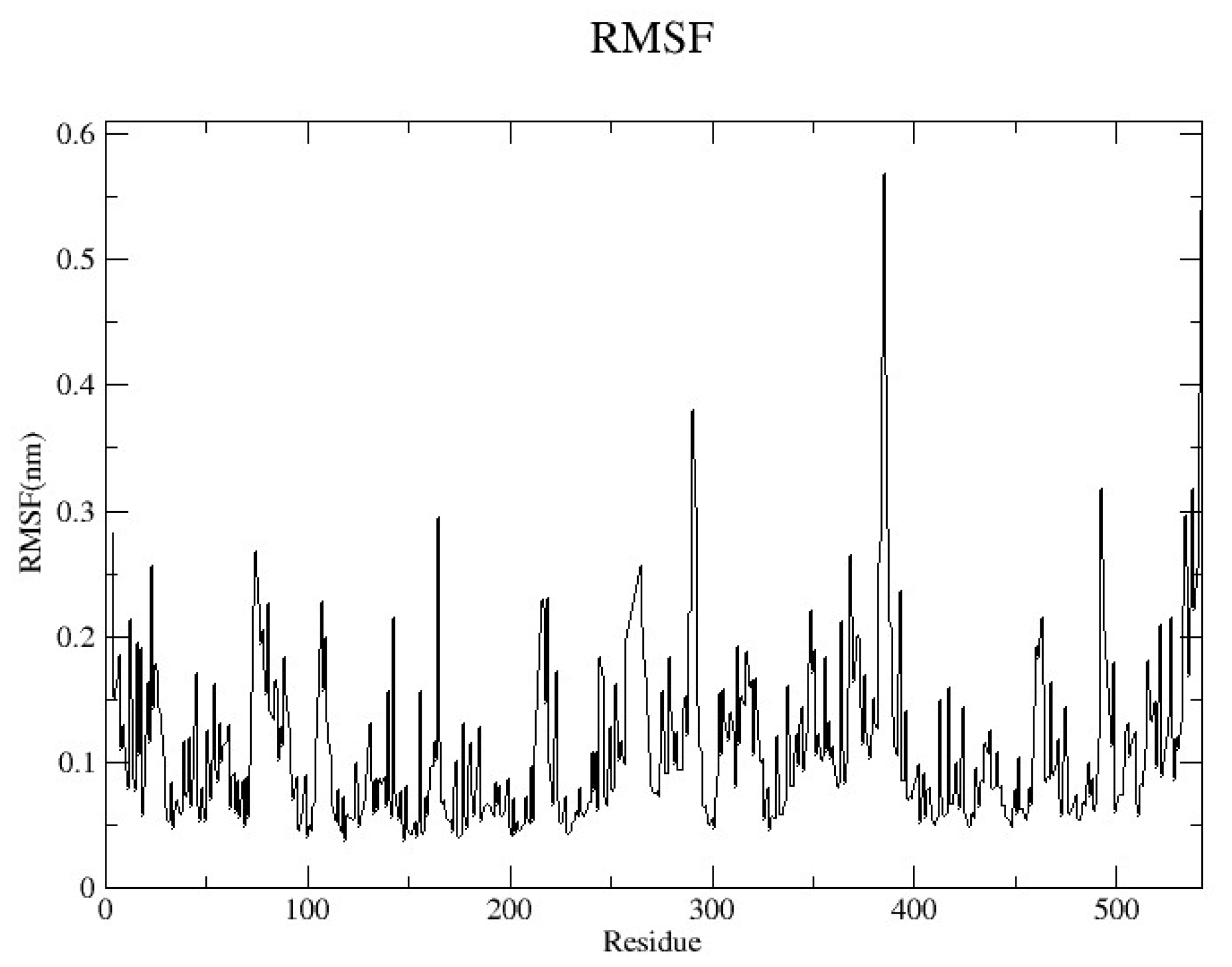
2.4.2. Root Mean Square Fluctuation (RMSF)
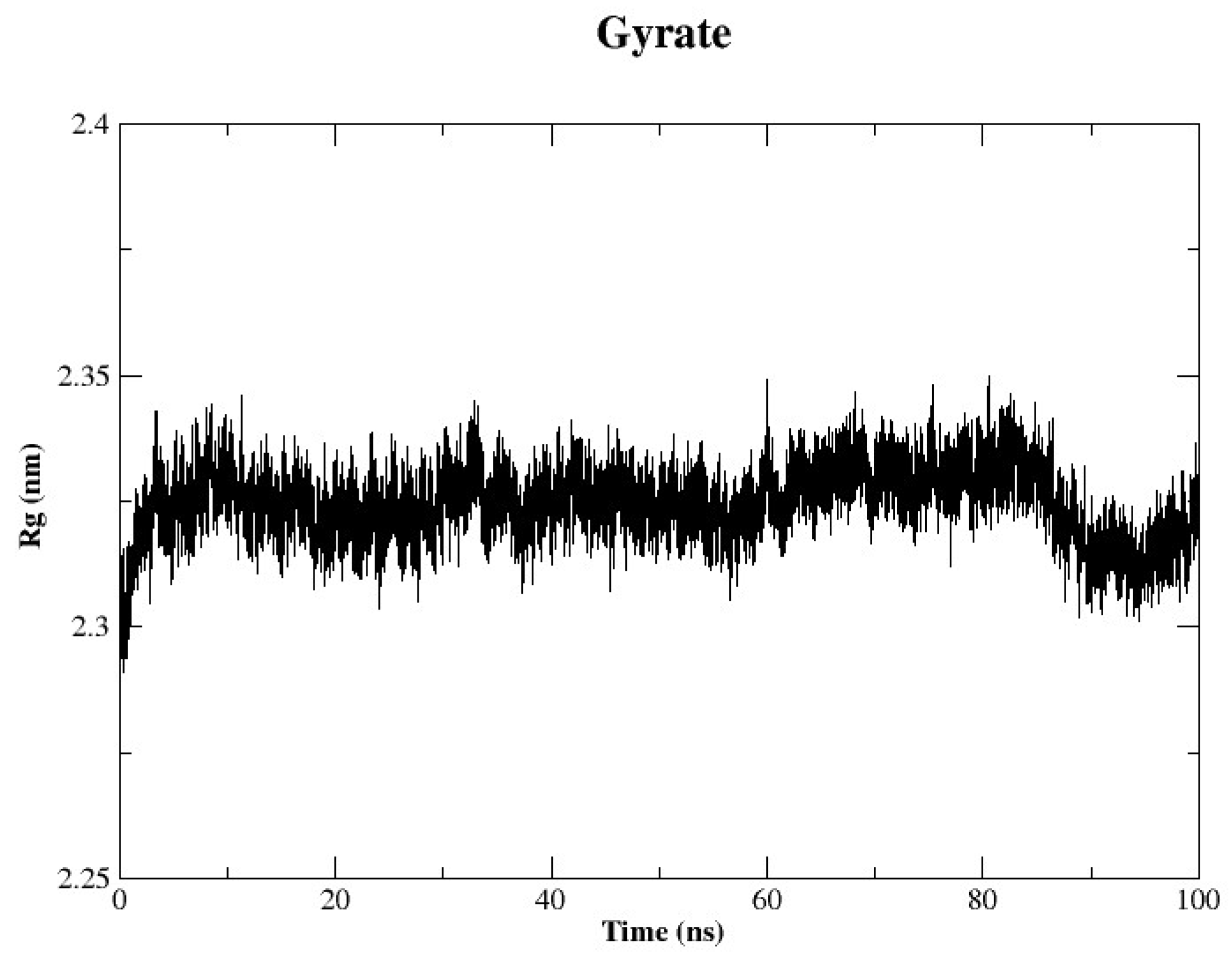
2.4.3. Radius of Gyration (Rg)
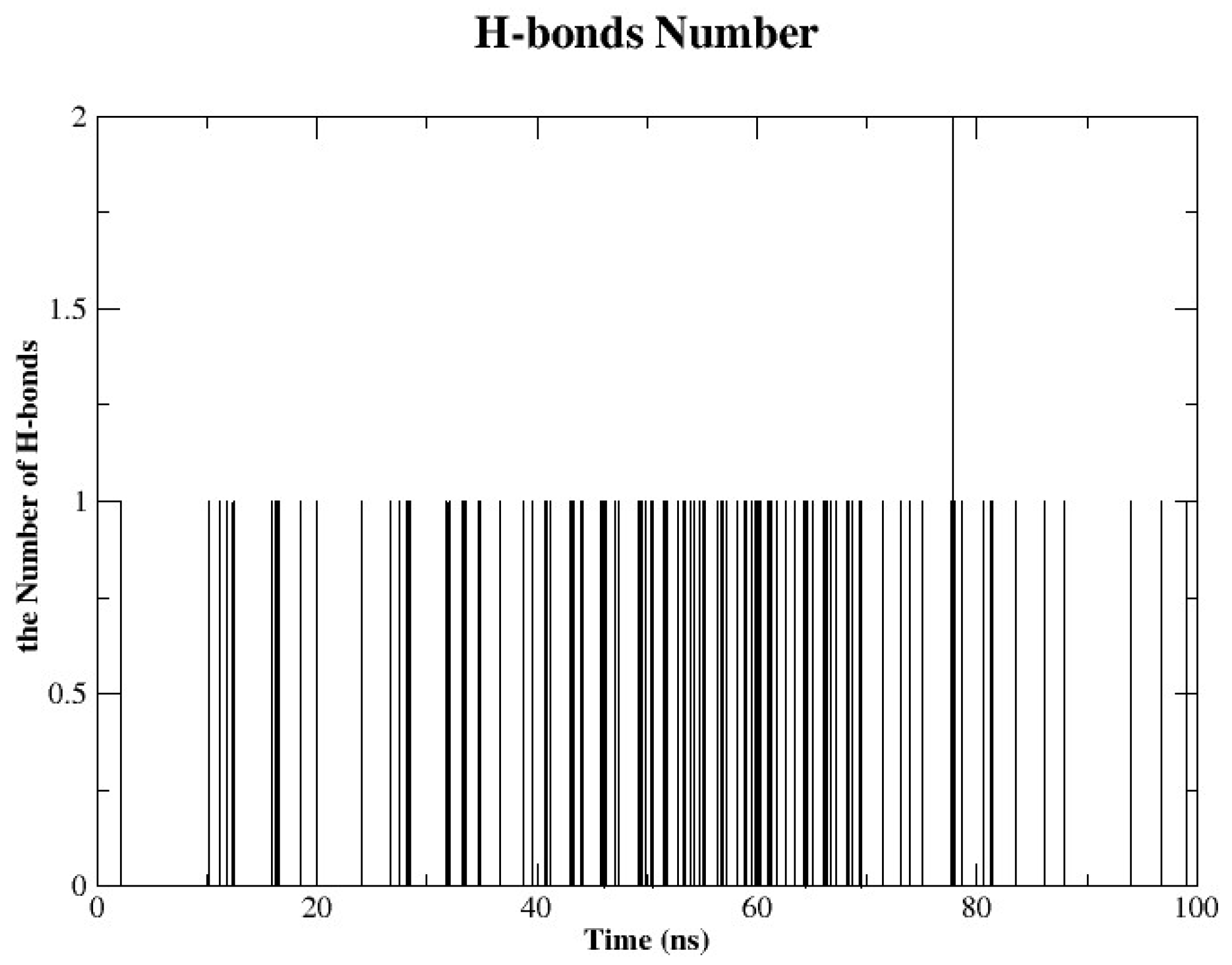
2.4.4. Hydrogen Bonds Analysis
3. Materials and Methods
3.1. Materials
3.2. Experimental Procedures
3.2.1. General Procedure of Preparation of 2a–2b
3.2.2. General Procedure for the Synthesis of Compounds 3a–3n and 4a–4n
3.3. Biological Assay Methods
AChE and BChE Inhibition Assays
3.4. Molecular Docking
Molecular Dynamics Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, Q.; Durga, P.; Wang, F.X.C.; Yao, H.-P.; Wang, M.-H. Pharmaceutical innovation and advanced biotechnology in the biotech-pharmaceutical industry for antibody–drug conjugate development. Drug Discov. Today 2024, 29, 104057. [Google Scholar] [CrossRef] [PubMed]
- Rowinska-Zyrek, M.; Salerno, M.; Kozlowski, H. Neurodegenerative diseases—Understanding their molecular bases and progress in the development of potential treatments. Coord. Chem. Rev. 2015, 284, 298–312. [Google Scholar] [CrossRef]
- Durães, F.; Pinto, M.; Sousa, E. Old Drugs as New Treatments for Neurodegenerative Diseases. Pharmaceuticals 2018, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Lamptey, R.N.L.; Chaulagain, B.; Trivedi, R.; Gothwal, A.; Layek, B.; Singh, J. A Review of the Common Neurodegenerative Disorders: Current Therapeutic Approaches and the Potential Role of Nanotherapeutics. Int. J. Mol. Sci. 2022, 23, 1851. [Google Scholar] [CrossRef]
- El Zowalaty, M.E.; Järhult, J.D. From SARS to COVID-19: A previously unknown SARS- related coronavirus (SARS-CoV-2) of pandemic potential infecting humans—Call for a One Health approach. One Health 2020, 9, 100124. [Google Scholar] [CrossRef]
- Krammer, F. SARS-CoV-2 vaccines in development. Nature 2020, 586, 516–527. [Google Scholar] [CrossRef]
- Uddin, M.S.; Al Mamun, A.; Kabir, M.T.; Jakaria, M.; Mathew, B.; Barreto, G.E.; Ashraf, G.M. Nootropic and Anti-Alzheimer’s Actions of Medicinal Plants: Molecular Insight into Therapeutic Potential to Alleviate Alzheimer’s Neuropathology. Mol. Neurobiol. 2019, 56, 4925–4944. [Google Scholar] [CrossRef]
- Balfour, J.E.; O’Rourke, N. Older Adults with Alzheimer’s Disease, Comorbid Arthritis and Prescription of Psychotropic Medications. Pain Res. Manag. 2003, 8, 105459. [Google Scholar] [CrossRef]
- Bitencourt-Ferreira, G.; Veit-Acosta, M.; de Azevedo, W.F. Hydrogen Bonds in Protein-Ligand Complexes. In Docking Screens for Drug Discovery; de Azevedo, W.F., Jr., Ed.; Springer: New York, NY, USA, 2019; pp. 93–107. [Google Scholar]
- Atri, A. The Alzheimer’s Disease Clinical Spectrum: Diagnosis and Management. Med. Clin. N. Am. 2019, 103, 263–293. [Google Scholar] [CrossRef]
- Prince, M.; Comas-Herrera, A.; Knapp, M.; Guerchet, M.; Karagiannidou, M. Improving Healthcare for People Living with Dementia: Coverage, Quality and Costs Now and in the Future; World Alzheimer Report; Alzheimer’s Disease International: London, UK, 2016. [Google Scholar]
- Ngandu, T.; Mangialasche, F.; Kivipelto, M. Chapter 1—The Epidemiology and Prevention of Alzheimer’s Disease and Projected Burden of Disease. In Global Clinical Trials for Alzheimer’s Disease; Bairu, M., Weiner, M.W., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 3–20. [Google Scholar]
- Tomaskova, H.; Jitka, K.; Richard, C.; Ondrej, D.; Kuca, K. Prediction of population with Alzheimer’s disease in the European Union using a system dynamics model. Neuropsychiatr. Dis. Treat. 2016, 12, 1589–1598. [Google Scholar]
- Talesa, V.N. Acetylcholinesterase in Alzheimer’s disease. Mech. Ageing Dev. 2001, 122, 1961–1969. [Google Scholar] [CrossRef]
- Hardy, J. The amyloid hypothesis for Alzheimer’s disease: A critical reappraisal. J. Neurochem. 2009, 110, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Grundke-Iqbal, I.; Iqbal, K.; Tung, Y.-C.; Quinlan, M.; Wisniewski, H.M.; Binder, L.I. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc. Natl. Acad. Sci. USA 1986, 83, 4913–4917. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kaur, M.; Kukreja, H.; Chugh, R.; Silakari, O.; Singh, D. Acetylcholinesterase inhibitors as Alzheimer therapy: From nerve toxins to neuroprotection. Eur. J. Med. Chem. 2013, 70, 165–188. [Google Scholar] [CrossRef] [PubMed]
- Cacabelos, R.; Torrellas, C.; Teijido, O.; Carril, J.C. Pharmacogenetic considerations in the treatment of Alzheimer’s disease. Pharmacogenomics 2016, 17, 1041–1074. [Google Scholar] [CrossRef]
- Noetzli, M.; Eap, C.B. Pharmacodynamic, pharmacokinetic and pharmacogenetic aspects of drugs used in the treatment of Alzheimer’s disease. Clin. Pharmacokinet. 2013, 52, 225–241. [Google Scholar] [CrossRef]
- Silva, T.; Reis, J.; Teixeira, J.; Borges, F. Alzheimer’s disease, enzyme targets and drug discovery struggles: From natural products to drug prototypes. Ageing Res. Rev. 2014, 15, 116–145. [Google Scholar] [CrossRef]
- Lin, K.; Zhang, X.; Dai, X.; Ma, L.; Bozorov, K.; Guo, H.; Huang, G.; Cao, J. Synthesis and anticancer activity of podophyllotoxin derivatives. Chem. Nat. Compd. 2021, 57, 1010–1018. [Google Scholar] [CrossRef]
- Chen, L.; Obul, M.; Bozorov, K.; Zhao, J. Synthesis and Antiviral Activity of New Derivatives of Rupestonic Acid. Chem. Nat. Compd. 2021, 57, 854–860. [Google Scholar] [CrossRef]
- Aibibula, P.; Aisa, H.A.; Bozorov, K.; Zhao, J. Synthesis and Cytotoxic Activity of a New Derivative of Rupestonic Acid. Chem. Nat. Compd. 2023, 59, 1102–1108. [Google Scholar] [CrossRef]
- Fitzgerald, J.; Johns, S.; Lamberton, J.; Redcliffe, A. 6,7,8,9-Tetrahydropyridoquinazolines, a new class of alkaloids from Mackinlaya species (Araliaceae). Aust. J. Chem. 1966, 19, 151–159. [Google Scholar] [CrossRef]
- Nasrullaev, A.; Bozorov, K.; Bobakulov, K.; Zhao, J.; Nie, L.F.; Turgunov, K.K.; Elmuradov, B.; Aisa, H.A. Synthesis, characterization, and antimicrobial activity of novel hydrazone-bearing tricyclic quinazolines. Res. Chem. Intermed. 2019, 45, 2287–2300. [Google Scholar] [CrossRef]
- Kuryazov, R.S.; Mukhamedov, N.S.; Dushamov, D.A.; Okmanov, R.Y.; Shakhidoyatov, K.M.; Tashkhodzaev, B. Quinazolines. 3*. synthesis of 6-bromo-8-chloro- sulfonylquinazoline- 2,4(1H,3H)-dione and its interaction with nucleophilic reagents. Chem. Heterocycl. Compd. 2010, 46, 585–591. [Google Scholar] [CrossRef]
- Sharma, G.; Laxman, S.; Murthy, Y.; Lakshmi, K.; Babu, G.; Ramji, M. Synthesis of novel deoxyvasicinone analogs and their anti-bacterial studies. Int. J. Ayurveda Pharm. Chem. 2012, 1, 328–333. [Google Scholar]
- Shakhidoyatov, K.M.; Elmuradov, B.Z. Tricyclic quinazoline alkaloids: Isolation, synthesis, chemical modification, and biological activity. Chem. Nat. Compd. 2014, 50, 781–800. [Google Scholar] [CrossRef]
- Zhong, H.-J.; Leung, K.-H.; Lin, S.; Chan, D.S.-H.; Han, Q.-B.; Chan, S.L.-F.; Ma, D.-L.; Leung, C.-H. Discovery of deoxyvasicinone derivatives as inhibitors of NEDD8-activating enzyme. Methods 2015, 71, 71–76. [Google Scholar] [CrossRef]
- Bozorov, K.A.; Mamadalieva, N.; Elmuradov, B.Z.; Triggiani, D.; Egamberdieva, D.; Tiezzi, A.; Aisa, H.; Shakhidoyatov, K.M. Synthesis of Substituted Thieno [2, 3-d] pyrimidin-4-ones and Their Testing for Evaluation of Cytotoxic Activity on Mammalian Cell Models. J. Chem. 2013, 2013, 976715. [Google Scholar] [CrossRef]
- Elmuradov, B.Z.; Bozorov, K.A.; Shakhidoyatov, K.M. Thieno [2, 3-d] pyrimidin-4-ones 1. Condensation of 2,3-dimethyl-and 2, 3-tri-, 2, 3-tetra-, and 2,3-pentamethylene-7,8-dihydro-pyrrolo [1, 2-a] thieno [2, 3-d] pyriminidin-4 (6H)-ones with aromatic aldehydes and furfural. Chem. Heterocycl. Compd. 2011, 46, 1393–1399. [Google Scholar] [CrossRef]
- Liu, F.; Hou, X.; Nie, L.F.; Bozorov, K.; Decker, M.; Huang, G. A Convenient One-pot Synthesis of 2, 3-Disubstituted Thieno [2, 3-d] pyrimidin-4 (3H)-ones from 2H-Thieno [2, 3-d][1, 3] oxazine-2, 4 (1H)-diones, Aromatic Aldehydes and Amines. SynOpen 2018, 2, 0207–0212. [Google Scholar] [CrossRef]
- Ruzi, Z.; Bozorov, K.; Nie, L.; Zhao, J.; Aisa, H.A. Discovery of novel (E)-1-methyl-9-(3-methylbenzylidene)-6,7,8,9-tetrahydropyrazolo [3, 4-d] pyrido [1, 2-a] pyrimidin-4 (1H)-one as DDR2 kinase inhibitor: Synthesis, molecular docking, and anticancer properties. Bioorg. Chem. 2023, 135, 106506. [Google Scholar] [CrossRef]
- Bozorov, K.; Zhao, J.Y.; Aisa, H.A. Recent advances in ipso-nitration reactions. Arkivoc 2016, 2017, 41–66. [Google Scholar] [CrossRef]
- Song, B.; Nie, L.; Bozorov, K.; Kuryazov, R.; Aisa, H.A.; Zhao, J. Parallel synthesis of condensed pyrimidine-thiones and their antitumor activities. Res. Chem. Intermed. 2023, 49, 1327–1348. [Google Scholar] [CrossRef]
- Song, B.; Nie, L.; Bozorov, K.; Kuryazov, R.; Zhao, J.; Aisa, H.A. Design, combinatorial synthesis and cytotoxic activity of 2-substituted furo[2,3-d]pyrimidinone and pyrrolo[2,3-d]pyrimidinone library. Mol. Divers. 2023, 27, 1767. [Google Scholar] [CrossRef] [PubMed]
- Darras, F.H.; Kling, B.; Heilmann, J.; Decker, M. Neuroprotective tri-and tetracyclic BChE inhibitors releasing reversible inhibitors upon carbamate transfer. ACS Med. Chem. Lett. 2012, 3, 914–919. [Google Scholar] [CrossRef]
- Darras, F.H.; Wehle, S.; Huang, G.; Sotriffer, C.A.; Decker, M. Amine substitution of quinazolinones leads to selective nanomolar AChE inhibitors with ‘inverted’binding mode. Bioorganic Med. Chem. 2014, 22, 4867–4881. [Google Scholar] [CrossRef]
- Decker, M.; Krauth, F.; Lehmann, J. Novel tricyclic quinazolinimines and related tetracyclic nitrogen bridgehead compounds as cholinesterase inhibitors with selectivity towards butyrylcholinesterase. Bioorganic Med. Chem. 2006, 14, 1966–1977. [Google Scholar] [CrossRef]
- Ma, F.; Du, H. Novel deoxyvasicinone derivatives as potent multitarget-directed ligands for the treatment of Alzheimer’s disease: Design, synthesis, and biological evaluation. Eur. J. Med. Chem. 2017, 140, 118–127. [Google Scholar] [CrossRef]
- Du, H.; Liu, X.; Xie, J.; Ma, F. Novel Deoxyvasicinone–Donepezil Hybrids as Potential Multitarget Drug Candidates for Alzheimer’s Disease. ACS Chem. Neurosci. 2019, 10, 2397–2407. [Google Scholar] [CrossRef]
- Darras, F.H.; Pockes, S.; Huang, G.; Wehle, S.; Strasser, A.; Wittmann, H.-J.; Nimczick, M.; Sotriffer, C.A.; Decker, M. Synthesis, biological evaluation, and computational studies of Tri-and tetracyclic nitrogen-bridgehead compounds as potent dual-acting AChE inhibitors and h H3 receptor antagonists. ACS Chem. Neurosci. 2014, 5, 225–242. [Google Scholar] [CrossRef]
- Murtazaeva, Z.; Nasrullaev, A.; Buronov, A.; Gaybullaev, S.; Nie, L.; Numonov, S.; Khushnazarov, Z.; Turgunov, D.; Kuryazov, R.; Zhao, J.; et al. Imidazole Hybrids: A Privileged Class of Heterocycles in Medicinal Chemistry with New Insights into Anticancer Activity. Molecules 2025, 30, 2245. [Google Scholar] [CrossRef]
- Zeng, Y.; Nie, L.; Liu, L.; Bozorov, K.; Zhao, J. Design, synthesis, biological evaluation of a new tricyclicthiazolopy-rimidinone derivatives as acetylcholinesterase inhibitors. J. Heterocycl. Chem. 2024, 61, 1542–1553. [Google Scholar] [CrossRef]
- Lu, T.; Nie, L.; Tang, D.; Bozorov, K.; Zhao, J.; Aisa, H.A. Synthesis of tricyclic pyrazolopyrimidine arylidene ester derivatives and their cytotoxic and molecular docking evaluations. J. Heterocycl. Chem. 2024, 61, 651–668. [Google Scholar] [CrossRef]
- Bellina, F.; Carpita, A.; Rossi, R. Palladium Catalysts for the Suzuki Cross-Coupling Reaction: An Overview of Recent Advances. Synthesis 2004, 2004, 2419–2440. [Google Scholar] [CrossRef]
- Pérez Sestelo, J.; Sarandeses, L.A. Advances in Cross-Coupling Reactions. Molecules 2020, 25, 4500. [Google Scholar] [CrossRef] [PubMed]
- Farhang, M.; Akbarzadeh, A.R.; Rabbani, M.; Ghadiri, A.M. A retrospective-prospective review of Suzuki–Miyaura reaction: From cross-coupling reaction to pharmaceutical industry applications. Polyhedron 2022, 227, 116124. [Google Scholar] [CrossRef]
- Blangetti, M.; Rosso, H.; Prandi, C.; Deagostino, A.; Venturello, P. Suzuki-Miyaura Cross-Coupling in Acylation Reactions, Scope and Recent Developments. Molecules 2013, 18, 1188–1213. [Google Scholar] [CrossRef]
- Marshall, C.M.; Federice, J.G.; Bell, C.N.; Cox, P.B.; Njardarson, J.T. An Update on the Nitrogen Heterocycle Compositions and Properties of U.S. FDA-Approved Pharmaceuticals (2013–2023). J. Med. Chem. 2024, 67, 11622–11655. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Li, J.; Liao, Q.; Shao, M.; Zhang, Q.; Zhang, Y.; Cai, T.; Zhu, W.; Xu, Z. Enhanced Bioactivity of Natural Products by Halogenation: A Database Survey and Quantum Chemistry Calculation Study. J. Med. Chem. 2025, 68, 10486–10496. [Google Scholar] [CrossRef]
- Compain, G.; Martin-Mingot, A.; Maresca, A.; Thibaudeau, S.; Supuran, C.T. Superacid synthesis of halogen containing N-substituted-4-aminobenzene sulfonamides: New selective tumor-associated carbonic anhydrase inhibitors. Bioorganic Med. Chem. 2013, 21, 1555–1563. [Google Scholar] [CrossRef]
- Worek, F.; Eyer, P.; Thiermann, H. Determination of acetylcholinesterase activity by the Ellman assay: A versatile tool for in vitro research on medical countermeasures against organophosphate poisoning. Drug Test. Anal. 2012, 4, 282–291. [Google Scholar] [CrossRef]
- de Almeida, R.B.M.; de Almeida Luz, R.L.S.; Leite, F.H.A.; Botura, M.B. A Review on the In Vitro Evaluation of the Anticholinesterase Activity Based on Ellman’s Method. Mini Rev. Med. Chem. 2022, 22, 1803–1813. [Google Scholar] [PubMed]
- Windisch, M.; Hutter-Paier, B.; Jerkovic, L.; Imbimbo, B.; Villetti, G. The protective effect of ganstigmine against amyloid beta25–35 neurotoxicity on chicken cortical neurons is independent from the cholinesterase inhibition. Neurosci. Lett. 2003, 341, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Tang, X.C. Neuroprotective effects of huperzine A: New therapeutic targets for neurodegenerative disease. Trends Pharmacol. Sci. 2006, 27, 619–625. [Google Scholar] [CrossRef]
- Qian, Z.M.; Ke, Y. Huperzine A: Is it an Effective Disease-Modifying Drug for Alzheimer’s Disease? Front. Aging Neurosci. 2014, 6, 216. [Google Scholar] [CrossRef] [PubMed]
- Wagstaff, A.J.; McTavish, D. Tacrine. Drugs Aging 1994, 4, 510–540. [Google Scholar] [CrossRef]
- Mitra, S.; Muni, M.; Shawon, N.J.; Das, R.; Emran, T.B.; Sharma, R.; Chandran, D.; Islam, F.; Hossain, M.J.; Safi, S.Z.; et al. Tacrine Derivatives in Neurological Disorders: Focus on Molecular Mechanisms and Neurotherapeutic Potential. Oxid. Med. Cell. Longev. 2022, 2022, 7252882. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Olson, A.J. Using AutoDock for Ligand-Receptor Docking. Curr. Protoc. Bioinform. 2008, 24, 8.14.1–8.14.40. [Google Scholar] [CrossRef]
- Li, Q.; Xing, S.; Chen, Y.; Liao, Q.; Xiong, B.; He, S.; Lu, W.; Liu, Y.; Yang, H.; Li, Q. Discovery and biological evaluation of a novel highly potent selective butyrylcholinsterase inhibitor. J. Med. Chem. 2020, 63, 10030–10044. [Google Scholar] [CrossRef] [PubMed]
- Harel, M.; Schalk, I.; Ehret-Sabatier, L.; Bouet, F.; Goeldner, M.; Hirth, C.; Axelsen, P.; Silman, I.; Sussman, J. Quaternary ligand binding to aromatic residues in the active-site gorge of acetylcholinesterase. Proc. Natl. Acad. Sci. USA 1993, 90, 9031–9035. [Google Scholar] [CrossRef]
- Gorelov, S.; Titov, A.; Tolicheva, O.; Konevega, A.; Shvetsov, A. Determination of Hydrogen Bonds in GROMACS: A New Implementation to Overcome Memory Limitation. J. Chem. Inf. Model. 2024, 64, 6241–6246. [Google Scholar] [CrossRef]
- Sasidharan, S.; Gosu, V.; Tripathi, T.; Saudagar, P. Molecular Dynamics Simulation to Study Protein Conformation and Ligand Interaction. In Protein Folding Dynamics and Stability: Experimental and Computational Methods; Saudagar, P., Tripathi, T., Eds.; Springer: Singapore, 2023; pp. 107–127. [Google Scholar]
- Shukla, R.; Tripathi, T. Molecular Dynamics Simulation of Protein and Protein–Ligand Complexes. In Computer-Aided Drug Design; Singh, D.B., Ed.; Springer: Singapore, 2020; pp. 133–161. [Google Scholar]
- Martínez, L. Automatic Identification of Mobile and Rigid Substructures in Molecular Dynamics Simulations and Fractional Structural Fluctuation Analysis. PLoS ONE 2015, 10, e0119264. [Google Scholar] [CrossRef] [PubMed]
- Lobanov, M.Y.; Bogatyreva, N.S.; Galzitskaya, O.V. Radius of gyration as an indicator of protein structure compactness. Mol. Biol. 2008, 42, 623–628. [Google Scholar] [CrossRef]
- Panigrahi, S.K. Strong and weak hydrogen bonds in protein-ligand complexes of kinases: A comparative study. Amino Acids 2008, 34, 617–633. [Google Scholar] [CrossRef]
- Klebe, G. Protein–Ligand Interactions as the Basis for Drug Action. In Drug Design: From Structure and Mode-of-Action to Rational Design Concepts; Klebe, G., Ed.; Springer: Berlin/Heidelberg, Germany, 2024; pp. 39–65. [Google Scholar]
- Zeng, Y.; Nie, L.; Liu, L.; Niu, C.; Li, Y.; Bozorov, K.; Zhao, J.; Shen, J.; Aisa, H.A. Design, synthesis, in vitro evaluation of a new pyrrolo[1,2-a]thiazolo[5,4-d]pyrimidinone derivatives as cholinesterase inhibitors against Alzheimer’s disease. J. Heterocycl. Chem. 2022, 59, 1086–1101. [Google Scholar] [CrossRef]



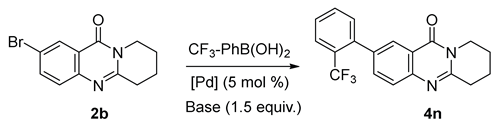 | ||||||
|---|---|---|---|---|---|---|
| No. | Catalyst a | Base b | Solvent | Temperature | Time | Yield (%) |
| 1 | PdCl2(PPh3)2 | K2CO3 | PhCH3 | 110 °C | 8 h | 48 |
| 2 | Pd(AcO)2 | K2CO3 | PhCH3 | 110 °C | 8 h | 45 |
| 3 | Pd2(DBA)3 | K2CO3 | PhCH3 | 110 °C | 8 h | 38 |
| 4 | Pd(PPh3)4 | K2CO3 | PhCH3 | 110 °C | 8 h | 56 |
| 5 | Pd(PPh3)4 | Cs2CO3 | PhCH3 | 110 °C | 8 h | 61 |
| 6 | Pd(PPh3)4 | t-BuOK | PhCH3 | 110 °C | 8 h | 51 |
| 7 | Pd(PPh3)4 | K2CO3 | 1,4-dioxane | 100 °C | 8 h | 26 |
| 8 | Pd(PPh3)4 | Cs2CO3 | 1,4-dioxane | 100 °C | 8 h | 32 |
| 9 | Pd(PPh3)4 | t-BuOK | 1,4-dioxane | 100 °C | 8 h | 24 |
| 10 | Pd(PPh3)4 | Cs2CO3 | PhCH3:H2O:EtOH = 1:1:1 | 90 °C | 8 h | 62 |
| 11 | Pd(PPh3)4 | Cs2CO3 | PhCH3:H2O = 3:1 | 110 °C | 8 h | 96 |
| 12 | Pd(PPh3)4 | Cs2CO3 | PhCH3:H2O = 5:1 | 110 °C | 8 h | 70 |
| No. | IC50 c, μM | Selectivity (BChE/AChE) d | No. | IC50 c, μM | Selectivity (BChE/AChE) d | ||
|---|---|---|---|---|---|---|---|
| AChE a | BChE b | AChE a | BChE b | ||||
| 3a | >50 | >50 | – e | 4a | >50 | >50 | – |
| 3b | 34.76 ± 1.21 | 15.52 ± 0.41 | 0.5 | 4b | 34.59 ± 1.27 | >50 | – |
| 3c | >50 | >50 | – | 4c | >50 | >50 | – |
| 3d | 16.21 ± 0.39 | >50 | – | 4d | 33.24 ± 1.08 | >50 | – |
| 3e | >50 | >50 | – | 4e | >50 | >50 | – |
| 3f | 28.71 ± 0.69 | 22.79 ± 0.49 | 0.8 | 4f | 6.39 ± 0.31 | 10.74 ± 0.64 | 1.7 |
| 3g | 27.92 ± 0.72 | 22.2 ± 0.48 | 0.8 | 4g | 21.28 ± 0.88 | 22.93 ± 0.76 | 1.1 |
| 3h | 17.7 ± 0.84 | 41.84 ± 1.28 | 2.4 | 4h | 42.67 ± 1.80 | 42.3 ± 1.23 | 1 |
| 3i | 33.18 ± 1.01 | >50 | – | 4i | 11.12 ± 0.73 | >50 | – |
| 3j | >50 | 11.06 ± 0.37 | – | 4j | >50 | >50 | – |
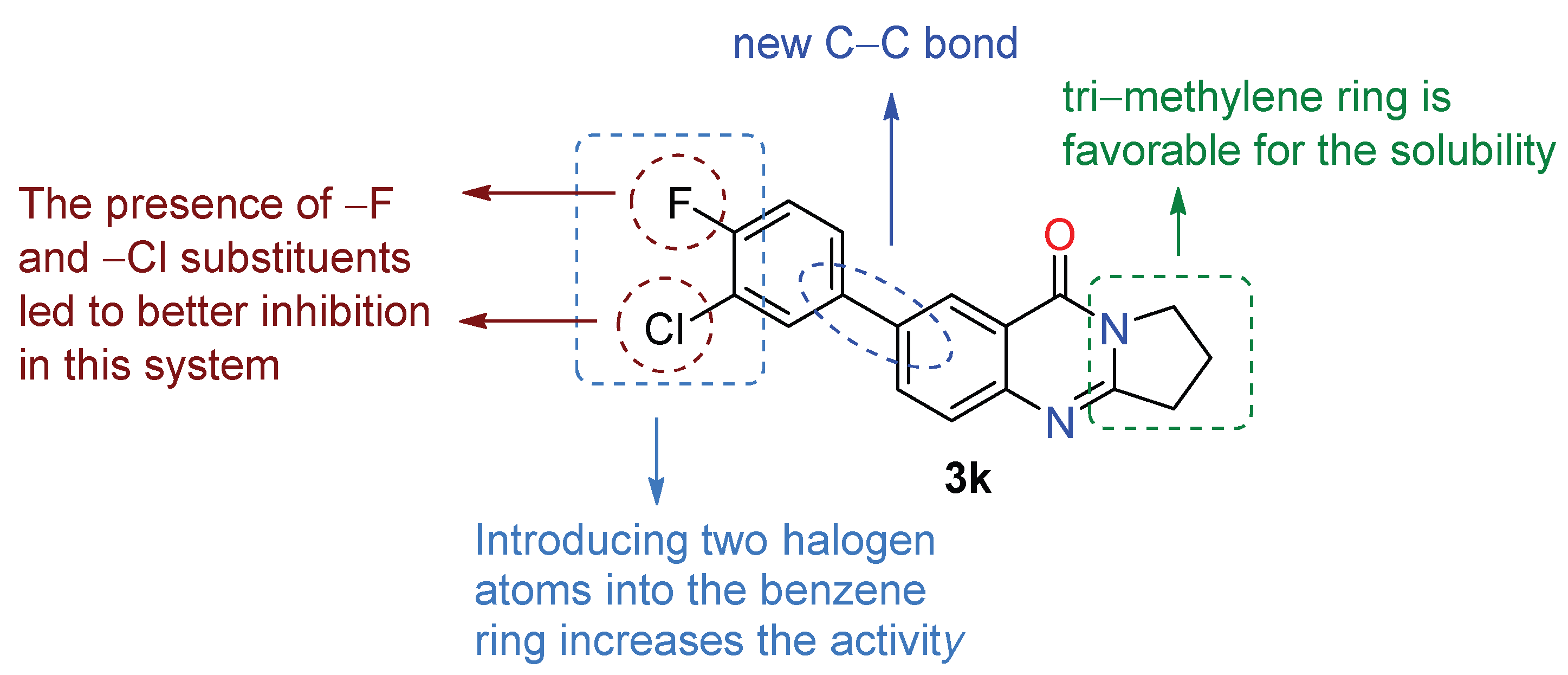
| 3k | 6.084 ± 0.26 | 29.01 ± 0.74 | 4.8 | 4k | >50 | >50 | – |
| 3l | 14.74 ± 0.48 | 28.53 ± 0.46 | 2.0 | 4l | >50 | 46.61 ± 1.45 | – |
| 3m | >50 | 31.98 ± 0.72 | – | 4m | >50 | >50 | – |
| 3n | 35.86 ± 1.03 | 22.94 ± 0.57 | 0.64 | 4n | >50 | 43.19 ± 1.27 | – |
| Huperzine A | 0.25 ± 0.01 | – | – | 0.25 ± 0.01 | – | – | |
| Tacrine | 0.037 ± 0.00 | 5.24 ± 0.02 | – | 0.037 ± 0.00 | 5.24 ± 0.02 | – | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turgunov, D.; Nie, L.; Nasrullaev, A.; Murtazaeva, Z.; Wang, B.; Kholmurodova, D.; Kuryazov, R.; Zhao, J.; Bozorov, K.; Aisa, H.A. Synthesis of Novel 7-Phenyl-2,3-Dihydropyrrolo[2,1-b]Quinazolin-9(1H)-ones as Cholinesterase Inhibitors Targeting Alzheimer’s Disease Through Suzuki–Miyaura Cross-Coupling Reaction. Molecules 2025, 30, 2791. https://doi.org/10.3390/molecules30132791
Turgunov D, Nie L, Nasrullaev A, Murtazaeva Z, Wang B, Kholmurodova D, Kuryazov R, Zhao J, Bozorov K, Aisa HA. Synthesis of Novel 7-Phenyl-2,3-Dihydropyrrolo[2,1-b]Quinazolin-9(1H)-ones as Cholinesterase Inhibitors Targeting Alzheimer’s Disease Through Suzuki–Miyaura Cross-Coupling Reaction. Molecules. 2025; 30(13):2791. https://doi.org/10.3390/molecules30132791
Chicago/Turabian StyleTurgunov, Davron, Lifei Nie, Azizbek Nasrullaev, Zarifa Murtazaeva, Bianlin Wang, Dilafruz Kholmurodova, Rustamkhon Kuryazov, Jiangyu Zhao, Khurshed Bozorov, and Haji Akber Aisa. 2025. "Synthesis of Novel 7-Phenyl-2,3-Dihydropyrrolo[2,1-b]Quinazolin-9(1H)-ones as Cholinesterase Inhibitors Targeting Alzheimer’s Disease Through Suzuki–Miyaura Cross-Coupling Reaction" Molecules 30, no. 13: 2791. https://doi.org/10.3390/molecules30132791
APA StyleTurgunov, D., Nie, L., Nasrullaev, A., Murtazaeva, Z., Wang, B., Kholmurodova, D., Kuryazov, R., Zhao, J., Bozorov, K., & Aisa, H. A. (2025). Synthesis of Novel 7-Phenyl-2,3-Dihydropyrrolo[2,1-b]Quinazolin-9(1H)-ones as Cholinesterase Inhibitors Targeting Alzheimer’s Disease Through Suzuki–Miyaura Cross-Coupling Reaction. Molecules, 30(13), 2791. https://doi.org/10.3390/molecules30132791










