Synthesis and Characterization of 1-Hydroxy-5-Methyltetrazole and Its Energetic Salts
Abstract
1. Introduction
2. Results and Discussion
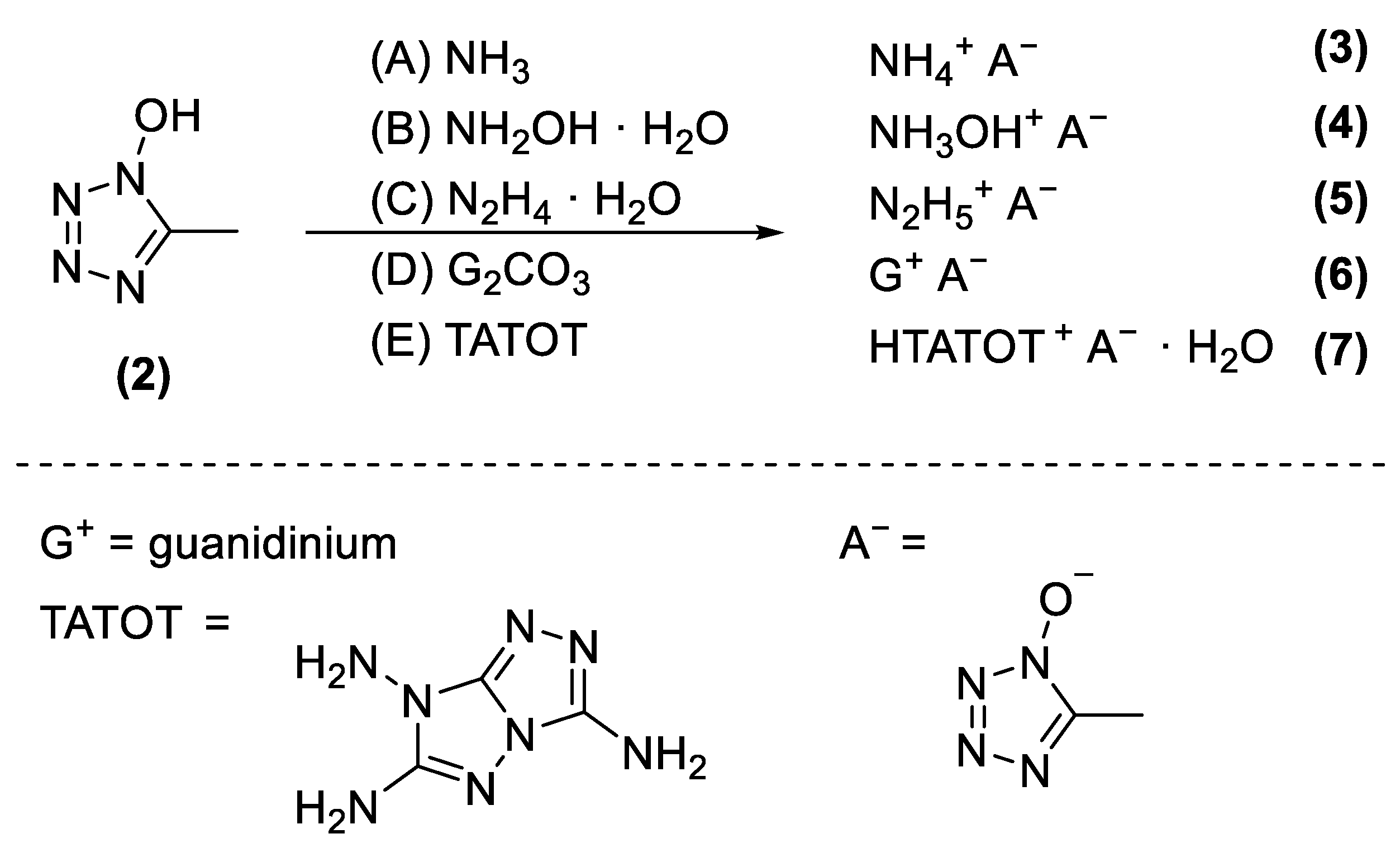
2.1. Synthesis
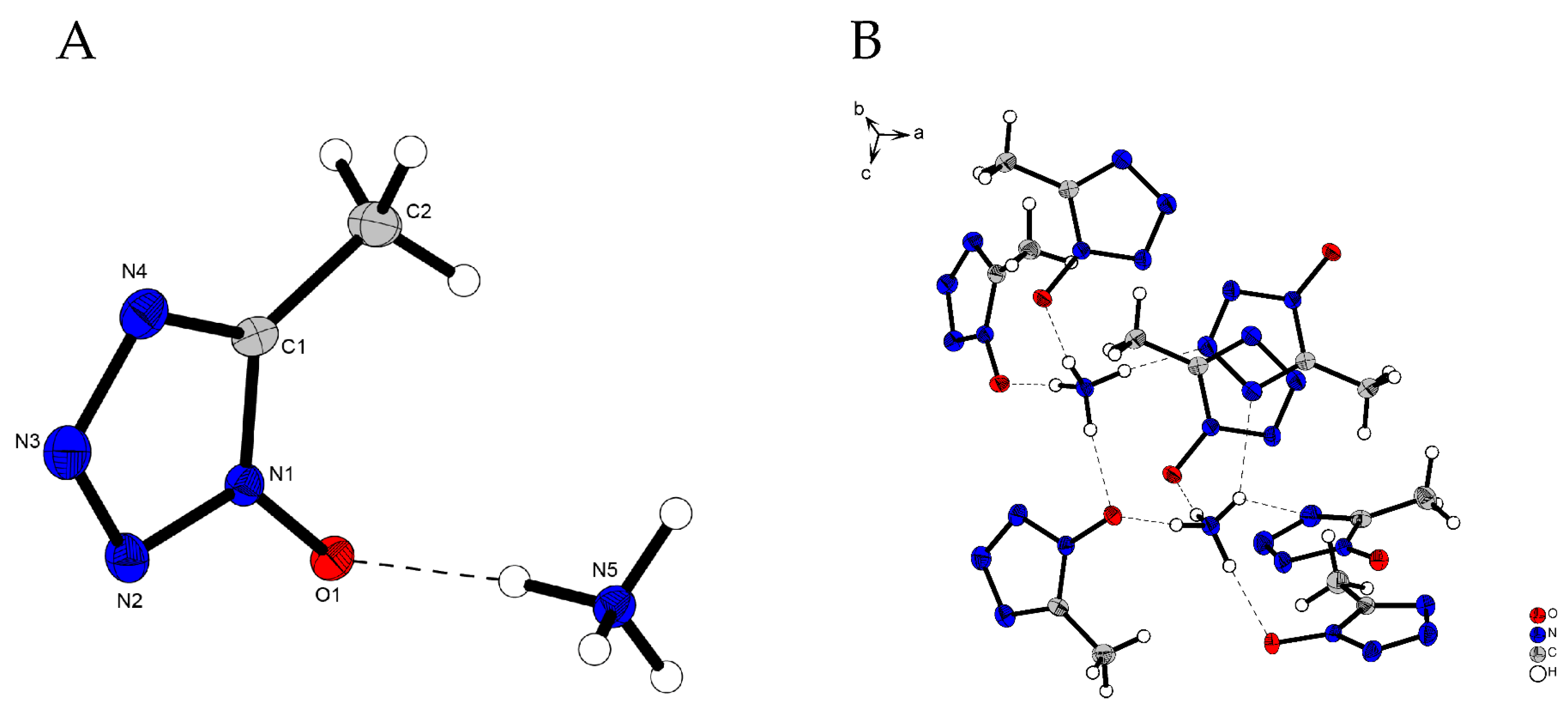
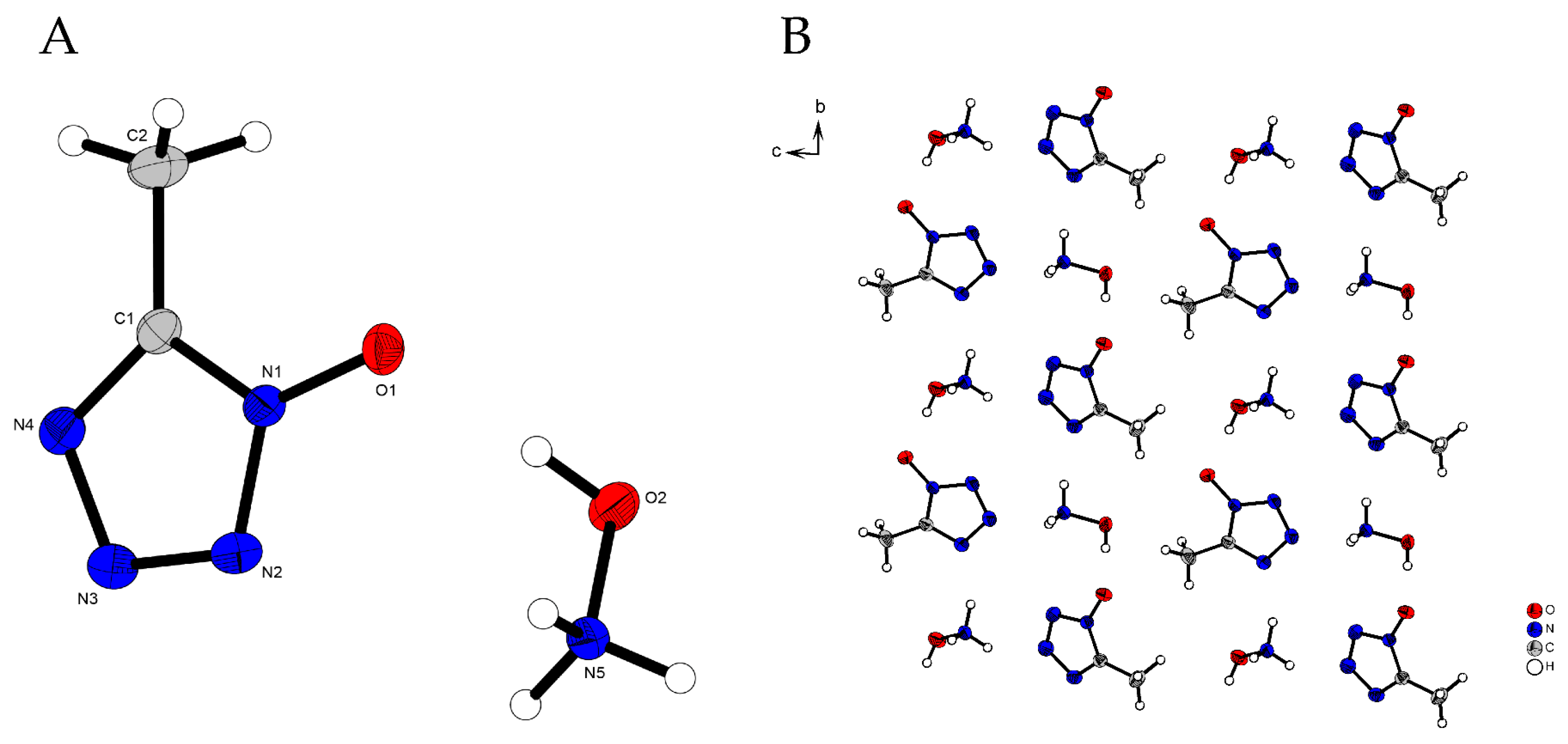
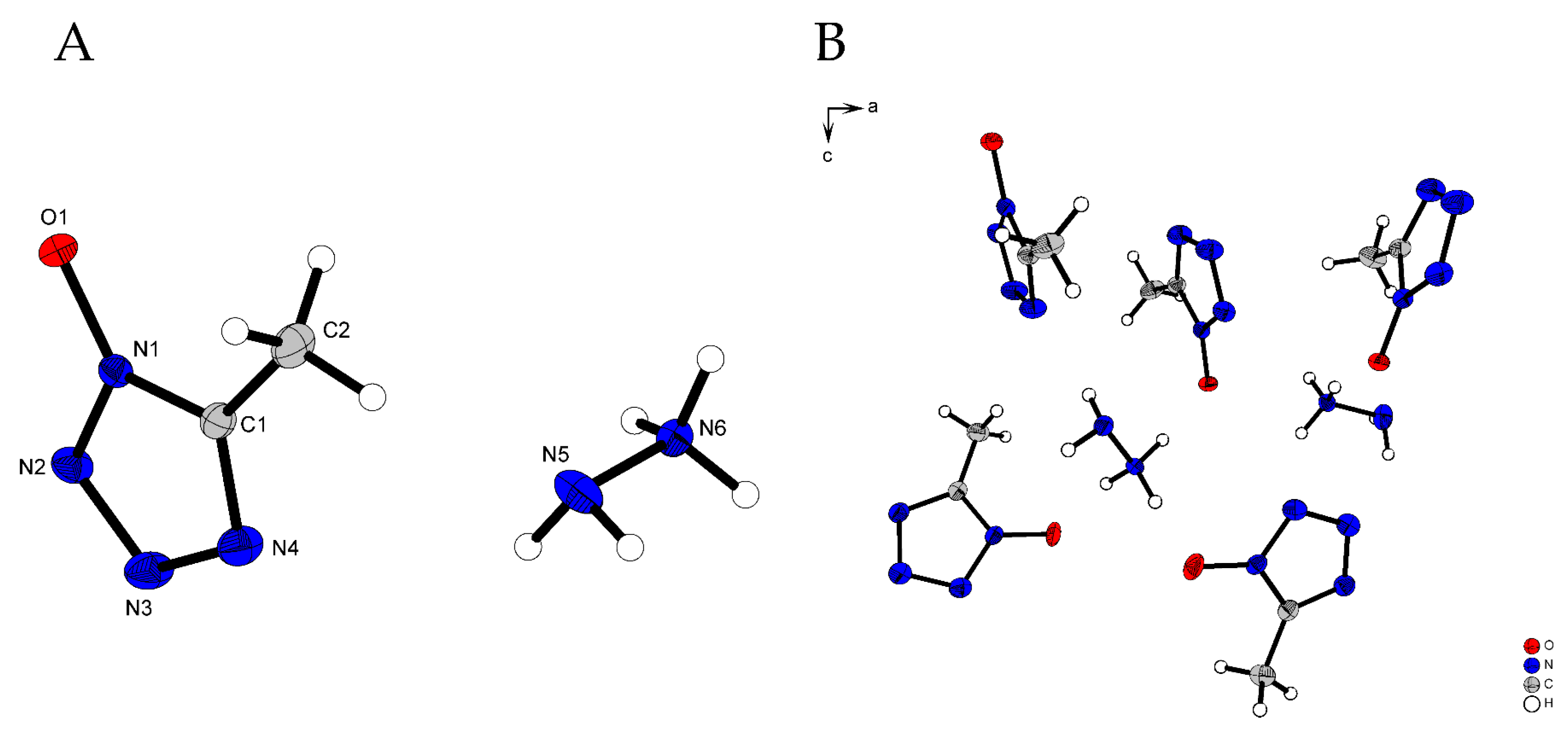
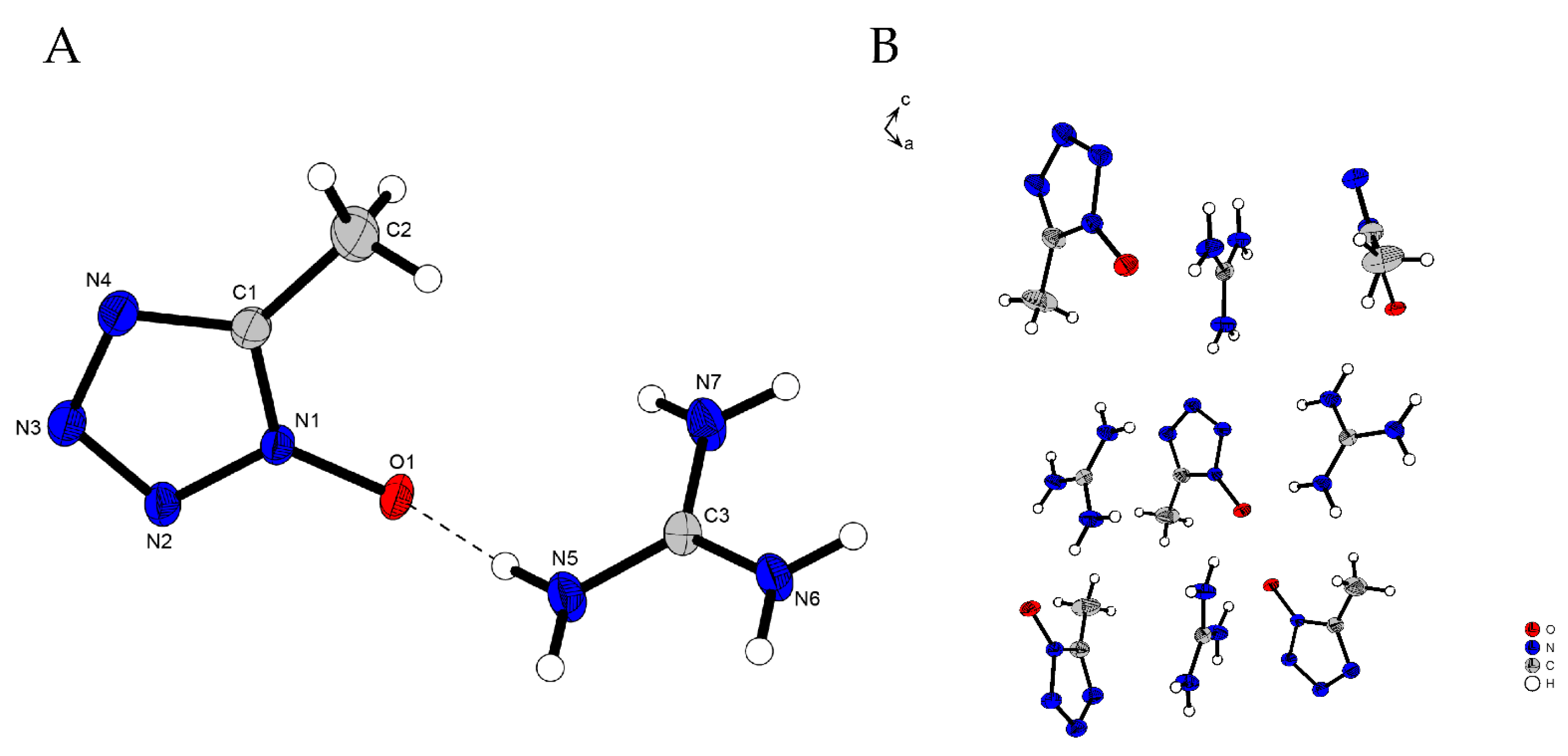
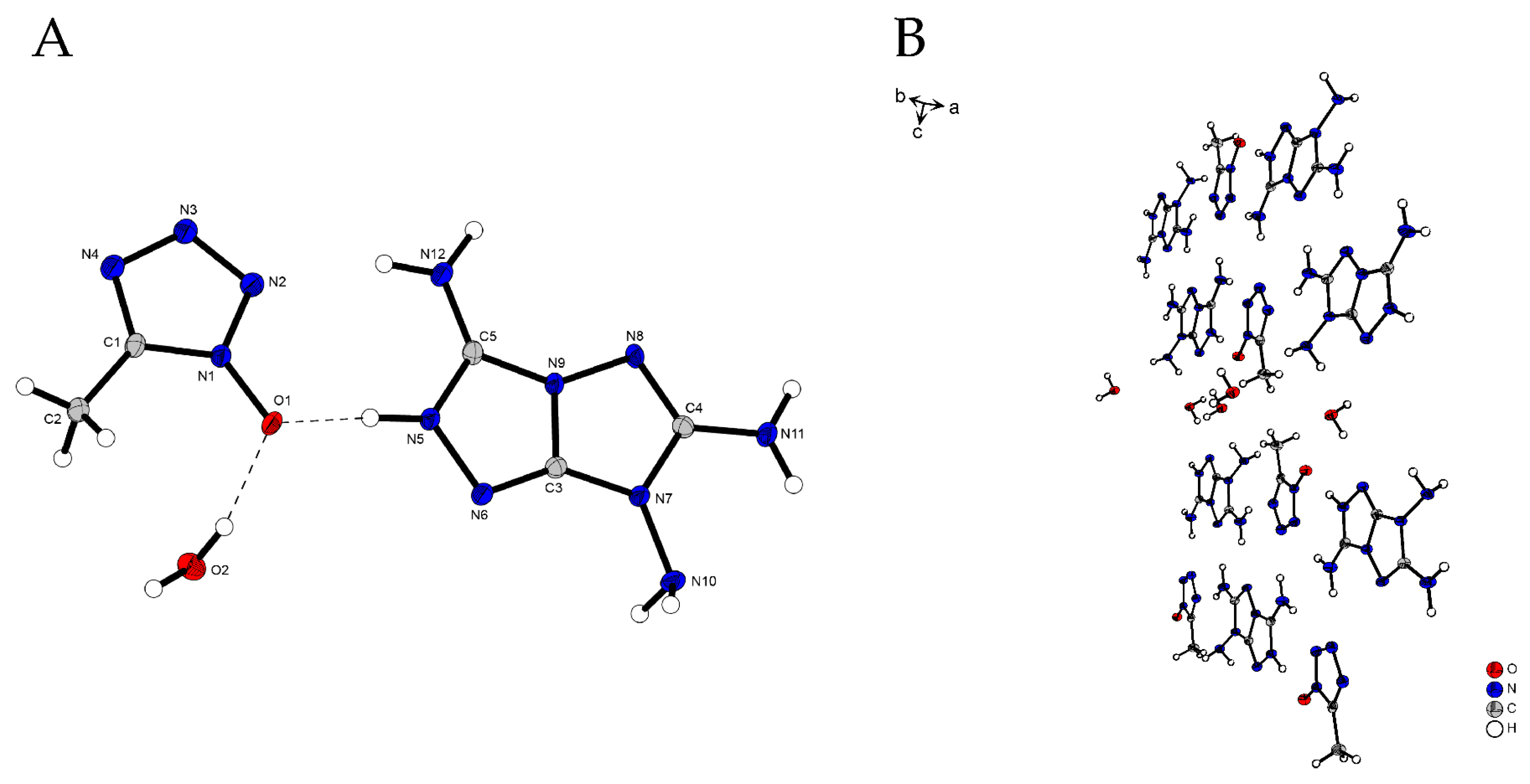
2.2. X-Ray Diffraction
2.3. Physicochemical Properties
2.4. Toxicity
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klapötke, T.M. Chemistry of High-Energy Materials, 6th ed.; De Gruyter: Berlin, Germany; Boston, MA, UAA, 2022. [Google Scholar]
- Benz, M.; Delage, A.; Klapötke, T.M.; Kofen, M.; Stierstorfer, J. A rocky road toward a suitable TNT replacement—A closer look at three promising azoles. Propellants Explos. Pyrotech. 2023, 48, e202300042. [Google Scholar] [CrossRef]
- Conder, J.M.; La Point, T.W.; Steevens, J.A.; Lotufo, G.R. Recommendations for the assessment of TNT toxicity in sediment. Environ. Toxicol. Chem. 2004, 23, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Bradley, P.M.; Chapelle, F.H.; Landmeyer, J.E.; Schumacher, J.G. Microbial Transformation of Nitroaromatics in Surface Soils and Aquifer Materials. Appl. Environ. Microbiol. 1994, 60, 2170–2175. [Google Scholar] [CrossRef]
- Pichtel, J. Distribution and Fate of Military Explosives and Propellants in Soil: A Review. Appl. Environ. Soil Sci. 2012, 1, 617236. [Google Scholar] [CrossRef]
- Bhadra, R.; Wayment, D.G.; Williams, R.K.; Barman, S.N.; Stone, M.B.; Hughes, J.B.; Shanks, J.V. Studies on plant-mediated fate of the explosives RDX and HMX. Chemosphere 2001, 44, 1259–1264. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- Zhang, J.; Yin, P.; Pan, G.; Wang, Z.; Zhang, J.; Mitchell, L.A.; Parrish, D.A.; Shreeve, J.N.M. 5-(4-Azidofurazan-3-yl)-1-hydroxytetrazole and its derivatives: From green primary to secondary explosives. New J. Chem. 2019, 43, 12684–12689. [Google Scholar] [CrossRef]
- Demko, Z.P.; Sharpless, K.B. Preparation of 5-Substituted 1H-Tetrazoles from Nitriles in Water. J. Org. Chem. 2001, 66, 7945–7950. [Google Scholar] [CrossRef]
- Vorona, S.; Artamonova, T.; Zevatskii, Y.; Myznikov, L. An Improved Protocol for the Preparation of 5-Substituted Tetrazoles from Organic Thiocyanates and Nitriles. Synthesis 2014, 46, 781–786. [Google Scholar]
- Babu, A.; Sinha, A. Catalytic Tetrazole Synthesis via [3+2] Cycloaddition of NaN3 to Organonitriles Promoted by Co(II)-complex: Isolation and Characterization of a Co(II)-diazido Intermediate. ACS Omega 2024, 9, 21626–21636. [Google Scholar] [CrossRef]
- Finnegan, W.G.; Henry, R.A.; Lofquist, R. An Improved Synthesis of 5-Substituted Tetrazoles. J. Am. Chem. Soc. 1958, 80, 3908–3911. [Google Scholar] [CrossRef]
- Wang, T.; Xu, L.; Dong, J. FSO2N3-Enabled Synthesis of Tetrazoles from Amidines and Guanidines. Org. Lett. 2023, 25, 6222–6227. [Google Scholar] [CrossRef]
- Stepanov, A.I.; Sannikov, V.S.; Dashko, D.V.; Roslyakov, A.G.; Astrat’ev, A.A.; Stepanova, E.V. A new preparative method and some chemical properties of 4-R-furazan-3-carboxylic acid amidrazones. Chem. Heterocycl. Compd. 2015, 51, 350–360. [Google Scholar] [CrossRef]
- Oberhummer, W. Die Reaktion aliphatischer Iminoäther mit Hydrazin. Monatsh. Chem. 1933, 63, 285–300. [Google Scholar] [CrossRef]
- Zhang, J.-G.; Li, J.-Y.; Zang, Y.; Shu, Y.-J.; Zhang, T.-L.; Yang, L.; Power, P.P. Synthesis and Characterization of a Novel Energetic Complex [Cd(DAT)6](NO3)2 (DAT = 1,5-diamino-tetrazole) with High Nitrogen Content. Z. Anorg. Allg. Chem. 2010, 636, 1147–1151. [Google Scholar] [CrossRef]
- Bauer, L.; Benz, M.; Klapötke, T.M.; Pignot, C.; Stierstorfer, J. Combining the most suitable energetic tetrazole and triazole moieties: Synthesis and characterization of 5-(1-hydroxy-3-nitro-1,2,4-triazol-5-yl)-1-hydroxy-tetrazole and its nitrogen-rich ionic derivatives. Mater. Adv. 2022, 3, 3945–3951. [Google Scholar] [CrossRef]
- Klapötke, T.M.; Kofen, M.; Schmidt, L.; Stierstorfer, J.; Wurzenberger, M.H.H. Selective Synthesis and Characterization of the Highly Energetic Materials 1-Hydroxy-5H-tetrazole (CHN4O), its Anion 1-Oxido-5H-tetrazolate (CN4O−) and Bis(1-hydroxytetrazol-5-yl)triazene. Chem. Asian J. 2021, 16, 3001–3012. [Google Scholar] [CrossRef]
- Klapötke, T.M.; Piercey, D.G.; Stierstorfer, J. The Taming of CN7−: The Azidotetrazolate 2-Oxide Anion. Chem. Eur. J. 2011, 17, 13068–13077. [Google Scholar] [CrossRef]
- Fischer, N.; Fischer, D.; Klapötke, T.M.; Piercey, D.G.; Stierstorfer, J. Pushing the limits of energetic materials—The synthesis and characterization of dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate. J. Mater. Chem. 2012, 22, 20418–20422. [Google Scholar] [CrossRef]
- Begtrup, M.; Vedsø, P. Preparation of N-hydroxyazoles by oxidation of azoles. J. Chem. Soc. Perkin Trans. 1995, 1, 243–247. [Google Scholar] [CrossRef]
- Giles, R.G.; Lewis, N.J.; Oxley, P.W.; Quick, J.K. Facile synthesis of novel 2-hydroxytetrazole derivatives via selective N-2 oxidation. Tetrahedron Lett. 1999, 40, 6093–6094. [Google Scholar] [CrossRef]
- Göbel, M.; Karaghiosoff, K.; Klapötke, T.M.; Piercey, D.G.; Stierstorfer, J. Nitrotetrazolate-2N-oxides and the Strategy of N-Oxide Introduction. J. Am. Chem. Soc. 2010, 132, 17216–17226. [Google Scholar] [CrossRef]
- Plenkiewicz, J. Rearrangement of azidoximes to tetrazole derivatives. Tetrahedron Lett. 1975, 16, 341–342. [Google Scholar] [CrossRef]
- Lal, S.; Staples, R.J.; Shreeve, J.N.M. Design and synthesis of phenylene-bridged isoxazole and tetrazole-1-ol based energetic materials of low sensitivity. Dalton Trans. 2023, 52, 3449–3457. [Google Scholar] [CrossRef]
- Zhao, S.; Ali, A.S.; Kong, X.; Zhang, Y.; Liu, X.; Skidmore, M.A.; Forsyth, C.M.; Savage, G.P.; Wu, D.; Xu, Y.; et al. 1-Benzyloxy-5-phenyltetrazole derivatives highly active against androgen receptor-dependent prostate cancer cells. Eur. J. Med. Chem. 2023, 246, 114982. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, Z.; Hao, W.; Guo, Z.; Wang, Y.; Peng, R.; Jin, B. N2H62+ Energetic Salts: A Promising Strategy for Increasing the Density and Balance Safety and Energy of Explosives. Cryst. Growth Des. 2023, 23, 4499–4505. [Google Scholar] [CrossRef]
- Bettinetti, G.F.; Maffei. The action of hydrazoic acid on nitrolic acids. S. Ann. Chim. 1956, 46, 812–815. [Google Scholar]
- Eberhardt, L.J.; Benz, M.; Stierstorfer, J.; Klapötke, T.M. Synthesis and Characterization of 1-Hydroxy-5-Methyltetrazole and its Energetic Salts. In Proceedings of the 27th Seminar New Trends in Research of Energetic Materials, Pardubice, Czech Republic, 2–4 April 2025. [Google Scholar]
- Mihina, J.S.; Herbst, R.M. The Reaction of Nitriles with Hydrazoic Acid: Synthesis of Monosubstituted Tetrazoles. J. Org. Chem. 1950, 15, 1082–1092. [Google Scholar] [CrossRef]
- Abrishami, F.; Ebrahimikia, M.; Rafiee, F. Synthesis of 5-substituted 1H-tetrazoles using a recyclable heterogeneous nanonickel ferrite catalyst. Appl. Organomet. Chem. 2015, 29, 730–735. [Google Scholar] [CrossRef]
- Sudhakar, K.; Rao, B.P.C.; Kumar, B.P.; Suresh, M.; Ravi, S. Syntheses of 5-Substituted 1H-tetrazoles Catalyzed by Reusable Cu(II)-NaY Zeolite from Nitriles. Asian J. Chem. 2017, 29, 864–866. [Google Scholar] [CrossRef]
- Movaheditabar, P.; Javaherian, M.; Nobakht, V. CuO/aluminosilicate as an efficient heterogeneous nanocatalyst for the synthesis and sequential one-pot functionalization of 5-substituted-1H-tetrazoles. React. Kinet. Mech. Cat. 2017, 122, 217–228. [Google Scholar] [CrossRef]
- Ohno, Y.; Akutsu, Y.; Arai, M.; Tamura, M.; Matsunaga, T. 5-Methyl-1H-tetrazole. Acta Crystallog. C 1999, 55, 1014–1016. [Google Scholar] [CrossRef]
- Zhang, X.-M.; Zhao, Y.-F.; Wu, H.-S.; Batten, S.R.; Ng, S.W. Syntheses and structures of metal tetrazole coordination polymers. Dalton Trans. 2006, 26, 3170–3178. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Sasahara, Y.; Matsumoto, S.; Kumasaki, M. Synthesis, crystallographic characterization, and thermal analyses of five 5-methyl-1H-tetrazole salts. Sci. Technol. Energ. Ma. 2024, 85, 53–59. [Google Scholar]
- Reichel & Partner GmbH. Available online: https://www.rp-mespro.de/de (accessed on 20 June 2025).
- United Nations. Test Methods According to the UN Recommendations on the Transport of Dangerous Goods, Manual of Test and Criteria, 4th ed.; United Nations Publication: New York, NY, USA; Geneva, Switzerland, 2003. [Google Scholar]
- Shen, C.; Xu, Y.-G.; Lu, M. A series of high-energy coordination polymers with 3,6-bis(4-nitroamino-1,2,5-oxadiazol-3-yl)-1,4,2,5-dioxadiazine, a ligand with multi-coordination sites, high oxygen content and detonation performance: Syntheses, structures, and performance. J. Mater. Chem. A 2017, 5, 18854–18861. [Google Scholar] [CrossRef]
- Banerjee, P.; Kemmler, E.; Dunkel, M.; Preissner, R. ProTox 3.0: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2024, 52, 513–520. [Google Scholar] [CrossRef]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018, 46, 257–263. [Google Scholar] [CrossRef]
- ProTox 3.0. Available online: https://tox.charite.de/protox3/ (accessed on 26 June 2025).
- CrysAlisPro Software; Version 171.33.41; Rigaku Corporation: Wroclaw, Poland, 2009; Available online: http://www.rigaku.com (accessed on 20 June 2025).
- Sheldrick, G.M. SHELXT Integrated space-group and structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- SCALE3 ABSPACK—An Oxford Diffraction Program; Version 1.0.4; Rigaku Corporation: Wroclaw, Poland, 2009.
- APEX3; Version 2018.7-2; Bruker AXS Inc.: Madison, WI, USA, 2018.
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Revision A.04; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Ochterski, J.W.; Petersson, G.A.; Montgomery, J.A., Jr. A complete basis set model chemistry. V. Extensions to six or more heavy atoms. J. Chem. Phys. 1996, 104, 2598–2619. [Google Scholar] [CrossRef]
- Montgomery, J.A.; Frisch, M.J.; Ochterski, J.W.; Petersson, G.A. A complete basis set model chemistry. VII. Use of the minimum population localization method. J. Chem. Phys. 2000, 12, 6532–6542. [Google Scholar]
- Curtiss, L.A.; Raghavachari, K.; Redfern, P.C.; Pople, J.A. Assessment of Gaussian-2 and density functional theories for the computation of enthalpies of formation. J. Chem. Phys. 1997, 106, 1063–1079. [Google Scholar] [CrossRef]
- Byrd, E.F.C.; Rice, B.M. Improved Prediction of Heats of Formation of Energetic Materials Using Quantum Mechanical Calculations. Phys. Chem. 2006, 110, 1005–1013. [Google Scholar] [CrossRef]
- Rice, B.M.; Pai, S.V.; Hare, J. Predicting heats of formation of energetic materials using quantum mechanical calculations. Comb. Flame 1999, 118, 445–458. [Google Scholar] [CrossRef]
- Lindstrom, P.J.; Mallard, W.G. NIST Standard Reference Database Number 69. Available online: http://webbook.nist.gov/chemistry/ (accessed on 26 June 2020).
- Westwell, M.S.; Searle, M.S.; Wales, D.J.; Williams, D.H.; Trouton, F. Empirical Correlations between Thermodynamic Properties and Intermolecular Forces. J. Am. Chem. Soc. 1995, 117, 5013–5015. [Google Scholar] [CrossRef]
- Trouton, F. IV. On molecular latent heat. Philos. Mag. 1884, 18, 54–57. [Google Scholar] [CrossRef]
- Jenkins, H.D.B.; Roobottom, H.K.; Passmore, J.; Glasser, L. Relationships among Ionic Lattice Energies, Molecular (Formula Unit) Volumes, and Thermochemical Radii. Inorg. Chem. 1999, 38, 3609–3620. [Google Scholar] [CrossRef]
- Jenkins, H.D.B.; Tudela, D.; Glasser, L. Lattice potential energy estimation for complex ionic salts from density measurements. Inorg. Chem. 2002, 41, 2364–2367. [Google Scholar] [CrossRef]
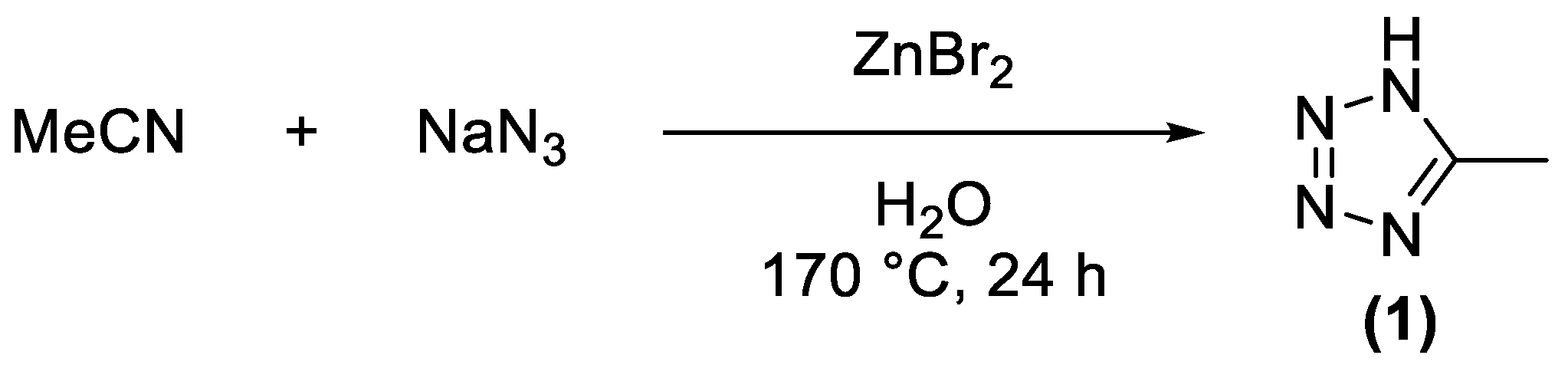
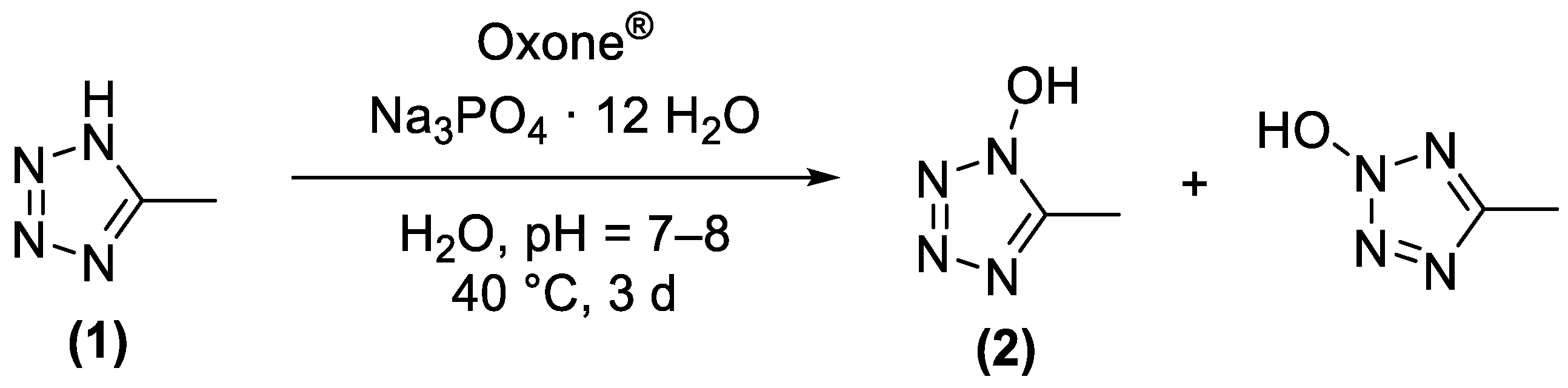

| 2 | 3 | 4 | 5 | 6 | TNT | |
|---|---|---|---|---|---|---|
| Formula | C2H4N4O | C2H7N5O | C2H7N5O2 | C2H8N6O | C3H9N7O | C7H5N3O6 |
| M [g mol−1] | 100.08 | 117.11 | 133.11 | 132.13 | 159.15 | 227.13 |
| IS [J] (a) | 3 | >40 | >40 | >40 | >40 | 15 |
| FS [N] (b) | 160 | 360 | 288 | 360 | >360 | >360 |
| ρ (298 K) [g cm−3] (c) | 1.465 | 1.460 | 1.461 | 1.404 | 1.367 | 1.65 |
| N [%] (d) | 55.9 | 59.8 | 52.6 | 63.6 | 61.6 | 18.5 |
| Ω [%] (e) | −48.0 | −61.5 | −42.1 | −60.6 | −65.4 | −24.7 |
| Tendo [°C] (f) | 146 | 166 | 139 | 105 | 68, 182 | 80 |
| Tdec [°C] (g) | 194 | 229 | 141 | 224 | 256 | 290 |
| ΔfH° [kJ mol−1] (h) | 230.5 | 177.5 | 245.0 | 335.6 | 164.4 | −59 |
| Explo5 V7.01.01 | ||||||
| −ΔEx U0 [kJ kg−1] (i) | 4483 | 3966 | 5393 | 4823 | 3008 | 4363 |
| Tdet [K] (j) | 2966 | 2459 | 3217 | 2817 | 2098 | 3165 |
| V0 [L kg−1] (k) | 776 | 859 | 867 | 884 | 593 | 593 |
| PCJ [kbar] (l) | 186 | 212 | 230 | 219 | 156 | 186 |
| Vdet [m s−1] (m) | 7343 | 7982 | 8065 | 8109 | 7090 | 6817 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eberhardt, L.J.; Benz, M.; Stierstorfer, J.; Klapötke, T.M. Synthesis and Characterization of 1-Hydroxy-5-Methyltetrazole and Its Energetic Salts. Molecules 2025, 30, 2766. https://doi.org/10.3390/molecules30132766
Eberhardt LJ, Benz M, Stierstorfer J, Klapötke TM. Synthesis and Characterization of 1-Hydroxy-5-Methyltetrazole and Its Energetic Salts. Molecules. 2025; 30(13):2766. https://doi.org/10.3390/molecules30132766
Chicago/Turabian StyleEberhardt, Lukas J., Maximilian Benz, Jörg Stierstorfer, and Thomas M. Klapötke. 2025. "Synthesis and Characterization of 1-Hydroxy-5-Methyltetrazole and Its Energetic Salts" Molecules 30, no. 13: 2766. https://doi.org/10.3390/molecules30132766
APA StyleEberhardt, L. J., Benz, M., Stierstorfer, J., & Klapötke, T. M. (2025). Synthesis and Characterization of 1-Hydroxy-5-Methyltetrazole and Its Energetic Salts. Molecules, 30(13), 2766. https://doi.org/10.3390/molecules30132766







